Divergent Avian Influenza H10 Viruses from Sympatric Waterbird Species in Italy: Zoonotic Potential Assessment by Molecular Markers
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Virus Isolation
- (i)
- During bird-ringing activities carried out in the Laguna di Orbetello Oasis and Lago di Burano World Wildlife Fund Oasis—protected wetlands placed on the west coast of Central Italy in the Tuscany region, about 140 km north of Rome—seven cloacal swabs were collected between 1994 and 2006 from two Eurasian coots (Fulica atra) and five mallards (Anas platyrhynchos). These samples were individually processed and inoculated according to standard procedures in specific pathogen-free (SPF)-embryonated chicken eggs for virus isolation [20], followed by influenza A virus detection and characterisation by hemagglutination (HA) assay [21], enzyme-linked immunosorbent assay (ELISA) [22], RT-PCR [23], and finally subtyped by hemagglutination inhibition (HI) and neuraminidase inhibition (NI) tests [21].
- (ii)
- During the AIV Surveillance Plan implemented in the Emilia-Romagna Region of northern Italy, one pool of 10 cloacal swabs obtained from a group of 2000 free-range mallards reared in an open-air breeding farm (Anas platyrhynchos domestic farm), and one cloacal swab obtained from an injured wild mallard (recovered in the upper valley of the Senio River), were processed to be inoculated into SPF-embryonated chicken eggs [20]. The harvested allantoic fluids were tested by the HA assay [21] and an ELISA specific for the detection of influenza A virus nucleoprotein [22]. Allantoic fluids that tested positive by both HA and ELISA were sent to the Italian National Reference Laboratory for Avian Influenza and Newcastle Disease (Legnaro, PD) for antigenic subtype and pathotype characterisation.
2.2. Sample Processing and Testing
2.2.1. RNA Extraction and Whole Genome Sequencing
2.2.2. Sequence and Phylogenetic Analyses
2.2.3. Neuraminidase Inhibitors and NA Inhibition Test
3. Results
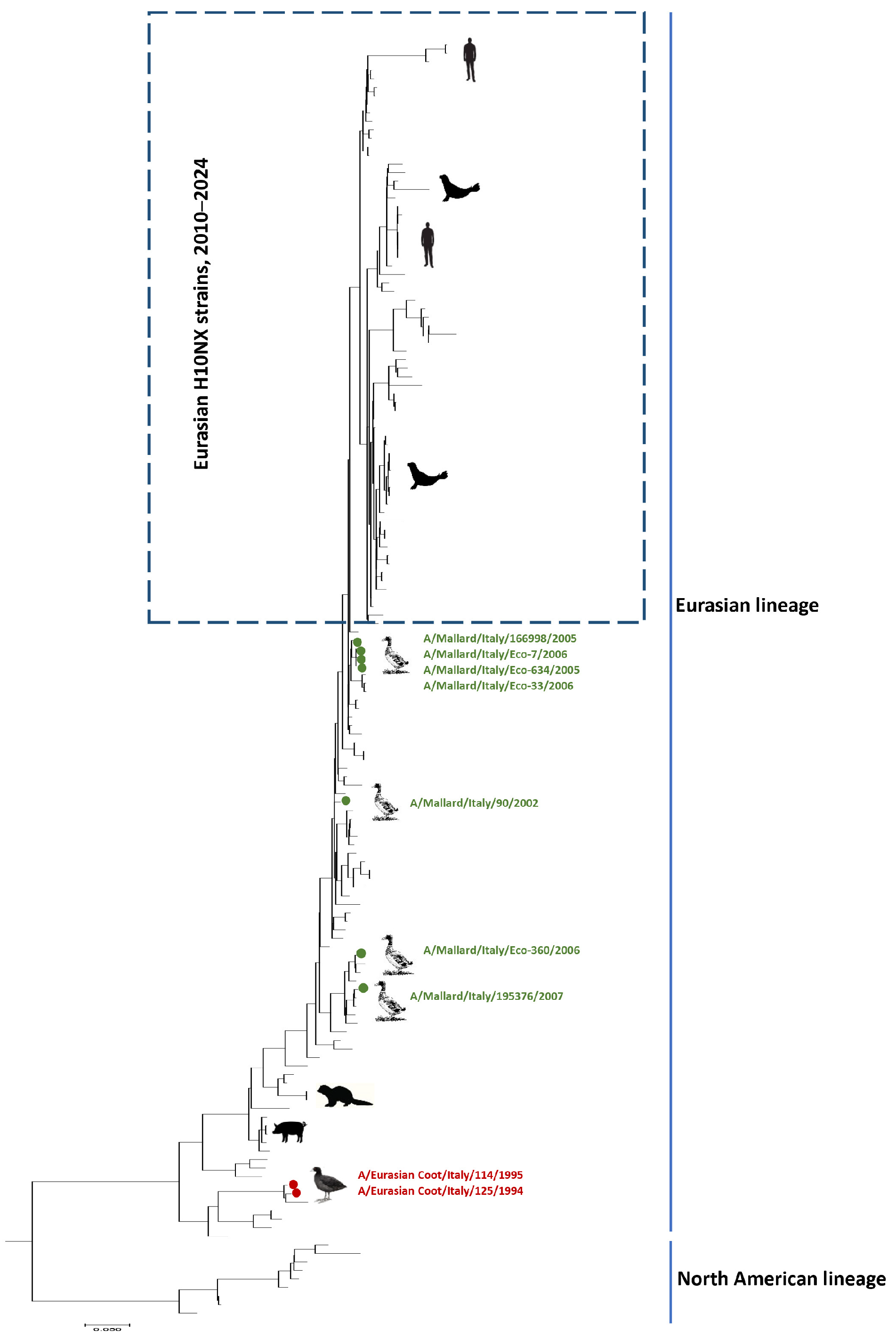
3.1. HA Phylogenetic Analysis
3.2. Molecular Characterisation of the H10 AIVs
3.2.1. Nucleotide Identity
3.2.2. HA Gene
3.2.3. NA Gene
3.2.4. Internal Protein Genes
3.3. Antiviral Susceptibility by Phenotypic Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Protein | Mutation/ Motif | Strains with Mutation | Phenotypic Effect/Subtype Tested | Reference |
|---|---|---|---|---|
| PB2 | R340K | A/Eurasian Coot/It/125/1994, A/Eurasian Coot/It/114/1995, A/Mallard/It/Eco-634/2005, A/Mallard/It/Eco-7/2006, and A/Mallard/It/Eco-33/2006 | Increased virulence in mice | [105] |
| K389R | All | Increased polymerase activity and replication in mammalian cell line/H7N9 | [106] | |
| A588V | A/Eurasian Coot/It/125/1994 and A/Eurasian Coot/It/114/1995 | Increased polymerase activity and replication in avian and mammalian cell line; increased virulence in mice/H7N9, H9N2, and H10N8 | [105] | |
| V598T | All | Increased polymerase activity and replication in mammalian cell line; increased virulence in mice/H7N9 | [106] | |
| L89V, G309D | All | Increased polymerase activity and replication in mammalian cell line; increased virulence in mice/H5N1 | [107] | |
| L89V, G309D, T339K, R477G, I495V, K627E, and A676T | All | Increased polymerase activity and replication in mammalian cell line; increased virulence in mice/H5N1 | [107] | |
| PB1 | D3V | All | Increased polymerase activity and replication in avian and mammalian cell line/H5N1 | [108] |
| D622G | All | Increased polymerase activity and virulence in mice/H5N1 | [109] | |
| PA | S37A | All | Increased polymerase activity in mammalian cell line/H7N9 | [110] |
| N383D | All | Increased polymerase activity in avian and mammalian cell line/H5N1 | [111] | |
| N409S | All | Increased polymerase activity in avian and mammalian cell line/H7N9 | [110] | |
| NP | M105V | A/Mallard/It/166998/2005, A/Mallard/It/Eco-634/2005, A/Mallard/It/Eco-7/2006, A/Mallard/It/Eco-33/2006, A/Mallard/It/Eco-360/2006, and A/Mallard/It/195376/2007 | Increased virulence in chickens/H5N1 | [112] |
| I109T | A/Mallard/It/Eco-33/2006 | Increased polymerase activity and viral replication in chickens (but not in ducks), and increased virulence in chickens/H5N1 | [112] | |
| A184K | All | Increased replication in avian cells virulence in chickens and enhanced IFN response/H5N1 | [113] | |
| M1 | N30D | All | Increased virulence in mice/H5N1 | [114] |
| I43M | All | Increased virulence in mice, ducks, and chickens/H5N1 | [115] | |
| T215A | All | Increased virulence in mice/H5N1 | [114] | |
| NS1 | P42S | A/Eurasian Coot/It/125/1994, A/Eurasian Coot/It/114/1995, A/Mallard/It/90/2002, A/Mallard/It/166998/2005, A/Mallard/It/Eco-634/2005, A/Mallard/It/Eco-7/2006, A/Mallard/It/Eco-33/2006, and A/Mallard/It/Eco-360/2006 | Increased virulence in mice and pigs/H5N1 | [116] |
