Natural Infection of Omicron BA.5.2 in Patients Provides Broad Immune Responses Against SARS-CoV-2
Abstract
1. Introduction
2. Statistical Analysis
3. Results
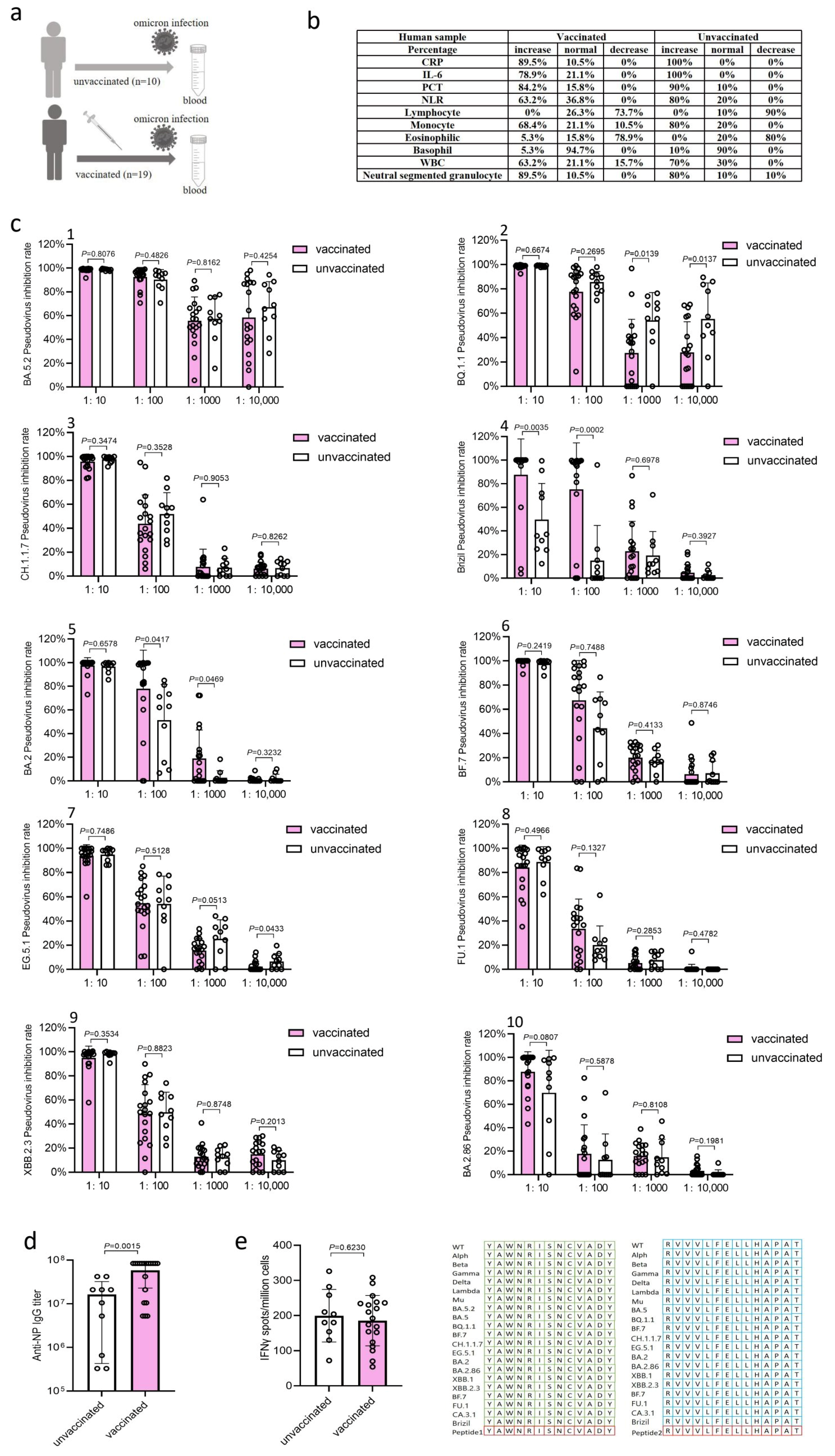
3.1. Clinical Symptoms and Inflammatory Response Markers in Patients
3.2. BA.5.2 Infection Induced Robust Neutralization Antibody Responses
3.3. BA.5.2 Infection Induced High Levels of IgG Antibody Responses
3.4. BA.5.2 Infection Induced T Cell Immune Responses (IFN-γ)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, B.; He, L.H.; Bao, Y.J.; Chen, Y.Y.; Lu, G.Z.; Zhang, Y.; Xu, Y.J.; Su, B.; Xu, J.; Wang, Y.; et al. Repeated vaccination of inactivated SARS-CoV-2 vaccine dampens neutralizing antibodies against Omicron variants in breakthrough infection. Cell Res. 2023, 33, 258–261. [Google Scholar] [PubMed]
- Cai, J.; Deng, X.W.; Yang, J.; Sun, K.Y.; Liu, H.C.; Chen, Z.Y.; Peng, C.; Chen, X.H.; Wu, Q.H.; Zou, J.Y.; et al. Modeling transmission of SARS-CoV-2 Omicron in China. Nat. Med. 2022, 28, 1468–1475. [Google Scholar] [PubMed]
- Wang, Z.C.; Chan, K.; Adrienne, N.; Guo, Y. An equitable route forward from China’s ‘zero COVID’ policy. Nat. Med. 2023, 29, 514–515. [Google Scholar]
- National Health Commission. 2023. Available online: http://www.nhc.gov.cn/xcs/kpzs/202302/b4ea6edabacf4061a0bde4fff649d35e.shtml (accessed on 10 January 2023).
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.M.; Gong, F.Y.; Han, Y.; Qiu, Y.; Wang, J.L.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China:A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar]
- Huang, C.L.; Wang, Y.M.; Li, X.W.; Ren, L.L.; Zhao, J.P.; Hu, Y.; Zhang, L.; Fan, G.H.; Xu, J.Y.; Gu, X.Y.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar]
- Yang, X.; Yu, Y.; Xu, J.Q.; Shu, H.Q.; Xia, J.A.; Liu, H.; Wu, Y.R.; Zhang, L.; Yu, Z.; Fang, M.H.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China:A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Tan, L.; Wang, Q.; Zhang, D.Y.; Ding, J.Y.; Huang, Q.C.; Tang, Y.Q.; Wang, Q.S.; Miao, H.M. Lymphopenia predicts disease severity of COVID-19: A descriptive and predictive study. Signal Transduct. Target. Ther. 2020, 5, 33. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, F.; Zhou, F.X.; Li, H.; Ge, W.Y.; Gan, R.; Nie, H.; Li, B.; Wang, Y.D.; Wu, M.; et al. Metagenomic analysis reveals oropharyngeal microbiota alterations in patients with COVID-19. Signal Transduct. Target. Ther. 2021, 6, 191. [Google Scholar] [CrossRef]
