Spatiotemporal Characteristics of Bacterial Communities in Estuarine Mangrove Sediments in Zhejiang Province, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sample Collection
2.2. Determination of Environmental Parameters
2.3. DNA Extraction, PCR Amplification, and Sequencing
2.4. Data Analysis
3. Results
3.1. Environmental Characteristics of Ao River Estuary Mangrove Sediments
3.2. Bacterial Diversity of Ao River Estuary Mangrove Sediments
3.3. Bacterial Community Characteristics by Season and Plants’ Age
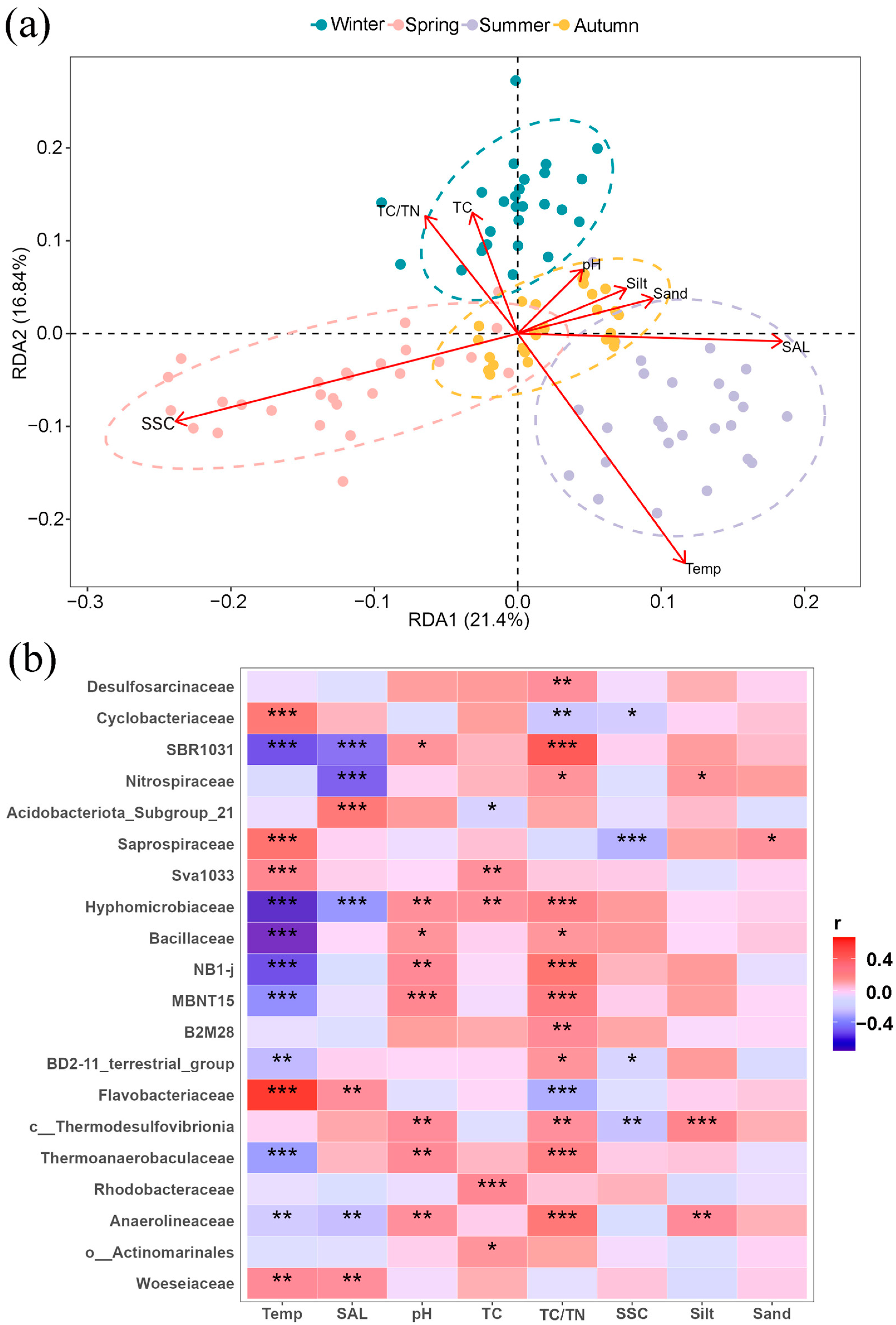
3.4. Impacts of Environmental Parameters on Bacterial Community and Functional Prediction Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, X.; Dai, Z.; Yuan, R.; Guo, Z.; Xi, H.; He, Z.; Wei, M. Effects of Salinity on Assembly Characteristics and Function of Microbial Communities in the Phyllosphere and Rhizosphere of Salt-Tolerant Avicennia marina Mangrove Species. Microbiol. Spectr. 2023, 11, e0300022. [Google Scholar] [CrossRef] [PubMed]
- Sandilyan, S.; Kathiresan, K. Mangrove conservation: A global perspective. Biodivers. Conserv. 2012, 21, 3523–3542. [Google Scholar] [CrossRef]
- Tang, J.; Ye, S.; Chen, X.; Yang, H.; Sun, X.; Wang, F.; Wen, Q.; Chen, S. Coastal blue carbon: Concept, study method, and the application to ecological restoration. Sci. China Earth Sci. 2018, 61, 637–646. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Gu, J.-D. Ecological responses, adaptation and mechanisms of mangrove wetland ecosystem to global climate change and anthropogenic activities. Int. Biodeterior. Biodegrad. 2021, 162, 105248. [Google Scholar] [CrossRef]
- Holguin, G.; Vazquez, P.; Bashan, Y. The role of sediment microorganisms in the productivity, conservation, and rehabilitation of mangrove ecosystems: An overview. Biol. Fertil. Soils 2001, 33, 265–278. [Google Scholar] [CrossRef]
- Saleem, M.; Hu, J.; Jousset, A. More Than the Sum of Its Parts: Microbiome Biodiversity as a Driver of Plant Growth and Soil Health. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 145–168. [Google Scholar] [CrossRef]
