Recycling of Undigested Proteins Provided by the Host to the Large Intestine Microbiota: Implication for Intestinal Bacterial Anabolism, Growth, and Physiology
Abstract
1. Introduction
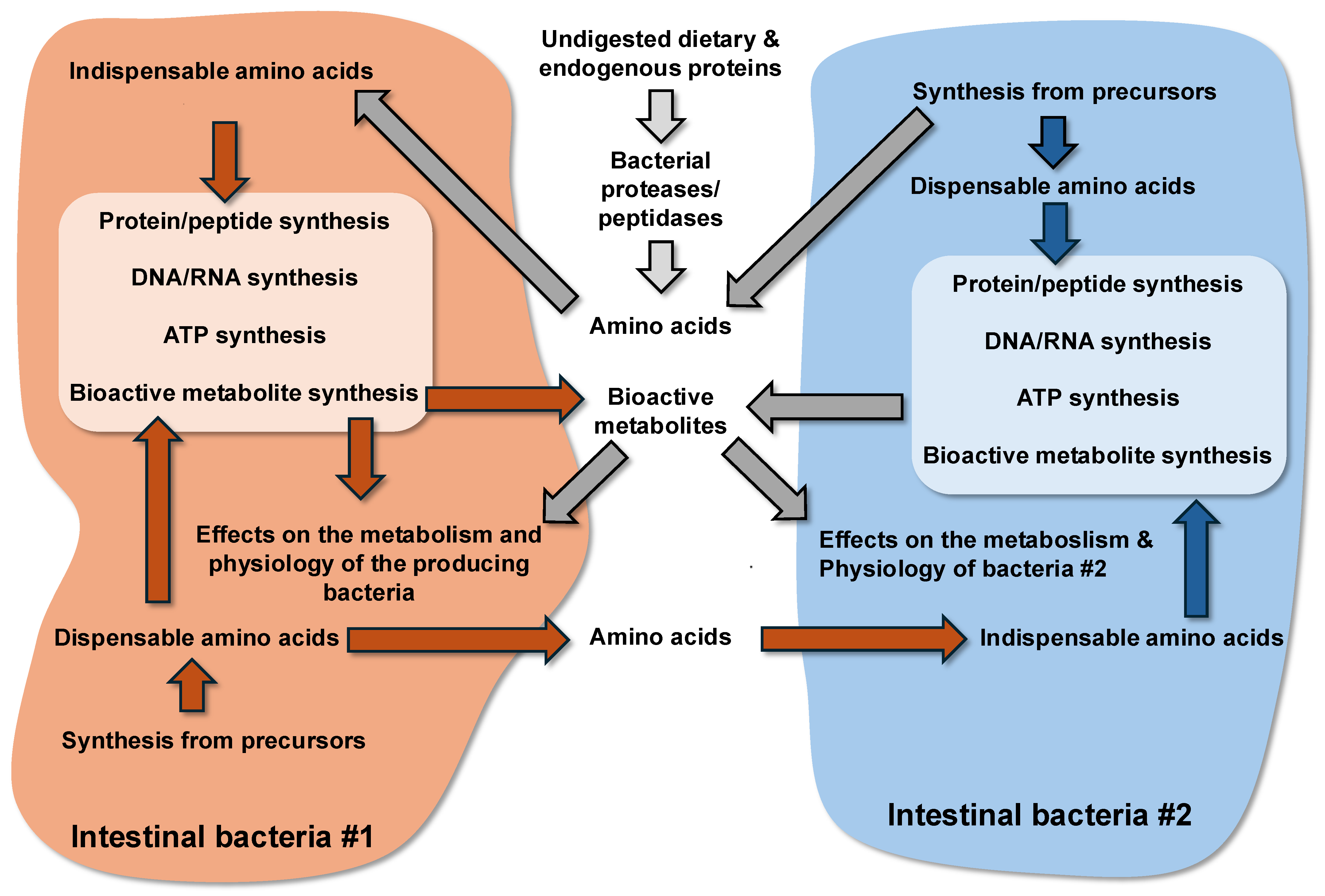
2. Origin of Proteins Available for the Large Intestine Microbiota
3. Metabolism of Proteins by the Large Intestine Bacteria
3.1. Degradation of Proteins by the Bacterial Proteases and Peptidases, and Transport of Peptides and Amino Acids in Bacteria
3.2. The Indispensable Amino Acids for Intestinal Bacterial Species
3.3. Utilization of Amino Acids for Synthesis of Macromolecules in Bacteria
3.4. Utilization of Amino Acids for the Synthesis of ATP in Bacteria
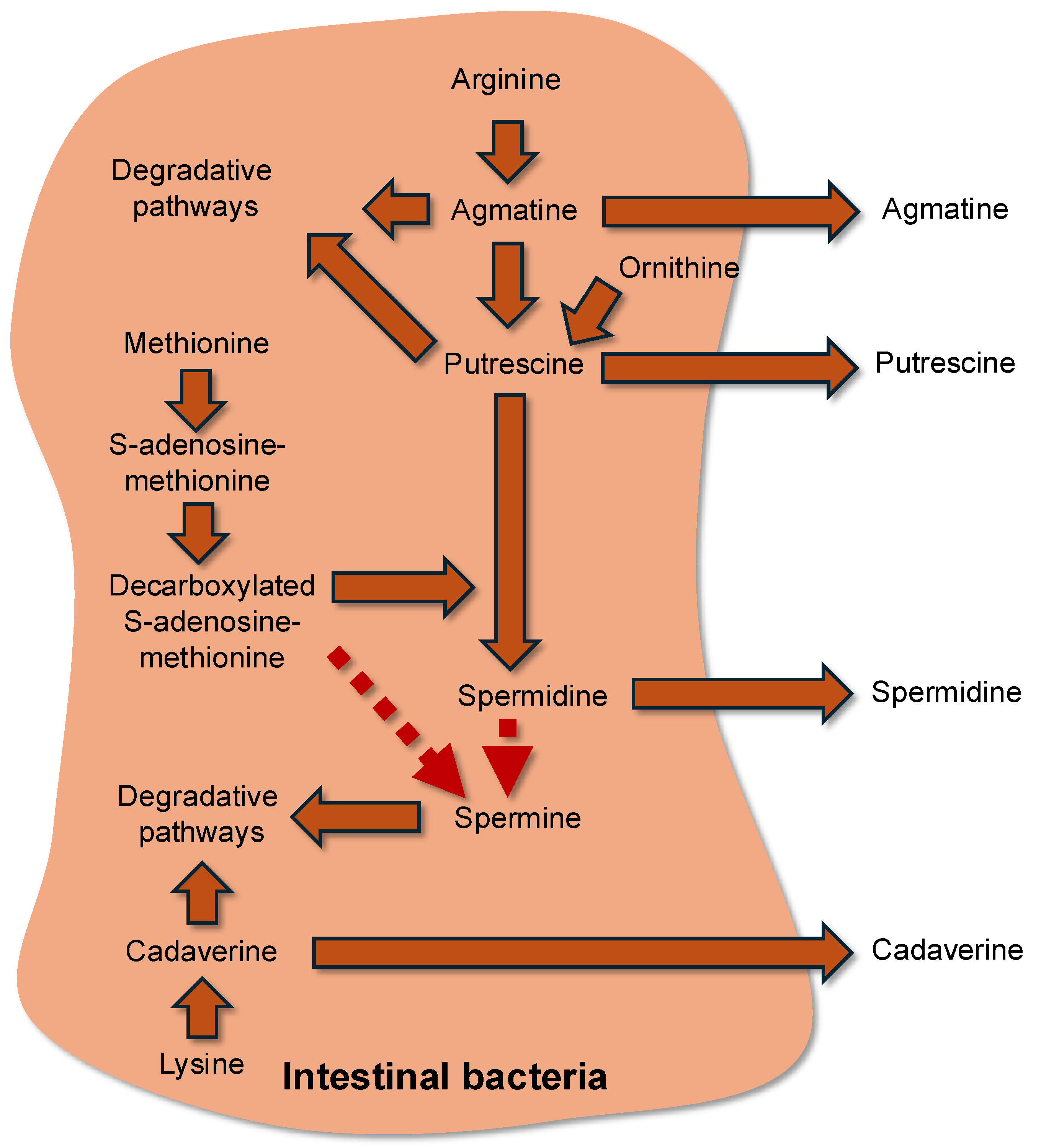
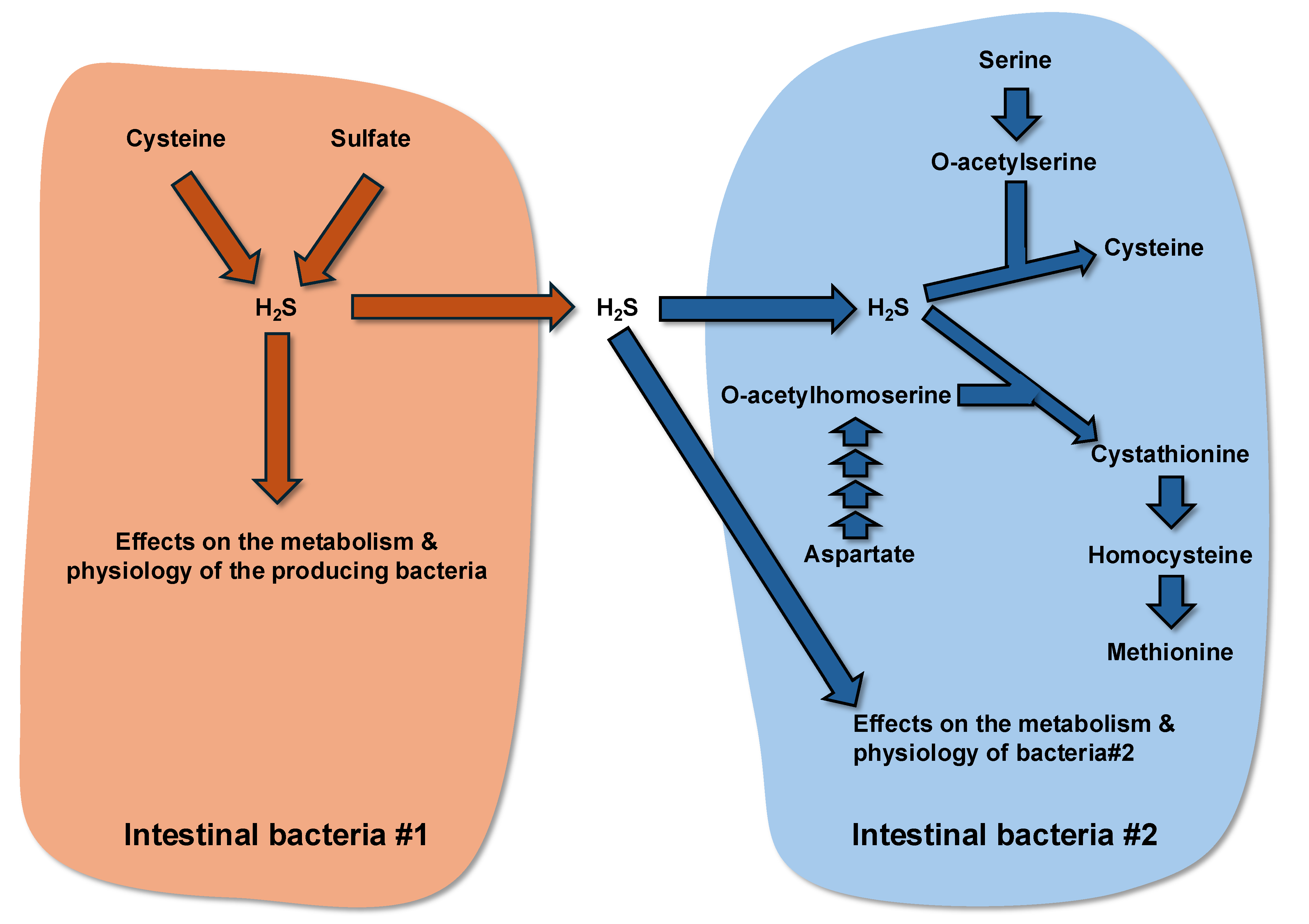
3.5. Amino Acid Utilization for the Synthesis of Bioactive Metabolites in Bacteria and Effects of These Compounds on Bacterial Growth and Physiology
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blachier, F. Metabolism of Alimentary Compounds by the Intestinal Microbiota and Health; Springer Nature: Cham, Switzerland, 2023. [Google Scholar]
- Schleifer, K.H. Classification of bacteria and archaea: Past, present and future. Syst. Appl. Microbiol. 2009, 32, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Matijašić, M.; Meštrović, T.; Paljetak, H.Č.; Perić, M.; Barešić, A.; Verbanac, D. Gut microbiota beyond bacteria-mycobiome, virome, archaeome, and eukaryotic parasites in IBD. Int. J. Mol. Sci. 2020, 21, 2668. [Google Scholar] [CrossRef] [PubMed]
- Rigottier-Gois, L. Dysbiosis in inflammatory bowel diseases: The oxygen hypothesis. ISME J. 2013, 7, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Carding, S.R.; Davis, N.; Hoyles, L. Review article: The human intestinal virome in health and disease. Aliment. Pharmacol. Ther. 2017, 46, 800–815. [Google Scholar] [CrossRef]
- Sausset, R.; Petit, M.A.; Gaboriau-Routhiau, V.; De Paepe, M. New insights into intestinal phages. Mucosal Immunol. 2020, 13, 205–215, Erratum in Mucosal Immunol. 2020, 13, 559. [Google Scholar] [CrossRef]
- Sartor, R.B.; Wu, G.D. Roles for Intestinal Bacteria, Viruses, and Fungi in Pathogenesis of Inflammatory Bowel Diseases and Therapeutic Approaches. Gastroenterology 2017, 152, 327–339. [Google Scholar] [CrossRef]
- Burgess, S.L.; Gilchrist, C.A.; Lynn, T.C.; Petri, W.A., Jr. Parasitic Protozoa and Interactions with the Host Intestinal Microbiota. Infect. Immun. 2017, 85, e00101-17. [Google Scholar] [CrossRef]
- Marteau, P.; Pochart, P.; Doré, J.; Béra-Maillet, C.; Bernalier, A.; Corthier, G. Comparative study of bacterial groups within the human cecal and fecal microbiota. Appl. Environ. Microbiol. 2001, 67, 4939–4942. [Google Scholar] [CrossRef]
- Stephen, A.M.; Cummings, J.H. The microbial contribution to human faecal mass. J. Med. Microbiol. 1980, 13, 45–56. [Google Scholar] [CrossRef]
- Stephen, A.M.; Wiggins, H.S.; Cummings, J.H. Effect of changing transit time on colonic microbial metabolism in man. Gut 1987, 28, 601–609. [Google Scholar] [CrossRef]
- Bharucha, A.E.; Anderson, B.; Bouchoucha, M. More movement with evaluating colonic transit in humans. Neurogastroenterol. Motil. 2019, 31, e13541. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–589. [Google Scholar] [CrossRef]
- Winter, S.E.; Bäumler, A.J. Why related bacterial species bloom simultaneously in the gut: Principles underlying the ‘Like will to like’ concept. Cell. Microbiol. 2014, 16, 179–184. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Cummings, J.H. The colonic flora, fermentation, and large bowel digestive function. In The Large Intestine: Physiology, Pathophysiology, and Disease; Phillips, S.F., Pemberton, J.H., Shorter, R.G., Eds.; Raven Press: New York, NY, USA, 1991. [Google Scholar]
- Windey, K.; De Preter, V.; Verbeke, K. Relevance of protein fermentation to gut health. Mol. Nutr. Food Res. 2012, 56, 184–196. [Google Scholar] [CrossRef]
- Korpela, K. Diet, Microbiota, and Metabolic Health: Trade-Off Between Saccharolytic and Proteolytic Fermentation. Annu. Rev. Food Sci. Technol. 2018, 9, 65–84. [Google Scholar] [CrossRef]
- Smith, E.A.; Macfarlane, G.T. Enumeration of human colonic bacteria producing phenolic and indolic compounds: Effects of pH, carbohydrate availability and retention time on dissimilatory aromatic amino acid metabolism. J. Appl. Bacteriol. 1996, 81, 288–302. [Google Scholar] [CrossRef]
- Birkett, A.; Muir, J.; Phillips, J.; Jones, G.; O’Dea, K. Resistant starch lowers fecal concentrations of ammonia and phenols in humans. Am. J. Clin. Nutr. 1996, 63, 766–772. [Google Scholar] [CrossRef]
- Geboes, K.P.; De Hertogh, G.; De Preter, V.; Luypaerts, A.; Bammens, B.; Evenepoel, P.; Ghoos, Y.; Geboes, K.; Rutgeerts, P.; Verbeke, K. The influence of inulin on the absorption of nitrogen and the production of metabolites of protein fermentation in the colon. Br. J. Nutr. 2006, 96, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Hill, M.J.; Bone, E.S.; Branch, W.J.; Jenkins, D.J. The effect of meat protein and dietary fiber on colonic function and metabolism. II. Bacterial metabolites in feces and urine. Am. J. Clin. Nutr. 1979, 32, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Cummings, J.H.; Macfarlane, S.; Gibson, G.R. Influence of retention time on degradation of pancreatic enzymes by human colonic bacteria grown in a 3-stage continuous culture system. J. Appl. Bacteriol. 1989, 67, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Blachier, F. Amino acid metabolism for bacterial physiology. In The Evolutionary Journey of Amino Acids. From the Origin of Life to Human Metabolism; Springer Nature: Cham, Switzerland, 2025. [Google Scholar]
- Bröer, S. Intestinal Amino Acid Transport and Metabolic Health. Annu. Rev. Nutr. 2023, 43, 73–99. [Google Scholar] [CrossRef]
- Gaudichon, C.; Bos, C.; Morens, C.; Petzke, K.J.; Mariotti, F.; Everwand, J.; Benamouzig, R.; Daré, S.; Tomé, D.; Metges, C.C. Ileal losses of nitrogen and amino acids in humans and their importance to the assessment of amino acid requirements. Gastroenterology 2002, 123, 50–59. [Google Scholar] [CrossRef]
- Blachier, F.; Andriamihaja, M.; Kong, X.F. Fate of undigested proteins in the pig large intestine: What impact on the colon epithelium? Anim. Nutr. 2021, 9, 110–118. [Google Scholar] [CrossRef]
- Baglieri, A.; Mahe, S.; Zidi, S.; Huneau, J.F.; Thuillier, F.; Marteau, P.; Tome, D. Gastro-jejunal digestion of soya-bean-milk protein in humans. Br. J. Nutr. 1994, 72, 519–532. [Google Scholar] [CrossRef]
- Bos, C.; Juillet, B.; Fouillet, H.; Turlan, L.; Daré, S.; Luengo, C.; N’tounda, R.; Benamouzig, R.; Gausserès, N.; Tomé, D.; et al. Postprandial metabolic utilization of wheat protein in humans. Am. J. Clin. Nutr. 2005, 81, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.K.; Muir, J.G.; Gibson, P.R. Review article: Insights into colonic protein fermentation, its modulation and potential health implications. Aliment. Pharmacol. Ther. 2016, 43, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.A.; Sladen, G.E.; Dawson, A.M. Protein absorption and ammonia production: The effects of dietary protein and removal of the colon. Br. J. Nutr. 1976, 35, 61–65. [Google Scholar] [CrossRef]
- Kramer, P. The effect of varying sodium loads on the ileal excreta of human ileostomized subjects. J. Clin. Investig. 1966, 45, 1710–1718. [Google Scholar] [CrossRef]
- Smiddy, F.G.; Gregory, S.D.; Smith, I.B.; Goligher, J. Faecal loss of fluid, electrolytes, and nitrogen in colitis before and after ileostomy. Lancet 1960, 1, 14–19. [Google Scholar] [CrossRef]