- Jeyanathan, M.; Afkhami, S.; Smaill, F.; Miller, M.S.; Lichty, B.D.; Xing, Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020, 20, 615–632. [Google Scholar]
- Florian, K. The human antibody response to influenza A virus infection and vaccination. Nat. Rev. Immunol. 2019, 19, 383–397. [Google Scholar]
- Zeng, W.; Ma, H.; Ding, C.C.; Yang, Y.R.; Sun, Y.; Huang, X.X.; He, W.H.; Xiang, Y.; Gao, Y.; Jin, T.C. Characterization of SARS-CoV-2-specific antibodies in COVID-19 patients reveals highly potent neutralizing IgA. Signal Transduct. Target. Ther. 2021, 6, 35. [Google Scholar] [PubMed]
- Havervall, S.; Ulrika, M.; Julia, S.; Greilert-Norin, N.; Bacchus, P.; Nilsson, P.; Hober, S.; Gordon, M.; Blom, K.; Klingstrom, J.; et al. Anti-Spike Mucosal IgA Protection against SARS-CoV-2 Omicron Infection. N. Engl. J. Med. 2022, 387, 1333–1336. [Google Scholar]
- Yu, S.H.; Lin, Y.N.; Li, Y.; Chen, S.J.; Zhou, L.; Song, H.J.; Yang, C.P.; Zhang, H.Q.; Zhou, J.P.; Sun, S.C.; et al. Systemic immune profiling of Omicron-infected subjects inoculated with different doses of inactivated virus vaccine. Cell 2023, 186, 4615–4631. [Google Scholar] [PubMed]
- Rick, A.M.; Laurens, M.B.; Huang, Y.; Yu, C.C.; Martin, T.C.S.; Rodriguez, C.A.; Rostad, C.A.; Maboa, R.M.; Baden, L.R.; Sahly, H.M.E.; et al. Risk of COVID-19 after natural infection or vaccination. Ebiomedicine 2023, 96, 104799. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, Y.; Wu, B.H.; Xiao, M.Z.; Wang, Z.; Diao, T.Y.; Zeng, R.; Chen, L.; Lei, Y.S.; Long, P.P.; et al. Neutralization against SARS-CoV-2 Delta/Omicron variants and B cell response after inactivated vaccination among COVID-19 convalescents. Front. Med. 2023, 17, 747–757. [Google Scholar]
- Tudoran, C.; Tudoran, M.; Cut, T.G.; Lazureanu, V.E.; Bende, F.; Fofiu, R.; Enache, A.; Pescariu, S.A.; Novacescu, D. The Impact of Metabolic Syndrome and Obesity on the Evolution of Diastolic Dysfunction in Apparently Healthy Patients Suffering from Post-COVID-19 Syndrome. Biomedicines 2022, 10, 1519. [Google Scholar] [CrossRef]
- Barrios, M.H.; Nicholson, S.; Bull, R.A.; Martinello, M.; Rawlinson, W.; Mina, M.; Post, J.J.; Hudson, B.; Gilroy, N.; Lloyd, A.R.; et al. Comparative Longitudinal Serological Study of Anti-SARS-CoV-2 Antibody Profiles in People with COVID-19. Microorganisms 2023, 11, 1985. [Google Scholar] [CrossRef]
- Gazit, S.; Shlezinger, R.; Perez, G.; Lotan, R.; Peretz, A.; Ben-Tov, A.; Herzel, E.; Alapi, H.; Cohen, D.; Muhsen, K.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Naturally Acquired Immunity versus Vaccine-induced Immunity, Reinfections versus Breakthrough Infections: A Retrospective Cohort Study. Clin. Infect. Dis. 2022, 75, E545–E551. [Google Scholar]
