Viscosupplementation and Synovial Fluid Rheology: A Hidden Risk for Bacterial Biofilm Formation in Joint Infections?
Abstract
1. Introduction
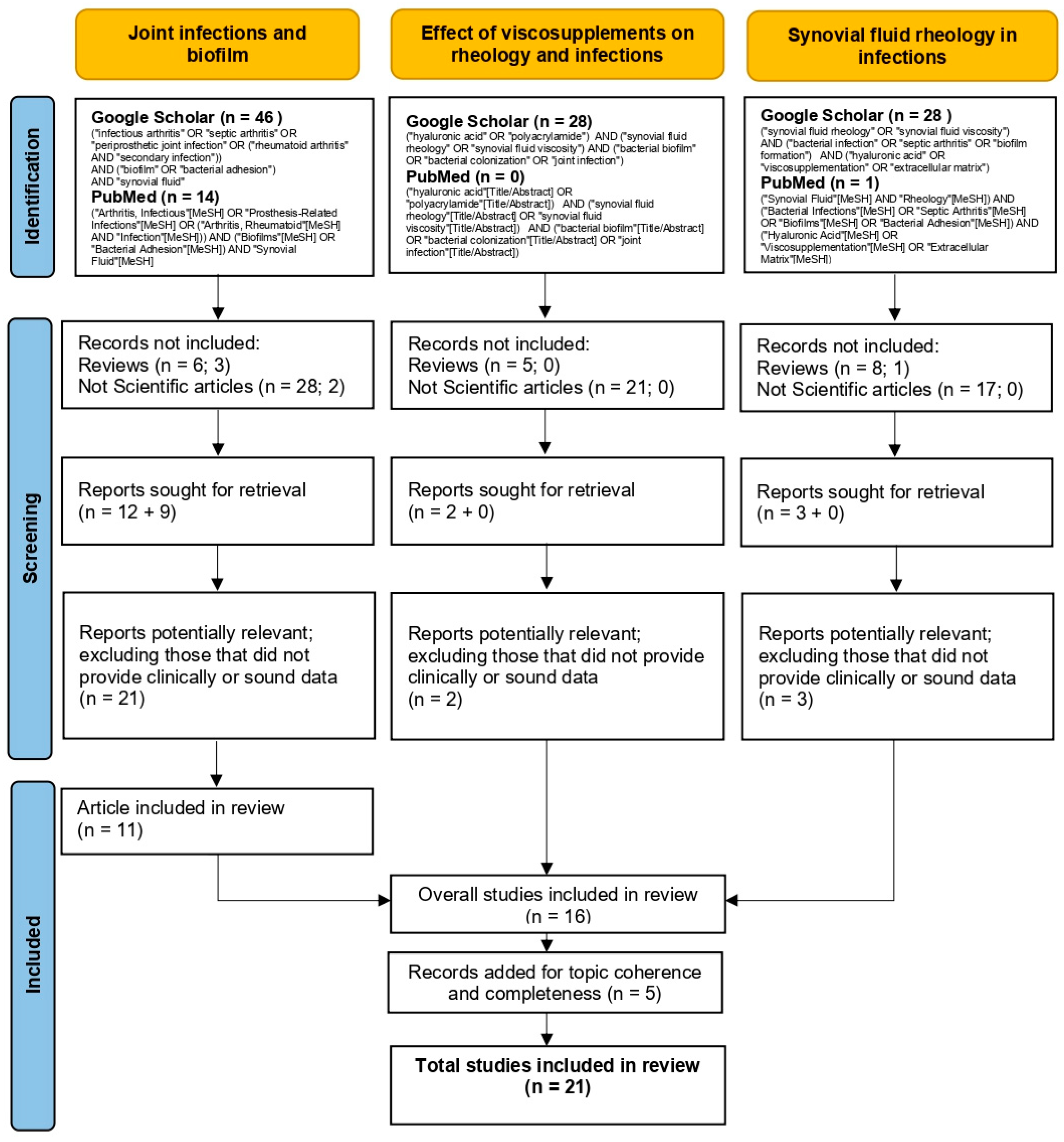
2. Materials and Methods
- Initial screening based on title and abstract to identify relevant studies.
- Full-text analysis to exclude articles lacking clinically relevant or methodologically robust data.
3. Synovial Fluid Rheology and Infection Susceptibility
3.1. Healthy Synovial Fluid Rheology: Viscosity and Elasticity
3.2. Rheology of Pathological Synovial Fluid in Infected Joints
- A reduction in HA concentration and molecular weight, shifting from (in healthy SF) to values as low as , resulting in a drastic loss of viscoelastic properties.