- Chacko, A.; Cummings, J.H. Nitrogen losses from the human small bowel: Obligatory losses and the effect of physical form of food. Gut 1988, 29, 809–815. [Google Scholar] [CrossRef]
- Dubuisson, C.; Lioret, S.; Touvier, M.; Dufour, A.; Calamassi-Tran, G.; Volatier, J.L.; Lafay, L. Trends in food and nutritional intakes of French adults from 1999 to 2007: Results from the INCA surveys. Br. J. Nutr. 2010, 103, 1035–1048. [Google Scholar] [CrossRef] [PubMed]
- Pasiakos, S.M.; Agarwal, S.; Lieberman, H.R.; Fulgoni, V.L., 3rd. Sources and Amounts of Animal, Dairy, and Plant Protein Intake of US Adults in 2007–2010. Nutrients 2015, 7, 7058–7069. [Google Scholar] [CrossRef] [PubMed]
- Blachier, F. Amino acid metabolism in the large intestine and physiological consequences. In The Evolutionary Journey of Amino Acids. From the Origin of Life to Human Metabolism; Springer Nature: Cham, Switzerland, 2025. [Google Scholar]
- Kaman, W.E.; Hays, J.P.; Endtz, H.P.; Bikker, F.J. Bacterial proteases: Targets for diagnostics and therapy. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1081–1087. [Google Scholar] [CrossRef]
- Portune, K.; Beaumont, M.; Davila, A.M.; Tomé, D.; Blachier, F.; Sanz, Y. Gut microbiota role in protein metabolism and health-related outcomes: The two side of the coin. Trends Food Sci. Technol. 2016, 57, 213–232. [Google Scholar] [CrossRef]
- Pessione, E. Lactic acid bacteria contribution to gut microbiota complexity: Lights and shadows. Front. Cell. Infect. Microbiol. 2012, 2, 86. [Google Scholar] [CrossRef]
- Liu, M.; Bayjanov, J.R.; Renckens, B.; Nauta, A.; Siezen, R.J. The proteolytic system of lactic acid bacteria revisited: A genomic comparison. BMC Genom. 2010, 11, 36. [Google Scholar] [CrossRef]
- Saier, M.H., Jr. Families of transmembrane transporters selective for amino acids and their derivatives. Microbiology 2000, 146, 1775–1795. [Google Scholar] [CrossRef]
- Tanaka, K.J.; Song, S.; Mason, K.; Pinkett, H.W. Selective substrate uptake: The role of ATP-binding cassette (ABC) importers in pathogenesis. Biochim. Biophys. Acta Biomembr. 2018, 1860, 868–877. [Google Scholar] [CrossRef]
- Hosie, A.H.; Poole, P.S. Bacterial ABC transporters of amino acids. Res. Microbiol. 2001, 152, 259–270. [Google Scholar] [CrossRef]
- Burkovski, A.; Krämer, R. Bacterial amino acid transport proteins: Occurrence, functions, and significance for biotechnological applications. Appl. Microbiol. Biotechnol. 2002, 58, 265–274. [Google Scholar] [CrossRef]
- Konings, W.N. The cell membrane and the struggle for life of lactic acid bacteria. Antonie Van Leeuwenhoek 2002, 82, 3–27. [Google Scholar] [CrossRef]
- Chen, Y.; Dinges, M.M.; Green, A.; Cramer, S.E.; Larive, C.K.; Lytle, C. Absorptive transport of amino acids by the rat colon. Am. J. Physiol. 2020, 318, G189–G202. [Google Scholar] [CrossRef] [PubMed]
- van der Wielen, N.; Moughan, P.J.; Mensink, M. Amino acid absorption in the large intestine of humans and porcine models. J. Nutr. 2017, 147, 1493–1498. [Google Scholar] [CrossRef] [PubMed]
- Fuller, M. Determination of protein and amino acid digestibility in foods including implications of gut microbial amino acid synthesis. Br. J. Nutr. 2012, 108, S238–S246. [Google Scholar] [CrossRef]
- Darragh, A.J.; Cranwell, P.D.; Moughan, P.J. Absorption of lysine and methionine from the proximal colon of the piglet. Br. J. Nutr. 1994, 71, 739–752. [Google Scholar] [CrossRef]
- Schaafsma, G. The protein digestibility-corrected amino acid score. J. Nutr. 2000, 130, 1865S–1867S. [Google Scholar] [CrossRef]
- Metges, C.C. Contribution of microbial amino acids to amino acid homeostasis of the host. J. Nutr. 2000, 130, 1857S–1864S. [Google Scholar] [CrossRef]
- Fuller, M.F.; Reeds, P.J. Nitrogen cycling in the gut. Annu. Rev. Nutr. 1998, 18, 385–411. [Google Scholar] [CrossRef]
- Niiyama, M.; Deguchi, E.; Kagota, K.; Namioka, S. Appearance of 15N-labeled intestinal microbial amino acids in the venous blood of the pig colon. Am. J. Vet. Res. 1979, 40, 716–718. [Google Scholar] [CrossRef]
- Nakanishi, T.; Hatanaka, T.; Huang, W.; Prasad, P.D.; Leibach, F.H.; Ganapathy, M.E.; Ganapathy, V. Na+- and Cl−-coupled active transport of carnitine by the amino acid transporter ATB(0,+) from mouse colon expressed in HRPE cells and Xenopus oocytes. J. Physiol. 2001, 532, 297–304. [Google Scholar] [CrossRef]
- Hatanaka, T.; Huang, W.; Nakanishi, T.; Bridges, C.C.; Smith, S.B.; Prasad, P.D.; Ganapathy, M.E.; Ganapathy, V. Transport of D-serine via the amino acid transporter ATB(0,+) expressed in the colon. Biochem. Biophys. Res. Commun. 2002, 291, 291–295. [Google Scholar] [CrossRef]
- Ugawa, S.; Sunouchi, Y.; Ueda, T.; Takahashi, E.; Saishin, Y.; Shimada, S. Characterization of a mouse colonic system B(0+) amino acid transporter related to amino acid absorption in colon. Am. J. Physiol. 2001, 281, G365–G370. [Google Scholar] [CrossRef] [PubMed]
- Blachier, F.; Mariotti, F.; Huneau, J.F.; Tomé, D. Effects of amino acid-derived luminal metabolites on the colonic epithelium and physiopathological consequences. Amino Acids 2007, 33, 547–562. [Google Scholar] [CrossRef] [PubMed]
- James, P.S.; Smith, M.W. Methionine transport by pig colonic mucosa measured during early post-natal development. J. Physiol. 1976, 262, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, F.V.; Smith, M.W. Different mechanisms for neutral amino acid uptake by new-born pig colon. J. Physiol. 1979, 286, 479–490. [Google Scholar] [CrossRef]
- Hou, Y.; Yin, Y.; Wu, G. Dietary essentiality of “nutritionally non-essential amino acids” for animals and humans. Exp. Biol. Med. 2015, 240, 997–1007. [Google Scholar] [CrossRef]
- Mathai, J.K.; Liu, Y.; Stein, H.H. Values for digestible indispensable amino acid scores (DIAAS) for some dairy and plant proteins may better describe protein quality than values calculated using the concept for protein digestibility-corrected amino acid scores (PDCAAS). Br. J. Nutr. 2017, 117, 490–499. [Google Scholar] [CrossRef]
- Detzner, J.; Pohlentz, G.; Müthing, J. Enterohemorrhagic Escherichia coli and a fresh view on Shiga toxin-binding glycosphingolipids of primary human kidney and colon epithelial cells and their toxin susceptibility. Int. J. Mol. Sci. 2022, 23, 6884. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Singhal, M.; Lin, Z.; Manzella, C.; Kumar, A.; Alrefai, W.A.; Dudeja, P.K.; Saksena, S.; Sun, J.; Gill, R.K. Infection with enteric pathogens Salmonella typhimurium and Citrobacter rodentium modulate TGF-beta/Smad signaling pathways in the intestine. Gut Microbes 2018, 9, 326–337. [Google Scholar]
- Jones, S.E.; Knight, K.L. Bacillus subtilis-mediated protection from Citrobacter rodentium-associated enteric disease requires espH and functional flagella. Infect. Immun. 2012, 80, 710–719. [Google Scholar] [CrossRef]
- Shrestha, A.; Mehdizadeh Gohari, I.; Li, J.; Navarro, M.; Uzal, F.A.; McClane, B.A. The biology and pathogenicity of Clostridium perfringens type F: A common human enteropathogen with a new(ish) name. Microbiol. Mol. Biol. Rev. 2024, 88, e0014023. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Ohtani, K.; Hirakawa, H.; Ohshima, K.; Yamashita, A.; Shiba, T.; Ogasawara, N.; Hattori, M.; Kuhara, S.; Hayashi, H. Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. Proc. Natl. Acad. Sci. USA 2002, 99, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Denou, E.; Rezzonico, E.; Panoff, J.M.; Arigoni, F.; Brüssow, H. A Mesocosm of Lactobacillus johnsonii, Bifidobacterium longum, and Escherichia coli in the mouse gut. DNA Cell. Biol. 2009, 28, 413–422. [Google Scholar] [CrossRef]
