Abstract
Urinary tract infections (UTIs) are a leading cause of illness in children and adults of all ages, with uropathogenic Escherichia coli (UPEC) being the primary agent responsible. During colonization and subsequent infection of the urinary tract (UT), UPEC requires the expression of genes associated with virulence, such as those that encode the fimbrial adhesins FimH, PapG, and CsgA, as well as the presence of the TosA protein and the flagellar appendages of the bacteria. However, for colonization and infection to be successful, UPEC must overcome the host’s immunological barriers, such as physical barriers, expressed peptides and proteins, and immune cells found in the UT. In this context, the UT functions as an integral system where these factors act to prevent the colonization of uropathogens. Significant genetic diversity exists among UPEC strains, and the clonal complex ST131 represents one of the key lineages. This lineage has a high content of virulence genes, multiple mechanisms of antibiotic resistance, and a high frequency of extended-spectrum β-lactamases (ESBLs). New knowledge regarding protein structures known as adhesins and their role in the infection process can help identify therapeutic targets and aid in the design of vaccines. These vaccines could be based on the development of chimeric fusion proteins (FimH + CsgA + PapG), which may significantly reduce the incidence of UTIs in pediatric and adult patients.
1. Introduction
General characteristics. Urinary tract infections (UTIs) are among the main causes of morbidity in all age groups worldwide [1,2]. In 2023, the Ministry of Health of Mexico reported that UTIs are the second leading cause of morbidity, with a total of 3,365,799 identified cases (https://epidemiologia.salud.gob.mx/anuario/2023/principales/nacional/grupo_edad.pdf; accessed 31 January 2025). Recent data from the “Global Burden of Disease Study 2019” revealed the incidence, mortality, and disability-adjusted life years (DALYs) associated with UTIs across 204 countries from 1990 to 2019 [3]. In addition, these studies were conducted at different sociodemographic levels and at different national, regional, sex, and age levels. An analysis of these studies showed that the absolute number of UTI cases increased by 60.40%, from 252.25 million (95% CI: 223.31–279.3) in 1990 to 404.61 million (95% CI: 359.43–446.55) in 2019 [3]. Identifying UTIs in children in a timely manner is essential for providing timely treatment. Prompt identification of UTIs in children is essential for effective treatment, particularly because cystitis and pyelonephritis can often be hard to distinguish in children under two years of age. In the United Kingdom, 80% of UTIs in children, mainly infants, can go unnoticed, owing to the presence of nonspecific signs such as fever of 38 °C or higher, irritability, vomiting, decreased appetite, lethargy [4], and improper collection of urine samples [5]. In recent years, recommendations have been made for the diagnosis, treatment, and follow-up of children with UTIs, as well as for imaging studies (renal and bladder ultrasound), to identify underlying urological anomalies and their consequences [4,5,6]. The guidelines for diagnosing UTIs in infants emphasize preventing renal scarring. Research has highlighted the long-term consequences of renal scarring, which may include reduced kidney function, hypertension, and even end-stage renal disease [7,8]. The timely diagnosis of UTIs in both children and adults should always be a priority. This approach allows for the identification of the pathogen, enabling prompt treatment and eradication. However, there are existing recommendations concerning the diagnosis and treatment of UTIs in pediatric patients. Recent studies have indicated that the urinary tract (UT) is a common source of infections in children of all ages [4]. These infections are risk factors for potential complications following UTIs, including congenital anomalies of the kidney and UT, as well as vesicointestinal dysfunction. It is crucial to consider a diagnosis of UTI in every child who presents with fever without an apparent source of infection. Several factors must be accounted for appropriate management, such as distinguishing between lower UT infections (lUTIs) and upper UTI (uUTIs), selecting the appropriate age-specific urine collection method, assessing risk factors, obtaining a positive urine culture based on the Kass–Sandford criterion, accurately identifying the uropathogen, and applying suitable treatment on the basis of clinical observations and laboratory findings. In conclusion, several important factors must be considered to effectively manage pediatric patients with UTIs [4]. In adult patients, UTIs pose a significant health issue, mainly due to therapeutic failures linked to infections caused by multidrug-resistant (MDR) and extreme drug-resistant (XDR) uropathogenic Escherichia coli (UPEC) strains. An MDR strain is defined as one that is resistant to at least one antibiotic from three or more different classes of antimicrobial agents. In contrast, an XDR strain is resistant to at least one antibiotic from all classes but susceptible no more than two classes of antibiotics [9]. In women, several risk factors contribute to the development of UTIs, including loss of estrogen, changes in the vaginal microbiota, and increased vaginal pH. The signs and symptoms of a UTI in adults are typically clear and are characterized by cystitis, which includes dysuria (painful urination), polyuria (increased urination), urgent urination, and suprapubic pain. In cases of pyelonephritis, additional symptoms such as fever exceeding 38 °C, lower back pain, and systemic symptoms may be present [10,11,12]. Laboratory analyses with positive urine cultures (considering the patient’s age), noninvasive urine sampling, clinical history, and evaluation are essential for an optimal diagnosis [13].
UTIs can be classified into two categories on the basis of their anatomical site: lower urinary tract infections (lUTIs) and upper urinary tract infections (uUTIs). lUTIs include conditions such as cystitis, whereas uUTIs include urethritis, pyelonephritis, and urosepsis. The uropathogens responsible for causing lUTI include Escherichia coli, Staphylococcus saprophyticus, Staphylococcus spp., Proteus spp., Klebsiella spp., Enterococcus spp., Pseudomonas spp., and various yeast species [14]. Notably, E. coli accounts for more than 80% of the uropathogens associated with uUTIs. Moreover, more than 80% of community-acquired UTIs and 20–40% of hospital-acquired UTIs are associated with UPEC [15]. Our working group reported that one of the main problems with UTIs in the pediatric population is the presence of MDR and XDR clinical strains of UPEC and the production of extended-spectrum beta-lactamase (ESBL) [16]. Different guidelines for the optimal management of UTIs in pediatric patients have been proposed in several studies [17]. First-line antibiotics, such as trimethoprim-sulfamethoxazole, amoxicillin-clavulanate, and cephalosporins (2nd- and 3rd-generation, cefixime, and cefotaxime), are mainly used to treat pediatric UTIs [18]. Antibiotics such as fosfomycin, nitrofurantoin, and gentamicin have also been used as alternative antibiotics for the treatment of UTIs [19].
1.1. UPEC Colonization of the UT
Colonization is the initial event that promotes the establishment of UPEC in the UT of the host, subsequently leading to a UTI. UPEC adheres to the bladder epithelium as part of the colonization process, allowing it to evade urine clearance, interact with the “umbrella cells” of the urinary epithelium, and, in some cases, undergo an intracellular invasion process [20]. UPEC can internalize and multiply within host cells during this intracellular invasion, helping it evade the immune response. This ability diminishes the effectiveness of antibiotics and promotes their persistence by forming intracellular bacterial communities (IBCs) and quiescent intracellular reservoirs (QIRs) [21]. IBCs play several critical roles in UTIs: (i) they help bacteria persist in UTIs; (ii) they protect bacteria from antibiotics and antimicrobial treatments; (iii) they shield bacteria from the host’s immune response; (iv) they promote cell exfoliation, facilitating exposure of the transitional epithelium; and (v) their reactivation enables UPEC to exit through filamentation, initiating a new infectious cycle. This process leads to rUTIs and supports bacterial persistence and reactivation once the triggering stimulus has ceased [15]. Our working group characterized a collection of 178 clinical strains of urinary E. coli (UEc) [22] and 129 clinical strains of UPEC belonging to serotype O25b (EcO25b) from children with UTIs. The UEc and EcO25b strains presented a high prevalence of genes that encode various fimbrial adhesins, such as fimH, papG, ecpA, and csgA [23]. These adhesins are associated mainly with the adhesion process, mediating the intimate interaction between bacteria and host cells [24]. Additionally, each adhesin recognizes specific receptors in the host urothelium: (i) the FimH adhesin recognizes mannosylated residues, (ii) PapG adhesin variants recognize globosides (GbO1 to GbO5) in the renal epithelium, and (iii) the CsgA adhesin binds to fibronectin and fibrinogen [24].
1.2. Fimbrial and Nonfimbrial Adhesins in UPEC
Fimbria types 1 and P participate in the uropathogenesis of E. coli via the Chaperone-Usher (CU) assembly mechanism [24,25]. In the CU system, chaperones are the proteins responsible for preventing the polymerization of structural proteins in the periplasm, keeping them soluble until their recognition by the accommodator protein, which catalyzes the correct folding of the pilins [25].
1.2.1. Type 1 Fimbriae
The expression of type 1 fimbriae has been linked to biofilm formation, adherence to and invasion of the uroepithelium, yeast agglutination, and immune response evasion by invading macrophages [26]. Type 1 fimbria contribute to the pathogenicity of E. coli, which is involved in the colonization of various niches from the intestine, genitals, and UT to other organs [27]. Type 1 fimbriae are encoded by the fimAICDFGH operon, which includes the regulatory genes fimB, fimE, and fimX and the fimS switch region. The FimA protein is a pilin that forms the fimbrial main subunit (filament), FimC is a periplasmic chaperone, and FimD is an accommodator protein. The FimI protein is a homolog of FimA; the function of FimI is unknown, but FimI is essential for the expression of fimbrial proteins [28].
The tip of the fimbria comprises the docking proteins FimF and FimG, which are involved in binding a single copy of the FimH adhesin [29]. The FimH adhesin protein contains two domains: the pilin domain, which interacts with the FimG protein, forming a flexible junction, and the lectin domain, which forms a binding pocket for a receptor that recognizes mannosylated residues [29]. The FimH adhesin exhibits genetic variability within the fimH gene, leading to the identification of several polymorphisms that give rise to different versions of the FimH protein. These polymorphisms can induce functional changes, impacting the ability of bacteria to adhere to various surfaces and tissues within their host [30,31]. A study involving E. coli strains collected from the intestinal mucosa of children with inflammatory bowel disease revealed that the amino acid sequence of the FimH protein is modified. These data suggest that commensal strains can adapt to changes in their microenvironment [32].
Although the exact number of polymorphisms in the fimH genes has not been determined, the high frequency of structural mutations in the fimbrial adhesins of extraintestinal pathogenic E. coli (ExPEC) indicates significant diversity in their sequences. This genetic variability of FimH enhances the adaptability of E. coli to different ecological niches and conditions within the host, thereby increasing its potential to cause infections [33]. An important polymorphism in UPEC/ST131 clones is FimH30. This polymorphism features an R166H mutation that weakens the interactions between the FimH domains. As a result, this change promotes stronger interactions with mannose and allows for the formation of high-affinity relaxed conformations. Compared with other variants, the expression of the FimH30 polymorphism in isogenic E. coli clones of the ST131 lineage enhances adherence and invasion to human cells and facilitates the formation of highly structured biofilms [34]. The expression of type 1 fimbrial proteins is regulated by two site-specific recombinases, FimB and FimE, which promote the inversion of the fimS switch region in response to stress factors that positively or negatively stimulate the expression of this fimbrial protein [35]. Receptors for the fimbrial adhesin FimH, which include mannosylated uroplakins (UPK1) and the α3β1 integrin, are rich in mannosylated residues and are widely distributed in the umbrella cells of the bladder epithelium. When FimH interacts with its receptors, it promotes the internalization of UPEC by reorganizing actin through the activation of RHO family GTPases [14]. Once inside, UPEC can form intracellular bacterial communities (IBCs) and quiescent intracellular reservoirs (QIRs), which are precursors to bacterial persistence in the UT [14]. Our working group described the frequency of type 1 fimbriae in UPEC strains from complicated UTIs (cUTIs) [92.1% (164/178)] and of clone ST131/O25b [95.2% (120/126)] in pediatric patients [22,23]. Our findings align with previous observations in commensal E. coli strains and other clinical samples, mainly due to the high conservation of type 1 fimbriae in this microorganism [36].
1.2.2. P Fimbriae
The distal part of pyelonephritis-associated fimbriae is anchored to the PapG adhesin, which interacts with glycosphingolipids consisting of Gal-α-(1,4) Gal residues. Three alleles have been described: PapGI, PapGII, and PapGIII, which bind to GbO3, GbO4 (both abundant in human uroepithelial cells), and GbO5 (abundant in canine cells but not in human cells) globoside variants, respectively [21]. PapG variations in clinical strains of UPEC are an adaptation mechanism of bacteria that allows high adaptability to colonize and cause infection in humans efficiently [37]. Recent studies have shown a strong association between the PapGII variant in clinical UPEC strains belonging to the B2 phylogenetic group and several medical conditions. Phylogroup B2 is recognized as a pathogenic group associated with UTIs and characterized by genes encoding various virulence factors, including adhesin genes, toxins, and iron acquisition systems [38,39,40]. Strains belonging to this phylogroup are also known for their ability to form biofilms [41], which increases their pathogenicity and increases the likelihood of severe infections. Furthermore, UPEC strains from phylogroup B2 have shown an increasing trend in the acquisition of multidrug resistance [42]. This variant is related to the development of pyelonephritis in adult women and children, acute prostatitis in men, and cases of bacteremia [43,44,45,46]. Additionally, the PapGIII variant has been linked to the occurrence of cystitis in women, men, and children [47,48]. In contrast, the PapGI variant has a low prevalence in humans with various clinical syndromes [49].