- An increased protein content (albumin, fibrinogen) that elevates friction and contributes to cartilage wear.
4. Managing HA Imbalance: Viscosupplement, a Therapeutic Challenge
4.1. Hyaluronic Acid Injections: History, Mechanism, and Clinical Use
- Cytokine inhibition: HA has been shown to inhibit pro-inflammatory mediators such as TNF-α, IL-1β, and IL-6, which contribute to cartilage degradation and pain sensitization while also modulating the senescence-associated secretory phenotype (SASP), a key driver of chronic joint inflammation [15,23,24].
HA Products in Clinical Use
- Low molecular weight (0.5–1.0 × 106 Da): shorter intra-articular retention but greater bioavailability (e.g., Suplasyn®, Fermathron®).
- Intermediate molecular weight (1.0–1.8 × 106 Da): prolonged residence time, with balanced viscosity and anti-inflammatory effects (e.g., Ostenil®, Orthovisc®).
- Inflammation-driven breakdown: oxidative stress and inflammatory cytokines accelerate HA depolymerization, reducing its retention in the joint space [34].
- Biofilm formation risk: degraded HA fragments can serve as bacterial adhesion sites, potentially promoting biofilm formation in septic joints [35].
4.2. Polyacrylamide Hydrogels: A New Alternative?
4.3. How Viscosupplementation Modulates Bacterial Proliferation and Biofilm Formation
- When SF becomes excessively diluted due to HA breakdown, the elastic modulus (G′) loses its plateau, the viscoelastic balance is disrupted, and bacterial motility increases. These changes favor bacterial dispersal within SF, promoting planktonic biofilm formation. The bacteria, now freely suspended in the low-viscosity SF, cluster into floating biofilm-like aggregates, exhibiting increased resistance to antibiotics and immune clearance, making their detection and eradication particularly challenging [8,46,47]. Studies indicate that such bacterial aggregation in SF can significantly influence the extent of colonization on prosthetic implants, further complicating treatment outcomes [48,49].
- Conversely, an excessive accumulation of HA, particularly following viscosupplementation, alters SF rheology in a manner that can also facilitate bacterial persistence [15,20]. When HA concentration increases beyond physiological levels (10 mg/mol or 5–10 mg/mL), SF transforms from a shear-thinning fluid into a viscoelastic solid-like material [48,49]. This shift is reflected in its rheological properties as the elastic modulus (G′) exceeds the viscous modulus (G″), meaning that the SF behaves more like a soft solid than a lubricating fluid [27,50]. High viscosity at low shear rates prevents effective fluid mobility, impairing joint lubrication rather than improving it. The polymeric network stabilizes, forming a dense meshwork that bacteria can exploit as an adhesion surface [51].
- ○
- Bacteria can anchor themselves within the SF matrix, shielded from immune clearance and antimicrobial penetration [4].
- ○
5. Clinical Evidence and Open Questions
5.1. Challenges in Diagnosing and Treating Biofilm-Associated Infections
5.2. Future Research Directions and Risk Mitigation Strategies
- Developing biofilm-resistant viscosupplements: investigating HA derivatives or polyacrylamide-based hydrogels (PAAGs) that mitigate bacterial adhesion while preserving joint lubrication.
- Assessing vascular implications of SF alterations: exploring the impact of viscosupplementation on systemic inflammation, endothelial health, and cardiovascular risks.
- Advancing diagnostic strategies for vascular involvement: investigating imaging techniques and biomarkers to detect early vascular changes in patients with chronic joint infections.
5.3. Future Perspectives and Patient Stratification
- Individuals with a history of joint infections, who may be more prone to biofilm-related complications.
- Patients undergoing immunosuppressive therapy, including rheumatoid arthritis patients on biologics and oncology patients.
