Genomic and Transcriptomic Characterization of Umatilla Virus Isolated and Identified from Mosquitoes in Ningxia, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Cells
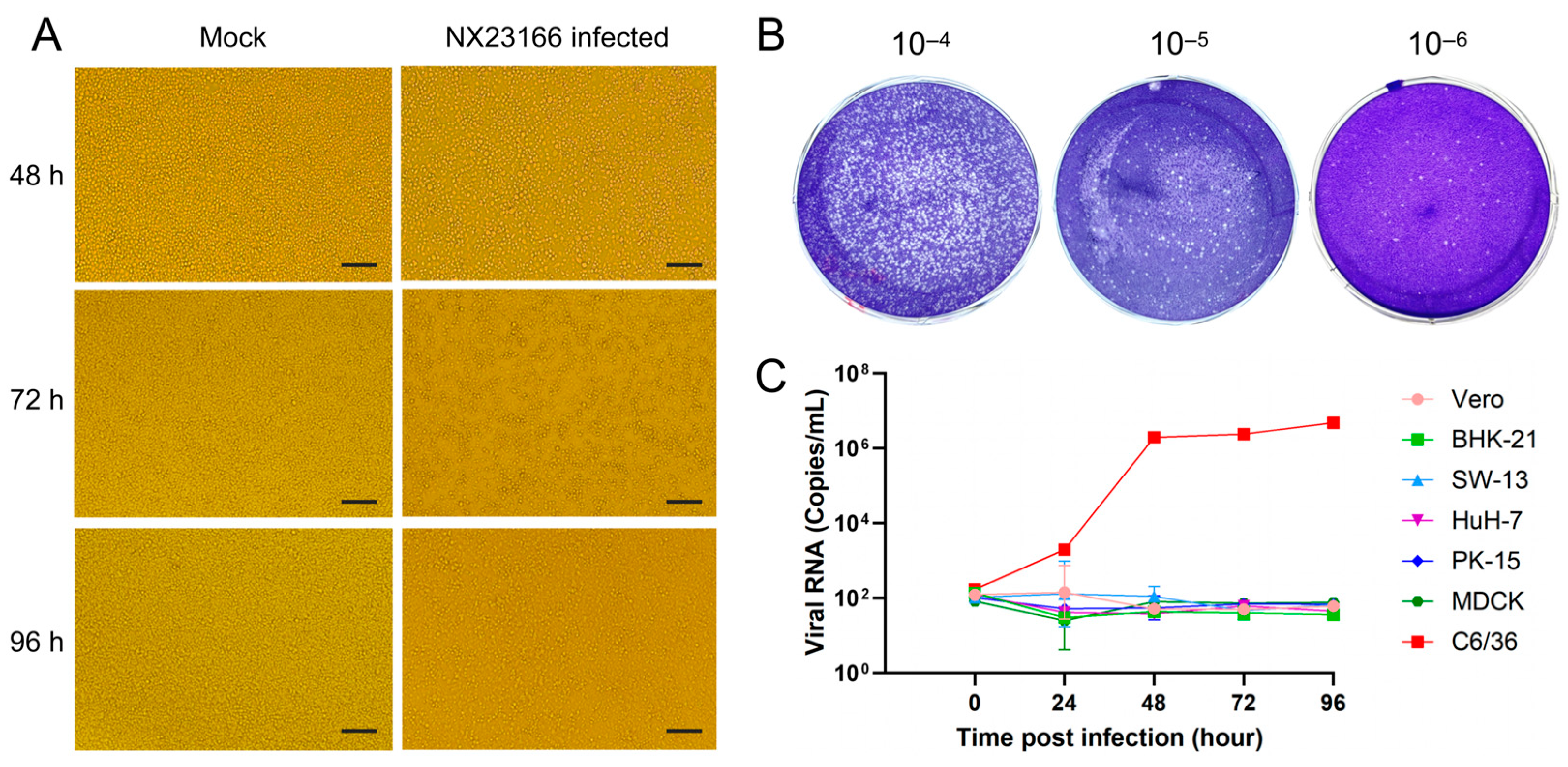
2.2. Virus Isolation and Cell Growth Kinetics
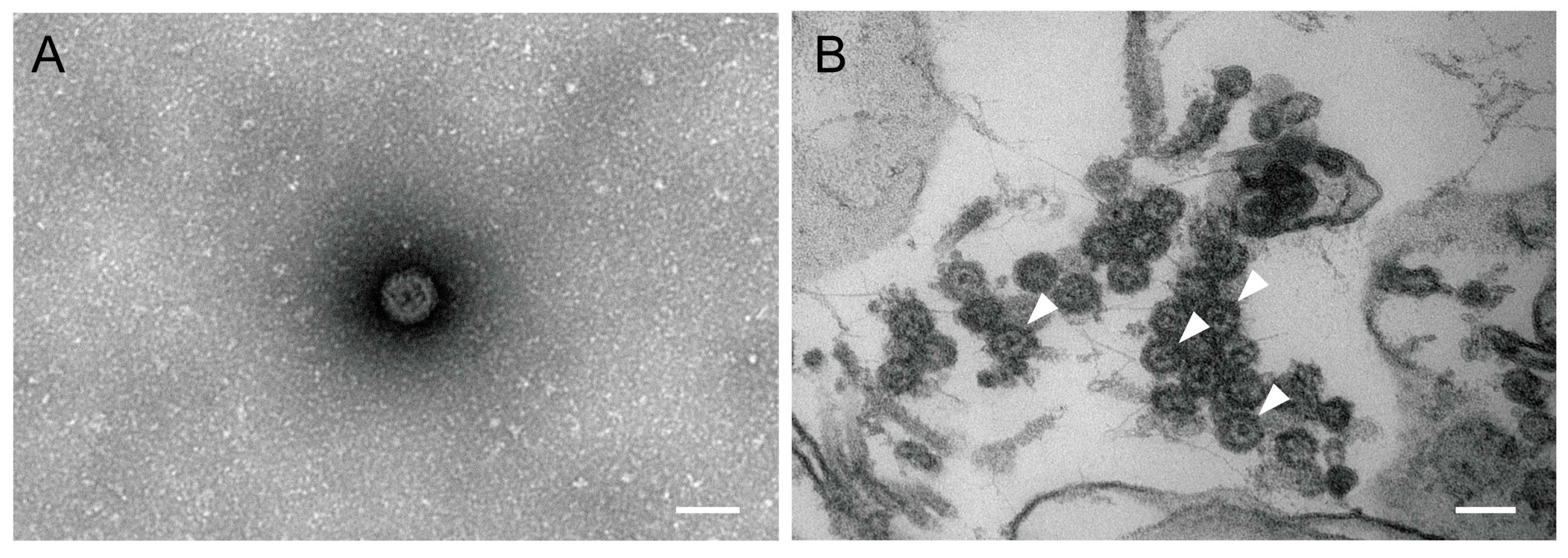
2.3. Virus Particle Electron Microscopy
2.4. Viral Titer Determination
2.5. Genome Sequence Determination
2.6. Sequence Alignment and Phylogenetic Analysis
2.7. Transcriptome Sequencing Analysis
3. Results
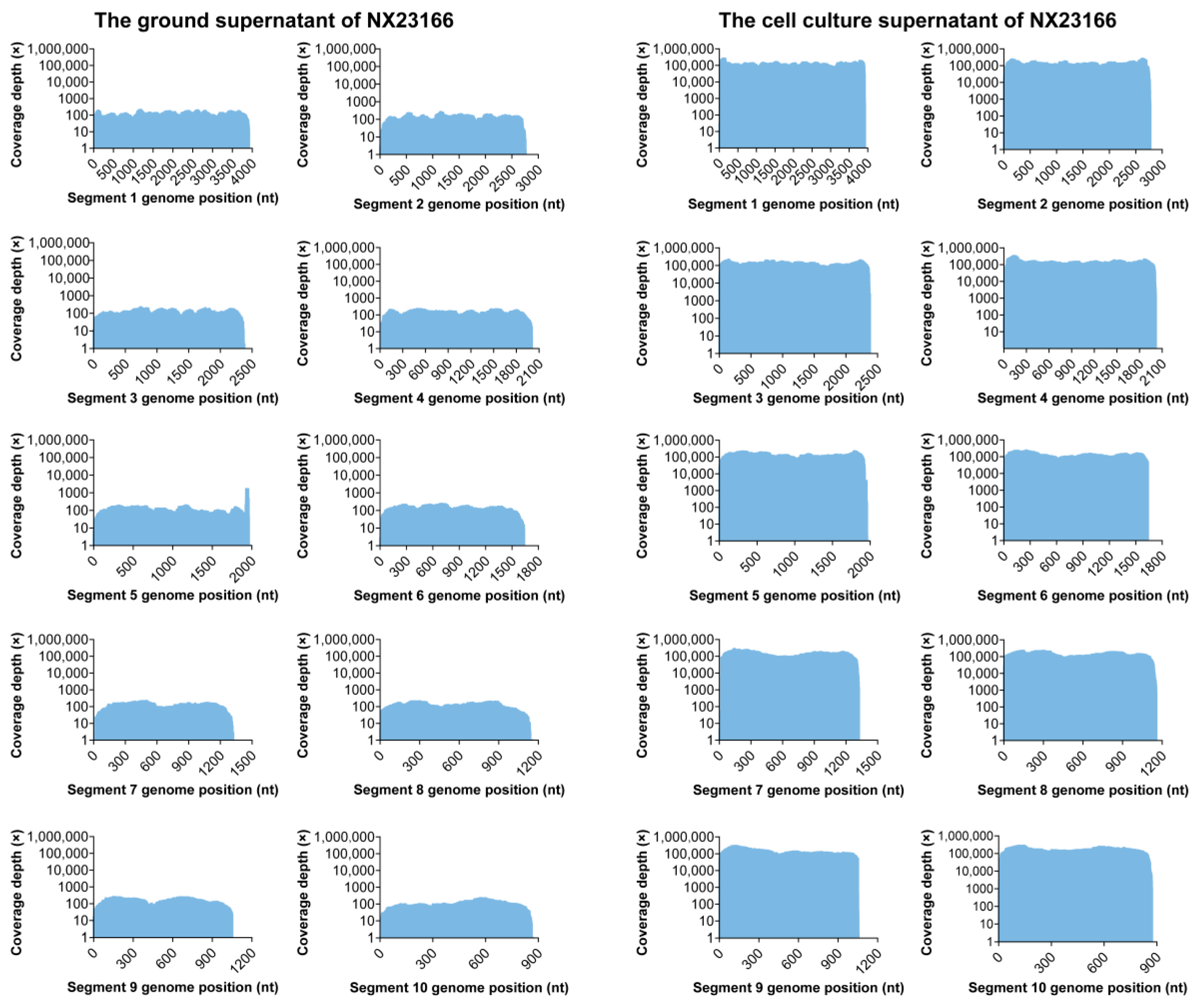
3.1. Viral Genome Sequencing and Assembly
3.2. Virus Isolation and Identification
3.3. Virus Electron Microscopy
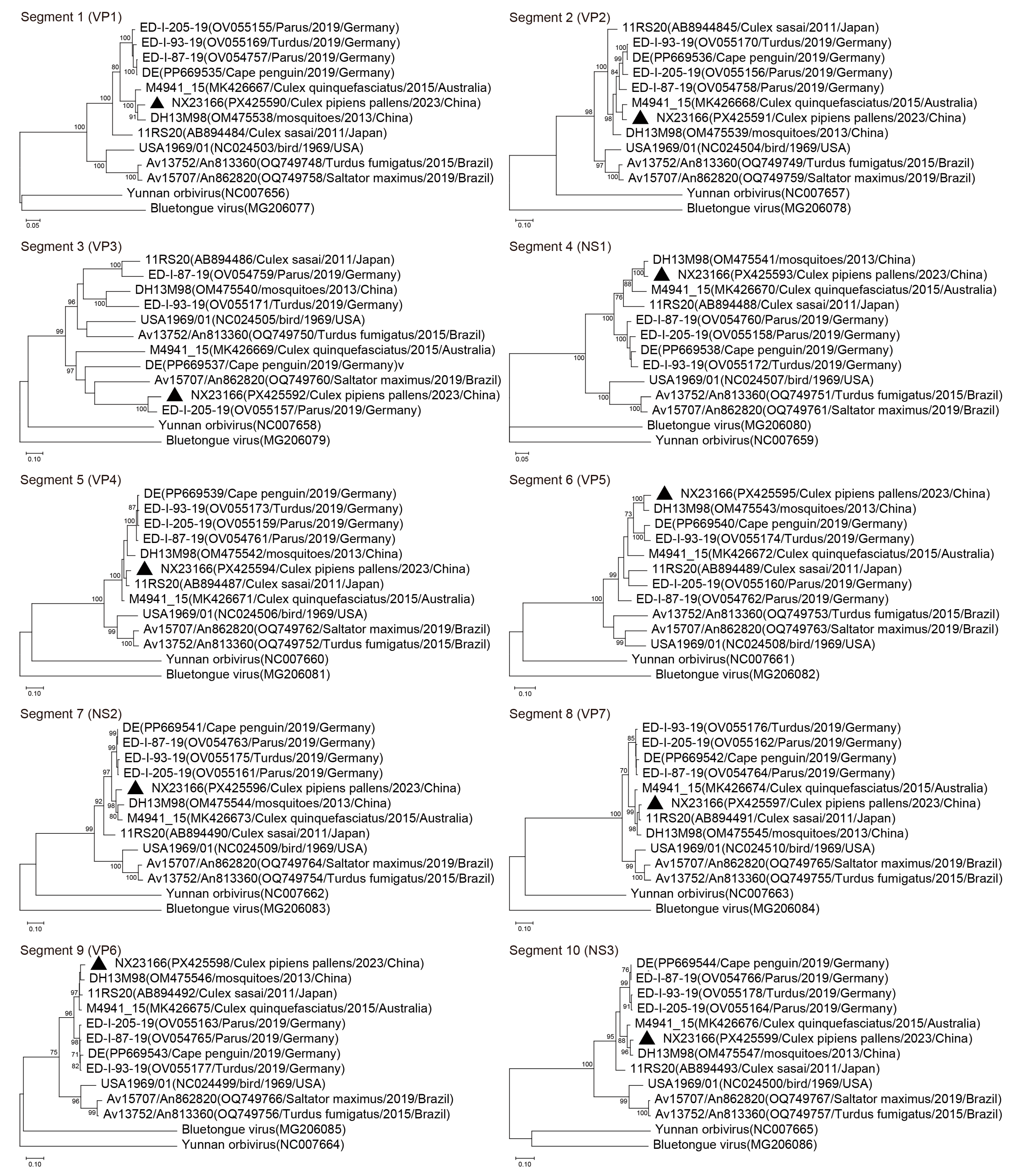
3.4. Phylogenetic Analysis
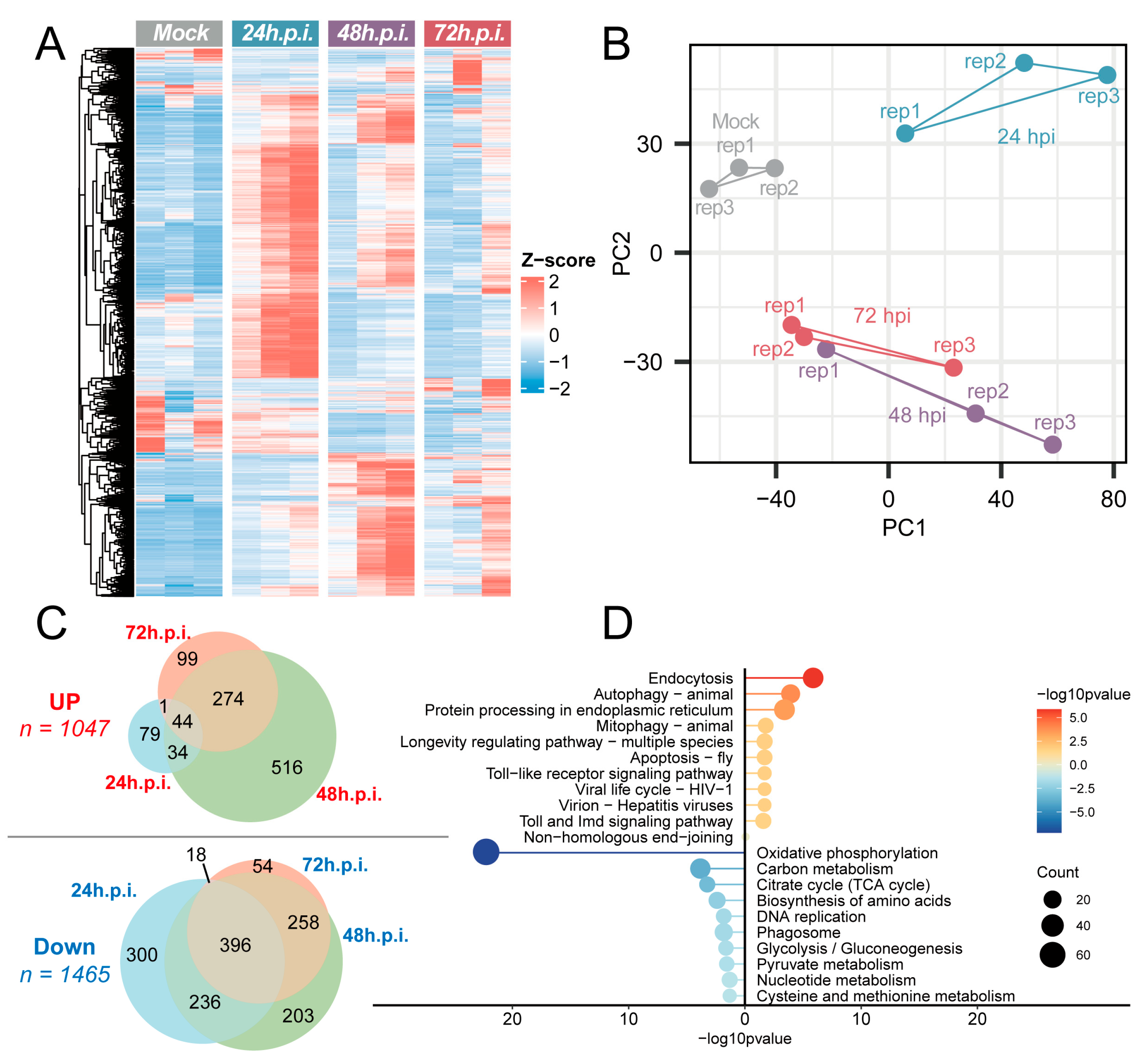
3.5. Transcriptomic Response to NX23166 (UMAV) Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UMAV | Umatilla virus |
| dsRNA | double-stranded RNA |
| NGS | next-generation sequencing |
| RT-qPCR | real-time quantitative polymerase chain reaction |
| CPE | cytopathic effect |
| PFU | plaque-forming unit |
| h.p.i. | hours post-infection |
| PCA | Principal component analysis |
References
- Karabatsos, N. Supplement to International Catalogue of Arboviruses including certain other viruses of vertebrates. Am. J. Trop. Med. Hyg. 1978, 27, 372–440. [Google Scholar] [CrossRef]
- Belaganahalli, M.N.; Maan, S.; Maan, N.S.; Nomikou, K.; Pritchard, I.; Lunt, R.; Kirkland, P.D.; Attoui, H.; Brownlie, J.; Mertens, P.P. Full genome sequencing and genetic characterization of Eubenangee viruses identify Pata virus as a distinct species within the genus Orbivirus. PLoS ONE 2012, 7, e31911. [Google Scholar] [CrossRef]
- Yang, Z.; Li, N.; He, Y.; Meng, J.; Wang, J. Genetic Characterization of DH13M98, Umatilla Virus, Isolated from Culex tritaeniorhynchus Giles in Yunnan Province, China. Vector Borne Zoonotic Dis. 2023, 23, 35–43. [Google Scholar] [CrossRef]