- Pridmore, R.D.; Berger, B.; Desiere, F.; Vilanova, D.; Barretto, C.; Pittet, A.C.; Zwahlen, M.C.; Rouvet, M.; Altermann, E.; Barrangou, R.; et al. The genome sequence of the probiotic intestinal bacterium Lactobacillus johnsonii NCC 533. Proc. Natl. Acad. Sci. USA 2004, 101, 2512–2517. [Google Scholar] [CrossRef]
- Young, K.T.; Davis, L.M.; Dirita, V.J. Campylobacter jejuni: Molecular biology and pathogenesis. Nat. Rev. Microbiol. 2007, 5, 665–679. [Google Scholar] [CrossRef]
- Fan, T.J.; Goeser, L.; Naziripour, A.; Redinbo, M.R.; Hansen, J.J. Enterococcus faecalis gluconate phosphotransferase system accelerates experimental colitis and bacterial killing by macrophages. Infect. Immun. 2019, 87, e00080-19. [Google Scholar] [CrossRef]
- Yu, X.J.; Walker, D.H.; Liu, Y.; Zhang, L. Amino acid biosynthesis deficiency in bacteria associated with human and animal hosts. Infect. Genet. Evol. 2009, 9, 514–517. [Google Scholar] [CrossRef]
- Gomes-Santos, A.C.; de Oliveira, R.P.; Moreira, T.G.; Castro-Junior, A.B.; Horta, B.C.; Lemos, L.; de Almeida, L.A.; Rezende, R.M.; Cara, D.C.; Oliveira, S.C.; et al. Hsp65-producing Lactococcus lactis prevents inflammatory intestinal disease in mice by IL-10- and TLR2-dependent pathways. Front. Immunol. 2017, 8, 30. [Google Scholar] [CrossRef]
- Bolotin, A.; Wincker, P.; Mauger, S.; Jaillon, O.; Malarme, K.; Weissenbach, J.; Ehrlich, S.D.; Sorokin, A. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 2001, 11, 731–753. [Google Scholar] [CrossRef]
- Godon, J.J.; Delorme, C.; Bardowski, J.; Chopin, M.C.; Ehrlich, S.D.; Renault, P. Gene inactivation in Lactococcus lactis: Branched-chain amino acid biosynthesis. J. Bacteriol. 1993, 175, 4383–4390. [Google Scholar] [CrossRef]
- Alsultan, A.; Walton, G.; Andrews, S.C.; Clarke, S.R. Staphylococcus aureus FadB is a dehydrogenase that mediates cholate resistance and survival under human colonic conditions. Microbiology 2023, 169, 001314. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.; Ohta, T.; Uchiyama, I.; Baba, T.; Yuzawa, H.; Kobayashi, I.; Cui, L.; Oguchi, A.; Aoki, K.; Nagai, Y.; et al. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet 2001, 357, 1225–1240. [Google Scholar] [CrossRef] [PubMed]
- Njenga, R.; Boele, J.; Öztürk, Y.; Koch, H.G. Coping with stress: How bacteria fine-tune protein synthesis and protein transport. J. Biol. Chem. 2023, 299, 105163. [Google Scholar] [CrossRef] [PubMed]
- Tollerson, R.; Ibba, M. Translational regulation of environmental adaptation in bacteria. J. Biol. Chem. 2020, 295, 10434–10445. [Google Scholar] [CrossRef]
- Macek, B.; Forchhammer, K.; Hardouin, J.; Weber-Ban, E.; Grangeasse, C.; Mijakovic, I. Protein post-translational modifications in bacteria. Nat. Rev. Microbiol. 2019, 17, 651–664. [Google Scholar] [CrossRef]
- Hu, Y.; Qing, Y.; Chen, J.; Liu, C.; Lu, J.; Wang, Q.; Zhen, S.; Zhou, H.; Huang, L.; Zhang, R. Prevalence, risk factors, and molecular epidemiology of intestinal carbapenem-resistant Pseudomonas aeruginosa. Microbiol. Spectr. 2021, 9, e0134421. [Google Scholar] [CrossRef]
- Zhang, J.; Hoedt, E.C.; Liu, Q.; Berendsen, E.; Teh, J.J.; Hamilton, A.; O’ Brien, A.W.; Ching, J.Y.L.; Wei, H.; Yang, K.; et al. Elucidation of Proteus mirabilis as a key bacterium in Crohn’s disease inflammation. Gastroenterology 2021, 160, 317–330.e11. [Google Scholar] [CrossRef]
- Charlier, D.; Nguyen Le Minh, P.; Roovers, M. Regulation of carbamoylphosphate synthesis in Escherichia coli: An amazing metabolite at the crossroad of arginine and pyrimidine biosynthesis. Amino Acids. 2018, 50, 1647–1661. [Google Scholar] [CrossRef]
- Leiva, L.E.; Zegarra, V.; Bange, G.; Ibba, M. At the crossroad of nucleotide dynamics and protein synthesis in bacteria. Microbiol. Mol. Biol. Rev. 2023, 87, e0004422. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Davila, A.M.; Blachier, F.; Gotteland, M.; Andriamihaja, M.; Benetti, P.H.; Sanz, Y.; Tomé, D. Intestinal luminal nitrogen metabolism: Role of the gut microbiota and consequences for the host. Pharmacol. Res. 2013, 68, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hetzel, M.; Boiangiu, C.D.; Buckel, W. Dehydration of (R)-2-hydroxyacyl-CoA to enoyl-CoA in the fermentation of alpha-amino acids by anaerobic bacteria. FEMS Microbiol. Rev. 2004, 28, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Buckel, W. Energy conservation in fermentations of anaerobic bacteria. Front. Microbiol. 2021, 12, 703525. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 2012, 95, 50–60. [Google Scholar] [CrossRef]
- Barker, H.A. Amino acid degradation by anaerobic bacteria. Annu. Rev. Biochem. 1981, 50, 23–40. [Google Scholar] [CrossRef]
- Pessione, A.; Lamberti, C.; Pessione, E. Proteomics as a tool for studying energy metabolism in lactic acid bacteria. Mol. Biosyst. 2010, 6, 1419–1430. [Google Scholar] [CrossRef]
- Fernández, M.; Zúñiga, M. Amino acid catabolic pathways of lactic acid bacteria. Crit. Rev. Microbiol. 2006, 32, 155–183. [Google Scholar] [CrossRef]
- Pereira, C.I.; Matos, D.; San Romão, M.V.; Crespo, M.T. Dual role for the tyrosine decarboxylation pathway in Enterococcus faecium E17: Response to an acid challenge and generation of a proton motive force. Appl. Environ. Microbiol. 2009, 75, 345–352. [Google Scholar] [CrossRef]
- Stickland, L.H. Studies in the metabolism of the strict anaerobes (Genus Clostridium): The reduction of proline by Cl. sporogenes. Biochem. J. 1935, 29, 288–290. [Google Scholar] [CrossRef]
- Marshall, A.; McGrath, J.W.; Graham, R.; McMullan, G. Food for thought-The link between Clostridioides difficile metabolism and pathogenesis. PLoS Pathog. 2023, 19, e1011034. [Google Scholar] [CrossRef]
- Pruss, K.M.; Enam, F.; Battaglioli, E.; DeFeo, M.; Diaz, O.R.; Higginbottom, S.K.; Fischer, C.R.; Hryckowian, A.J.; Van Treuren, W.; Dodd, D.; et al. Oxidative ornithine metabolism supports non-inflammatory C. difficile colonization. Nat. Metab. 2022, 4, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Pavao, A.; Graham, M.; Arrieta-Ortiz, M.L.; Immanuel, S.R.C.; Baliga, N.S.; Bry, L. Reconsidering the in vivo functions of Clostridial Stickland amino acid fermentations. Anaerobe 2022, 76, 102600. [Google Scholar] [CrossRef] [PubMed]
- Fonknechten, N.; Chaussonnerie, S.; Tricot, S.; Lajus, A.; Andreesen, J.R.; Perchat, N.; Pelletier, E.; Gouyvenoux, M.; Barbe, V.; Salanoubat, M.; et al. Clostridium sticklandii, a specialist in amino acid degradation: Revisiting its metabolism through its genome sequence. BMC Genom. 2010, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Blachier, F. Amino acid-derived bacterial metabolites in the colorectal luminal fluid: Effects on microbial communication, metabolism, physiology, and growth. Microorganisms 2023, 11, 1317. [Google Scholar] [CrossRef]
- Michael, A.J. Polyamines in eukaryotes, bacteria, and archaea. J. Biol. Chem. 2016, 291, 14896–14903. [Google Scholar] [CrossRef]
- Li, B.; Baniasadi, H.R.; Liang, J.; Phillips, M.A.; Michael, A.J. New routes for spermine biosynthesis. J. Biol. Chem. 2025, 301, 108390. [Google Scholar] [CrossRef]
- Shah, P.; Swiatlo, E. A multifaceted role for polyamines in bacterial pathogens. Mol. Microbiol. 2008, 68, 4–16. [Google Scholar] [CrossRef]
- Yoshida, M.; Kashiwagi, K.; Shigemasa, A.; Taniguchi, S.; Yamamoto, K.; Makinoshima, H.; Ishihama, A.; Igarashi, K. A unifying model for the role of polyamines in bacterial cell growth, the polyamine modulon. J. Biol. Chem. 2004, 279, 46008–46013. [Google Scholar] [CrossRef]