| C138F | All | Increased replication in mammalian cells and decreased interferon response/H5N1 | [117] | |
| V149A | All | Increased virulence and decreased interferon response in chickens/H5N1 | [117] | |
| L103F, I106M | A/Eurasian Coot/It/125/1994, A/Eurasian Coot/It/114/1995, A/Mallard/It/90/2002, A/Mallard/It/166998/2005, A/Mallard/It/Eco-634/2005, A/Mallard/It/Eco-7/2006, A/Mallard/It/Eco-33/2006, and A/Mallard/It/Eco-360/2006 | Increased virulence in mice | [118] | |
| K55E, K66E, C138F | A/Eurasian Coot/It/125/1994, A/Eurasian Coot/It/114/1995, A/Mallard/It/90/2002, A/Mallard/It/166998/2005, A/Mallard/It/Eco-634/2005, A/Mallard/It/Eco-7/2006, A/Mallard/It/Eco-33/2006, and A/Mallard/It/Eco-360/2006 | Increased replication in mammalian cells and decreased IF response/H5N1 | [117,119] |
References
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992, 56, 152–179. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.G.; Govorkova, E.A. Continuing challenges in influenza. Ann. N. Y. Acad. Sci. 2014, 1323, 115–139. [Google Scholar] [CrossRef] [PubMed]
- Donatelli, I.; Castrucci, M.R.; De Marco, M.A.; Delogu, M.; Webster, R.G. Human-Animal Interface: The Case for Influenza Interspecies Transmission. Adv. Exp. Med. Biol. 2017, 972, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Matrosovich, M.N.; Gambaryan, A.S.; Teneberg, S.; Piskarev, V.E.; Yamnikova, S.S.; Lvov, D.K.; Robertson, J.S.; Karlsson, K.A. Avian influenza A viruses differ from human viruses by recognition of sialyloligosaccharides and gangliosides and by a higher conservation of the HA receptor-binding site. Virology 1997, 233, 224–234. [Google Scholar] [CrossRef]
- Runstadler, J.A.; Puryear, W.B. The virus is out of the barn: The emergence of HPAI as a pathogen of avian and mammalian wildlife around the globe. Am. J. Vet. Res. 2024, 85, ajvr.24.01.0018. [Google Scholar] [CrossRef]
- World Organisation for Animal Health (WOAH). Terrestrial Animal Health Code, SECTION: 10. AVES, Chapter: 10.4. Infection with High Pathogenicity Avian Influenza Viruses. Available online: https://sont.woah.org/portal/tool?le=en (accessed on 1 October 2025).
- Abdelwhab, E.M.; Mettenleiter, T.C. Zoonotic Animal Influenza Virus and Potential Mixing Vessel Hosts. Viruses 2023, 15, 980. [Google Scholar] [CrossRef]
- Everest, H.; Billington, E.; Daines, R.; Burman, A.; Iqbal, M. The Emergence and Zoonotic Transmission of H10Nx Avian Influenza Virus Infections. mBio 2021, 12, e0178521. [Google Scholar] [CrossRef]
- Berg, M.; Englund, L.; Abusugra, I.A.; Klingeborn, B.; Linné, T. Close relationship between mink influenza (H10N4) and concomitantly circulating avian influenza viruses. Arch. Virol. 1990, 113, 61–71. [Google Scholar] [CrossRef]
- Si, Y.J.; Park, Y.R.; Baek, Y.G.; Park, M.J.; Lee, E.K.; Lee, K.N.; Kim, H.R.; Lee, Y.J.; Lee, Y.N. Pathogenesis and genetic characteristics of low pathogenic avian influenza H10 viruses isolated from migratory birds in South Korea during 2010–2019. Transbound. Emerg. Dis. 2022, 69, 2588–2599. [Google Scholar] [CrossRef]
- Pan American Health Organization. Avian Influenza Virus A (H10N7) Circulating Among Humans in Egypt. 2004. Available online: https://www.paho.org/en/documents/avian-influenza-virus-h10n7-circulating-among-humans-egypt-vol-2-no-18-7-may-2004 (accessed on 23 July 2025).
- Arzey, G.G.; Kirkland, P.D.; Arzey, K.E.; Frost, M.; Maywood, P.; Conaty, S.; Hurt, A.C.; Deng, Y.M.; Iannello, P.; Barr, I.; et al. Influenza virus A (H10N7) in chickens and poultry abattoir workers, Australia. Emerg. Infect. Dis. 2012, 18, 814–816. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, H.; Gao, R.; Zhang, J.; Wang, D.; Xiong, Y.; Fan, G.; Yang, F.; Li, X.; Zhou, J.; et al. Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: A descriptive study. Lancet 2014, 383, 714–721. [Google Scholar] [CrossRef]
- Xu, Y.; Cao, H.; Liu, H.; Sun, H.; Martin, B.; Zhao, Y.; Wang, Q.; Deng, G.; Xue, J.; Zong, Y.; et al. Identification of the source of A (H10N8) virus causing human infection. Infect. Genet. Evol. 2015, 30, 159–163. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; European Centre for Disease Prevention and Control; European Union Reference Laboratory for Avian Influenza; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Mirinavičiūtė, G.; Niqueux, É.; Ståhl, K.; Staubach, C.; et al. Avian influenza overview December 2023–March 2024. EFSA J. 2024, 22, e8754. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Weekly Bulletin, Communicable Disease Threats Report, 10–16 May 2025, Week 20. Available online: https://www.ecdc.europa.eu/en/publications-data/communicable-disease-threats-report-10-16-may-2025-week-20 (accessed on 23 July 2025).
