Antibacterial Effects of Bulgarian Oregano and Thyme Essential Oils Alone and in Combination with Antibiotics Against Klebsiella pneumoniae and Pseudomonas aeruginosa
Abstract
1. Introduction
2. Materials and Methods
2.1. Essential Oils
2.2. Microorganisms
2.3. GC-MS Analysis
2.4. Determination of the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.5. Disk-Difusion Assay
2.6. Assessment of the FIC Index
3. Results and Discussion
3.1. Public Health Implications
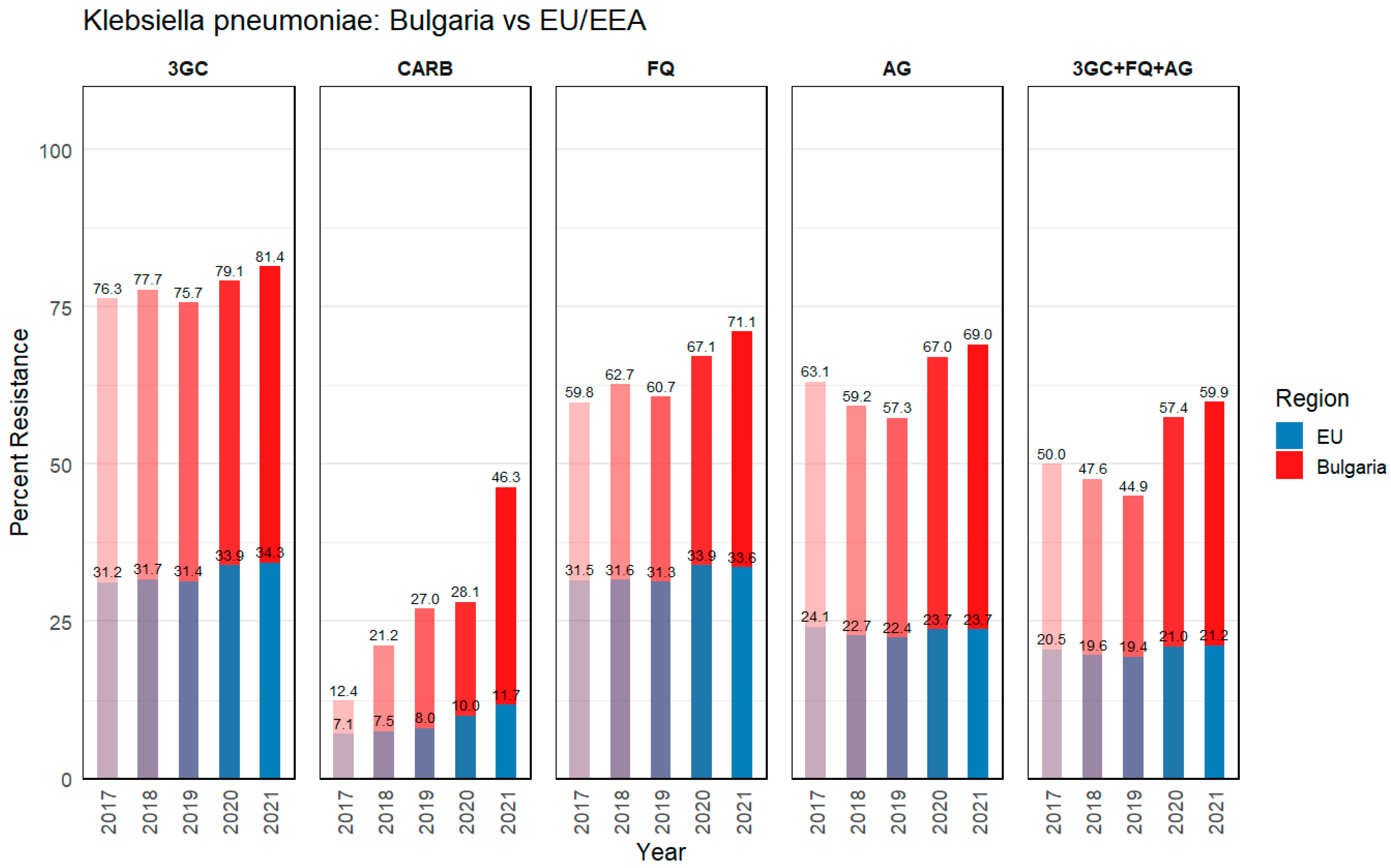
3.1.1. Klebsiella pneumoniae
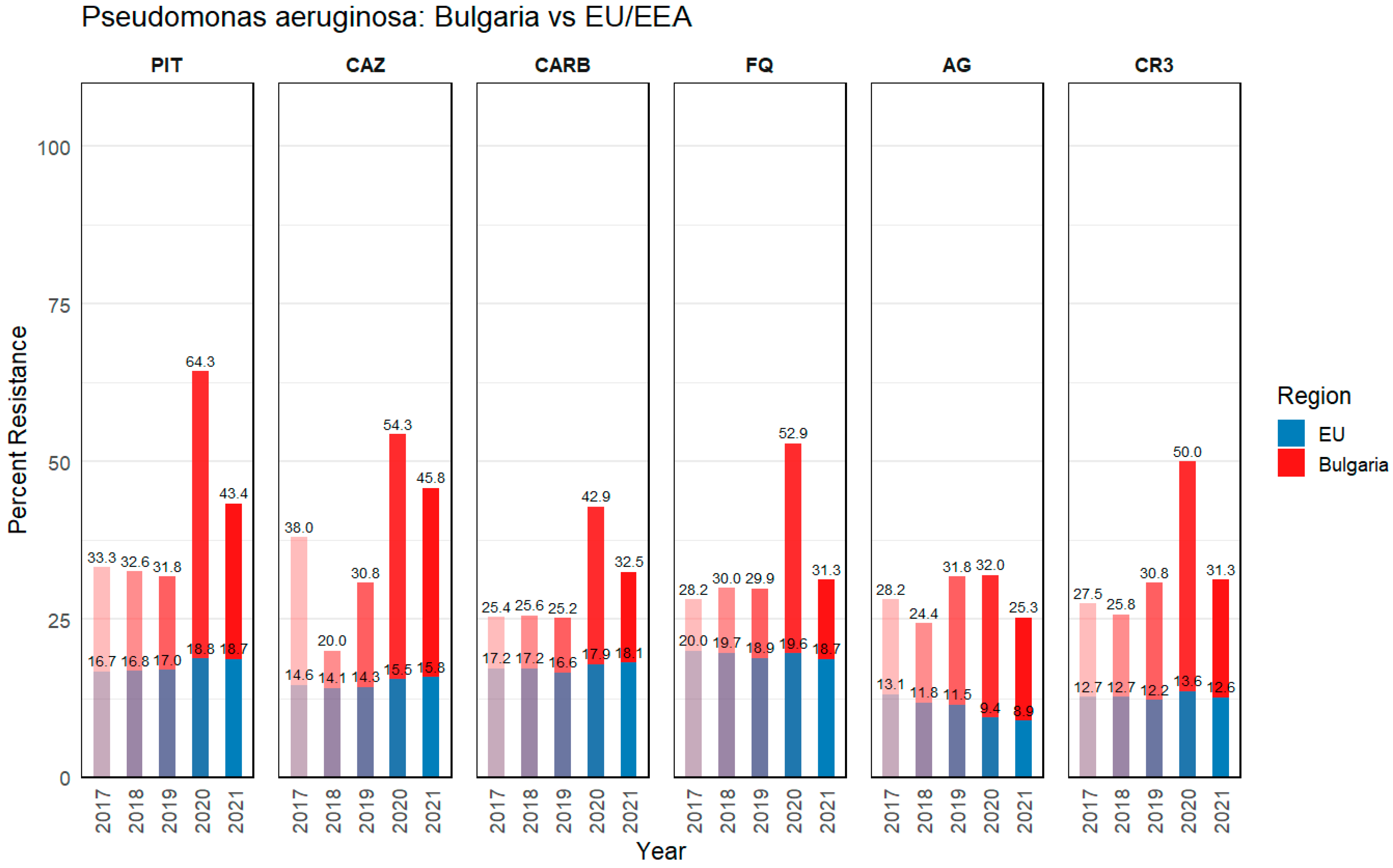
3.1.2. Pseudomonas aeruginosa
3.2. Antibiotic Sensitivity Profile of Clinical Isolates
3.3. Antibacterial Activity of Oregano and Thyme Essential Oils Alone and in Combination with Conventional Antibiotics Against K. pneumoniae and P. aeruginosa Strains
3.3.1. Monitoring the Sensitivity of Bacterial Strains When Combining Essential Oils with Antibiotic
3.3.2. Assessment of the FIC Index
3.3.3. Structure-Based Antibacterial Activity of Key Antibacterial Components Is EOs
3.4. Chemical Composition by GC-MS of Thyme and Oregano EOs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3GC | Third-generation cephalosporins |
| AG | Aminoglycosides |
| AMR | Antimicrobial resistance |
| ATCC | American Type Culture Collection |
| BPPL | Bacterial Priority Pathogens List |
| CARB | Carbapenems |
| CAUTI | Catheter-associated urinary tract infection |
| CAZ | Ceftazidime |
| CDC | Centers for Disease Control and Prevention |
| CFU | Colony-forming units |
| CIP | Ciprofloxacin |
| CLABSI | Central line-associated bloodstream infection |
| COVID-19 | Coronavirus disease 2019 |
| CR3 | Combined resistance to three or more antibiotic groups (among PIT, CAZ, CARB, FQ, and AG) |
| CRKP | Carbapenem-resistant K. pneumoniae |
| CRPA | Carbapenem-resistant P. aeruginosa |
| CTR | Ceftriaxone |
| DMSO | Dimethyl sulfoxide |
| EARS-Net | European Antimicrobial Resistance Surveillance Network |
| ECDC | European Centre for Disease Prevention and Control |
| EMA | European Medicines Agency |
| EO | Essential oil |