- Chattopadhyay, M.K.; Keembiyehetty, C.N.; Chen, W.; Tabor, H. Polyamines stimulate the level of the sigma38 subunit (RpoS) of Escherichia coli RNA polymerase, resulting in the induction of the glutamate decarboxylase-dependent acid response system via the gadE regulon. J. Biol. Chem. 2015, 290, 17809–17821. [Google Scholar] [CrossRef]
- Igarashi, K.; Kashiwagi, K. Effects of polyamines on protein synthesis and growth of Escherichia coli. J. Biol. Chem. 2018, 293, 18702–18709. [Google Scholar] [CrossRef]
- Igarashi, K.; Kashiwagi, K. Polyamine transport in bacteria and yeast. Biochem. J. 1999, 344, 633–642. [Google Scholar] [CrossRef]
- Driessen, A.J.; Smid, E.J.; Konings, W.N. Transport of diamines by Enterococcus faecalis is mediated by an agmatine-putrescine antiporter. J. Bacteriol. 1988, 170, 4522–4527. [Google Scholar] [CrossRef]
- Large, P.J. Enzymes and pathways of polyamine breakdown in microorganisms. FEMS Microbiol. Rev. 1992, 8, 249–262. [Google Scholar] [CrossRef]
- Chattopadhyay, M.K.; Tabor, C.W.; Tabor, H. Polyamines are not required for aerobic growth of Escherichia coli: Preparation of a strain with deletions in all of the genes for polyamine biosynthesis. J. Bacteriol. 2009, 191, 5549–5552. [Google Scholar] [CrossRef]
- Nakada, Y.; Itoh, Y. Identification of the putrescine biosynthetic genes in Pseudomonas aeruginosa and characterization of agmatine deiminase and N-carbamoylputrescine amidohydrolase of the arginine decarboxylase pathway. Microbiology 2003, 149, 707–714. [Google Scholar] [CrossRef]
- Hanfrey, C.C.; Pearson, B.M.; Hazeldine, S.; Lee, J.; Gaskin, D.J.; Woster, P.M.; Phillips, M.A.; Michael, A.J. Alternative spermidine biosynthetic route is critical for growth of Campylobacter jejuni and is the dominant polyamine pathway in human gut microbiota. J. Biol. Chem. 2011, 286, 43301–43312. [Google Scholar] [CrossRef] [PubMed]
- Chagneau, C.V.; Garcie, C.; Bossuet-Greif, N.; Tronnet, S.; Brachmann, A.O.; Piel, J.; Nougayrède, J.P.; Martin, P.; Oswald, E. The polyamine spermidine modulates the production of the bacterial genotoxin colibactin. mSphere 2019, 4, e00414-19. [Google Scholar] [CrossRef] [PubMed]
- Goforth, J.B.; Walter, N.E.; Karatan, E. Effects of polyamines on Vibrio cholerae virulence properties. PLoS ONE. 2013, 8, e60765. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.; Özkaya, Ö.; Kümmerli, R. Bacterial siderophores in community and host interactions. Nat. Rev. Microbiol. 2020, 18, 152–163. [Google Scholar] [CrossRef]
- Hamana, K.; Saito, T.; Okada, M.; Sakamoto, A.; Hosoya, R. Covalently linked polyamines in the cell wall peptidoglycan of Selenomonas, Anaeromusa, Dendrosporobacter, Acidaminococcus and Anaerovibrio belonging to the Sporomusa subbranch. J. Gen. Appl. Microbiol. 2002, 48, 177–180. [Google Scholar] [CrossRef]
- Samartzidou, H.; Mehrazin, M.; Xu, Z.; Benedik, M.J.; Delcour, A.H. Cadaverine inhibition of porin plays a role in cell survival at acidic pH. J. Bacteriol. 2003, 185, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kimura, B.; Takahashi, H.; Watanabe, T.; Obata, H.; Kai, A.; Morozumi, S.; Fujii, T. Lysine decarboxylase of Vibrio parahaemolyticus: Kinetics of transcription and role in acid resistance. J. Appl. Microbiol. 2008, 104, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Blachier, F.; Beaumont, M.; Andriamihaja, M.; Davila, A.M.; Lan, A.; Grauso, M.; Armand, L.; Benamouzig, R.; Tomé, D. Changes in the luminal environment of the colonic epithelial cells and physiopathological consequences. Am. J. Pathol. 2017, 187, 476–486. [Google Scholar] [CrossRef]
- Probert, H.M.; Gibson, G.R. Bacterial biofilms in the human gastrointestinal tract. Curr. Issues Intest. Microbiol. 2002, 3, 23–27. [Google Scholar]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef]
- Solano, C.; Echeverz, M.; Lasa, I. Biofilm dispersion and quorum sensing. Curr. Opin. Microbiol. 2014, 18, 96–104. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bassler, B.L. Bacterial quorum sensing in complex and dynamically changing environments. Nat. Rev. Microbiol. 2019, 17, 371–382. [Google Scholar] [CrossRef]
- Banerji, R.; Kanojiya, P.; Saroj, S.D. Role of interspecies bacterial communication in the virulence of pathogenic bacteria. Crit. Rev. Microbiol. 2020, 46, 136–146. [Google Scholar] [CrossRef]
- Burrell, M.; Hanfrey, C.C.; Murray, E.J.; Stanley-Wall, N.R.; Michael, A.J. Evolution and multiplicity of arginine decarboxylases in polyamine biosynthesis and essential role in Bacillus subtilis biofilm formation. J. Biol. Chem. 2010, 285, 39224–39238. [Google Scholar] [CrossRef] [PubMed]
- Prentice, J.A.; Bridges, A.A.; Bassler, B.L. Synergy between c-di-GMP and quorum-sensing signaling in Vibrio cholerae biofilm morphogenesis. J. Bacteriol. 2022, 204, e0024922. [Google Scholar] [CrossRef] [PubMed]
- Karatan, E.; Duncan, T.R.; Watnick, P.I. NspS, a predicted polyamine sensor, mediates activation of Vibrio cholerae biofilm formation by norspermidine. J. Bacteriol. 2005, 187, 7434–7443. [Google Scholar] [CrossRef]
- Lee, J.; Sperandio, V.; Frantz, D.E.; Longgood, J.; Camilli, A.; Phillips, M.A.; Michael, A.J. An alternative polyamine biosynthetic pathway is widespread in bacteria and essential for biofilm formation in Vibrio cholerae. J. Biol. Chem. 2009, 284, 9899–9907. [Google Scholar] [CrossRef]
- Sobe, R.C.; Bond, W.G.; Wotanis, C.K.; Zayner, J.P.; Burriss, M.A.; Fernandez, N.; Bruger, E.L.; Waters, C.M.; Neufeld, H.S.; Karatan, E. Spermine inhibits Vibrio cholerae biofilm formation through the NspS-MbaA polyamine signaling system. J. Biol. Chem. 2017, 292, 17025–17036. [Google Scholar] [CrossRef]
- Blachier, F.; Davila, A.M.; Mimoun, S.; Benetti, P.H.; Atanasiu, C.; Andriamihaja, M.; Benamouzig, R.; Bouillaud, F.; Tomé, D. Luminal sulfide and large intestine mucosa: Friend or foe? Amino Acids 2010, 39, 335–347. [Google Scholar] [CrossRef]
- Barton, L.L.; Ritz, N.L.; Fauque, G.D.; Lin, H.C. Sulfur cycling and the intestinal microbiome. Dig. Dis. Sci. 2017, 62, 2241–2257. [Google Scholar] [CrossRef]
- Basic, A.; Blomqvist, M.; Dahlén, G.; Svensäter, G. The proteins of Fusobacterium spp. involved in hydrogen sulfide production from L-cysteine. BMC Microbiol. 2017, 17, 61. [Google Scholar] [CrossRef]
- Linden, D.R. Hydrogen sulfide signaling in the gastrointestinal tract. Antioxid. Redox Signal. 2014, 20, 818–830. [Google Scholar] [CrossRef]
- Croix, J.A.; Carbonero, F.; Nava, G.M.; Russell, M.; Greenberg, E.; Gaskins, H.R. On the relationship between sialomucin and sulfomucin expression and hydrogenotrophic microbes in the human colonic mucosa. PLoS ONE 2011, 6, e24447. [Google Scholar] [CrossRef]
- Villanueva-Millan, M.J.; Leite, G.; Mathur, R.; Rezaie, A.; Fajardo, C.M.; de Freitas Germano, J.; Morales, W.; Sanchez, M.; Rivera, I.; Parodi, G.; et al. Hydrogen Sulfide and Methane on Breath Test Correlate with Human Small Intestinal Hydrogen Sulfide Producers and Methanogens. Dig. Dis. Sci. 2025, in press. [Google Scholar] [CrossRef]
- Gibson, G.R.; Macfarlane, G.T.; Cummings, J.H. Occurrence of sulphate-reducing bacteria in human faeces and the relationship of dissimilatory sulphate reduction to methanogenesis in the large gut. J. Appl. Bacteriol. 1988, 65, 103–111. [Google Scholar] [CrossRef]
- Ohge, H.; Furne, J.K.; Springfield, J.; Sueda, T.; Madoff, R.D.; Levitt, M.D. The effect of antibiotics and bismuth on fecal hydrogen sulfide and sulfate-reducing bacteria in the rat. FEMS Microbiol. Lett. 2003, 228, 137–142. [Google Scholar] [CrossRef]
