Chemical Alkaline Leaching and Alkaliphile-Driven Bioleaching: Advancing Metal Recovery from Ores
Abstract
1. Introduction
2. Alkaline Chemical Leaching
2.1. Alkaline Matrix
2.1.1. Sodium Hydroxide or Potassium Hydroxide
2.1.2. Glycine
2.1.3. Sodium Sulfide
2.1.4. Sodium Hypochlorite
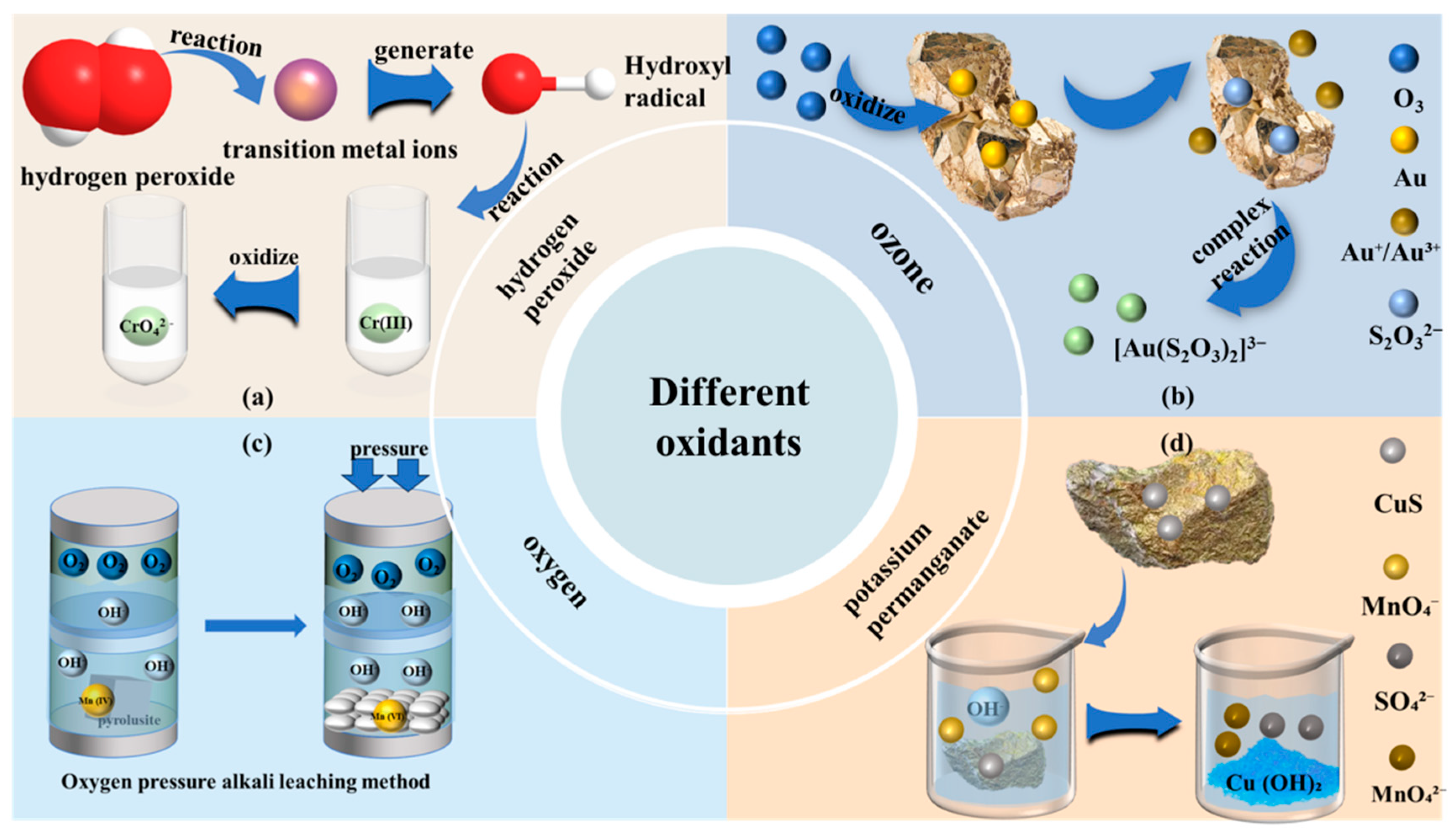
2.2. Oxidants
2.2.1. Hydrogen Peroxide
2.2.2. Ozone
2.2.3. Oxygen
2.2.4. Potassium Permanganate
2.3. Pressurized Operation
3. Bioleaching
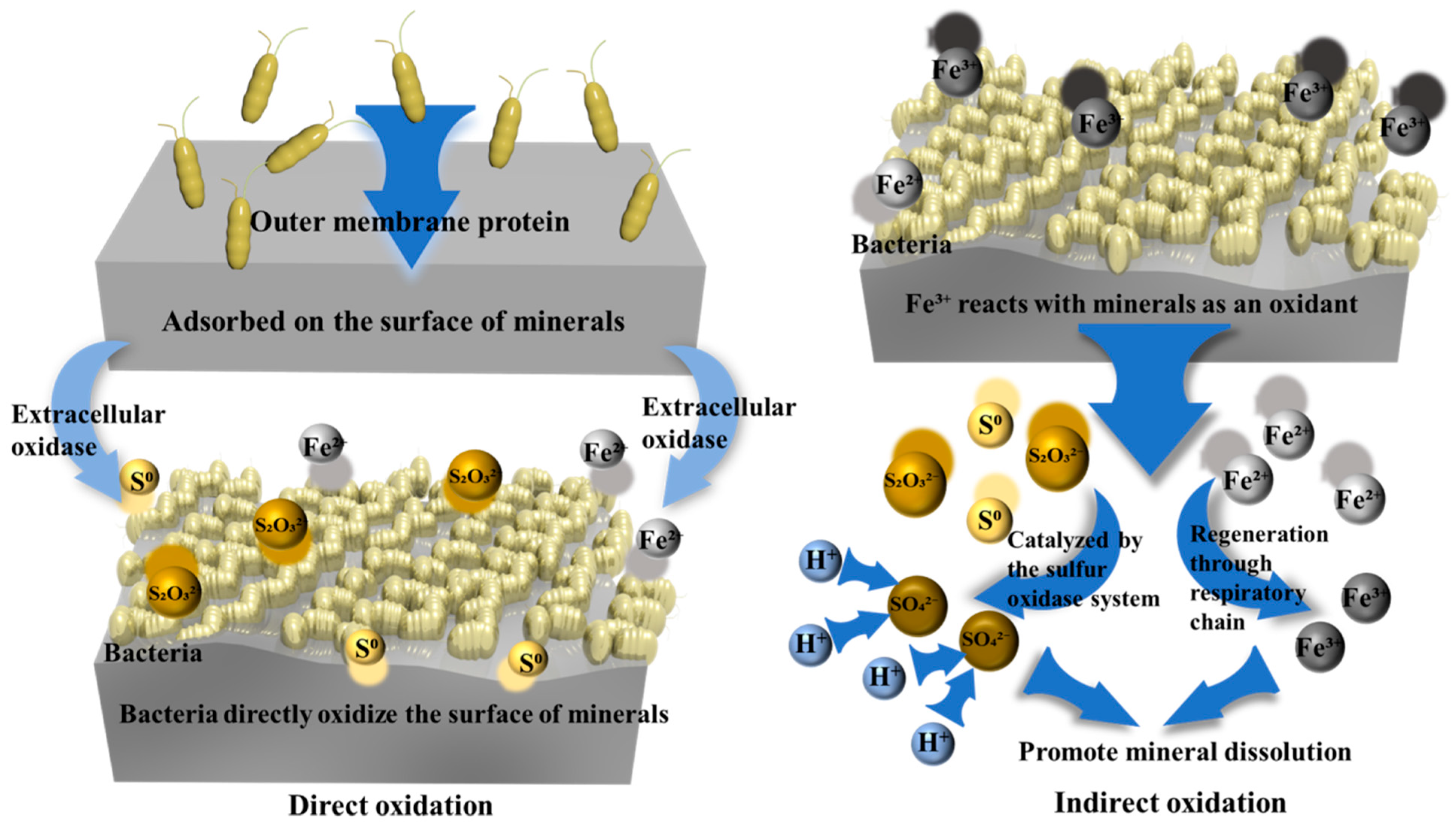
3.1. Acidophilic Microorganisms and Bioleaching Mechanisms
3.2. Alkaline-Tolerant Microorganisms and Bioleaching Mechanisms
3.2.1. Pseudomonas Genus
3.2.2. Actinomycetes
3.2.3. Alcaligenes Genus
4. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hasan, M.A.; Hossain, R.; Sahajwalla, V. Critical metals (Lithium and Zinc) recovery from battery waste, ores, brine, and steel dust: A review. Process Saf. Environ. Prot. 2023, 178, 976–994. [Google Scholar] [CrossRef]
- Saidi, A.; El Khawaja, R.; Boffito, D.C. A Review of Traditional and Intensified Hydrometallurgy Techniques to Remove Chromium and Vanadium from Solid Industrial Waste. ACS Eng. Au 2023, 4, 49–70. [Google Scholar] [CrossRef]
- Hubau, A.; Guezennec, A.G.; Joulian, C.; Falagán, C.; Dew, D.; Hudson-Edwards, K.A. Bioleaching to reprocess sulfidic polymetallic primary mining residues: Determination of metal leaching mechanisms. Hydrometallurgy 2020, 197, 105484. [Google Scholar] [CrossRef]
- Ma, A.Y.; Zheng, X.M.; Gao, L.; Li, K.Q.; Omran, M.; Chen, G. Investigations on the Thermodynamics Characteristics, Thermal and Dielectric Properties of Calcium-Activated Zinc-Containing Metallurgical Residues. Materials 2022, 15, 714. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.W.; Santos, R.M.; Monballiu, A.; Ghyselbrecht, K.; Martens, J.A.; Mattos, M.L.T.; Van Gerven, T.; Meesschaert, B. Effects of bioleaching on the chemical, mineralogical and morphological properties of natural and waste-derived alkaline materials. Miner. Eng. 2013, 48, 116–125. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, X.H.; Lv, W.G.; He, M.M.; Yan, W.Y.; Gao, W.F.; Ning, P.G.; Cao, H.B.; Sun, Z. An acid-free process to prepare battery grade nickel and cobalt sulfates from complex resources. Nat. Commun. 2025, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Li, J.H.; Sun, P.; Deng, Z.G.; Li, X.B.; Li, M.T.; Wei, C. Removing fluorine and chlorine from zinc oxide dust by wet alkaline washing and studying fluorine occurrence states. Int. J. Chem. React. Eng. 2025, 22, 1459–1467. [Google Scholar] [CrossRef]
