Riboflavin Production by Steady-State Continuous Cultures of Hyphopichia wangnamkhiaoensis in a Bubble Column Bioreactor
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strain and Its Preservation
2.2. Culture Medium
2.3. Inoculum Preparation
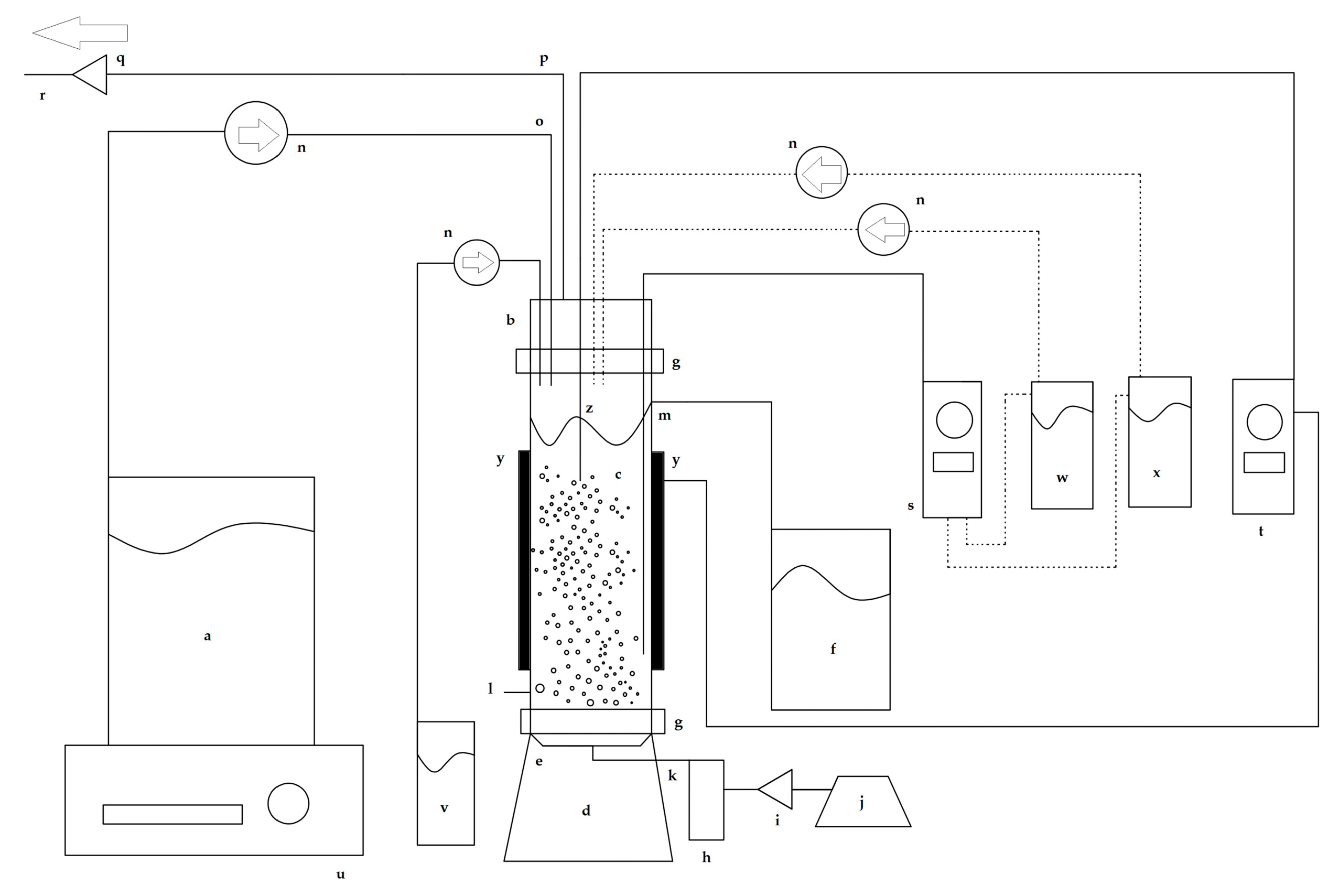
2.4. Bubble Column Bioreactor
2.5. Kinetics of Cell Growth, Glucose Consumption, and Riboflavin Production by H. wangnamkhiaoensis in a Chemostat
2.6. Analytical Methods
2.7. Determination of Kinetic Parameters
2.8. Data and Statistical Analyses
3. Results and Discussion
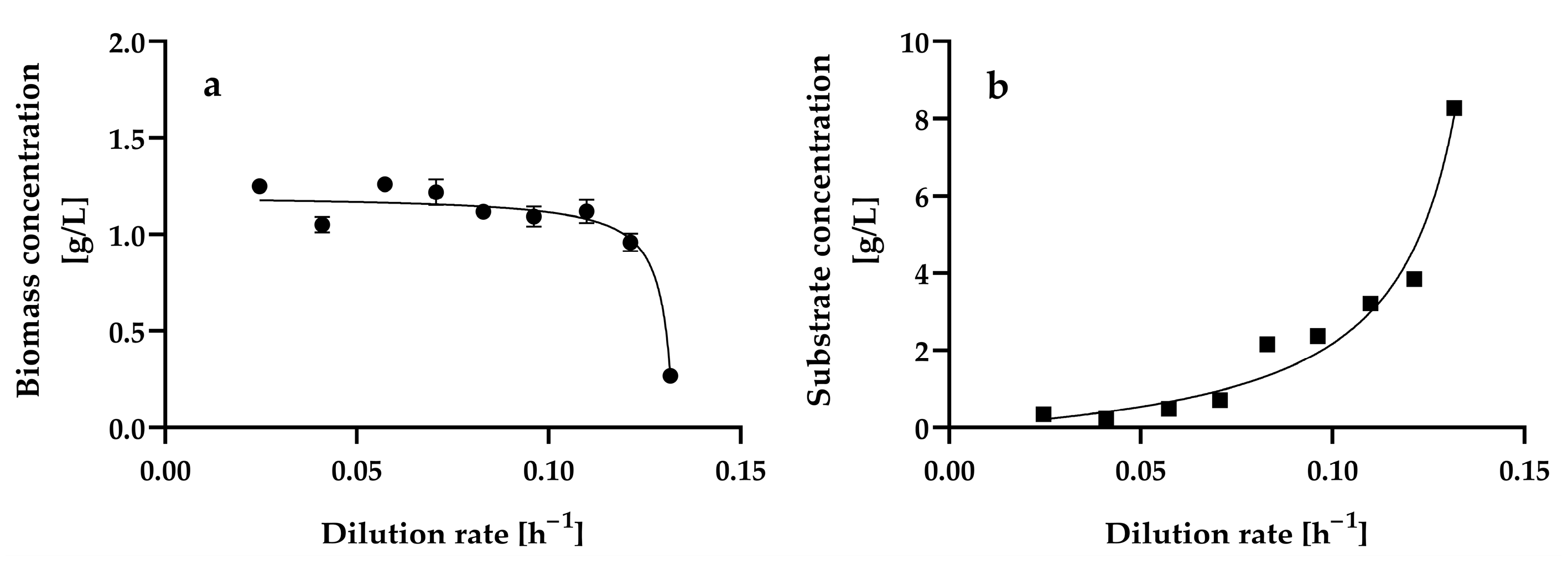
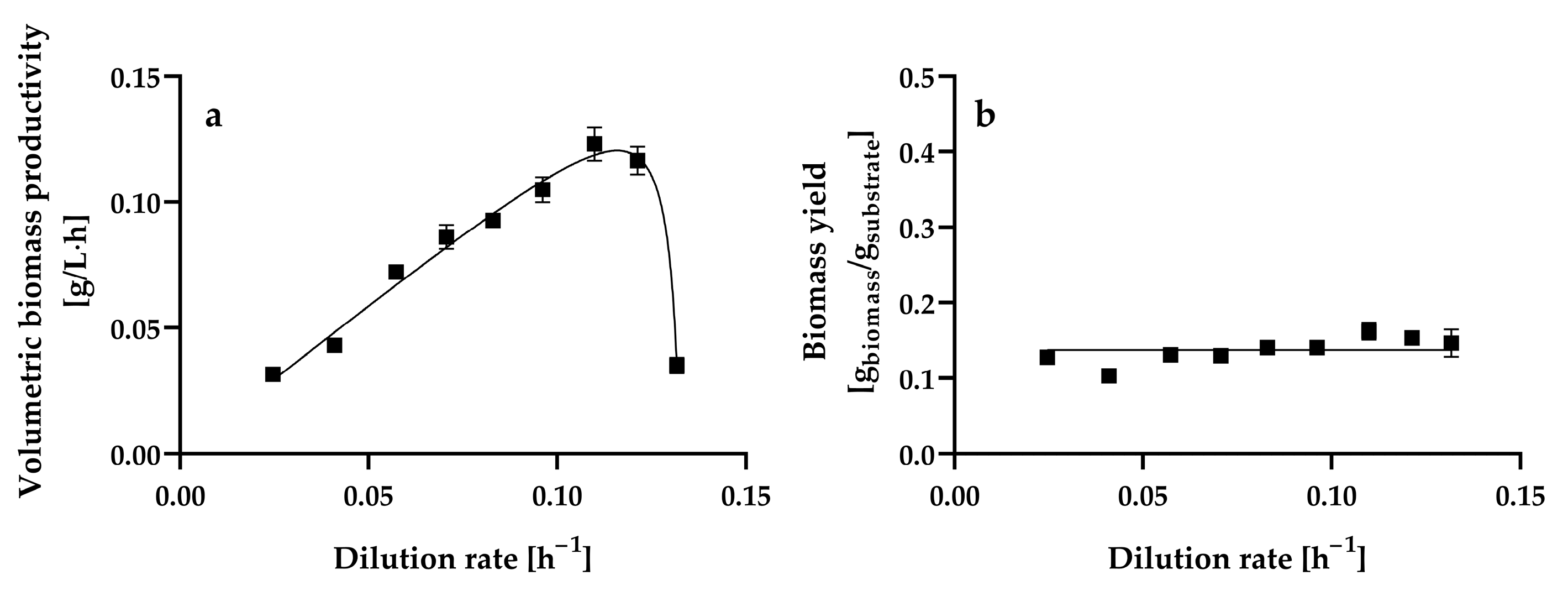
3.1. Effect of Dilution Rate on Cell Growth and Glucose Consumption by H. wangnamkhiaoensis
3.2. Determination of the H. wangnamkhiaoensis Maximum Specific Growth Rate (μmax), Cell Growth Model, Substrate Affinity Constant (Ks), Maximum Biomass Yield (Ymax), and Maintenance Energy Coefficient (m)
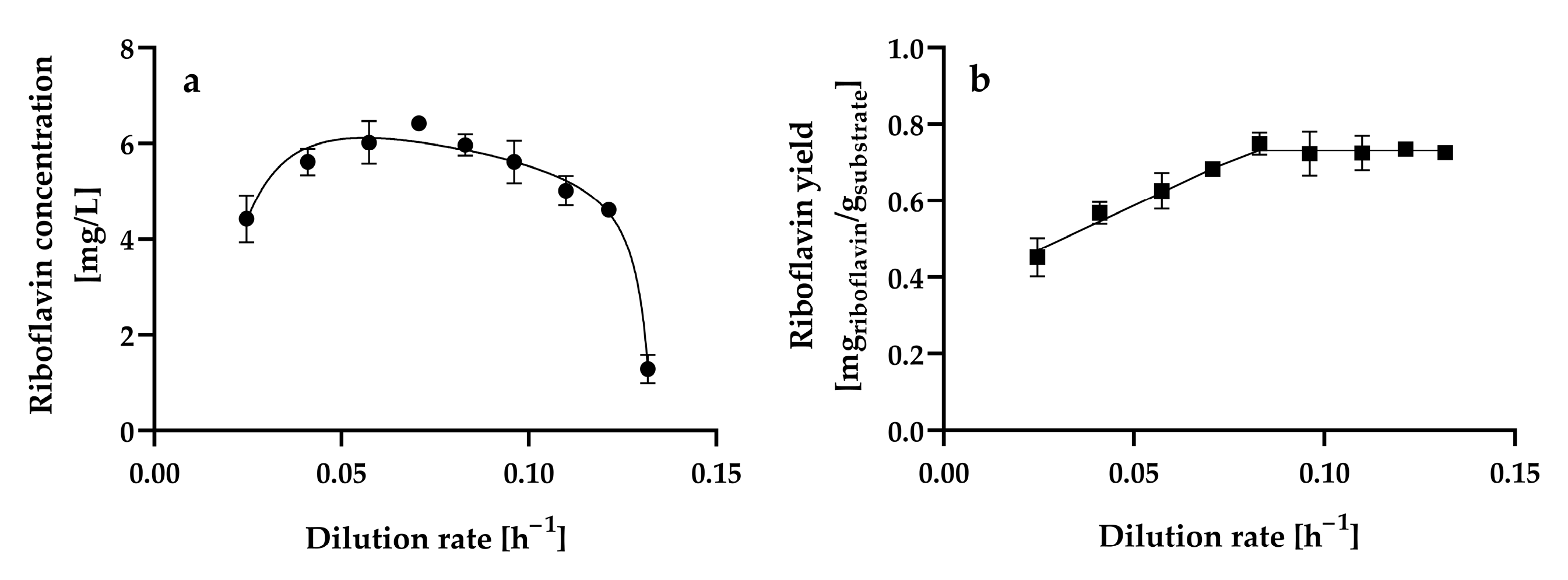
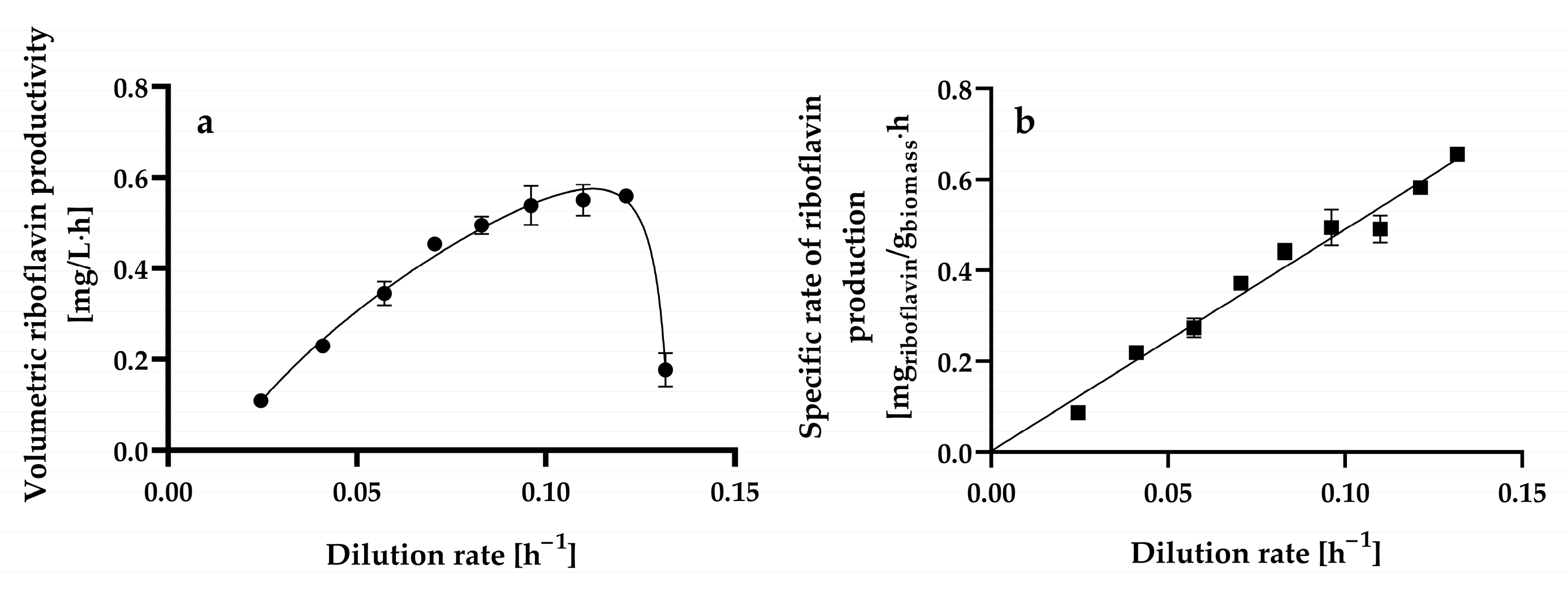
3.3. Effect of Dilution Rate on Riboflavin Production by H. wangnamkhiaoensis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lambertz, C.; Leopold, J.; Ammer, S.; Leiber, F.; Thesing, B.; Wild, C.; Damme, K. Demand-oriented riboflavin supply of organic broiler using a feed material from fermentation of Ashbya gossypii. Animal 2021, 15, 100003. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, S.K.; Park, E.Y.; Revuelta, J.L.; Becker, J.; Wittmann, C. Biotechnology of riboflavin. Appl. Microbiol. Biotechnol. 2016, 100, 2107–2119. [Google Scholar] [CrossRef] [PubMed]
- Stahmann, K.P.; Revuelta, J.L.; Seulberger, H. Three biotechnical processes using Ashbya gossypii, Candida famata, or Bacillus subtilis compete with chemical riboflavin production. Appl. Microbiol. Biotechnol. 2000, 53, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Suwannasom, N.; Kao, I.; Pruß, A.; Georgieva, R.; Bäumler, H. Riboflavin: The health benefits of a forgotten natural vitamin. Int. J. Mol. Sci. 2020, 21, 950. [Google Scholar] [CrossRef]
- Padonou, S.W.; Houngbédji, M.; Hounhouigan, M.H.; Chadare, F.J.; Hounhouigan, D.J. B-vitamins and heat processed fermented starchy and vegetable foods in sub-Saharan Africa: A review. J. Food Sci. 2023, 88, 3155–3188. [Google Scholar] [CrossRef]
