3.2. Chemical and Microbiological Composition of Must and UHPH Treatment
The results of the chemical characterization of grape juice after being clarified and submitted to the different treatments are shown in
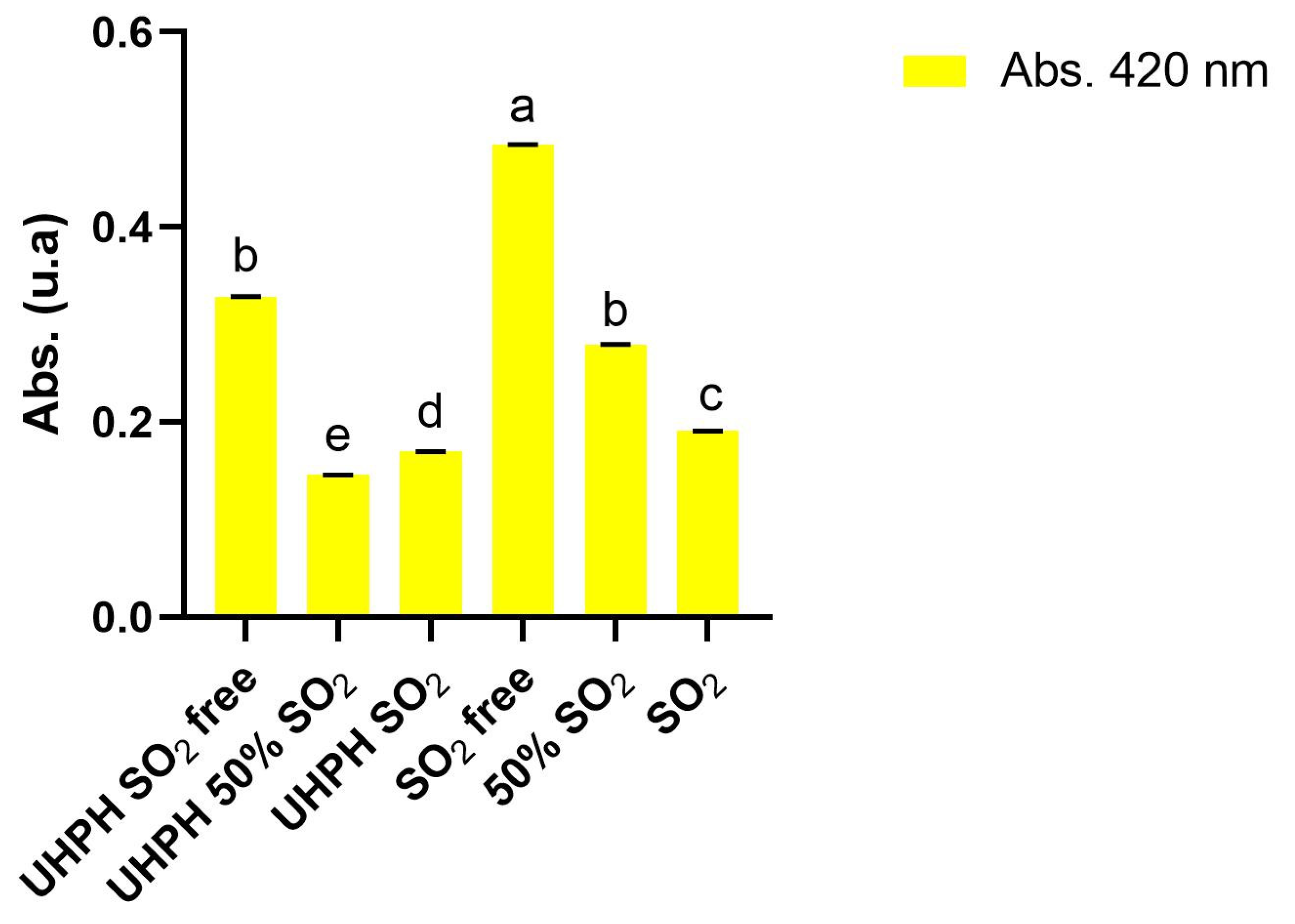
Table 2. No significant differences among treatments were found for degrees brix, glucose+fructose concentration, potential alcohol strength, total acidity, pH, ammonium and primary amino nitrogen, and L-malic acid. On the other hand, grape must color was significantly impacted by the UHPH technology and sulphur dioxide concentration. Higher absorbances at 420 nm were observed in samples not UHPH technology-treated and as the dose of sulphur dioxide was decreasing in all cases (
Figure 3). The addition of sulphur dioxide as well as the dose had a direct impact on absorbance at 420 nm for all the studied treatments. In the treatments in which UHPH technology was applied, the color of grapes must had lower intensities compared with those without UHPH treatment, with the UHPH treatment group having a lower yellow and total coloration (
Table 1 and
Figure 4). The effect of sulphur dioxide on the color intensity of musts and wines has been reported before [
39,
40], and it was also observed in this study. In the case of the UHPH technology group, this decrease in the total coloration of musts is due to the antioxidant capacity of must juice for enzymatic oxidations by polyphenol oxidases (PPOs) [
13]. Similar absorbances at 420 nm were obtained by UHPH-free SO
2 and 50% SO
2, reinforcing the idea that the present technology could help winemakers to reduce the use of sulphur dioxide without reducing the product quality and without a negative impact on organoleptic characteristics.
Microbiological concentrations of yeast, lactic acid bacteria, acetic acid bacteria, and
Brettanomyces bruxellensis were determined for all the different treatments. For all the treatments where UHPH technology was applied, there was no detection of any of the oenological microorganisms—yeast, acetic and lactic acid bacteria, and
Brettanomyces bruxellensis—by culture plate and qPCR. These results correspond to those obtained by other authors [
24]. In the case of must without the application of UHPH technology, 10
5 cells/mL for yeast, 10
2 cells/mL for acetic acid bacteria, 10
2 cells/mL for lactic bacteria populations were detected.
B. bruxellensis was not detected. These results agree with previously published data [
