Unlocking Nature’s Microbial Defenders: Genetic Mechanisms and Potential Against Monilinia spp. Pathogens
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Microorganisms
2.2. Evaluation of Morphological Characteristics of Microorganism Isolates
2.3. In Vitro Inhibition Assay for Monilinia spp.
2.4. Evaluation of Genetic Profiles of Potential Antagonists
3. Results
3.1. Morphology of Antagonistic Microorganisms
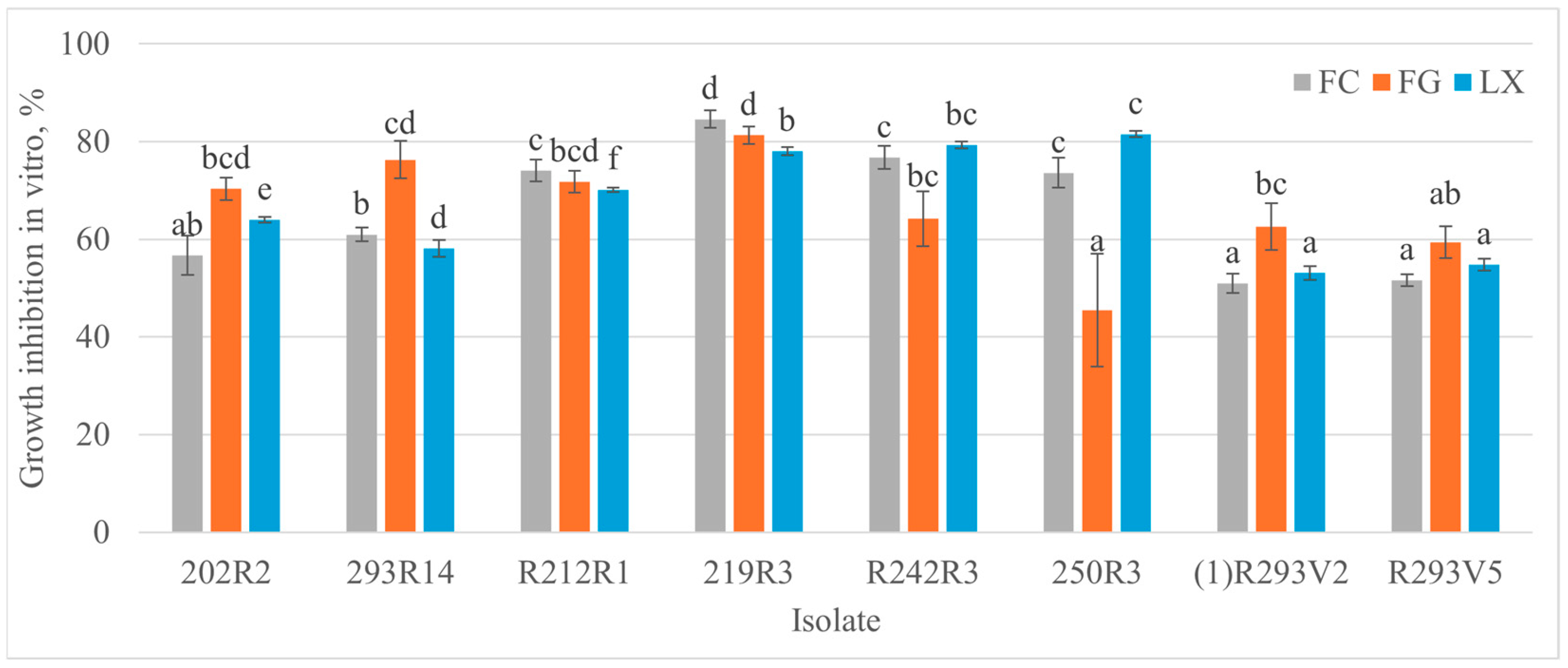
3.2. Antagonistic Activity of Microorganisms Against Monilinia spp. In Vitro
3.3. Genetic Characteristics of the Isolated Microbial Strains
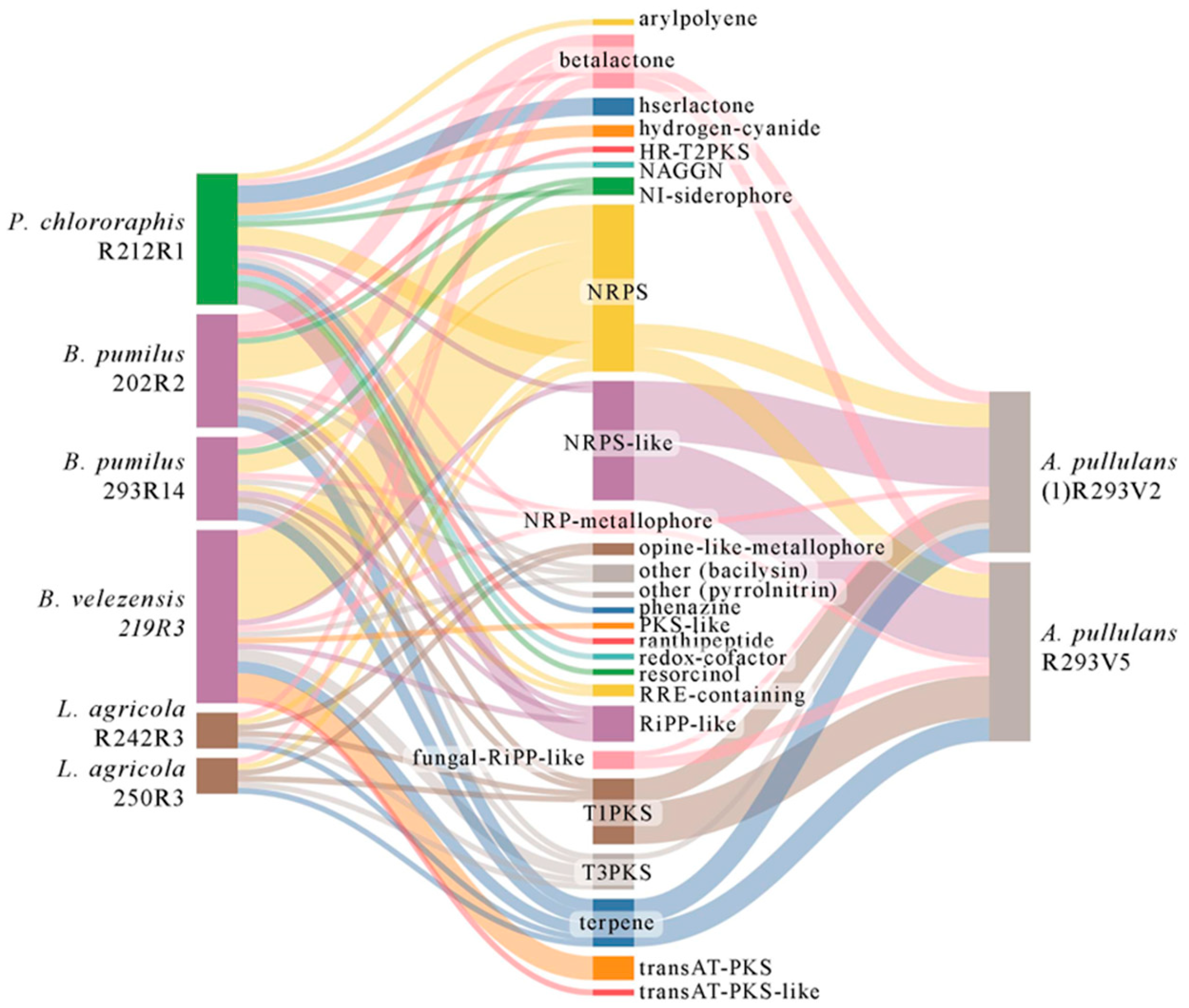
3.4. Genetic Background for Synthesizing Secondary Metabolites
3.5. Genes Linked with Plant Growth Promotion (PGP) and Antagonism
3.6. Pathogenicity and Virulence Annotation of Isolated Bacteria
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pageni, B.B.; Lupwayi, N.Z.; Akter, Z.; Larney, F.J.; Kawchuk, L.M.; Gan, Y. Plant growth-promoting and phytopathogen-antagonistic properties of bacterial endophytes from potato (Solanum tuberosum L.) cropping systems. Can. J. Plant Sci. 2014, 94, 835–844. [Google Scholar] [CrossRef]
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.S.; Patra, J.K. Endophytes: A treasure house of bioactive compounds of medicinal importance. Front. Microbiol. 2016, 7, 1538. [Google Scholar] [CrossRef]
- Ghadamgahi, F.; Tarighi, S.; Taheri, P.; Saripella, G.V.; Anzalone, A.; Kalyandurg, P.B.; Catara, V.; Ortiz, R.; Vetukuri, R.R. Plant growth-promoting activity of Pseudomonas aeruginosa FG106 and its ability to act as a biocontrol agent against potato, tomato and taro pathogens. Biology 2022, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G.; Moreno-Hagelsieb, G.; Orozco-Mosqueda Mdel, C.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Li, N.; Wang, S.; Ma, L.; Liu, Y.; Xiu, Y.; Li, X. Phosphate-solubilizing capacity of two bacteria strains and its effect on maize growth and the phosphorus fractions in red soil. J. Plant Nutr. Fertil. 2021, 27, 275–283. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Z.; Zhao, Q.; Yang, X.; Li, Y.; Zhou, H.; Zhao, M.; Zheng, H. Whole-Genome Analysis revealed the growth-promoting and biological control mechanism of the endophytic bacterial strain Bacillus halotolerans Q2H2, with strong antagonistic activity in potato plants. Front. Microbiol. 2024, 14, 1287921. [Google Scholar] [CrossRef]
- Garzón-Posse, F.; Quevedo-Acosta, Y.; Mahecha-Mahecha, C.; Acosta-Guzmán, P. Recent progress in the synthesis of naturally occurring siderophores. Eur. J. Org. Chem. 2019, 2019, 7747–7769. [Google Scholar] [CrossRef]
- Waksman, S.A.; Woodruff, B.H. The soil as a source of microorganisms antagonistic to disease-producing bacteria. J. Bacteriol. 1940, 40, 581–600. [Google Scholar]
