Manure-Amended One-Year-Reclamation Promoted Soil Bacterial Phylotypic and Phenotypic Shifts in a Typical Coal-Mining Area
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Sampling
2.2. Soil Biophysicochemical Properties Analyses
2.3. Soil Bacterial 16S rRNA Gene Quantification and Sequencing
2.4. Data Processing and Statistical Analyses
3. Results
3.1. Soil Biophysicochemical Properties
3.2. Bacterial Biomass and Alpha Diversity
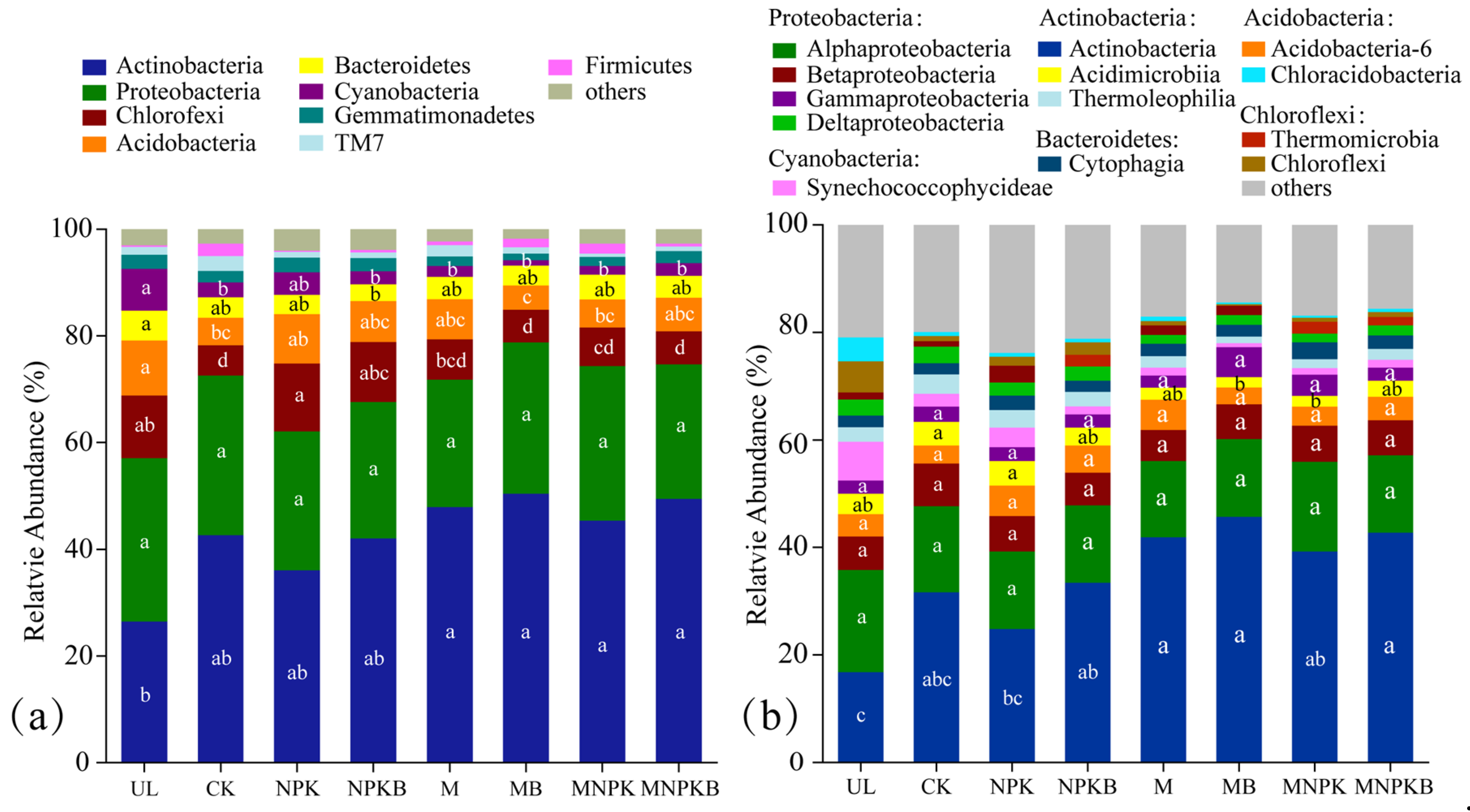
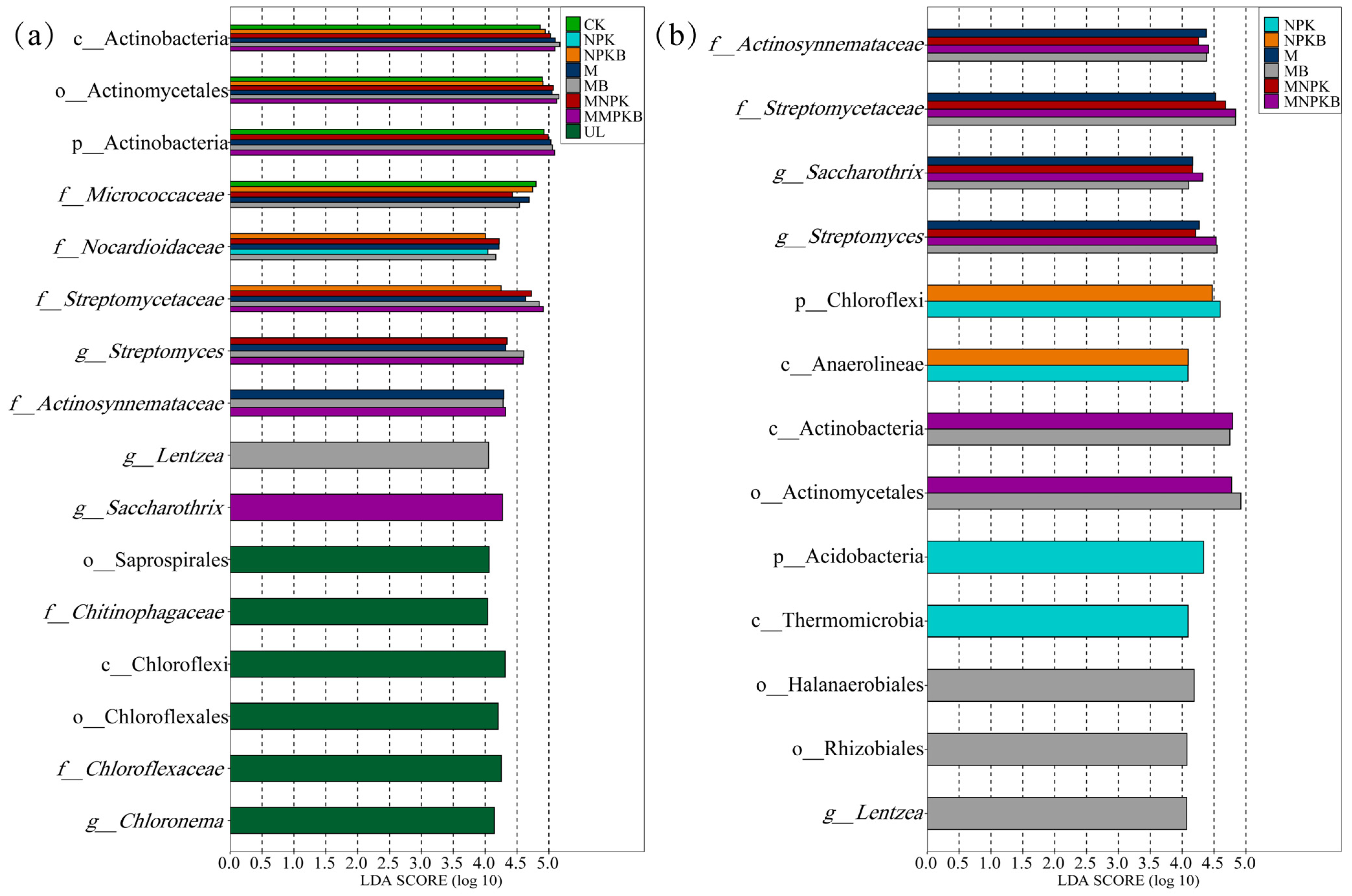
3.3. Bacterial Community Composition, Biomarkers and Phenotypes
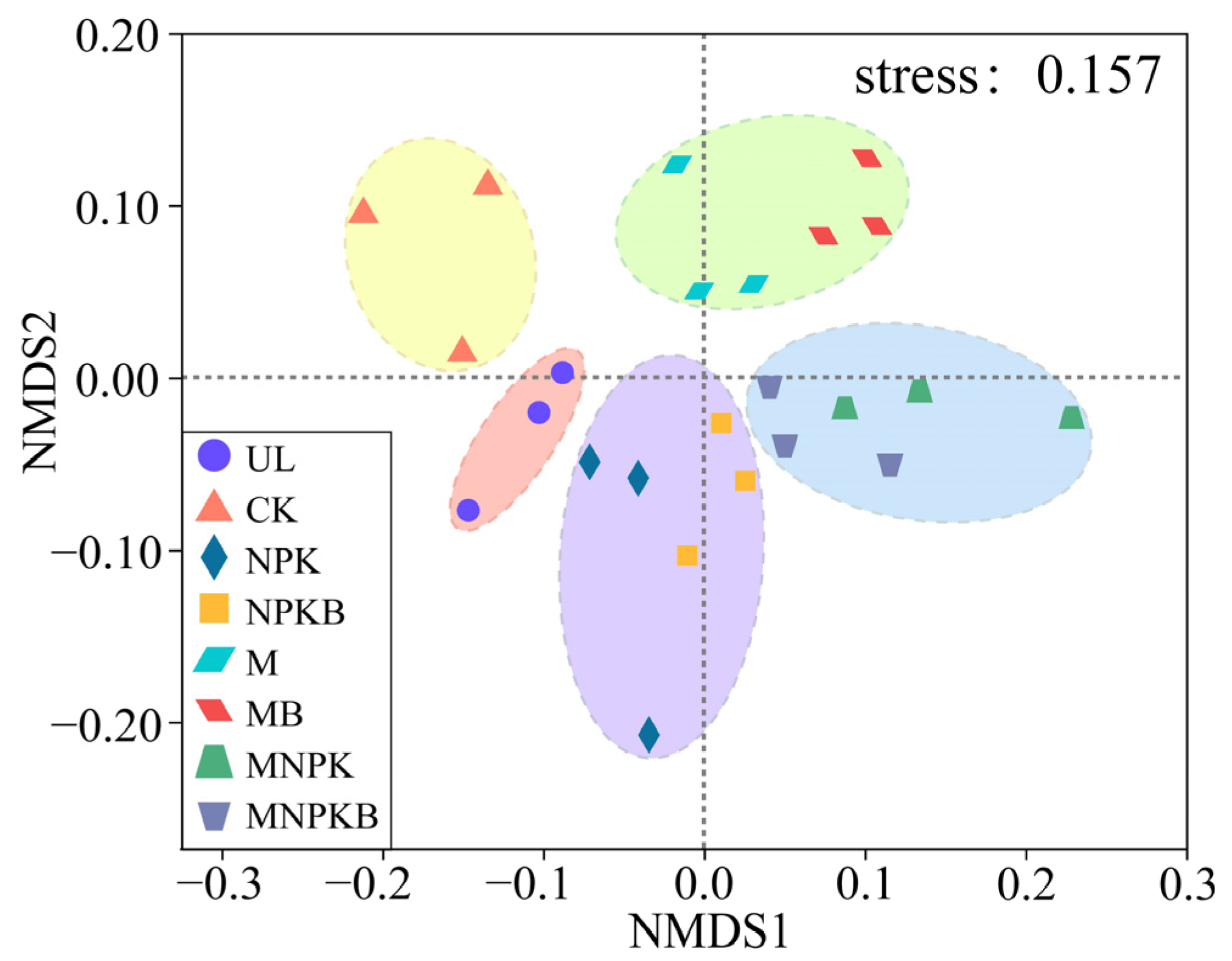
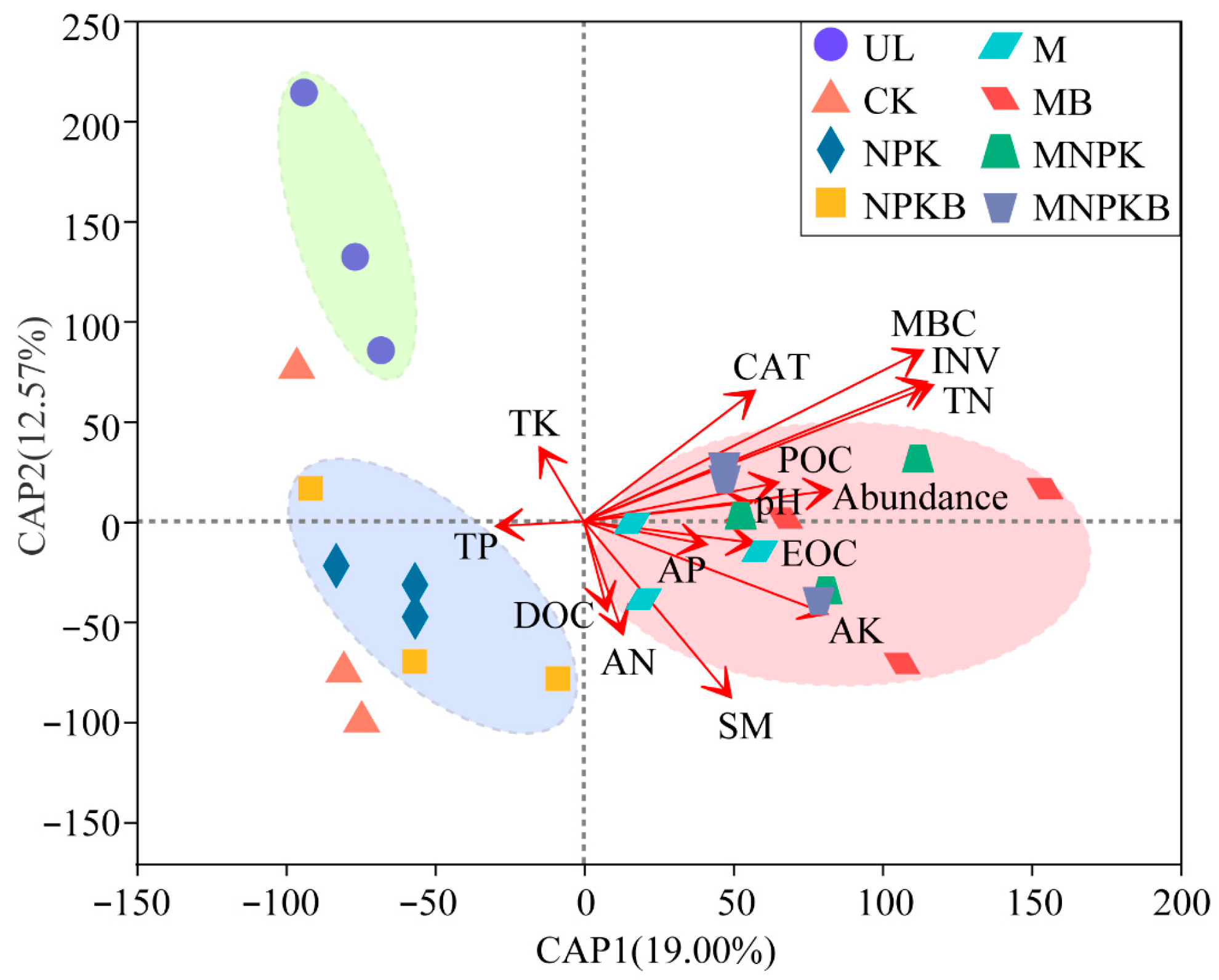
3.4. Bacterial Community Structure Differentiations and Driving Factors Among Different Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SM | Soil moisture |
| TN | Total nitrogen |
| AN | Alkaline nitrogen |
| TP | Total phosphorus |
| AP | Available phosphorus |
| TK | Total potassium |
| AK | Available potassium |
| SOC | Soil organic carbon |
| MBC | Microbial biomass carbon |
| EOC | Easily oxidized organic carbon |
| DOC | Dissolved organic carbon |
| POC | Particulate organic carbon |
| ALP | Alkaline phosphatase |
| URE | Urease |
| CAT | Catalase |
| INV | Invertase |
| UL | Uncultivated land |
| CK | Control without fertilization |
| NPK | Chemical fertilizer |
| NPKB | Co-application of chemical fertilizer and bacterial fertilizer |
| M | Manure |
| MB | Co-application of manure and bacterial fertilizer |
| MNPK | Co-application of chemical fertilizer and manure |
| MNPKB | Co-application of chemical fertilizer manure and bacterial fertilizer |
References
- Feng, Y.; Wang, J.; Bai, Z.; Reading, L. Effects of surface coal mining and land reclamation on soil properties: A review. Earth-Sci. Rev. 2019, 191, 12–25. [Google Scholar]
- Xiao, W.; Zhang, W.; Ye, Y.; Lv, X.; Yang, W. Is underground coal mining causing land degradation and significantly damaging ecosystems in semi-arid areas? A study from an Ecological Capital perspective. Land Degrad. Dev. 2020, 31, 1969–1989. [Google Scholar]
- Padhiary, M.; Kumar, R. Assessing the environmental impacts of agriculture, industrial operations, and mining on agro-ecosystems. In Smart Internet of Things for Environment and Healthcare; Springer Nature Switzerland: Cham, Switzerland, 2024; pp. 107–126. [Google Scholar]
- Zhang, M.; Wang, J.; Feng, Y. Temporal and spatial change of land use in a large-scale opencast coal mine area: A complex network approach. Land Use Policy 2019, 86, 375–386. [Google Scholar]