P fimbriae are encoded by the papBAHCDJKEFG operon and are clustered in the pathogenicity island (PAI) PAI-CFT073-pheV (PAI ICFT073). P fimbria transcription is regulated by the transcriptional repressor PapI and the transcriptional activator PapB [50]. The PapA pilin protein and the PapH protein form the major and minor subunits, respectively. PapC is the accommodator protein, PapD is the chaperone, and PapG is the adhesin [50]. Recently, the prevalence of pap alleles was reported to be 34.3% (61/178) for papGII and 1.7% (3/178) for papGIII in a collection of UEc strains [22]. Interestingly, a higher prevalence was observed in the EcO25b collection, associated with ST131 and phylogroup B2; 80.9% (102/126) amplified the papGII gene, and 2.4% (3/126) amplified the papGIII gene. In contrast, the papGI allele has not been identified in pediatric clinical strains of UPEC [23].
1.2.3. Curli Fimbriae
Amyloid structures such as curli fimbriae use an extracellular assembly mechanism called the nucleation/precipitation (NP) pathway, also known as the type VIII secretion system. A wide variety of microorganisms produce functional amyloid components as structural support that favors the integrity of biofilms, a mechanism that promotes the colonization of abiotic and biotic surfaces [51]. UPEC biofilm is essential for the persistence and recurrence of UTIs. Biofilms protect bacteria from harsh conditions, antimicrobial agents, and antibiotics, and they also safeguard bacteria from the host immune response [51]. These biofilms can easily form on biological surfaces and medical devices, such as urinary catheters and the uroepithelium [52]. Curli are amyloid fimbria present in most strains of E. coli, and their expression promotes the formation of biofilms in both pathogenic and commensal strains. In addition, curli-dependent biofilms can play a significant role in UTIsassociated with medical devices, such as catheters and urinary probes [53,54]. While many pathogenic, opportunistic, and commensal bacteria of the Enterobacteriaceae family members produce curli, our research group has demonstrated that curli fimbria functions as an accessory protein that enhances UT colonization in a C57BL/6 mouse model. These results indicate that infection with a curli-producing strain can cause more significant damage to the bladder and kidneys than the damage caused by the same strain in which the csgA gene is deleted [55]. Although curli fimbria are directly involved in the formation of biofilms by UPEC strains, type 1 fimbria-dependent biofilms have also been reported on abiotic surfaces [56]. These biofilms contribute to the persistence of catheter-associated UTIs (CAUTIs) [57] and are crucial factors in the development of UTIs [58]. Furthermore, type 1 fimbria facilitate colonization of the bladder epithelium and IBCs, contributing to persistent and recurrent UTI infections. Curli fimbriae are primarily composed of CsgA proteins, which have a fine structure with aggregation and adherence properties [59]. The genes encoding the proteins involved in curli biogenesis are located in the csgBAC operon, which is transcribed divergently. The csgA gene encodes the CsgA pilin, the structural protein of curli fimbriae. The csgB gene encodes the CsgB minor subunit, and the csgC gene encodes the CsgC periplasmic protein, the function of which is unknown. However, it has been proposed that the CsgC protein plays a role in the secretion of the major protein of curli [60]. Briefly, through the NP, the CsgA protein is secreted into the outer membrane in soluble form, where the nucleator or minor subunit CsgB polymerizes monomeric proteins (CsgA) to form the amyloid structure [54].
1.2.4. The Nonfimbrial Adhesin, TosA
TosA is a nonfimbrial adhesin with a molecular weight greater than 250 kDa that is closely related to UPEC. TosA is classified as a novel repeat-in-toxin (RTX) protein that is specifically expressed in the host UT and plays a crucial role in the virulence and survival of UPEC [61]. This adhesin has been associated with the phylogenetic group B2 and is a potential marker for strains with high content of virulence genes [62]. Interestingly, TosA is located in the outer membrane of E. coli but does not exhibit cytotoxic effects on mammalian cells. Instead, it enhances the adherence of E. coli to renal cell lines, contributing to pathogenesis by binding to receptors on the surfaces of host epithelial cells derived from the upper UT [63]. TosA facilitates three main functions: (i) adherence to host cells originating from the upper UT, (ii) survival during disseminated infections, and (iii) increased lethality during urosepsis [61,64]. Xicohtencatl et al., 2019, identified a relationship between strains carrying the gene that encodes the TosA protein, their virulence potential, resistance to both β-lactam and non-β-lactam antibiotics, and their enhanced adherence to the bladder cell line HTB-9 [62].
1.3. Immunity in UTIs
Three defense mechanisms against bacterial pathogens are present in the bladder: (1) physical barriers, (2) expressed peptides and proteins, and (3) immune cells [65]. The urothelium in the bladder is the first physical barrier and is made up of three to six layers of transitional epithelial cells. Umbrella cells constitute the first layer of cells in the lumen of the bladder and are coated with the protein uroplakin, which functions as a physical barrier to liquids, toxins, and microorganisms that do not express receptor proteins for uroplakin (UPK) and can adhere to the urothelium [66]. The umbrella cells are covered with mucus, composed of proteoglycans and glycosaminoglycans, and thus create a natural impermeable barrier [66]. The Tamm-Horsfall protein is a highly glycosylated protein, has many cysteines (disulfide bridges), is the most abundant glycoprotein in urine, and is produced exclusively in the tubular epithelial cells of the ascending limb of Henle [67]. The Tamm–Horsfall protein competes with uroplakin for interaction with FimH. This competition partially prevents the adherence of uropathogens that have fimbrial proteins, including FimH, which recognize and bind to mannosylated uroplakins. The structure of the Tamm–Horsfall protein is rich in mannose and contains disaccharides that bind to type I fimbriae, effectively competing with the mannose receptors on bladder epithelial cells (BECs). This action reduces the adhesion and colonization of UPEC in the bladder, facilitating its elimination through the urinary stream. Furthermore, the Tamm–Horsfall protein helps prevent excessive inflammation during bladder infection by inhibiting chemotaxis and reactive oxygen species (ROS) production by binding to the Ig-like lectin-9 (Siglec-9) receptor of neutrophils [68]. Secretory IgA (sIgA) is also produced in the UT; sIgA is produced by the plasma cells of the lamina propria and is subsequently secreted on the surface of the mucosa, in the intestine, and other organs [69]. The function of sIgA is to control the mucosal microbiota, limiting access to uropathogens and allergens; however, the function of sIgA in UTIs has not yet been fully elucidated [70]. The type and proportion of the immune cell population have been described; however, there are no reports describing the behavior of immune cells in the bladder. In contrast, other studies have investigated immune system behavior in murine models of bladder cancer [71]. Flow cytometry has been used to show that healthy murine bladders contain between 30,000 and 50,000 CD45+ cells, with 100-fold variation depending on organ dissociation [72,73]. Several studies have shown that the majority of immune cells in the bladder (70%) are antigen-presenting cells, where F4/80+CD64+ (40%) are the most abundant macrophages, followed by CD11b+ (15%) and CD130+ (5%) dendritic cells (DCs). The remaining 30% of immune cells are natural killer (NK) cells, mast cells (MCs), CD4 + ɑβ T cells, and γδ T cells [73].
Vaccines Targeting UTIs
Antibiotics are the primary treatment for UTIs, despite the emergence of MDR and XDR UPEC strains, which makes treatment difficult. The development of vaccines, potentially prophylactic vaccines, may be a good alternative to support the treatment of UTIs with antibiotics [74]. The Uromune vaccine was developed in Spain. Uromune is administered sublingually, with a required daily dose for two consecutive days. Each dose contains 100 μL of a culture of 108 inactivated bacteria (E. coli, Klebsiella pneumoniae, Proteus vulgaris, and Enterococcus faecalis). The efficacy of this vaccine for protection against UTIs is 63.3% to 81% over six months and 56.6% to 90.3% at 15 months [75].
The Urovaxom vaccine, also called OM-89, was developed in Switzerland and is presented in tablets containing 6 mg of lyophilized bacterial lysates from 18 different strains of E. coli. The indicated dose is one tablet daily for three months, with four subsequent booster doses. One tablet is administered daily during the first ten days of months 6 to 9. Several studies have shown an efficacy of 52.6% to 87.5% for protection against UTIs; however, side effects such as headaches, dizziness, nausea, erythema, and occasionally hair loss have been reported [76] (Table 1). Solco-Urovac is a vaccine that was developed in Germany. Solco-Urovac is administered as a vaginal suppository, and each suppository contains ten strains of different inactivated uropathogens (six different strains of E. coli, one strain of K. pneumoniae, one strain of Proteus mirabilis, one strain of Morganella morganii and one strain of E. faecalis). The indicated dose is one suppository per week for three weeks, with three booster doses involving the administration of one suppository per month. Studies have showed 22.2% to 25% protection against UTIs after six months of administration without boosters and 46% to 55.6% protection when boosters are administered. However, side effects, such as burning sensation, low grade fever, nauseas, vaginal rash, and vaginal bleeding, have been reported [77] (Table 1). ExPEC4V is a genetically modified vaccine developed by Johnson & Johnson that uses endotoxin A from Pseudomonas aeruginosa bound to four surface polysaccharides (serogroups O1A, O2, O6a, and O25b of E. coli). Intramuscular administration of ExPEC4V has 52% efficacy in preventing UTIs. The reported side effects of this vaccine are headache, dizziness, chills, diarrhea, limb pain, hyperhidrosis, and persistent pain at the injection site [78] (Table 1). Briefly, commercial vaccines for UTIs have demonstrated promising protection results; however, there are also reports of adverse outcomes with no noticeable improvement in patients [79]. A key advantage of these vaccines is their ability to reduce the number of UTI episodes, shorten the duration of antibiotic treatment, and help prevent the development of MDR strains that are tolerant to antibiotics. The use of vaccines in UTIs caused by MDR bacteria has been controversial, although more benefits have been obtained in reducing symptoms [78]. Importantly, their use for the general population is difficult owing to the high costs and the fact that they do not provide 100% protection. Unfortunately, the use of commercial vaccines in pediatric patients has not been accepted to date.

Table 1.
Commercial vaccines for the treatment of UTIs.
Importantly, current vaccines against UTIs have not provided significant protection and have caused various adverse effects. Recently, our working group developed a double and triple fusion of the main adhesins (FimH, PapG, and CsgA) of UPEC, with a focus on eradicating or reducing the incidence of UTIs. The dimeric protein FC [FimH(F) + CsgA(C)] and trimeric protein FCP [(FimH(F) + CsgA(C) + PapG(P)] can capacity to promote the release of 464.79 pg/mL and 521.24 pg/mL of IL-6, respectively [74]. Additionally, the FC protein triggered the release of 398.52 pg/mL of IL-8, and the FCP protein triggered the release of 450.40 pg/mL of IL-8. Inhibition data have shown that anti-FC and -FCP antibodies block the adhesion of CFT073 to HTB-5 cells by 73% and 46%, respectively [74]. Our results suggest that these fusion proteins can function as protective molecules against UTIs. Other studies have shown that the FimH and FliC proteins trigger high concentrations of IL-6 and IL-8 when coculture models of HTB-5 and HMC-1 cells are used; however, these cytokines are detected at low concentrations in the presence of the CsgA protein [80].
1.4. UPEC Genetic Diversity
Genetic diversity in UPEC strains has been determined mainly by pulsed-field gel electrophoresis (PFGE), multilocus sequence typing (MLST), and recently, by next-generation whole-genome sequencing (NGS) [81]. Sequence typing is an essential tool for the identification of clonal lineages and clonal complexes (CCs) in UPEC strains; the Achtman-Oxford MLST is the most widely used method [82,83]. Meta-analysis studies have revealed the presence of 20 sequence types (STs) (ST131, ST69, ST10, ST405, ST38, ST95, ST648, ST73, ST410, ST393, ST354, ST12, ST127, ST167, ST58, ST617, ST88, ST23, ST117, and ST1193) in extraintestinal ExPEC strains [60]. Our workgroup identified 14 STs associated with UPEC strains of serotypes O25b, ST73, ST93, ST120, ST405, ST998, ST10, ST62, ST443, ST117, ST421, ST69, ST10, ST38, and ST95, all of which were recovered from Mexican children with complicated UTIs [23].