- Attoui, H.; Mohd Jaafar, F. Zoonotic and emerging orbivirus infections. Rev. Sci. Tech. 2015, 34, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Dennis, S.J.; Meyers, A.E.; Hitzeroth, I.I.; Rybicki, E.P. African Horse Sickness: A Review of Current Understanding and Vaccine Development. Viruses 2019, 11, 844. [Google Scholar] [CrossRef]
- Maclachlan, N.J.; Zientara, S.; Wilson, W.C.; Richt, J.A.; Savini, G. Bluetongue and epizootic hemorrhagic disease viruses: Recent developments with these globally re-emerging arboviral infections of ruminants. Curr. Opin. Virol. 2019, 34, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Mohd Jaafar, F.; Belhouchet, M.; Belaganahalli, M.; Tesh, R.B.; Mertens, P.P.; Attoui, H. Full-genome characterisation of Orungo, Lebombo and Changuinola viruses provides evidence for co-evolution of orbiviruses with their arthropod vectors. PLoS ONE 2014, 9, e86392. [Google Scholar] [CrossRef] [PubMed]
- Belaganahalli, M.N.; Maan, S.; Maan, N.S.; Brownlie, J.; Tesh, R.; Attoui, H.; Mertens, P.P. Genetic characterization of the tick-borne orbiviruses. Viruses 2015, 7, 2185–2209. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Silva, S.; Cruz, A.C.; da Silva, E.; Wanzeller, A.L.; Carvalho, V.; Chiang, J.; Martins, L. First Isolation and Molecular Characterization of Umatilla Virus (Sedoreoviridae, Orbivirus) in Brazil. Viruses 2024, 16, 1050. [Google Scholar] [CrossRef]
- Belhouchet, M.; Mohd Jaafar, F.; Tesh, R.; Grimes, J.; Maan, S.; Mertens, P.P.; Attoui, H. Complete sequence of Great Island virus and comparison with the T2 and outer-capsid proteins of Kemerovo, Lipovnik and Tribec viruses (genus Orbivirus, family Reoviridae). J. Gen. Virol. 2010, 91, 2985–2993. [Google Scholar] [CrossRef]
- Belaganahalli, M.N.; Maan, S.; Maan, N.S.; Tesh, R.; Attoui, H.; Mertens, P.P. Umatilla virus genome sequencing and phylogenetic analysis: Identification of stretch lagoon orbivirus as a new member of the Umatilla virus species. PLoS ONE 2011, 6, e23605. [Google Scholar] [CrossRef]
