Enhancement of Lycopene Biosynthesis Using Self-Assembled Multi-Enzymic Protein Cages
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plasmid Construction
2.3. Expression and Assembly of Carboxysome Nanocage Scaffold
2.3.1. Expression and Assembly of mCherry-Labeled Carboxysome Shell-SC and mGFP-ST Cargo Protein
2.3.2. Construction of Carboxysome Nanocages for Immobilization of IPP Synthetic Enzymes
2.4. Fluorescence Microscopy
2.5. Transmission Electron Microscopy (TEM)
2.6. Carotenoid Extraction
2.7. Carotenoid Analysis Using HPLC
2.8. Statistics Analysis
3. Results
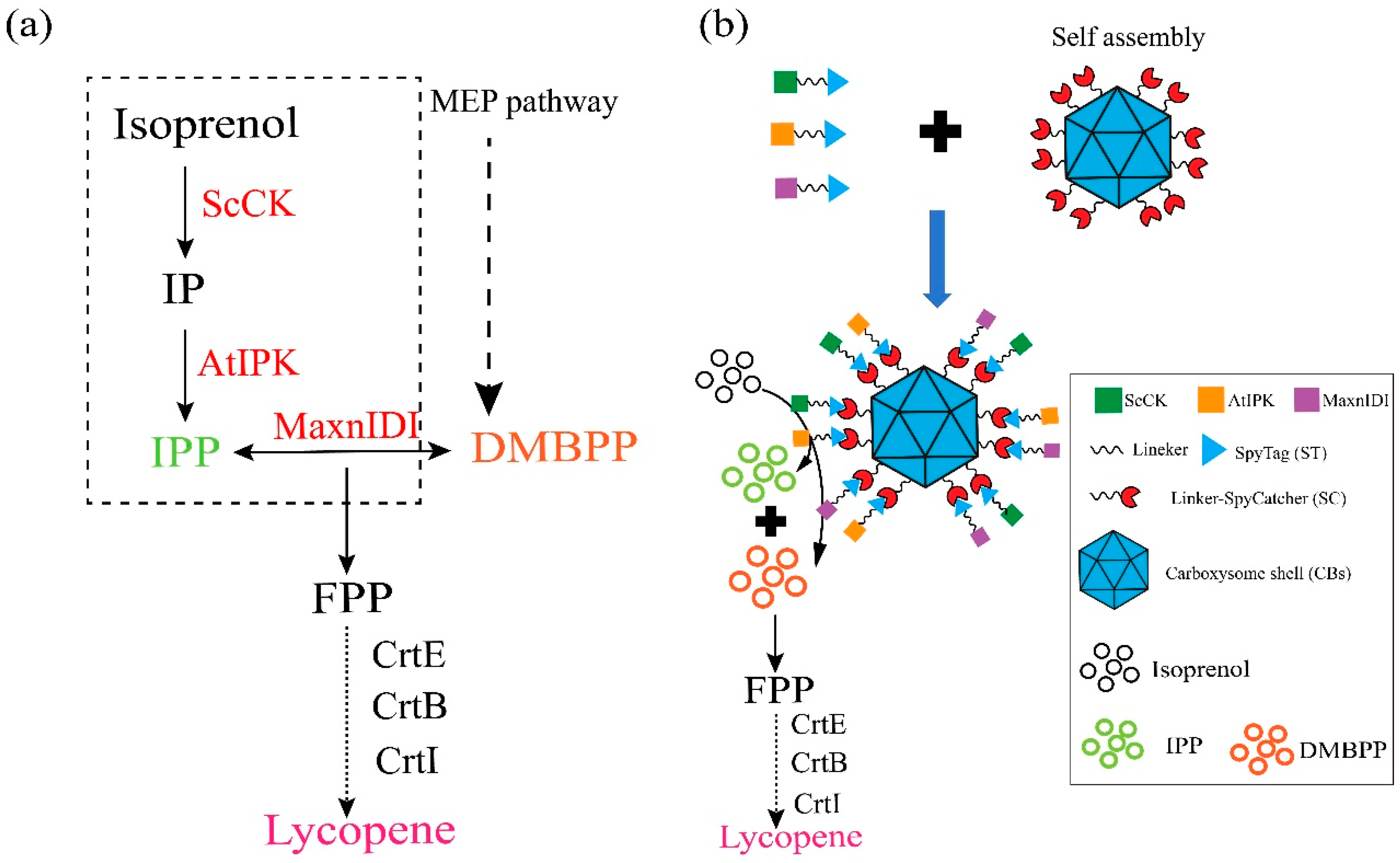
3.1. Construction of IPP Synthetic Multi-Enzyme Nanocage Using α-Carboxysome Shell Assembly
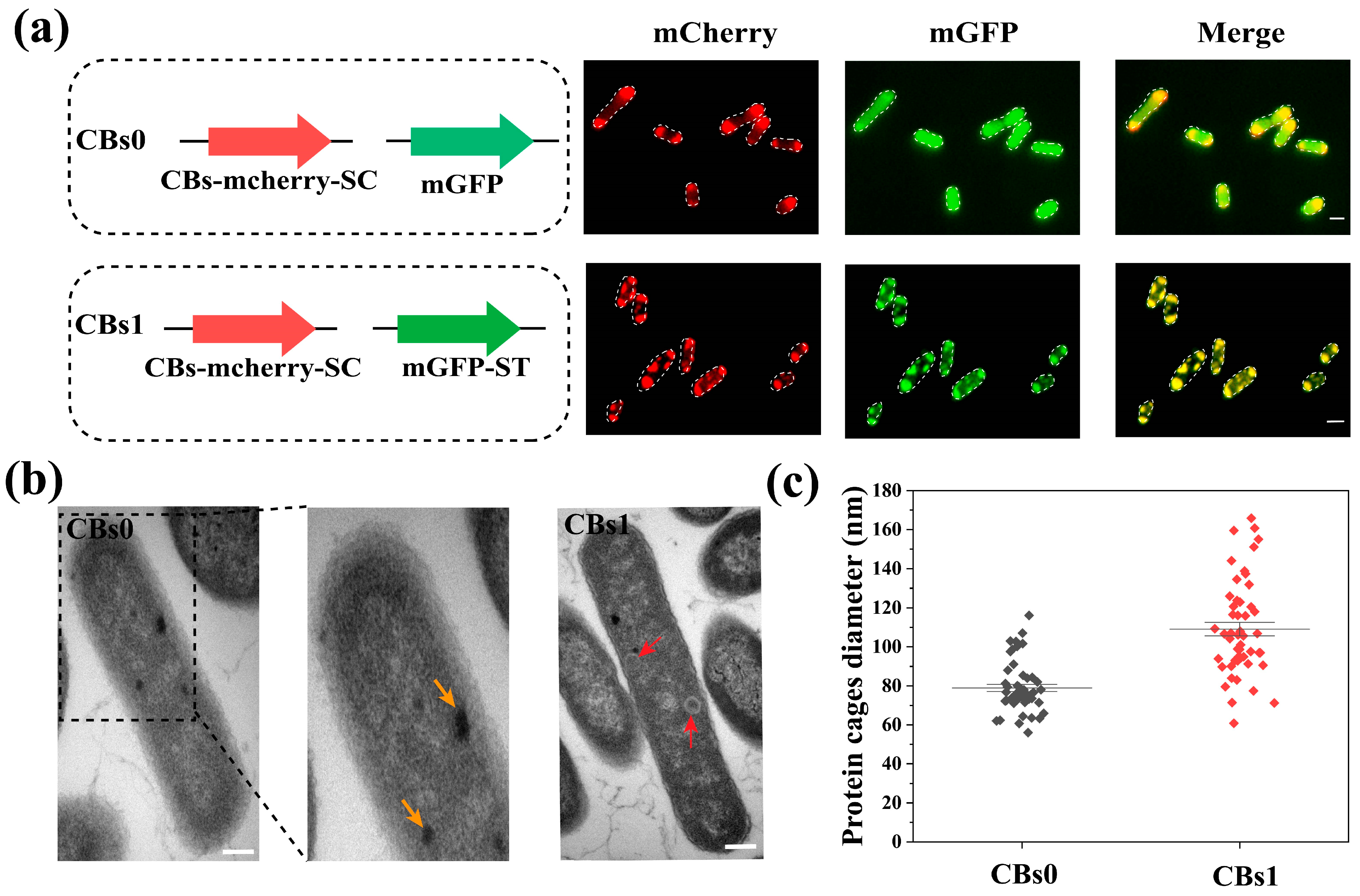
3.2. Expression and Characterization of Carboxysome Nanocage Scaffold in E. coli
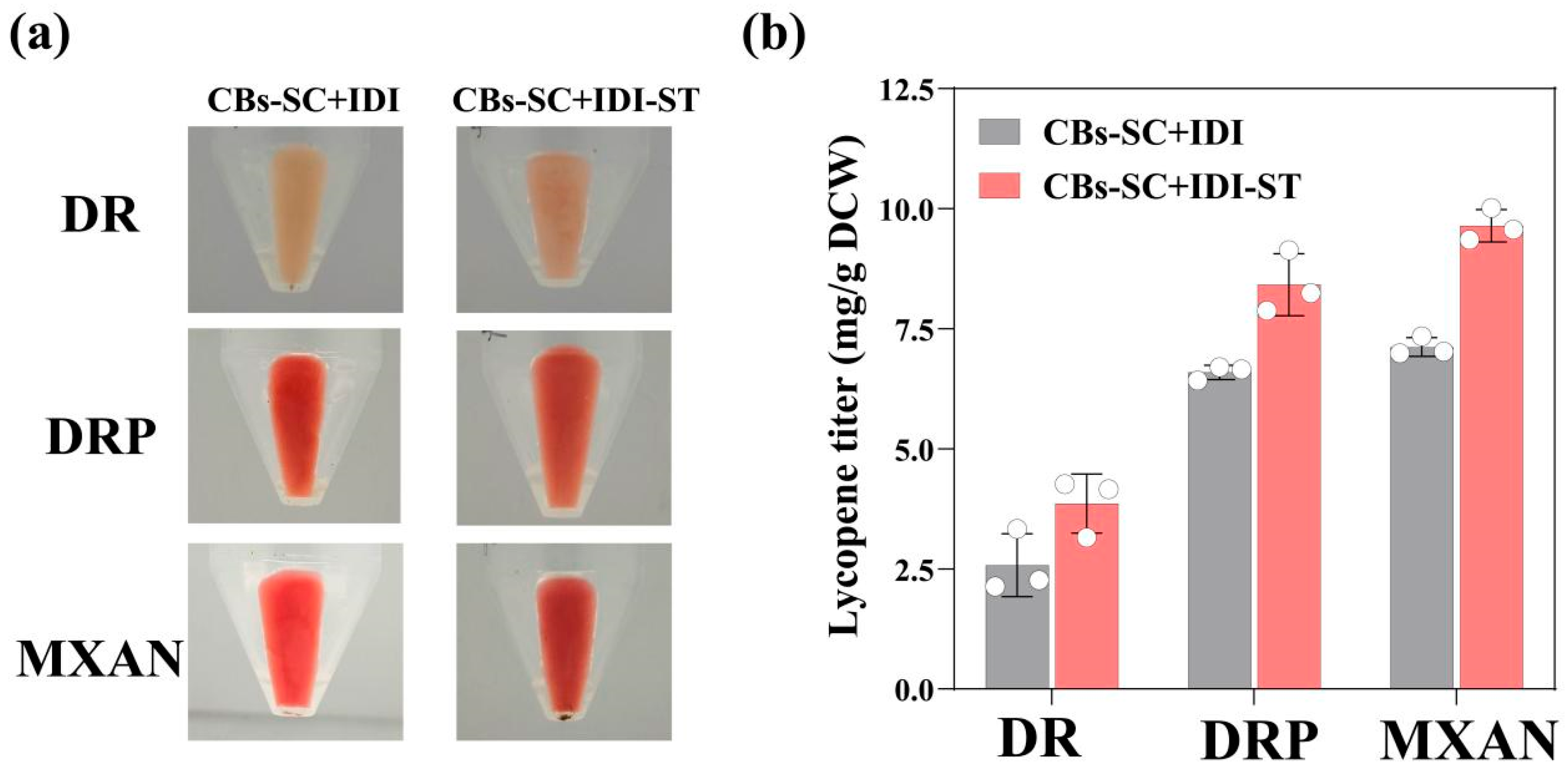
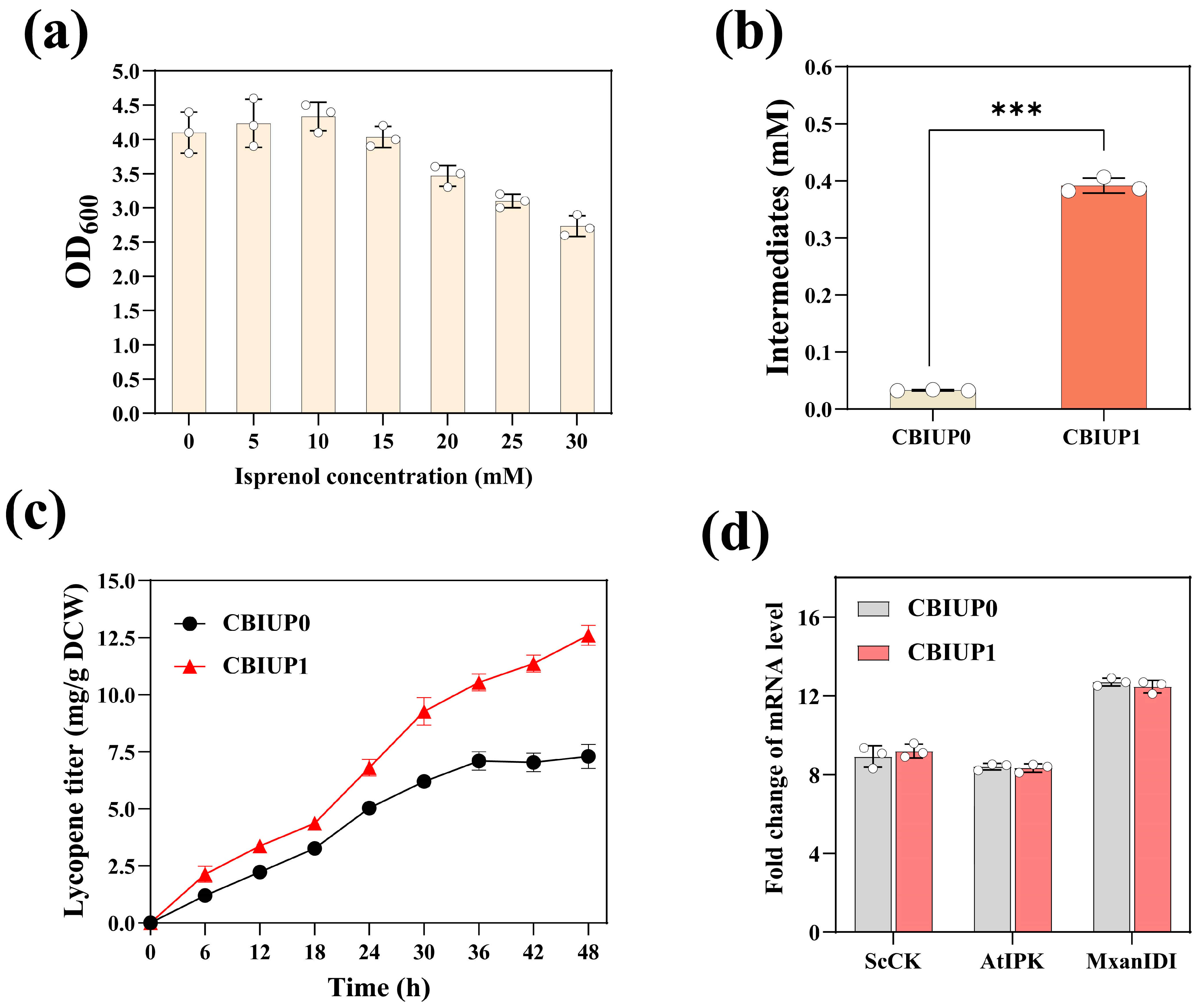
3.3. Immobilization of IPP Synthetic Enzymes on Nanocages Led to Increased Lycopene Production in E. coli
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greening, C.; Lithgow, T. Formation and Function of Bacterial Organelles. Nat. Rev. Microbiol. 2020, 18, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Ni, T.; Sun, Y.; Burn, W.; Al-Hazeem, M.M.J.; Zhu, Y.; Yu, X.; Liu, L.-N.; Zhang, P. Structure and Assembly of Cargo Rubisco in Two Native α-Carboxysomes. Nat. Commun. 2022, 13, 4299. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, C.; Sinha, S.; Chun, S.; Yeates, T.O.; Bobik, T.A. Diverse Bacterial Microcompartment Organelles. Microbiol. Mol. Biol. Rev. 2014, 78, 438–468. [Google Scholar] [CrossRef] [PubMed]
