Stoichiometric Responses of Soil Microbes and Enzymes to Altitudinal Gradients in Alpine Meadows
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Study Area and Site
2.2. Study Design and Field Sampling
2.3. Soil Analysis
2.3.1. Soil Physicochemical Analysis
2.3.2. Analysis of Soil Microbial Biomass and Extracellular Enzyme Activity
2.4. Calculations
2.4.1. Enzyme Stoichiometric Vector Analysis
2.4.2. Stoichiometric Homeostasis
2.5. Statistical Analysis
3. Results
3.1. Soil Stoichiometric Ratios
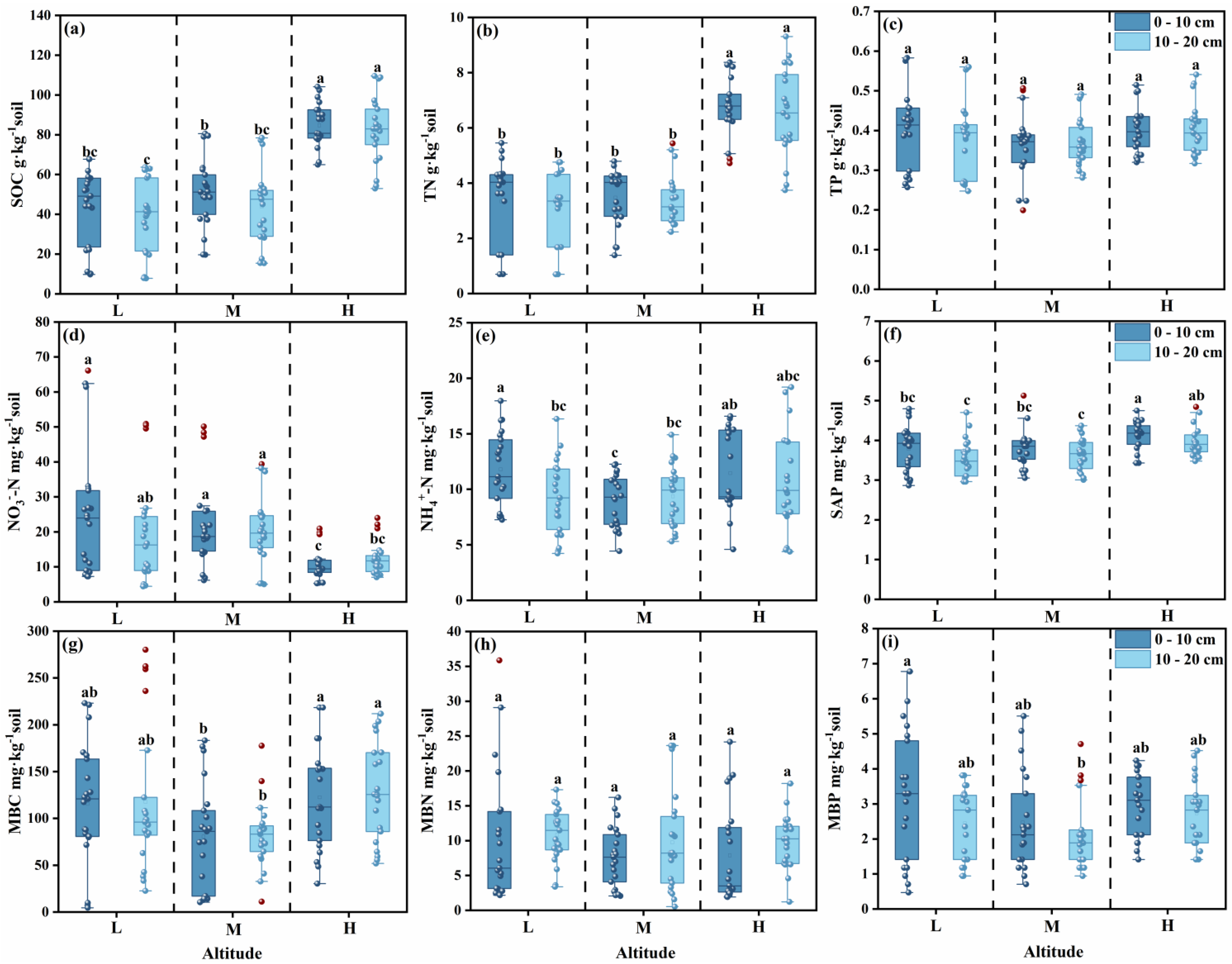
3.1.1. Soil Organic Carbon, Total Nitrogen, Total Phosphorus and Soil Available Nutrient
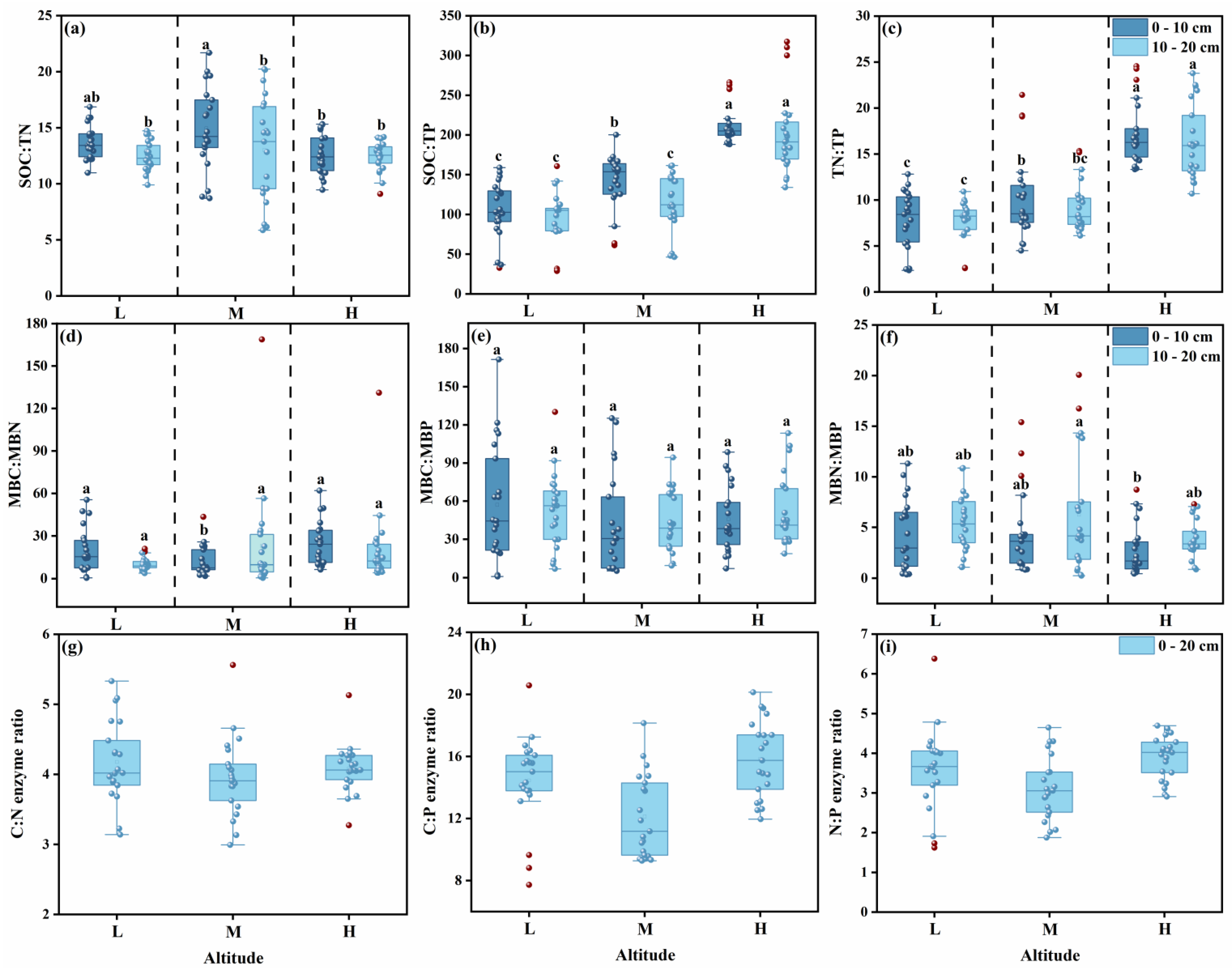
3.1.2. Stoichiometric Ratios
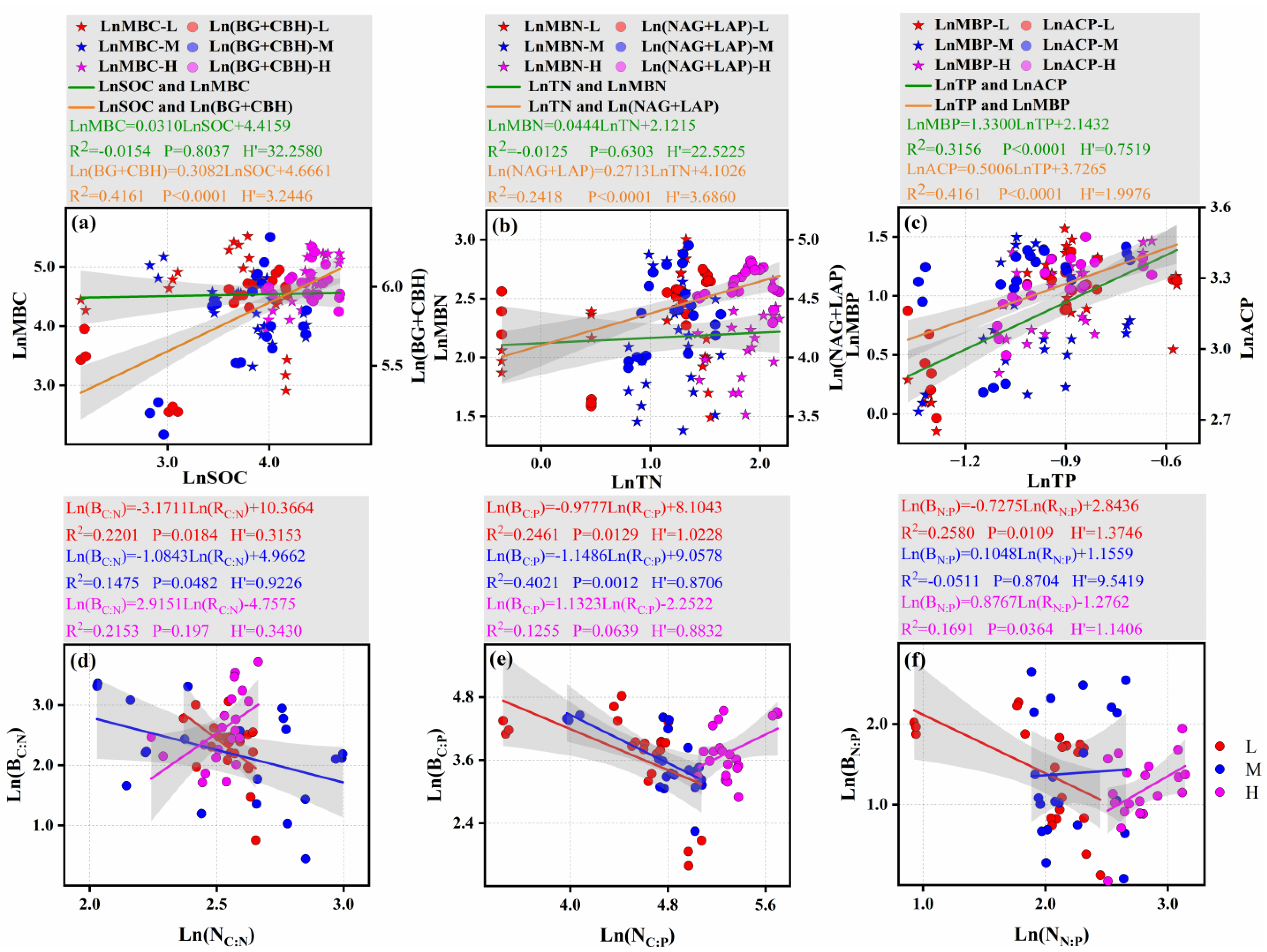
3.2. Microbial Biomass Stoichiometric Ratios
3.2.1. Microbial Biomass
3.2.2. Stoichiometric Ratios
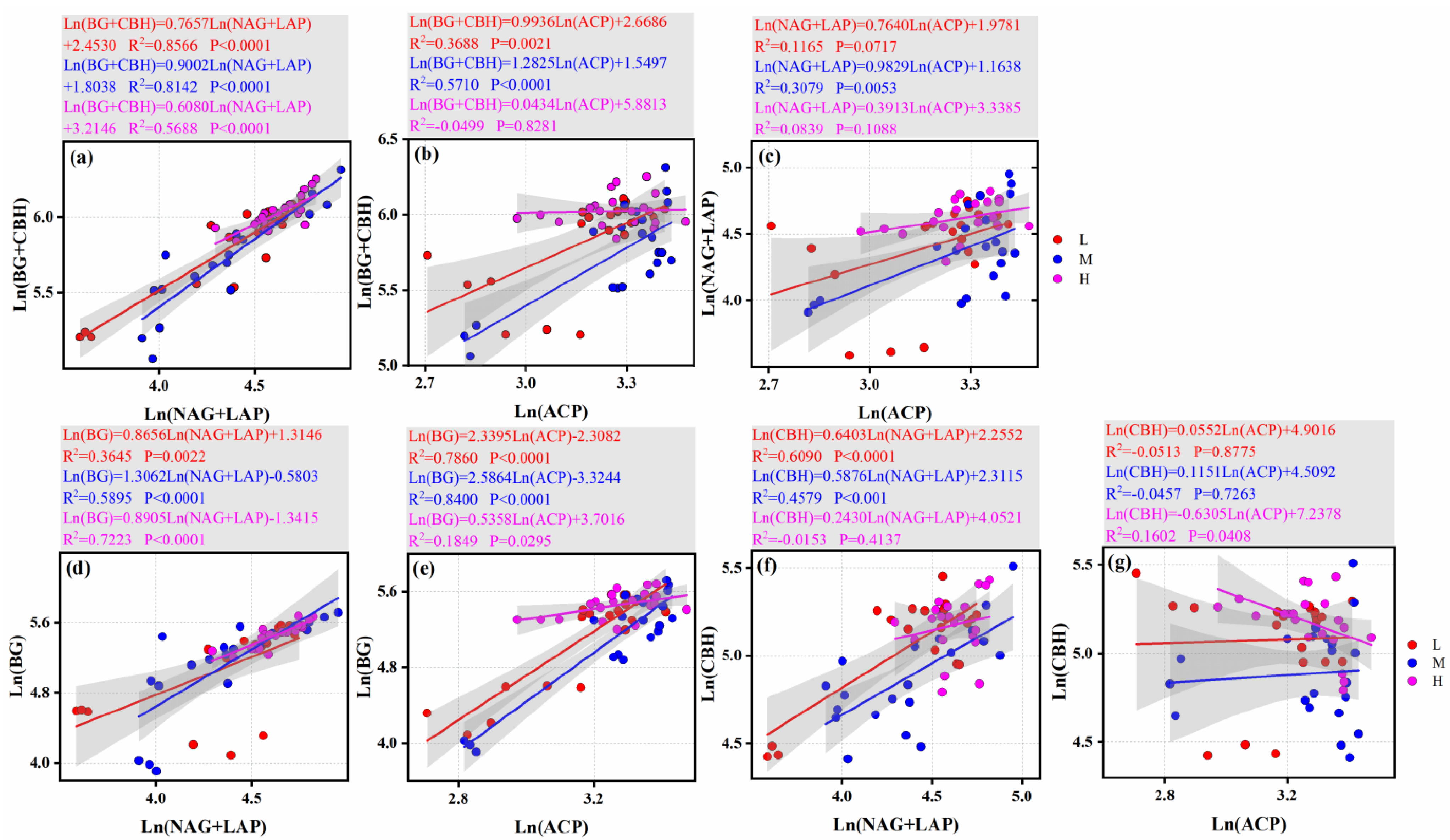
3.3. Characteristic of Extracellular Enzyme Activity Ratios
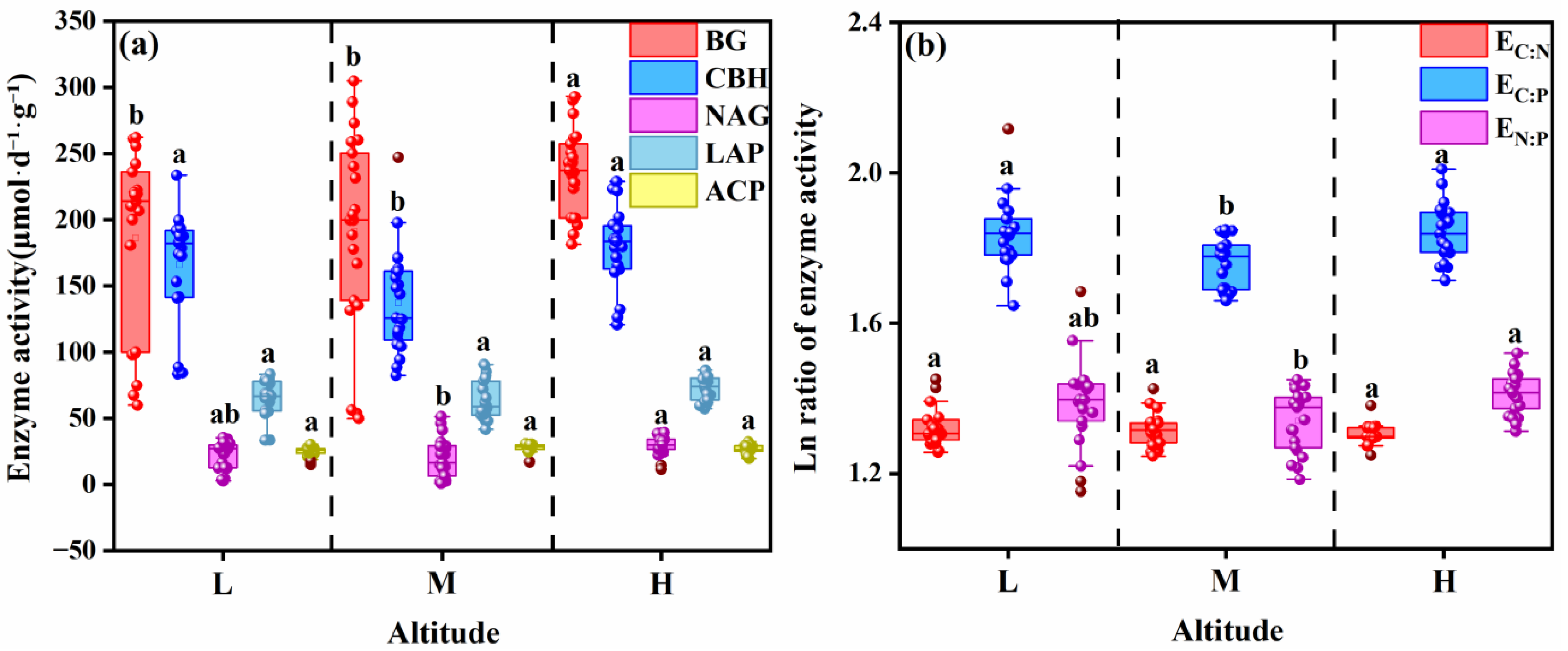
3.3.1. Extracellular Enzyme Activities
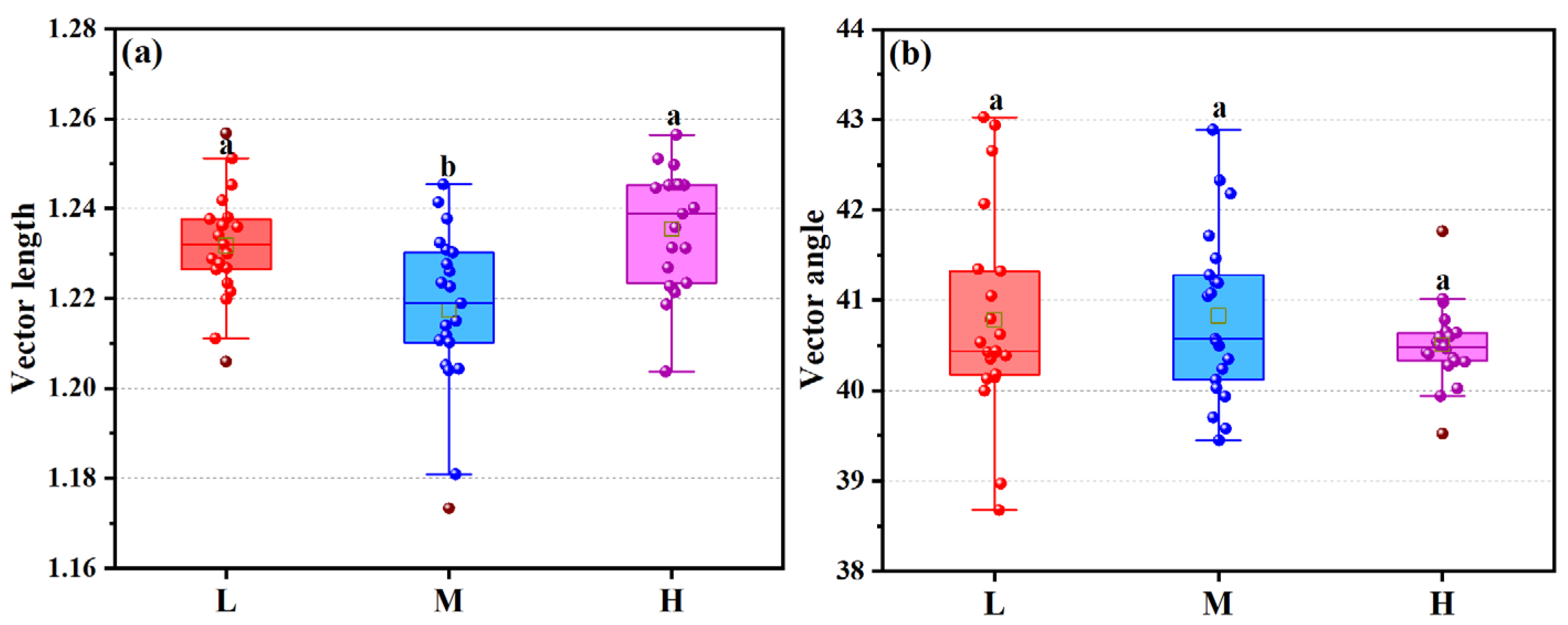
3.3.2. Enzyme Stoichiometric Ratios
3.4. Characteristics of Microbial Stoichiometric Homeostasis and Microbial Nutrient Limitation Under Different Altitudinal Gradients
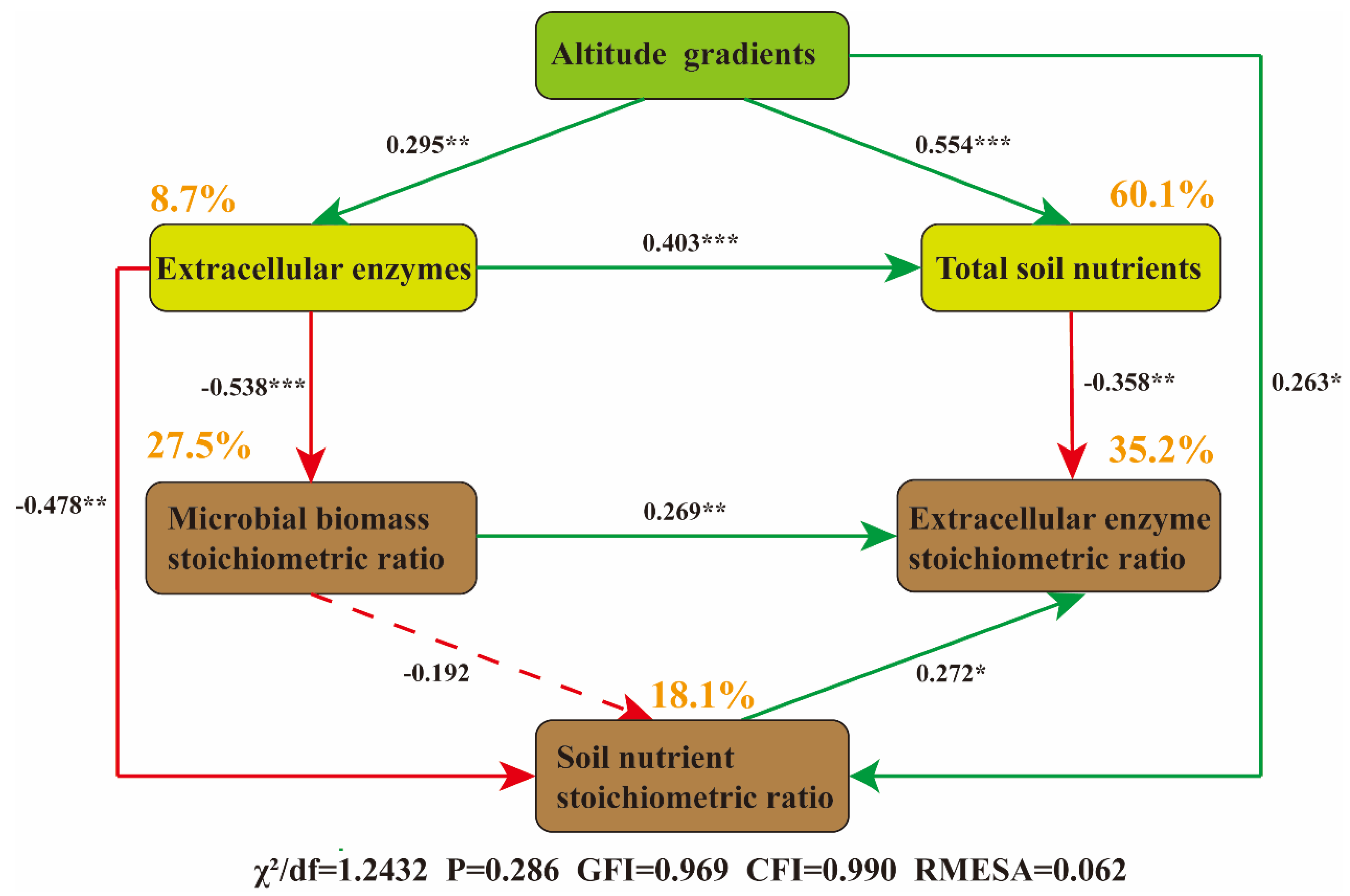
3.5. Driving Mechanism of Soil Extracellular Enzymes on Soil Nutrients
3.6. Integrated Microbial Adaptation Mechanism of Altitudinal Gradient on Soil Nutrient Stoichiometry
4. Discussion
4.1. Soil Nutrients and Enzyme Activities Along the Altitudinal Gradient
4.2. Microbial Nutrient Limitation Along the Altitudinal Gradient
4.3. Regulation Mechanism of Soil Nutrients on Extracellular Enzyme Activities from the Perspective of Microbial Adaptation Mechanisms
4.4. Association of Research Findings with Global Change, Research Limitations, and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bocharnikov, M.V. Climate-Related Gradients on Vegetation Diversity of The Altai-Sayan Orobiome (Southern Siberia). Geogr. Environ. Sustain. 2023, 15, 17–31. [Google Scholar] [CrossRef]
- Tegegn, M.G.; Berlie, A.B.; Utallo, A.U. Spatiotemporal variability and trends of intra-seasonal rainfall and temperature in the drought-prone districts of Northwestern Ethiopia. Discov. Sustain. 2024, 5, 230. [Google Scholar] [CrossRef]
- Wu, G.-L.; Liu, Y.; Wang, D.; Zhao, J. Divergent successions to shrubs- and forbs-dominated meadows decrease ecosystem multifunctionality of hillside alpine meadow. Catena 2024, 236, 107718. [Google Scholar] [CrossRef]
- A’Bear, A.D.; Boddy, L.; Kandeler, E.; Ruess, L.; Jones, T.H. Effects of isopod population density on woodland decomposer microbial community function. Soil Biol. Biochem. 2014, 77, 112–120. [Google Scholar] [CrossRef]