- Post-surgical cases, where residual inflammation and surgical implants may increase susceptibility to infection.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schurz, J.; Ribitsch, V. Rheology of synovial fluid. Biorheology 1987, 24, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Krakowski, P.; Rejniak, A.; Sobczyk, J.; Karpinski, R. Cartilage Integrity: A Review of Mechanical and Frictional Properties and Repair Approaches in Osteoarthritis. Healthcare 2024, 12, 1648. [Google Scholar] [CrossRef]
- Ouerfelli, N.; Vrinceanu, N.; Mliki, E.; Amin, K.A.; Snoussi, L.; Coman, D.; Mrabet, D. Rheological behavior of the synovial fluid: A mathematical challenge. Front. Mater. 2024, 11, 1386694. [Google Scholar] [CrossRef]
- Leonardo, M.A.; Aleksandra, G.; Randy, H.E.; Bryant, S.L.; Jay, G.D.; Schmidt, T.A.; Trifkovic, M. Scale-Dependent Rheology of Synovial Fluid Lubricating Macromolecules. Small 2024, 20, e2306207. [Google Scholar]
- Imagama, T.; Tokushige, A.; Seki, K.; Seki, T.; Nakashima, D.; Ogasa, H.; Sakai, T.; Taguchi, T. Early diagnosis of septic arthritis using synovial fluid presepsin: A preliminary study. J. Infect. Chemother. 2019, 25, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Strootmann, T.; Spitzbarth, I.; Della Tommasa, S.; Brehm, W.; Koller, G.; Troillet, A. Synovial Fluid Analysis and Microscopic Assessment of Macrophage Quantities and Morphology in Equine Septic Arthritis. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 2022, 50, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Balazs, E.A.; Denlinger, J.L. Viscosupplementation: A new concept in the treatment of osteoarthritis. J. Rheumatol. Suppl. 1993, 39, 3–9. [Google Scholar] [PubMed]
- Bidossi, A.; Bottagisio, M.; Savadori, P.; De Vecchi, E. Identification and Characterization of Planktonic Biofilm-Like Aggregates in Infected Synovial Fluids From Joint Infections. Front. Microbiol. 2020, 11, 1368. [Google Scholar] [CrossRef]
- del Pozo, J.L.; Patel, R. The challenge of treating biofilm-associated bacterial infections. Clin. Pharmacol. Ther. 2007, 82, 204–209. [Google Scholar] [CrossRef]
- La Gatta, A.; Stellavato, A.; Vassallo, V.; Di Meo, C.; Toro, G.; Iolascon, G.; Schiraldi, C. Hyaluronan and Derivatives: An In Vitro Multilevel Assessment of Their Potential in Viscosupplementation. Polymers 2021, 13, 3208. [Google Scholar] [CrossRef]
- Bernetti, A.; Agostini, F.; Paoloni, M.; Raele, M.V.; Fari, G.; Megna, M.; Mangone, M. Could Hyaluronic Acid Be Considered as a Senomorphic Agent in Knee Osteoarthritis? A Systematic Review. Biomedicines 2023, 11, 2858. [Google Scholar] [CrossRef] [PubMed]
- Mikzinski, P.; Kraus, K.; Widelski, J.; Paluch, E. Modern Microbiological Methods to Detect Biofilm Formation in Orthopedy and Suggestions for Antibiotic Therapy, with Particular Emphasis on Prosthetic Joint Infection (PJI). Microorganisms 2024, 12, 1198. [Google Scholar] [CrossRef]
- Knott, S.; Curry, D.; Zhao, N.; Metgud, P.; Dastgheyb, S.S.; Purtill, C.; Harwood, M.; Chen, A.F.; Schaer, T.P.; Otto, M.; et al. Staphylococcus aureus Floating Biofilm Formation and Phenotype in Synovial Fluid Depends on Albumin, Fibrinogen, and Hyaluronic Acid. Front. Microbiol. 2021, 12, 655873. [Google Scholar] [CrossRef]