1.4.1. Main STs in UPEC
ST131 lineage. ST131 is an important lineage of UPEC that is recognized globally and is associated with a wide range of virulence genes, high antibiotic resistance, and ESBLs [82,84,85]. The ST131 clone exemplifies the complex evolution that microorganisms can undergo, evolving from a commensal state to a multidrug-resistant state. Whole-genome sequencing studies have provided valuable insights into point mutations, critical genes selected due to antibiotic resistance, the presence of specific plasmids, genomic islands, and compensatory mutations [86]. ST131 clones responsible for UTIs are high-risk clones for resistance to multiple antimicrobial determinants and have MDR and XDR phenotypes [87]. High-risk clones are distributed globally and associated with several antimicrobial resistance determinants, which aid their transmission and persistence in hosts [88].
In addition, ST131 clones contain the blaCTX-M-15 gene, which confers resistance to cephalosporins. ST131 clones has been associated mainly with clinical UPEC strains belonging to serogroups O25b and O16. A specific subclone of UPEC known as ST131/O16, which is part of the ST131 clonal lineage, has emerged as a variant. This subclone has been identified in the fecal samples of both healthy individuals and patients suffering from cystitis and pyelonephritis in China [71,89]. High virulence has been observed in the ST131-O16 clones, associated with subcutaneous sepsis in murine models. This virulence is linked to a high frequency of the fimH gene and the presence of specific mutations in the gyrA gene (S83L and D87N) and the parC gene (S80I), which confer fluoroquinolone resistance [89,90]. Additionally, the fimH41 allele has been correlated with increased virulence in vitro [91]. In Nigeria, the ST131/O16 clone has been identified in patients diagnosed with sepsis [92].
The UPEC/ST131 clones of serogroup O25b (UPEC/ST131/O25b) is the most prevalent clone identified in adult women in UTIs across China. This clone is resistant to fluoroquinolones due to specific mutations in the gyrA (S83L, D87N, and A93E/G) and parC (S80I and E84N) genes, as reported by Zhong et al. (2019) [90,93]. Additionally, mutations in the gyrB gene have also been associated with fluoroquinolone resistance [94]. Furthermore, the ST131-O25b clone has been observed to carry the fimH30, fimH41, and fimH27 alleles, according to findings by Dahbi et al. (2014) [95].
Single-nucleotide polymorphism (SNP) studies have revealed three alleles or variants of fimH (H30, H30-R, and H30-Rx) in clones of UPEC/ST131 [96,97]. Interestingly, clones of ST131 have diversified into three clades (A, B, and C) on the basis of variations in the fimbrial adhesin gene fimH [96,97]. Clade A corresponds to the fimH41 variant, which has been suggested to have originated in Southeast Asia. Clade B is characterized by the fimH22 variant, whereas clade C is characterized by the fimH30 variant, both of which originated in North America [96]. Additionally, clade C includes two subclades: subclade C1, which contains the H30-R subclone, and subclade C2, which includes the H30-Rx subclone [97,98]. Strains with fluoroquinolone resistance and carrying the blaCTX-M-15 gene were identified in subclone H30. Subclone H30-R is is loaded with strains that are resistant mainly to fluoroquinolones, and subclone H30-Rx contains mainly the blaCTX-M-15 gene [98,99]. The C1/H30-R subclade is defined by double mutations in the gyrA (S83L and D87N) and parC (S80I and E84V) genes [93], along with the presence of the blaCTX-M-14 or blaCTX-M-27 genes. Within the C1/H30-R subclade, there is a sublineage called C1-M27, which is specifically characterized by the blaCTX-M-27 gene and the F1:A2:B20 plasmid [100]. In contrast, the C2/H30-Rx subclade also presents mutations in the gyrA and parC genes, but it is associated with the blaCTX-M-15 gene and the F2:A1:B-plasmid. Additionally, the C2/H30-Rx subclade harbors several virulence genes (iutA, afa, dra, and kpsII), the K100 capsule, multidrug resistance genes, and genes responsible for ESBL production [96,101,102]. Serogroup O25b is associated mainly with ST131, is widely distributed throughout the world, and is a high producer of ESBLs in children and adults [23,103,104,105,106,107,108,109,110]. In addition, the genes encoding class C β-lactamases, DHA-4 and CYM-42, are involved in UTI processes in Tunisia [111] (Figure 1).
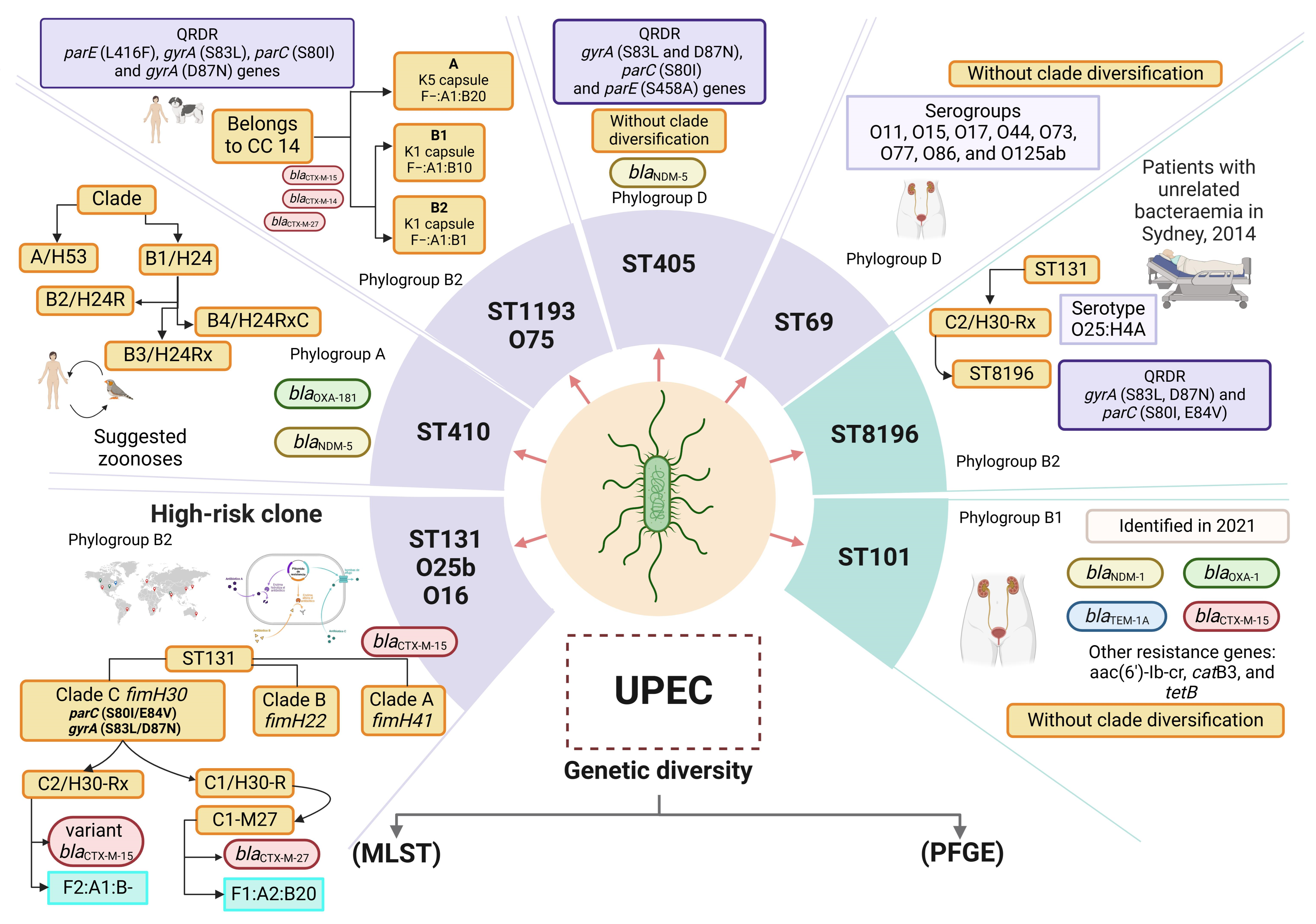
Figure 1.
Genetic diversity of UPEC. Sequence type (ST) analysis using multilocus sequence typing (MLST) and pulsotype analysis with pulsed-field gel electrophoresis (PFGE) are two widely used methods for the analysis of genetic diversity in UPEC. PFGE pulsotype analysis has allowed the evaluation of the bacterial genome of UPEC with macrorestriction assays using specific cuts with restriction enzymes. However, pulsotypes are highly diverse, and pulsotyping cannot be used to determine the characteristics of the various clonal lineages of UPEC. In addition, ST analysis by MLST has been used to determine the associations of virulence and resistance characteristics with the most important clonal lineages of UPEC. The ST8196 clone has been reported as a sublineage within ST131, which shares the same ancestor. The most important ST lineages in UPEC are shown in light purple squares, and the recently reported STs are shown in light green squares. The blaCTX genes are indicated in light red ovals, the blaOXA genes are indicated in light green ovals, the blaNDM genes are indicated in light yellow ovals, and the blaTEM genes are indicated in light blue ovals. Each ST shows the representative characteristics of each lineage/ST, serogroup, subclone, and phylogenetic group and the presence of genes associated with the antibiotic hydrolysis resistance mechanism. Two clonal lineages have been identified among the clones from ST410: clade A/fimH53 and clade B/fimH24. The B/H24 lineage has been further divided into three subclades: B2/H24R (which exhibit fluoroquinolone resistance); B3/H24Rx (which carries the blaCTX-M-15 gene); and B4/H24RxC (which contains the blaOXA-181 gene) [83]. The ST1193 clone has diversified into two clades: ST1193-A (characterized by the K5 capsule and F−:A1:B20 plasmids) and ST1193-B (features the K1 capsule). The ST1193-B clade is further divided into subclades ST1193-B1 (with the plasmid F−:A1:B10) and ST1193-B2 (with the plasmid F−:A1:B1 [112]. The ST8196 clone is associated with phylogroup B2 and has the serotype O25:H4A. It is derived from the ST131/C2-H30Rx clone [113]. Design of this study. Created with http://www.Biorender.com.
ST1193 Lineage
The emerging ST1193 lineage was first identified in 2007 and has been associated with systemic infections and UTIs, as well as with companion animals and the environment [114]. This clone has been isolated from fecal matter and is associated with bloodstream infections and UTIs [115]. In the United States, UPEC clones with ST1193 from the urine of adults (<40 years) were found to be resistant to fluoroquinolones, trimethoprim/sulfamethoxazole, and tetracyclines [116]. In Japan in 2020, ESBL-positive UPEC strains (blaCTX-M-27, blaCTX-M-14, and blaCTX-M-15) were associated with ST1193 [117]. ST1193 belongs to clonal complex 14 (CC14), which is related with the serogroup O75 clonal group; is a nonlactose fermenter; is related to phylogenetic group B2; and is resistant to quinolones [114,118,119], maintaining a correlation with ST131 clones [119,120,121]. Several virulence genes associated with ST1193 clones have been described, such as papA (pili P main subunit), iha (adhesin and siderophore), fimH (type 1 fimbriae adhesin), sat (secreted autotransporter toxin), vat (vacuolizing autotransporter toxin), fyuA (yersiniabactin), kpsMII (capsule synthesis), usp (uropathogenic specific protein), ompT (outer membrane protein), and malX K1/K5 (production of K1- and K5-type polysaccharide capsules) [115]. The UPEC/ST1193 clones from uncomplicated UTIs (unUTIs) of women in Spain is resistant to fluoroquinolones and belongs to the O75:H4 serotype [120]. In Mexico, UPEC/ST1193 clones have been observed in women with rUTIs of several years’ duration [121]. The genomes of these strains contained chromosomal mutations that conferred resistance to fluoroquinolones (gyrA, S83L, D87N, parC S80I, and parE L416F) [122]. The genes chuA (heme group receptor), fyuA (yersiniabctin), gad (glutamic acid dehydratase), iha (adhesin and siderophore), irp2 (regulation of iron acquisition), iucC (aerobactin biosynthesis), iutA (aerobactin), kpsE (polysaccharide transport), kpsMII K1 (capsule synthesis serogroup K1), neuC (UDP-GlcNAc 2-epimerase), ompT (outer membrane protein), papA_F43 (main subunit of pili P F43), sat (secreted autotransporter toxin), senB (shiga-like toxin), sitA (iron transport system), terC (tellurite resistance protein), tratT (complement resistance protein), usp (uropathogenic specific protein), vat (vacuolizing autotransporter toxin), and fcV (iron and cobalt transport protein) have been identified [112].