- De Marco, M.A.; Campitelli, L.; Foni, E.; Raffini, E.; Barigazzi, G.; Delogu, M.; Guberti, V.; Di Trani, L.; Tollis, M.; Donatelli, I. Influenza surveillance in birds in Italian wetlands (1992–1998): Is there a host restricted circulation of influenza viruses in sympatric ducks and coots? Vet. Microbiol. 2004, 98, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Delogu, M.; De Marco, M.A.; Di Trani, L.; Raffini, E.; Cotti, C.; Puzelli, S.; Ostanello, F.; Webster, R.G.; Cassone, A.; Donatelli, I. Can preening contribute to influenza A virus infection in wild waterbirds? PLoS ONE 2010, 5, e11315. [Google Scholar] [CrossRef]
- De Marco, M.A.; Delogu, M.; Facchini, M.; Di Trani, L.; Boni, A.; Cotti, C.; Graziosi, G.; Venturini, D.; Regazzi, D.; Ravaioli, V.; et al. Serologic Evidence of Occupational Exposure to Avian Influenza Viruses at the Wildfowl/Poultry/Human Interface. Microorganisms 2021, 9, 2153. [Google Scholar] [CrossRef]
- Council of European Union. Council Directive 92/40/EEC of 19 May 1992 Introducing Community Measures for the Control of Avian Influenza. Off. J. L 1992, 167, 1–16. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A31992L0040 (accessed on 22 January 2025).
- World Health Organization. WHO Manual on Animal Influenza Diagnosis and Surveillance. 2002. Available online: https://apps.who.int/iris/bitstream/handle/10665/68026/WHO_CDS_CSR_NCS_2002.5.pdf (accessed on 26 July 2021).
- Siebinga, J.T.; de Boer, G.F. Influenza A viral nucleoprotein detection in isolates from human and various animal species. Arch. Virol. 1988, 100, 75–87. [Google Scholar] [CrossRef]
- Fouchier, R.A.; Bestebroer, T.M.; Herfst, S.; Van Der Kemp, L.; Rimmelzwaan, G.F.; Osterhaus, A.D. Detection of influenza A viruses from different species by PCR amplification of conserved sequences in the matrix gene. J. Clin. Microbiol. 2000, 38, 4096–4101. [Google Scholar] [CrossRef]
- Zhou, B.; Donnelly, M.E.; Scholes, D.T.; St George, K.; Hatta, M.; Kawaoka, Y.; Wentworth, D.E. Single-reaction genomic amplification accelerates sequencing and vaccine production for classical and Swine origin human influenza a viruses. J. Virol. 2009, 83, 10309–10313. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Shepard, S.S.; Meno, S.; Bahl, J.; Wilson, M.M.; Barnes, J.; Neuhaus, E. Viral deep sequencing needs an adaptive approach: IRMA, the iterative refinement meta-assembler. BMC Genom. 2016, 17, 708, Erratum in BMC Genom. 2016, 17, 801. https://doi.org/10.1186/s12864-016-3138-8. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis pro-gram for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Puzelli, S.; Facchini, M.; Di Martino, A.; Fabiani, C.; Lackenby, A.; Zambon, M.; Donatelli, I. Evaluation of the antiviral drug susceptibility of influenza viruses in Italy from 2004/05 to 2009/10 epidemics and from the recent 2009 pandemic. Antivir. Res. 2011, 90, 205–212. [Google Scholar] [CrossRef]
- World Health Organization. Meetings of the WHO working group on surveillance of influenza antiviral susceptibility—Geneva, November 2011 and June 2012. Wkly. Epidemiol. Rec. 2012, 87, 369–374. [Google Scholar]
- Berhane, Y.; Joseph, T.; Lung, O.; Embury-Hyatt, C.; Xu, W.; Cottrell, P.; Raverty, S. Isolation and Characterization of Novel Reassortant Influenza A(H10N7) Virus in a Harbor Seal, British Columbia, Canada. Emerg. Infect. Dis. 2022, 28, 1480–1484. [Google Scholar] [CrossRef]
- Lv, X.; Tian, J.; Li, X.; Bai, X.; Li, Y.; Li, M.; An, Q.; Song, X.; Xu, Y.; Sun, H.; et al. H10Nx avian influenza viruses detected in wild birds in China pose potential threat to mammals. One Health 2023, 16, 100515. [Google Scholar] [CrossRef]
- Hao, M.; Wu, J.; Ji, L.; Zhao, Y.; Zhang, S.; Guan, Y.; Li, L.; Yang, W.; Zhang, Y.; Chen, J. Pathogenicity, transmissibility, and receptor binding of a human-isolated influenza A (H10N5) virus. mBio 2025, 16, e0073125. [Google Scholar] [CrossRef] [PubMed]
- Suttie, A.; Deng, Y.M.; Greenhill, A.R.; Dussart, P.; Horwood, P.F.; Karlsson, E.A. Inventory of molecular markers affecting biological characteristics of avian influenza A viruses. Virus Genes 2019, 55, 739–768. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Belser, J.A.; Yang, H.; Pulit-Penaloza, J.A.; Pappas, C.; Brock, N.; Zeng, H.; Creager, H.M.; Stevens, J.; Maines, T.R. Identification of key hemagglutinin residues responsible for cleavage, acid stability, and virulence of fifth-wave highly pathogenic avian influenza A(H7N9) viruses. Virology 2019, 535, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Yang, H.-Y.; Zhang, B.-J.; Jia, H.-L.; Tien, P. Analysis of a point mutation in H5N1 avian influenza virus hemagglutinin in relation to virus entry into live mammalian cells. Arch. Virol. 2008, 153, 2253–2261. [Google Scholar] [CrossRef]
- Wang, W.; Lu, B.; Zhou, H.; Suguitan, A.L., Jr.; Cheng, X.; Subbarao, K.; Kemble, G.; Jin, H. Glycosylation at 158N of the hemagglutinin protein and receptor binding specificity synergistically affect the antigenicity and immunogenicity of a live attenuated H5N1 A/Vietnam/1203/2004 vaccine virus in ferrets. J. Virol. 2010, 84, 6570–6577. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Wei, C.J.; Kong, W.P.; Wu, L.; Xu, L.; Smith, D.F.; Nabel, G.J. Immunization by avian H5 influenza hemagglutinin mutants with altered receptor binding specificity. Science 2007, 317, 825–828. [Google Scholar] [CrossRef]
- Naughtin, M.; Dyason, J.C.; Mardy, S.; Sorn, S.; von Itzstein, M.; Buchy, P. Neuraminidase inhibitor sensitivity and receptor-binding specificity of Cambodian clade 1 highly pathogenic H5N1 influenza virus. Antimicrob. Agents Chemother. 2011, 55, 2004–2010. [Google Scholar] [CrossRef]
- Kongchanagul, A.; Suptawiwat, O.; Kanrai, P.; Uiprasertkul, M.; Puthavathana, P.; Auewarakul, P. Positive selection at the receptor-binding site of haemagglutinin H5 in viral sequences derived from human tissues. J. Gen. Virol. 2008, 89, 1805–1810. [Google Scholar] [CrossRef]