- Hu, Z.; Li, G.; Xia, J.; Feng, Z.; Han, J.; Chen, Z.; Wang, W.; Li, G. Coupling of underground coal mining and mine reclamation for farmland protection and sustainable mining. Resour. Policy 2023, 84, 103756. [Google Scholar]
- Wang, X.; Li, Y.; Wei, Y.; Meng, H.; Cao, Y.; Lead, J.; Hong, J. Effects of fertilization and reclamation time on soil bacterial communities in coal mining subsidence areas. Sci. Total Environ. 2020, 739, 139882. [Google Scholar]
- Yahaya, S.M.; Mahmud, A.A.; Abdullahi, M.; Haruna, A. Recent advances in the chemistry of nitrogen, phosphorus and potassium as fertilizers in soil: A review. Pedosphere 2023, 33, 385–406. [Google Scholar]
- Luo, G.; Li, L.; Friman, V.-P.; Guo, J.; Guo, S.; Shen, Q.; Ling, N. Organic amendments increase crop yields by improving microbe-mediated soil functioning of agroecosystems: A meta-analysis. Soil Biol. Biochem. 2018, 124, 105–115. [Google Scholar]
- Tiong, Y.W.; Sharma, P.; Xu, S.; Bu, J.; An, S.; Foo, J.B.L.; Wee, B.K.; Wang, Y.; Lee, J.T.E.; Zhang, J. Enhancing sustainable crop cultivation: The impact of renewable soil amendments and digestate fertilizer on crop growth and nutrient composition. Environ. Pollut. 2024, 342, 123132. [Google Scholar]
- Tahat, M.M.; Alananbeh, K.M.; Othman, Y.A.; Leskovar, D.I. Soil health and sustainable agriculture. Sustainability 2020, 12, 4859. [Google Scholar] [CrossRef]
- Cao, Y.; Zhou, B.; Wang, X.; Meng, H.; Zhang, J.; Li, L.; Hong, J. Different fertilization treatments in coal mining-affected soils change bacterial populations and enable soil reclamation. Ann. Microbiol. 2020, 70, 47. [Google Scholar]
- Zhang, J.; Xie, Y.; Wei, Y.; Meng, H.; Cao, Y.; Qin, J.; Hong, J. Effects of fertilisation on microbial communities in short-term coal mine reclamation. Soil Res. 2020, 58, 779–789. [Google Scholar]
- Li, L.; Li, T.; Meng, H.; Xie, Y.; Zhang, J.; Hong, J. Effects of seven-year fertilization reclamation on bacterial community in a coal mining subsidence area in Shanxi, China. Int. J. Environ. Res. Public Health 2021, 18, 12504. [Google Scholar] [CrossRef]
- Meng, H.; Wang, S.; Zhang, J.; Wang, X.; Qiu, C.; Hong, J. Effects of coal-derived compound fertilizers on soil bacterial community structure in coal mining subsidence areas. Front. Microbiol. 2023, 14, 1187572. [Google Scholar]