- Rebenda, D.; Vrbka, M.; Cipek, P.; Toropitsyn, E.; Necas, D.; Pravda, M.; Hartl, M. On the Dependence of Rheology of Hyaluronic Acid Solutions and Frictional Behavior of Articular Cartilage. Materials 2020, 13, 2659. [Google Scholar] [CrossRef]
- Bidossi, A.; Bottagisio, M.; De Grandi, R.; De Vecchi, E. Ability of adhesion and biofilm formation of pathogens of periprosthetic joint infections on titanium-niobium nitride (TiNbN) ceramic coatings. J. Orthop. Surg. Res. 2020, 15, 90. [Google Scholar] [CrossRef]
- Brannan, S.R.; Jerrard, D.A. Synovial fluid analysis. J. Emerg. Med. 2006, 30, 331–339. [Google Scholar] [CrossRef]
- Li, H.; Fu, J.; Erlong, N.; Li, R.; Xu, C.; Hao, L.; Chen, J.; Chai, W. Characterization of periprosthetic environment microbiome in patients after total joint arthroplasty and its potential correlation with inflammation. BMC Infect. Dis. 2023, 23, 423. [Google Scholar] [CrossRef]
- Lenski, M.; Scherer, M.A. Synovial IL-6 as inflammatory marker in periprosthetic joint infections. J. Arthroplast. 2014, 29, 1105–1109. [Google Scholar] [CrossRef]
- Deirmengian, C.; Kardos, K.; Kilmartin, P.; Cameron, A.; Schiller, K.; Parvizi, J. Combined measurement of synovial fluid alpha-Defensin and C-reactive protein levels: Highly accurate for diagnosing periprosthetic joint infection. J. Bone Jt. Surg. Am. 2014, 96, 1439–1445. [Google Scholar] [CrossRef]
- Staats, A.; Li, D.; Sullivan, A.C.; Stoodley, P. Biofilm formation in periprosthetic joint infections. Ann. Jt. 2021, 6, 43. [Google Scholar] [CrossRef]
- Henrotin, Y.; Raman, R.; Richette, P.; Bard, H.; Jerosch, J.; Conrozier, T.; Chevalier, X.; Migliore, A. Consensus statement on viscosupplementation with hyaluronic acid for the management of osteoarthritis. Semin. Arthritis Rheum. 2015, 45, 140–149. [Google Scholar] [CrossRef]
- Fari, G.; Mancini, R.; Dell’Anna, L.; Ricci, V.; Della Tommasa, S.; Bianchi, F.P.; Ladisa, I.; De Serio, C.; Fiore, S.; Donati, D.; et al. Medial or Lateral, That Is the Question: A Retrospective Study to Compare Two Injection Techniques in the Treatment of Knee Osteoarthritis Pain with Hyaluronic Acid. J. Clin. Med. 2024, 13, 1141. [Google Scholar] [CrossRef] [PubMed]
- Casale, M.; Moffa, A.; Vella, P.; Sabatino, L.; Capuano, F.; Salvinelli, B.; Lopez, M.A.; Carinci, F.; Salvinelli, F. Hyaluronic acid: Perspectives in dentistry. A systematic review. Int. J. Immunopathol. Pharmacol. 2016, 29, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Lee, J.Y.; Shim, K.S.; Lee, S.; Min, K.; Bae, J.H.; Kim, H.J.; Park, K.; Song, H.R. Attenuation of inflammation and cartilage degradation by sulfasalazine-containing hyaluronic acid on osteoarthritis rat model. Int. J. Biol. Macromol. 2018, 114, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Lin, C.Y.; Wang, H.S.; Lyu, S.R. Matrix metalloproteases and tissue inhibitors of metalloproteinases in medial plica and pannus-like tissue contribute to knee osteoarthritis progression. PLoS ONE 2013, 8, e79662. [Google Scholar] [CrossRef]