In Vietnam, ST1193 clones from sepsis patients and from fecal matter presented a high frequency of genes encoding adhesins papG (type P fimbriae adhesin), fimH (type 1 fimbriae adhesin), and csgA (curli fimbriae adhesin); toxins sat (secreted autotransporter toxin), usp (uropathogenic specific protein), and senB (shiga-like toxin); siderophores fepA (enterobactin receptor), chuA (heme group receptor), fyuA (yersiniabctin), irp1 (yersiniabactin biosynthesis), sitA (iron and manganese transport), iucA (aerobactin biosynthesis), and iutA (aerobactin); and invasins ibeB (invasine), vat (vacuolizing autotransporter toxin), and hlyE (hemolysin) [123]. ST1193 clones also contain the IncF−:A1:B10 plasmid located within the C1 cluster, as well as the IncF1:A2:B20 plasmid in ST131 clones, both of which contain the conjugation module, indicating that the ST1193 clone is a self-transferable plasmid [123]. The similarity of STs in several countries (Vietnam, Australia, the United States, Denmark, and Singapore) indicates that there is a high transfer of genes and plasmids that contribute to the pathogenesis of these bacteria [88,115,124,125].
Genome sequence data have shown that Mexican strains of UPEC identified as ST1193 lack the genes fimH (type 1 fimbriae adhesin), papG (type P fimbriae adhesin), hlyA (hemolysin), and satA (secreted autotransporter toxin). However, these strains harbor four plasmids (small cryptic, nonconjugative mobilization, conjugative mobilization, and resistance plasmids) associated with virulence and resistance [126]. Interestingly, the ST1193 clone has high activity in promoting adherence to the human bladder cell line HTB-5 and nonbiofilm formation [126] (Figure 1). ST131 and ST1193 clones of UPEC pose a significant risk to human health because of their resistance to multiple antibiotics and widespread global distribution. Both clones developed this resistance through mutations in the QRDR region, which is responsible for quinolone resistance. Additionally, they can carry IncF plasmids and virulence factors, contributing to their prevalence among E. coli isolates [112,127].
ST73 Lineage
The prevalence of UPEC clinical strains identified with ST73 is significant, especially in the United Kingdom, Spain, Switzerland, and Portugal. The pathogenic potential of these clones has been linked to the production of ESBLs that confer multidrug resistance and can cause sequelae of urosepsis and bacteremia [128]. In Mexico, 7.81% of ST73 clones have been reported from Mexican children with cUTIs [23]. ST73 clones belong to phylogroup B2 and are associated with four main serotypes (O6:H1, O2:H1, O18:H1, and O25:H1) and several virulence genes: fimH (type 1 fimbriae adhesin), papG (type P fimbriae adhesin), csgA (curli fimbriae adhesin), expA (synthesis and secretion of exopolysaccharides), fdeC (fructose transport protein), iut (aerobactin transport protein), fyuA (yersiniabactin), chuA (heme group receptor), hma (siderophore), sit (sit iron transport system), eit (iron-induced enterotoxin), and ire (siderophore) [128,129]. Significant variations among strains of different serogroups, including ST73/O25 clone, have revealed many toxin genes, such as cnf1 (cytotoxic necrotizing factor), hlyABCDE (hemolysin), and sat (secreted autotransporter toxin), and genes that promote bloodstream dissemination and invasion, pic (proinflammatory cytokine-induced cytotoxin), tsh (temperature-sensitive hemagglutinin), iss (serum resistance protein), ibe (blood–brain barrier invasion protein), hek (hemagglutinating adhesin), and tia (subtilase) [128]. Interestingly, the tcp gene associated with immune modulation via inhibition of Toll/IL-1 receptor signaling was identified in ST73/O6 clones [129]. Furthermore, ST73 clones contain class 1 integrons (intl1) and blaOXA-1-type β-lactamases [129].
ST405 Lineage
The ST 405 clone associated with phylogroup D, are resistant to quinolones (gyrA, S83L and D87N; parC, S80I; and parE, S458A) [130] and to ESBLs (blaCTX-M-14 and blaCTX-M-15); however, some ST405 clones harbor the blaCTX-M-27 gene, as has been identified in other STs. ST405 clones shares virulence and multidrug resistance characteristics with clones belonging to ST131/O25b [131] (Figure 1).
ST69 Lineage
Clones belonging to ST69 are associated with phylogenetic group D; are resistant to trimethoprim/sulfamethoxazole; are considered endemic; and have a wide variety of serogroups, such as O11, O15, O17, O44, O73, O77, O86, O125ab, and O25b [5,132]. Our workgroup revealed that ST69 clones was highly prevalent in children with cUTIs, with high loads of virulence genes (fimH, csgA, papGII, ecpA, iutD, fyuA, and hlyA) and resistance genes (MDR and XDR), as well as 21.81% of the strains belonging to serogroup O25 [23] (Figure 1).
Other STs
ST8196 is a new ST identified in clones from patients with bacteremia that phylogenetically shares a common ancestor with ST131 [113]. Clones belonging to ST101 carry the blaNDM-7 gene and are associated with the resistance genes blaOXA-1, blaTEM-1A, blaCTX-M-15, aac(6′)-Ib-cr, catB3, and tetB [133]. As of 2019, 29 variants of NDM-type enzymes have been reported, which are distributed mainly in countries such as China and India [134,135,136]. Briefly, phylogenetic studies have shown that the adaptation of new STs in E. coli, with virulence and resistance determinants, represents a health problem, and epidemiological studies at the molecular level are needed. The ST410 clone has been identified mainly in ExPEC and is becoming increasingly significant as a high-risk clone. Research indicates a high frequency of transmission between species [88,137]. Whole-genome sequencing of 10 isolates from both animals and humans revealed a high degree of genetic similarity, distinguished by a low number of SNPs. The ST410 clone has been identified predominantly in ExPEC and is increasingly recognized as a high-risk clone. Research has demonstrated a significant frequency of interspecies transmission (humans, poultry, companion animals, and sewage) in Germany [137,138]. The ST410 clone has been reported in several countries, including China, Italy, Denmark, and Ghana [136,139,140,141]. Clones of ST410 carry the blaOXA-181 gene, an OXA-48-type carbapenemase that confers resistance to penicillins and carbapenems. The blaOXA-181 gene is believed to have originated from Shewanella xiamenensis, which is an environmental bacterium [142]. In addition, there are reports of its identification from a natural water source in Singapore with resistance to carbapenems, as well as its similarity with isolates in Thailand. Furthermore, the IS26 insertion sequence has been mentioned as a mediator of the acquisition of resistance through the blaNDM-5 gene [143] (Figure 1).
2. Conclusions
UTIs continue to have a high impact worldwide due to the increased difficulty of antimicrobial treatment caused by the emergence of MDR and XDR strains. The acquisition of antibiotic resistance genes and the dissemination of virulence factors have facilitated recurrence (Figure 2). A variety of clinical treatment alternatives to antibiotic therapy have been developed, such as the use of vaccines, although the protective efficacy of these vaccines has not been achieved. Moreover, the high diversity of serogroups has limited the vaccine protection spectrum, and these vaccines have been associated with multiple side effects, prolonged use times, and high costs. Therefore, it is imperative to study the genetic diversity of UPEC and the immune response that develops in the host during a UTI to develop new and better treatment options that reduce the impact of UTI recurrence and persistence.
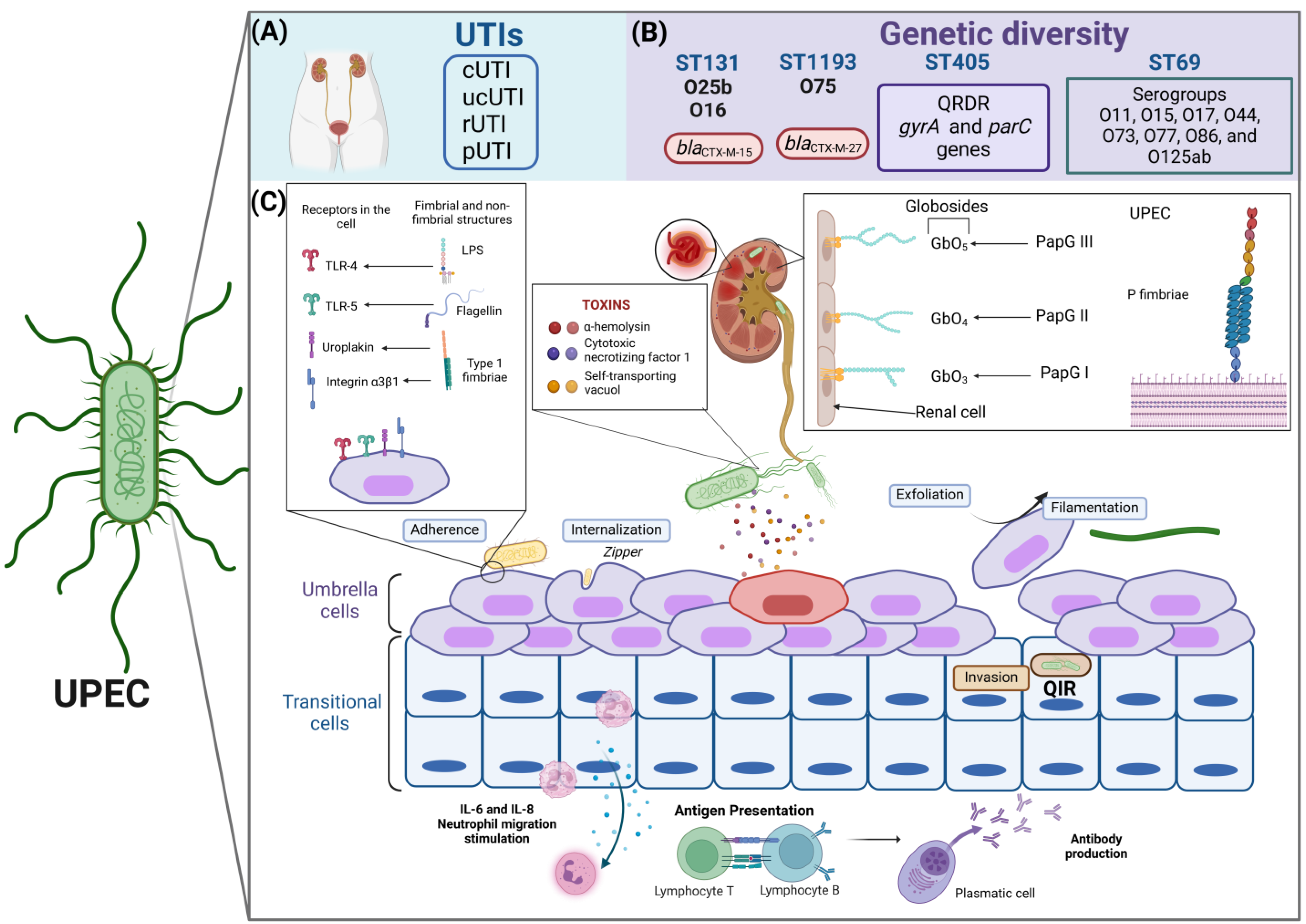
Figure 2.
Characteristics of the pathogenic cycle of UPEC, type of infection, and genetic diversity. (A) UPEC is the main uropathogen associated with urinary tract infections (UTIs) and can be recovered from different clinical stages, such as the complicated (cUTI), uncomplicated (unUTI), recurrent (rUTI), and persistent (pUTI) stages. (B) UPEC shows high clonality, highlighting that the high-risk clones, such as ST131/O25b, ST131/O16, and ST1193/O75, and other clones that have been frequently reported in isolates of urinary origin are ST69 and ST405. UPEC clones present particular characteristics of antibiotic resistance, which allows their identification as epidemiological risk clones. (C) The pathogenesis of UPEC in the bladder epithelium begins with the specific adherence of UPEC type 1 fimbriae to the mannosylated residues of the uroplakin receptors and integrin α3β1-type receptors on the surface of the umbrella cells of the urothelium. Curli fimbriae, which are recognized by fibronectin and laminin in the cell, also participate. Through a rearrangement of the actin filaments, UPEC is internalized in superficial cells or umbrella cells via a zipper-type mechanism. Once inside the cell, UPEC can form an intracellular bacterial community (IBC) or carry out a filamentation process in which UPEC maintains bacterial growth but does not divide so that it can form filaments, which facilitate greater contact with the umbrella cells and allow a new cycle of infection to begin. Exfoliation of the urothelium occurs naturally or is favored by external signals caused by UPEC, such as the release of α-hemolysin (HlyA), cytotoxic necrotizing factor 1 (Cnf-1), and vacuolating self-transporting toxin (Sat), which cause cell damage, inducing apoptosis and excessive exfoliation. After the exfoliation process, UPEC can infect transitional epithelial cells, forming quiescent intracellular reservoirs (QIRs). The nonfimbrial structures of UPEC, such as lipopolysaccharides and surface glucans, are recognized by TLR-4 receptors on the surface of the bladder, and TLR-5 recognizes flagellin. P fimbriae are essential virulence factors for colonizing the renal epithelium and are also recognized by the major histocompatibility complex type 1 of antigen-presenting cells. The P fimbriae variants PapGI, PapGII, and PapGIII have affinities for globosides in renal cells. Finally, UPEC can evade the host immune response and the action of antimicrobials during its pathogenic cycle, which is thus associated with recurrent and persistent infections. UPEC synthesizes the flagellum to colonize the upper UI in an ascending manner, reaching the kidney, where it can cause pyelonephritis and, subsequently, reach the hematogenous route to cause urosepsis or septicemia, created with http://www.Biorender.com (design in this study).