- Fedorovych, D.V.; Dmytruk, K.V.; Sibirny, A.A. Recent advances in construction of the efficient producers of riboflavin and flavin nucleotides (FMN, FAD) in the yeast Candida famata. In Flavins and Flavoproteins: Methods and Protocols; Humana Press Incorporated: New York, NY, USA, 2021; Volume 2280, pp. 15–30. [Google Scholar] [CrossRef]
- Liu, C.; Xia, M.; Fang, H.; Xu, F.; Wang, S.; Zhang, D. De novo engineering riboflavin production Bacillus subtilis by overexpressing the downstream genes in the purine biosynthesis pathway. Microb. Cell Factories 2024, 23, 159. [Google Scholar] [CrossRef]
- Zhao, G.; Dong, F.; Lao, X.; Zheng, H. Strategies to increase the production of biosynthetic riboflavin. Mol. Biotechnol. 2021, 63, 909–918. [Google Scholar] [CrossRef]
- Ruchala, J.; Fedorovych, D.; Wojdyla, D.; Dmytruk, K.V.; Sibirny, A.A. Riboflavin Overproduction in Yeasts and Filamentous Fungi. In Biotechnology of Yeasts and Filamentous Fungi. Grand Challenges in Biology and Biotechnology; Sibirny, A.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2025; pp. 263–289. [Google Scholar] [CrossRef]
- Lim, S.H.; Choi, J.S.; Park, E.Y. Microbial production of riboflavin using riboflavin overproducers, Ashbya gossypii, Bacillus subtilis, and Candida famate: An overview. Biotechnol. Bioprocess Eng. 2001, 6, 75–88. [Google Scholar] [CrossRef]
- Zhang, J.-R.; Ge, Y.-Y.; Liu, P.-H.; Wu, D.-T.; Liu, H.-Y.; Li, H.-B.; Corke, H.; Gan, R.-Y. Biotechnological strategies of riboflavin biosynthesis in microbes. Engineering 2022, 12, 115–127. [Google Scholar] [CrossRef]
- Abbas, C.A.; Sibirny, A.A. Genetic control of biosynthesis and transport of riboflavin and flavin nucleotides and construction of robust biotechnological producers. Microbiol. Mol. Biol. Rev. 2011, 75, 321–360. [Google Scholar] [CrossRef]
- Averianova, L.A.; Balabanova, L.A.; Son, O.M.; Podvolotskaya, A.B.; Tekutyeva, L.A. Production of vitamin B2 (riboflavin) by microorganisms: An overview. Front. Bioeng. Biotechnol. 2020, 8, 570828. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Nava, R.A.; Chávez-Camarillo, G.M.; Cristiani-Urbina, E. Kinetics of riboflavin production by Hyphopichia wangnamkhiaoensis under varying nutritional conditions. Int. J. Mol. Sci. 2024, 25, 9430. [Google Scholar] [CrossRef] [PubMed]