- Jia, L.P.; Huang, J.J.; Ma, Z.L.; Liu, X.H.; Chen, X.Y.; Li, J.T.; He, L.H.; Zhao, Z.W. Research and development trends of hydrometallurgy: An overview based on Hydrometallurgy literature from 1975 to 2019. Trans. Nonferrous Met. Soc. China 2020, 30, 3147–3160. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Stanforth, R. Production of Zn powder by alkaline treatment of smithsonite Zn-Pb ores. Hydrometallurgy 2000, 56, 237–249. [Google Scholar] [CrossRef]
- Zekavat, M.; Yoozbashizadeh, H.; Khodaei, A. Leaching of Antimony from Stibnite Ore in KOH Solution for Sodium Pyroantimonate Production: Systematic Optimization and Kinetic Study. JOM 2021, 73, 903–912. [Google Scholar] [CrossRef]
- Tanda, B.C.; Eksteen, J.J.; Oraby, E.A. An investigation into the leaching behaviour of copper oxide minerals in aqueous alkaline glycine solutions. Hydrometallurgy 2017, 167, 153–162. [Google Scholar] [CrossRef]
- Oraby, E.A.; Eksteen, J.J.; O’Connor, G.M. Gold leaching from oxide ores in alkaline glycine solutions in the presence of permanganate. Hydrometallurgy 2020, 198, 105527. [Google Scholar] [CrossRef]
- Barragán-Mantilla, S.P.; Gascó, G.; Almendros, P.; Méndez, A. Insights into the use of green leaching systems based on glycine for the selective recovery of copper. Miner. Eng. 2024, 206, 108534. [Google Scholar] [CrossRef]
- Huang, Y.K.; Wang, D.S.; Liu, H.T.; Fan, G.X.; Peng, W.J.; Cao, Y.J. Selective complexation leaching of copper from copper smelting slag with the alkaline glycine solution: An effective recovery method of copper from secondary resource. Sep. Purif. Technol. 2023, 326, 124619. [Google Scholar] [CrossRef]
- Oraby, E.; Li, H.; Deng, Z.X.; Eksteen, J. Selective extraction of Ni and Co from a pyrrhotite-rich flotation slime using an alkaline glycine-based leach system. Miner. Eng. 2023, 203, 108330. [Google Scholar] [CrossRef]
- Aghazadeh, S.; Abdollahi, H.; Gharabaghi, M.; Mirmohammadi, M. Selective leaching of antimony from tetrahedrite rich concentrate using alkaline sulfide solution with experimental design: Optimization and kinetic studies. J. Taiwan Inst. Chem. Eng. 2021, 119, 298–312. [Google Scholar] [CrossRef]
- Guo, X.Y.; Xu, Z.P.; Li, D.; Tian, Q.H.; Xu, R.Z.; Zhang, Z. Recovery of tellurium from high tellurium-bearing materials by alkaline sulfide leaching followed by sodium sulfite precipitation. Hydrometallurgy 2017, 171, 355–361. [Google Scholar] [CrossRef]