3. Perspectives
The pathogenic cycle of UPEC in the urinary tract begins with the expression of various colonization factors, which are essential as a first step for pathogenicity. The adhesins CsgA, FimH, and PapG are commonly found across different UPEC lineages and play crucial roles in the initial colonization process of the urinary tract. This step is fundamental to the pathogenic cycle of bacteria. Therefore, a cutting-edge therapeutic strategy should focus on creating a chimeric fusion protein that combines these three adhesins. This fusion protein could serve as a specific vaccine against UPEC UTIs by generating protective memory antibodies that reduce or block initial adherence to the bladder epithelium (Figure 2).
Author Contributions
J.D.G.-G., L.M.C.-A. and J.X.-C. designed and wrote the manuscript. S.A.O., A.C.-C., R.H.-C., M.F.-E., S.R.-G., J.A.-G. and J.X.-C. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The authors disclose that they received financial support for the publication of this research article. This study was carried out with the support of Federal Funds (grant numbers HIM/2021/031FF SSA. 1720, HIM/2021/030FF SSA. 1730, HIM/2022/014 SSA. 1777, and HIM/2022/048FF SSA. 1815) from the Hospital Infantil de México “Federico Gómez”. This work was also supported by the “Consejo Nacional de Humanidades, Ciencias y Tecnologías (CONAHCYT)” (grant number 319563) from the “Announcement for Basic Science and/or Frontier Science, Modality: Paradigms and Controversies of Science 2022”.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
Jesús David García-García and Laura M. Contreras-Alvarado completed their doctoral studies at the “Programa de Doctorado en Ciencias en Biomedicina y Biotecnología Molecular de la Escuela Nacional de Ciencias Biológicas del Instituto Politécnico Nacional-IPN” with registration numbers A210150 and A210149, respectively. Jesús David García-García and Laura M. Contreras-Alvarado also received support from a doctoral scholarship from SECIHTI (Secretaría de Ciencia, Humanidades, Tecnología e Innovación) of Mexico with CVU 958410 and 963336, respectively. We also acknowledge Marco Antonio Flores Oropeza for his opinion on this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Leung, A.K.C.; Wong, A.H.C.; Leung, A.A.M.; Hon, K.L. Urinary Tract Infection in Children. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 2–18. [Google Scholar] [CrossRef]
- Flores-Mireles, A.; Hreha, T.N.; Hunstad, D.A. Pathophysiology, Treatment, and Prevention of Catheter-Associated Urinary Tract Infection. Top. Spinal Cord. Inj. Rehabil. 2019, 25, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, H.; Zheng, Y.; Qu, S.; Wang, H.; Yi, F. Disease burden and long-term trends of urinary tract infections: A worldwide report. Front. Public Health 2022, 10, 888205. [Google Scholar]
- Buettcher, M.; Trueck, J.; Niederer-Loher, A.; Heininger, U.; Agyeman, P.; Asner, S.; Berger, C.; Bielicki, J.; Kahlert, C.; Kottanattu, L.; et al. Swiss consensus recommendations on urinary tract infections in children. Eur. J. Pediatr. 2021, 180, 663–674. [Google Scholar]
- de Souza da-Silva, A.P.; de Sousa, V.S.; de Araújo Longo, L.G.; Caldera, S.; Lemos Baltazar, I.C.; Regina Bonelli, R.; Santoro-Lopes, G.; Woodland Riley, L.; Meurer Moreira, B. Prevalence of fluoroquinolone-resistant and broad-spectrum cephalosporin-resistant community-acquired urinary tract infections in Rio de Janeiro: Impact of Escherichia coli genotypes ST69 and ST131. Infect. Genet. Evol. 2020, 85, 104452. [Google Scholar]
- Yang, S.S.; Tsai, J.D.; Kanematsu, A.; Han, C.H. Asian guidelines for urinary tract infection in children. J. Infect. Chemother. 2021, 27, 1543–1554. [Google Scholar] [PubMed]
- Park, Y.S. Renal scar formation after urinary tract infection in children. Korean J. Pediatr. 2012, 55, 367–370. [Google Scholar] [CrossRef]
- Pleniceanu, O.; Twig, G.; Tzur, D.; Sherman, G.; Afek, A.; Erlich, T.; Keinan-Boker, L.; Skorecki, K.; Vivante, A.; Calderon-Margalit, R. Acute pyelonephritis in children and the risk of end-stage kidney disease. J. Nephrol. 2021, 34, 1757–1765. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar]
- Dickson, K.; Zhou, J.; Lehmann, C. Lower Urinary Tract Inflammation and Infection: Key Microbiological and Immunological Aspects. J. Clin. Med. 2024, 13, 315. [Google Scholar] [CrossRef]
- Nelson, Z.; Aslan, A.T.; Beahm, N.P.; Blyth, M.; Capiello, M.; Casaus, D.; Dominguez, F.; Egbert, S.; Hanretty, A.; Khadem, T.; et al. Guidelines for the Prevention, Diagnosis, and Management of Urinary Tract Infections in Pediatrics and Adults: A WikiGuidelines Group Consensus Statement. JAMA Netw. Open 2024, 7, e2444495. [Google Scholar] [CrossRef]
- Lodise, T.P.; Nowak, M.; Rodriguez, M. The 30-Day Economic Burden of Newly Diagnosed Complicated Urinary Tract Infections in Medicare Fee-for-Service Patients Who Resided in the Community. Antibiotics 2022, 11, 578. [Google Scholar] [CrossRef]
- ’t Hoen, L.A.; Bogaert, G.; Radmayr, C.; Dogan, H.S.; Nijman, R.J.M.; Quaedackers, J.; Rawashdeh, Y.F.; Silay, M.S.; Tekgul, S.; Bhatt, N.R.; et al. Update of the EAU/ESPU guidelines on urinary tract infections in children. J. Pediatr. Urol. 2021, 17, 200–207. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Terlizzi, M.E.; Gribaudo, G.; Maffei, M.E. UroPathogenic Escherichia coli (UPEC) Infections: Virulence Factors, Bladder Responses, Antibiotic, and Non-antibiotic Antimicrobial Strategies. Front. Microbiol. 2017, 8, 1566. [Google Scholar] [CrossRef]
- Ochoa, S.A.; Cruz-Córdova, A.; Luna-Pineda, V.M.; Reyes-Grajeda, J.P.; Cázares-Domiínguez, V.; Escalona, G.; Sepúlveda-González, M.E.; López-Montiel, F.; Arellano-Galindo, J.; López-Martínez, B.; et al. Multidrug- and Extensively Drug-Resistant Uropathogenic Escherichia coli Clinical Strains: Phylogenetic Groups Widely Associated with Integrons Maintain High Genetic Diversity. Front. Microbiol. 2016, 7, 2042. [Google Scholar] [CrossRef]
- Nicolle, L.E.; Gupta, K.; Bradley, S.F.; Colgan, R.; P DeMuri, G.; Drekonja, D.; O Eckert, L.; E Geerlings, S.; Koves, V.; M Hooten, T.; et al. Clinical Practice Guideline for the Management of Asymptomatic Bacteriuria: 2019 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2019, 68, 1611–1615. [Google Scholar] [CrossRef]
- Hughes, T.; Juliebø-Jones, P.; Saada, L.; Saeed, K.; Somani, B.K. Recurrent urinary tract infections in adults: A practical guide. Br. J. Hosp. Med. 2021, 82, 1–11. [Google Scholar] [CrossRef]
- Harding, C.; Mossop, H.; Homer, T.; Chadwick, T.; King, W.; Carnell, S.; Lecouturier, J.; Abouhajar, A.; Vale, L.; Watson, G.; et al. Alternative to prophylactic antibiotics for the treatment of recurrent urinary tract infections in women: Multicentre, open label, randomised, non-inferiority trial. BMJ 2022, 376, e068229. [Google Scholar] [CrossRef]
- Whelan, S.; Lucey, B.; Finn, K. Uropathogenic Escherichia coli (UPEC)-Associated Urinary Tract Infections: The Molecular Basis for Challenges to Effective Treatment. Microorganisms 2023, 11, 2169. [Google Scholar] [CrossRef]
- Lupo, F.; Ingersoll, M.A.; Pineda, M.A. The glycobiology of uropathogenic E. coli infection: The sweet and bitter role of sugars in urinary tract immunity. Immunology 2021, 164, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Luna-Pineda, V.M.; Ochoa, S.A.; Cruz-Córdova, A.; Cázares-Domínguez, V.; Reyes Grajeda, J.P.; Flores-Oropeza, M.A.; Arellando-Galindo, J.; Castro-Hernández, R.; Flores-Encarnación, M.; Ramírez-Vargas, A.; et al. Correction: Features of urinary Escherichia coli isolated from children with complicated and uncomplicated urinary tract infections in Mexico. PLoS ONE 2018, 13, e0208285. [Google Scholar]
- Contreras-Alvarado, L.M.; Zavala-Vega, S.; Cruz-Córdova, A.; Reyes-Grajeda, J.P.; Escalona Venegas, G.; Flores, F.; Alcazar-López, V.; Arellano-Galindo, J.; Hernández Catdro, R.; Castro-Escarpulli, G.; et al. Molecular Epidemiology of Multidrug-Resistant Uropathogenic Escherichia coli O25b Strains Associated with Complicated Urinary Tract Infection in Children. Microorganisms 2021, 9, 2299. [Google Scholar] [CrossRef]
- Chahales, P.; Thanassi, D.G. Structure, Function, and Assembly of Adhesive Organelles by Uropathogenic Bacteria. Microbiol. Spectr. 2015, 3, 277–329. [Google Scholar] [CrossRef]
- Wurpel, D.J.; Beatson, S.A.; Totsika, M.; Petty, N.K.; Schembri, M.A. Chaperone-usher fimbriae of Escherichia coli. PLoS ONE 2013, 8, e52835. [Google Scholar]
- Bokil, N.J.; Totsika, M.; Carey, A.J.; Stacey, K.J.; Hancock, V.; Saunders, B.M.; Ravasi, T.; Ulett, G.C.; Schermbri, M.A.; Sweet, M.J. Intramacrophage survival of uropathogenic Escherichia coli: Differences between diverse clinical isolates and between mouse and human macrophages. Immunobiology 2011, 216, 1164–1171. [Google Scholar] [CrossRef]
- Weichhart, T.; Haidinger, M.; Hörl, W.H.; Säemann, M.D. Current concepts of molecular defence mechanisms operative during urinary tract infection. Eur. J. Clin. Investig. 2008, 38 (Suppl. S2), 29–38. [Google Scholar]
- Zyla, D.S.; Wiegand, T.; Bachmann, P.; Zdanowicz, R.; Giese, C.; Meier, B.H.; Waksman, G.; Hospenthal, M.K.; Glockshuber, R. The assembly platform FimD is required to obtain the most stable quaternary structure of type 1 pili. Nat. Commun. 2024, 15, 3032. [Google Scholar]
- Knight, S.D.; Bouckaert, J. Structure, function, and assembly of type 1 fimbriae. Top. Curr. Chem. 2009, 288, 67–107. [Google Scholar]
- Dwyer, B.E.; Newton, K.L.; Kisiela, D.; Sokurenko, E.V.; Clegg, S. Single nucleotide polypmorphisms of fimH associated with adherence and biofilm formation by serovars of Salmonella enterica. Microbiology 2011, 157 Pt 11, 3162–3171. [Google Scholar]
- Yoshida, M.; Thiriet-Rupert, S.; Mayer, L.; Beloin, C.; Ghigo, J.M. Selection for nonspecific adhesion is a driver of FimH evolution increasing Escherichia coli biofilm capacity. Microlife 2022, 3, uqac001. [Google Scholar] [CrossRef] [PubMed]