- Amornrattanapan, P. Riboflavin production by Candida tropicalis isolated from seawater. Sci. Res. Essays 2013, 8, 43–47. [Google Scholar]
- Andreieva, Y.; Lyzak, O.; Liu, W.; Kang, Y.; Dmytruk, K.; Sibirny, A. SEF1 and VMA1 genes regulate riboflavin biosynthesis in the flavinogenic yeast Candida famata. Cytol. Genet. 2020, 54, 379–385. [Google Scholar] [CrossRef]
- Kurtzman, C.P. New species and a new combination in the Hyphopichia and Yarrowia yeast clades. Antonie Leeuwenhoek 2005, 88, 121–130. [Google Scholar] [CrossRef]
- Liu, S.; Hu, W.; Wang, Z.; Chen, T. Production of riboflavin and related cofactors by biotechnological processes. Microb. Cell Factories 2020, 19, 31. [Google Scholar] [CrossRef]
- Revuelta, J.L.; Ledesma-Amaro, R.; Lozano-Martinez, P.; Díaz-Fernández, D.; Buey, R.M.; Jiménez, A. Bioproduction of riboflavin: A bright yellow history. J. Ind. Microbiol. Biotechnol. 2017, 44, 659–665. [Google Scholar] [CrossRef]
- Russo, P.; Capozzi, V.; Arena, M.P.; Spadaccino, G.; Dueñas, M.T.; López, P.; Fiocco, D.; Spano, G. Riboflavin-overproducing strains of Lactobacillus fermentum for riboflavin-enriched bread. Appl. Microbiol. Biotechnol. 2014, 98, 3691–3700. [Google Scholar] [CrossRef]
- Petrovska, Y.; Lyzak, O.; Ruchala, J.; Dmytruk, K.; Sibirny, A. Co-overexpression of RIB1 and RIB6 increases riboflavin production in the yeast Candida famata. Fermentation 2022, 8, 141. [Google Scholar] [CrossRef]
- Chávez-Camarillo, G.M.; Lopez-Nuñez, P.V.; Jiménez-Nava, R.A.; Aranda-García, E.; Cristiani-Urbina, E. Production of extracellular α-amylase by single-stage steady-state continuous cultures of Candida wangnamkhiaoensis in an airlift bioreactor. PLoS ONE 2022, 17, e0264734. [Google Scholar] [CrossRef]