- Xing, Y.; Qiu, J.; Chen, J.; Cheng, D.; Yin, Q.; Chen, X.; Xu, L.; Zheng, P. Unveiling hidden interactions: Microorganisms, enzymes, and mangroves at different stages of succession in the Shankou Mangrove Nature Reserve, China. Sci. Total Environ. 2024, 923, 171340. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Yu, X.; Hu, R.; Luo, Z.; Liu, X.; Zheng, X.; Xiao, F.; Peng, Y.; He, Q.; Tian, Y.; et al. Diversity, function and assembly of mangrove root-associated microbial communities at a continuous fine-scale. NPJ Biofilms Microbiomes 2020, 6, 52. [Google Scholar] [CrossRef]
- Baker, B.J.; Lazar, C.S.; Teske, A.P.; Dick, G.J. Genomic resolution of linkages in carbon, nitrogen, and sulfur cycling among widespread estuary sediment bacteria. Microbiome 2015, 3, 14. [Google Scholar] [CrossRef]
- Rashid, M.I.; Mujawar, L.H.; Shahzad, T.; Almeelbi, T.; Ismail, I.M.I.; Oves, M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 2016, 183, 26–41. [Google Scholar] [CrossRef]
- Kwon, S.K. Marine Glycobiology: Principles and Applications; CRC Press: Boca Raton, FL, USA, 2016; pp. 393–399. [Google Scholar]
- Tam, N.F.Y.; Guo, C.L.; Yau, W.Y.; Wong, Y.S. Preliminary study on biodegradation of phenanthrene by bacteria isolated from mangrove sediments in Hong Kong. Mar. Pollut. Bull. 2002, 45, 316–324. [Google Scholar] [CrossRef]
- Tam, N.F.Y. Effects of wastewater discharge on microbial populations and enzyme activities in mangrove soils. Environ. Pollut. 1998, 102, 233–242. [Google Scholar] [CrossRef]
- Al-Sayed, H.A.; Ghanem, E.H.; Saleh, K.M. Bacterial community and some physico-chemical characteristics in a subtropical mangrove environment in Bahrain. Mar. Pollut. Bull. 2005, 50, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Sidhu, V.; Rong, Y.; Zheng, Y. Pesticide Pollution in Agricultural Soils and Sustainable Remediation Methods: A Review. Curr. Pollut. Rep. 2018, 4, 240–250. [Google Scholar] [CrossRef]
- Silliman, B.R.; Gedan, K.B. Using Facilitation Theory to Enhance Mangrove Restoration. AMBIO A J. Hum. Environ. 2009, 38, 109. [Google Scholar] [CrossRef]
- Cong, J.; Yang, Y.; Liu, X.; Lu, H.; Liu, X.; Zhou, J.; Li, D.; Yin, H.; Ding, J.; Zhang, Y. Analyses of soil microbial community compositions and functional genes reveal potential consequences of natural forest succession. Sci. Rep. 2015, 5, 10007. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.M.; Tang, H.Y.; Liu, Y.; Zhang, M.Q.; Jiang, S.S.; Yang, F.; Li, X.Y.; Wang, C.Y. Resource status and protection strategies of mangroves in China. J. Coast. Conserv. 2021, 25, 42. [Google Scholar] [CrossRef]