- Iebba, V.; Conte, M.P.; Lepanto, M.S.; Di Nardo, G.; Santangelo, F.; Aloi, M.; Totino, V.; Checchi, M.P.; Longhi, C.; Cucchiara, S.; et al. Microevolution in fimH gene of mucosa-associated Escherichia coli strains isolated from pediatric patients with inflammatory bowel disease. Infect. Immun. 2012, 80, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Weissman, S.J.; Chattopadhyay, S.; Aprikian, P.; Obata-Yasuoka, M.; Yarova-Yarovaya, Y.; Stapleton, A.; Ba-Thein, W.; Dykhuizen, D.; Johnson, J.R.; Sokurenko, E.V. Clonal analysis reveals high rate of structural mutations in fimbrial adhesins of extraintestinal pathogenic Escherichia coli. Mol Microbiol. 2006, 59, 975–988. [Google Scholar] [CrossRef]
- Qin, J.; Wilson, K.A.; Sarkar, S.; Heras, B.; O’Mara, M.L.; Totsika, M. Conserved FimH mutations in the global Escherichia coli ST131 multi-drug resistant lineage weaken interdomain interactions and alter adhesin function. Comput. Struct. Biotechnol. J. 2022, 20, 4532–4541. [Google Scholar] [CrossRef] [PubMed]
- Schwan, W.R. Regulation of fim genes in uropathogenic Escherichia coli. World J. Clin. Infect. Dis. 2011, 1, 17–25. [Google Scholar] [CrossRef]
- Schwartz, D.J.; Kalas, V.; Pinkner, J.S.; Chen, S.L.; Spaulding, C.N.; Dodson, K.W.; Hultgren, S.J. Positively selected FimH residues enhance virulence during urinary tract infection by altering FimH conformation. Proc. Natl. Acad. Sci. USA 2013, 110, 15530–15537. [Google Scholar] [CrossRef]
- Habouria, H.; Bessaiah, H.; Pokharel, P.; Dhakal, S.; Maris, S.; Buron, J.; Houle, S.; Dozois, C.M. A Newly Identified Group of P-like (PL) Fimbria Genes from Extraintestinal Pathogenic Escherichia coli (ExPEC) Encode Distinct Adhesin Subunits and Mediate Adherence to Host Cells. Appl. Environ. Microbiol. 2022, 88, e0142121. [Google Scholar] [CrossRef]
- Nojoomi, F.; Ghasemian, A. The relation of phylogroups, serogroups, virulence factors and resistance pattern of Escherichia coli isolated from children with septicemia. New Microbes New Infect. 2019, 29, 100517. [Google Scholar] [CrossRef]
- Čurová, K.; Slebodníková, R.; Kmeťová, M.; Hrabovský, V.; Maruniak, M.; Liptáková, E.; Siegfried, L. Virulence, phylogenetic background and antimicrobial resistance in Escherichia coli associated with extraintestinal infections. J. Infect. Public Health 2020, 13, 1537–1543. [Google Scholar] [CrossRef]
- Clark, J.R.; Maresso, A.M. Comparative Pathogenomics of Escherichia coli: Polyvalent Vaccine Target Identification through Virulome Analysis. Infect. Immun. 2021, 89, e0011521. [Google Scholar] [CrossRef]
- Baldiris-Avila, R.; Montes-Robledo, A.; Buelvas-Montes, Y. Phylogenetic Classification, Biofilm-Forming Capacity, Virulence Factors, and Antimicrobial Resistance in Uropathogenic Escherichia coli (UPEC). Curr. Microbiol. 2020, 77, 3361–3370. [Google Scholar] [CrossRef] [PubMed]
- Hyun, M.; Lee, J.Y.; Kim, H.A. Differences of virulence factors, and antimicrobial susceptibility according to phylogenetic group in uropathogenic Escherichia coli strains isolated from Korean patients. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 77. [Google Scholar]
- Lin, W.H.; Zhang, Y.Z.; Liu, P.Y.; Chen, P.S.; Wang, S.; Kuo, P.Y.; Thuy, T.T.D.; Duong, T.T.T.; Wen, L.L.; Hsieh, Y.H.; et al. Distinct Characteristics of Escherichia coli Isolated from Patients with Urinary Tract Infections in a Medical Center at a Ten-Year Interval. Pathogens 2021, 10, 1156. [Google Scholar] [CrossRef] [PubMed]
- Biggel, M.; Xavier, B.B.; Johnson, J.R.; Nielsen, K.L.; Frimodt-Møller, N.; Matheeussen, V.; Goossens, H.; Moons, P.; Van Puyvelde, S. Horizontally acquired papGII-containing pathogenicity islands underlie the emergence of invasive uropathogenic Escherichia coli lineages. Nat. Commun. 2020, 11, 5968. [Google Scholar]
- Mitsumori, K.; Terai, A.; Yamamoto, S.; Ishitoya, S.; Yoshida, O. Virulence characteristics of Escherichia coli in acute bacterial prostatitis. J. Infect. Dis. 1999, 180, 1378–1381. [Google Scholar]
- Otto, G.; Magnusson, M.; Svensson, M.; Braconier, J.; Svanborg, C. pap genotype and P fimbrial expression in Escherichia coli causing bacteremic and nonbacteremic febrile urinary tract infection. Clin. Infect. Dis. 2001, 32, 1523–1531. [Google Scholar]
- Sarowska, J.; Futoma-Koloch, B.; Jama-Kmiecik, A.; Frej-Madrzak, M.; Ksiazczyk, M.; Bugla-Ploskonska, G.; Choroszy-Krol, I. Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: Recent reports. Gut Pathog. 2019, 11, 10. [Google Scholar]
- Johnson, J.R.; Johnson, C.E.; Maslow, J.N. Clinical and bacteriologic correlates of the papG alleles among Escherichia coli strains from children with acute cystitis. Pediatr. Infect. Dis. J. 1999, 18, 446–451. [Google Scholar]
- Johnson, J.R.; Russo, T.A. Molecular epidemiology of extraintestinal pathogenic (uropathogenic) Escherichia coli. Int. J. Med. Microbiol. 2005, 295, 383–404. [Google Scholar]
- Du, M.; Yuan, Z.; Werneburg, G.T.; Henderson, N.S.; Chauhan, H.; Kovach, A.; Zhao, G.; Johl, J.; Li, H.; Thanassi, D.G. Processive dynamics of the usher assembly platform during uropathogenic Escherichia coli P pilus biogenesis. Nat. Commun. 2021, 12, 5207. [Google Scholar]
- Bhoite, S.; van Gerven, N.; Chapman, M.R.; Remaut, H. Curli Biogenesis: Bacterial Amyloid Assembly by the Type VIII Secretion Pathway. EcoSal Plus. 2019, 8, 10–1128. [Google Scholar] [CrossRef]
- Kot, B.; Wicha, J.; Piechota, M.; Wolska, K.; Gruzewska, A. Antibiofilm activity of trans-cinnamaldehyde, p-coumaric, and ferulic acids on uropathogenic Escherichia coli. Turk. J. Med. Sci. 2015, 45, 919–924. [Google Scholar] [CrossRef]
- Tomasek, K.; Leithner, A.; Glatzova, I.; Lukesch, M.S.; Guet, C.C.; Sixt, M. Type 1 piliated uropathogenic Escherichia coli hijack the host immune response by binding to CD14. Elife 2022, 11, e78995. [Google Scholar] [CrossRef]
- Oh, Y.J.; Hubauer-Brenner, M.; Gruber, H.J.; Cui, Y.; Traxler, L.; Siligan, C.; Park, S.; Hinterdorfer, P. Curli mediate bacterial adhesion to fibronectin via tensile multiple bonds. Sci. Rep. 2016, 6, 33909. [Google Scholar] [CrossRef]
- Luna-Pineda, V.M.; Moreno-Fierros, L.; Cázares-Domínguez, V.; Ilhuicatzi-Alvarado, D.; Ochoa, S.A.; Cruz-Córdova, A.; Valencia-Mayoral, P.; Rodríguez-Leviz, A.; Xicohtencatl-Cortes, J. Curli of Uropathogenic Escherichia coli Enhance Urinary Tract Colonization as a Fitness Factor. Front. Microbiol. 2019, 10, 2063. [Google Scholar] [CrossRef]
- Blumer, C.; Kleefeld, A.; Lehnen, D.; Heintz, M.; Dobrindt, U.; Nagy, G.; Michaelis, K.; Emödy, L.; Polen, T.; Rachel, R.; et al. Regulation of type 1 fimbriae synthesis and biofilm formation by the transcriptional regulator LrhA of Escherichia coli. Microbiology 2005, 151 Pt 10, 3287–3298. [Google Scholar] [CrossRef]
- Reisner, A.; Maierl, M.; Jörger, M.; Krause, R.; Berger, D.; Haid, A.; Tesic, D.; Zechner, E.L. Type 1 fimbriae contribute to catheter-associated urinary tract infections caused by Escherichia coli. J. Bacteriol. 2014, 196, 931–939. [Google Scholar]
- Zamani, H.; Salehzadeh, A. Biofilm formation in uropathogenic Escherichia coli: Association with adhesion factor genes. Turk. J. Med. Sci. 2018, 48, 162–167. [Google Scholar] [CrossRef]
- Yan, Z.; Yin, M.; Chen, J.; Li, X. Assembly and substrate recognition of curli biogenesis system. Nat. Commun. 2020, 11, 241. [Google Scholar] [CrossRef]
- Hawthorne, W.; Rouse, S.; Sewell, L.; Matthews, S.J. Structural insights into functional amyloid inhibition in Gram -ve bacteria. Biochem. Soc. Trans. 2016, 44, 1643–1649. [Google Scholar] [CrossRef]
- Vigil, P.D.; Wiles, T.J.; Engstrom, M.D.; Prasov, L.; Mulvey, M.A.; Mobley, H.L. The repeat-in-toxin family member TosA mediates adherence of uropathogenic Escherichia coli and survival during bacteremia. Infect. Immun. 2012, 80, 493–505. [Google Scholar] [PubMed]
- Xicohtencatl-Cortes, J.; Cruz-Córdova, A.; Cázares-Domínguez, V.; Escalona-Venegas, G.; Zavala-Vega, S.; Arellano-Galindo, J.; Romo-Castillo, M.; Hernández-Castro, R.; Ochoa, S.A.; Luna-Pineda, V.M. Uropathogenic Escherichia coli strains harboring tosA gene were associated to high virulence genes and a multidrug-resistant profile. Microb. Pathog. 2019, 134, 103593. [Google Scholar]
- Spurbek, R.R.; Mobley, H.T.L. Section III: Escherichia coli virulence factors. In Escherichia coli, 2nd ed.; Donnenberg, M.S., Ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 275–304. [Google Scholar]
- Vigil, P.D.; Stapleton, A.E.; Johnson, J.R.; Hooton, T.M.; Hodges, A.P.; He, Y.; Mobley, H.L. Presence of putative repeat-in-toxin gene tosA in Escherichia coli predicts successful colonization of the urinary tract. mBio 2011, 2, e00066-11. [Google Scholar]
- Ingersoll, M.A.; Albert, M.L. From infection to immunotherapy: Host immune responses to bacteria at the bladder mucosa. Mucosal Immunol. 2013, 6, 1041–1053. [Google Scholar] [PubMed]
- Jafari, N.V.; Rohn, J.L. The urothelium: A multi-faceted barrier against a harsh environment. Mucosal Immunol. 2022, 15, 1127–1142. [Google Scholar]
- Bleyer, A.J.; Kmoch, S. Tamm Horsfall Glycoprotein and Uromodulin: It Is All about the Tubules! Clin. J. Am. Soc. Nephrol. 2016, 11, 6–8. [Google Scholar] [PubMed]
- Mo, B.; Sendker, J.; Herrmann, F.; Nowak, S.; Hensel, A. Aqueous extract from Equisetum arvense stimulates the secretion of Tamm-Horsfall protein in human urine after oral intake. Phytomedicine. 2022, 104, 154302. [Google Scholar]
- Zhong, Z.; Nan, K.; Weng, M.; Yue, Y.; Zhou, W.; Wang, Z.; Chu, Y.; Liu, R.; Miao, C. Pro- and Anti- Effects of Immunoglobulin A- Producing B Cell in Tumors and Its Triggers. Front. Immunol. 2021, 12, 765044. [Google Scholar]
- Masajtis-Zagajewska, A.; Nowicki, M. New markers of urinary tract infection. Clin. Chim. Acta 2017, 471, 286–291. [Google Scholar] [CrossRef]
- Zychlinsky-Scharff, A.; Rousseau, M.; Lacerda-Mariano, L.; Canton, T.; Consiglio, C.R.; Albert, M.L.; Fontes, M.; Duffy, D.; Ingersoll, M.A. Sex differences in IL-17 contribute to chronicity in male versus female urinary tract infection. JCI Insight 2019, 5, e122998. [Google Scholar]
- Mora-Bau, G.; Platt, A.M.; van-Rooijen, N.; Randolph, G.J.; Albert, M.L.; Ingersoll, M.A. Macrophages Subvert Adaptive Immunity to Urinary Tract Infection. PLoS Pathog. 2015, 11, e1005044. [Google Scholar] [CrossRef] [PubMed]
- Steinert, E.M.; Schenkel, J.M.; Fraser, K.A.; Beura, L.K.; Manlove, L.S.; Igyártó, B.Z.; Southern, P.J.; Masopust, D. Quantifying Memory CD8 T Cells Reveals Regionalization of Immunosurveillance. Cell 2015, 161, 737–749. [Google Scholar] [PubMed]