- Yamada, S.; Suzuki, Y.; Suzuki, T.; Le, M.Q.; Nidom, C.A.; Sakai-Tagawa, Y.; Muramoto, Y.; Ito, M.; Kiso, M.; Horimoto, T.; et al. Haemagglutinin mutations responsible for the binding of H5N1 influenza A viruses to human-type receptors. Nature 2006, 444, 378–382. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Shinya, K.; Deng, G.; Jiang, Y.; Li, Z.; Guan, Y.; Tian, G.; Li, Y.; Shi, J.; et al. Identification of amino acids in HA and PB2 critical for the transmission of H5N1 avian influenza viruses in a mammalian host. PLoS Pathog. 2009, 5, e1000709. [Google Scholar] [CrossRef]
- Chen, L.M.; Blixt, O.; Stevens, J.; Lipatov, A.S.; Davis, C.T.; Collins, B.E.; Cox, N.J.; Paulson, J.C.; Donis, R.O. In vitro evolution of H5N1 avian influenza virus toward human-type receptor specificity. Virology 2012, 422, 105–113, Erratum in Virology 2012, 424, 154. https://doi.org/10.1016/j.virol.2011.12.014. [Google Scholar] [CrossRef]
- Han, P.; Hu, Y.; Sun, W.; Zhang, S.; Li, Y.; Wu, X.; Yang, Y.; Zhu, Q.; Jiang, T.; Li, J.; et al. Mouse lung-adapted mutation of E190G in hemagglutinin from H5N1 influenza virus contributes to attenuation in mice. J. Med. Virol. 2015, 87, 1816–1822. [Google Scholar] [CrossRef]
- Watanabe, Y.; Ibrahim, M.S.; Ellakany, H.F.; Kawashita, N.; Mizuike, R.; Hiramatsu, H.; Sriwilaijaroen, N.; Takagi, T.; Suzuki, Y.; Ikuta, K. Acquisition of human-type receptor binding specificity by new H5N1 influenza virus sublineages during their emergence in birds in Egypt. PLoS Pathog. 2011, 7, e1002068. [Google Scholar] [CrossRef] [PubMed]
- Behera, A.K.; Chandra, I.; Cherian, S.S. Molecular dynamics simulation of the effects of single (S221P) and double (S221P and K216E) mutations in the hemagglutinin protein of influenza A H5N1 virus: A study on host receptor specificity. J. Biomol. Struct. Dyn. 2016, 34, 2054–2067. [Google Scholar] [CrossRef] [PubMed]
- Chutinimitkul, S.; van Riel, D.; Munster, V.J.; van den Brand, J.M.; Rimmelzwaan, G.F.; Kuiken, T.; Osterhaus, A.D.; Fouchier, R.A.; de Wit, E. In vitro assessment of attachment pattern and replication efficiency of H5N1 influenza A viruses with altered receptor specificity. J. Virol. 2010, 84, 6825–6833. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.; Blixt, O.; Tumpey, T.M.; Taubenberger, J.K.; Paulson, J.C.; Wilson, I.A. Structure and receptor specificity of the hemagglutinin from an H5N1 influenza virus. Science 2006, 312, 404–410. [Google Scholar] [CrossRef]
- Herfst, S.; Schrauwen, E.J.; Linster, M.; Chutinimitkul, S.; de Wit, E.; Munster, V.J.; Sorrell, E.M.; Bestebroer, T.M.; Burke, D.F.; Smith, D.J.; et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science 2012, 336, 1534–1541. [Google Scholar] [CrossRef]
- Linster, M.; van Boheemen, S.; de Graaf, M.; Schrauwen, E.J.A.; Lexmond, P.; Mänz, B.; Bestebroer, T.M.; Baumann, J.; van Riel, D.; Rimmelzwaan, G.F.; et al. Identification, characterization, and natural selection of mutations driving airborne transmission of A/H5N1 virus. Cell 2014, 157, 329–339. [Google Scholar] [CrossRef]
- Wessels, U.; Abdelwhab, E.M.; Veits, J.; Hoffmann, D.; Mamerow, S.; Stech, O.; Stech, J. A dual motif in the hemagglutinin of H5N1 goose/Guangdong-like highly pathogenic avian influenza virus strains is conserved from their early evolution and increases both membrane fusion pH and virulence. J. Virol. 2018, 92, e01068-18. [Google Scholar] [CrossRef]
- Abdelwhab, E.-S.M.; Veits, J.; Breithaupt, A.; Gohrbandt, S.; Ziller, M.; Teifke, J.P.; Stech, J.; Mettenleiter, T.C. Prevalence of the C-terminal truncations of NS1 in avian influenza A viruses and effect on virulence and replication of a highly pathogenic H7N1 virus in chickens. Virulence 2016, 7, 546–557. [Google Scholar] [CrossRef]
- Reed, M.L.; Yen, H.L.; DuBois, R.M.; Bridges, O.A.; Salomon, R.; Webster, R.G.; Russell, C.J. Amino acid residues in the fusion peptide pocket regulate the pH of activation of the H5N1 influenza virus hemagglutinin protein. J. Virol. 2009, 83, 3568–3580. [Google Scholar] [CrossRef][Green Version]
- Krenn, B.M.; Egorov, A.; Romanovskaya-Romanko, E.; Wolschek, M.; Nakowitsch, S.; Ruthsatz, T.; Kiefmann, B.; Morokutti, A.; Humer, J.; Geiler, J.; et al. HA2 mutation increases the infectivity and immunogenicity of a live attenuated H5N1 intranasal influenza vaccine candidate lacking NS1. PLoS ONE 2011, 6, e18577. [Google Scholar] [CrossRef]
- Ilyushina, N.A.; Govorkova, E.A.; Gray, T.E.; Bovin, N.V.; Webster, R.G. Human-like receptor specificity does not affect the neuraminidase-inhibitor susceptibility of H5N1 influenza viruses. PLoS Pathog. 2008, 4, e1000043. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.L.; Aldridge, J.R.; Boon, A.C.; Ilyushina, N.A.; Salomon, R.; Hulse-Post, D.J.; Marjuki, H.; Franks, J.; Boltz, D.A.; Bush, D.; et al. Changes in H5N1 influenza virus hemagglutinin receptor binding domain affect systemic spread. Proc. Natl. Acad. Sci. USA 2009, 106, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Bouwman, K.M.; McBride, R.; Grant, O.C.; Woods, R.J.; Verheije, M.H.; Paulson, J.C.; de Vries, R.P. Enhanced human-type receptor binding by ferret-transmissible H5N1 with a K193T mutation. J. Virol. 2018, 92, e02016-17. [Google Scholar] [CrossRef] [PubMed]
- Maines, T.R.; Chen, L.M.; Van Hoeven, N.; Tumpey, T.M.; Blixt, O.; Belser, J.A.; Gustin, K.M.; Pearce, M.B.; Pappas, C.; Stevens, J.; et al. Effect of receptor binding domain mutations on receptor binding and transmissibility of avian influenza H5N1 viruses. Virology 2011, 413, 139–147. [Google Scholar] [CrossRef]
- Guo, H.; de Vries, E.; McBride, R.; Dekkers, J.; Peng, W.; Bouwman, K.M.; Nycholat, C.; Verheije, M.H.; Paulson, J.C.; van Kuppeveld, F.J.; et al. Highly pathogenic influenza A(H5Nx) viruses with altered H5 receptor-binding specificity. Emerg. Infect. Dis. 2017, 23, 220–231. [Google Scholar] [CrossRef]