- Rowan, F.E.; Docherty, N.G.; Coffey, J.C.; O’Connell, P.R. Sulphate-reducing bacteria and hydrogen sulphide in the aetiology of ulcerative colitis. Br. J. Surg. 2009, 96, 151–158. [Google Scholar] [CrossRef]
- Kushkevych, I.; Dordević, D.; Alberfkani, M.I.; Gajdács, M.; Ostorházi, E.; Vítězová, M.; Rittmann, S.K.R. NADH and NADPH peroxidases as antioxidant defense mechanisms in intestinal sulfate-reducing bacteria. Sci. Rep. 2023, 13, 13922. [Google Scholar] [CrossRef]
- Florin, T.; Neale, G.; Gibson, G.R.; Christl, S.U.; Cummings, J.H. Metabolism of dietary sulphate: Absorption and excretion in humans. Gut 1991, 32, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.; Cochrane, S. Alteration of sulfate and hydrogen metabolism in the human colon by changing intestinal transit rate. Am. J. Gastroenterol. 2007, 102, 624–633. [Google Scholar] [CrossRef]
- Blachier, F.; Andriamihaja, M.; Larraufie, P.; Ahn, E.; Lan, A.; Kim, E. Production of hydrogen sulfide by the intestinal microbiota and epithelial cells and consequences for the colonic and rectal mucosa. Am. J. Physiol. 2021, 320, G125–G135, Erratum in Am. J. Physiol. 2021, 320, G484. [Google Scholar] [CrossRef]
- Bouillaud, F.; Blachier, F. Mitochondria and sulfide: A very old story of poisoning, feeding, and signaling? Antioxid. Redox Signal. 2011, 15, 379–391. [Google Scholar] [CrossRef]
- Jørgensen, J.; Mortensen, P.B. Hydrogen sulfide and colonic epithelial metabolism: Implications for ulcerative colitis. Dig. Dis. Sci. 2001, 46, 1722–1732. [Google Scholar] [CrossRef]
- Suarez, F.; Furne, J.; Springfield, J.; Levitt, M. Production and elimination of sulfur-containing gases in the rat colon. Am. J. Physiol. 1998, 274, G727–G733. [Google Scholar] [CrossRef]
- Jensen, B.; Fago, A. Reactions of ferric hemoglobin and myoglobin with hydrogen sulfide under physiological conditions. J. Inorg. Biochem. 2018, 182, 133–140. [Google Scholar] [CrossRef]
- Andriamihaja, M.; Lan, A.; Beaumont, M.; Grauso, M.; Gotteland, M.; Pastene, E.; Cires, M.J.; Carrasco-Pozo, C.; Tomé, D.; Blachier, F. Proanthocyanidin-containing polyphenol extracts from fruits prevent the inhibitory effect of hydrogen sulfide on human colonocyte oxygen consumption. Amino Acids 2018, 50, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Oldham, K.E.A.; Prentice, E.J.; Summers, E.L.; Hicks, J.L. Serine acetyltransferase from Neisseria gonorrhoeae; structural and biochemical basis of inhibition. Biochem. J. 2022, 479, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Hicks, J.L.; Mullholland, C.V. Cysteine biosynthesis in Neisseria species. Microbiology 2018, 164, 1471–1480. [Google Scholar] [CrossRef]
- Yao, S.; Zhao, Z.; Wang, W.; Liu, X. Bifidobacterium longum: Protection against inflammatory bowel disease. J. Immunol. Res. 2021, 2021, 8030297. [Google Scholar] [CrossRef]
- Schell, M.A.; Karmirantzou, M.; Snel, B.; Vilanova, D.; Berger, B.; Pessi, G.; Zwahlen, M.C.; Desiere, F.; Bork, P.; Delley, M.; et al. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc. Natl. Acad. Sci. USA 2002, 99, 14422–14427. [Google Scholar] [CrossRef]
- Rodionov, D.A.; Vitreschak, A.G.; Mironov, A.A.; Gelfand, M.S. Comparative genomics of the methionine metabolism in Gram-positive bacteria: A variety of regulatory systems. Nucleic Acids Res. 2004, 32, 3340–3353. [Google Scholar] [CrossRef]
- Ferla, M.P.; Patrick, W.M. Bacterial methionine biosynthesis. Microbiology 2014, 160, 1571–1584. [Google Scholar] [CrossRef]
- Saini, V.; Chinta, K.C.; Reddy, V.P.; Glasgow, J.N.; Stein, A.; Lamprecht, D.A.; Rahman, M.A.; Mackenzie, J.S.; Truebody, B.E.; Adamson, J.H.; et al. Hydrogen sulfide stimulates Mycobacterium tuberculosis respiration, growth and pathogenesis. Nat. Commun. 2020, 11, 557. [Google Scholar] [CrossRef]
- Borisov, V.B.; Forte, E. Impact of hydrogen sulfide on mitochondrial and bacterial bioenergetics. Int. J. Mol. Sci. 2021, 22, 12688. [Google Scholar] [CrossRef] [PubMed]
- Melo, A.M.; Teixeira, M. Supramolecular organization of bacterial aerobic respiratory chains: From cells and back. Biochim. Biophys. Acta. 2016, 1857, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Forte, E.; Borisov, V.B.; Falabella, M.; Colaço, H.G.; Tinajero-Trejo, M.; Poole, R.K.; Vicente, J.B.; Sarti, P.; Giuffrè, A. The terminal oxidase cytochrome bd promotes sulfide-resistant bacterial respiration and growth. Sci. Rep. 2016, 6, 23788. [Google Scholar] [CrossRef]
- Nastasi, M.R.; Caruso, L.; Giordano, F.; Mellini, M.; Rampioni, G.; Giuffrè, A.; Forte, E. Cyanide insensitive oxidase confers hydrogen sulfide and nitric oxide tolerance to Pseudomonas aeruginosa aerobic respiration. Antioxidants 2024, 13, 383. [Google Scholar] [CrossRef]
- Bachenheimer, A.G.; Bennett, E.O. The sensitivity of mixed population of bacteria to inhibitors. I. The mechanism by which Desulfovibrio desulfuricans protects Ps. aeruginosa from the toxicity of mercurials. Antonie Van Leeuwenhoek 1961, 27, 180–188. [Google Scholar] [CrossRef]
- Stutzenberger, F.J.; Bennett, E.O. Sensitivity of mixed populations of Staphylococcus aureus and Escherichia coli to mercurials. Appl. Microbiol. 1965, 13, 570–574. [Google Scholar] [CrossRef]
- Shatalin, K.; Shatalina, E.; Mironov, A.; Nudler, E. H2S: A universal defense against antibiotics in bacteria. Science 2011, 334, 986–990. [Google Scholar] [CrossRef]
- Pal, V.K.; Bandyopadhyay, P.; Singh, A. Hydrogen sulfide in physiology and pathogenesis of bacteria and viruses. IUBMB Life 2018, 70, 393–410. [Google Scholar] [CrossRef]
- Mironov, A.; Seregina, T.; Nagornykh, M.; Luhachack, L.G.; Korolkova, N.; Lopes, L.E.; Kotova, V.; Zavilgelsky, G.; Shakulov, R.; Shatalin, K.; et al. Mechanism of H2S-mediated protection against oxidative stress in Escherichia coli. Proc. Natl. Acad. Sci. USA 2017, 114, 6022–6027. [Google Scholar] [CrossRef]
- Shukla, P.; Khodade, V.S.; SharathChandra, M.; Chauhan, P.; Mishra, S.; Siddaramappa, S.; Pradeep, B.E.; Singh, A.; Chakrapani, H. “On demand” redox buffering by H2S contributes to antibiotic resistance revealed by a bacteria-specific H2S donor. Chem. Sci. 2017, 8, 4967–4972. [Google Scholar] [CrossRef]
- Shatalin, K.; Nuthanakanti, A.; Kaushik, A.; Shishov, D.; Peselis, A.; Shamovsky, I.; Pani, B.; Lechpammer, M.; Vasilyev, N.; Shatalina, E.; et al. Inhibitors of bacterial H2S biogenesis targeting antibiotic resistance and tolerance. Science 2021, 372, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.Y.; Ong, K.X.; Surendran, S.T.; Sinha, A.; Lai, J.J.H.; Chen, J.; Liang, J.; Tay, L.K.S.; Cui, L.; Loo, H.L.; et al. Hydrogen Sulfide Sensitizes Acinetobacter baumannii to Killing by Antibiotics. Front. Microbiol. 2020, 11, 1875. [Google Scholar] [CrossRef] [PubMed]
- Stacy, A.; Andrade-Oliveira, V.; McCulloch, J.A.; Hild, B.; Oh, J.H.; Perez-Chaparro, P.J.; Sim, C.K.; Lim, A.I.; Link, V.M.; Enamorado, M.; et al. Infection trains the host for microbiota-enhanced resistance to pathogens. Cell 2021, 184, 615–627.e17. [Google Scholar] [CrossRef]
- Motta, J.P.; Flannigan, K.L.; Agbor, T.A.; Beatty, J.K.; Blackler, R.W.; Workentine, M.L.; Da Silva, G.J.; Wang, R.; Buret, A.G.; Wallace, J.L. Hydrogen sulfide protects from colitis and restores intestinal microbiota biofilm and mucus production. Inflamm. Bowel Dis. 2015, 21, 1006–1017. [Google Scholar] [CrossRef]
- Chen, Y.W.; Camacho, M.I.; Chen, Y.; Bhat, A.H.; Chang, C.; Peluso, E.A.; Wu, C.; Das, A.; Ton-That, H. Genetic determinants of hydrogen sulfide biosynthesis in Fusobacterium nucleatum are required for bacterial fitness, antibiotic sensitivity, and virulence. mBio 2022, 13, 0193622. [Google Scholar] [CrossRef]