- Peng, S.; Liu, W.; Xu, G.; Pei, X.; Millerick, K.; Duan, B. A meta-analysis of soil microbial and physicochemical properties following native forest conversion. Catena 2021, 204, 105447. [Google Scholar] [CrossRef]
- Acosta, B.; Sánchez-Jardón, L.; del Pozo, A.; García-Ibáñez, E.; Casado, M.A.; Montalvo, J.; Pineda, F.D. Grassland species composition and morpho-functional traits along an altitudinal gradient in a Mediterranean environment: Relationship with soil water availability and evaporative dynamic. Acta Oecol. 2008, 34, 26–37. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Bai, E.; Piao, S.; Chen, N.; Zhao, G.; Zheng, Z.; Zhu, Y. The stimulatory effect of elevated CO2 on soil respiration is unaffected by N addition. Sci. Total Environ. 2022, 813, 151907. [Google Scholar] [CrossRef]
- Dolker, T.; Mukherjee, A.; Agrawal, S.B.; Agrawal, M. Responses of a semi-natural grassland community of tropical region to elevated ozone: An assessment of soil dynamics and biomass accumulation. Sci. Total Environ. 2020, 718, 137141. [Google Scholar] [CrossRef]
- Du, H.; Pan, J.; Zhang, C.; Yang, X.; Wang, C.; Lin, X.; Li, M. Analogous assembly mechanisms and functional guilds govern prokaryotic communities in mangrove ecosystems of China and South America. Microbiol. Spectr. 2023, 11, e0157723. [Google Scholar] [CrossRef]
- Zheng, X.; Lin, C.; Guo, B.; Yu, J.; Ding, H.; Peng, S.; Sveen, T.R.; Zhang, Y. Effects of re-vegetation restoration on soil bacterial community structure in degraded land in subtropical China. Eur. J. Soil Biol. 2020, 98, 103184. [Google Scholar] [CrossRef]
- Ren, C.; Zhou, Z.; Guo, Y.; Yang, G.; Zhao, F.; Wei, G.; Han, X.; Feng, L.; Feng, Y.; Ren, G. Contrasting patterns of microbial community and enzyme activity between rhizosphere and bulk soil along an elevation gradient. Catena 2021, 196, 104921. [Google Scholar] [CrossRef]
- Xing, J.; Chen, W.; Song, C.; Li, X.; Wang, Q.; Song, Y. N deposition affects litter decomposition in evergreen broad-leaf forests by reducing soil enzyme activities and altering the structure of microbial communities. Plant Soil 2025, 1–17. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Chen, L.; Yang, H.; Wang, G.; Wang, C.; Dong, X. Effects of excessive nitrogen on nitrogen uptake and transformation in the wetland soils of Liaohe estuary, northeast China. Sci. Total Environ. 2021, 791, 148228. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xu, Z.; Wang, R.; Zhang, Y.; Yao, F.; Zhang, Y.; Turco, R.F.; Jiang, Y.; Zou, H.; Li, H. Variations in soil microbial community composition and enzymatic activities in response to increased N deposition and precipitation in Inner Mongolian grassland. Appl. Soil Ecol. 2017, 119, 275–285. [Google Scholar] [CrossRef]