- Webster, R.G.; Rott, R. Influenza virus A pathogenicity: The pivotal role of hemagglutinin. Cell 1987, 50, 665–666. [Google Scholar] [CrossRef]
- Imai, M.; Watanabe, T.; Hatta, M.; Das, S.C.; Ozawa, M.; Shinya, K.; Zhong, G.; Hanson, A.; Katsura, H.; Watanabe, S.; et al. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 2012, 486, 420–428. [Google Scholar] [CrossRef]
- Dortmans, J.C.; Dekkers, J.; Wickramasinghe, I.N.; Verheije, M.H.; Rottier, P.J.; van Kuppeveld, F.J.; de Vries, E.; de Haan, C.A. Adaptation of novel H7N9 influenza A virus to human receptors. Sci. Rep. 2013, 3, 3058. [Google Scholar] [CrossRef]
- Xu, R.; de Vries, R.P.; Zhu, X.; Nycholat, C.M.; McBride, R.; Yu, W.; Paulson, J.C.; Wilson, I.A. Preferential recognition of avian-like receptors in human influenza A H7N9 viruses. Science 2013, 342, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Ramos, I.; Krammer, F.; Hai, R.; Aguilera, D.; Bernal-Rubio, D.; Steel, J.; García-Sastre, A.; Fernandez-Sesma, A. H7N9 influenza viruses interact preferentially with α2,3-linked sialic acids and bind weakly to α2,6-linked sialic acids. J. Gen. Virol. 2013, 94, 2417–2423. [Google Scholar] [CrossRef] [PubMed]
- de Vries, R.P.; Peng, W.; Grant, O.C.; Thompson, A.J.; Zhu, X.; Bouwman, K.M.; Paulson, J.C. Three mutations switch H7N9 influenza to human-type receptor specificity. PLoS Pathog. 2017, 13, e1006390. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.; Raman, R.; Jayaraman, A.; Viswanathan, K.; Sasisekharan, R. Quantitative description of glycan-receptor binding of influenza A virus H7 hemagglutinin. PLoS ONE 2013, 8, e49597, Correction in PLoS ONE 2013, 8. https://doi.org/10.1371/annotation/c3b40a6f-a4be-4117-8ce2-a1b9b873e87c. [Google Scholar] [CrossRef]
- Jin, F.; Dong, X.; Wan, Z.; Ren, D.; Liu, M.; Geng, T.; Zhang, J.; Gao, W.; Shao, H.; Qin, A.; et al. A single mutation N166D in hemagglutinin affects antigenicity and pathogenesis of H9N2 avian influenza virus. Viruses 2019, 11, 709. [Google Scholar] [CrossRef]
- Teng, Q.; Xu, D.; Shen, W.; Liu, Q.; Rong, G.; Li, X.; Yan, L.; Yang, J.; Chen, H.; Yu, H.; et al. A single mutation at position 190 in hemagglutinin enhances binding affinity for human-type sialic acid receptor and replication of H9N2 avian influenza virus in mice. J. Virol. 2016, 90, 9806–9825. [Google Scholar] [CrossRef]
- Wan, H.; Perez, D.R. Amino acid 226 in the hemagglutinin of H9N2 influenza viruses determines cell tropism and replication in human airway epithelial cells. J. Virol. 2007, 81, 5181–5191. [Google Scholar] [CrossRef]
- Ramirez-Nieto, G.; Monne, I.; Stevens, J.; Cattoli, G.; Capua, I.; Chen, L.M.; Donis, R.O.; Busch, J.; Paulson, J.C.; Brockwell, C.; et al. Replication and transmission of H9N2 influenza viruses in ferrets: Evaluation of pandemic potential. PLoS ONE 2008, 3, e2923. [Google Scholar] [CrossRef]
- Sorrell, E.M.; Wan, H.; Araya, Y.; Song, H.; Perez, D.R. Minimal molecular constraints for respiratory droplet transmission of an avian-human H9N2 influenza A virus. Proc. Natl. Acad. Sci. USA 2009, 106, 7565–7570. [Google Scholar] [CrossRef]
- Wang, F.; Qi, J.; Bi, Y.; Zhang, W.; Wang, M.; Zhang, B.; Wang, M.; Liu, J.; Yan, J.; Shi, Y.; et al. Adaptation of avian influenza A (H6N1) virus from avian to human receptor-binding preference. EMBO J. 2015, 34, 1661–1673. [Google Scholar] [CrossRef]
- de Vries, R.P.; Tzarum, N.; Peng, W.; Thompson, A.J.; Ambepitiya Wickramasinghe, I.N.; de la Pena, A.T.T.; van Breemen, M.J.; Bouwman, K.M.; Zhu, X.; McBride, R.; et al. A single mutation in Taiwanese H6N1 influenza hemagglutinin switches binding to human-type receptors. EMBO Mol. Med. 2017, 9, 1314–1325. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Ma, S.; Kong, H.; Deng, G.; Shi, J.; Liu, L.; Chen, H. Identification of a key amino acid in hemagglutinin that increases human-type receptor binding and transmission of an H6N2 avian influenza virus. Microbes Infect. 2017, 19, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Qi, J.; Xiao, H.; Bi, Y.; Zhang, W.; Xu, Y.; Wang, F.; Shi, Y.; Gao, G.F. Avian-to-Human Receptor-Binding Adaptation by Influenza A Virus Hemagglutinin H4. Cell Rep. 2017, 20, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Ren, Z.; Zhao, Y.; Cheng, K.; Sun, W.; Zhang, X.; Wu, J.; He, H.; Xia, X.; Gao, Y. PB2 and hemagglutinin mutations confer a virulent phenotype on an H1N2 avian influenza virus in mice. Arch. Virol. 2019, 164, 2023–2029. [Google Scholar] [CrossRef]
- Tzarum, N.; de Vries, R.P.; Peng, W.; Thompson, A.J.; Bouwman, K.M.; McBride, R.; Yu, W.; Zhu, X.; Verheije, M.H.; Paulson, J.C.; et al. The 150-Loop Restricts the Host Specificity of Human H10N8 Influenza Virus. Cell Rep. 2017, 19, 235–245. [Google Scholar] [CrossRef]
- Zhang, H.; de Vries, R.P.; Tzarum, N.; Zhu, X.; Yu, W.; McBride, R.; Paulson, J.C.; Wilson, I.A. A human-infecting H10N8 influenza virus retains a strong preference for avian-type receptors. Cell Host Microbe 2015, 17, 377–384. [Google Scholar] [CrossRef]
- Lu, X.; Qi, J.; Shi, Y.; Wang, M.; Smith, D.F.; Heimburg-Molinaro, J.; Zhang, Y.; Paulson, J.C.; Xiao, H.; Gao, G.F. Structure and receptor binding specificity of hemagglutinin H13 from avian influenza A virus H13N6. J. Virol. 2013, 87, 9077–9085. [Google Scholar] [CrossRef]
- Colman, P.M.; Hoyne, P.A.; Lawrence, M.C. Sequence and structure alignment of paramyxovirus hemagglutinin-neuraminidase with influenza virus neuraminidase. J. Virol. 1993, 67, 2972–2980. [Google Scholar] [CrossRef]
- WHO. Summary of Neuraminidase (NA) Amino Acid Substitutions Assessed for Their Effects on Inhibition by NA Inhibitors (NAIs) Among Avian Influenza Viruses of Group 1 (N1, N4, N5, N8 subtypes) and Group 2 (N2, N3, N6, N7, N9 subtypes) NAs. Available online: https://cdn.who.int/media/docs/default-source/influenza/avwg/avian-nai-marker-who-table_07-08-2024_updated_final-version.pdf?sfvrsn=bc0d1e9a_5 (accessed on 23 July 2025).