- Sun, J.; Wang, X.; Gao, Y.; Li, S.; Hu, Z.; Huang, Y.; Fan, B.; Wang, X.; Liu, M.; Qiao, C.; et al. H2S scavenger as a broad-spectrum strategy to deplete bacteria-derived H2S for antibacterial sensitization. Nat. Commun. 2024, 15, 9422. [Google Scholar] [CrossRef]
- Sudhamsu, J.; Crane, B.R. Bacterial nitric oxide synthases: What are they good for? Trends Microbiol. 2009, 17, 212–218. [Google Scholar] [CrossRef]
- Adak, S.; Aulak, K.S.; Stuehr, D.J. Direct evidence for nitric oxide production by a nitric-oxide synthase-like protein from Bacillus subtilis. J. Biol. Chem. 2002, 277, 16167–16171. [Google Scholar] [CrossRef]
- Morita, H.; Yoshikawa, H.; Sakata, R.; Nagata, Y.; Tanaka, H. Synthesis of nitric oxide from the two equivalent guanidino nitrogens of L-arginine by Lactobacillus fermentum. J. Bacteriol. 1997, 179, 7812–7815. [Google Scholar] [CrossRef]
- Sellars, M.J.; Hall, S.J.; Kelly, D.J. Growth of Campylobacter jejuni supported by respiration of fumarate, nitrate, nitrite, trimethylamine-N-oxide, or dimethyl sulfoxide requires oxygen. J. Bacteriol. 2002, 184, 4187–4196. [Google Scholar] [CrossRef]
- Weingarten, R.A.; Grimes, J.L.; Olson, J.W. Role of Campylobacter jejuni respiratory oxidases and reductases in host colonization. Appl. Environ. Microbiol. 2008, 74, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Silvestrini, M.C.; Falcinelli, S.; Ciabatti, I.; Cutruzzolà, F.; Brunori, M. Pseudomonas aeruginosa nitrite reductase (or cytochrome oxidase): An overview. Biochimie 1994, 76, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Daims, H.; Lebedeva, E.V.; Pjevac, P.; Han, P.; Herbold, C.; Albertsen, M.; Jehmlich, N.; Palatinszky, M.; Vierheilig, J.; Bulaev, A.; et al. Complete nitrification by Nitrospira bacteria. Nature 2015, 528, 504–509. [Google Scholar] [CrossRef]
- Zheng, Y.; Ke, J.; Song, J.; Li, X.; Kuang, R.; Wang, H.; Li, S.; Li, Y. Correlation between daily physical activity and intestinal microbiota in perimenopausal women. Sports Med. Health Sci. 2024, 7, 230–236. [Google Scholar] [CrossRef]
- Kastner, J.; Pfeffel, F.; Rajek, A.; Pezawas, T.; Hiesmayr, M.; Eichler, H.G. Nitric oxide concentration in the gas phase of the gastrointestinal tract in man. Eur. J. Clin. Investig. 1997, 27, 992–996. [Google Scholar] [CrossRef]
- Richardson, A.R.; Payne, E.C.; Younger, N.; Karlinsey, J.E.; Thomas, V.C.; Becker, L.A.; Navarre, W.W.; Castor, M.E.; Libby, S.J.; Fang, F.C. Multiple targets of nitric oxide in the tricarboxylic acid cycle of Salmonella enterica serovar typhimurium. Cell Host Microbe 2011, 10, 33–43. [Google Scholar] [CrossRef]
- Stern, A.M.; Zhu, J. An introduction to nitric oxide sensing and response in bacteria. Adv. Appl. Microbiol. 2014, 87, 187–220. [Google Scholar]
- Zhao, G.; Guo, Y.Y.; Yao, S.; Shi, X.; Lv, L.; Du, Y.L. Nitric oxide as a source for bacterial triazole biosynthesis. Nat. Commun. 2020, 11, 1614. [Google Scholar] [CrossRef]
- Caranto, J.D. The emergence of nitric oxide in the biosynthesis of bacterial natural products. Curr. Opin. Chem. Biol. 2019, 49, 130–138. [Google Scholar] [CrossRef]
- Choules, M.P.; Wolf, N.M.; Lee, H.; Anderson, J.R.; Grzelak, E.M.; Wang, Y.; Ma, R.; Gao, W.; McAlpine, J.B.; Jin, Y.Y.; et al. Rufomycin targets ClpC1 proteolysis in Mycobacterium tuberculosis and M. abscessus. Antimicrob. Agents Chemother. 2019, 63, e02204-18. [Google Scholar] [CrossRef]
- Anantharaman, S.; Guercio, D.; Mendoza, A.G.; Withorn, J.M.; Boon, E.M. Negative regulation of biofilm formation by nitric oxide sensing proteins. Biochem. Soc. Trans. 2023, 51, 1447–1458. [Google Scholar] [CrossRef]
- Poh, W.H.; Rice, S.A. Recent Developments in nitric oxide donors and delivery for antimicrobial and anti-biofilm applications. Molecules 2022, 27, 674. [Google Scholar] [CrossRef]
- Ueno, T.; Fischer, J.T.; Boon, E.M. Nitric oxide enters quorum sensing via the H-NOX signaling pathway in Vibrio parahaemolyticus. Front. Microbiol. 2019, 10, 2108. [Google Scholar] [CrossRef]
- Keszthelyi, D.; Troost, F.J.; Masclee, A.A. Understanding the role of tryptophan and serotonin metabolism in gastrointestinal function. Neurogastroenterol. Motil. 2009, 21, 1239–1249. [Google Scholar] [CrossRef]
- Lee, J.H.; Wood, T.K.; Lee, J. Roles of indole as an interspecies and interkingdom signaling molecule. Trends Microbiol. 2015, 23, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef] [PubMed]
- Rattanaphan, P.; Mittraparp-Arthorn, P.; Srinoun, K.; Vuddhakul, V.; Tansila, N. Indole signaling decreases biofilm formation and related virulence of Listeria monocytogenes. FEMS Microbiol. Lett. 2020, 367, fnaa116. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Attila, C.; Cirillo, S.L.; Cirillo, J.D.; Wood, T.K. Indole and 7-hydroxyindole diminish Pseudomonas aeruginosa virulence. Microb. Biotechnol. 2009, 2, 75–90. [Google Scholar] [CrossRef]
- Nikaido, E.; Giraud, E.; Baucheron, S.; Yamasaki, S.; Wiedemann, A.; Okamoto, K.; Takagi, T.; Yamaguchi, A.; Cloeckaert, A.; Nishino, K. Effects of indole on drug resistance and virulence of Salmonella enterica serovar Typhimurium revealed by genome-wide analyses. Gut Pathog. 2012, 4, 5. [Google Scholar] [CrossRef]
- Oh, S.; Go, G.W.; Mylonakis, E.; Kim, Y. The bacterial signalling molecule indole attenuates the virulence of the fungal pathogen Candida albicans. J. Appl. Microbiol. 2012, 113, 622–628. [Google Scholar] [CrossRef]
- Talapko, J.; Juzbašić, M.; Matijević, T.; Pustijanac, E.; Bekić, S.; Kotris, I.; Škrlec, I. Candida albicans-The virulence factors and clinical manifestations of infection. J. Fungi 2021, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Libudzisz, Z. Influence of phenol, p-cresol and indole on growth and survival of intestinal lactic acid bacteria. Anaerobe 2006, 12, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Ledala, N.; Malik, M.; Rezaul, K.; Paveglio, S.; Provatas, A.; Kiel, A.; Caimano, M.; Zhou, Y.; Lindgren, J.; Krasulova, K.; et al. Bacterial indole as a multifunctional regulator of Klebsiella oxytoca complex enterotoxicity. mBio 2022, 13, e0375221. [Google Scholar] [CrossRef] [PubMed]
- Högenauer, C.; Langner, C.; Beubler, E.; Lippe, I.T.; Schicho, R.; Gorkiewicz, G.; Krause, R.; Gerstgrasser, N.; Krejs, G.J.; Hinterleitner, T.A. Klebsiella oxytoca as a causative organism of antibiotic-associated hemorrhagic colitis. N. Engl. J. Med. 2006, 355, 2418–2426. [Google Scholar] [CrossRef]
- Bone, E.; Tamm, A.; Hill, M. The production of urinary phenols by gut bacteria and their possible role in the causation of large bowel cancer. Am. J. Clin. Nutr. 1976, 29, 1448–1454. [Google Scholar] [CrossRef]
- Saito, Y.; Sato, T.; Nomoto, K.; Tsuji, H. Identification of phenol- and p-cresol-producing intestinal bacteria by using media supplemented with tyrosine and its metabolites. FEMS Microbiol. Ecol. 2018, 94, fiy125, Erratum in FEMS Microbiol. Ecol. 2020, 96, fiz195. [Google Scholar]
- Harrison, M.A.; Kaur, H.; Wren, B.W.; Dawson, L.F. Production of p-cresol by Decarboxylation of p-HPA by All Five Lineages of Clostridioides difficile Provides a Growth Advantage. Front. Cell. Infect. Microbiol. 2021, 11, 757599. [Google Scholar] [CrossRef]
- Passmore, I.J.; Letertre, M.P.M.; Preston, M.D.; Bianconi, I.; Harrison, M.A.; Nasher, F.; Kaur, H.; Hong, H.A.; Baines, S.D.; Cutting, S.M.; et al. Para-cresol production by Clostridium difficile affects microbial diversity and membrane integrity of Gram-negative bacteria. PLoS Pathog. 2018, 14, e1007191. [Google Scholar] [CrossRef]