- Cannon, G.C.; Bradburne, C.E.; Aldrich, H.C.; Baker, S.H.; Heinhorst, S.; Shively, J.M. Microcompartments in Prokaryotes: Carboxysomes and Related Polyhedra. Appl. Environ. Microbiol. 2001, 67, 5351–5361. [Google Scholar] [CrossRef]
- Metskas, L.A.; Ortega, D.; Oltrogge, L.M.; Blikstad, C.; Lovejoy, D.R.; Laughlin, T.G.; Savage, D.F.; Jensen, G.J. Rubisco Forms a Lattice inside Alpha-Carboxysomes. Nat. Commun. 2022, 13, 4863. [Google Scholar] [CrossRef]
- Bobik, T.A.; Xu, Y.; Jeter, R.M.; Otto, K.E.; Roth, J.R. Propanediol Utilization Genes (Pdu) of Salmonella Typhimurium: Three Genes for the Propanediol Dehydratase. J. Bacteriol. 1997, 179, 6633–6639. [Google Scholar] [CrossRef]
- Kerfeld, C.A.; Aussignargues, C.; Zarzycki, J.; Cai, F.; Sutter, M. Bacterial Microcompartments. Nat. Rev. Microbiol. 2018, 16, 277–290. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, Q.; Yang, M.; Dykes, G.F.; Weetman, S.L.; Xin, W.; He, H.-L.; Liu, L.-N. Probing the Internal pH and Permeability of a Carboxysome Shell. Biomacromolecules 2022, 23, 4339–4348. [Google Scholar] [CrossRef]
- Li, T.; Jiang, Q.; Huang, J.; Aitchison, C.M.; Huang, F.; Yang, M.; Dykes, G.F.; He, H.-L.; Wang, Q.; Sprick, R.S.; et al. Reprogramming Bacterial Protein Organelles as a Nanoreactor for Hydrogen Production. Nat. Commun. 2020, 11, 5448. [Google Scholar] [CrossRef]