- Xu, Z.; Hu, Z.; Jiang, L.; Qiu, T.; Li, W.; Anthony, M.A. Contrasting wood carbon quality of angiosperms and gymnosperms drives fungal-mediated decomposition responses to nutrient enrichment. For. Ecol. Manag. 2025, 593, 122910. [Google Scholar] [CrossRef]
- Hu, M.; Yan, R.; Wu, H.; Ni, R.; Zhang, D.; Zou, S. Linking soil phosphorus availability and phosphatase functional genes to coastal marsh erosion: Implications for nutrient cycling and wetland restoration. Sci. Total Environ. 2023, 898, 165559. [Google Scholar] [CrossRef]
- Saleem, M.; Pervaiz, Z.H.; Traw, M.B. Theories, Mechanisms and Patterns of Microbiome Species Coexistence in an Era of Climate Change. In Microbiome Community Ecology; SpringerBriefs in Ecology; Springer International Publishing: Cham, Switzerland, 2015; pp. 13–53. ISBN 9783319116648. [Google Scholar] [CrossRef]
- King, A.E.; Hofmockel, K.S. Diversified cropping systems support greater microbial cycling and retention of carbon and nitrogen. Agric. Ecosyst. Environ. 2017, 240, 66–76. [Google Scholar] [CrossRef]
- Drahorad, S.; Felix-Henningsen, P.; Siemens, J.; Marschner, B.; Heinze, S. Patterns of enzyme activities and nutrient availability within biocrusts under increasing aridity in Negev desert. Ecosphere 2022, 13, e4051. [Google Scholar] [CrossRef]
- Johnson, C.; Delattre, H.; Hayes, C.; Soyer, O.S. An Evolutionary Systems Biology View on Metabolic System Structure and Dynamics. In Evolutionary Systems Biology; Springer Nature Switzerland: Cham, Switzerland, 2021; pp. 159–196. ISBN 9783030717360. [Google Scholar] [CrossRef]
- Wang, W.; Wang, W.; Yu, S.; Zhang, H.; Yang, J.; Li, X. Structural Characteristics and Driving Factors of Rhizosphere Microbial Communities in the Rhizosphere of Six Stipa Species Across the Ningxia Steppe. Sustainability 2024, 16, 10273. [Google Scholar] [CrossRef]
- Yang, H.X.L. Retention of fragmented fine woody debris enhances soil health, microbial activity, and ecosystem functions in urban forests of Northeast China. Plant Soil 2025, 1–19. [Google Scholar] [CrossRef]
- Yao, F.; Qi, W.; Cao, Y.; Liang, J.; Liu, X.; Liu, Z.; Lv, Y.; Wei, W.; Xu, W.; Yu, Y.; et al. The effects of a combination of maize/peanut intercropping and residue return on soil microbial nutrient limitation in maize fields. Appl. Soil Ecol. 2025, 206, 105874. [Google Scholar] [CrossRef]
- Sui, X.; Bao, X.; Xie, H.; Ba, X.; Yu, Y.; Yang, Y.; He, H.; Liang, C.; Zhang, X. Contrasting seasonal effects of legume and grass cover crops as living mulch on the soil microbial community and nutrient metabolic limitations. Agric. Ecosyst. Environ. 2025, 380, 109374. [Google Scholar] [CrossRef]