- Gubareva, L.V.; Robinson, M.J.; Bethell, R.C.; Webster, R.G. Catalytic and framework mutations in the neuraminidase active site of influenza viruses that are resistant to 4-guanidino-Neu5Ac2en. J. Virol. 1997, 71, 3385–3390. [Google Scholar] [CrossRef]
- Mishin, V.P.; Hayden, F.G.; Gubareva, L.V. Susceptibilities of antiviral-resistant influenza viruses to novel neuraminidase inhibitors. Antimicrob. Agents Chemother. 2005, 49, 4515–4520. [Google Scholar] [CrossRef]
- Kode, S.S.; Pawar, S.D.; Cherian, S.S.; Tare, D.S.; Bhoye, D.; Keng, S.S.; Mullick, J. Selection of avian influenza A (H9N2) virus with reduced susceptibility to neuraminidase inhibitors oseltamivir and zanamivir. Virus Res. 2019, 265, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Tepper, V.; Nykvist, M.; Gillman, A.; Skog, E.; Wille, M.; Lindstrom, H.S.; Tang, C.; Lindberg, R.H.; Lundkvist, A.; Jarhult, J.D. Influenza A/H4N2 mallard infection experiments further indicate zanamivir as less prone to induce environmental resistance development than oseltamivir. J. Gen. Virol. 2020, 101, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Gillman, A.; Muradrasoli, S.; Soderstrom, H.; Nordh, J.; Brojer, C.; Lindberg, R.H.; Latorre-Margalef, N.; Waldenstrom, J.; Olsen, B.; Jarhult, J.D. Resistance mutation R292K is induced in influenza A(H6N2) virus by exposure of infected mallards to low levels of oseltamivir. PLoS ONE 2013, 8, e71230. [Google Scholar] [CrossRef] [PubMed]
- Bialy, D.; Shelton, H. Functional neuraminidase inhibitor resistance motifs in avian influenza A (H5Nx) viruses. Antivir. Res. 2020, 182, 104886. [Google Scholar] [CrossRef]
- Orozovic, G.; Orozovic, K.; Lennerstrand, J.; Olsen, B. Detection of resistance mutations to antivirals oseltamivir and zanamivir in avian influenza A viruses isolated from wild birds. PLoS ONE 2011, 6, e16028. [Google Scholar] [CrossRef]
- Song, M.S.; Marathe, B.M.; Kumar, G.; Wong, S.S.; Rubrum, A.; Zanin, M.; Choi, Y.K.; Webster, R.G.; Govorkova, E.A.; Webby, R.J. Unique Determinants of Neuraminidase Inhibitor Resistance among N3, N7, and N9 Avian Influenza Viruses. J. Virol. 2015, 89, 10891–10900. [Google Scholar] [CrossRef][Green Version]
- Choi, W.S.; Jeong, J.H.; Kwon, J.J.; Ahn, S.J.; Lloren, K.K.S.; Kwon, H.I.; Chae, H.B.; Hwang, J.; Kim, M.H.; Kim, C.J. Screening for Neuraminidase Inhibitor Resistance Markers among Avian Influenza Viruses of the N4, N5, N6, and N8 Neuraminidase Subtypes. J. Virol. 2017, 92, e01580-17. [Google Scholar] [CrossRef]
- Svyatchenko, S.V.; Goncharova, N.I.; Marchenko, V.Y.; Kolosova, N.P.; Shvalov, A.N.; Kovrizhkina, V.L.; Durymanov, A.G.; Onkhonova, G.S.; Tregubchak, T.V.; Susloparov, I.M.; et al. An influenza A(H5N8) virus isolated during an outbreak at a poultry farm in Russia in 2017 has an N294S substitution in the neuraminidase and shows reduced susceptibility to oseltamivir. Antivir. Res. 2021, 191, 105079. [Google Scholar] [CrossRef]
- Xie, R.; Wang, W.; Gao, Y.; Liu, W.; Yue, B.; Liu, S.; Fan, W.; Song, S.; Yan, L. Evolution and mammalian adaptation of H3 and H10 subtype avian influenza viruses in wild birds in Yancheng Wetland of China. Vet. Microbiol. 2023, 279, 109669. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Swayne, D.E.; Thomas, C.; Rameix-Welti, M.A.; Naffakh, N.; Warnes, C.; Altholtz, M.; Donis, R.; Subbarao, K. Neuraminidase stalk length and additional glycosylation of the hemagglutinin influence the virulence of influenza H5N1 viruses for mice. J. Virol. 2009, 83, 4704–4708. [Google Scholar] [CrossRef]
- Li, J.; Zu Dohna, H.; Cardona, C.J.; Miller, J.; Carpenter, T.E. Emergence and genetic variation of neuraminidase stalk deletions in avian influenza viruses. PLoS ONE 2011, 6, e14722. [Google Scholar] [CrossRef] [PubMed]
- Munier, S.; Larcher, T.; Cormier-Aline, F.; Soubieux, D.; Su, B.; Guigand, L.; Labrosse, B.; Cherel, Y.; Quéré, P.; Marc, D.; et al. A genetically engineered waterfowl influenza virus with a deletion in the stalk of the neuraminidase has increased virulence for chickens. J. Virol. 2010, 84, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.W.; Munier, S.; Larcher, T.; Soubieux, D.; Ledevin, M.; Esnault, E.; Tourdes, A.; Croville, G.; Guérin, J.L.; Quéré, P.; et al. Length variations in the NA stalk of an H7N1 influenza virus have opposite effects on viral excretion in chickens and ducks. J. Virol. 2012, 86, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Xiao, H.; Chen, Q.; Wu, Y.; Fu, L.; Quan, C.; Wong, G.; Liu, J.; Haywood, J.; Liu, Y.; et al. Changes in the Length of the Neuraminidase Stalk Region Impact H7N9 Virulence in Mice. J. Virol. 2016, 90, 2142–2149. [Google Scholar] [CrossRef]
- Sorrell, E.M.; Song, H.; Pena, L.; Perez, D.R. A 27-amino-acid deletion in the neuraminidase stalk supports replication of an avian H2N2 influenza A virus in the respiratory tract of chickens. J. Virol. 2010, 84, 11831–11840. [Google Scholar] [CrossRef]
- Obenauer, J.C.; Denson, J.; Mehta, P.K.; Su, X.; Mukatira, S.; Finkelstein, D.B.; Xu, X.; Wang, J.; Ma, J.; Fan, Y.; et al. Large-scale sequence analysis of avian influenza isolates. Science 2006, 311, 1576–1580. [Google Scholar] [CrossRef]
- Lan, Y.; Zhang, Y.; Dong, L.; Wang, D.; Huang, W.; Xin, L.; Yang, L.; Zhao, X.; Li, Z.; Wang, W.; et al. A comprehensive surveillance of adamantane resistance among human influenza A virus isolated from mainland China between 1956 and 2009. Antivir. Ther. 2010, 15, 853–859. [Google Scholar] [CrossRef]
- WHO. Summary of Polymerase Acid Protein (PA) Amino Acid Substitutions for Their Effects on PA Inhibitor (PAI) Baloxavir Susceptibility. Available online: https://cdn.who.int/media/docs/default-source/influenza/laboratory---network/quality-assurance/antiviral-susceptibility-influenza/pa-marker-who-table_07-08-2024_updated_final-version.pdf?sfvrsn=5307d6fe_2 (accessed on 23 July 2025).