- Lawrence, A.D.; Frank, S.; Newnham, S.; Lee, M.J.; Brown, I.R.; Xue, W.-F.; Rowe, M.L.; Mulvihill, D.P.; Prentice, M.B.; Howard, M.J.; et al. Solution Structure of a Bacterial Microcompartment Targeting Peptide and Its Application in the Construction of an Ethanol Bioreactor. ACS Synth. Biol. 2014, 3, 454–465. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J.; Zhang, Y.; Liu, T.; Lu, X.; Men, D.; Zhang, X.-E. Auxiliary Module Promotes the Synthesis of Carboxysomes in E. Coli to Achieve High-Efficiency CO2 Assimilation. ACS Synth. Biol. 2021, 10, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Yeates, T.O.; Kerfeld, C.A.; Heinhorst, S.; Cannon, G.C.; Shively, J.M. Protein-Based Organelles in Bacteria: Carboxysomes and Related Microcompartments. Nat. Rev. Microbiol. 2008, 6, 681–691. [Google Scholar] [CrossRef]
- Frey, R.; Mantri, S.; Rocca, M.; Hilvert, D. Bottom-up Construction of a Primordial Carboxysome Mimic. J. Am. Chem. Soc. 2016, 138, 10072–10075. [Google Scholar] [CrossRef] [PubMed]
- Rae, B.D.; Long, B.M.; Badger, M.R.; Price, G.D. Functions, Compositions, and Evolution of the Two Types of Carboxysomes: Polyhedral Microcompartments That Facilitate CO2 Fixation in Cyanobacteria and Some Proteobacteria. Microbiol. Mol. Biol. Rev. 2013, 77, 357–379. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yan, X.; Aigner, H.; Bracher, A.; Nguyen, N.D.; Hee, W.Y.; Long, B.M.; Price, G.D.; Hartl, F.U.; Hayer-Hartl, M. Rubisco Condensate Formation by CcmM in β-Carboxysome Biogenesis. Nature 2019, 566, 131–135. [Google Scholar] [CrossRef]
- Cameron, J.C.; Wilson, S.C.; Bernstein, S.L.; Kerfeld, C.A. Biogenesis of a Bacterial Organelle: The Carboxysome Assembly Pathway. Cell 2013, 155, 1131–1140. [Google Scholar] [CrossRef]