| EU/EEA | European Union/European Economic Area |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| FQ | Fluoroquinolones |
| GC-MS | Gas chromatography-mass spectrometry |
| GEN | Gentamicin |
| GDP | Gross domestic product |
| HAI | Healthcare-associated infection |
| ICU | Intensive care unit |
| IFRA | International Fragrance Association |
| K. pneumoniae | Klebsiella pneumoniae |
| MBC | Minimum bactericidal concentration |
| MDR | Multidrug-resistant |
| MIC | Minimum inhibitory concentration |
| MSD | Mass selective detector |
| NIST | National Institute of Standards and Technology |
| P. aeruginosa | Pseudomonas aeruginosa |
| PDR | Pandrug-resistant |
| PIT | Piperacillin–tazobactam |
| QS | Quorum sensing |
| RI | Retention index |
| RT | Retention time |
| SSI | Surgical site infection |
| TA-EO | Thymus algeriensis essential oil |
| TIC | Total ion current |
| WHO | World Health Organization |
| XDR | Extensively drug-resistant |
References
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Aguilar, G.R.; Mestrovic, T.; Smith, G.; Han, C. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar]
- World Health Organization. WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance, to Guide Research, Development, and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Costanzo, V.; Roviello, G.N. The Potential Role of Vaccines in Preventing Antimicrobial Resistance (AMR): An Update and Future Perspectives. Vaccines 2023, 11, 333. [Google Scholar] [CrossRef] [PubMed]
- Ben Selma, W.; Farouk, A.; Ban, Z.; Ferjeni, M.; Alsulami, T.; Ali, H.; Boukadida, J. Thymus algeriensis essential oil: Phytochemical investigation, bactericidal activity, synergistic effect with colistin, molecular docking, and dynamics analysis against Gram-negative bacteria resistant to colistin. Heliyon 2024, 10, e38281. [Google Scholar] [CrossRef]