41]. Sulphur dioxide concentration has a direct effect on the initial populations, especially of bacteria, but without the possibility the UHPH technology has for obtaining sterilized must.
3.4. Chemical, Microbiological, and Sensory Composition of Wines After Alcoholic Fermentation
According to the basic parameters, no significant differences were found in parameters like alcohol, volatile acidity, total acidity, pH, and the L-malic and L-lactic acids of the studied wines coming from musts treated or not treated by UHPH with different SO
2 concentrations (
Table 3). On the other hand, significant differences were detected in color and oxidation-related parameters. Absorbance at 420 nm, often used as an indicator of oxidative browning (40), was significantly lower in the UHPH-treated samples with partial or SO
2-free treatments (0.075–0.077) compared to the SO
2 without UHPH treatment (0.131), suggesting a protective potential effect of UHPH against oxidative browning. The UHPH SO
2-free sample showed lower oxidation (0.104) than the full SO
2 treatment without UHPH technology, indicating that UHPH may reduce the need for SO
2 under certain conditions. Absorbance at 420 nm was highest in the SO
2-treated sample (0.131), followed by the 50% SO
2 sample (0.105), while all UHPH treatments showed significantly lower intensities, especially the UHPH 50% SO
2 sample (0.075), suggesting that UHPH may lead the prevention of polyphenol oxidation [
13]. In terms of CIE Lab color parameters, all wines maintained very high Luminosity (L*) values (>97), reflecting the expected brightness of young wines. The b* coordinates (yellow–blue axis) were lower in the UHPH 50% SO
2 and UHPH SO
2 treatments than wine with SO
2, indicating a lower oxidative effect and UHPH mitigation of browning reactions. UHPH treatment independent of SO
2 concentrations had an important and significant impact on wine turbidity, with the UHPH SO
2-free treatment showing low turbidity (1.0 NTU), while free or SO
2-containing wines without UHPH showed higher turbidity (>7.0 NTU). These results suggest that UHPH may improve the clarity of wines. The Folin–Ciocalteu index, which is an estimate of the total phenolic content, was highest in the SO
2-free and SO
2 50% treatments, 6.11 and 5.08, respectively, with samples obtained with UHPH treatment showing lower values, suggesting a partial degradation or structural modification of phenols [
27,
42].