- Bo, H.; Li, Z.; Jin, D.; Xu, M.; Zhang, Q. Fertilizer management methods affect bacterial community structure and diversity in the maize rhizosphere soil of a coal mine reclamation area. Ann. Microbiol. 2023, 73, 24. [Google Scholar]
- Bo, H.; Li, Z.; Wang, W.; Zhang, R.; Wang, H.; Jin, D.; Xu, M.; Zhang, Q. Combining organic and inorganic fertilization enhances soil enzyme activity, the bacterial community, and molecular ecological network complexity in coal mine reclamation areas. Agronomy 2024, 14, 1427. [Google Scholar] [CrossRef]
- Dangi, S.R.; Stahl, P.D.; Wick, A.F.; Ingram, L.J.; Buyer, J.S. Soil microbial community recovery in reclaimed soils on a surface coal mine site. Soil Sci. Soc. Am. J. 2012, 76, 915–924. [Google Scholar]
- Cui, Y.; Fang, L.; Guo, X.; Wang, X.; Wang, Y.; Li, P.; Zhang, Y.; Zhang, X. Responses of soil microbial communities to nutrient limitation in the desert-grassland ecological transition zone. Sci. Total Environ. 2018, 642, 45–55. [Google Scholar]
- Shang, Y.; Wu, M.; Zhang, J.; Meng, H.; Hong, J.; Hao, X.; Lead, J.R.; Wang, X. Nutrient enhanced reclamation promoted growth, diversity and activities of carbon fixing microbes in a coal-mining subsistence land. Soil Sci. Environ. 2023, 2, 2. [Google Scholar]
- Wang, S.; Li, Y.; Zhang, J.; Wang, X.; Hong, J.; Qiu, C.; Meng, H. Transcriptome profiling analysis of phosphate-solubilizing mechanism of pseudomonas strain W134. Microorganisms 2022, 10, 1998. [Google Scholar] [CrossRef]
- Jiao, J.; An, X.; Xu, C.; Li, T.; Meng, H.; Zhang, J.; Hao, X. Effects of organic fertilizer combined with Pseudomonas fluorescein on maize yield and phosphorus activity in reclaimed soil. Trans. Chin. Soc. Agric. Eng. 2024, 40, 79–87. [Google Scholar]
- Bao, S. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2013. [Google Scholar]
- Cambardella, C.A.; Elliott, E. Particulate soil organic-matter changes across a grassland cultivation sequence. Soil Sci. Soc. Am. J. 1992, 56, 777–783. [Google Scholar]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Johnson, J.L.; Temple, K.L. Some variables affecting the measurement of “catalase activity” in soil. Soil Sci. Soc. Am. J. 1964, 28, 207–209. [Google Scholar]
- Cang, L.; Zhou, D.-M.; Wang, Q.-Y.; Wu, D.-Y. Effects of electrokinetic treatment of a heavy metal contaminated soil on soil enzyme activities. J. Hazard. Mater. 