- Dong, H.; You, Y.; Wang, N.; Wang, M.; Song, T.; He, Y.; Zou, Y.; He, Y.; Peng, T.; Mei, L. Development of amphipathic derivatives of thymol and carvacrol as potent broad-spectrum antibacterial agents. Eur. J. Med. Chem. 2024, 276, 116716. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Ucha, J.C.; Martínez-Guitián, M.; Lasarte-Monterrubio, C.; Conde-Pérez, K.; Arca-Suárez, J.; Álvarez-Fraga, L.; Pérez, A.; Crecente-Campo, J.; Alonso, M.J.; Bou, G.; et al. Syzygium aromaticum (clove) and Thymus zygis (thyme) essential oils increase susceptibility to colistin in the nosocomial pathogens Acinetobacter baumannii and Klebsiella pneumoniae. Biomed. Pharmacother. 2020, 130, 110606. [Google Scholar] [CrossRef]
- Lister Philip, D.; Wolter Daniel, J.; Hanson Nancy, D. Antibacterial-Resistant Pseudomonas aeruginosa: Clinical Impact and Complex Regulation of Chromosomally Encoded Resistance Mechanisms. Clin. Microbiol. Rev. 2009, 22, 582–610. [Google Scholar] [CrossRef]
- Cendra Gascón, M.d.M.; Torrents Serra, E. Pseudomonas aeruginosa biofilms and their partners in crime. Biotechnol. Adv. 2021, 49, 1–15. [Google Scholar]
- Pesingi, P.V.; Singh, B.R.; Pesingi, P.K.; Bhardwaj, M.; Singh, S.V.; Kumawat, M.; Sinha, D.K.; Gandham, R.K. MexAB-OprM efflux pump of Pseudomonas aeruginosa offers resistance to carvacrol: A herbal antimicrobial agent. Front. Microbiol. 2019, 10, 2664. [Google Scholar]
- ECDC. Antimicrobial Resistance in the EU/EEA (EARS-Net); European Centre for Disease Prevention and Control: Solna, Sweden, 2022. [Google Scholar]
- Janda, J.M.; Abbott, S.L. The changing face of the family Enterobacteriaceae (Order: “Enterobacterales”): New members, taxonomic issues, geographic expansion, and new diseases and disease syndromes. Clin. Microbiol. Rev. 2021, 34, e00174-20. [Google Scholar] [CrossRef]
- Karampatakis, T.; Tsergouli, K.; Roilides, E. Infection control measures against multidrug-resistant Gram-negative bacteria in children and neonates. Future Microbiol. 2023, 18, 751–765. [Google Scholar]
- Mestrovic, T.; Aguilar, G.R.; Swetschinski, L.R.; Ikuta, K.S.; Gray, A.P.; Weaver, N.D.; Han, C.; Wool, E.E.; Hayoon, A.G.; Hay, S.I. The burden of bacterial antimicrobial resistance in the WHO European region in 2019: A cross-country systematic analysis. Lancet Public Health 2022, 7, e897–e913. [Google Scholar]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar]