- Wang, M.; Cao, W.; Guan, Q.; Wu, G.; Wang, F. Assessing changes of mangrove forest in a coastal region of southeast China using multi-temporal satellite images. Estuar. Coast. Shelf Sci. 2018, 207, 283–292. [Google Scholar] [CrossRef]
- O’Connell, D.P.; Fusi, M.; Djamaluddin, R.; Rajagukguk, B.B.; Bachmid, F.; Kitson, J.J.N.; Dunnett, Z.; Trianto, A.; Tjoa, A.B.; Diele, K.; et al. Assessing mangrove restoration practices using species-interaction networks. Restor. Ecol. 2021, 30, e13546. [Google Scholar] [CrossRef]
- Dar, S.A.; Kleerebezem, R.; Stams, A.J.M.; Kuenen, J.G.; Muyzer, G. Competition and coexistence of sulfate-reducing bacteria, acetogens and methanogens in a lab-scale anaerobic bioreactor as affected by changing substrate to sulfate ratio. Appl. Microbiol. Biotechnol. 2008, 78, 1045–1055. [Google Scholar] [CrossRef]
- Sun, D.; Huang, Y.; Wang, Z.; Tang, X.; Ye, W.; Cao, H.; Shen, H. Soil microbial community structure, function and network along a mangrove forest restoration chronosequence. Sci. Total Environ. 2023, 913, 169704. [Google Scholar] [CrossRef]
- Aaron, M. Ellison. Mangrove Restoration: Do We Know Enough? Restor. Ecol. 2000, 8, 219–229. [Google Scholar] [CrossRef]
- Pérez-Cobas, A.E.; Gomez-Valero, L.; Buchrieser, C. Metagenomic approaches in microbial ecology: An update on whole-genome and marker gene sequencing analyses. Microb. Genom. 2020, 6, mgen000409. [Google Scholar] [CrossRef]
- Selvaraj, S.; Kannan, M.R.; Pattapulavar, V.; Godwin Christopher, J.; Sasikumar, S. Mangrove Microbiome; Muthusamy, S., Manikkam, R., Venugopal, G., Eds.; Springer: Singapore, 2025; pp. 169–284. [Google Scholar]
- Lin, G.; He, Y.; Lu, J.; Chen, H.; Feng, J. Seasonal variations in soil physicochemical properties and microbial community structure influenced by Spartina alterniflora invasion and Kandelia obovata restoration. Sci. Total Environ. 2021, 797, 149213. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhuang, J.; Wang, J.; Fan, G.; Feng, M.; Zhang, S. Soil bacterial communities of three types of plants from ecological restoration areas and plant-growth promotional benefits of Microbacterium invictum (strain X-18). Front. Microbiol. 2022, 13, 926037. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, T.; Cai, L.; Li, Z.; Chen, S.; Zhu, Y.; Huang, X.; Li, C. Analysis of Soil Bacterial Commnity Structure Characteristics in Wetland of Riparian Vegetation Buffer Zone in Ao River Basin, Zhejiang Province. Acta Agric. Jiangxi 2023, 35, 69–73. [Google Scholar]