- Attoui, H.; Mendez-Lopez, M.R.; Rao, S.; Hurtado-Alendes, A.; Lizaraso-Caparo, F.; Mohd Jaafar, F.; Samuel, A.R.; Belhouchet, M.; Pritchard, L.I.; Melville, L.; et al. Peruvian horse sickness virus and Yunnan orbivirus, isolated from vertebrates and mosquitoes in Peru and Australia. Virology 2009, 394, 298–310. [Google Scholar] [CrossRef]
- Attoui, H.; Jaafar, F.M.; Belhouchet, M.; Aldrovandi, N.; Tao, S.; Chen, B.; Liang, G.; Tesh, R.B.; de Micco, P.; de Lamballerie, X. Yunnan orbivirus, a new orbivirus species isolated from Culex tritaeniorhynchus mosquitoes in China. J. Gen. Virol. 2005, 86, 3409–3417. [Google Scholar] [CrossRef]
- Maclachlan, N.J.; Guthrie, A.J. Re-emergence of bluetongue, African horse sickness, and other orbivirus diseases. Vet. Res. 2010, 41, 35. [Google Scholar] [CrossRef]
- Mirolo, M.; de le Roi, M.; von Dörnberg, K.; Kaiser, F.; Fayyad, A.; Puff, C.; Voigt, U.; Siebert, U.; Ludlow, M.; Baumgärtner, W.; et al. Umatilla Virus in Zoo-Dwelling Cape Penguins with Hepatitis, Germany. Emerg. Infect. Dis. 2024, 30, 2643–2646. [Google Scholar] [CrossRef]
- Cowled, C.; Palacios, G.; Melville, L.; Weir, R.; Walsh, S.; Davis, S.; Gubala, A.; Lipkin, W.I.; Briese, T.; Boyle, D. Genetic and epidemiological characterization of Stretch Lagoon orbivirus, a novel orbivirus isolated from Culex and Aedes mosquitoes in northern Australia. J. Gen. Virol. 2009, 90, 1433–1439. [Google Scholar] [CrossRef]
- Ejiri, H.; Kuwata, R.; Tsuda, Y.; Sasaki, T.; Kobayashi, M.; Sato, Y.; Sawabe, K.; Isawa, H. First isolation and characterization of a mosquito-borne orbivirus belonging to the species Umatilla virus in East Asia. Arch. Virol. 2014, 159, 2675–2685. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.L.; Wu, H.Y. Classification and Identification of Important Medical Insects of China; Henan Science and Technology Publishing House: Zhengzhou, China, 2003. [Google Scholar]