- Jiménez-Nava, R.A.; Zepeda-Vallejo, L.G.; Santoyo-Tepole, F.; Chávez-Camarillo, G.M.; Cristiani-Urbina, E. RP-HPLC separation and 1H NMR identification of a yellow fluorescent compound—Riboflavin (vitamin B2)—Produced by the yeast Hyphopichia wangnamkhiaoensis. Biomolecules 2023, 13, 1423. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, L.; Jin, Z.; Zhang, D. Microbial cell factories for green production of vitamins. Front. Bioeng. Biotechnol. 2021, 9, 661562. [Google Scholar] [CrossRef]
- Buzzini, P.; Rossi, J. Semi-continuous and continuous riboflavin production by calcium-alginate-immobilized Candida tropicalis in concentrated rectified grape must. World J. Microbiol. Biotechnol. 1998, 14, 377–381. [Google Scholar] [CrossRef]
- Enari, T.-M.; Kauppinen, V. Interaction of cobalt and iron in riboflavine production of Candida guilliermondii. Acta Chem. Scand. 1961, 15, 1513–1516. [Google Scholar] [CrossRef]
- Schlösser, T.; Wiesenburg, A.; Gätgens, C.; Funke, A.; Viets, U.; Vijayalakshmi, S.; Nieland, S.; Stahmann, K.P. Growth stress triggers riboflavin overproduction in Ashbya gossypii. Appl. Microbiol. Biotechnol. 2007, 76, 569–578. [Google Scholar] [CrossRef]
- Stahmann, K.P.; Arst, H.N.; Althöfer, H.; Revuelta, J.L.; Monschau, N.; Schlüpen, C.; Gätgens, C.; Wiesenburg, A.; Schlösser, T. Riboflavin, overproduced during sporulation of Ashbya gossypii, protects its hyaline spores against ultraviolet light. Environ. Microbiol. 2001, 3, 545–550. [Google Scholar] [CrossRef]
- Dauner, M.; Sonderegger, M.; Hochuli, M.; Szyperski, T.; Wüthrich, K.; Hohmann, H.-P.; Sauer, U.; Bailey, J.E. Intracellular carbon fluxes in riboflavin-producing Bacillus subtilis during growth on two-carbon substrate mixtures. Appl. Environ. Microbiol. 2002, 68, 1760–1771. [Google Scholar] [CrossRef]
- Sauer, U.; Hatzimanikatis, V.; Hohmann, H.P.; Manneberg, M.; van Loon, A.P.; Bailey, J.E. Physiology and metabolic fluxes of wild-type and riboflavin-producing Bacillus subtilis. Appl. Environ. Microbiol. 1996, 62, 3687–3696. [Google Scholar] [CrossRef]
- Zamboni, N.; Mouncey, N.; Hohmann, H.-P.; Sauer, U. Reducing maintenance metabolism by metabolic engineering of respiration improves riboflavin production by Bacillus subtilis. Metab. Eng. 2003, 5, 49–55. [Google Scholar] [CrossRef]