2009, 172, 1602–1607. [Google Scholar] [PubMed]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Rasche, F.; Knapp, D.; Kaiser, C.; Koranda, M.; Kitzler, B.; Zechmeister-Boltenstern, S.; Richter, A.; Sessitsch, A. Seasonality and resource availability control bacterial and archaeal communities in soils of a temperate beech forest. ISME J. 2011, 5, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wei, L.; Lv, W.; Zhang, H.; Zhang, Y.; Zhang, H.; Zhang, H.; Zhu, Z.; Ge, T.; Zhang, W. Long-term bioorganic and organic fertilization improved soil quality and multifunctionality under continuous cropping in watermelon. Agric. Ecosyst. Environ. 2024, 359, 108721. [Google Scholar]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar]
- Zhao, Z.-B.; He, J.-Z.; Geisen, S.; Han, L.-L.; Wang, J.-T.; Shen, J.-P.; Wei, W.-X.; Fang, Y.-T.; Li, P.-P.; Zhang, L.-M. Protist communities are more sensitive to nitrogen fertilization than other microorganisms in diverse agricultural soils. Microbiome 2019, 7, 1–16. [Google Scholar]
- Dai, J.; Yan, R.; Wei, Z.; Bai, Y.; Zhang, S.; Wang, T.; Sun, S. Effects of short-term fertilization on soil microorganisms in a mown Leymus chinensis meadow. Chin. J. Ecol. 2017, 36, 2431. [Google Scholar]
- Li, Y.; Liu, X.; Zhang, L.; Xie, Y.; Cai, X.; Wang, S.; Lian, B. Effects of short-term application of chemical and organic fertilizers on bacterial diversity of cornfield soil in a karst area. J. Soil Sci. Plant Nutr. 2020, 20, 2048–2058. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, H.; Zhang, R.; Huang, Z.; Yang, C. Impacts of pseudomonas fluorescent bacterial fertilizer addition on the soil environment and fruit yield under water stress in greenhouse grape. Front. Microbiol. 2025, 16, 1540628. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Wang, H.; He, X.; Zhang, Z.; Li, M.; Zhang, Q.; Zhu, H.; Xu, S.; Yang, P. Eight years of manure fertilization favor copiotrophic traits in paddy soil microbiomes. Eur. J. Soil Biol. 2021, 106, 103352. [Google Scholar] [CrossRef]
- Ye, G.; Banerjee, S.; He, J.-Z.; Fan, J.; Wang, Z.; Wei, X.; Hu, H.-W.; Zheng, Y.; Duan, C.; Wan, S. Manure application increases microbiome complexity in soil aggregate fractions: Results of an 18-year field experiment. Agric. Ecosyst. Environ. 2021, 307, 107249. [Google Scholar] [CrossRef]
- Kaiser, C.; Kilburn, M.R.; Clode, P.L.; Fuchslueger, L.; Koranda, M.; Cliff, J.B.; Solaiman, Z.M.; Murphy, D.V. Exploring the transfer of recent plant photosynthates to soil microbes: Mycorrhizal pathway vs direct root exudation. New Phytol. 2015, 205, 1537–1551. [Google Scholar]