- Luna-Pineda, V.M.; Ochoa, S.; Cruz-Córdova, A.; Cázares-Domínguez, V.; Vélez-González, F.; Hernández-Castro, R.; Xicohtencatl-Cortes, J. Infecciones del tracto urinario, inmunidad y vacunación [Urinary tract infections, immunity, and vaccination]. Bol. Med. Hosp. Infant. Mex. 2018, 75, 67–78. [Google Scholar] [PubMed]
- Yang, B.; Foley, S. First experience in the UK of treating women with recurrent urinary tract infections with the bacterial vaccine Uromune®. BJU Int. 2018, 121, 289–292. [Google Scholar]
- Wagenlehner, F.M.; Ballarini, S.; Pilatz, A.; Weidner, W.; Lehr, L.; Naber, K.G. A Randomized, Double-Blind, Parallel-Group, Multicenter Clinical Study of Escherichia coli-Lyophilized Lysate for the Prophylaxis of Recurrent Uncomplicated Urinary Tract Infections. Urol. Int. 2015, 95, 167–176. [Google Scholar] [CrossRef]
- Hopkins, W.J.; Elkahwaji, J.; Beierle, L.M.; Leverson, G.E.; Uehling, D.T. Vaginal mucosal vaccine for recurrent urinary tract infections in women: Results of a phase 2 clinical trial. J. Urol. 2007, 177, 1349–1591. [Google Scholar]
- Huttner, A.; Hatz, C.; van den Dobbelsteen, G.; Abbanat, D.; Hornacek, A.; Frölich, R.; Dreyer, A.M.; Martin, P.; Davies, T.; Fae, K.; et al. Safety, immunogenicity, and preliminary clinical efficacy of a vaccine against extraintestinal pathogenic Escherichia coli in women with a history of recurrent urinary tract infection: A randomised, single-blind, placebo-controlled phase 1b trial. Lancet Infect. Dis. 2017, 17, 528–537. [Google Scholar]
- Nestler, S.; Peschel, C.; Horstmann, A.H.; Vahlensieck, W.; Fabry, W.; Neisius, A. Prospective multicentre randomized double-blind placebo-controlled parallel group study on the efficacy and tolerability of StroVac® in patients with recurrent symptomatic uncomplicated bacterial urinary tract infections. Int. Urol. Nephrol. 2023, 55, 9–16. [Google Scholar]
- Vega-Hernández, R.; Ochoa, S.A.; Valle-Rios, R.; Jaimes-Ortega, G.A.; Arellano-Galindo, J.; Aparicio-Ozores, G.; Ibarra, J.A.; Hernández-Castro, R.; Cruz-Córdova, A.; Xicohtencatl-Cortes, J. Flagella, Type I Fimbriae and Curli of Uropathogenic Escherichia coli Promote the Release of Proinflammatory Cytokines in a Coculture System. Microorganisms 2021, 9, 2233. [Google Scholar] [CrossRef]
- Quinn, T.P.; Erb, I.; Richardson, M.F.; Crowley, T.M. Understanding sequencing data as compositions: An outlook and review. Bioinformatics 2018, 34, 2870–2878. [Google Scholar]
- Manges, A.R.; Geum, H.M.; Guo, A.; Edens, T.J.; Fibke, C.D.; Pitout, J.D.D. Global Extraintestinal Pathogenic Escherichia coli (ExPEC) Lineages. Clin. Microbiol. Rev. 2019, 32, e00135-18. [Google Scholar] [CrossRef] [PubMed]
- Spratt, B.G. Exploring the concept of clonality in bacteria. Methods Mol. Biol. 2004, 266, 323–352. [Google Scholar] [PubMed]
- Ali, I.; Rafaque, Z.; Ahmed, I.; Tariq, F.; Graham, S.E.; Salzman, E.; Foxman, B.; Dasti, J.I. Phylogeny, sequence-typing and virulence profile of uropathogenic Escherichia coli (UPEC) strains from Pakistan. BMC Infect. Dis. 2019, 19, 620. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wyrsch, E.R.; Elankumaran, P.; Dolejska, M.; Marenda, M.S.; Browning, G.F.; Bushell, R.N.; McKinnon, J.; Chowdhury, P.R.; Hitchick, N.; et al. Genomic comparisons of Escherichia coli ST131 from Australia. Microb. Genom. 2021, 7, 000721. [Google Scholar] [CrossRef]
- Pitout, J.D.D.; Finn, T.J. The evolutionary puzzle of Escherichia coli ST131. Infect. Genet. Evol. 2020, 81, 104265. [Google Scholar] [CrossRef]
- Kocsis, B.; Gulyás, D.; Szabó, D. Emergence and Dissemination of Extraintestinal Pathogenic High-Risk International Clones of Escherichia coli. Life 2022, 12, 2077. [Google Scholar] [CrossRef]
- Roer, L.; Overballe-Petersen, S.; Hansen, F.; Schønning, K.; Wang, M.; Røder, B.L.; Hansen, D.S.; Justesen, U.S.; Andersen, L.P.; Fulgsang-Damgaard, D.; et al. Escherichia coli Sequence Type 410 Is Causing New International High-Risk Clones. mSphere 2018, 3, e00337-18. [Google Scholar] [CrossRef]
- Zhong, Y.M.; Liu, W.E.; Liang, X.H.; Li, Y.M.; Jian, Z.J.; Hawkey, P.M. Emergence and spread of O16-ST131 and O25b-ST131 clones among faecal CTX-M-producing Escherichia coli in healthy individuals in Hunan Province, China. J. Antimicrob. Chemother. 2015, 70, 2223–2227. [Google Scholar] [CrossRef]
- Zhong, Y.M.; Liu, W.E.; Meng, Q.; Li, Y. Escherichia coli O25b-ST131 and O16-ST131 causing urinary tract infection in women in Changsha, China: Molecular epidemiology and clinical characteristics. Infect. Drug Resist. 2019, 12, 2693–2702. [Google Scholar] [CrossRef]
- Mora, A.; Dahbi, G.; López, C.; Mamani, R.; Marzoa, J.; Dion, S.; Picard, B.; Blanco, M.; Alonso, M.P.; Denamur, E.; et al. Virulence patterns in a murine sepsis model of ST131 Escherichia coli clínicas isolates belonging to serotypes O25b:H4 and O16:H5 are associated to specific virotypes. PLoS ONE 2014, 9, e87025. [Google Scholar] [CrossRef]
- Afolayan, A.O.; Aboderin, A.O.; Oaikhena, A.O.; Odih, E.E.; Ogunleye, V.O.; Adeyemo, A.T.; Adeyemo, A.T.; Bejide, O.S.; Underwood, A.; Argimón, S.; et al. An ST131 clade and a phylogroup A clade bearing an O101-like O-antigen cluster predominate among bloodstream Escherichia coli isolates from South-West Nigeria hospitals. Microb. Genom. 2022, 8, mgen000863. [Google Scholar] [CrossRef] [PubMed]
- Varughese, L.R.; Rajpoot, M.; Goyal, S.; Mehra, R.; Chhokar, V.; Beniwal, V. Analytical profiling of mutations in quinolone resistance determining region of gyrA gene among UPEC. PLoS ONE 2018, 13, e0190729. [Google Scholar] [CrossRef] [PubMed]
- Perault, A.; Turlan, C.; Eynard, N.; Vallé, Q.; Bousquet-Mélou, A.; Giraud, E. Repeated Exposure of Escherichia coli to High Ciprofloxacin Concentrations Selects gyrB Mutants That Show Fluoroquinolone-Specific Hyperpersistence. Front. Microbiol. 2022, 13, 908296. [Google Scholar]
- Dahbi, G.; Mora, A.; Mamani, R.; López, C.; Alonso, M.P.; Marzoa, J.; Blanco, M.; Herrera, A.; Viso, S.; García-Garrote, F.; et al. Molecular epidemiology and virulence of Escherichia coli O16:H5-ST131: Comparison with H30 and H30-Rx subclones of O25b:H4-ST131. Int. J. Med. Microbiol. 2014, 304, 1247–1257. [Google Scholar] [CrossRef]
- Stoesser, N.; Sheppard, A.E.; Pankhurst, L.; De Maio, N.; Moore, C.E.; Sebra, R.; Turner, P.; Anson, L.W.; Kasarskis, A.; Batty, E.M.; et al. Evolutionary History of the Global Emergence of the Escherichia coli Epidemic Clone ST131. mBio 2016, 7, e02162. [Google Scholar]
- Pitout, J.D.; DeVinney, R. Escherichia coli ST131: A multidrug-resistant clone primed for global domination. F1000Research 2017, 6, 195. [Google Scholar]
- Price, L.B.; Johnson, J.R.; Aziz, M.; Clabots, C.; Johnston, B.; Tchesnokova, V.; Nordstrom, L.; Billig, M.; Chattopadhyay, S.; Stegger, M.; et al. The epidemic of extended-spectrum-β-lactamase-producing Escherichia coli ST131 is driven by a single highly pathogenic subclone, H30-Rx. mBio 2013, 4, e00377-13. [Google Scholar] [PubMed]
- Banerjee, R.; Johnson, J.R. A new clone sweeps clean: The enigmatic emergence of Escherichia coli sequence type 131. Antimicrob. Agents Chemother. 2014, 58, 4997–5004. [Google Scholar]
- Tóth, K.; Damjanova, I.; Laczkó, L.; Buzgó, L.; Lesinszki, V.; Ungvári, E.; Jánvári, L.; Hanczvikkel, A.; Tóth, Á.; Szabó, D. Genomic Epidemiology of C2/H30Rx and C1-M27 Subclades of Escherichia coli ST131 Isolates from Clinical Blood Samples in Hungary. Antibiotics 2024, 13, 363. [Google Scholar] [CrossRef]
- Olsen, R.H.; Bisgaard, M.; Löhren, U.; Robineau, B.; Christensen, H. Extended-spectrum β-lactamase-producing Escherichia coli isolated from poultry: A review of current problems, illustrated with some laboratory findings. Avian Pathol. 2014, 43, 199–208. [Google Scholar] [CrossRef]
- Teramae, M.; Osawa, K.; Shigemura, K.; Kitagawa, K.; Shirakawa, T.; Fujisawa, M.; Miyara, T. Prevalence of Quinolone Resistance of Extended-Spectrum β-Lactamase-Producing Escherichia coli with ST131-fimH30 in a City Hospital in Hyogo, Japan. Int. J. Mol. Sci. 2019, 20, 5162. [Google Scholar] [CrossRef] [PubMed]
- Namaei, M.H.; Yousefi, M.; Ziaee, M.; Salehabadi, A.; Ghannadkafi, M.; Amini, E.; Askari, P. First Report of Prevalence of CTX-M-15-Producing Escherichia coli O25b/ST131 from Iran. Microb. Drug Resist. 2017, 23, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Giedraitienė, A.; Vitkauskienė, A.; Pavilonis, A.; Patamsytė, V.; Genel, N.; Decre, D.; Arlet, G. Prevalence of O25b-ST131 clone among Escherichia coli strains producing CTX-M-15, CTX-M-14 and CTX-M-92 β-lactamases. Infect. Dis. 2017, 49, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Yun, K.W.; Lee, M.K.; Kim, W.; Lim, I.S. Uropathogenic Escherichia coli ST131 in urinary tract infections in children. Korean J. Pediatr. 2017, 60, 221–226. [Google Scholar] [CrossRef]
- Kim, Y.A.; Lee, K.; Chung, J.E. Risk factors and molecular features of sequence type (ST) 131 extended-Spectrum-β-lactamase-producing Escherichia coli in community-onset female genital tract infections. BMC Infect. Dis. 2018, 18, 250. [Google Scholar] [CrossRef]
- Hojabri, Z.; Darabi, N.; Arab, M.; Saffari, F.; Pajand, O. Clonal diversity, virulence genes content and subclone status of Escherichia coli sequence type 131: Comparative analysis of E. coli ST131 and non-ST131 isolates from Iran. BMC Microbiol. 2019, 19, 117. [Google Scholar]
- Demirci, M.; Ünlü, Ö.; İstanbullu-Tosun, A. Detection of O25b-ST131 clone, CTX-M-1 and CTX-M-15 genes via real-time PCR in Escherichia coli strains in patients with UTIs obtained from a university hospital in Istanbul. J. Infect. Public Health 2019, 12, 640–644. [Google Scholar] [CrossRef]
- Alqasim, A.; Abu, J.A.; Alyousef, A.A. Prevalence and molecular characteristics of sequence type 131 clone among clinical uropathogenic Escherichia coli isolates in Riyadh, Saudi Arabia. Saudi J. Biol. Sci. 2020, 27, 296–302. [Google Scholar] [CrossRef]
- Muller, A.; Gbaguidi-Haore, H.; Cholley, P.; Hocquet, D.; Sauget, M.; Bertrand, X. Hospital-diagnosed infections with Escherichia coli clonal group ST131 are mostly acquired in the community. Sci. Rep. 2021, 11, 5702. [Google Scholar] [CrossRef]