- Fari, G.; Megna, M.; Scacco, S.; Ranieri, M.; Raele, M.V.; Chiaia Noya, E.; Macchiarola, D.; Bianchi, F.P.; Carati, D.; Panico, S.; et al. Hemp Seed Oil in Association with beta-Caryophyllene, Myrcene and Ginger Extract as a Nutraceutical Integration in Knee Osteoarthritis: A Double-Blind Prospective Case-Control Study. Medicina 2023, 59, 191. [Google Scholar] [CrossRef]
- Wobig, M.; Bach, G.; Beks, P.; Dickhut, A.; Runzheimer, J.; Schwieger, G.; Vetter, G.; Balazs, E. The role of elastoviscosity in the efficacy of viscosupplementation for osteoarthritis of the knee: A comparison of hylan G-F 20 and a lower-molecular-weight hyaluronan. Clin. Ther. 1999, 21, 1549–1562. [Google Scholar] [CrossRef]
- Levy, D.M.; Petersen, K.A.; Scalley Vaught, M.; Christian, D.R.; Cole, B.J. Injections for Knee Osteoarthritis: Corticosteroids, Viscosupplementation, Platelet-Rich Plasma, and Autologous Stem Cells. Arthroscopy 2018, 34, 1730–1743. [Google Scholar] [CrossRef]
- Highley, C.B.; Prestwich, G.D.; Burdick, J.A. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr. Opin. Biotechnol. 2016, 40, 35–40. [Google Scholar] [CrossRef]
- Guidolin, D.; Franceschi, F. Viscosupplementation with high molecular weight native hyaluronan. Focus on a 1500–2000 KDa fraction (Hyalubrix(R)). Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3326–3338. [Google Scholar]
- Sun, S.F.; Hsu, C.W.; Lin, H.S.; Liou, I.H.; Chen, Y.H.; Hung, C.L. Comparison of Single Intra-Articular Injection of Novel Hyaluronan (HYA-JOINT Plus) with Synvisc-One for Knee Osteoarthritis: A Randomized, Controlled, Double-Blind Trial of Efficacy and Safety. J. Bone Jt. Surg. Am. 2017, 99, 462–471. [Google Scholar] [CrossRef]
- Legre-Boyer, V. Viscosupplementation: Techniques, indications, results. Orthop. Traumatol. Surg. Res. 2015, 101, S101–S108. [Google Scholar] [CrossRef]
- Pestrak, M.J.; Gupta, T.T.; Dusane, D.H.; Guzior, D.V.; Staats, A.; Harro, J.; Horswill, A.R.; Stoodley, P. Correction: Investigation of synovial fluid induced Staphylococcus aureus aggregate development and its impact on surface attachment and biofilm formation. PLoS ONE 2020, 15, e0233534. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.W. Role of hyaluronan in atherosclerosis: Current knowledge and open questions. Matrix Biol. 2019, 78–79, 324–336. [Google Scholar] [CrossRef]
- Ibberson, C.B.; Parlet, C.P.; Kwiecinski, J.; Crosby, H.A.; Meyerholz, D.K.; Horswill, A.R. Hyaluronan Modulation Impacts Staphylococcus aureus Biofilm Infection. Infect. Immun. 2016, 84, 1917–1929. [Google Scholar] [CrossRef] [PubMed]
- Bonnevie, E.D.; Galesso, D.; Secchieri, C.; Bonassar, L.J. Frictional characterization of injectable hyaluronic acids is more predictive of clinical outcomes than traditional rheological or viscoelastic characterization. PLoS ONE 2019, 14, e0216702. [Google Scholar] [CrossRef]
- de Clifford, L.T.; Lowe, J.N.; McKellar, C.D.; McGowan, C.; David, F. A Double-Blinded Positive Control Study Comparing the Relative Efficacy of 2.5% Polyacrylamide Hydrogel (PAAG) Against Triamcinolone Acetonide (TA) And Sodium Hyaluronate (HA) in the Management of Middle Carpal Joint Lameness in Racing Thoroughbreds. J. Equine Vet. Sci. 2021, 107, 103780. [Google Scholar] [CrossRef]