- Andreev, K.; Jones, J.C.; Seiler, P.; Kandeil, A.; Webby, R.J.; Govorkova, E.A. Genotypic and phenotypic susceptibility of emerging avian influenza A viruses to neuraminidase and cap-dependent endonuclease inhibitors. Antiviral Res. 2024, 229, 105959. [Google Scholar] [CrossRef]
- Xiao, C.; Ma, W.; Sun, N.; Huang, L.; Li, Y.; Zeng, Z.; Wen, Y.; Zhang, Z.; Li, H.; Li, Q.; et al. PB2-588 V promotes the mammalian adaptation of H10N8, H7N9 and H9N2 avian influenza viruses. Sci. Rep. 2016, 6, 19474. [Google Scholar] [CrossRef]
- Hu, M.; Yuan, S.; Zhang, K.; Singh, K.; Ma, Q.; Zhou, J.; Chu, H.; Zheng, B.J. PB2 substitutions V598T/I increase the virulence of H7N9 influenza A virus in mammals. Virology 2017, 501, 92–101. [Google Scholar] [CrossRef]
- Li, J.; Ishaq, M.; Prudence, M.; Xi, X.; Hu, T.; Liu, Q.; Guo, D. Single mutation at the amino acid position 627 of PB2 that leads to increased virulence of an H5N1 avian influenza virus during adaptation in mice can be compensated by multiple mutations at other sites of PB2. Virus Res. 2009, 144, 123–129. [Google Scholar] [CrossRef]
- Elgendy, E.M.; Arai, Y.; Kawashita, N.; Daidoji, T.; Takagi, T.; Ibrahim, M.S.; Nakaya, T.; Watanabe, Y. Identification of polymerase gene mutations that affect viral replication in H5N1 influenza viruses isolated from pigeons. J. Gen. Virol. 2017, 98, 6–17. [Google Scholar] [CrossRef]
- Feng, X.; Wang, Z.; Shi, J.; Deng, G.; Kong, H.; Tao, S.; Li, C.; Liu, L.; Guan, Y.; Chen, H. Glycine at Position 622 in PB1 Contributes to the Virulence of H5N1 Avian Influenza Virus in Mice. J. Virol. 2015, 90, 1872–1879. [Google Scholar] [CrossRef]
- Yamayoshi, S.; Yamada, S.; Fukuyama, S.; Murakami, S.; Zhao, D.; Uraki, R.; Watanabe, T.; Tomita, Y.; Macken, C.; Neumann, G.; et al. Virulence-affecting amino acid changes in the PA protein of H7N9 influenza A viruses. J. Virol. 2014, 88, 3127–3134. [Google Scholar] [CrossRef]
- Song, J.; Xu, J.; Shi, J.; Li, Y.; Chen, H. Synergistic Effect of S224P and N383D Substitutions in the PA of H5N1 Avian Influenza Virus Contributes to Mammalian Adaptation. Sci. Rep. 2015, 5, 10510. [Google Scholar] [CrossRef]
- Tada, T.; Suzuki, K.; Sakurai, Y.; Kubo, M.; Okada, H.; Itoh, T.; Tsukamoto, K. NP body domain and PB2 contribute to increased virulence of H5N1 highly pathogenic avian influenza viruses in chickens. J. Virol. 2011, 85, 1834–1846. [Google Scholar] [CrossRef] [PubMed]
- Wasilenko, J.L.; Sarmento, L.; Pantin-Jackwood, M.J. A single substitution in amino acid 184 of the NP protein alters the replication and pathogenicity of H5N1 avian influenza viruses in chickens. Arch. Virol. 2009, 154, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Deng, G.; Song, J.; Tian, G.; Suo, Y.; Jiang, Y.; Guan, Y.; Bu, Z.; Kawaoka, Y.; Chen, H. Two amino acid residues in the matrix protein M1 contribute to the virulence difference of H5N1 avian influenza viruses in mice. Virology 2009, 384, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Nao, N.; Kajihara, M.; Manzoor, R.; Maruyama, J.; Yoshida, R.; Muramatsu, M.; Miyamoto, H.; Igarashi, M.; Eguchi, N.; Sato, M.; et al. A Single Amino Acid in the M1 Protein Responsible for the Different Pathogenic Potentials of H5N1 Highly Pathogenic Avian Influenza Virus Strains. PLoS ONE 2015, 10, e0137989. [Google Scholar] [CrossRef]
- Jiao, P.; Tian, G.; Li, Y.; Deng, G.; Jiang, Y.; Liu, C.; Liu, W.; Bu, Z.; Kawaoka, Y.; Chen, H. A single-amino-acid substitution in the NS1 protein changes the pathogenicity of H5N1 avian influenza viruses in mice. J. Virol. 2008, 82, 1146–1154, Erratum in J. Virol. 2008, 82, 4190. https://doi.org/10.1128/JVI.00376-08. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Chen, Q.; Zhang, X.; Sun, Y.; Bi, Y.; Zhang, S.; Gu, J.; Li, J.; Liu, D.; et al. Three amino acid substitutions in the NS1 protein change the virus replication of H5N1 influenza virus in human cells. Virology 2018, 519, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Kuo, R.L.; Krug, R.M. Influenza a virus polymerase is an integral component of the CPSF30-NS1A protein complex in infected cells. J. Virol. 2009, 83, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Spesock, A.; Malur, M.; Hossain, M.; Chen, L.M.; Njaa, B.L.; Davis, C.T.; Lipatov, A.S.; York, I.A.; Krug, R.M.; Donis, R.O. The virulence of 1997 H5N1 influenza viruses in the mouse model is increased by correcting a defect in their NS1 proteins. J. Virol. 2011, 85, 7048–7058. [Google Scholar] [CrossRef] [PubMed]
- Takashita, E.; Morita, H.; Nagata, S.; Shirakura, M.; Fujisaki, S.; Miura, H.; Takayama, I.; Arita, T.; Suzuki, Y.; Yamaoka, M.; et al. Influenza Virus Surveillance Group of Japan. Antiviral Susceptibilities of Avian Influenza A(H5), A(H7), and A(H9) Viruses Isolated in Japan. J. Infect. Dis. 2022, 75, 398–402. [Google Scholar] [CrossRef]