Even though all musts treated by UHPH were sterilized to remove all oenological microorganisms, they can still be present after the alcoholic fermentation for several reasons. On one hand, yeasts inoculated during the alcoholic fermentation can be found in final wines. Moreover, microorganisms can be found on the equipment in the cellar (yeasts and lactic and acetic acid bacteria), that can contaminate the wine during the winemaking process. For this reason, the population conditions after alcoholic fermentation, clarification, and bottling were controlled. Details of all microbiological results after bottling are shown in
Table 4. The results show that culturable populations of yeast, acetic acid bacteria, lactic acid bacteria, and
Brettanomyces bruxellensis were below the detection limit (<1 CFU/mL) for all samples treated by UHPH (
Table 4). In the case of samples untreated by UHPH, culturable yeast and acetic acid bacteria were detected in the 50% SO
2 treatment. The populations of acetic acid bacteria, lactic acid bacteria, and
Brettanomyces bruxelensis detected by RT-PCR were also lower than the detection limits (<20 cells/mL) for all the samples treated by UHPH. Only in the case of yeast, different populations (between 103 and 118 cells/mL) were found in all the samples. The RT-PCR technique detects the presence of DNA, which may come from either live or dead cells, so the population found can be either viable but not cultivable or dead. With regards to the samples not treated by UHPH technology and analyzed by RT-PCR, different populations of yeast were detected (from 109 to 22.825 cells/mL) and acetic acid bacteria at 56 cells/mL for 50% SO
2 treatment. Wines produced from must treated with UHPH did not have microorganisms that came from the grape, but, like the non-treated wines, were susceptible to contamination during the winemaking process, as is shown in the case of 50% SO
2 for yeasts. Therefore, the UHPH treatment reduces the sources of growth of non-desirable microorganisms, making wines more stable from a microbiological point of view.
The analysis of fermentative volatile compound groups after the alcoholic fermentation and oxidation conditions shows different behaviors depending on the studied treatment. Ultra-High-Pressure Homogenization (UHPH) and sulphur dioxide addition at different concentrations did not produce significant differences (
p < 0.05) among samples in total ethyl esters, total acetates, and total fatty acids (
Figure 5). This result suggests that, prior to oxidative stress, both UHPH and SO
2 had a limited impact on the concentration of major aroma-active esters, alcohols, and fatty acids. However, a slight decrease in acetate esters was noted in the SO
2-treated samples compared to the SO
2-free samples, potentially reflecting early-stage ester hydrolysis or transformation [
43]. These results indicate the neutral effect of UHPH treatment on the fermentative aromas produced by yeast during alcoholic fermentation. These results are in accordance with the results obtained by [
44].
In terms of the oxidation markers of wines after alcoholic fermentation, the main aromatic compounds associated were analyzed and data considered the sum of all the compounds of each family: alkenals, strecker aldehydes, lactones, ketones, and aldehydes (
Table 5). The alkenals family were formed by (E)-2-hexenal, (E)-2-heptenal, (E)-2-octenal, and (E)-2-nonenal, the strecker aldehydes family by 3-methylbutanal, 2-phenylacetaldehyde, and methional, the lactone family by γ- nonalactona and 3-methyl-2,4-nonadiona, furans by 3-hydroxy-4,5-dimethylfuran-2(5H)-one, and the aldehydes family by benzaldehyde.
Comparable values were obtained between the UHPH SO2-free and SO2 treatments, exhibiting comparable levels of oxidative aromas. These results indicate the potential of the UHPH technique to produce wines without SO2 with the same oxidation level as wines with the conventional use of SO2. The wine with a lower concentration of oxidative aromatic compounds was obtained with a conventional dose of SO2, and the wine with a higher oxidative aroma intensity was SO2-free, both without the use of UHPH.