- Yu, L.Q.; Han, K.; Chen, H.; Wang, R.C.; Zhang, S.B.; Ma, J.T.; Yin, Q.K.; Cui, Q.Q.; Nie, K.; Fu, S.H. A Survey of Six Mosquito-Borne Viruses in the Ningxia Hui Autonomous Region in 2023. Chin. J. Virol. 2025, 41, 507–514. [Google Scholar] [CrossRef]
- Hu, D.; Wu, C.; Wang, R.; Yao, X.; Nie, K.; Lyu, Q.; Fu, S.; Yin, Q.; Su, W.; Li, F.; et al. Persistence of Tembusu Virus in Culex tritaeniorhynchus in Yunnan Province, China. Pathogens 2023, 12, 490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, F.; Liu, A.; Lin, X.; Fu, S.; Song, J.; Liu, G.; Shao, N.; Tao, Z.; Wang, Q.; et al. Identification and genetic analysis of Kadipiro virus isolated in Shandong province, China. Virol. J. 2018, 15, 64. [Google Scholar] [CrossRef]
- Yang, Y.H.; Li, J.X.; Wang, R.C.; Yin, Q.K.; Fu, S.H.; Nie, K.; Cui, Q.Q.; Xu, S.T.; Wei, Q.; Li, F.; et al. Isolation and characterization of a novel coltivirus from Haemaphysalis concinna ticks in Northeastern China. Infect. Med. 2025, 4, 100179. [Google Scholar] [CrossRef] [PubMed]
- Chen, S. Ultrafast one-pass FASTQ data preprocessing, quality control, and deduplication using fastp. Imeta 2023, 2, e107. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.M.; Luo, R.; Sadakane, K.; Lam, T.W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Oberc, C.; Li, P.C.H. Next-generation DNA sequencing of Panax samples revealed new genotypes: Burrows-Wheeler Aligner, Python-based abundance and clustering analysis. Heliyon 2024, 10, e29104. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H.M.; Guo, X.X.; Song, X.; Wang, H.Y.; Cheng, P.; Wang, H.F.; Wang, H.W.; Gong, M.Q. Molecular cloning and expression analysis of cuticular protein gene CpCPR117 in Culex pipiens pallens. Chin. J. Vector Biol. Control 2021, 32, 436–440. [Google Scholar] [CrossRef]