- Labeda, D.; Goodfellow, M.; Brown, R.; Ward, A.; Lanoot, B.; Vanncanneyt, M.; Swings, J.; Kim, S.-B.; Liu, Z.; Chun, J. Phylogenetic study of the species within the family Streptomycetaceae. Antonie Leeuwenhoek 2012, 101, 73–104. [Google Scholar] [CrossRef]
- Labeda, D.P. The Family Actinosynnemataceae. In The Prokaryotes; Springer: New York, NY, USA, 2006; pp. 654–668. [Google Scholar]
- Thompson, C.; Kieser, T.; Ward, J.; Hopwood, D. Physical analysis of antibiotic-resistance genes from Streptomyces and their use in vector construction. Gene 1982, 20, 51–62. [Google Scholar] [CrossRef]
- Zhou, X.; Qiao, M.; Wang, F.-H.; Zhu, Y.-G. Use of commercial organic fertilizer increases the abundance of antibiotic resistance genes and antibiotics in soil. Environ. Sci. Pollut. Res. 2017, 24, 701–710. [Google Scholar] [CrossRef]
- Xie, W.Y.; Shen, Q.; Zhao, F. Antibiotics and antibiotic resistance from animal manures to soil: A review. Eur. J. Soil Sci. 2018, 69, 181–195. [Google Scholar] [CrossRef]
- Xiong, W.; Wang, M.; Dai, J.; Sun, Y.; Zeng, Z. Application of manure containing tetracyclines slowed down the dissipation of tet resistance genes and caused changes in the composition of soil bacteria. Ecotoxicol. Environ. Saf. 2018, 147, 455–460. [Google Scholar] [CrossRef]
- Udikovic-Kolic, N.; Wichmann, F.; Broderick, N.A.; Handelsman, J. Bloom of resident antibiotic-resistant bacteria in soil following manure fertilization. Proc. Natl. Acad. Sci. USA 2014, 111, 15202–15207. [Google Scholar] [CrossRef]
- Francioli, D.; Schulz, E.; Lentendu, G.; Wubet, T.; Buscot, F.; Reitz, T. Mineral vs. organic amendments: Microbial community structure, activity and abundance of agriculturally relevant microbes are driven by long-term fertilization strategies. Front. Microbiol. 2016, 7, 1446. [Google Scholar]
- Zhang, Y.; Shen, H.; He, X.; Thomas, B.W.; Lupwayi, N.Z.; Hao, X.; Thomas, M.C.; Shi, X. Fertilization shapes bacterial community structure by alteration of soil pH. Front. Microbiol. 2017, 8, 1325. [Google Scholar]
- Muneer, M.A.; Hou, W.; Li, J.; Huang, X.; ur Rehman Kayani, M.; Cai, Y.; Yang, W.; Wu, L.; Ji, B.; Zheng, C. Soil pH: A key edaphic factor regulating distribution and functions of bacterial community along vertical soil profiles in red soil of pomelo orchard. BMC Microbiol. 2022, 22, 38. [Google Scholar] [CrossRef]
- Ventura, M.; Canchaya, C.; Tauch, A.; Chandra, G.; Fitzgerald, G.F.; Chater, K.F.; van Sinderen, D. Genomics of Actinobacteria: Tracing the evolutionary history of an ancient phylum. Microbiol. Mol. Biol. Rev. 2007, 71, 495–548. [Google Scholar]