- Henriksen, M.; Overgaard, A.; Hartkopp, A.; Bliddal, H. Intra-articular 2.5% polyacrylamide hydrogel for the treatment of knee osteoarthritis: An observational proof-of-concept cohort study. Clin. Exp. Rheumatol. 2018, 36, 1082–1085. [Google Scholar]
- Pavan, M.; Galesso, D.; Menon, G.; Renier, D.; Guarise, C. Hyaluronan derivatives: Alkyl chain length boosts viscoelastic behavior to depolymerization. Carbohydr. Polym. 2013, 97, 321–326. [Google Scholar] [CrossRef]
- La Gatta, A.; De Rosa, M.; Marzaioli, I.; Busico, T.; Schiraldi, C. A complete hyaluronan hydrodynamic characterization using a size exclusion chromatography-triple detector array system during in vitro enzymatic degradation. Anal. Biochem. 2010, 404, 21–29. [Google Scholar] [CrossRef]
- La Gatta, A.; Ricci, G.; Stellavato, A.; Cammarota, M.; Filosa, R.; Papa, A.; D’Agostino, A.; Portaccio, M.; Delfino, I.; De Rosa, M.; et al. Hyaluronan hydrogels with a low degree of modification as scaffolds for cartilage engineering. Int. J. Biol. Macromol. 2017, 103, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Konttinen, Y.T.; Li, T.F.; Mandelin, J.; Ainola, M.; Lassus, J.; Virtanen, I.; Santavirta, S.; Tammi, M.; Tammi, R. Hyaluronan synthases, hyaluronan, and its CD44 receptor in tissue around loosened total hip prostheses. J. Pathol. 2001, 194, 384–390. [Google Scholar] [CrossRef]
- Chao, D.; Tran, H.; Hogan, Q.H.; Pan, B. Analgesic dorsal root ganglion field stimulation blocks both afferent and efferent spontaneous activity in sensory neurons of rats with monosodium iodoacetate-induced osteoarthritis. Osteoarthr. Cartil. 2022, 30, 1468–1481. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Luo, X.; Wang, X.; Ma, J.; Liu, F.; Yang, Q.; Yang, J.; Wang, X. Complications and Treatment Strategy After Breast Augmentation by Polyacrylamide Hydrogel Injection: Summary of 10-Year Clinical Experience. Aesthetic Plast. Surg. 2018, 42, 402–409. [Google Scholar] [CrossRef]
- Dastgheyb, S.S.; Hammoud, S.; Ketonis, C.; Liu, A.Y.; Fitzgerald, K.; Parvizi, J.; Purtill, J.; Ciccotti, M.; Shapiro, I.M.; Otto, M.; et al. Staphylococcal persistence due to biofilm formation in synovial fluid containing prophylactic cefazolin. Antimicrob. Agents Chemother. 2015, 59, 2122–2128. [Google Scholar] [CrossRef]
- Drago, L.; Fidanza, A.; Giannetti, A.; Ciuffoletti, A.; Logroscino, G.; Romanò, C.L. Bacteria Living in Biofilms in Fluids: Could Chemical Antibiofilm Pretreatment of Culture Represent a Paradigm Shift in Diagnostics? Microorganisms 2024, 12, 259. [Google Scholar] [PubMed]
- Taha, M.; Arnaud, T.; Lightly, T.J.; Peters, D.; Wang, L.; Chen, W.; Cook, B.W.M.; Theriault, S.S.; Abdelbary, H. Combining bacteriophage and vancomycin is efficacious against MRSA biofilm-like aggregates formed in synovial fluid. Front. Med. 2023, 10, 1134912. [Google Scholar] [CrossRef]
- Dastgheyb, S.; Parvizi, J.; Shapiro, I.M.; Hickok, N.J.; Otto, M. Effect of biofilms on recalcitrance of staphylococcal joint infection to antibiotic treatment. J. Infect. Dis. 2015, 211, 641–650. [Google Scholar] [CrossRef]