- Smith, A.P.; Marín-Spiotta, E.; Balser, T. Successional and seasonal variations in soil and litter microbial community structure and function during tropical postagricultural forest regeneration: A multiyear study. Glob. Change Biol. 2015, 21, 3532–3547. [Google Scholar] [CrossRef] [PubMed]
- Jiao, P.-Y.; Guo, W.; Chen, Z.-L.; Liu, X.; Hu, Y.-L.; Wang, Y.-Z. Soil Enzyme Stoichiometric Characteristics of Pinus massonianaPlantations at Different Stand Ages in Mid-subtropical Areas. Huan Jing Ke Xue Huanjing Kexue 2022, 43, 1059–1068. [Google Scholar] [CrossRef]
- Zhou, C.; Dun, X.; Tang, Q.; Long, Y.; Du, J.; Bao, M. Elevation-dependent shifts in soil phosphorus forms and phosphorus-solubilizing microbial diversity suggest enhanced bioavailable phosphorus cycling with rising temperatures. Microbiol. Spectr. 2025, 13, e01300–e01324. [Google Scholar] [CrossRef]
- Bai, J.; Sturchio, M.A.; Luo, Y.; Lin, G.; Cheng, X. Species turnover and climates co-dominate the carbon–water relationship in grasslands along an elevational gradient. Funct. Ecol. 2025, 39, 2123–2134. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Bremner, J.; Mulvaney, C. Nitrogen—Total. In Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties, 2nd ed.; Soil Science Society of America, Inc.: Madison, WI, USA, 1982; pp. 595–624. ISBN 9780891180722. [Google Scholar] [CrossRef]
- Olsen, S.R.; Sommers, L.E. Phosphorous. In Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Wiley: Hoboken, NJ, USA, 1982; pp. 403–430. ISBN 9780891180722. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Jenkinson, D. Measuring soil microbial biomass. Soil Biol. Biochem. 2004, 36, 5–7. [Google Scholar] [CrossRef]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Sinsabaugh, R.L.; Hill, B.H.; Weintraub, M.N. Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Biol. Biochem. 2016, 93, 1–7. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, X.; Zhang, X.; Ju, W.; Duan, C.; Guo, X.; Wang, Y.; Fang, L. Soil moisture mediates microbial carbon and phosphorus metabolism during vegetation succession in a semiarid region. Soil Biol. Biochem. 2020, 147, 107814. [Google Scholar] [CrossRef]
- Luo, H.; Yu, J.; Li, R.; Gu, J.-D.; Luo, L.; Zhang, Y.; He, Y.; Xiao, Y.; Deng, S.; Zhang, Y.; et al. Microbial biomass C:N:P as a better indicator than soil and ecoenzymatic C:N:P for microbial nutrient limitation and C dynamics in Zoige Plateau peatland soils. Int. Biodeterior. Biodegrad. 2022, 175, 105492. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Wang, Z.; Long, A.; Ji, X.; Gong, X.; Jiang, Y.; Qi, H. Straw return increased maize phosphorus uptake and grain yield by alleviating rhizosphere soil microbial metabolism limitation: Insights from ecoenzymatic stoichiometry. Plant Soil 2025, 1–9. [Google Scholar] [CrossRef]