- Liu, R.Q.; Zhong, C.Y.; Lin, S.Y.; He, D.D.; Sun, W. Depression Mechanism of Sodium Sulfide in Flotation Separation of Molybdenite and Bismuthinite. Miner. Process. Extr. Metall. Rev. 2022, 43, 739–746. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, H.; Cao, Z. Molybdenum removal from copper ore concentrate by sodium hypochlorite leaching. Min. Sci. Technol. 2010, 21, 61–64. [Google Scholar] [CrossRef]
- Hernández, M.C.; Benavente, O.; Roca, A.; Melo, E.; Quezada, V. Selective Leaching of Arsenic from Copper Concentrates in Hypochlorite Medium. Minerals 2023, 13, 1372. [Google Scholar] [CrossRef]
- Curreli, L.; Ghiani, M.; Surracco, M.; Orrù, G. Beneficiation of a gold bearing enargite ore by flotation and As leaching with Na-hypochlorite. Miner. Eng. 2005, 18, 849–854. [Google Scholar] [CrossRef]
- Montoya, A.; Reyes, J.L.; Reyes, I.A.; Cruz, R.; Lázaro, I.; Rodríguez, I. Effect of sodium hypochlorite as a depressant for copper species in Cu-Mo flotation separation. Miner. Eng. 2023, 201, 108166. [Google Scholar] [CrossRef]
- Peng, H.; Guo, J.; Lv, L.; Huang, H.; Li, B. Recovery of chromium by calcium-roasting, sodium-roasting, acidic leaching, alkaline leaching and sub-molten technology: A review. Environ. Chem. Lett. 2020, 19, 1383–1393. [Google Scholar] [CrossRef]
- Nicol, M.J. The role and use of hydrogen peroxide as an oxidant in the leaching of minerals. II. alkaline solutions. Hydrometallurgy 2020, 194, 105365. [Google Scholar] [CrossRef]
- Nwaila, G.T.; Notole, V.; Alex, S.; Ghorbani, Y. Optimizing Gold Recovery from Witwatersrand-Type Ores Using Alkaline Glycine Leaching and Conditional Simulation. Nat. Resour. Res. 2025, 34, 1365–1381. [Google Scholar] [CrossRef]
- Suyantara, W.G.P.; Berdakh, D.; Miki, H.; Hirajima, T.; Sasaki, K.; Ochi, D.; Aoki, Y. Effect of hydrogen peroxide on selective flotation of chalcocite and enargite. Int. J. Min. Sci. Technol. 2023, 33, 703–716. [Google Scholar] [CrossRef]
- Trujic, S.; Popovic, M.P.; Conic, V.; Janosevic, M.; Alimpic, F.; Bajic, D.; Milenkovic-Andelkovic, A.; Abramovic, F. Ozone/Thiosulfate-Assisted Leaching of Cu and Au from Old Flotation Tailings. Molecules 2025, 30, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Hou, W.; Wang, H.Q.; Hu, E.M.; Lei, Z.W.; Hu, F.; Zhou, W.; Wang, Q.L. Oxidative leaching of sandstone uranium ore assisted by ozone micro-nano bubbles. J. Radioanal. Nucl. Chem. 2022, 331, 1645–1658. [Google Scholar] [CrossRef]