- Berendsen, R.L.; Vismans, G.; Yu, K.; Song, Y.; de Jonge, R.; Burgman, W.P. Disease-induced assemblage of a plant-beneficial bacterial consortium. ISME J. 2018, 12, 1496–1507. [Google Scholar] [CrossRef]
- Babalola, O.O. Beneficial bacteria of agricultural importance. Biotechnol. Lett. 2010, 32, 1559–1570. [Google Scholar] [CrossRef]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Arrebola, E.; Tienda, S.; Vida, C.; de Vicente, A.; Cazorla, F.M. Fitness features involved in the biocontrol interaction of Pseudomonas chlororaphis with host plants: The case study of PcPCL1606. Front. Microbiol. 2019, 10, 719. [Google Scholar] [CrossRef] [PubMed]
- Boro, M.; Sannyasi, S.; Chettri, D.; Verma, A.K. Microorganisms in biological control strategies to manage microbial plant pathogens: A review. Arch. Microbiol. 2022, 204, 666. [Google Scholar] [CrossRef] [PubMed]
- Arrebola, E.; Jacobs, R.; Korsten, L. Iturin A is the principal inhibitor in the biocontrol activity of Bacillus amyloliquefaciens PPCB004 against postharvest fungal pathogens. J. Appl. Microbiol. 2010, 108, 386–395. [Google Scholar] [CrossRef]
- Zeriouh, H.; Romero, D.; Garcia-Gutierrez, L.; Cazorla, F.M.; de Vicente, A.; Perez-Garcia, A. The Iturin-like lipopeptides are essential components in the biological control arsenal of Bacillus subtilis against bacterial diseases of cucurbits. Mol. Plant-Microbe Interact. 2011, 24, 1540–1552. [Google Scholar] [CrossRef]
- Bonaterra, A.; Badosa, E.; Daranas, N.; Francés, J.; Roselló, G.; Montesinos, E. Bacteria as biological control agents of plant diseases. Microorganisms 2022, 10, 1759. [Google Scholar] [CrossRef]
- Zhou, J.; Xie, Y.; Liao, Y.; Li, X.; Li, Y.; Li, S.; Ma, X.; Lei, S.; Lin, F.; Jiang, W.; et al. Characterization of a Bacillus velezensis strain isolated from Bolbostemmatis rhizoma displaying strong antagonistic activities against a variety of rice pathogens. Front. Microbiol. 2022, 13, 983781. [Google Scholar] [CrossRef]
- Berrie, A.M.; Holb, I. Brown rot diseases. In Compendium of Apple and Pear Diseases and Pests, 2nd ed.; Sutton, T.B., Aldwinckle, H.S., Agnello, A.M., Walgenbach, J.F., Eds.; American Phytopathological Society: St Paul, MN, USA, 2014; pp. 43–45. [Google Scholar]
- Tsalgatidou, P.C.; Boutsika, A.; Papageorgiou, A.G.; Dalianis, A.; Michaliou, M.; Chatzidimopoulos, M.; Delis, C.; Tsitsigiannis, D.I.; Paplomatas, E.; Zambounis, A. Global transcriptome analysis of the peach (Prunus persica) in the interaction system of fruit–chitosan–Monilinia fructicola. Plants 2024, 13, 567. [Google Scholar] [CrossRef]
- Fenta, L.; Mekonnen, H. Microbial biofungicides as a substitute for chemical fungicides in the control of phytopathogens: Current perspectives and research directions. Scientifica 2024, 2024, 5322696. [Google Scholar] [CrossRef]
- Hrustic, J.; Medic, O.; Beric, T.; Mihajlovic, M.; Milijaševic-Marčic, S.; Stankovic, S.; Pešic, B. Suppression of Monilinia brown rot by Bacillus spp. strains. Agronomy 2023, 13, 2839. [Google Scholar] [CrossRef]