- Zamboni, N.; Fischer, E.; Muffler, A.; Wyss, M.; Hohmann, H.-P.; Sauer, U. Transient expression and flux changes during a shift from high to low riboflavin production in continuous cultures of Bacillus subtilis. Biotechnol. Bioeng. 2005, 89, 219–232. [Google Scholar] [CrossRef]
- Ariff, A.B.; Nelofer, R.; Abdul Rahman, R.N.Z.R.A.; Basri, M. Kinetics and modelling of batch fermentation for the production of organic solvent tolerant and thermostable lipase by recombinant E. coli/Organik çözücü toleranslı ve ısıya dayanıklı rekombinan E. coli lipaz üretiminin kinetiği ve grup fermentasyonu modellemesi. Turk. J. Biochem. 2015, 40, 298–309. [Google Scholar] [CrossRef]
- Ariyajaroenwong, P.; Laopaiboon, P.; Salakkam, A.; Srinophakun, P.; Laopaiboon, L. Kinetic models for batch and continuous ethanol fermentation from sweet sorghum juice by yeast immobilized on sweet sorghum stalks. J. Taiwan Inst. Chem. Eng. 2016, 66, 210–216. [Google Scholar] [CrossRef]
- Boden, R.; Hutt, L.P. Determination of kinetic parameters and metabolic modes using the chemostat. In Consequences of Microbial Interactions with Hydrocarbons, Oils, and Lipids: Biodegradation and Bioremediation; Steffan, R.J., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 363–404. [Google Scholar] [CrossRef]
- Nieto-Taype, M.A.; Garcia-Ortega, X.; Albiol, J.; Montesinos-Seguí, J.L.; Valero, F. Continuous cultivation as a tool toward the rational bioprocess development with Pichia pastoris cell factory. Front. Bioeng. Biotechnol. 2020, 8, 632. [Google Scholar] [CrossRef]
- Rastädter, K.; Wurm, D.J.; Spadiut, O.; Quehenberger, J. Physiological characterization of Sulfolobus acidocaldarius in a controlled bioreactor environment. Int. J. Environ. Res. Public Health 2021, 18, 5532. [Google Scholar] [CrossRef]
- Karamerou, E.E.; Webb, C. Cultivation modes for microbial oil production using oleaginous yeasts—A review. Biochem. Eng. J. 2019, 151, 107322. [Google Scholar] [CrossRef]
- Li, T.; Chen, X.B.; Chen, J.C.; Wu, Q.; Chen, G.-Q. Open and continuous fermentation: Products, conditions and bioprocess economy. Biotechnol. J. 2014, 9, 1503–1511. [Google Scholar] [CrossRef]
- Richter, H.; Martin, M.E.; Angenent, L.T. A two-stage continuous fermentation system for conversion of syngas into ethanol. Energies 2013, 6, 3987–4000. [Google Scholar] [CrossRef]