- Hunter, E. On the Leaching Behavior of Uranium-Bearing Resources in Carbonate-Bicarbonate Solution by Gaseous Oxidants. Ph.D. Thesis, Colorado School of Mines, Golden, CO, USA, 2013. [Google Scholar]
- Wang, J.X.; Faraji, F.; Ghahreman, A. Evaluation of ozone as an efficient and sustainable reagent for chalcopyrite leaching: Process optimization and oxidative mechanism. J. Ind. Eng. Chem. 2021, 104, 333–344. [Google Scholar] [CrossRef]
- Luo, F.L.; Xie, H.Y.; Jin, H.X.; Li, C.Z.; Ma, L.R.; Wang, D.L. Oxidative Leaching of Low-Grade Pyrolusite in Alkaline Solutions to Produce Potassium Manganate. Min. Metall. Explor. 2023, 40, 81–94. [Google Scholar] [CrossRef]
- Liu, W.; Huang, C.; Han, J.W.; Qin, W.Q. Removal and reuse of arsenic from arsenic-bearing purified residue by alkaline pressure oxidative leaching and reduction of As (V). Hydrometallurgy 2021, 199, 105541. [Google Scholar] [CrossRef]
- Guo, S.H.; Kuang, J.Z.; Wang, L. Interfacial reaction mechanisms of potassium permanganate and sodium persulfate in separating stibnite from arsenopyrite. Surf. Interfaces 2025, 64, 106359. [Google Scholar] [CrossRef]
- Wang, M.S.; Wei, C.; Fan, G.; Li, M.T.; Deng, Z.G.; Wang, S.F. Selective extraction of Mo from a Ni-Mo ore using pressure alkaline leaching. Hydrometallurgy 2015, 153, 6–11. [Google Scholar] [CrossRef]
- Jiang, L.S.; Leng, H.G.; Han, B.S. Dissolution and Passivation Mechanism of Chalcopyrite during Pressurized Water Leaching. Minerals 2023, 13, 996. [Google Scholar] [CrossRef]
- McDonald, R.G.; Muir, D.M. Pressure oxidation leaching of chalcopyrite Part II: Comparison of medium temperature kinetics and products and effect of chloride ion. Hydrometallurgy 2007, 86, 206–220. [Google Scholar] [CrossRef]
- Petersen, J. From understanding the rate limitations of bioleaching mechanisms to improved bioleach process design. Hydrometallurgy 2023, 221, 106148. [Google Scholar] [CrossRef]
- Nnaemeka, I.C.; O, C.T.; Nonso, U.C.; Ikechukwu, O.M.; Anezichukwu, A.F.; Ikechukwu, N.A.; M, O.; Chukwudi, E.B.; Chisom, M.K.; Ifeanyichukwu, O.T.; et al. Examining the efficiency of microbe-assisted metal extraction: A review of bio-hydrometallurgical leaching techniques. Hybrid J. 2025, 9, 100407. [Google Scholar] [CrossRef]