- Gilbertie, J.M.; Schaer, T.P.; Schubert, A.G.; Jacob, M.E.; Menegatti, S.; Ashton Lavoie, R.; Schnabel, L.V. Platelet-rich plasma lysate displays antibiofilm properties and restores antimicrobial activity against synovial fluid biofilms in vitro. J. Orthop. Res. 2020, 38, 1365–1374. [Google Scholar] [CrossRef]
- Cicognani, M.; Rossi, S.; Vecchi, G.; Giori, A.M.; Ferrari, F. DoE-Assisted Development of a Novel Glycosaminoglycan-Based Injectable Formulation for Viscosupplementation. Pharmaceutics 2020, 12, 681. [Google Scholar] [CrossRef]
- Nicholls, M.; Manjoo, A.; Shaw, P.; Niazi, F.; Rosen, J. A Comparison Between Rheological Properties of Intra-articular Hyaluronic Acid Preparations and Reported Human Synovial Fluid. Adv. Ther. 2018, 35, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Giarritiello, F.; Romano, C.L.; Lob, G.; Benevenia, J.; Tsuchiya, H.; Zappia, E.; Drago, L. Enhancing Pathogen Detection in Implant-Related Infections through Chemical Antibiofilm Strategies: A Comprehensive Review. Antibiotics 2024, 13, 678. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.M. Non-surface Attached Bacterial Aggregates: A Ubiquitous Third Lifestyle. Front. Microbiol. 2020, 11, 557035. [Google Scholar] [CrossRef]
- Hoiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef]
- Perez, K.; Patel, R. Biofilm-like aggregation of Staphylococcus epidermidis in synovial fluid. J. Infect. Dis. 2015, 212, 335–336. [Google Scholar] [CrossRef]
- Drago, L.; Romano, D.; Fidanza, A.; Giannetti, A.; Erasmo, R.; Mavrogenis, A.F.; Romano, C.L. Dithiotreitol pre-treatment of synovial fluid samples improves microbiological counts in peri-prosthetic joint infection. Int. Orthop. 2023, 47, 1147–1152. [Google Scholar] [CrossRef]
- Tsikopoulos, K.; Christofilos, S.I.; Kitridis, D.; Sidiropoulos, K.; Stoikos, P.N.; Gravalidis, C.; Givissis, P.; Papaioannidou, P. Is sonication superior to dithiothreitol in diagnosis of periprosthetic joint infections? A meta-analysis. Int. Orthop. 2022, 46, 1215–1224. [Google Scholar] [CrossRef]
- Rimoldi, S.G.; De Vecchi, E.; Pagani, C.; Zambelli, A.; Di Gregorio, A.; Bosisio, E.; Vanelli, P.; Scrofani, R.; Gismondo, M.R.; Cagnoni, G.; et al. Use of Dithiothreitol to Dislodge Bacteria from the Biofilm on an Aortic Valve in the Operating Theatre: A Case of Infective Endocarditis Caused by Staphylococcus aureus and Proteus mirabilis. Ann. Thorac. Surg. 2016, 102, e357–e359. [Google Scholar] [CrossRef]
- Peng, J.; Lu, Y.; Song, J.; Vallance, B.A.; Jacobson, K.; Yu, H.B.; Sun, Z. Direct Clinical Evidence Recommending the Use of Proteinase K or Dithiothreitol to Pretreat Sputum for Detection of SARS-CoV-2. Front. Med. 2020, 7, 549860. [Google Scholar] [CrossRef]