- Vulcanescu, D.D.; Bagiu, I.C.; Avram, C.R.; Oprisoni, L.A.; Tanasescu, S.; Sorescu, T.; Susan, R.; Susan, M.; Sorop, V.B.; Diaconu, M.M. Bacterial Infections, Trends, and Resistance Patterns in the Time of the COVID-19 Pandemic in Romania—A Systematic Review. Antibiotics 2024, 13, 1219. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Surveillance in Europe 2023—2021 Data; European Centre for Disease Prevention and Control and World Health Organization: Stockholm, Sweden, 2023; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Antimicrobial%20resistance%20surveillance%20in%20Europe%202023%20-%202021%20data.pdf (accessed on 5 March 2025).
- Vazquez, N.M.; Moreno, S.; Galván, E.M. Exposure of multidrug-resistant Klebsiella pneumoniae biofilms to 1,8-cineole leads to bacterial cell death and biomass disruption. Biofilm 2022, 4, 100085. [Google Scholar] [CrossRef]
- van Vuuren, S.F.; Suliman, S.; Viljoen, A.M. The antimicrobial activity of four commercial essential oils in combination with conventional antimicrobials. Lett. Appl. Microbiol. 2009, 48, 440–446. [Google Scholar] [CrossRef]
- Mohammadzamani, Z.; Khorshidi, A.; Khaledi, A.; Shakerimoghaddam, A.; Moosavi, G.A.; Piroozmand, A. Inhibitory effects of Cinnamaldehyde, Carvacrol, and honey on the expression of exoS and ampC genes in multidrug-resistant Pseudomonas aeruginosa isolated from burn wound infections. Microb. Pathog. 2020, 140, 103946. [Google Scholar] [CrossRef]
- Dorman, H.J.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, F.; Ji, B.P.; Pei, R.S.; Xu, N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2020, 60, 3042–3053. [Google Scholar] [CrossRef] [PubMed]
- IFRA. International Fragrance Association (IFRA). Available online: https://ifrafragrance.org (accessed on 26 February 2025).
- EMA. European Medicines Agency. Available online: https://www.ema.europa.eu/en/search?f%5B0%5D=ema_search_categories%3A83&f%5B1%5D=ema_search_categories%3A85&f%5B2%5D=ema_search_entity_is_document%3ADocument&f%5B3%5D=herbal_status%3A78&f%5B4%5D=herbal_status%3A82&landing_from=73303&search_api_fulltext=aetheroleum (accessed on 26 February 2025).