- Abt, M.C.; McKenney, P.T.; Pamer, E.G. Clostridium difficile colitis: Pathogenesis and host defence. Nat. Rev. Microbiol. 2016, 14, 609–620. [Google Scholar] [CrossRef]
- Hafiz, S.; Oakley, C.L. Clostridium difficile: Isolation and characteristics. J. Med. Microbiol. 1976, 9, 129–136. [Google Scholar] [CrossRef]
- Dawson, L.F.; Donahue, E.H.; Cartman, S.T.; Barton, R.H.; Bundy, J.; McNerney, R.; Minton, N.P.; Wren, B.W. The analysis of para-cresol production and tolerance in Clostridium difficile 027 and 012 strains. BMC Microbiol. 2011, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.T.; Cox, R.P.; Jensen, B.B. 3-Methylindole (skatole) and indole production by mixed populations of pig fecal bacteria. Appl. Environ. Microbiol. 1995, 61, 3180–3184. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.T.; Carlson, J.R. Microbial metabolites of tryptophan in the intestinal tract with special reference to skatole. Am. J. Clin. Nutr. 1979, 32, 173–178. [Google Scholar] [CrossRef]
- Whitehead, T.R.; Price, N.P.; Drake, H.L.; Cotta, M.A. Catabolic pathway for the production of skatole and indoleacetic acid by the acetogen Clostridium drakei, Clostridium scatologenes, and swine manure. Appl. Environ. Microbiol. 2008, 74, 1950–1953. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, Y.; Oh, S.; Oh, S.; Chun, T.; Kim, S.H. Inhibitory effect of skatole (3-methylindole) on enterohemorrhagic Escherichia coli O157:H7 ATCC 43894 biofilm formation mediated by elevated endogenous oxidative stress. Lett. Appl. Microbiol. 2014, 58, 454–461. [Google Scholar] [CrossRef]
- Morbach, S.; Krämer, R. Structure and function of the betaine uptake system BetP of Corynebacterium glutamicum: Strategies to sense osmotic and chill stress. J. Mol. Microbiol. Biotechnol. 2005, 10, 143–153. [Google Scholar] [CrossRef]
- Nau-Wagner, G.; Opper, D.; Rolbetzki, A.; Boch, J.; Kempf, B.; Hoffmann, T.; Bremer, E. Genetic control of osmoadaptive glycine betaine synthesis in Bacillus subtilis through the choline-sensing and glycine betaine-responsive GbsR repressor. J. Bacteriol. 2012, 194, 2703–2714. [Google Scholar] [CrossRef]
- Zou, H.; Chen, N.; Shi, M.; Xian, M.; Song, Y.; Liu, J. The metabolism and biotechnological application of betaine in microorganism. Appl. Microbiol. Biotechnol. 2016, 100, 3865–3876. [Google Scholar] [CrossRef]
- Wargo, M.J. Homeostasis and catabolism of choline and glycine betaine: Lessons from Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2013, 79, 2112–2120. [Google Scholar] [CrossRef]
- Ku, J.W.; Gan, Y.H. Modulation of bacterial virulence and fitness by host glutathione. Curr. Opin. Microbiol. 2019, 47, 8–13. [Google Scholar] [CrossRef]
- Masip, L.; Veeravalli, K.; Georgiou, G. The many faces of glutathione in bacteria. Antioxid. Redox Signal. 2006, 8, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M.; De las Rivas, B.; Marcobal, A.; Muñoz, R. Updated molecular knowledge about histamine biosynthesis by bacteria. Crit. Rev. Food Sci. Nutr. 2008, 48, 697–714. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.M.T. Gut bacteria and neurotransmitters. Microorganisms. 2022, 10, 1838. [Google Scholar] [CrossRef]
- Molenaar, D.; Bosscher, J.S.; ten Brink, B.; Driessen, A.J.; Konings, W.N. Generation of a proton motive force by histidine decarboxylation and electrogenic histidine/histamine antiport in Lactobacillus buchneri. J. Bacteriol. 1993, 175, 2864–2870. [Google Scholar] [CrossRef]
- Trip, H.; Mulder, N.L.; Lolkema, J.S. Improved acid stress survival of Lactococcus lactis expressing the histidine decarboxylation pathway of Streptococcus thermophilus CHCC1524. J. Biol. Chem. 2012, 287, 11195–11204. [Google Scholar] [CrossRef]
- Nugent, S.G.; Kumar, D.; Rampton, D.S.; Evans, D.F. Intestinal luminal pH in inflammatory bowel disease: Possible determinants and implications for therapy with aminosalicylates and other drugs. Gut 2001, 48, 571–577. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Claus, H.; Decker, H. Bacterial tyrosinases. Syst. Appl. Microbiol. 2006, 29, 3–14. [Google Scholar] [CrossRef]
- Asano, Y.; Hiramoto, T.; Nishino, R.; Aiba, Y.; Kimura, T.; Yoshihara, K.; Koga, Y.; Sudo, N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. 2012, 303, G1288–G1295. [Google Scholar] [CrossRef]
- Belay, T.; Sonnenfeld, G. Differential effects of catecholamines on in vitro growth of pathogenic bacteria. Life Sci. 2002, 71, 447–456. [Google Scholar] [CrossRef]
- Dichtl, S.; Demetz, E.; Haschka, D.; Tymoszuk, P.; Petzer, V.; Nairz, M.; Seifert, M.; Hoffmann, A.; Brigo, N.; Würzner, R.; et al. Dopamine is a siderophore-like iron chelator that promotes Salmonella enterica serovar Typhimurium virulence in mice. mBio 2019, 10, e02624-18. [Google Scholar] [CrossRef]
- Knecht, L.D.; O’Connor, G.; Mittal, R.; Liu, X.Z.; Daftarian, P.; Deo, S.K.; Daunert, S. Serotonin activates bacterial quorum sensing and enhances the virulence of Pseudomonas aeruginosa in the host. EBioMedicine 2016, 9, 161–169, Erratum in EBioMedicine 2017, 23, 195. [Google Scholar] [CrossRef] [PubMed]
- Boyanova, L. Stress hormone epinephrine (adrenaline) and norepinephrine (noradrenaline) effects on the anaerobic bacteria. Anaerobe 2017, 44, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Lustri, B.C.; Sperandio, V.; Moreira, C.G. Bacterial chat: Intestinal metabolites and signals in host-microbiota-pathogen interactions. Infect. Immun. 2017, 85, e00476-17. [Google Scholar] [CrossRef]
- O’Donnell, P.M.; Aviles, H.; Lyte, M.; Sonnenfeld, G. Enhancement of in vitro growth of pathogenic bacteria by norepinephrine: Importance of inoculum density and role of transferrin. Appl. Environ. Microbiol. 2006, 72, 5097–5099. [Google Scholar] [CrossRef]
- Kim, J.; Lee, M.H.; Kim, M.S.; Kim, G.H.; Yoon, S.S. Probiotic properties and optimization of gamma-aminobutyric acid production by Lactiplantibacillus plantarum FBT215. J. Microbiol. Biotechnol. 2022, 32, 783–791. [Google Scholar] [CrossRef]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. gamma-aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef]
- Nomura, M.; Nakajima, I.; Fujita, Y.; Kobayashi, M.; Kimoto, H.; Suzuki, I.; Aso, H. Lactococcus lactis contains only one glutamate decarboxylase gene. Microbiology 1999, 145, 1375–1380. [Google Scholar] [CrossRef]
- Otaru, N.; Ye, K.; Mujezinovic, D.; Berchtold, L.; Constancias, F.; Cornejo, F.A.; Krzystek, A.; de Wouters, T.; Braegger, C.; Lacroix, C.; et al. GABA Production by human intestinal Bacteroides spp.: Prevalence, regulation, and role in acid stress tolerance. Front. Microbiol. 2021, 12, 656895. [Google Scholar] [CrossRef]
- Feehily, C.; Karatzas, K.A. Role of glutamate metabolism in bacterial responses towards acid and other stresses. J. Appl. Microbiol. 2013, 114, 11–24. [Google Scholar] [CrossRef]
- Prieto, M.A.; García, J.L. Identification of the 4-hydroxyphenylacetate transport gene of Escherichia coli W: Construction of a highly sensitive cellular biosensor. FEBS Lett. 1997, 414, 293–297. [Google Scholar]
- YujiaLiu Shi, C.; Zhang, G.; Zhan, H.; Liu, B.; Li, C.; Wang, L.; Wang, H.; Wang, J. Antimicrobial mechanism of 4-hydroxyphenylacetic acid on Listeria monocytogenes membrane and virulence. Biochem. Biophys. Res. Commun. 2021, 572, 145–150. [Google Scholar] [CrossRef]
- Dai, Z.L.; Wu, G.; Zhu, W.Y. Amino acid metabolism in intestinal bacteria: Links between gut ecology and host health. Front. Biosci. 2011, 16, 1768–1786. [Google Scholar] [CrossRef]