- Santos, P.D.; Ziegler, U.; Szillat, K.P.; Szentiks, C.A.; Strobel, B.; Skuballa, J.; Merbach, S.; Grothmann, P.; Tews, B.A.; Beer, M.; et al. In action-an early warning system for the detection of unexpected or novel pathogens. Virus Evol. 2021, 7, veab085. [Google Scholar] [CrossRef]
- Tangudu, C.S.; Charles, J.; Hurt, S.L.; Dunphy, B.M.; Smith, R.C.; Bartholomay, L.C.; Blitvich, B.J. Skunk River virus, a novel orbivirus isolated from Aedes trivittatus in the United States. J. Gen. Virol. 2019, 100, 295–300. [Google Scholar] [CrossRef]
- Georgopoulou, I.; Tsiouris, V. The potential role of migratory birds in the transmission of zoonoses. Vet. Ital. 2008, 44, 671–677. [Google Scholar]
- Belaganahalli, M.N.; Maan, S.; Maan, N.S.; Nomikou, K.; Guimera, M.; Brownlie, J.; Tesh, R.; Attoui, H.; Mertens, P.P. Full genome sequencing of Corriparta virus, identifies California mosquito pool virus as a member of the Corriparta virus species. PLoS ONE 2013, 8, e70779. [Google Scholar] [CrossRef]
- Cooper, E.; Anbalagan, S.; Klumper, P.; Scherba, G.; Simonson, R.R.; Hause, B.M. Mobuck virus genome sequence and phylogenetic analysis: Identification of a novel Orbivirus isolated from a white-tailed deer in Missouri, USA. J. Gen. Virol. 2014, 95, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xing, D.; Su, D.; Wang, D.; Gao, H.; Lan, C.; Gu, Z.; Zhao, T.; Li, C. Transcriptome Analysis of Responses to Dengue Virus 2 Infection in Aedes albopictus (Skuse) C6/36 Cells. Viruses 2021, 13, 343. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Gao, S.; Tian, Z.; Guo, Y.; Kang, D.; Xing, S.; Zhang, G.; Liu, G.; Luo, J.; Chang, H.; et al. Transcriptome analysis of responses to bluetongue virus infection in Aedes albopictus cells. BMC Microbiol. 2019, 19, 121. [Google Scholar] [CrossRef] [PubMed]
- Brackney, D.E.; Scott, J.C.; Sagawa, F.; Woodward, J.E.; Miller, N.A.; Schilkey, F.D.; Mudge, J.; Wilusz, J.; Olson, K.E.; Blair, C.D.; et al. C6/36 Aedes albopictus cells have a dysfunctional antiviral RNA interference response. PLoS Negl. Trop. Dis. 2010, 4, e856. [Google Scholar] [CrossRef]