- Franco-Sierra, N.D.; Posada, L.F.; Santa-Maria, G.; Romero-Tabarez, M.; Villegas-Escobar, V.; Alvarez, J.C. Bacillus subtilis EA-CB0575 genome reveals clues for plant growth promotion and potential for sustainable agriculture. Funct. Integr. Genom. 2020, 20, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Coulson, T.J.D.; Patten, C.L. Complete genome sequence of Enterobacter cloacae UW5, a Rhizobacterium capable of high levels of Indole-3-acetic acid production. Genome Announc. 2015, 3, e00843-15. [Google Scholar] [CrossRef] [PubMed]
- Asaf, S.; Khan, A.L.; Khan, M.A.; Al-Harrasi, A.; Lee, I.J. Complete genome sequencing and analysis of endophytic Sphingomonas sp. LK11 and its potential in plant growth. 3 Biotech 2018, 8, 389. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Ullah, N.; Janjua, H.A. In vitro evaluation and genome mining of Bacillus subtilis strain RS10 reveals its biocontrol and plant growth-promoting potential. Agriculture 2021, 11, 1273. [Google Scholar] [CrossRef]
- Kolytaitė, A.; Vaitiekūnaitė, D.; Antanynienė, R.; Baniulis, D.; Frercks, B. Monilinia fructigena suppressing and plant growth promoting endophytic Pseudomonas spp. bacteria isolated from plum. Microorganisms 2022, 10, 2402. [Google Scholar] [CrossRef]
- Powers, E.M. Efficacy of the Ryu nonstaining KOH technique for rapidly determining Gram reactions of food-borne and waterborne bacteria and yeasts. Appl. Environ. Microbiol. 1995, 61, 3756–3758. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Manni, M.; Berkeley, M.R.; Seppey, M.; Zdobnov, E.M. BUSCO: Assessing genomic data quality and beyond. Curr. Protoc. 2021, 1, e323. [Google Scholar] [CrossRef]
- NCBI Resource Coordinators. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2018, 46, D8–D13. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 1, 27–30. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; van Weezel, G.P.; Medema, M.H.; Weber, T. antiSMASH 6.0: Improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021, 48, W29–W35. [Google Scholar] [CrossRef] [PubMed]
- Alcock, B.P.; Huynh, W.; Chalil, R.; Smith, K.W.; Raphenya, A.R.; Wlodarski, M.A.; Edalatmand, A.; Petkau, A.; Syed, S.A.; Tsang, K.K.; et al. CARD 2023: Expanded curation, support for machine learning, and resistome prediction at the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2023, 51, D690–D699. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, S.; Voldby, L.M.; Møller, A.F.; Lund, O. PathogenFinder—Distinguishing friend from foe using bacterial whole genome sequence data. PLoS ONE 2013, 8, e77302. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, D.; Jin, Q.; Chen, L.; Yang, J. VFDB 2019: A comparative pathogenomic platform with an interactive web interface. Nucleic Acids Res. 2019, 47, D687–D692. [Google Scholar] [CrossRef]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]