- Chemaitelly, H.; Ayoub, H.H.; Coyle, P.; Patrick, T.; Mohammad, R.H.; Hadi, M.Y.; Asmaa, A.T.; Zaina, A.K.; Einas, A.K.; Andrew, J.; et al. Differential protection against SARS-CoV-2 reinfection pre- and post-Omicron. Nature 2025, 1, 5. [Google Scholar] [CrossRef]
- Wang, P.; Lau, S.Y.; Deng, S.F.; Lotan, R.; Peretz, A.; Ben-Tov, A.; Herzel, E.; Alapi, H.; Cohen, D.; Muhsen, K.; et al. Characterization of an attenuated SARS-CoV-2 variant with a deletion at the S1/S2 junction of the spike protein. Nat. Commun. 2021, 12, 2790. [Google Scholar]
- Liu, Y.; Zhang, X.N.; Liu, J.Y.; Xia, H.J.; Zou, J.; Muruato, A.E.; Periasamy, S.; Kurhade, C.; Plante, J.A.; Bopp, N.E.; et al. A live-attenuated SARS-CoV-2 vaccine candidate with accessory protein deletions. Nat. Commun. 2022, 13, 4337. [Google Scholar]
- Okutani, M.S.; Okamura, S.; Gis, T.; Sasaki, H.; Lee, S.; Kashiwabara, A.; Goto, S.; Matsumoto, M.; Yamawaki, M.; Miyazaki, T.; et al. Immunogenicity and safety of a live-attenuated SARS-CoV-2 vaccine candidate based on multiple attenuation mechanisms. Elife 2025, 13, RP97532. [Google Scholar]
- Adler, J.M.; Vidal, R.M.; Voss, A.; Kunder, S.; Nascimento, M.; Abdelgawad, A.; Langner, C.; Vladimirova, D.; Osterrieder, N.; Gruber, A.D.; et al. A non-transmissible live attenuated SARS-CoV-2 vaccine. Mol. Ther. 2023, 31, 2391–2407. [Google Scholar]
- Adler, J.M.; Vidal, R.M.; Langner, C.; Vladimirova, D.; Abdelgawad, A.; Kunecova, D.; Lin, X.Y.; Nouailles, G.; Voss, G.; Kunder, S.; et al. An intranasal live-attenuated SARS-CoV-2 vaccine limits virus transmission. Nat. Commun. 2024, 15, 995. [Google Scholar] [PubMed]
- Trimpert, J.; Dietert, K.; Firsching, T.C.; Ebert, N.; Thao, T.T.N.; Vladimirova, D.; Kaufer, S.; Labroussaa, F.; Abdelgawad, A.; Conradie, A.; et al. Development of safe and highly protective live-attenuated SARS-CoV-2 vaccine candidates by genome recoding. Cell Rep. 2021, 36, 109493. [Google Scholar]
- Novailles, G.; Adler, J.M.; Pennitz, P.; Peidli, S.; Alves, L.G.T.; Baumgardt, M.; Bushe, J.; Voss, A.; Langenhagen, A.; Langner, C.; et al. Live-attenuated vaccine sCPD9 elicits superior mucosal and systemic immunity to SARS-CoV-2 variants in hamsters. Nat. Microbiol. 2023, 8, 860–874. [Google Scholar]