- Jia, B.J.; Yu, J.M. The Research Status and Development Trend of Microbial Flocculant. Phys. Procedia 2012, 24, 425–428. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Lee, M.H.; Park, H.J.; Lee, J.U. Bioleaching of arsenic and heavy metals from mine tailings by pure and mixed cultures of Acidithiobacillus, spp. J. Ind. Eng. Chem. 2015, 21, 451–458. [Google Scholar] [CrossRef]
- Roberto, F.F.; Schippers, A. Progress in bioleaching: Part B, applications of microbial processes by the minerals industries. Appl. Microbiol. Biotechnol. 2022, 106, 5913–5928. [Google Scholar] [CrossRef]
- Jones, S.; Santini, J.M. Mechanisms of bioleaching: Iron and sulfur oxidation by acidophilic microorganisms. Essays Biochem. 2023, 67, 685–699. [Google Scholar] [CrossRef]
- Behera, S.K.; Mulaba-Bafubiandi, A.F. Advances in microbial leaching processes for nickel extraction from lateritic minerals—A review. Korean J. Chem. Eng. 2015, 32, 1447–1454. [Google Scholar] [CrossRef]
- Crundwell, F.K. How do bacteria interact with minerals? Hydrometallurgy 2003, 71, 75–81. [Google Scholar] [CrossRef]
- Koizhanova, A.; Magomedov, D.; Abdyldayev, N.; Kamalov, E.; Yerdenova, M.; Bakrayeva, A. Copper Extraction from Complex Waste Dumps by Biochemical Leaching Method. J. Ecol. Eng. 2022, 23, 283–290. [Google Scholar] [CrossRef]
- Marrero, J.; Coto, O.; Goldmann, S.; Graupner, T.; Schippers, A. Recovery of Nickel and Cobalt from Laterite Tailings by Reductive Dissolution under Aerobic Conditions Using Acidithiobacillus Species. Environ. Sci. Technol. 2015, 49, 6674–6682. [Google Scholar] [CrossRef]
- Stanković, S.; Martin, M.; Goldmann, S.; Gäbler, H.-E.; Ufer, K.; Haubrich, F.; Moutinho, V.F.; Giese, E.C.; Neumann, R.; Stropper, J.L.; et al. Effect of mineralogy on Co and Ni extraction from Brazilian limonitic laterites via bioleaching and chemical leaching. Miner. Eng. 2022, 184, 107604. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, X.; He, H.; Tang, J.; Tao, X.; Huang, H.; Haider, R.; Ali, M.I.; Jamal, A.; Huang, Z. Bioleaching Coal Gangue with a Mixed Culture of Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans. Minerals 2021, 11, 1043. [Google Scholar] [CrossRef]
- Sukla, L.B.; Pattanaik, A.; Samal, D.P.K.; Pradhan, D. Microbial Leaching for Recovery of Nickel and Cobalt from Lateritic Ore: A Review. In Ni-Co 2021: The 5th International Symposium on Nickel and Cobalt. The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2021; pp. 207–217. [Google Scholar] [CrossRef]