- Endo, A.; Nakamura, S.; Konishi, K.; Nakagawa, J.; Tochio, T. Variations in prebiotic oligosaccharide fermentation by intestinal lactic acid bacteria. Int. J. Food Sci. Nutr. 2016, 67, 125–132. [Google Scholar] [CrossRef]
- Liong, M.T.; Shah, N.P. Production of organic acids from fermentation of mannitol, fructooligosaccharide and inulin by a cholesterol removing Lactobacillus acidophilus strain. J. Appl. Microbiol. 2005, 99, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Goocher, C.R.; Woodside, E.E.; Kocholaty, W. The influence of 2,4-dinitrophenol on the oxidation of acetate and succinate by Escherichia coli. J. Bacteriol. 1954, 67, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Lemma, E.; Hägerhäll, C.; Geisler, V.; Brandt, U.; von Jagow, G.; Kröger, A. Reactivity of the Bacillus subtilis succinate dehydrogenase complex with quinones. Biochim. Biophys Acta 1991, 1059, 281–285. [Google Scholar] [CrossRef]
- Adolph, C.; McNeil, M.B.; Cook, G.M. Impaired succinate oxidation prevents growth and influences drug susceptibility in Mycobacterium tuberculosis. mBio. 2022, 13, e0167222. [Google Scholar] [CrossRef]
- Hartman, T.; Weinrick, B.; Vilchèze, C.; Berney, M.; Tufariello, J.; Cook, G.M.; Jacobs, W.R., Jr. Succinate dehydrogenase is the regulator of respiration in Mycobacterium tuberculosis. PLoS Pathog. 2014, 10, e1004510. [Google Scholar] [CrossRef]
- Ferreyra, J.A.; Wu, K.J.; Hryckowian, A.J.; Bouley, D.M.; Weimer, B.C.; Sonnenburg, J.L. Gut microbiota-produced succinate promotes C. difficile infection after antibiotic treatment or motility disturbance. Cell Host Microbe 2014, 16, 770–777. [Google Scholar] [CrossRef]
- Auria, E.; Deschamps, J.; Briandet, R.; Dupuy, B. Extracellular succinate induces spatially organized biofilm formation in Clostridioides difficile. Biofilm 2023, 5, 100125. [Google Scholar] [CrossRef] [PubMed]
- Hagihara, M.; Ariyoshi, T.; Kuroki, Y.; Eguchi, S.; Higashi, S.; Mori, T.; Nonogaki, T.; Iwasaki, K.; Yamashita, M.; Asai, N.; et al. Clostridium butyricum enhances colonization resistance against Clostridioides difficile by metabolic and immune modulation. Sci. Rep. 2021, 11, 15007. [Google Scholar] [CrossRef] [PubMed]
- Shaulov, Y.; Shimokawa, C.; Trebicz-Geffen, M.; Nagaraja, S.; Methling, K.; Lalk, M.; Weiss-Cerem, L.; Lamm, A.T.; Hisaeda, H.; Ankri, S. Escherichia coli mediated resistance of Entamoeba histolytica to oxidative stress is triggered by oxaloacetate. PLoS Pathog. 2018, 14, e1007295. [Google Scholar] [CrossRef] [PubMed]
- Guillén, N. Pathogenicity and virulence of Entamoeba histolytica, the agent of amoebiasis. Virulence 2023, 14, 2158656. [Google Scholar] [CrossRef]
- Kassem, I.I.; Candelero-Rueda, R.A.; Esseili, K.A.; Rajashekara, G. Formate simultaneously reduces oxidase activity and enhances respiration in Campylobacter jejuni. Sci. Rep. 2017, 7, 40117. [Google Scholar] [CrossRef]
- Voskuhl, L.; Brusilova, D.; Brauer, V.S.; Meckenstock, R.U. Inhibition of sulfate-reducing bacteria with formate. FEMS Microbiol. Ecol. 2022, 98, fiac003. [Google Scholar] [CrossRef]
- Koestler, B.J.; Fisher, C.R.; Payne, S.M. Formate promotes Shigella intercellular spread and virulence gene expression. mBio 2018, 9, e01777-18. [Google Scholar] [CrossRef]
- Liu, S.Q. Practical implications of lactate and pyruvate metabolism by lactic acid bacteria in food and beverage fermentations. Int. J. Food Microbiol. 2003, 83, 115–131. [Google Scholar] [CrossRef]
- Sheridan, P.O.; Louis, P.; Tsompanidou, E.; Shaw, S.; Harmsen, H.J.; Duncan, S.H.; Flint, H.J.; Walker, A.W. Distribution, organization and expression of genes concerned with anaerobic lactate utilization in human intestinal bacteria. Microb. Genom. 2022, 8, 000739. [Google Scholar] [CrossRef]
- Weghoff, M.C.; Bertsch, J.; Müller, V. A novel mode of lactate metabolism in strictly anaerobic bacteria. Environ. Microbiol. 2015, 17, 670–677. [Google Scholar] [CrossRef]
- Thauer, R.K. Citric-acid cycle, 50 years on. Modifications and an alternative pathway in anaerobic bacteria. Eur. J. Biochem. 1988, 176, 497–508. [Google Scholar] [CrossRef]
- Zampieri, M.; Hörl, M.; Hotz, F.; Müller, N.F.; Sauer, U. Regulatory mechanisms underlying coordination of amino acid and glucose catabolism in Escherichia coli. Nat. Commun. 2019, 10, 3354. [Google Scholar] [CrossRef]
- Sarantinopoulos, P.; Makras, L.; Vaningelgem, F.; Kalantzopoulos, G.; De Vuyst, L.; Tsakalidou, E. Growth and energy generation by Enterococcus faecium FAIR-E 198 during citrate metabolism. Int. J. Food Microbiol. 2003, 84, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Elías, E.J.; McKinney, J.D. Carbon metabolism of intracellular bacteria. Cell Microbiol. 2006, 8, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Underhill, S.A.M.; Cabeen, M.T. Redundancy in Citrate and cis-Aconitate Transport in Pseudomonas aeruginosa. J. Bacteriol. 2022, 204, e0028422. [Google Scholar] [CrossRef] [PubMed]
- Mortera, P.; Pudlik, A.; Magni, C.; Alarcón, S.; Lolkema, J.S. Ca2+-citrate uptake and metabolism in Lactobacillus casei ATCC 334. Appl. Environ. Microbiol. 2013, 79, 4603–4612. [Google Scholar] [CrossRef]
- Pfenninger-Li, X.D.; Dimroth, P. NADH formation by Na(+)-coupled reversed electron transfer in Klebsiella pneumoniae. Mol. Microbiol. 1992, 6, 1943–1948. [Google Scholar] [CrossRef]
- Ramos, A.; Poolman, B.; Santos, H.; Lolkema, J.S.; Konings, W.N. Uniport of anionic citrate and proton consumption in citrate metabolism generates a proton motive force in Leuconostoc oenos. J. Bacteriol. 1994, 176, 4899–4905. [Google Scholar] [CrossRef]
- Pudlik, A.M.; Lolkema, J.S. Citrate uptake in exchange with intermediates in the citrate metabolic pathway in Lactococcus lactis IL1403. J. Bacteriol. 2011, 193, 706–714. [Google Scholar] [CrossRef]
- Sheldon, J.R.; Marolda, C.L.; Heinrichs, D.E. TCA cycle activity in Staphylococcus aureus is essential for iron-regulated synthesis of staphyloferrin A, but not staphyloferrin B: The benefit of a second citrate synthase. Mol. Microbiol. 2014, 92, 824–839. [Google Scholar] [CrossRef]
- Beaumont, M.; Portune, K.J.; Steuer, N.; Lan, A.; Cerrudo, V.; Audebert, M.; Dumont, F.; Mancano, G.; Khodorova, N.; Andriamihaja, M.; et al. Quantity and source of dietary protein influence metabolite production by gut microbiota and rectal mucosa gene expression: A randomized, parallel, double-blind trial in overweight humans. Am. J. Clin. Nutr. 2017, 106, 1005–1019. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blachier, F.; Kong, X. Recycling of Undigested Proteins Provided by the Host to the Large Intestine Microbiota: Implication for Intestinal Bacterial Anabolism, Growth, and Physiology. Microorganisms 2025, 13, 2690. https://doi.org/10.3390/microorganisms13122690
Blachier F, Kong X. Recycling of Undigested Proteins Provided by the Host to the Large Intestine Microbiota: Implication for Intestinal Bacterial Anabolism, Growth, and Physiology. Microorganisms. 2025; 13(12):2690. https://doi.org/10.3390/microorganisms13122690
Chicago/Turabian StyleBlachier, François, and Xiangfeng Kong. 2025. "Recycling of Undigested Proteins Provided by the Host to the Large Intestine Microbiota: Implication for Intestinal Bacterial Anabolism, Growth, and Physiology" Microorganisms 13, no. 12: 2690. https://doi.org/10.3390/microorganisms13122690
APA StyleBlachier, F., & Kong, X. (2025). Recycling of Undigested Proteins Provided by the Host to the Large Intestine Microbiota: Implication for Intestinal Bacterial Anabolism, Growth, and Physiology. Microorganisms, 13(12), 2690. https://doi.org/10.3390/microorganisms13122690