- Wu, Y.; Cai, P.; Jing, X.; Niu, X.; Ji, D.; Ashry, N.M.; Gao, C.; Huang, Q. Soil biofilm formation enhances microbial community diversity and metabolic activity. Environ. Int. 2019, 132, 105116. [Google Scholar]
- Brzezińska, M.; Włodarczyk, T.; Stępniewski, W.; Przywara, G. Soil aeration status and catalase activity. Acta Agrophys. 2005, 5, 555–565. [Google Scholar]
- Dini-Andreote, F.; de Cássia Pereira e Silva, M.; Triado-Margarit, X.; Casamayor, E.O.; Van Elsas, J.D.; Salles, J.F. Dynamics of bacterial community succession in a salt marsh chronosequence: Evidences for temporal niche partitioning. ISME J. 2014, 8, 1989–2001. [Google Scholar] [PubMed]
- Feng, Y.; Guo, Z.; Zhong, L.; Zhao, F.; Zhang, J.; Lin, X. Balanced fertilization decreases environmental filtering on soil bacterial community assemblage in North China. Front. Microbiol. 2017, 8, 2376. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xun, W.; Huang, T.; Zhang, G.; Gao, J.; Ran, W.; Li, D.; Shen, Q.; Zhang, R. Alteration of the soil bacterial community during parent material maturation driven by different fertilization treatments. Soil Biol. Biochem. 2016, 96, 207–215. [Google Scholar]
- Frankeberger, W.; Johanson, J. Method of measuring invertase activity in soils. Plant Soil 1983, 74, 301–311. [Google Scholar]
- Ciarkowska, K.; Sołek-Podwika, K.; Wieczorek, J. Enzyme activity as an indicator of soil-rehabilitation processes at a zinc and lead ore mining and processing area. J. Environ. Manag. 2014, 132, 250–256. [Google Scholar]
- Samuel, A.D.; Bungau, S.; Fodor, I.K.; Tit, D.M.; Blidar, C.F.; David, A.T.; Melinte, C.E. Effects of liming and fertilization on the dehydrogenase and catalase activities. Rev. Chim. 2019, 70, 3464–3468. [Google Scholar] [CrossRef]
- Hepşen Türkay, F.Ş.; Durmuş, M.; Yakupoğlu, T. Exploring Catalase Activity as A Biological Indicator in Degraded Soils. Anadolu J. Agric. Sci. 2024, 39, 401–417. [Google Scholar]
- Chaubey, O.; Bohre, P.; Singhal, P. Impact of bio-reclamation of coal mine spoil on nutritional and microbial characteristics—A case study. Int. J. Bio-Sci. Bio-Technol. 2012, 4, 69–79. [Google Scholar]
- Jing, Z.; Wang, J.; Zhu, Y.; Feng, Y. Effects of land subsidence resulted from coal mining on soil nutrient distributions in a loess area of China. J. Clean. Prod. 2018, 177, 350–361. [Google Scholar]
- Li, Y.; Wen, H.; Chen, L.; Yin, T. Succession of bacterial community structure and diversity in soil along a chronosequence of reclamation and re-vegetation on coal mine spoils in China. PLoS ONE 2014, 9, e115024. [Google Scholar]