- Berenjian, A.; Mahdinia, E.; Demirci, A. Sustainable menaquinone-7 production through continuous fermentation in biofilm bioreactors. Bioprocess Biosyst. Eng. 2024, 47, 1107–1116. [Google Scholar] [CrossRef]
- Brethauer, S.; Wyman, C.E. Review: Continuous hydrolysis and fermentation for cellulosic ethanol production. Bioresour. Technol. 2010, 101, 4862–4874. [Google Scholar] [CrossRef]
- Pirt, S.J.; Callow, D.S. Studies of the growth of Penicillium chrysogenum in continuous flow culture with reference to penicillin production. J. Appl. Bacteriol. 1960, 23, 87–98. [Google Scholar] [CrossRef]
- Chávez-Camarillo, G.M.; Santiago-Flores, U.M.; Mena-Vivanco, A.; Morales-Barrera, L.; Cortés-Acosta, E.; Cristiani-Urbina, E. Transient responses of Wickerhamia sp. yeast continuous cultures to qualitative changes in carbon source supply: Induction and catabolite repression of α-amylase synthesis. Ann. Microbiol. 2018, 68, 625–635. [Google Scholar] [CrossRef]
- Monod, J. Recherches sur la Croissance des Cultures Bactériennes; Hermann: Paris, France, 1942. [Google Scholar]
- Monod, J. The growth of bacterial cultures. Annu. Rev. Microbiol. 1949, 3, 371–394. [Google Scholar] [CrossRef]
- Berlanga, M.; Guerrero, R. Living together in biofilms: The microbial cell factory and its biotechnological implications. Microb. Cell Factories 2016, 15, 165. [Google Scholar] [CrossRef] [PubMed]
- Galíndez-Mayer, C.J.J.; Ruíz-Ordaz, N. Bioingeniería. Fundamentos Biocinéticos Para el Diseño de Procesos Fermentativos; Vargas-Chávez, J., Ed.; Escuela Nacional de Ciencias Biológicas: Mexico City, Mexico, 1994. [Google Scholar]
- Wang, D.I.C.; Cooney, C.L.; Demain, A.L.; Dunnill, P.; Humprey, A.E.; Lilly, M.D. Continuous culture. In Fermentation and Enzyme Technology; John Wiley & Sons Inc.: Chichester, UK, 1979; pp. 98–137. [Google Scholar]
- Tempest, D.W. Dynamics of microbial growth. In Essays in Microbiology; Norris, J.R., Richmond, M.H., Eds.; John Wiley & Sons, Limited: Chichester, UK, 1978; pp. 7–32. [Google Scholar]
- Moser, A. Bioprocess Kinetics. In Bioprocess Technology; Moser, A., Ed.; Springer: New York, NY, USA, 1988; pp. 197–306. [Google Scholar] [CrossRef]