- Iancu, C.V.; Morris, D.M.; Dou, Z.; Heinhorst, S.; Cannon, G.C.; Jensen, G.J. Organization, Structure, and Assembly of α-Carboxysomes Determined by Electron Cryotomography of Intact Cells. J. Mol. Biol. 2010, 396, 105–117. [Google Scholar] [CrossRef]
- Oltrogge, L.M.; Chaijarasphong, T.; Chen, A.W.; Bolin, E.R.; Marqusee, S.; Savage, D.F. Multivalent Interactions between CsoS2 and Rubisco Mediate α-Carboxysome Formation. Nat. Struct. Mol. Biol. 2020, 27, 281–287. [Google Scholar] [CrossRef]
- Li, T.; Chang, P.; Chen, W.; Shi, Z.; Xue, C.; Dykes, G.F.; Huang, F.; Wang, Q.; Liu, L.-N. Nanoengineering Carboxysome Shells for Protein Cages with Programmable Cargo Targeting. ACS Nano 2024, 18, 7473–7484. [Google Scholar] [CrossRef]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef]
- Klaus, O.; Hilgers, F.; Nakielski, A.; Hasenklever, D.; Jaeger, K.E.; Axmann, I.M.; Drepper, T. Engineering phototrophic bacteria for the production of terpenoids. Curr. Opin. Biotechnol. 2022, 77, 102764. [Google Scholar] [CrossRef]
- Chandran, S.S.; Kealey, J.T.; Reeves, C.D. Microbial Production of Isoprenoids. Process Biochem. 2011, 46, 1703–1710. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, C.; Lindley, N.D. Metabolic Engineering Strategies for Sustainable Terpenoid Flavor and Fragrance Synthesis. J. Agric. Food Chem. 2020, 68, 10252–10264. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-J.; Wang, Y.-Z.; Wang, L.-R.; Shi, T.-Q.; Sun, X.-M.; Huang, H. Advanced Strategies for the Synthesis of Terpenoids in Yarrowia Lipolytica. J. Agric. Food Chem. 2021, 69, 2367–2381. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.; Lee, S.-M.; Um, Y.; Kim, Y.; Sim, S.J.; Choi, J.; Woo, H.M. Direct Conversion of CO2 to α-Farnesene Using Metabolically Engineered Synechococcus Elongatus PCC 7942. J. Agric. Food Chem. 2017, 65, 10424–10428. [Google Scholar] [CrossRef]