- Jiang, H.; Kan, X.; Ding, C.; Sun, Y. The Multi-Faceted Role of Autophagy During Animal Virus Infection. Front. Cell Infect. Microbiol. 2022, 12, 858953. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, Y.; Huang, J.; Li, J. Mitophagy in Antiviral Immunity. Front. Cell Dev. Biol. 2021, 9, 723108. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Kircheis, R.; Planz, O. The Role of Toll-like Receptors (TLRs) and Their Related Signaling Pathways in Viral Infection and Inflammation. Int. J. Mol. Sci. 2023, 24, 6701. [Google Scholar] [CrossRef]
- Wilson, D.F. Oxidative phosphorylation: Regulation and role in cellular and tissue metabolism. J. Physiol. 2017, 595, 7023–7038. [Google Scholar] [CrossRef]
- Rothan, H.A.; Fang, S.; Mahesh, M.; Byrareddy, S.N. Zika Virus and the Metabolism of Neuronal Cells. Mol. Neurobiol. 2019, 56, 2551–2557. [Google Scholar] [CrossRef] [PubMed]
- Gerresheim, G.K.; Roeb, E.; Michel, A.M.; Niepmann, M. Hepatitis C Virus Downregulates Core Subunits of Oxidative Phosphorylation, Reminiscent of the Warburg Effect in Cancer Cells. Cells 2019, 8, 1410. [Google Scholar] [CrossRef] [PubMed]
| UMAV | The Primer and Probe Sequences (5′–3′) |
|---|---|
| Forward | TCCATGACTCTTGAGCCTGT |
| Reverse | TGTTTCAATCCTTGCACCGC |
| Probe | HEX-TGTCCGGATTCGTTGGCCCTCCA-BHQ2 |
| Segment | Genes | Next-Generation Sequencing | Putative Protein | |||
|---|---|---|---|---|---|---|
| The Ground Supernatant (bp) | Reads | The Cell Culture Supernatant (bp) | Reads | |||
| 1 | VP1 | 3912 | 4503 | 3928 | 4,066,811 | RNA dependent RNA Polymerase (Pol) |
| 2 | VP2 | 2764 | 3400 | 2780 | 3,223,049 | Major subcore protein (T2) |
| 3 | VP3 | 2381 | 2735 | 2381 | 2,615,661 | Outer capsid protein (OCP1) |
| 4 | NS1 | 1999 | 2667 | 2018 | 2,477,484 | Tubule protein (TuP) |
| 5 | VP4 | 1978 | 3865 | 1994 | 2,244,156 | Minor core protein-capping enzyme (Cap) |