- Veen, G.F.; Olff, H.; Duyts, H.; Van Der Putten, W.H. Vertebrate herbivores influence soil nematodes by modifying plant communities. Ecology 2010, 91, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Njiru, L.G.; Yegon, J.R.; Mwithiga, G.; Micheni, A.; Gitari, N.J.; Mairura, F.S. Restoring soil nutrient stocks using local inputs, tillage and sorghum-green gram intercropping strategies for drylands in Eastern Kenya. Heliyon 2023, 9, e20926. [Google Scholar] [CrossRef] [PubMed]
- Lefcheck, J.S.; Freckleton, R. piecewiseSEM: Piecewise structural equation modelling inr for ecology, evolution, and systematics. Methods Ecol. Evol. 2015, 7, 573–579. [Google Scholar] [CrossRef]
- Guan, Z.-H.; Jia, B.; Niu, Z.-q.; Mou, X.-M.; Chen, J.; Li, F.-C.; Wu, Y.-N.; Ning, S.; Yakov, K.; Li, X.G. Humidity controls soil organic carbon accrual in grassland on the Qinghai–Tibet Plateau. Soil Biol. Biochem. 2025, 201, 109655. [Google Scholar] [CrossRef]
- Cui, H.; Chen, S.; Song, H.; Liu, Z.; Chen, J.; Zhang, A.; Xiao, S.; Jiang, X.; Yang, Z.; Li, X.; et al. Contrasting mechanisms of non-vascular and vascular plants on spatial turnover in multifunctionality in the Antarctic continent. J. Ecol. 2024, 112, 1624–1637. [Google Scholar] [CrossRef]
- Bicharanloo, B.; Bagheri Shirvan, M.; Dijkstra, F.A. Decoupled cycling of carbon, nitrogen, and phosphorus in a grassland soil along a hillslope mediated by clay and soil moisture. Catena 2022, 219, 106648. [Google Scholar] [CrossRef]
- Nielsen, P.L.; Andresen, L.C.; Michelsen, A.; Schmidt, I.K.; Kongstad, J. Seasonal variations and effects of nutrient applications on N and P and microbial biomass under two temperate heathland plants. Appl. Soil Ecol. 2009, 42, 279–287. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Hai, X.; Shangguan, Z.; Deng, L. Dynamics of soil microbial C:N:P stoichiometry and its driving mechanisms following natural vegetation restoration after farmland abandonment. Sci. Total Environ. 2019, 693, 133613. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Chen, X.; Jing, X.; Zhu, B. A meta-analysis of soil extracellular enzyme activities in response to global change. Soil Biol. Biochem. 2018, 123, 21–32. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, S.; Shen, H.; Zhao, M.; Xu, L.; Xing, A.; Fang, J.; Sayer, E. Soil extracellular enzyme activity and stoichiometry in China’s forests. Funct. Ecol. 2020, 34, 1461–1471. [Google Scholar] [CrossRef]