- Chen, Q. Development history and discussion of mangrove forest in Zhejiang Province. Zhejiang Agric. Sci. 2019, 60, 1177–1181. [Google Scholar]
- Yu, Y.; Shui, B.-N.; Lú, C.-C.; Li, B.; Li, X.-L.; Wei, Z.; Hu, C.-Y. Analysis of the Characteristics and Sources of Organic Carbon Burial in Mangrove Wetland Sediments of Maoyan Island. China Environ. Sci. 2024, 44, 4539–4546. [Google Scholar]
- Faé, S.G.; Montes, F.; Bazilevskaya, E.; Añó, R.M.; Kemanian, A.R. Making Soil Particle Size Analysis by Laser Diffraction Compatible with Standard Soil Texture Determination Methods. Soil Sci. Soc. Am. J. 2019, 83, 1244–1252. [Google Scholar] [CrossRef]
- Shang, S.; Hu, S.; Liu, X.; Zang, Y.; Chen, J.; Gao, N.; Li, L.; Wang, J.; Liu, L.; Xu, J.; et al. Effects of Spartina alterniflora invasion on the community structure and diversity of wetland soil bacteria in the Yellow River Delta. Ecol. Evol. 2022, 12, e8905. [Google Scholar] [CrossRef]
- Jiang, C.; Diao, X.; Wang, H.; Ma, S. Diverse and abundant antibiotic resistance genes in mangrove area and their relationship with bacterial communities—A study in Hainan Island, China. Environ. Pollut. 2021, 276, 116704. [Google Scholar] [CrossRef] [PubMed]
- Michael, H.; Beiko, R.G. 16S rRNA Gene Analysis with QIIME2. Methods Mol. Biol. 2018, 1849, 113–129. [Google Scholar] [CrossRef]
- Balvočiūtė, M.; Huson, D.H. SILVA, RDP, Greengenes, NCBI and OTT—How do these taxonomies compare? BMC Genom. 2017, 18, 114. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Guo, F.; Ju, F.; Zhang, T. Shifts in the microbial community, nitrifiers and denitrifiers in the biofilm in a full-scale rotating biological contactor. Environ. Sci. Technol. 2014, 48, 8044–8052. [Google Scholar] [CrossRef]
- Wang, L.; Jian, X.; Mei, H.; Shen, X.; Fu, H. Clay composition heterogeneity in sediments from mountainous catchments with contrasting bedrock lithology in SE China coast. Catena 2024, 247, 108470. [Google Scholar] [CrossRef]