- Kato, S.; Ohkuma, M. A Single Bacterium Capable of Oxidation and Reduction of Iron at Circumneutral pH. Microbiol. Spectrum 2021, 9, e0016121. [Google Scholar] [CrossRef]
- Liu, R.H.; Zhou, H.B. Growth in ever-increasing acidity condition enhanced the adaptation and bioleaching ability of Leptospirillum ferriphilum. Int. Microbiol. 2022, 25, 541–550. [Google Scholar] [CrossRef]
- Pakostova, E.; Grail, B.M.; Johnson, D.B. Indirect oxidative bioleaching of a polymetallic black schist sulfide ore. Miner. Eng. 2017, 106, 102–107. [Google Scholar] [CrossRef]
- Hetz, S.A.; Schippers, A. Aerobic Bioleaching of Six Brazilian Laterite Ores with Acidithiobacillus thiooxidans, Sulfobacillus species and Archaea at Various Conditions. J. Sustain. Metall. 2025. [Google Scholar] [CrossRef]
- Gu, G.-H.; Hu, K.-T.; Li, S.-K. Bioleaching of chalcopyrite by Leptospirillum ferriphilum. J. Cent. South Univ. 2013, 20, 178–183. [Google Scholar] [CrossRef]
- López-Martínez, A.; Martínez-Prado, M.A.; Núñez-Ramírez, D.M.; Medina-Torres, L.; Rojas-Contreras, J.A.; Anguiano-Vega, G.A.; Soto-Cruz, N.O. Acidophilic bacteria for metal extraction: Biotechnological characteristics and applications. Braz. J. Chem. Eng. 2025, 42, 31–52. [Google Scholar] [CrossRef]
- Dey, S. Microbial Resources of Alkaline Bauxite Residue and Their Possible Exploitation in Remediation and Rehabilitation. Geomicrobiol. J. 2022, 39, 219–232. [Google Scholar] [CrossRef]
- Han, P.; Liu, T.; Zheng, Y.; Song, R.; Nan, T.; Yang, X.; Huang, L.; Yuan, Y. A Mycorrhizal Bacteria Strain Isolated From Polyporus umbellatus Exhibits Broad-Spectrum Antifungal Activity. Front. Plant Sci. 2022, 13, 954160. [Google Scholar] [CrossRef]
- Williamson, A.J.; Folens, K.; Matthijs, S.; Cortes, Y.P.; Varia, J.; Du Laing, G.; Boon, N.; Hennebel, T. Selective metal extraction by biologically produced siderophores during bioleaching from low-grade primary and secondary mineral resources. Miner. Eng. 2021, 163, 106774. [Google Scholar] [CrossRef]
- Boguta, P.; Sokołowska, Z. Zinc Binding to Fulvic acids: Assessing the Impact of pH, Metal Concentrations and Chemical Properties of Fulvic Acids on the Mechanism and Stability of Formed Soluble Complexes. Molecules 2020, 25, 1297. [Google Scholar] [CrossRef]
- Shivlata, L.; Satyanarayana, T. Thermophilic and alkaliphilic Actinobacteria: Biology and potential applications. Front. Microbiol. 2015, 6, 1014. [Google Scholar] [CrossRef]