- Zuccarini, P.; Asensio, D.; Ogaya, R.; Sardans, J.; Peñuelas, J. Effects of seasonal and decadal warming on soil enzymatic activity in a P-deficient Mediterranean shrubland. Glob. Change Biol. 2020, 26, 3698–3714. [Google Scholar] [CrossRef]
- Malik, A.A.; Martiny, J.B.H.; Brodie, E.L.; Martiny, A.C.; Treseder, K.K.; Allison, S.D. Defining trait-based microbial strategies with consequences for soil carbon cycling under climate change. ISME J. 2020, 14, 1–9. [Google Scholar] [CrossRef]
- Yang, T.; Li, X.; Hu, B.; Li, F.; Wei, D.; Wang, Z.; Huang, L.; Bao, W. Climate and soil properties shape latitudinal patterns of soil extracellular enzyme activity and stoichiometry: Evidence from Southwest China. Appl. Soil Ecol. 2024, 197, 105319. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, R.; Vilonen, L.; Qu, Y.; Fu, X.; Shi, B.; Cui, H.; Gao, W.; Cai, H.; Sun, W. Grazing and nitrogen addition restructure the spatial heterogeneity of soil microbial community structure and enzymatic activities. Funct. Ecol. 2021, 35, 2763–2777. [Google Scholar] [CrossRef]
- Thomas, F.M.; Molitor, F.; Werner, W. Lignin and cellulose concentrations in roots of Douglas fir and European beech of different diameter classes and soil depths. Trees 2013, 28, 309–315. [Google Scholar] [CrossRef]
- Tan, B.; Yin, R.; Yang, W.; Zhang, J.; Xu, Z.; Liu, Y.; He, S.; Zhou, W.; Zhang, L.; Li, H.; et al. Soil fauna show different degradation patterns of lignin and cellulose along an elevational gradient. Appl. Soil Ecol. 2020, 155, 103673. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, B.; Li, J.; Cheng, X. Soil microbial relative resource limitation exhibited contrasting seasonal patterns along an elevational gradient in Yulong Snow Mountain. Funct. Ecol. 2023, 37, 1328–1338. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Zhao, W.; Liu, K.; Chen, Y.; Wang, F.; Mao, G.; Ji, M. Distinct genes and microbial communities involved in nitrogen cycling between monsoon- and westerlies-dominated Tibetan glaciers. Nat. Commun. 2025, 16, 5926. [Google Scholar] [CrossRef] [PubMed]
- Lie, Z.; Huang, W.; Liu, X.; Zhou, G.; Yan, J.; Li, Y.; Huang, C.; Wu, T.; Fang, X.; Zhao, M.; et al. Warming leads to more closed nitrogen cycling in nitrogen-rich tropical forests. Glob. Change Biol. 2021, 27, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Niu, D.; Gherardi, L.A.; Liu, Y.; Wang, Y.; Elser, J.J.; Fu, H. Linkages of stoichiometric imbalances to soil microbial respiration with increasing nitrogen addition: Evidence from a long-term grassland experiment. Soil Biol. Biochem. 2019, 138, 107580. [Google Scholar] [CrossRef]