- Price, C.L.; Parker, J.E.; Warrilow, A.G.; Kelly, D.E.; Kelly, S.L. Azole fungicides—Understanding resistance mechanisms in agricultural fungal pathogens. Pest. Manag. Sci. 2015, 71, 1054–1058. [Google Scholar] [CrossRef]
- Selim, R.E.; Khalil, M.S. Strobilurins: New group of fungicides. J. Plant Sci. Phytopathol. 2021, 5, 63–64. [Google Scholar] [CrossRef]
- Chen, F.; Everhart, S.E.; Bryson, P.K.; Luo, C.; Song, X.; Liu, X.; Schnabel, G. Fungicide-induced transposon movement in Monilinia fructicola. Fungal Genet. Biol. 2015, 85, 38–44. [Google Scholar] [CrossRef]
- Martini, C.; Mari, M. Monilinia fructicola, Monilinia laxa (Monilinia Rot, Brown Rot). In Postharvest Decay; Bautista-Baños, S., Ed.; Academic Press: Cambridge, MA, USA, 2014; Chapter 7; pp. 233–265. ISBN 9780124115521. [Google Scholar] [CrossRef]
- Jayawardana, M.A.; Fernando, W.G.D. The mechanisms of developing fungicide resistance in Fusarium graminearum causing Fusarium head blight and fungicide resistance management. Pathogens 2024, 13, 1012. [Google Scholar] [CrossRef]
- Luo, C.-X.; Schnabel, G. Adaptation to fungicides in Monilinia fructicola isolates with different fungicide resistance phenotypes. Phytopathology 2008, 98, 230–238. [Google Scholar] [CrossRef]
- Van Lenteren, J.C.; Bolckmans, K.; Köhl, J. Biological control using invertebrates and microorganisms: Plenty of new opportunities. BioControl 2018, 63, 39–59. [Google Scholar] [CrossRef]
- Shi, A.; Fan, F.; Broach, J.R. Microbial adaptive evolution. J. Indust. Microbiol. Biotechnol. 2022, 49, kuab076. [Google Scholar] [CrossRef]
- Lu, J.R.; Liu, G.H. Lysinibacillus agricola sp. nov., isolated from soil. Arch. Microbiol. 2021, 203, 4173–4178. [Google Scholar] [CrossRef] [PubMed]
- Jamal, Q.M.S.; Ahmad, V. Lysinibacilli: A biological factories intended for bio-insecticidal, bio-control, and bioremediation activities. J. Fungi 2022, 8, 1288. [Google Scholar] [CrossRef]
- Rabbee, M.F.; Ali, M.S.; Choi, J.; Hwang, B.S.; Jeong, S.C.; Baek, K.H. Bacillus velezensis: A valuable member of bioactive molecules within plant microbiomes. Molecules 2019, 16, 1046. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Dai, G.; Zhang, Y.; Bian, X. Microbial chassis engineering drives heterologous production of complex secondary metabolites. Biotech. Adv. 2022, 59, 107966. [Google Scholar] [CrossRef]
- Samson, R.A.; Hoekstra, E.S.; Frisvad, J.C. Introduction to Food and Airborne Fungi, 7th ed.; Centralalbureau voor Schimmellcultures; Institute of the Royal Netherlands Academy of Arts and Sciences: Utrecht, The Netherlands, 2004; 389p. [Google Scholar]
- Bozoudi, D.; Tsaltas, D. The multiple and versatile roles of Aureobasidium pullulans in the vitivinicultural sector. Fermentation 2018, 4, 85. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Wang, C.; Liu, S. Transcriptomic and metabolomic analyses reveal the antifungal mechanism of the compound phenazine-1-carboxamide on Rhizoctonia solani AG1IA. Front. Plant Sci. 2022, 13, 1041733. [Google Scholar] [CrossRef]