- Pandey, A.K.; Singh, P.; Tripathi, N.N. Chemistry and bioactivities of essential oils of some Ocimum species: An overview. Asian Pac. J. Trop. Biomed. 2014, 4, 682–694. [Google Scholar] [CrossRef]
- Al-Harrasi, A.; Bhatia, S.; Behl, T.; Kaushik, D.; Ahmed, M.M.; Anwer, K. Antibacterial mechanism of action of essential oils. In Role of Essential Oils in the Management of COVID-19; CRC Press: Boca Raton, FL, USA, 2022; pp. 227–237. [Google Scholar]
- Shariati, A.; Noei, M.; Askarinia, M.; Khoshbayan, A.; Farahani, A.; Chegini, Z. Inhibitory effect of natural compounds on quorum sensing system in Pseudomonas aeruginosa: A helpful promise for managing biofilm community. Front. Pharmacol. 2024, 15, 1350391. [Google Scholar] [CrossRef]
- Taibi, M.; Elbouzidi, A.; Haddou, M.; Loukili, E.H.; Bellaouchi, R.; Asehraou, A.; Douzi, Y.; Addi, M.; Salamatullah, A.M.; Nafidi, H.-A.; et al. Chemical Profiling, Antibacterial Efficacy, and Synergistic Actions of Ptychotis verticillata Duby Essential Oil in Combination with Conventional Antibiotics. Nat. Prod. Commun. 2024, 19, 1934578X231222785. [Google Scholar] [CrossRef]
- Dhaouadi, S.; Ghorbel, S.K.B.; Bouglita, W.; Chaari, S.; Dhifi, W.; Khrouf, R.; Cherif, A.; Elandoulsi, R.B. The potency of Cupressus sempervirens and Eucalyptus globulus Essential Oils Against Antibiotic-Resistant Escherichia coli and Mammaliicoccus sciuri from Diseased Animals in Tunisia. Curr. Microbiol. 2024, 82, 14. [Google Scholar] [CrossRef] [PubMed]
- Uddin Mahamud, A.; Nahar, S.; Ashrafudoulla, M.; Park, S.H.; Ha, S.D. Insights into antibiofilm mechanisms of phytochemicals: Prospects in the food industry. Crit. Rev. Food Sci. Nutr. 2024, 64, 1736–1763. [Google Scholar] [CrossRef]
- Szabó, M.A.; Varga, G.Z.; Hohmann, J.; Schelz, Z.; Szegedi, E.; Amaral, L.; Molnár, J. Inhibition of quorum-sensing signals by essential oils. Phytother. Res. 2010, 24, 782–786. [Google Scholar] [CrossRef]
- Walczak, M.; Michalska-Sionkowska, M.; Olkiewicz, D.; Tarnawska, P.; Warżyńska, O. Potential of Carvacrol and Thymol in Reducing Biofilm Formation on Technical Surfaces. Molecules 2021, 26, 2723. [Google Scholar] [CrossRef]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Carol Stream 2005, 16, 65–120. [Google Scholar]
- EUCAST. European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID): Terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. Clin. Microbiol. Infect. 2003, 9, 1–7. [Google Scholar]
- Hudzicki, J. Kirby-Bauer disk diffusion susceptibility test protocol. Am. Soc. Microbiol. 2009, 15, 1–23. [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 15.0. 2025. Available online: https://www.eucast.org (accessed on 19 February 2025).