- Schippers, A.; Bosecker, K.; Willscher, S.; Spröer, C.; Schumann, P.; Kroppenstedt, R.M. Nocardiopsis metallicus sp. nov., a metalleaching actinomycete isolated from an alkaline slag dump. Int. J. Syst. Evol. Microbiol. 2002, 52, 2291–2295. [Google Scholar] [CrossRef] [PubMed]
- Lwin, H.W.; Dattelbaum, J.D. Isolation and Whole Genome Sequence Analysis of Alcaligenes and Chromobacterium Strains with Antimicrobial Activity Against ESKAPE Pathogen Relatives. J. Genom. 2025, 13, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Eltokhy, M.A.; Saad, B.T.; Eltayeb, W.N.; El-Ansary, M.R.; Aboshanab, K.M.; Ashour, M.S.E. A Metagenomic Nanopore Sequence Analysis Combined with Conventional Screening and Spectroscopic Methods for Deciphering the Antimicrobial Metabolites Produced by Alcaligenes faecalis Soil Isolate MZ921504. Antibiotics 2021, 10, 1382. [Google Scholar] [CrossRef]
- Pineda, Y.S.; Devries, S.L.; Steiner, N.C.; Block-Cora, K.A. Bioleaching of Gold in Mine Tailings by Alcaligenes faecalis. Minerals 2023, 13, 410. [Google Scholar] [CrossRef]
- Whitworth, A.J.; Vaughan, J.; Southam, G.; Van der Ent, A.; Nkrumah, P.N.; Ma, X.D.; Parbhakar-Fox, A. Review on metal extraction technologies suitable for critical metal recovery from mining and processing wastes. Miner. Eng. 2022, 182, 107537. [Google Scholar] [CrossRef]
- Mishra, S.; Panda, S.; Akcil, A.; Dembele, S.; Agcasulu, I. A Review on Chemical versus Microbial Leaching of Electronic Wastes with Emphasis on Base Metals Dissolution. Minerals 2021, 11, 1255. [Google Scholar] [CrossRef]
- Moradkhani, M.; Yaghmaei, S.; Nejad, Z.G. Biodegradation of Cyanide under Alkaline Conditions by a Strain of Pseudomonas putida Isolated from Gold Mine Soil and Optimization of Process Variables through Response Surface Methodology (RSM). Period. Polytech.-Chem. Eng. 2018, 62, 265–273. [Google Scholar] [CrossRef]
- Newsome, L.; Falagán, C. The Microbiology of Metal Mine Waste: Bioremediation Applications and Implications for Planetary Health. GeoHealth 2021, 5, e2020GH000380. [Google Scholar] [CrossRef] [PubMed]
- Kara, I.T.; Kremser, K.; Wagland, S.T.; Coulon, F. Bioleaching metal-bearing wastes and by-products for resource recovery: A review. Environ. Chem. Lett. 2023, 21, 3329–3350. [Google Scholar] [CrossRef]


| Serial Number | Types of Substances and Bacterial Strains | Property Classification | Oxidizable Metal | References |
|---|---|---|---|---|