- Ratjen, F.; Waters, V.; Klingel, M.; McDonald, N.; Dell, S.; Leahy, T.R.; Yau, Y.; Grasemann, H. Changes in airway inflammation during pulmonary exacerbations in patients with cystic fibrosis and primary ciliary dyskinesia. Eur. Respir. J. 2016, 47, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Umemoto, D.; Hara, S.; Nishioka, H. Infective endocarditis and septic arthritis caused by Corynebacteriumstriatum. J. Infect. Chemother. 2024, 30, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Abideen, Z.U.; Bhatti, R.M.; Khalid, F.; Jaan, A.; Ahmed, Z. Tricuspid Valve Endocarditis: A Disguise In Multifocal Septic Arthritis. Cureus 2020, 12, e11375. [Google Scholar] [CrossRef] [PubMed]
- Dri, E.; Lampas, E.; Lazaros, G.; Lazarou, E.; Theofilis, P.; Tsioufis, C.; Tousoulis, D. Inflammatory Mediators of Endothelial Dysfunction. Life 2023, 13, 1420. [Google Scholar] [CrossRef]
- Mochizuki, S.; Vink, H.; Hiramatsu, O.; Kajita, T.; Shigeto, F.; Spaan, J.A.; Kajiya, F. Role of hyaluronic acid glycosaminoglycans in shear-induced endothelium-derived nitric oxide release. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H722–H726. [Google Scholar] [CrossRef]
- Conrozier, T.; Raman, R.; Diracoglu, D.; Montfort, J.; Bard, H.; Baron, D.; Goncalves, B.; Richette, P.; Migliore, A.; Chevalier, X.; et al. EUROVISCO Consensus Guidelines for the Use of Hyaluronic Acid Viscosupplementation in Knee Osteoarthritis Based on Patient Characteristics. Cartilage 2024, 19476035241271970. [Google Scholar] [CrossRef]
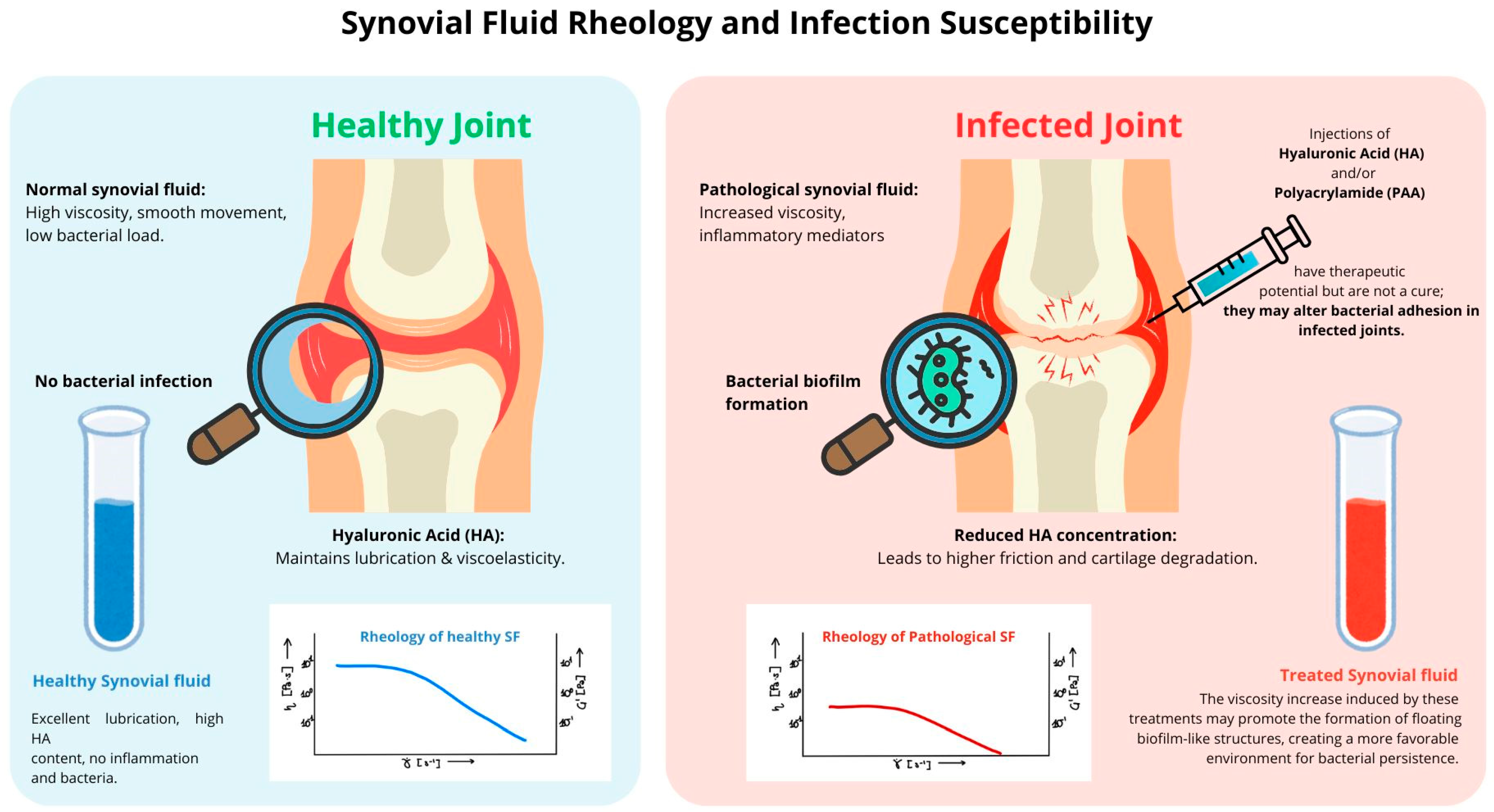
| Scheme | HA Concentration and MW | Rheological Behavior | Biofilm Fromation |
|---|---|---|---|
| Pathological synovial fluid (untreated) | Low HA concentration (<1–2 mg/mL), MW~ | Low viscosity, loss of shear-thinning, behaves as Newtonian fluid | Planktonic bacterial formation, increased bacterial motility and dispersion |
| Healthy synovial fluid | Normal HA concentration (2–4 mg/mL), MW~ | Non-Newtonian fluid, shear-thinning, viscoelastic balance | Minimal bacterial adhesion, homeostatic joint environment |
| Pathological synovial fluid (overtreated) | Excessive HA concentration (>5–10 mg/mL), MW remains high or increases | Solid-like behavior, reduced shear-thinning, increased elasticity | Potential biofilm adhesion due to high-viscosity and surface-like behavior |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giarritiello, F.; De La Motte, L.R.; Drago, L. Viscosupplementation and Synovial Fluid Rheology: A Hidden Risk for Bacterial Biofilm Formation in Joint Infections? Microorganisms 2025, 13, 700. https://doi.org/10.3390/microorganisms13040700
Giarritiello F, De La Motte LR, Drago L. Viscosupplementation and Synovial Fluid Rheology: A Hidden Risk for Bacterial Biofilm Formation in Joint Infections? Microorganisms. 2025; 13(4):700. https://doi.org/10.3390/microorganisms13040700
Chicago/Turabian StyleGiarritiello, Fabiana, Luigi Regenburgh De La Motte, and Lorenzo Drago. 2025. "Viscosupplementation and Synovial Fluid Rheology: A Hidden Risk for Bacterial Biofilm Formation in Joint Infections?" Microorganisms 13, no. 4: 700. https://doi.org/10.3390/microorganisms13040700
APA StyleGiarritiello, F., De La Motte, L. R., & Drago, L. (2025). Viscosupplementation and Synovial Fluid Rheology: A Hidden Risk for Bacterial Biofilm Formation in Joint Infections? Microorganisms, 13(4), 700. https://doi.org/10.3390/microorganisms13040700