The compounds that most strongly contribute to the high levels of oxidative aromas in wines with insufficient oxidation protection are aldehydes, particularly 2-phenylacetaldehyde and benzaldehyde, as shown in
Table 5. These compounds are typically associated with notes of faded flowers, honey, and bitter almond. ANOVA results revealed significant differences in their concentrations among treatments: untreated or partially treated wines exhibited the highest levels, whereas SO
2 and/or UHPH significantly reduced them. Overall, these aldehydes emerge as key contributors to the oxidative aroma profile, explaining the stronger sensory perception of oxidative notes in inadequately protected wines.
As shown in
Figure 6a,b, the formation of oxidative aromas after fermentation was relatively low in wines treated only with SO
2 (SO
2), with values comparable to those obtained exclusively with UHPH (UHPH SO
2-free). All other combinations displayed higher levels of initial oxidative aromas, with the SO
2-free treatment showing the highest concentration. After the oxidation process, the SO
2-free oxi. treatment showed the highest increase in oxidative aromas, followed by all other treatments without the use of UHPH (50% SO
2 oxi. and SO
2 oxi.). All treatments which combined the use of UHPH and sulphur dioxide (UHPH 50% SO
2 oxi. and UHPH SO
2 oxi.) showed lower concentrations of oxidative aromas.
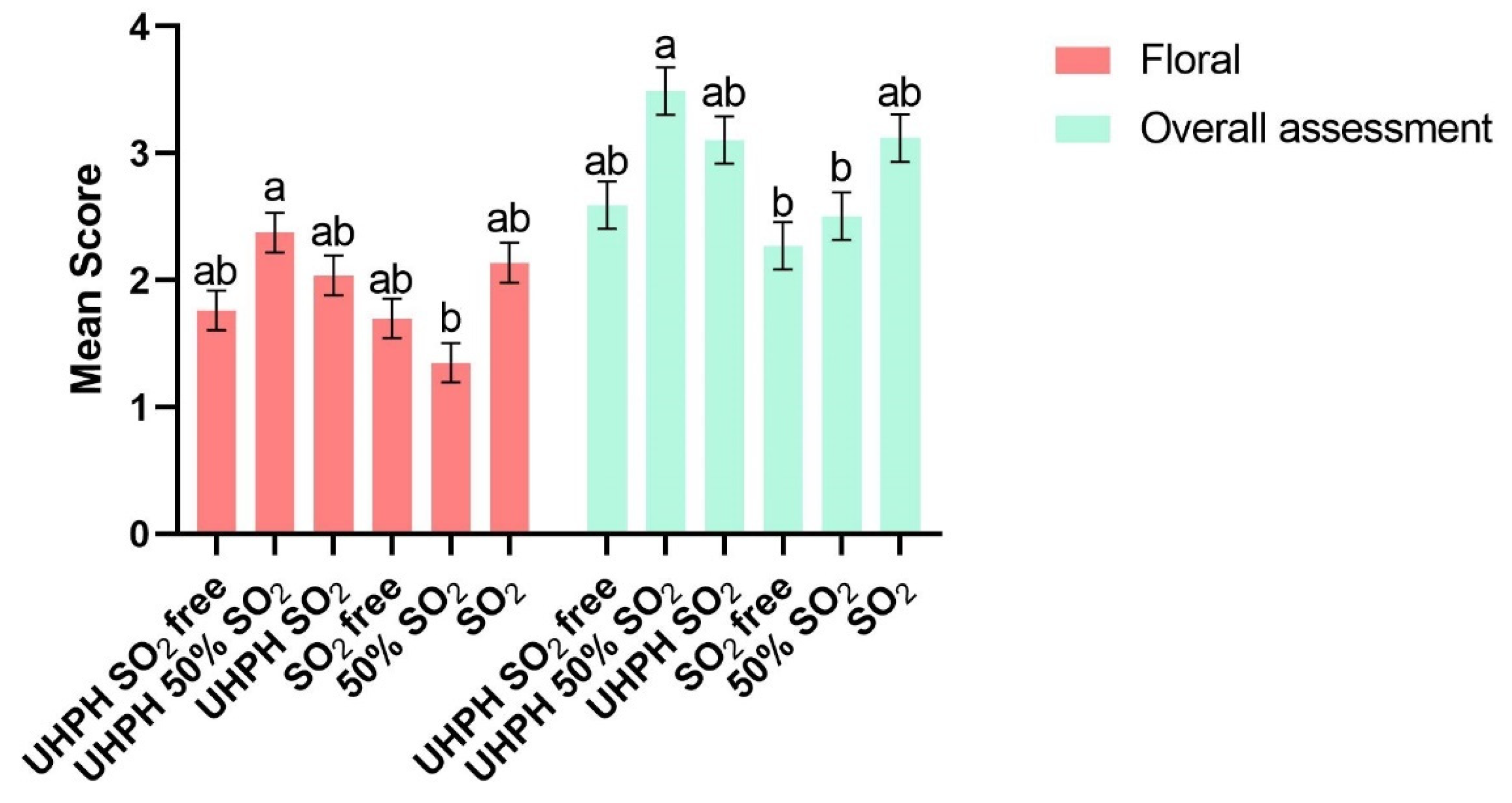
A total of nineteen sensorial parameters were analyzed by the tasting panel. Between them, significant differences were found in two parameters, that being floral and overall assessment attributes (
Figure 7). The best-rated wine by floral and overall assessment attributes was, in both cases, UHPH 50% SO