- Trimpert, J.; Adler, J.M.; Eschke, K.; Abdelgawad, A.; Firsching, T.C.; Ebert, N.; Thao, T.T.N.; Gruber, A.D.; Thiel, V.; Osterrieder, N.; et al. Live attenuated virus vaccine protects against SARS-CoV-2 variants of concern B.1.1.7 (Alpha) and B.1.351 (Beta). Sci. Adv. 2021, 7, eabk0172. [Google Scholar]
- Wang, Y.; Yang, C.; Song, Y.T.; Coleman, J.R.; Stawowczyk, M.; Tafrova, J.; Tasker, S.; Boltz, D.; Baker, R.; Garcia, L.; et al. Scalable live-attenuated SARS-CoV-2 vaccine candidate demonstrates preclinical safety and efficacy. Proc. Natl. Acad. Sci. USA 2021, 118, e2102775118. [Google Scholar] [CrossRef]
- Liu, X.; Ng, W.H.; Zusinaite, E.; Freitas, J.; Taylor, A.; Yerragunta, V.; Aavula, S.M.; Gorriparthi, S.; Ponsekaran, S.; Bonda, R.L.; et al. A single-dose intranasal live-attenuated codon deoptimized vaccine provides broad protection against SARS-CoV-2 and its variants. Nat. Commun. 2024, 15, 7225. [Google Scholar]
- Seo, S.H.; Jang, Y. Cold-Adapted Live Attenuated SARS-CoV-2 Vaccine Completely Protects Human ACE2 Transgenic Mice from SARS-CoV-2 Infection. Vaccines 2020, 8, 584. [Google Scholar]
- Pacheco-García, U.; Serafín-López, J. Indirect Dispersion of SARS-CoV-2 Live-Attenuated Vaccine and Its Contribution to Herd Immunity. Vaccines 2023, 11, 655. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.C.H.; Ng, W.H.; King, N.J.C.; Mahalingam, S. Can live-attenuated SARS-CoV-2 vaccine contribute to stopping the pandemic? PLoS Pathog. 2022, 18, e1010821. [Google Scholar]
- Zhao, J.; Wang, L.; Schank, M.; Dang, X.D.; Lu, Z.Y.; Cao, D.C.; Khanal, S.S.; Nguyen, L.N.; Nguyen, L.N.T.; Zhang, J.Y.; et al. SARS-CoV-2 specific memory T cell epitopes identified in COVID-19-recovered subjects. Virus Res. 2021, 304, 198508. [Google Scholar]
- Verhagen, J.; van der Meijden, E.D.; Lang, V.; Kremer, A.E.; Volkl, S.; Mackensen, A.; Aigner, M.; Kremer, A.N. Human CD4+ T cells specific for dominant epitopes of SARS-CoV-2 Spike and Nucleocapsid proteins with therapeutic potential. Clin. Exp. Immunol. 2021, 205, 363–378. [Google Scholar] [PubMed]
- Peng, Y.; Mentzer, A.J.; Liu, G.; Yao, X.; Yin, Z.X.; Dong, D.D.; Dejnirattisai, W.; Rostron, T.; Supasa, P.; Liu, C.; et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat. Immunol. 2020, 21, 1336–1345. [Google Scholar]
- Low, J.S.; Vaqueirinho, D.; Mele, F.; Foglierini, M.; Jerak, J.; Perotti, M.; Jarrossay, D.; Jovic, S.; Perez, L.; Cacciatore, R.; et al. Clonal analysis of immunodominance and cross-reactivity of the CD4 T cell response to SARS-CoV-2. Science 2021, 372, 1336–1341. [Google Scholar]