- Meadows, A.L.; Hawkins, K.M.; Tsegaye, Y.; Antipov, E.; Kim, Y.; Raetz, L.; Dahl, R.H.; Tai, A.; Mahatdejkul-Meadows, T.; Xu, L.; et al. Rewriting Yeast Central Carbon Metabolism for Industrial Isoprenoid Production. Nature 2016, 537, 694–697. [Google Scholar] [CrossRef]
- Quílez Del Moral, J.F.; Pérez, Á.; Barrero, A.F. Chemical Synthesis of Terpenoids with Participation of Cyclizations plus Rearrangements of Carbocations: A Current Overview. Phytochem. Rev. 2020, 19, 559–576. [Google Scholar] [CrossRef]
- Tian, B.; Hua, Y. Carotenoid Biosynthesis in Extremophilic Deinococcus–Thermus bacteria. Trends Microbiol. 2010, 18, 512–520. [Google Scholar] [CrossRef]
- Li, L.; Liu, Z.; Jiang, H.; Mao, X. Biotechnological Production of Lycopene by Microorganisms. Appl. Microbiol. Biotechnol. 2020, 104, 10307–10324. [Google Scholar] [CrossRef]
- Zhang, X.-K.; Nie, M.-Y.; Chen, J.; Wei, L.-J.; Hua, Q. Multicopy Integrants of Crt Genes and Co-Expression of AMP Deaminase Improve Lycopene Production in Yarrowia Lipolytica. J. Biotechnol. 2019, 289, 46–54. [Google Scholar] [CrossRef]
- Chatzivasileiou, A.O.; Ward, V.; Edgar, S.M.; Stephanopoulos, G. Two-Step Pathway for Isoprenoid Synthesis. Proc. Natl. Acad. Sci. USA 2019, 116, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Clomburg, J.M.; Qian, S.; Tan, Z.; Cheong, S.; Gonzalez, R. The Isoprenoid Alcohol Pathway, a Synthetic Route for Isoprenoid Biosynthesis. Proc. Natl. Acad. Sci. USA 2019, 116, 12810–12815. [Google Scholar] [CrossRef] [PubMed]