- Ramette, A. Multivariate analyses in microbial ecology. FEMS Microbiol. Ecol. 2007, 62, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Li, R.; Wu, S.; Xie, S. The distribution of sediment bacterial community in mangroves across China was governed by geographic location and eutrophication. Mar. Pollut. Bull. 2019, 140, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Sanka Loganathachetti, D.; Sadaiappan, B.; Poosakkannu, A.; Muthuraman, S. Pyrosequencing-Based Seasonal Observation of Prokaryotic Diversity in Pneumatophore-Associated Soil of Avicennia marina. Curr. Microbiol. 2016, 72, 68–74. [Google Scholar] [CrossRef]
- Chang, Y.; Li, X.; Wang, P.Y.; Klingbeil, K.; Li, W.; Zhang, F.; Burchard, H. Salinity mixing in a tidal multi-branched estuary with huge and variable run off. J. Hydrol. 2024, 634, 131094. [Google Scholar] [CrossRef]
- Rath, K.M.; Fierer, N.; Murphy, D.V.; Rousk, J. Linking bacterial community composition to soil salinity along environmental gradients. ISME J. 2019, 13, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhou, T.; Huang, G.; Xiong, Y. Soil microbial community and associated functions response to salt stresses: Resistance and resilience. Sci. Total Environ. 2024, 954, 176475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Sun, J.; Lin, B.; Huang, G. Seasonal hydrodynamic interactions between tidal waves and river flows in the Yangtze Estuary. J. Mar. Syst. 2018, 186, 17–28. [Google Scholar] [CrossRef]
- Guo, Z.; Li, Y.; Shao, M.; Sun, T.; Lin, M.; Zhang, T.; Hu, K.; Jiang, H.; Guan, X. Succession and environmental response of sediment bacterial communities in the Liao River Estuary at the centenary scale. Mar. Environ. Res. 2023, 188, 105980. [Google Scholar] [CrossRef]
- Xie, X.F.; Xiang, Q.; Wu, T.; Jiang, G.J.; Sun, X.M.; Zhu, M.; Pu, L.J. Progress and prospect of soil microorganisms and their influencing factors in coastal wetland ecosystem. Acta Ecol. Sin. 2021, 41, 1–12. [Google Scholar] [CrossRef]
- Sun, X.; Lin, Y.-L.; Li, B.-L.; Huang, L.-F. Analysis and function prediction of soil microbial communities of Cynomorium songaricum in two daodi-origins. Acta Pharm. Sin. 2020, 55, 1334. [Google Scholar] [CrossRef]
- Miao, L.Z.; Wang, P.F.; Hou, J.; Yao, Y.; Liu, Z.; Liu, S.; Li, T. Distinct community structure and microbial functions of biofilms colonizing microplastics. Sci. Total Environ. 2019, 650, 2395–2402. [Google Scholar] [CrossRef]