- Chen, Y.L.; Ding, J.Z.; Peng, Y.F.; Li, F.; Yang, G.B.; Liu, L.; Qin, S.Q.; Fang, K.; Yang, Y.H. Patterns and drivers of soil microbial communities in Tibetan alpine and global terrestrial ecosystems. J. Biogeogr. 2016, 43, 2027–2039. [Google Scholar] [CrossRef]
- Zhang, C.; Lei, S.; Wu, H.; Liao, L.; Wang, X.; Zhang, L.; Liu, G.; Wang, G.; Fang, L.; Song, Z. Simplified microbial network reduced microbial structure stability and soil functionality in alpine grassland along a natural aridity gradient. Soil Biol. Biochem. 2024, 191, 109366. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; He, L.; Xu, X.; Wang, J.; Ren, C.; Guo, Y.; Zhao, F. Metagenomic highlight contrasting elevational pattern of bacteria- and fungi-derived compound decompositions in forest soils. Plant Soil 2023, 490, 617–629. [Google Scholar] [CrossRef]
- Sun, T.; Wang, Y.; Guo, Y.; Jing, X.; Feng, W. Contrasting elevational patterns of microbial carbon and nutrient limitation in soil from alpine meadow to desert. Catena 2023, 223, 106901. [Google Scholar] [CrossRef]
- Ramin, K.I.; Allison, S.D. Bacterial Tradeoffs in Growth Rate and Extracellular Enzymes. Front. Microbiol. 2019, 10, 2956. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Liptzin, D. C:N:P stoichiometry in soil: Is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Follstad Shah, J.J. Ecoenzymatic stoichiometry of recalcitrant organic matter decomposition: The growth rate hypothesis in reverse. Biogeochemistry 2010, 102, 31–43. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef]
- Dong, L.; Zeng, W.; Wang, A.; Tang, J.; Yao, X.; Wang, W. Response of Soil Respiration and Its Components to Warming and Dominant Species Removal along an Elevation Gradient in Alpine Meadow of the Qinghai–Tibetan Plateau. Environ. Sci. Technol. 2020, 54, 10472–10482. [Google Scholar] [CrossRef]
- Liu, S.; Ru, J.; Guo, X.; Gao, Q.; Deng, S.; Lei, J.; Jian, S.; Changchun, Z.; Shiqiang, W.; Yang, Y. Altered precipitation and nighttime warming reshape the vertical distribution of soil microbial communities. Msystems 2025, 10, e0124824. [Google Scholar] [CrossRef]
- Sieradzki, E.T.; Nuccio, E.E.; Pett-Ridge, J.; Firestone, M.K. Expression of macromolecular organic nitrogen degrading enzymes identifies potential mediators of soil organic N availability to an annual grass. ISME J. 2023, 17, 967–975. [Google Scholar] [CrossRef] [PubMed]
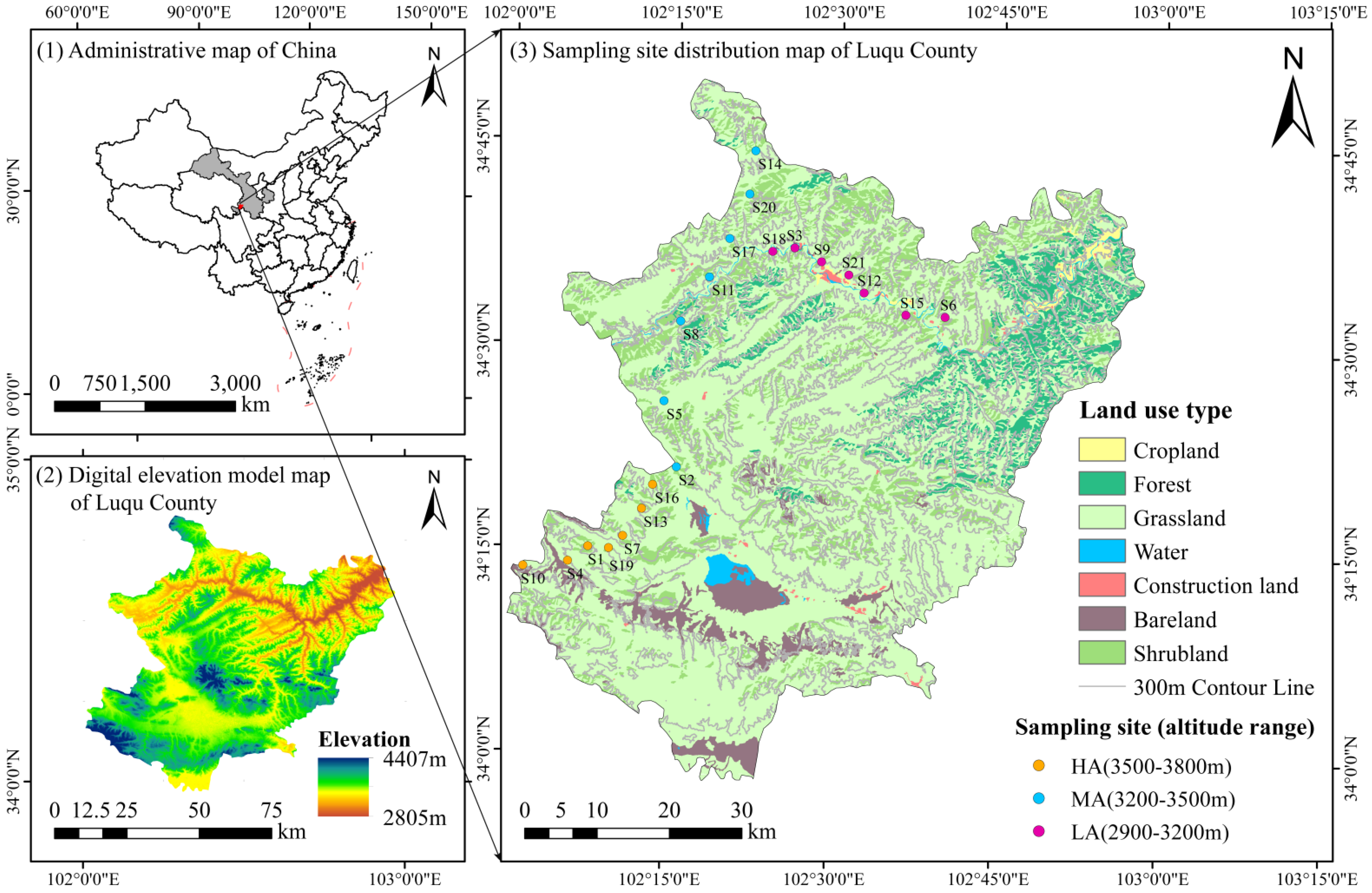
| Sample Number | Dominant Species | Altitude/masl | Longitude and Latitude |
|---|---|---|---|
| S1 | Festuca ovina | 3749 | 102°7′40″ E, 34°15′5″ N |
| S2 | Elymus dahuricus | 3453 | 102°15′35″ E, 34°21′4″ N |
| S3 | Elymus dahuricus | 3122 | 102°25′57″ E, 34°37′23″ N |
| S4 | Elymus dahuricus, Anemone vitifolia | 3646 | 102°5′50″ E, 34°13′59″ N |