- Lund, S.; Hall, R.; Williams, G.J. An Artificial Pathway for Isoprenoid Biosynthesis Decoupled from Native Hemiterpene Metabolism. ACS Synth. Biol. 2019, 8, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Rico, J.; Duquesne, K.; Petit, J.-L.; Mariage, A.; Darii, E.; Peruch, F.; De Berardinis, V.; Iacazio, G. Exploring Natural Biodiversity to Expand Access to Microbial Terpene Synthesis. Microb. Cell Fact. 2019, 18, 23. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, J.; Yang, Q.; Yang, J. Metabolic Engineering Escherichia Coli for the Production of Lycopene. Molecules 2020, 25, 3136. [Google Scholar] [CrossRef]
- Huang, G.; Li, J.; Lin, J.; Duan, C.; Yan, G. Multi-Modular Metabolic Engineering and Efflux Engineering for Enhanced Lycopene Production in Recombinant Saccharomyces Cerevisiae. J. Ind. Microbiol. Biotechnol. 2024, 51, kuae015. [Google Scholar] [CrossRef]
- Xu, X.; Liu, J.; Lu, Y.; Lan, H.; Tian, L.; Zhang, Z.; Xie, C.; Jiang, L. Pathway Engineering of Saccharomyces Cerevisiae for Efficient Lycopene Production. Bioprocess. Biosyst. Eng. 2021, 44, 1033–1047. [Google Scholar] [CrossRef]
- Jin, K.; Xia, H.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Liu, L. Compartmentalization and Transporter Engineering Strategies for Terpenoid Synthesis. Microb. Cell Fact. 2022, 21, 92. [Google Scholar] [CrossRef]
- Vranová, E.; Coman, D.; Gruissem, W. Network Analysis of the MVA and MEP Pathways for Isoprenoid Synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef]