- Jiang, X.T.; Peng, X.; Deng, G.H.; Sheng, H.F.; Wang, Y.; Zhou, H.W.; Tam, N.F.Y. Illumina Sequencing of 16S rRNA Tag Revealed Spatial Variations of Bacterial Communities in a Mangrove Wetland. Microb. Ecol. 2013, 66, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Basak, P.; Pramanik, A.; Sengupta, S.; Nag, S.; Bhattacharyya, A.; Roy, D.; Pattanayak, R.; Ghosh, A.; Chattopadhyay, D.; Bhattacharyya, M. Bacterial diversity assessment of pristine mangrove microbial community from Dhulibhashani, Sundarbans using 16S rRNA gene tag sequencing. Genom. Data 2016, 7, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Satyanarayana, T.; Das, S.K.; Johri, B.N. Microbial Diversity in Ecosystem Sustainability and Biotechnological Applications; Springer: Singapore, 2019; pp. 319–353. [Google Scholar]
- Zhou, Z.; Tran, P.Q.; Kieft, K.; Anantharaman, K. Genome diversification in globally distributed novel marine Proteobacteria is linked to environmental adaptation. ISME J. 2020, 14, 2060–2077. [Google Scholar] [CrossRef]
- Ghiglione, J.F.; Murray, A.E. Pronounced summer to winter differences and higher wintertime richness in coastal Antarctic marine bacterioplankton. Environ. Microbiol. 2012, 14, 617–629. [Google Scholar] [CrossRef]
- Ladau, J.; Sharpton, T.; Finucane, M.; Jospin, G.; Kembel, S.W.; O’Dwyer, J.; Koeppel, A.F.; Green, J.L.; Pollard, K.S. Global marine bacterial diversity peaks at high latitudes in winter. ISME J. 2013, 7, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhou, T.; Xu, C.; Shen, D.; Xu, S.; Lin, C. Spatial and Temporal Variation in Microbial Diversity and Community Structure in a Contaminated Mangrove Wetland. Appl. Sci. 2020, 10, 5850. [Google Scholar] [CrossRef]
- Klier, J.; Dellwig, O.; Leipe, T.; Jürgens, K.; Herlemann, D.P.R. Benthic Bacterial Community Composition in the Oligohaline-Marine Transition of Surface Sediments in the Baltic Sea Based on rRNA Analysis. Front. Microbiol. 2018, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Xiao, N.; Wang, Q.; Wu, H. Seasonal variation of bacterial community diversity in Yangshan Port area. Acta Ecol. Sin. 2016, 36, 7758–7767. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, K.; Guo, Z.; Liu, X.; Bian, R.; Sun, B.; Li, J.; Chen, J. An antagonistic effect of elevated CO2 and warming on soil N2O emissions related to nitrifier and denitrifier communities in a Chinese wheat field. Plant Soil 2022, 470, 97–110. [Google Scholar] [CrossRef]