- Veljovic, K.; Tesevic, V.; Mitrovic, H.; Stankovic, M. Essential oil of Origanum minutiflorum exhibits anti-inflammatory and antioxidative effects in human bronchial cells and antimicrobial activity on lung pathogens. J. Herb. Med. 2023, 39, 100651. [Google Scholar] [CrossRef]
- da Silva, A.R.P.; Costa, M.d.S.; Araújo, N.J.S.; de Freitas, T.S.; dos Santos, A.T.L.; Gonçalves, S.A.; da Silva, V.B.; Andrade-Pinheiro, J.C.; Tahim, C.M.; Lucetti, E.C.P.; et al. Antibacterial activity and antibiotic-modifying action of carvacrol against multidrug-resistant bacteria. Adv. Sample Prep. 2023, 7, 100072. [Google Scholar] [CrossRef]
- Fadli, M.; Saad, A.; Sayadi, S.; Chevalier, J.; Mezrioui, N.-E.; Pagès, J.-M.; Hassani, L. Antibacterial activity of Thymus maroccanus and Thymus broussonetii essential oils against nosocomial infection—Bacteria and their synergistic potential with antibiotics. Phytomedicine 2012, 19, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Ergüden, B. Phenol group of terpenoids is crucial for antibacterial activity upon ion leakage. Lett. Appl. Microbiol. 2021, 73, 438–445. [Google Scholar]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef]
- Moghrovyan, A.; Sahakyan, N. Antimicrobial activity and mechanisms of action of Origanum vulgare L. essential oil: Effects on membrane-associated properties. AIMS Biophys. 2024, 11, 508–526. [Google Scholar] [CrossRef]
- Saad, N.Y.; Muller, C.D.; Lobstein, A. Major bioactivities and mechanism of action of essential oils and their components. Flavour Fragr. J. 2013, 28, 269–279. [Google Scholar]
- Tsitlakidou, P.; Tasopoulos, N.; Chatzopoulou, P.; Mourtzinos, I. Current status, technology, regulation and future perspectives of essential oils usage in the food and drink industry. J. Sci. Food Agric. 2023, 103, 6727–6751. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4.1. ed.; Allured Publishing: Carol Steam, IL, USA, 2017; ISBN 978-1-932633-21-4. [Google Scholar]
- Hussain, A.I.; Anwar, F.; Nigam, P.S.; Sarker, S.D.; Moore, J.E.; Rao, J.R.; Mazumdar, A. Antibacterial activity of some Lamiaceae essential oils using resazurin as an indicator of cell growth. LWT Food Sci. Technol. 2011, 44, 1199–1206. [Google Scholar] [CrossRef]
- Mourabiti, F.; Derdak, R.; El Amrani, A.; Momen, G.; Timinouni, M.; Soukri, A.; El Khalfi, B.; Zouheir, Y. The antimicrobial effectiveness of Rosmarinus officinalis, Lavandula angustifolia, and Salvia officinalis essential oils against Klebsiella pneumoniae and Pseudomonas aeruginosa in vitro and in silico. S. Afr. J. Bot. 2024, 168, 112–123. [Google Scholar] [CrossRef]
| Bacterial Strains | Clinical Isolates | Antibiotic Class | |||
|---|---|---|---|---|---|
| 3GC | AG | FQ | |||
| CTR 30 | CAZ 10 | GEN 10 | CIP 5 | ||
| P. aeruginosa | PA2 | - | S | - | I |
| PA12 | - | I | - | I | |
| PA13 | - | I | - | I | |
| K. pneumoniae | KP17 | R | S | S | S |
| KP18 | I | I | S | R | |
| Antimicrobial Agent | Oregano EO Conc. % (v/v) | Thyme EO Conc. % (v/v) | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| K. pneumoniae ATCC13883 | 0.625 | 0.625 | 0.625 | 1.250 |