- Nagegowda, D.A. Plant Volatile Terpenoid Metabolism: Biosynthetic Genes, Transcriptional Regulation and Subcellular Compartmentation. FEBS Lett. 2010, 584, 2965–2973. [Google Scholar] [CrossRef]
- Kang, W.; Ma, X.; Kakarla, D.; Zhang, H.; Fang, Y.; Chen, B.; Zhu, K.; Zheng, D.; Wu, Z.; Li, B.; et al. Organizing Enzymes on Self-Assembled Protein Cages for Cascade Reactions. Angew. Chem. Int. Ed. 2022, 61, e202214001. [Google Scholar] [CrossRef]
- Hsia, Y.; Bale, J.B.; Gonen, S.; Shi, D.; Sheffler, W.; Fong, K.K.; Nattermann, U.; Xu, C.; Huang, P.-S.; Ravichandran, R.; et al. Design of a Hyperstable 60-Subunit Protein Icosahedron. Nature 2016, 535, 136–139. [Google Scholar] [CrossRef]
- Bruun, T.U.J.; Andersson, A.-M.C.; Draper, S.J.; Howarth, M. Engineering a Rugged Nanoscaffold To Enhance Plug-and-Display Vaccination. ACS Nano 2018, 12, 8855–8866. [Google Scholar] [CrossRef]
- Misawa, N. Pathway Engineering for Functional Isoprenoids. Curr. Opin. Biotechnol. 2011, 22, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, B.; Fierer, J.O.; Celik, E.; Chittock, E.C.; Schwarz-Linek, U.; Moy, V.T.; Howarth, M. Peptide Tag Forming a Rapid Covalent Bond to a Protein, through Engineering a Bacterial Adhesin. Proc. Natl. Acad. Sci. USA 2012, 109, E690–E697. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Lee, Y.; Kim, J.; Lee, S.; Lee, J.; Kim, J.; Jung, K.; Shin, Y.; Keasling, J.D.; Kim, S. Enhanced Lycopene Production in Escherichia Coli Engineered to Synthesize Isopentenyl Diphosphate and Dimethylallyl Diphosphate from Mevalonate. Biotech. Bioeng. 2006, 94, 1025–1032. [Google Scholar] [CrossRef]