- Li, J.; Xin, Z.; Yan, J.; Li, H.; Chen, J.; Ding, G. Physicochemical and microbiological assessment of soil quality on a chronosequence of a mine reclamation site. Eur. J. Soil Sci. 2018, 69, 1056–1067. [Google Scholar]
- Ngugi, M.R.; Dennis, P.G.; Neldner, V.J.; Doley, D.; Fechner, N.; McElnea, A. Open-cut mining impacts on soil abiotic and bacterial community properties as shown by restoration chronosequence. Restor. Ecol. 2018, 26, 839–850. [Google Scholar]
- Xu, C.; Zhang, H.; Li, J.; Liu, Y.; Su, C. Microbial diversity drives soil multifunctionality along reclamation chronosequence in an opencast coal mine. Land Degrad. Dev. 2014, 35, 1985–1999. [Google Scholar] [CrossRef]
- Sokolov, D.A.; Androkhanov, V.A.; Abakumov, E.V. Soil formation in technogenic landscapes: Trends, results, and representation in the current classifications. Vestn. Tomsk Gos. Univ. Biol. 2021, 56, 6–32. [Google Scholar] [CrossRef]



| Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|
| UL | CK | NPK | NPKB | M | MB | MNPK | MNPKB | |
| SM | 0.17 ± 0.02 abc | 0.19 ± 0.02 a | 0.15 ± 0.01 bcd | 0.15 ± 0.01 cd | 0.18 ± 0.01 ab | 0.19 ± 0.01 a | 0.15 ± 0.01 cd | 0.14 ± 0.01 d |
| pH | 8.27 ± 0.09 a | 7.86 ± 0.49 a | 7.85 ± 0.42 a | 8.09 ± 0.04 a | 8.15 ± 0.06 a | 8.10 ± 0.03 a | 8.14 ± 0.13 a | 8.01 ± 0.28 a |
| TN (g/kg) | 0.27 ± 0.04 d | 0.32 ± 0.06 cd | 0.41 ± 0.08 c | 0.57 ± 0.06 b | 0.56 ± 0.01 b | 0.77 ± 0.03 a | 0.59 ± 0.06 b | 0.62 ± 0.06 b |
| AN (mg/kg) | 304.05 ± 0.001 ab | 344.59 ± 11.50 a | 302.85 ± 30.84 ab | 151.43 ± 52.37 c | 315.97 ± 145.67 a | 331.47 ± 69.02 a | 174.08 ± 99.02 bc | 162.16 ± 38.25 c |
| TP (g/kg) | 0.38 ± 0.001 a | 0.69 ± 0.46 a | 0.39 ± 0.20 a | 0.34 ± 0.19 a | 0.35 ± 0.28 a | 0.52 ± 0.18 a | 0.39 ± 0.20 a | 0.33 ± 0.24 a |
| AP (g/kg | 5.09 ± 0.01 d | 10.93 ± 0.26 bc | 17.68 ± 5.80 a | 12.72 ± 1.88 ab | 12.14 ± 0.80 ab | 12.08 ± 1.50 ab | 18.65 ± 7.16 a | 9.59 ± 2.03 bc |
| TK (g/kg) | 5.61 ± 0.02 a | 3.72 ± 0.74 bc | 2.88 ± 1.40 c | 4.10 ± 0.66 bc | 3.20 ± 0.03 bc | 4.45 ± 0.05 ab | 4.01 ± 0.70 bc | 4.03 ± 0.64 bc |
| AK (mg/kg) | 80.06 ± 9.21 ab | 74.71 ± 8.34 b | 77.39 ± 8.34 b | 70.71 ± 6.12 b | 86.73 ± 10.08 ab | 98.75 ± 18.51 a | 86.74 ± 15.17 ab | 72.05 ± 6.94 b |
| SOC (g/kg) | 1.74 ± 0.03 e | 1.55 ± 0.08 e | 2.07 ± 0.17 d | 2.62 ± 0.34 c | 3.35 ± 0.24 ab | 3.64 ± 0.16 a | 3.12 ± 0.04 b | 3.31 ± 0.21 ab |