- Bertani, I.; Zampieri, E.; Bez, C.; Volante, A.; Venturi, V.; Monaco, S. Isolation and characterization of Pseudomonas chlororaphis strain ST9; Rhizomicrobiota and in planta studies. Plants 2021, 10, 1466. [Google Scholar] [CrossRef]
- Mari, M.; Martini, C.; Guidarelli, M.; Neri, F. Postharvest biocontrol of Monilinia laxa, Monilinia fructicola and Monilinia fructigena on stone fruit by two Aureobasidium pullulans strains. Biol. Contr. 2012, 60, 132–140. [Google Scholar] [CrossRef]
- Naureen, Z.; Rehman, N.U.; Hussain, H.; Hussain, J.; Gilani, S.A.; Al Housni, S.K.; Mabood, F.; Khan, A.L.; Farooq, S.; Abbas, G.; et al. Exploring the potentials of Lysinibacillus sphaericus ZA9 for plant growth promotion and biocontrol activities against phytopathogenic fungi. Front. Microbiol. 2017, 8, 1477. [Google Scholar] [CrossRef]
- Islam, T.; Rabbee, M.F.; Choi, J.; Baek, K.H. Biosynthesis, molecular regulation, and application of bacilysin produced by Bacillus species. Metabolites 2022, 27, 397. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Zhong, Z.; Chao, S.; Liu, L.; Chen, M.; Feng, X.; Wu, H. Antifungal effect of Bacillus velezensis ZN-S10 against plant pathogen Colletotrichum changpingense and its inhibition mechanism. Int. J. Mol. Sci. 2023, 24, 16694. [Google Scholar] [CrossRef] [PubMed]
- Scales, B.S.; Dickson, R.P.; LiPuma, J.J.; Huffnagle, G.B. Microbiology, genomics, and clinical significance of the Pseudomonas fluorescens species complex, an unappreciated colonizer of humans. Clin. Microbiol. Rev. 2014, 27, 927–948. [Google Scholar] [CrossRef]
- Anderson, A.J.; Young Cheol, K. Biopesticides produced by plant-probiotic Pseudomonas chlororaphis isolates. Crop Prot. 2018, 105, 62–69, ISSN 0261-2194. [Google Scholar] [CrossRef]
- Jong, H.; Arshed, S.; Yu, J.; Cornish, D.; Vanneste, J.L.; Reglinski, T.; Elmer, P.A.G.; Templeton, M.D. A high-quality genome assembly for Aureobasidium pullulans CG163, the active ingredient in Aureo Gold, a bio-bactericide registered for control of bacterial canker of kiwifruit caused by Pseudomonas syringae pv. actinidiae Biovar 3. PhytoFrontiers 2024, 4, 486–490. [Google Scholar]
- Cheng, M.; Zhao, S.; Liu, H.; Liu, Y.; Lin, C.; Song, J.; Thawai, C.; Charoensettasilp, S.; Yang, Q. Functional analysis of a chaetoglobosin A biosynthetic regulator in Chaetomium globosum. Fungal Biol. 2021, 125, 201–210. [Google Scholar] [CrossRef]
- Pudova, D.S.; Toymentseva, A.A.; Gogoleva, N.E.; Shagimardanova, E.I.; Mardanova, A.M.; Sharipova, M.R. Comparative genome analysis of two Bacillus pumilus strains producing high level of extracellular hydrolases. Genes 2022, 13, 409. [Google Scholar] [CrossRef]