- George, K.W.; Thompson, M.G.; Kim, J.; Baidoo, E.E.K.; Wang, G.; Benites, V.T.; Petzold, C.J.; Chan, L.J.; Yilmaz, S.; Turhanen, P.; et al. Integrated analysis of isopentenyl pyrophosphate (IPP) toxicity in isoprenoid-producing Escherichia coli. Metab. Eng. 2018, 47, 60–72. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, N.; Lazar, Z.; Chatzivasileiou, A.; Ward, V.; Chen, J.; Zhou, J.; Stephanopoulos, G. Enhancing Isoprenoid Synthesis in Yarrowia Lipolytica by Expressing the Isopentenol Utilization Pathway and Modulating Intracellular Hydrophobicity. Metab. Eng. 2020, 61, 344–351. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Yao, Y.; Zhang, F.; Yu, N.; Wang, B.; Tian, B. Enhancement of Lycopene Biosynthesis Using Self-Assembled Multi-Enzymic Protein Cages. Microorganisms 2025, 13, 747. https://doi.org/10.3390/microorganisms13040747
Zhou Y, Yao Y, Zhang F, Yu N, Wang B, Tian B. Enhancement of Lycopene Biosynthesis Using Self-Assembled Multi-Enzymic Protein Cages. Microorganisms. 2025; 13(4):747. https://doi.org/10.3390/microorganisms13040747
Chicago/Turabian StyleZhou, Yulong, Yonghua Yao, Furong Zhang, Ning Yu, Binqiang Wang, and Bing Tian. 2025. "Enhancement of Lycopene Biosynthesis Using Self-Assembled Multi-Enzymic Protein Cages" Microorganisms 13, no. 4: 747. https://doi.org/10.3390/microorganisms13040747
APA StyleZhou, Y., Yao, Y., Zhang, F., Yu, N., Wang, B., & Tian, B. (2025). Enhancement of Lycopene Biosynthesis Using Self-Assembled Multi-Enzymic Protein Cages. Microorganisms, 13(4), 747. https://doi.org/10.3390/microorganisms13040747