| KP17 | 0.039 | 0.039 | 0.156 | 0.312 |
| KP18 | 0.039 | 0.039 | 0.156 | 0.156 |
| P. aeruginosa ATCC27853 | 1.250 | 1.250 | 2.500 | 5.000 |
| PA2 | 0.156 | 0.625 | 2.500 | 2.500 |
| PA12 | 0.625 | 2.500 | 5.000 | 5.000 |
| PA13 | 0.078 | 0.078 | 1.205 | 1.250 |
| Antimicrobial Agent/Bacterial Strains | CTR S ≥ 2 7 R < 24 | CTR + Oregano EO | CTR + Thyme EO | GEN S ≥ 17 R < 17 | GEN + Oregano EO | GEN + Thyme EO | CIP S ≥ 25 R < 22 | CIP + Oregano EO | CIP + Thyme EO | CAZ S ≥ 22 R < 19 | CAZ + Oregano EO | CAZ + Thyme EO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K. pneumoniae ATCC13883 | 35.0 ± 0.0 | 35.0 ± 0.0 | 35.0 ± 0.0 | 20.3 ± 0.6 | 30.3 ± 0.6 | 24.7 ± 0.6 | 43.7 ± 1.5 | 45.7 ± 0.5 | 44.7 ± 1.5 | 22.7 ± 0.6 | 23.7 ± 0.6 | 23.0 ± 0.0 |
| KP17 | 22.0 ± 0.0 | 30.0 ± 2.0 | 26.0 ± 1.0 | 22.0 ± 0.0 | 22.3 ± 1.2 | 21.0 ± 1.7 | 28.7 ± 0.5 | 31.3 ± 1.5 | 28.0 ± 1.0 | 21.7 ± 0.6 | 24.7 ± 0.6 | 21.7 ± 0.6 |
| KP18 | 24.3 ± 0.6 | 24.7 ± 0.6 | 24.3 ± 0.6 | 22.0 ± 0.0 | 22.7 ± 0.6 | 18.3 ± 0.6 | 19.3 ± 0.5 | 24.0 ± 1.5 | 15.3 ± 0.5 | 20.0 ± 0.0 | 20.0 ± 0.0 | 20.0 ± 0.0 |
| Antimicrobial Agent/Bacterial Strains | CIP S ≥ 50 R < 26 | CIP + Oregano EO | CIP + Thyme EO | CAZ S ≥ 50 R < 17 | CAZ + Oregano EO | CAZ + Thyme EO |
|---|---|---|---|---|---|---|
| P. aeruginosa ATCC27853 | 51.3 ± 0.6 | 52.7 ± 0.6 | 52.3 ± 0.6 | 55.0 ± 0.0 | 58.3 ± 0.6 | 54.7 ± 0.6 |
| PA2 | 36.0 ± 2.0 | 55.0 ± 1.0 | 41.3 ± 1.2 | 50.7 ± 0.6 | 54.0 ± 1.0 | 49.7 ± 0.6 |
| PA12 | 39.0 ± 1.0 | 51.7 ± 2.3 | 39.0 ± 1.0 | 22.3 ± 0.6 | 23.7 ± 0.6 | 22.3 ± 0.6 |
| PA13 | 40.3 ± 1.5 | 41.0 ± 1.7 | 39.3 ± 0.6 | 21.0 ± 1.0 | 23.3 ± 1.2 | 21.3 ± 1.2 |
| Strain | MICa (Thyme EO) | A | FICA | MICb (CIP) | B | FICB | FICI |
|---|---|---|---|---|---|---|---|
| KP18 | 0.156 | 0.625 | 4 | 8 | 16 | 2 | 6 |
| Name | Oregano | Thyme | RT | RI |
|---|---|---|---|---|
| α-Thujene | nd | 1.34 | 9.11 | 922 |
| α-Pinene | 0.32 | 1.26 | 9.32 | 929 |
| Camphene | 0.13 | 1.11 | 9.84 | 945 |
| β-Pinene | nd | 0.15 | 10.75 | 974 |
| 1-Octen-3-ol | nd | 0.2 | 10.97 | 980 |
| β-Myrcene | 0.69 | 0.88 | 11.23 | 988 |
| α-Phellandrene | 0.12 | nd | 11.71 | 1004 |
| α-Terpinene | 1.03 | 1.13 | 12.06 | 1015 |
| p-Cymene | 2.82 | 21.05 | 12.31 | 1023 |
| Limonene | nd | 0.5 | 12.47 | 1027 |
| γ-Terpinene | 2.64 | 12.37 | 13.41 | 1057 |
| Sabinene hydrate | nd | 0.57 | 13.79 | 1069 |
| β-Linalool | 3.41 | 2.03 | 14.74 | 1099 |
| Camphor | nd | 1.01 | 16.12 | 1145 |
| Borneol | 0.48 | 1.79 | 16.9 | 1171 |
| Terpinen-4-ol | nd | 0.87 | 17.16 | 1180 |
| α-Terpineol | 0.48 | nd | 17.63 | 1195 |
| Thymol methyl ether | nd | 0.14 | 18.58 | 1228 |
| Carvacrol, methyl ether | nd | 1.33 | 18.84 | 1237 |
| Bornyl acetate | nd | 0.66 | 20.2 | 1286 |
| Thymol | 1.13 | 45.74 | 20.37 | 1293 |
| Carvacrol | 84.2 | 2.01 | 20.75 | 1301 |
| β-Caryophyllene | 0.74 | 2.79 | 23.77 | 1420 |
| Aromadendrene | 0.09 | nd | 24.23 | 1438 |
| β-Bisabolene | 1.13 | nd | 25.93 | 1506 |
| γ-Cadinene | nd | 0.12 | 26.07 | 1512 |
| δ-Cadinene | nd | 0.18 | 26.19 | 1517 |
| Caryophyllene oxide | 0.11 | 0.48 | 27.73 | 1581 |
| Essential Oil | Dominant Compound(s) | Reference |