- Dobrzyński, J.; Jakubowska, Z.; Dybek, B. Potential of Bacillus pumilus to directly promote plant growth. Front. Microbiol. 2022, 13, 1069053. [Google Scholar] [CrossRef]
- Dobrzyński, J.; Jakubowska, Z.; Kulkova, I.; Kowalczyk, P.; Kramkowski, K. Biocontrol of fungal phytopathogens by Bacillus pumilus. Front. Microbiol. 2023, 14, 1194606. [Google Scholar] [CrossRef]
- Rabbee, M.F.; Hwang, B.S.; Baek, K.H. Bacillus velezensis: A beneficial biocontrol agent or facultative phytopathogen for sustainable agriculture. Agronomy 2023, 13, 840. [Google Scholar] [CrossRef]
- Zajc, J.; Cernosa, A.; Francesco, A.J.; Castoria, R.; Curtis, F.D.; Lima, G. Characterization of Aureobasidium pullulans isolates selected as biocontrol agents against fruit decay pathogens. Fungal Genom. Biol. 2020, 10, 163. [Google Scholar]
- Liu, F.; Hu, M.; Tan, X.X.; Li, C.; Wang, S. Pseudomonas chlororaphis L5 and Enterobacter asburiae L95 biocontrol Dickeya soft rot diseases by quenching virulence factor modulating quorum sensing signal. Microb. Biotechnol. 2023, 16, 2145–2160. [Google Scholar] [CrossRef] [PubMed]
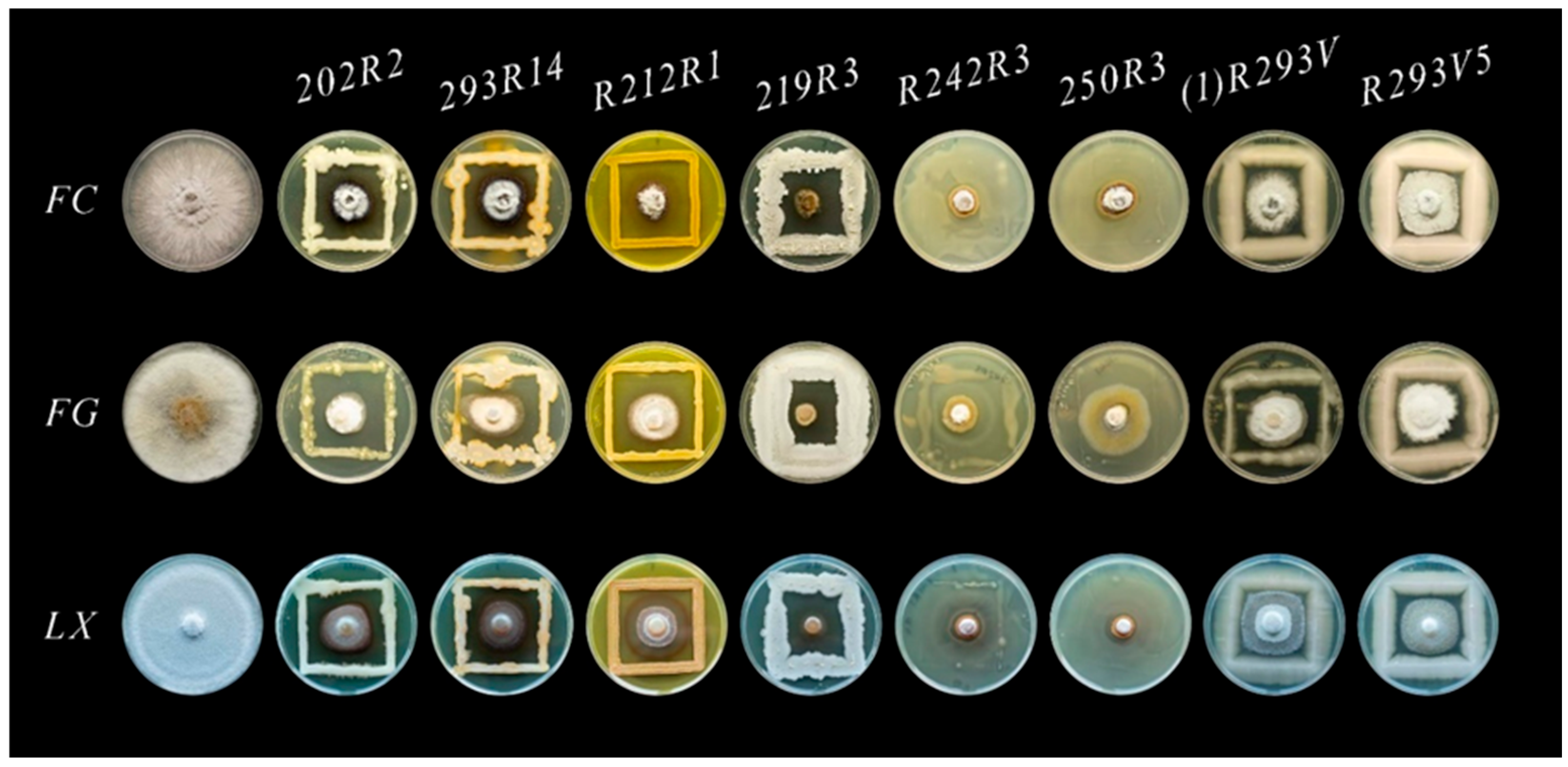
| Morphotype | A | B | C | D | E | |||
|---|---|---|---|---|---|---|---|---|
| Isolate | 202R2 | 293R14 | R212R1 | 219R3 | R242R3 | 250R3 | (1)R293V2 | R293V5 |
| Source | soil | soil | soil | soil | soil | soil | fruits | fruits |
| Color | creamy | orange | white | creamy | creamy | |||
| Form | circular | circular | circular | irregular | circular | |||
| Margin | entire | undulate | undulate | entire | ||||
| Opacity | opaque | translucent | opaque | |||||
| Appearance | non-glistening | glistening | non-glistening | glistening | glistening | |||
| Elevation | flat | convex | flat | flat | ||||
| Consistency | butyrous | mucoid | butyrous | butyrous | ||||
| Smoothness | coarse | smooth | coarse | smooth | smooth | |||
| Gram | + | + | − | + | + | + | n.a. | |
| Characteristics of Microorganisms—Antagonists of Monilinia spp. | |||||||
|---|---|---|---|---|---|---|---|
| B. pumilus | B. pumilus | B. velezensis | P. chlororaphis subsp. chlororaphis | L. agricola | L. agricola | A. pullulans | A. pullulans |
| Accession number in NCBI database and isolate strain in current research | |||||||
| SAMN 44109744 | SAMN 44109745 | SAMN 44109747 | SAMN 44109746 | SAMN 44109750 | SAMN 44109751 | SAMN 44109752 | SAMN 44109754 |
| 202R2 | 293R14 | 219R3 | R212R1 | R242R3 | 250R3 | (1)R293V2 | R293V5 |
| Mapping statistics with the reference genome (accession number in NCBI and identity with the reference genome (%) | |||||||
| NC_009848 | NC_009725 | NZ_CP144767 | NZ_CP067341 | NZ_CP067341 | GCF_000721785 | ||
| 95.65 | 95.64 | 98.87 | 95.11 | 90.65 | 90.71 | 97.06 | 97.00 |
| Statistics of sequencing data and annotation Genome size (bp) | |||||||
| 3,733,453 | 3,721,408 | 3,976,132 | 6,995,087 | 4,901,210 | 4,923,664 | 30,364,197 | 28,396,646 |