- Dziri, O.; Dziri, R.; Maraoub, A.; Chouchani, C. Characterization of O25b-ST131 Escherichia coli Clone Producing CTX-M-15, DHA-4, and CMY-42 in Urinary Tract Infections in a Tunisian Island. Microb. Drug Resist. 2020, 26, 741–746. [Google Scholar] [CrossRef]
- Pitout, J.D.D.; Peirano, G.; Chen, L.; DeVinney, R.; Matsumura, Y. Escherichia coli ST1193: Following in the Footsteps of E. coli ST131. Antimicrob. Agents Chemother. 2022, 66, e0051122. [Google Scholar] [CrossRef]
- Hastak, P.; Fourment, M.; Darling, A.E.; Gottlieb, T.; Cheong, E.; Merlino, J.; Myers, G.S.A.; Djordjevic, S.P.; Roy Chowdhury, P. Escherichia coli ST8196 is a novel, locally evolved, and extensively drug resistant pathogenic lineage within the ST131 clonal complex. Emerg. Microbes Infect. 2020, 9, 1780–1792. [Google Scholar] [CrossRef] [PubMed]
- White, R.T.; Ashcroft, M.M.; Bauer, M.J.; Bell, J.; Butkiewicz, D.; Alvarez-Fraga, L.; Gibson, J.S.; Kidsley, A.K.; Mollinger, J.L.; Peter, K.M.; et al. The complete genome sequence of five pre-2013 Escherichia coli sequence type (ST)1193 strains reveal insights into an emerging pathogen. Access Microbiol. 2024, 6, 000894.v3. [Google Scholar] [CrossRef]
- Platell, J.L.; Trott, D.J.; Johnson, J.R.; Heisig, P.; Heisig, A.; Clabots, C.R.; Johnston, B.; Cobbold, R.N. Prominence of an O75 clonal group (clonal complex 14) among non-ST131 fluoroquinolone-resistant Escherichia coli causing extraintestinal infections in humans and dogs in Australia. Antimicrob. Agents Chemother. 2012, 56, 3898–3904. [Google Scholar] [CrossRef]
- Tchesnokova, V.L.; Rechkina, E.; Larson, L.; Ferrier, K.; Weaver, J.L.; Schroeder, D.W.; She, R.; Butler-Wu, S.M.; Aguero-Rosenfeld, M.E.; Zerr, D.; et al. Rapid and Extensive Expansion in the United States of a New Multidrug-resistant Escherichia coli Clonal Group, Sequence Type 1193. Clin. Infect. Dis. 2019, 68, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, Y.; Sato, T.; Tsukamoto, N.; Nakajima, C.; Suzuki, Y.; Takahashi, S.; Yokota, S.I. Corrigendum to Clonal/subclonal changes and accumulation of CTX-M-type β-lactamase genes in fluoroquinolone-resistant Escherichia coli ST131 and ST1193 strains isolated during the past 12 years, Japan. J. Glob. Antimicrob. Resist. 2023, 32, 195. [Google Scholar] [CrossRef] [PubMed]
- Fuzi, M.; Sokurenko, E. Commensal Fitness Advantage May Contribute to the Global Dissemination of Multidrug-Resistant Lineages of Bacteria-The Case of Uropathogenic E. coli. Pathogens 2023, 12, 1150. [Google Scholar] [CrossRef]
- Johnson, T.J.; Elnekave, E.; Miller, E.A.; Munoz-Aguayo, J.; Flores Figueroa, C.; Johnston, B.; Nielson, D.W.; Logue, C.M.; Johnson, J.R. Phylogenomic Analysis of Extraintestinal Pathogenic Escherichia coli Sequence Type 1193, an Emerging Multidrug-Resistant Clonal Group. Antimicrob. Agents Chemother. 2018, 63, e01913-18. [Google Scholar] [CrossRef]
- Wu, J.; Lan, F.; Lu, Y.; He, Q.; Li, B. Molecular Characteristics of ST1193 Clone among Phylogenetic Group B2 Non-ST131 Fluoroquinolone-Resistant Escherichia coli. Front. Microbiol. 2017, 8, 2294. [Google Scholar] [CrossRef]
- Chang, J.; Yu, J.; Lee, H.; Ryu, H.; Park, K.; Park, Y.J. Prevalence and characteristics of lactose non-fermenting Escherichia coli in urinary isolates. J. Infect. Chemother. 2014, 20, 738–740. [Google Scholar] [CrossRef]
- García-Meniño, I.; Lumbreras, P.; Lestón, L.; Álvarez-Álvarez, M.; García, V.; Hammerl, J.A.; Fernández, J.; Mora, A. Occurrence and Genomic Characterization of Clone ST1193 Clonotype 14-64 in Uncomplicated Urinary Tract Infections Caused by Escherichia coli in Spain. Microbiol. Spectr. 2022, 10, e0004122. [Google Scholar]
- Nguyen, Q.; Nguyen, T.T.N.; Pham, P.; Chau, V.; Nguyen, L.P.H.; Nguyen, T.D.; Ha, T.T.; Le, N.T.Q.; Vu, D.T.; Baker, S.; et al. Genomic insights into the circulation of pandemic fluoroquinolone-resistant extra-intestinal pathogenic Escherichia coli ST1193 in Vietnam. Microb. Genom. 2021, 7, 000733. [Google Scholar] [CrossRef]
- Pataki, B.Á.; Matamoros, S.; van der Putten, B.C.L.; Remondini, D.; Giampieri, E.; Aytan-Aktug, D.; Hendriksen, R.S.; Lund, O.; Csabai, I.; Schultsz, C.; et al. Understanding and predicting ciprofloxacin minimum inhibitory concentration in Escherichia coli with machine learning. Sci. Rep. 2020, 10, 15026. [Google Scholar]
- Heng, S.T.; Chen, S.L.; Wong, J.G.X.; Lye, D.C.; Ng, T.M. No association between resistance mutations, empiric antibiotic, and mortality in ceftriaxone-resistant Escherichia coli and Klebsiella pneumoniae bacteremia. Sci. Rep. 2018, 8, 12785. [Google Scholar] [CrossRef] [PubMed]
- Flores-Oropeza, M.A.; Ochoa, S.A.; Cruz-Córdova, A.; Chavez-Tepecano, R.; Martínez-Peñafiel, E.; Rembao-Bojórquez, D.; Zavala-Vega, S.; Hernández-Castro, R.; Flores-Encarnacion, M.; Arellano-Galindo, J.; et al. Comparative genomic analysis of uropathogenic Escherichia coli strains from women with recurrent urinary tract infection. Front. Microbiol. 2024, 14, 1340427. [Google Scholar] [CrossRef]
- García-Meniño, I.; García, V.; Lumbreras-Iglesias, P.; Fernández, J.; Mora, A. Fluoroquinolone resistance in complicated urinary tract infections: Association with the increased occurrence and diversity of Escherichia coli of clonal complex 131, together with ST1193. Front. Cell Infect. Microbiol. 2024, 14, 1351618. [Google Scholar]
- Aurich, S.; Wolf, S.A.; Prenger-Berninghoff, E.; Thrukonda, L.; Semmler, T.; Ewers, C. Genotypic Characterization of Uropathogenic Escherichia coli from Companion Animals: Predominance of ST372 in Dogs and Human-Related ST73 in Cats. Antibiotics 2023, 13, 38. [Google Scholar] [CrossRef]
- Bogema, D.R.; McKinnon, J.; Liu, M.; Hitchick, N.; Miller, N.; Venturini, C.; Iredell, J.; Darling, A.E.; Roy Chowdury, P.; Djordjevic, S.P. Whole-genome analysis of extraintestinal Escherichia coli sequence type 73 from a single hospital over a 2 year period identified different circulating clonal groups. Microb. Genom. 2020, 6, e000255. [Google Scholar]
- Yao, M.; Zhu, Q.; Zou, J.; Shenkutie, A.M.; Hu, S.; Qu, J.; He, Z.; Leung, P.H.M. Genomic Characterization of a Uropathogenic Escherichia coli ST405 Isolate Harboring blaCTX-M-15-Encoding IncFIA-FIB Plasmid, blaCTX-M-24-Encoding IncI1 Plasmid, and Phage-Like Plasmid. Front. Microbiol. 2022, 13, 845045. [Google Scholar]
- Matsumura, Y.; Yamamoto, M.; Nagao, M.; Ito, Y.; Takakura, S.; Ichiyama, S.; Kyoto-Shiga Clinical Microbiology Study Group. Association of fluoroquinolone resistance, virulence genes, and IncF plasmids with extended-spectrum-β-lactamase-producing Escherichia coli sequence type 131 (ST131) and ST405 clonal groups. Antimicrob. Agents Chemother. 2013, 57, 4736–4742. [Google Scholar]
- Riley, L.W. Pandemic lineages of extraintestinal pathogenic Escherichia coli. Clin. Microbiol. Infect. 2014, 20, 380–390. [Google Scholar] [CrossRef]
- Ramakrishnan, V.; Marialouis, X.A.; Sankarasubramanian, J.; Santhanam, A.; Balakrishnan, A.S. Whole Genomic analysis of a clinical isolate of Uropathogenic Escherichia coli strain of Sequence Type-101 carrying the drug resistance NDM-7 in IncX3 plasmid. Bioinformation 2021, 17, 126–131. [Google Scholar]
- Dadashi, M.; Yaslianifard, S.; Hajikhani, B.; Kabir, K.; Owlia, P.; Goudarzi, M.; Hakemivala, M.; Darban-Sarokhalil, D. Frequency distribution, genotypes and prevalent sequence types of New Delhi metallo-β-lactamase-producing Escherichia coli among clinical isolates around the world: A review. J. Glob. Antimicrob. Resist. 2019, 19, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Ayibieke, A.; Sato, W.; Mahazu, S.; Prah, I.; Addow-Thompson, J.; Ohashi, M.; Suzuki, T.; Iwanaga, S.; Ablordey, A.; Saito, R. Molecular characterisation of the NDM-1-encoding plasmid p2189-NDM in an Escherichia coli ST410 clinical isolate from Ghana. PLoS ONE 2018, 13, e0209623. [Google Scholar] [CrossRef] [PubMed]
- Bi, R.; Kong, Z.; Qian, H.; Jiang, F.; Kang, H.; Gu, B.; Ma, P. High Prevalence of blaNDM Variants Among Carbapenem-Resistant Escherichia coli in Northern Jiangsu Province, China. Front. Microbiol. 2018, 9, 2704. [Google Scholar] [CrossRef]
- Schaufler, K.; Semmler, T.; Wieler, L.H.; Wöhrmann, M.; Baddam, R.; Ahmed, N.; Müller, K.; Kola, A.; Fruth, A.; Ewers, C.; et al. Clonal spread and interspecies transmission of clinically relevant ESBL-producing Escherichia coli of ST410--another successful pandemic clone? FEMS Microbiol. Ecol. 2016, 92, fiv155. [Google Scholar]
- Falgenhauer, L.; Imirzalioglu, C.; Ghosh, H.; Gwozdzinski, K.; Schmiedel, J.; Gentil, K.; Bauerfeind, R.; Kämpfer, P.; Seifert, H.; Michael, G.B.; et al. Circulation of clonal populations of fluoroquinolone-resistant CTX-M-15-producing Escherichia coli ST410 in humans and animals in Germany. Int. J. Antimicrob. Agents 2016, 47, 457–465. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, Y.; Wu, W.; Xie, Y.; Wang, X.; Zhang, X.; Chen, X.; Zong, Z. First Report of OXA-181-Producing Escherichia coli in China and Characterization of the Isolate Using Whole-Genome Sequencing. Antimicrob. Agents Chemother. 2015, 59, 5022–5025. [Google Scholar]
- Piazza, A.; Comandatore, F.; Romeri, F.; Pagani, C.; Floriano, A.M.; Ridolfo, A.; Antona, C.; Brilli, M.; Mattioni Marchetti, V.; Bandi, C.; et al. First Report of an ST410 OXA-181 and CTX-M-15 Coproducing Escherichia coli Clone in Italy: A Whole-Genome Sequence Characterization. Microb. Drug Resist. 2018, 24, 1207–1209. [Google Scholar] [CrossRef]
- Mahazu, S.; Prah, I.; Ayibieke, A.; Sato, W.; Hayashi, T.; Suzuki, T.; Iwanaga, S.; Ablordey, A.; Saito, R. Possible Dissemination of Escherichia coli Sequence Type 410 Closely Related to B4/H24RxC in Ghana. Front. Microbiol. 2021, 12, 770130. [Google Scholar] [CrossRef]
- Potron, A.; Poirel, L.; Nordmann, P. Origin of OXA-181, an emerging carbapenem-hydrolyzing oxacillinase, as a chromosomal gene in Shewanella xiamenensis. Antimicrob. Agents Chemother. 2011, 55, 4405–4407. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Guo, S.; Schlundt, J.; Kwa, A.L. Identification and genomic characterization of a blaNDM-5-harbouring MDR plasmid in a carbapenem-resistant Escherichia coli ST410 strain isolated from a natural water environmental source. JAC-Antimicrob. Resist. 2022, 4, dlac071. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).