|---|---|---|
| Thymus algeriensis | Carvacrol (68%), p-Cymene (9%), γ-Terpinene (3%), α-Terpinyl acetate (2%), Linalool (1%) | [4] |
| Thymus vulgaris | Thymol (10–64%), Carvacrol (2–11%), γ-Terpinene (2–31%), p-Cymene (10–56%) | [22] |
| Thymol (52%), p-Cymene (18%), Carvacrol (4%), 1,8-Cineole (6%), α-Terpineol (3%) | [48] | |
| Origanum minutiflorum | Carvacrol (78%), Linalool (5%), p-Cymene (4%), γ-Terpinene (2%), (E)-Caryophyllene (2%) | [39] |
| Origanum vulgare | Carvacrol (Trace-80%), Thymol (Trace-64%), γ-Terpinene (2–52%), p-Cymene (Trace-52%) | [22] |
| Melissa officinalis | Citronellal (21%), Geraniol (17%), β-Citronellol (12%), Caryophyllene Oxide (1%), α-Cadinol (3%) | [48] |
| Rosmarinus officinalis | 1,8-Cineole (48%), Camphor (10%), α-Pinene (10%), Borneol (8%) | [49] |
| 1,8-Cineole (29%), Camphor (17%), α-Pinene (12%), β-Pinene (6%), Limonene (5%) | [48] | |
| Lavandula angustifolia | Linalool (30%), 1-Dodecene (33%) | [49] |
| Linalool (25%), Linalyl Acetate (23%), 1,8-Cineole (3%), Terpinen-4-ol (5%), β-Farnesene (3%) | [48] | |
| Salvia officinalis | Thujone isomers (25%), Camphor (22%) | [49] |
| α-Thujone (16%), β-Thujone (11%), 1,8-Cineole (27%), Borneol (10%), Camphor (8%) | [48] | |
| Syzygium aromaticum | Thujone (25%), Camphor (22%), 1,8-Cineole (Eucalyptol) (11%), Thujone-Trans (7%), Caryophyllene (4%) | [6] |
| Pogostemon cablin | Patchouli Alcohol (23%), α-Guaiene (14%), γ-Patchoulene (9%), β-Patchoulene (7%), α-Bulnesene (17%) | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihaylova, S.; Tsvetkova, A.; Stamova, S.; Ermenlieva, N.; Tsankova, G.; Georgieva, E.; Peycheva, K.; Panayotova, V.; Voynikov, Y. Antibacterial Effects of Bulgarian Oregano and Thyme Essential Oils Alone and in Combination with Antibiotics Against Klebsiella pneumoniae and Pseudomonas aeruginosa. Microorganisms 2025, 13, 843. https://doi.org/10.3390/microorganisms13040843
Mihaylova S, Tsvetkova A, Stamova S, Ermenlieva N, Tsankova G, Georgieva E, Peycheva K, Panayotova V, Voynikov Y. Antibacterial Effects of Bulgarian Oregano and Thyme Essential Oils Alone and in Combination with Antibiotics Against Klebsiella pneumoniae and Pseudomonas aeruginosa. Microorganisms. 2025; 13(4):843. https://doi.org/10.3390/microorganisms13040843
Chicago/Turabian StyleMihaylova, Silviya, Antoaneta Tsvetkova, Sylvia Stamova, Neli Ermenlieva, Gabriela Tsankova, Emiliya Georgieva, Katya Peycheva, Veselina Panayotova, and Yulian Voynikov. 2025. "Antibacterial Effects of Bulgarian Oregano and Thyme Essential Oils Alone and in Combination with Antibiotics Against Klebsiella pneumoniae and Pseudomonas aeruginosa" Microorganisms 13, no. 4: 843. https://doi.org/10.3390/microorganisms13040843
APA StyleMihaylova, S., Tsvetkova, A., Stamova, S., Ermenlieva, N., Tsankova, G., Georgieva, E., Peycheva, K., Panayotova, V., & Voynikov, Y. (2025). Antibacterial Effects of Bulgarian Oregano and Thyme Essential Oils Alone and in Combination with Antibiotics Against Klebsiella pneumoniae and Pseudomonas aeruginosa. Microorganisms, 13(4), 843. https://doi.org/10.3390/microorganisms13040843