| 6 | VP5 | 1639 | 2161 | 1639 | 1,819,865 | Outer capsid protein (OCP2) |
| 7 | NS2 | 1308 | 1555 | 1324 | 1,623,891 | Viral inclusion body protein (ViP) |
| 8 | VP7 | 1156 | 1328 | 1157 | 1,379,620 | Major core-surface protein (T13) |
| 9 | VP6 | 1052 | 1588 | 1072 | 1,284,522 | Minor core protein-helicase enzyme (Hel) |
| 10 | NS3 | 867 | 858 | 873 | 1,247,249 | Virus release protein (VRP) |
| Virus Strains | Nucleotide Sequence Identity (%) | |||||||||
| NX23166 | Seg1 | Seg2 | Seg3 | Seg4 | Seg5 | Seg6 | Seg7 | Seg8 | Seg9 | Seg10 |
| DH13M98 | 94.87 | 90.11 | 55.67 | 97.16 | 90.40 | 94.46 | 93.41 | 95.67 | 96.64 | 97.11 |
| M4941_15 | 95.06 | 95.26 | 53.28 | 89.36 | 94.67 | 84.67 | 93.88 | 95.41 | 96.93 | 95.36 |
| ED-I-87-19 | 88.12 | 90.34 | 52.94 | 84.67 | 90.34 | 84.91 | 91.98 | 89.90 | 91.75 | 89.11 |
| ED-I-93-19 | 88.30 | 89.72 | 53.80 | 85.23 | 90.41 | 86.58 | 91.25 | 89.74 | 91.94 | 88.89 |
| ED-I-205-19 | 88.09 | 89.67 | 87.75 | 84.36 | 90.34 | 84.90 | 91.90 | 89.61 | 91.55 | 88.75 |
| DE | 88.20 | 89.40 | 56.21 | 83.73 | 89.75 | 86.70 | 91.76 | 88.76 | 91.26 | 87.68 |
| 11RS20 | 85.65 | 87.51 | 54.46 | 86.38 | 95.95 | 82.76 | 85.88 | 98.01 | 96.64 | 87.98 |
| Av13752/An813360 | 75.26 | 78.30 | 53.52 | 71.70 | 76.00 | 74.72 | 72.02 | 80.96 | 73.03 | 66.91 |
| Av15707/An862820 | 75.26 | 78.24 | 56.61 | 72.40 | 76.85 | 76.66 | 71.61 | 81.80 | 73.64 | 66.50 |
| USA1969/01 | 76.38 | 78.23 | 53.73 | 71.96 | 72.04 | 75.18 | 72.07 | 81.89 | 74.38 | 67.89 |
| Virus Strains | Amino Acid Sequence Identity (%) | |||||||||
| NX23166 | VP1 | VP2 | VP3 | NS1 | VP4 | VP5 | NS2 | VP7 | VP6 | NS3 |
| DH13M98 | 99.00 | 99.24 | 49.24 | 98.95 | 97.04 | 99.26 | 99.29 | 99.48 | 96.25 | 98.25 |
| M4941_15 | 99.00 | 99.67 | 47.44 | 98.50 | 97.73 | 96.67 | 99.53 | 99.74 | 97.12 | 98.60 |
| ED-I-87-19 | 98.16 | 99.24 | 47.38 | 96.68 | 97.02 | 97.41 | 99.05 | 97.10 | 91.64 | 97.53 |