| MBC (mg/kg) | 9.85 ± 0.45 f | 17.06 ± 1.50 e | 19.72 ± 0.58 d | 28.26 ± 1.88 c | 37.78 ± 2.29 b | 38.51 ± 0.99 ab | 37.45 ± 0.82 b | 40.75 ± 1.44 a |
| DOC (g/kg) | 0.08 ± 0.01 a | 0.09 ± 0.02 a | 0.08 ± 0.01 a | 0.07 ± 0.01 a | 0.08 ± 0.01 a | 0.09 ± 0.01 a | 0.08 ± 0.01 a | 0.08 ± 0.01 a |
| EOC (g/kg) | 1.58 ± 0.22 a | 1.48 ± 0.58 a | 1.34 ± 0.35 a | 1.51 ± 0.36 a | 1.93 ± 0.46 a | 1.54 ± 0.30 a | 1.65 ± 0.69 a | 1.47 ± 0.12 a |
| POC (g/kg) | 0.24 ± 0.23 a | 0.28 ± 0.15 a | 0.52 ± 0.12 a | 0.46 ± 0.07 a | 0.60 ± 0.18 a | 0.75 ± 0.36 a | 0.71 ± 0.57 a | 0.59 ± 0.03 a |
| ALP (μg/g) | 1.88 ± 0.13 e | 2.50 ± 1.07 e | 5.11 ± 0.79 d | 6.07 ± 0.70 cd | 9.48 ± 0.43 b | 11.21 ± 1.53 a | 7.13 ± 0.19 c | 9.11 ± 0.73 b |
| CAT (mL/g) | 2.15 ± 0.06 a | 1.52 ± 0.22 d | 1.65 ± 0.03 cd | 1.78 ± 0.03 bc | 2.22 ± 0.05 a | 2.10 ± 0.08 a | 1.97 ± 0.26 ab | 2.18 ± 0.10 a |
| INV (mg/g) | 1.40 ± 0.12 cd | 1.23 ± 0.16 d | 1.35 ± 0.09 cd | 1.54 ± 0.13 bc | 1.66 ± 0.06 b | 1.88 ± 0.06 a | 1.53 ± 0.18 bc | 1.66 ± 0.06 b |
| URE (mg/g) | 0.11 ± 0.02 d | 0.10 ± 0.01 d | 0.22 ± 0.02 d | 0.48 ± 0.06 c | 0.98 ± 0.11 b | 1.53 ± 0.27 a | 0.99 ± 0.11 b | 1.17 ± 0.18 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Shang, Y.; Bai, S.; Fan, M.; Sui, X.; Meng, H.; Hao, X.; Wang, X.; Liu, Y.; Li, Y.; et al. Manure-Amended One-Year-Reclamation Promoted Soil Bacterial Phylotypic and Phenotypic Shifts in a Typical Coal-Mining Area. Microorganisms 2025, 13, 699. https://doi.org/10.3390/microorganisms13040699
Zhang H, Shang Y, Bai S, Fan M, Sui X, Meng H, Hao X, Wang X, Liu Y, Li Y, et al. Manure-Amended One-Year-Reclamation Promoted Soil Bacterial Phylotypic and Phenotypic Shifts in a Typical Coal-Mining Area. Microorganisms. 2025; 13(4):699. https://doi.org/10.3390/microorganisms13040699
Chicago/Turabian StyleZhang, Hongjuan, Yanmeng Shang, Shuning Bai, Meihua Fan, Xiaolong Sui, Huisheng Meng, Xianjun Hao, Xiangying Wang, Yulin Liu, Yi Li, and et al. 2025. "Manure-Amended One-Year-Reclamation Promoted Soil Bacterial Phylotypic and Phenotypic Shifts in a Typical Coal-Mining Area" Microorganisms 13, no. 4: 699. https://doi.org/10.3390/microorganisms13040699
APA StyleZhang, H., Shang, Y., Bai, S., Fan, M., Sui, X., Meng, H., Hao, X., Wang, X., Liu, Y., Li, Y., Hong, J., & Zhang, J. (2025). Manure-Amended One-Year-Reclamation Promoted Soil Bacterial Phylotypic and Phenotypic Shifts in a Typical Coal-Mining Area. Microorganisms, 13(4), 699. https://doi.org/10.3390/microorganisms13040699