| GC content (%) | |||||||
| 41.55 | 41.52 | 46.36 | 62.69 | 36.74 | 36.66 | 50.26 | 50.33 |
| Gene length (bp) | |||||||
| 3,312,802 | 3,313,672 | 3,520,942 | 6,125,621 | 3,922,538 | 3,964,603 | 21,770,735 | 20,714,412 |
| Genes % in genome | |||||||
| 88.73 | 89.04 | 88.55 | 87.57 | 80.03 | 80.52 | 71.70 | 72.95 |
| Total number of genes | |||||||
| 3764 | 3753 | 3835 | 6233 | 4776 | 4764 | 8337 | 5784 |
| Transfer RNA/Ribosomal RNA (5S, 16S, 23S)/Transfer-messenger RNA | |||||||
| 70/4/1 | 70/6/1 | 83/5/1 | 61/4/1 | 80/8/1 | 75/5/1 | 158/59/N | 156/59—N |
| Noncoding RNA | |||||||
| 16 | 14 | 20 | 62 | 180 | 156 | 78 | 76 |
| Repeat region/CRISPR | |||||||
| 3/3 | 1/1 | N/N | N/N | 3/2 | N/N | N/N | N/N |
| Genes assigned according to NR | |||||||
| 3425 | 3654 | 3489 | 5672 | 4346 | 4335 | 6886 | 6256 |
| Genes assigned according to GO | |||||||
| 3321 | 3415 | 3356 | 4387 | 4091 | 4210 | 4856 | 3066 |
| Genes assigned according to KEGG | |||||||
| 3078 | 3002 | 3321 | 4128 | 4005 | 3845 | 3798 | 3088 |
| PGP Activities Description | B. pumilus | B. velezensis | P. chlororaphis | L. agricola | A. pullulans | |||
|---|---|---|---|---|---|---|---|---|
| 202R2 | 293R14 | 219R3 | R212R1 | R242R3 | R250R3 | (1)R293V2 | R293V5 | |
| Phosphate metabolism | pstA, pstC, pstS | pstA, pstB, pstC, pstS, iolU, mmsA | pstA, pstB, pstC, pstS, gdh, hxlB, glpX, pfk, hxlA, kdgK, rbsK | pstA, pstB, pstS, iolG, iolE, iolD, mmsA, iolC | pstA, pstB, pstC, pstS, mmsA | pstA, pstB, pstC, pstS, mmsA | pstB, iolG, pstA, pstC, pstS, iolU | pstB, iolG, pstA, pstC, iolU |
| Nitrogen fixation | nifS, nifU | nifS, nifU | nifS, salA, sufU | - | nifU | nifU | - | - |
| Nitrogen metabolism | gltB, ltD, glnA | gltB, ltD, glnA | nasE, nasD, gudB, rocG, narG, narH, narI, glnA, cah, gltB | cynS, cynT, arcC, gltB, gltD, glnA, nirB, nirD, norB | cynT | cynT, glnA | ncd2, nirB, mbtB, cah, cynT, nirD, glnA, gltB, gltD | ncd2, nirB, mbtB, cah, cynT, glnA, gltB |
| Siderophore | menF, entE, ntA | fhuC, enF | menF | - | fhuC, menF | fhuC, menF | fhuC, fhuG, fhuB, fhuD | fhuC, fhuG, huB, fhuD |
| IAA production | trpA, rpB, trpC, rpD, trpE | trpA, rpB, trpC, rpD, trpE | trpA, trpB, trpC, trpD, trpE, trpF | trpA, trpB, trpC, trpD, trpE, trpF | trpA, trpB, trpE | trpA, trpB, trpE | trpA, trpB, trpD, trpE | trpA, trpE |
| Hydrolase | eglS | gmuD, ganB | eglS | - | - | - | trpE, eglS, pqsH, pelF | eglS, pqsH, pelF |
| Chitinase activity | - | - | ydhD | - | - | - | - | sleL, ydhD |
| Biofilm | trpE, flgM, fliA, csrA | trpE, flgM, fliA, csrA | tasA, bslA, bslB, trpE, flgM, csrA | crp, cpdA, trpG, trpE, flgM | trpE, fliA | trpE, fliA, csrA | tasA, bslA, bslB | tasA, bslA, bslB |
| 2,3-butanediol | ilvE, ilvA, ilvD, ilvC, ilvB | ilvE, ilvA, ilvD, ilvC, ilvB | ilvE, ilvA, ilvD, ilvC, ilvB | ilvE, ilvA, ilvD, ilvC, ilvB | ilvA, ilvD, ilvC, ilvB | ilvA, ilvD, ilvC, ilvB | ilvY, ilvA, ilvD, ilvC, ilvH | ilvK, ilvE, ilvY, ilvD, ilvC, ilvH, ilvB |