2. As these results show, the combination of the UHPH technology with lower doses of sulphur dioxide could be a good option for wineries to produce wines with a lower concentration of sulphur dioxide without the loss of sensory characteristics and flavors. No significant differences were found for wines produced without the use of sulphur dioxide in floral and overall assessment attributes.
3.5. Effect of UHPH Technology Combined with SO2 Concentrations After Forced Aging
After the forced oxidation processes, analyses of the basic chemical characteristics, fermentative aromas, and oxidative aromas were carried out to evaluate the impact of the new technology combined with different doses of SO
2 on the capacity of wines to fight against the accelerated oxidation process. According to the basic characterization, no significant differences were found between alcoholic degree, volatile acidity, total acidity, pH, and L-malic and L-lactic acid. The greatest impact of the accelerated oxidation on the basic parameters were in color characteristics, shown in
Table 6 and
Figure 8. Oxidation caused an increase in absorbance at 420 nm, resulting in a progressive darkening of the wine. This effect was particularly pronounced in untreated wines and those treated only with UHPH, which reached the highest values in all color bands. In contrast, wines treated with SO
2, both individually and in combination with UHPH (even at reduced doses of 50%), showed significantly lower values, closer to those of the initial wine. These results confirm that SO
2 is an effective protector against oxidative browning and that its combination with UHPH enhances this effect, allowing color stability to be maintained even with lower concentrations of sulphur dioxide.