| 1 | Sodium hydroxide or potassium hydroxide | Alkaline matrix | Zinc oxide ore, stibnite | [9,10] |
| 2 | Glycine | Alkaline matrix | Cuprite, Low-sulfide oxidized gold ores | [11,12] |
| 3 | Sodium sulfide | Alkaline matrix | Lead-zinc ores, Tetrahedrite-rich concentrates containing copper and antimony | [16,17] |
| 4 | Sodium hypochlorite | Alkaline matrix | Copper concentrates, tennantite, Gold-bearing enargite | [19,20,21] |
| 5 | Hydrogen peroxide | Oxidants | Chromium-containing slag | [23,24,25] |
| 6 | Ozone | Oxidants | Pyrite, Uranium ore | [27,28] |
| 7 | Oxygen | Oxidants | Manganese ore, Arsenic-bearing residues | [31,32] |
| 8 | Potassium permanganate | Oxidants | copper sulfide ores | [12,33] |
| 9 | Iron- and sulfur-oxidizing bacteria | Acidophilic microorganisms | Sulfide minerals, laterite ore | [44,45,46,47,49] |
| 10 | Obligate iron-oxidizing bacteria | Acidophilic microorganisms | Sulfide minerals | [50,51,52] |
| 11 | Obligate sulfur-oxidizing bacteria | Acidophilic microorganisms | Sulfide minerals, laterite ore | [40,53,54] |
| 12 | Pseudomonas genus | Alkaline-tolerant microorganisms | Zinc-containing minerals | [57,58,59] |
| 13 | Actinomycetes | Alkaline-tolerant microorganisms | siliceous alkaline slag | [60,61] |
| 14 | Alcaligenes genus | Alkaline-tolerant microorganisms | gold ore | [62,63,64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Qi, X.; Yu, W.; Guan, Q.; Bu, Y.; Zhu, J.; Gu, G.; Li, T.; Zhang, C. Chemical Alkaline Leaching and Alkaliphile-Driven Bioleaching: Advancing Metal Recovery from Ores. Microorganisms 2025, 13, 2577. https://doi.org/10.3390/microorganisms13112577
Zhou S, Qi X, Yu W, Guan Q, Bu Y, Zhu J, Gu G, Li T, Zhang C. Chemical Alkaline Leaching and Alkaliphile-Driven Bioleaching: Advancing Metal Recovery from Ores. Microorganisms. 2025; 13(11):2577. https://doi.org/10.3390/microorganisms13112577
Chicago/Turabian StyleZhou, Shuang, Xianglong Qi, Weijian Yu, Qingjun Guan, Yongjie Bu, Jianyu Zhu, Guohua Gu, Tiantao Li, and Chenyang Zhang. 2025. "Chemical Alkaline Leaching and Alkaliphile-Driven Bioleaching: Advancing Metal Recovery from Ores" Microorganisms 13, no. 11: 2577. https://doi.org/10.3390/microorganisms13112577
APA StyleZhou, S., Qi, X., Yu, W., Guan, Q., Bu, Y., Zhu, J., Gu, G., Li, T., & Zhang, C. (2025). Chemical Alkaline Leaching and Alkaliphile-Driven Bioleaching: Advancing Metal Recovery from Ores. Microorganisms, 13(11), 2577. https://doi.org/10.3390/microorganisms13112577