- Bhatti, A.A.; Haq, S.; Bhat, A.R. Actinomycetes benefaction role in soil and plant health. Microb. Pathog. 2017, 111, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Dang, P.; Li, C.; Lu, C.; Zhang, M.; Huang, T.; Wan, C.; Wang, H.; Chen, Y.; Qin, X.; Liao, Y. Effect of fertilizer management on the soil bacterial community in agroecosystems across the globe. Agric. Ecosyst. Environ. 2022, 326, 107795. [Google Scholar] [CrossRef]
- Collins, P.D.; Jacobsen, J.B. Optimizing a Bacillus subtilis isolate for biological control of sugar beet cercospora leaf spot. Biol. Control. 2003, 26, 153–161. [Google Scholar] [CrossRef]
- An, X.; Wang, Z.; Teng, X.; Zhou, R.; Wang, X.; Xu, M.; Lian, B. Rhizosphere bacterial diversity and environmental function prediction of wild salt-tolerant plants in coastal silt soil. Ecol. Indic. 2022, 134, 108503. [Google Scholar] [CrossRef]
- Rietz, D.; Haynes, R. Effects of irrigation-induced salinity and sodicity on soil microbial activity. Soil Biol. Biochem. 2003, 35, 845–854. [Google Scholar] [CrossRef]
- Estrelles, E.; Biondi, E.; Galiè, M.; Mainardi, F.; Hurtado, A.; Soriano, P. Aridity level, rainfall pattern and soil features as key factors in germination strategies in salt-affected plant communities. J. Arid. Environ. 2015, 117, 1–9. [Google Scholar] [CrossRef]
- Yan, N.; Marschner, P.; Cao, W.; Zuo, C.; Qin, W. Influence of salinity and water content on soil microorganisms. Int. Soil Water Conserv. Res. 2015, 3, 316–323. [Google Scholar] [CrossRef]
- Boyrahmadi, M.; Raiesi, F. Plant roots and species moderate the salinity effect on microbial respiration, biomass, and enzyme activities in a sandy clay soil. Biol. Fertil. Soils 2018, 54, 509–521. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, Q.; Li, J.; Jian, S.; Ren, H. Mangrove succession enriches the sediment microbial community in South China. Sci. Rep. 2016, 6, 27468. [Google Scholar] [CrossRef] [PubMed]
- Mandic-Mulec, I.; Stefanic, P.; van Elsas, J.D. Ecology of Bacillaceae. Microbiol. Spectr. 2015, 3, TBS-0017-2013. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, L.; Liu, Y.; Guo, Y.; Tang, S.; Ren, J. Land use shapes the microbial community structure by altering soil aggregates and dissolved organic matter components. J. Integr. Agric. 2025, 24, 827–844. [Google Scholar] [CrossRef]