- Sinclair, C.G.; Kristiansen, B. Fermentation Kinetics and Modelling; Taylor & Francis: New York, NY, USA, 1987. [Google Scholar]
- Rieger, M.; Käppeli, O.; Fiechter, A. The role of limited respiration in the incomplete oxidation of glucose by Saccharomyces cerevisiae. Microbiology 1983, 129, 653–661. [Google Scholar] [CrossRef]
- Postma, E.; Scheffers, W.A.; Van Dijken, J.P. Kinetics of growth and glucose transport in glucose-limited chemostat cultures of Saccharomyces cerevisiae CBS 8066. Yeast 1989, 5, 159–165. [Google Scholar] [CrossRef]
- Toda, K.; Yabe, I.; Yamagata, T. Kinetics of biphasic growth of yeast in continuous and fed-batch cultures. Biotechnol. Bioeng. 1980, 22, 1805–1827. [Google Scholar] [CrossRef]
- Verduyn, C.; Stouthamer, A.H.; Scheffers, W.A.; van Dijken, J.P. A theoretical evaluation of growth yields of yeasts. Antonie Leeuwenhoek 1991, 59, 49–63. [Google Scholar] [CrossRef]
- Verduyn, C. Physiology of yeasts in relation to biomass yields. Antonie Leeuwenhoek 1991, 60, 325–353. [Google Scholar] [CrossRef]
- Blanch, H.W.; Clark, D.S. Biochemical Engineering; Marcel Dekker Inc.: New York, NY, USA, 1997. [Google Scholar]
- Herbert, D.; Elsworth, R.; Telling, R.C. The continuous culture of bacteria: A theoretical and experimental study. J. Gen. Microbiol. 1956, 14, 601–622. [Google Scholar] [CrossRef]
- Atkinson, B.; Mavituna, F. Biochemical Engineering and Biotechnology Handbook, 2nd ed.; Stockton Press: New York, NY, USA, 1991. [Google Scholar]
- Leuenberger, H.G.W. Cultivation of Saccharomyces cerevisiae in continuous culture. II. Influence of the crabtree effect on the growth characteristics of Saccharomyces cerevisiae grown in a glucose limited chemostat. Arch. Mikrobiol. 1972, 83, 347–358. [Google Scholar] [CrossRef]
- O’Neil, D.G.; Lyberatos, G. Dynamic model development for a continuous culture of Saccharomyces cerevisiae. Biotechnol. Bioeng. 1990, 36, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Papagianni, M.; Boonpooh, Y.; Mattey, M.; Kristiansen, B. Substrate inhibition kinetics of Saccharomyces cerevisiae in fed-batch cultures operated at constant glucose and maltose concentration levels. J. Ind. Microbiol. Biotechnol. 2007, 34, 301–309. [Google Scholar] [CrossRef] [PubMed]