| Predicted Virulence Factors | B. pumilus | B. velezensis | P. chlororaphis | L. agricola | ||
|---|---|---|---|---|---|---|
| 202R2 | 293R14 | 219R3 | R212R1 | R242R3 | 250R3 | |
| Acid resistance | 0 | 0 | 1 | 0 | 0 | 0 |
| Adherence | 2 | 2 | 2 | 73 | 3 | 5 |
| Antimicrobial activity | 0 | 0 | 0 | 6 | 0 | 0 |
| Antiphagocytosis | 1 | 1 | 2 | 24 | 2 | 1 |
| Biofilm formation | 0 | 0 | 0 | 4 | 0 | 0 |
| Biosurfactant | 0 | 0 | 0 | 1 | 0 | 0 |
| Cell surface components | 0 | 0 | 2 | 0 | 0 | 1 |
| Copper uptake | 0 | 0 | 0 | 1 | 0 | 0 |
| Efflux pump | 0 | 0 | 0 | 1 | 0 | 0 |
| Enzyme | 1 | 1 | 0 | 1 | 0 | 0 |
| Glycosylation system | 0 | 0 | 0 | 1 | 0 | 1 |
| Immune evasion | 10 | 11 | 18 | 8 | 4 | 1 |
| Intracellular survival | 0 | 0 | 0 | 0 | 1 | 1 |
| Invasion | 1 | 1 | 1 | 1 | 0 | 0 |
| Iron acquisition | 5 | 5 | 5 | 0 | 0 | 0 |
| Iron uptake | 2 | 2 | 1 | 31 | 7 | 7 |
| Lipid and fatty acid metabolism | 1 | 1 | 0 | 1 | 1 | 1 |
| Magnesium uptake | 0 | 0 | 0 | 1 | 0 | 0 |
| Others | 0 | 0 | 0 | 2 | 0 | 0 |
| Peptidoglycan modification | 1 | 1 | 0 | 0 | 0 | 1 |
| Protease | 0 | 0 | 0 | 2 | 0 | 0 |
| Quorum sensing | 0 | 0 | 0 | 5 | 0 | 0 |
| Regulation | 2 | 2 | 2 | 7 | 3 | 3 |
| Secretion system | 1 | 1 | 1 | 28 | 2 | 2 |
| Serum resistance | 0 | 0 | 0 | 1 | 0 | 0 |
| Serum and immune evasion | 0 | 0 | 0 | 0 | 1 | 0 |
| Stress adaptation | 0 | 0 | 1 | 4 | 1 | 1 |
| Surface protein anchoring | 2 | 2 | 1 | 0 | 1 | 1 |
| Toxin | 1 | 2 | 2 | 8 | 3 | 6 |
| Predicted VF genes | 30 | 32 | 39 | 211 | 29 | 32 |
| Total genes | 3845 | 3842 | 4029 | 6212 | 4811 | 5020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolytaitė, A.; Mažeikienė, I.; Kurgonaitė, M.; Raklevičiūtė, S.; Paškevičiūtė, G.; Frercks, B. Unlocking Nature’s Microbial Defenders: Genetic Mechanisms and Potential Against Monilinia spp. Pathogens. Microorganisms 2025, 13, 818. https://doi.org/10.3390/microorganisms13040818
Kolytaitė A, Mažeikienė I, Kurgonaitė M, Raklevičiūtė S, Paškevičiūtė G, Frercks B. Unlocking Nature’s Microbial Defenders: Genetic Mechanisms and Potential Against Monilinia spp. Pathogens. Microorganisms. 2025; 13(4):818. https://doi.org/10.3390/microorganisms13040818
Chicago/Turabian StyleKolytaitė, Augustina, Ingrida Mažeikienė, Monika Kurgonaitė, Saulė Raklevičiūtė, Gabija Paškevičiūtė, and Birutė Frercks. 2025. "Unlocking Nature’s Microbial Defenders: Genetic Mechanisms and Potential Against Monilinia spp. Pathogens" Microorganisms 13, no. 4: 818. https://doi.org/10.3390/microorganisms13040818
APA StyleKolytaitė, A., Mažeikienė, I., Kurgonaitė, M., Raklevičiūtė, S., Paškevičiūtė, G., & Frercks, B. (2025). Unlocking Nature’s Microbial Defenders: Genetic Mechanisms and Potential Against Monilinia spp. Pathogens. Microorganisms, 13(4), 818. https://doi.org/10.3390/microorganisms13040818