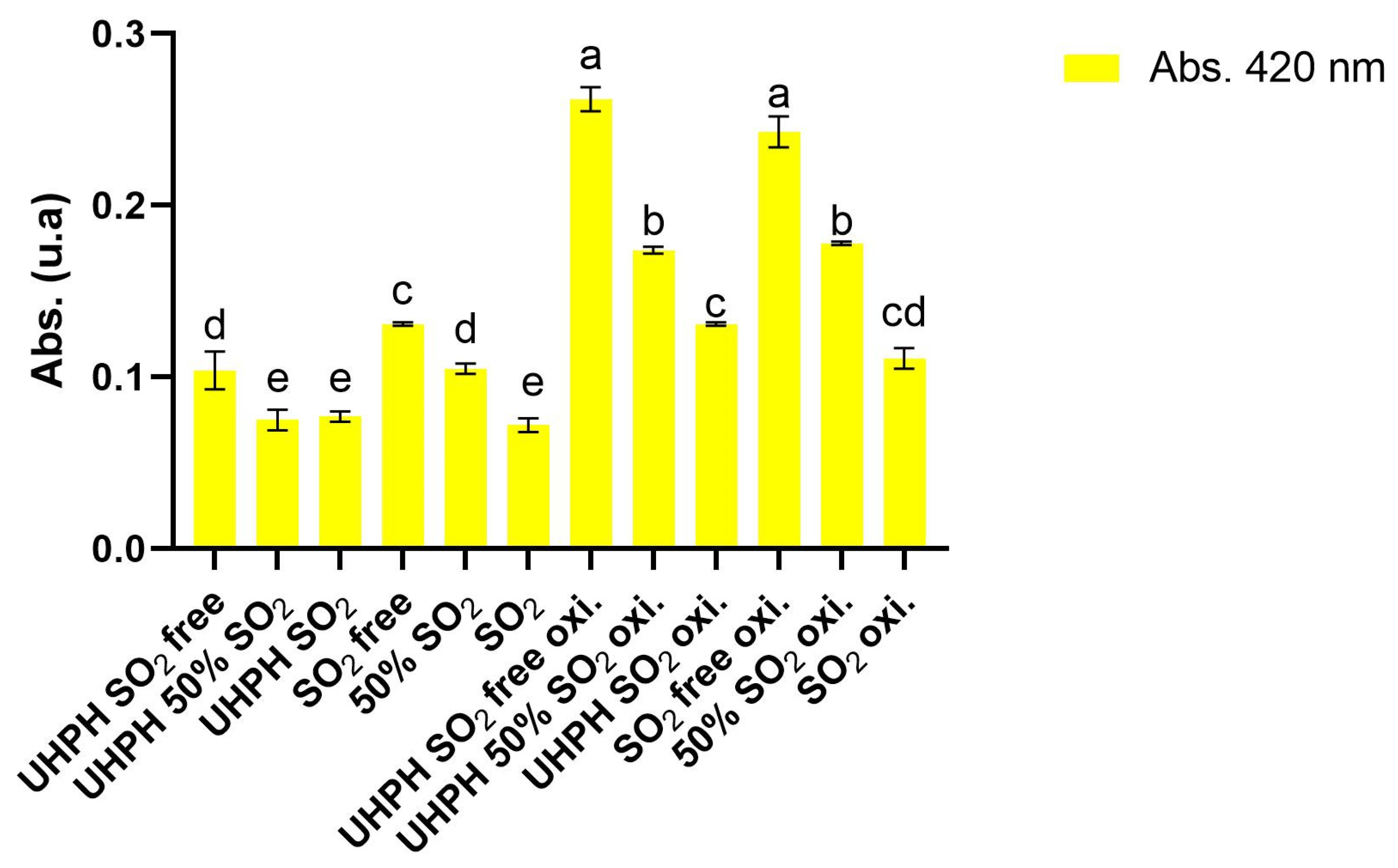
Focusing only on the values after the forced oxidation process, the colors of the wines reveal significant differences related to oxidation depending on the treatment strategy used (UHPH, free, partial, and full use of SO2). Absorbance 420 nm, as an oxidative browning indicator, was highest in the UHPH SO2-free and SO2-free treatments (0.262 and 0.243, respectively), confirming their greater susceptibility to oxidation degradation. Simultaneously, samples treated with UHPH SO2 and SO2 without UHPH showed the lowest 420 nm values (0.131 and 0.111, respectively), reflecting higher oxidation resistance. These results align with the known antioxidant effect of SO2 and suggest that UHPH could be used as additional protection against oxidation processes once wines are bottled. Oxidative polymerization and browning were more pronounced in the absence of SO2. The L* values in the CIE Lab coordinates indicate a clear difference in brightness, with the samples of UHPH SO2 and SO2 showing higher values (>98), higher visual brightness, and less browning. On the other hand, UHPH SO2-free and SO2-free samples had significantly lower L* values, confirming their oxidation level due to the low concentration or absence of SO2. Regarding the b* coordinate, which indicates the color preservation, the highest values were, notably, shown for the UHPH SO2-free and SO2-free treatments (15.39 and 14.38, respectively), and the lowest values were found in the UHPH SO2 and SO2 treatments (8.82 and 7.15, respectively). No significant differences were found for the a* CIE Lab coordinate (red–green axis).