| ED-I-93-19 | 98.08 | 98.80 | 48.85 | 96.70 | 96.24 | 97.60 | 99.05 | 97.13 | 91.64 | 97.20 |
| ED-I-205-19 | 98.08 | 99.01 | 94.00 | 96.36 | 96.72 | 96.11 | 99.05 | 97.09 | 91.35 | 96.72 |
| DE | 98.23 | 98.87 | 51.49 | 96.49 | 96.17 | 97.23 | 99.01 | 96.77 | 90.96 | 97.98 |
| 11RS20 | 97.55 | 98.92 | 48.03 | 96.71 | 97.35 | 94.82 | 94.34 | 99.74 | 95.68 | 97.55 |
| Av13752/An813360 | 89.33 | 95.35 | 49.15 | 78.88 | 86.35 | 86.43 | 79.62 | 91.83 | 64.62 | 66.79 |
| Av15707/An862820 | 89.10 | 94.75 | 50.89 | 80.00 | 86.18 | 88.17 | 79.42 | 92.16 | 65.99 | 67.44 |
| USA1969/01 | 89.50 | 95.12 | 47.79 | 79.64 | 80.18 | 89.09 | 80.19 | 91.15 | 66.28 | 69.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, K.; Yang, Y.; Wang, L.; Yu, L.; Wang, R.; Gu, X.; Li, F.; Yin, Q.; Fu, S.; Nie, K.; et al. Genomic and Transcriptomic Characterization of Umatilla Virus Isolated and Identified from Mosquitoes in Ningxia, China. Microorganisms 2025, 13, 2717. https://doi.org/10.3390/microorganisms13122717
Han K, Yang Y, Wang L, Yu L, Wang R, Gu X, Li F, Yin Q, Fu S, Nie K, et al. Genomic and Transcriptomic Characterization of Umatilla Virus Isolated and Identified from Mosquitoes in Ningxia, China. Microorganisms. 2025; 13(12):2717. https://doi.org/10.3390/microorganisms13122717
Chicago/Turabian StyleHan, Kun, Yuhong Yang, Long Wang, Liqin Yu, Ruichen Wang, Xiaoyu Gu, Fan Li, Qikai Yin, Shihong Fu, Kai Nie, and et al. 2025. "Genomic and Transcriptomic Characterization of Umatilla Virus Isolated and Identified from Mosquitoes in Ningxia, China" Microorganisms 13, no. 12: 2717. https://doi.org/10.3390/microorganisms13122717
APA StyleHan, K., Yang, Y., Wang, L., Yu, L., Wang, R., Gu, X., Li, F., Yin, Q., Fu, S., Nie, K., Cui, Q., Xu, S., & Wang, H. (2025). Genomic and Transcriptomic Characterization of Umatilla Virus Isolated and Identified from Mosquitoes in Ningxia, China. Microorganisms, 13(12), 2717. https://doi.org/10.3390/microorganisms13122717