- van Bodegom, P. Microbial maintenance: A critical review on its quantification. Microb. Ecol. 2007, 53, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Pirt, S.J. The maintenance energy of bacteria in growing cultures. Proc. R. Soc. Lond. B Biol. Sci. 1965, 163, 224–231. [Google Scholar] [CrossRef]
- Luedeking, R.; Piret, E.L. A kinetic study of the lactic acid fermentation. Batch process at controlled pH. J. Biochem. Microbiol. Technol. Eng. 1959, 1, 393–412. [Google Scholar] [CrossRef]
- Mu, Y.; Yang, H.-Y.; Wang, Y.-Z.; He, C.-S.; Zhao, Q.-B.; Wang, Y.; Yu, H.-Q. The maximum specific hydrogen-producing activity of anaerobic mixed cultures: Definition and determination. Sci. Rep. 2014, 4, 5239. [Google Scholar] [CrossRef]
- Thierie, J. Computing and interpreting specific production rates in a chemostat in steady state according to the Luedeking-Piret model. Appl. Biochem. Biotechnol. 2013, 169, 477–492. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Nava, R.A.; Chávez-Camarillo, G.M.; Cristiani-Urbina, E. Riboflavin Production by Steady-State Continuous Cultures of Hyphopichia wangnamkhiaoensis in a Bubble Column Bioreactor. Microorganisms 2025, 13, 817. https://doi.org/10.3390/microorganisms13040817
Jiménez-Nava RA, Chávez-Camarillo GM, Cristiani-Urbina E. Riboflavin Production by Steady-State Continuous Cultures of Hyphopichia wangnamkhiaoensis in a Bubble Column Bioreactor. Microorganisms. 2025; 13(4):817. https://doi.org/10.3390/microorganisms13040817
Chicago/Turabian StyleJiménez-Nava, Raziel Arturo, Griselda Ma. Chávez-Camarillo, and Eliseo Cristiani-Urbina. 2025. "Riboflavin Production by Steady-State Continuous Cultures of Hyphopichia wangnamkhiaoensis in a Bubble Column Bioreactor" Microorganisms 13, no. 4: 817. https://doi.org/10.3390/microorganisms13040817
APA StyleJiménez-Nava, R. A., Chávez-Camarillo, G. M., & Cristiani-Urbina, E. (2025). Riboflavin Production by Steady-State Continuous Cultures of Hyphopichia wangnamkhiaoensis in a Bubble Column Bioreactor. Microorganisms, 13(4), 817. https://doi.org/10.3390/microorganisms13040817