In contrast, the forced oxidation process reduced the levels of volatile ethyl esters and acetates across all treatments (
Table 7). Notably, the UHPH SO
2-free and SO
2-free samples showed the largest decreases in both ethyl esters and acetates, suggesting an increased susceptibility to oxidative degradation without the presence of sulphur dioxide. In these treatments, the UHPH SO
2-free treatment exhibited a dramatic drop in total ethyl esters (from 30.874 to 9.198 µg/L), highlighting the protective role of SO
2 and the limited antioxidant capacity of UHPH without any other antioxidant agent preserving ethyl ester content under stress treatments like accelerating aging. Samples treated with SO
2 or 50% SO
2, in both the UHPH and conventional contexts, maintained significantly higher levels of ethyl esters during forced oxidation, with reductions between 25 and 75% of total ethyl esters compounds. These results indicated that partial SO
2 addition, combined with UHPH, can offer a synergistic protective effect, being one of the most significant results of the present study. Total alcohol levels remained stable across treatments, with oxidative processes yielding no significant differences in alcohol concentrations between the SO
2, 50% SO
2, and SO
2-free treatments, implying that alcohol compounds are less propitious to oxidative degradation than other compounds [
32]. The percentage of total aroma loss for treatments without SO
2 for the UHPH and no-UHPH treatments were 63% and 56%, respectively, 25% and 21% for the 50% SO
2 UHPH and no-UHPH treatments, respectively, and finally, 35% and 28% for the UHPH SO
2 and SO
2 treatments. Overall, these findings suggest that while UHPH alone may not be enough for preventing the oxidative loss of key aroma compounds, its combination with reduced SO
2 doses or the conventional use of SO
2 could be a viable enological strategy to preserve wine aroma under oxidative stress, contributing to the development of more sustainable wine processing alternatives.
In relation to oxidative aromas, it was observed that they reached their highest values in wines without the application of suphur dioxide or UHPH, as we can see in
Figure 6 and
Table 5. In contrast, the combined treatment of UHPH and SO
2 at 50% showed the greatest resistance to oxidation at the aromatic level, followed by the same treatment with SO
2 at 100%. Overall, these results show that the application of UHPH in combination with SO
2 is an effective strategy for delaying the appearance of oxidative notes, while also allowing for a reduction in the dose of sulfites without compromising the sensory stability of the wine.
As was expected, the accelerated oxidation process had an important effect on the concentration of oxidation markers for all the wines (
Table 5).
Figure 6b shows the oxidative aroma levels in the wines after forced oxidation. A marked increase was observed in the untreated wine (SO
2-free oxid.), followed by the wine containing only 50% SO
2 without UHPH. Intermediate values were found in wines treated with SO
2 alone or UHPH alone. In contrast, the lowest concentrations were detected in wines treated with both techniques, as well as in those treated with 50% SO
2 combined with UHPH. These results indicate that the combined application of SO
2 and UHPH provides the greatest protection against oxidative aroma development, while SO
2 and UHPH applied individually confer a similar, yet insufficient, level of protection to maintain a low oxidative profile. After oxidation, wines showed significantly higher concentrations of aldehydes (2-phenylacetaldehyde and 3-methylbutanal) and lactones, as confirmed by the ANOVA analyses. Among the aldehydes, the highest levels were observed in oxidized wines without SO
2 and without UHPH treatment, whereas the application of SO
2, UHPH, or their combination resulted in significantly lower concentrations. A similar trend was observed for γ-nonalactone: wines lacking protective treatments accumulated the highest amounts, while those treated with SO
2 and/or UHPH exhibited reduced levels. These results demonstrate that both SO
2 addition and UHPH processing mitigate the oxidative formation of aldehydes and lactones, thereby preventing the development of off-aromas such as dried fig, peach, or coconut notes and contributing to a more stable aromatic profile.
This study demonstrates that the combined application of SO2 (at both 100% and 50%) with UHPH was the most effective strategy, leading to the lowest concentrations of oxidative aroma compounds and providing greater resistance to their formation over time. When applied individually, SO2 or UHPH also reduced aldehyde and lactone accumulation, although their protective effect was less sustained compared to the combined treatment. In contrast, wines produced without any treatment consistently exhibited the highest levels of oxidative aromas. Finally, this study highlights the importance of combining strategies to replace or remove sulphur dioxide in winemaking to obtain high-quality wines. The effects of UHPH technology from a sensory and volatile perspective had not been studied yet in relation to their potential for higher stability and shelf life.