- Colares, B.G.; Melo, M.M.V. Relating microbial community structure and environmental variables in mangrove sediments inside Rhizophora mangle L. habitats. Appl. Soil Ecol. 2013, 64, 171–177. [Google Scholar] [CrossRef]
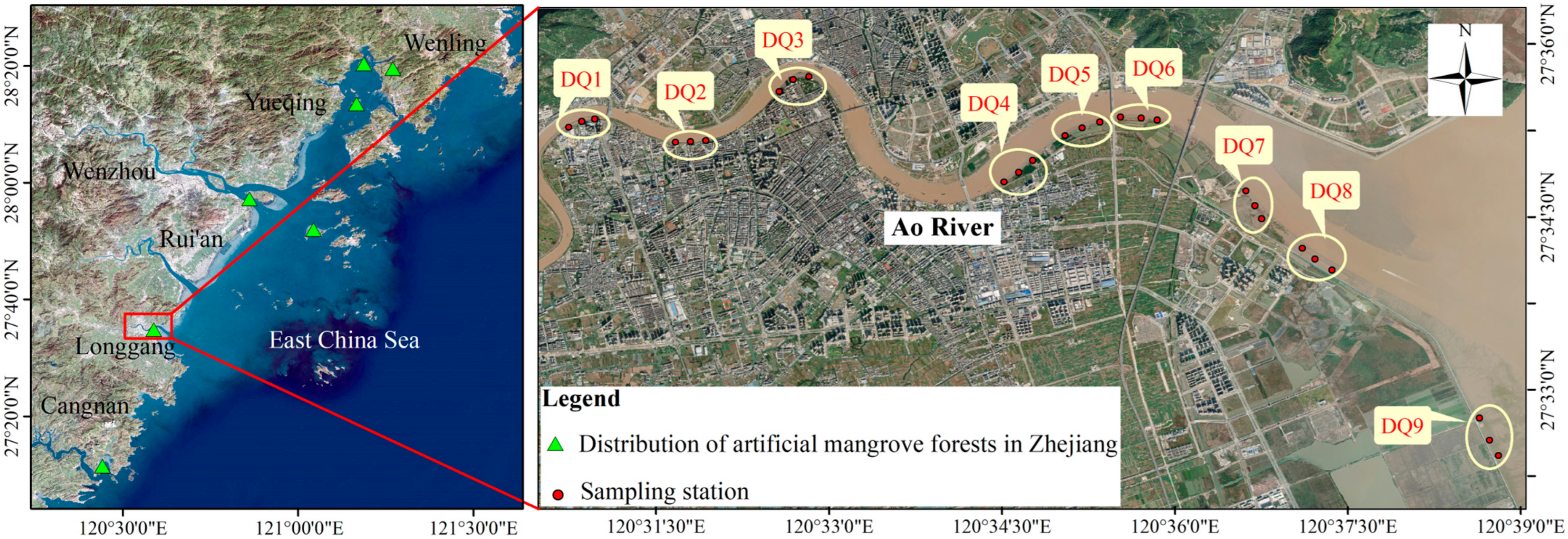
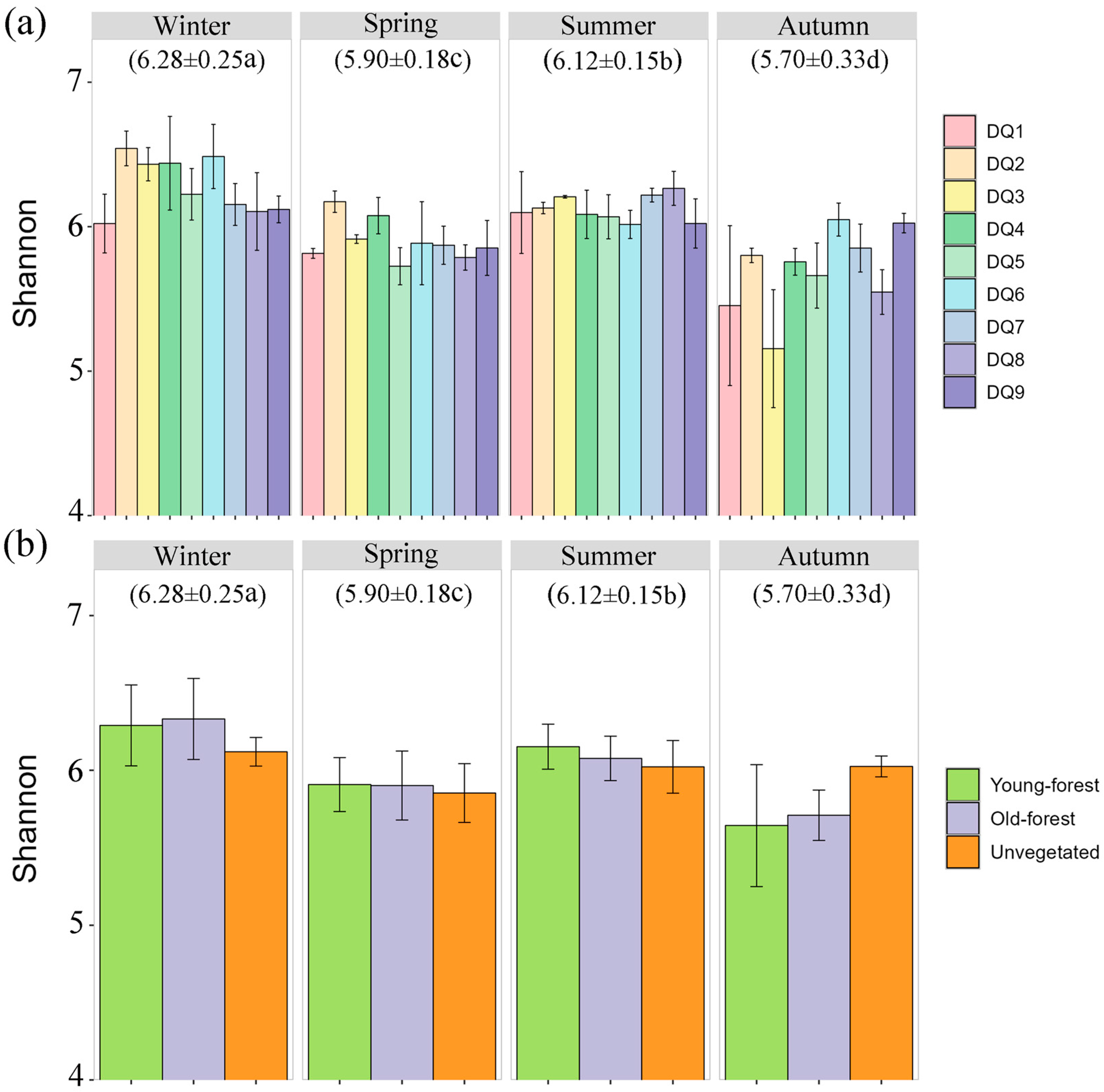


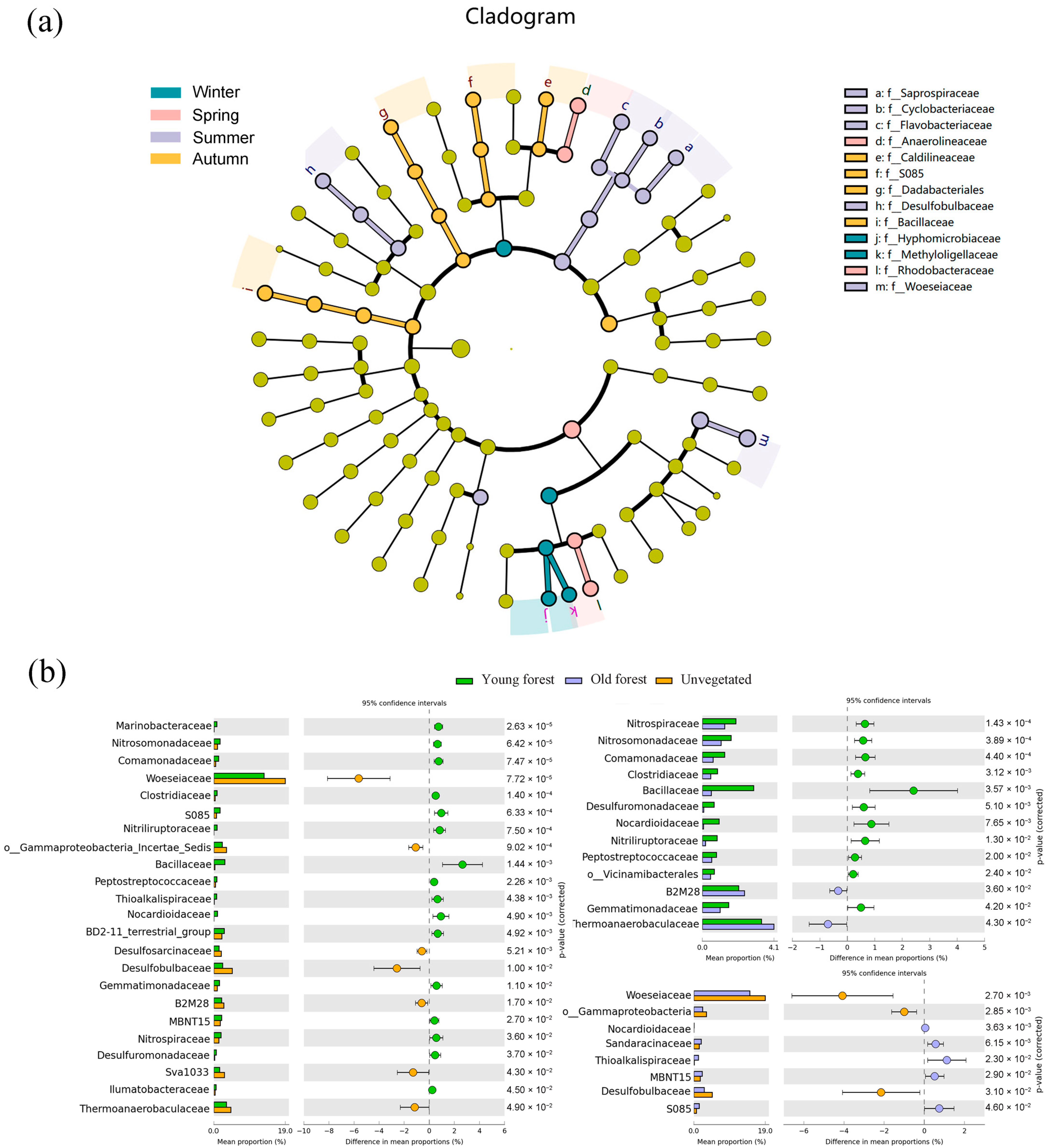
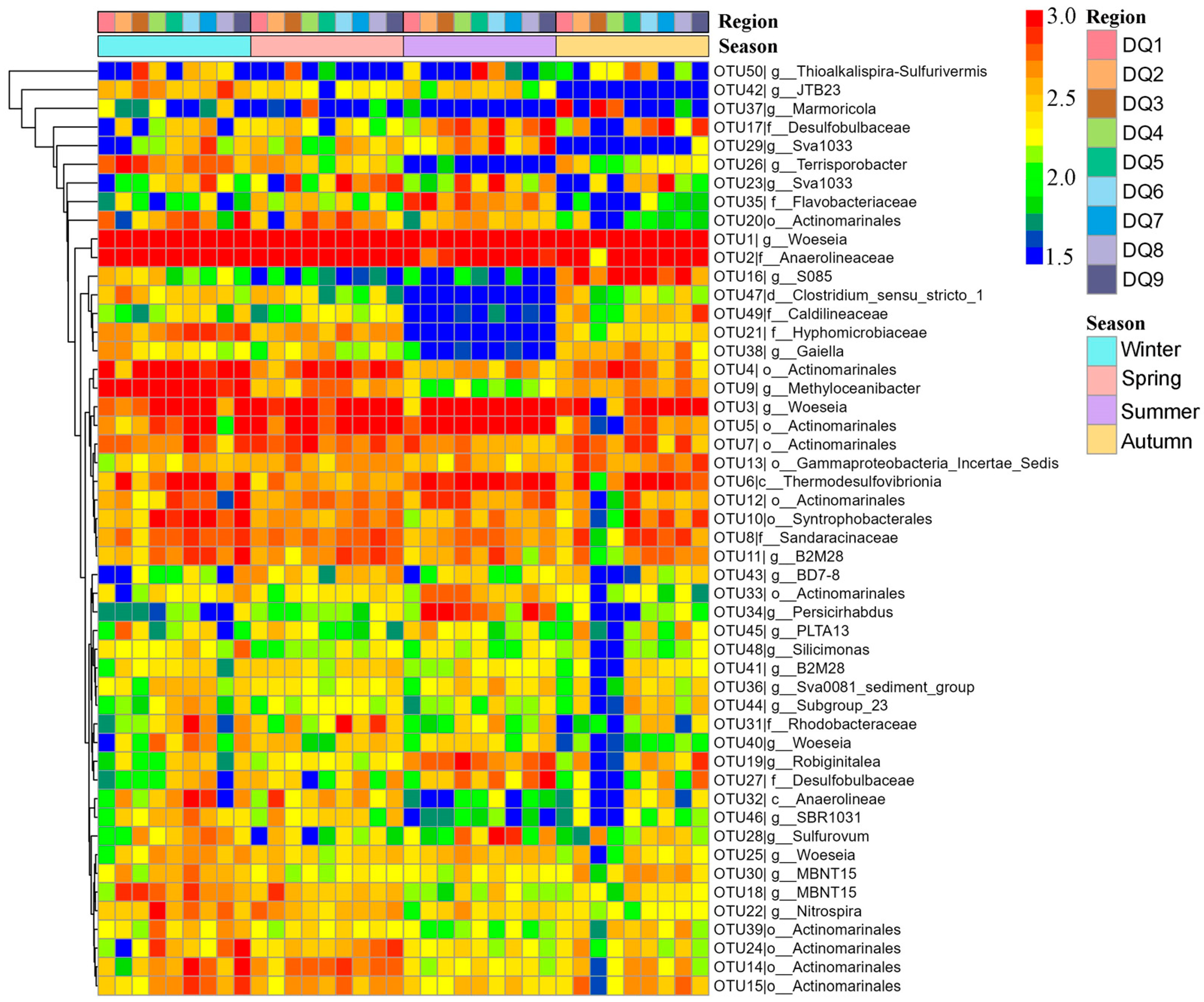
| Parameter | Winter 2021 | Spring 2022 | Summer 2022 | Autumn 2022 |
|---|---|---|---|---|
| Temperature (°C) | 14.39 ± 0.62 d | 21.97 ± 1.03 b | 30.02 ± 0.93 a | 21.16 ± 0.71 c |
| Salinity (ppt) | 15.24 ± 5.67 c | 10.79 ± 5.47 d | 24.64 ± 7.44 b | 29.01 ± 2.77 a |
| pH | 7.87 ± 0.31 a | 7.54 ± 0.45 b | 7.52 ± 0.23 b | 7.66 ± 0.29 ab |
| Total carbon (g/kg) | 12.52 ± 0.60 a | 12.21 ± 0.88 a | 11.97 ± 0.82 a | 11.88 ± 0.89 a |
| Total nitrogen (g/kg) | 0.93 ± 0.12 a | 0.97 ± 0.13 a | 1.01 ± 0.83 a | 0.97 ± 0.13 a |
| Total carbon/total nitrogen (%) | 13.71 ± 1.09 a | 12.79 ± 1.10 b | 12.04 ± 0.69 b | 12.29 ± 1.51 b |
| Soluble salt content (g/kg) | 12.59 ± 3.55 c | 46.29 ± 26.97 a | 10.19 ± 4.91 c | 27.39 ± 8.77 b |
| Clay content (%) | 28.88 ± 3.40 b | 36.04 ± 5.25 a | 29.99 ± 3.85 b | 35.08 ± 3.48 a |
| Silt content (%) | 69.12 ± 3.91 a | 63.59 ± 4.96 b | 67.88 ± 3.86 a | 63.67 ± 3.05 b |
| Sand content (%) | 1.99 ± 1.19 a | 0.37 ± 0.64 b | 2.13 ± 1.29 a | 1.25 ± 2.19 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, L.; He, M.; Jiang, S.; Li, X.; Shui, B. Spatiotemporal Characteristics of Bacterial Communities in Estuarine Mangrove Sediments in Zhejiang Province, China. Microorganisms 2025, 13, 859. https://doi.org/10.3390/microorganisms13040859
Yao L, He M, Jiang S, Li X, Shui B. Spatiotemporal Characteristics of Bacterial Communities in Estuarine Mangrove Sediments in Zhejiang Province, China. Microorganisms. 2025; 13(4):859. https://doi.org/10.3390/microorganisms13040859
Chicago/Turabian StyleYao, Liqin, Maoqiu He, Shoudian Jiang, Xiangfu Li, and Bonian Shui. 2025. "Spatiotemporal Characteristics of Bacterial Communities in Estuarine Mangrove Sediments in Zhejiang Province, China" Microorganisms 13, no. 4: 859. https://doi.org/10.3390/microorganisms13040859
APA StyleYao, L., He, M., Jiang, S., Li, X., & Shui, B. (2025). Spatiotemporal Characteristics of Bacterial Communities in Estuarine Mangrove Sediments in Zhejiang Province, China. Microorganisms, 13(4), 859. https://doi.org/10.3390/microorganisms13040859