| S5 | Elymus dahuricus | 3459 | 102°14′16″ E, 34°25′54″ N |
| S6 | Elymus dahuricus | 3081 | 102°39′54″ E, 34°32′32″ N |
| S7 | Hippophae rhamnoides, Festuca ovina, Elymus dahuricus | 3692 | 102°10′50″ E, 34°15′55″ N |
| S8 | Festuca ovina | 3413 | 102°15′37″ E, 34°31′47″ N |
| S9 | Festuca ovina | 3139 | 102°28′25″ E, 34°36′24″ N |
| S10 | Elymus dahuricus | 3762 | 102°1′46″ E, 34°13′32″ N |
| S11 | Elymus dahuricus | 3436 | 102°18′1″ E, 34°35′5″ N |
| S12 | Leontopodium nanum | 3092 | 102°32′25″ E, 34°34′11″ N |
| S13 | Anemone vitifolia | 3723 | 102°12′30″ E, 34°17′57″ N |
| S14 | Potentilla fruticosa | 3389 | 102°22′7″ E, 34°44′26″ N |
| S15 | Taraxacum mongolicum, Lycium barbarum, Hippophae rhamnoides | 2996 | 102°36′18″ E, 34°32′38″ N |
| S16 | Potentilla fruticosa | 3722 | 102°13′26″ E, 34°19′44″ N |
| S17 | Elymus dahuricus | 3316 | 102°19′55″ E, 34°37′56″ N |
| S18 | Juniperus formosana | 3128 | 102°23′54″ E, 34°37′5″ N |
| S19 | Lycium barbarum | 3759 | 102°9′34″ E, 34°15′ N |
| S20 | Hippophae rhamnoides, Gentiana scabra | 3317 | 102°21′40″ E, 34°41′15″ N |
| S21 | Rhodiola crenulata, Lycium barbarum | 3141 | 102°30′58″ E, 34°35′30″ N |
| Enzyme | Abbreviation | Function | Substrate | Time (h) |
|---|---|---|---|---|
| β-1,4-glucosidase | BG | EC3.2.1.21 | ρNP-β-D-glucopyranoside | 4 |
| β-1,4-cellobiohydrolase | CBH | EC3.2.1.91 | 4-ρNP-β-D-cellobioside | 4 |
| β-1,4-N-acetylglucosaminidase | NAG | EC3.2.1.14 | ρNP-N-acetyl-β-D-glucosaminide | 4 |
| Leucine aminopeptidase | LAP | EC 3.4.11.1 | Leucine-ρ-nitroanilide | 4 |
| acid phosphatase | ACP | EC 3.1.3.1 | ρNP-phosphate | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wu, J.; Ma, W.; Zhou, Z.; Zhang, H.; Cai, L.; Lin, D. Stoichiometric Responses of Soil Microbes and Enzymes to Altitudinal Gradients in Alpine Meadows. Microorganisms 2025, 13, 2692. https://doi.org/10.3390/microorganisms13122692
Li Y, Wu J, Ma W, Zhou Z, Zhang H, Cai L, Lin D. Stoichiometric Responses of Soil Microbes and Enzymes to Altitudinal Gradients in Alpine Meadows. Microorganisms. 2025; 13(12):2692. https://doi.org/10.3390/microorganisms13122692
Chicago/Turabian StyleLi, Yongqian, Jun Wu, Wenjun Ma, Zhengqian Zhou, Hongming Zhang, Liqun Cai, and Dong Lin. 2025. "Stoichiometric Responses of Soil Microbes and Enzymes to Altitudinal Gradients in Alpine Meadows" Microorganisms 13, no. 12: 2692. https://doi.org/10.3390/microorganisms13122692
APA StyleLi, Y., Wu, J., Ma, W., Zhou, Z., Zhang, H., Cai, L., & Lin, D. (2025). Stoichiometric Responses of Soil Microbes and Enzymes to Altitudinal Gradients in Alpine Meadows. Microorganisms, 13(12), 2692. https://doi.org/10.3390/microorganisms13122692