- Knierman, M.D.; Lannan, M.B.; Spindler, L.J.; McMillian, C.L.; Konrad, R.J.; Siegel, R.W. The Human Leukocyte Antigen Class II Immunopeptidome of the SARS-CoV-2 Spike Glycoprotein. Cell Rep. 2020, 33, 108454. [Google Scholar]
- Chen, J.; Wang, P.; Yuan, L.; Zhang, L.; Zhang, L.M.; Zhao, H.; Chen, C.J.; Wang, X.J.; Han, J.L.; Chen, Y.D.; et al. A live attenuated virus-based intranasal COVID-19 vaccine provides rapid, prolonged, and broad protection against SARS-CoV-2. Sci. Bull. 2022, 67, 1372–1387. [Google Scholar]
- Chevalier, M.F.; Bobisse, S.; Costa-Nunes, C.; Cesson, V.; Jichlinski, P.; Speiser, D.E.; Harari, A.; Coukos, G.; Romero, P.; Nardelli-Haefliger, D.; et al. High-throughput monitoring of human tumor-specific T-cell responses with large peptide pools. Oncoimmunology 2015, 4, e1029702. [Google Scholar]
- Wang, J.; Li, K.; Mei, X.; Cao, J.P.; Zhong, J.Y.; Huang, P.Y.; Luo, Q.; Li, G.C.; Wei, R.; Zhong, N.S.; et al. SARS-CoV-2 vaccination-infection pattern imprints and diversifies T cell differentiation and neutralizing response against Omicron subvariants. Cell Discov. 2022, 8, 136. [Google Scholar]
- Li, L.; Feng, T.; Shen, Q.; Shi, X.S.; Wei, Z.G.; Chen, W.Z.; Yang, F.; Zhu, Y.T.; Zhang, C.X.; Zhang, S.; et al. Infection of Omicron BA.5.2 in patients provides broad immune responses against SARS-CoV-2. Res. Sq. 2023. Preprint. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Feng, T.; Shen, Q.; Shi, X.; Wei, Z.; Chen, W.; Yang, F.; Zhu, Y.; Zhang, C.; Zhang, S.; et al. Natural Infection of Omicron BA.5.2 in Patients Provides Broad Immune Responses Against SARS-CoV-2. Microorganisms 2025, 13, 746. https://doi.org/10.3390/microorganisms13040746
Li L, Feng T, Shen Q, Shi X, Wei Z, Chen W, Yang F, Zhu Y, Zhang C, Zhang S, et al. Natural Infection of Omicron BA.5.2 in Patients Provides Broad Immune Responses Against SARS-CoV-2. Microorganisms. 2025; 13(4):746. https://doi.org/10.3390/microorganisms13040746
Chicago/Turabian StyleLi, Le, Tang Feng, Quan Shen, Xiaoshan Shi, Zhigong Wei, Wanze Chen, Fan Yang, Yueting Zhu, Chengxin Zhang, Shuang Zhang, and et al. 2025. "Natural Infection of Omicron BA.5.2 in Patients Provides Broad Immune Responses Against SARS-CoV-2" Microorganisms 13, no. 4: 746. https://doi.org/10.3390/microorganisms13040746
APA StyleLi, L., Feng, T., Shen, Q., Shi, X., Wei, Z., Chen, W., Yang, F., Zhu, Y., Zhang, C., Zhang, S., Zhang, Q., Fu, S., Wang, N., Tian, W.-x., Liu, J., & Si, L. (2025). Natural Infection of Omicron BA.5.2 in Patients Provides Broad Immune Responses Against SARS-CoV-2. Microorganisms, 13(4), 746. https://doi.org/10.3390/microorganisms13040746