- Liu, K.; Qi, X.; Bao, C.; Wang, X.; Liu, X. Novel H10N3 avian influenza viruses: A potential threat to public health. Lancet Microbe 2024, 5, e417. [Google Scholar] [CrossRef]
- Bodewes, R.; Bestebroer, T.M.; van der Vries, E.; Verhagen, J.H.; Herfst, S.; Koopmans, M.P.; Fouchier, R.A.; Pfankuche, V.M.; Wohlsein, P.; Siebert, U.; et al. Avian Influenza A(H10N7) virus-associated mass deaths among harbor seals. Emerg. Infect. Dis. 2015, 21, 720–722. [Google Scholar] [CrossRef]
- De Marco, M.A.; Binazzi, A.; Melis, P.; Cotti, C.; Bonafede, M.; Delogu, M.; Tomao, P.; Vonesch, N. Occupational Risk from Avian Influenza Viruses at Different Ecological Interfaces Between 1997 and 2019. Microorganisms 2025, 13, 1391. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zenatello, M.; Baccetti, N.; Borghesi, F. Risulati dei Censimenti Degli Uccelli Acquatici Svernanti in Italia. Distribuzione, Stima e Trend Delle Popolazioni nel 2001–2010. ISPRA, Serie Rapporti, 206/2014. Available online: https://www.isprambiente.gov.it/files/pubblicazioni/rapporti/R_206_14_Uccelli_acquatici_svernanti_def.pdf (accessed on 10 April 2025).
- Brichetti, P.; Fracasso, G. Germano Reale (Anas platyrhynchos). In Ornitologia Italiana. Gavidae—Falconidae; Alberto Perdisa Editore: Bologna, Italy, 2003; Volume 1, pp. 204–208. [Google Scholar]
- Brichetti, P.; Fracasso, G. Folaga (Fulica atra). In Ornitologia Italiana. Tetraonidae—Scolopacidae; Alberto Perdisa Editore: Bologna, Italy, 2004; Volume 2, pp. 109–114. [Google Scholar]
- Kawaoka, Y.; Chambers, T.M.; Sladen, W.L.; Webster, R.G. Is the gene pool of influenza viruses in shorebirds and gulls different from that in wild ducks? Virology 1988, 163, 247–250. [Google Scholar] [CrossRef]
- McBride, D.S.; Lauterbach, S.E.; Li, Y.T.; Smith, G.J.D.; Killian, M.L.; Nolting, J.M.; Su, Y.C.F.; Bowman, A.S. Genomic Evidence for Sequestration of Influenza A Virus Lineages in Sea Duck Host Species. Viruses 2021, 13, 172. [Google Scholar] [CrossRef]
- Hill, N.J.; Bishop, M.A.; Trovão, N.S.; Ineson, K.M.; Schaefer, A.L.; Puryear, W.B.; Zhou, K.; Foss, A.D.; Clark, D.E.; MacKenzie, K.G.; et al. Ecological divergence of wild birds drives avian influenza spillover and global spread. PLoS Pathog. 2022, 18, e1010062. [Google Scholar] [CrossRef]
- De Marco, M.A.; Foni, E.; Campitelli, L.; Raffini, E.; Delogu, M.; Donatelli, I. Long-term monitoring for avian influenza viruses in wild bird species in Italy. Vet. Res. Commun. 2003, 27 (Suppl. S1), 107–114. [Google Scholar] [CrossRef] [PubMed]
- Matrosovich, M.N.; Matrosovich, T.Y.; Gray, T.; Roberts, N.A.; Klenk, H.D. Human and avian influenza viruses target different cell types in cultures of human airway epithelium. Proc. Natl. Acad. Sci. USA 2004, 101, 4620–4624. [Google Scholar] [CrossRef] [PubMed]
- Hatta, M.; Gao, P.; Halfmann, P.; Kawaoka, Y. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science 2001, 293, 1840–1842. [Google Scholar] [CrossRef]
- Yang, J.; Yang, L.; Zhu, W.; Wang, D.; Shu, Y. Epidemiological and Genetic Characteristics of the H3 Subtype Avian Influenza Viruses in China. China CDC Wkly. 2021, 3, 929–936. [Google Scholar] [CrossRef]
- Long, J.S.; Howard, W.A.; Núñez, A.; Moncorgé, O.; Lycett, S.; Banks, J.; Barclay, W.S. The effect of the PB2 mutation 627K on highly pathogenic H5N1 avian influenza virus is dependent on the virus lineage. J. Virol. 2013, 87, 9983–9996. [Google Scholar] [CrossRef]
- Jones, J.C.; Yen, H.L.; Adams, P.; Armstrong, K.; Govorkova, E.A. Influenza antivirals and their role in pandemic preparedness. Antivir. Res. 2023, 210, 105499. [Google Scholar] [CrossRef]
- Qi, W.; Zhou, X.; Shi, W.; Huang, L.; Xia, W.; Liu, D.; Li, H.; Chen, S.; Lei, F.; Cao, L.; et al. Genesis of the novel human-infecting influenza A(H10N8) virus and potential genetic diversity of the virus in poultry, China. Eurosurveillance 2014, 19, 20841. [Google Scholar] [CrossRef]
- WHO. Summary of Neuraminidase (NA) Amino Acid Substitutions Assessed for Their Effects on Inhibition by Neuraminidase Inhibitors (NAIs). Available online: https://cdn.who.int/media/docs/default-source/influenza/laboratory---network/quality-assurance/human-nai-marker-table_for-publication_final_20240918.pdf?sfvrsn=c6d153ec_3 (accessed on 23 July 2025).
- Taniguchi, K.; Noshi, T.; Omoto, S.; Sato, A.; Shishido, T.; Matsuno, K.; Okamatsu, M.; Krauss, S.; Webby, R.J.; Sakoda, Y.; et al. The impact of PA/I38 substitutions and PA polymorphisms on the susceptibility of zoonotic influenza A viruses to baloxavir. Arch. Virol. 2024, 169, 29. [Google Scholar] [CrossRef]
- Jetz, W.; Thomas, G.H.; Joy, J.B.; Hartmann, K.; Mooers, A.O. The global diversity of birds in space and time. Nature 2012, 491, 444–448. [Google Scholar] [CrossRef]
- Elbe, S.; Buckland-Merrett, G. Data, disease and diplomacy: GISAID’s innovative contribution to global health. Glob. Chall. 2017, 1, 33–46. [Google Scholar] [CrossRef]
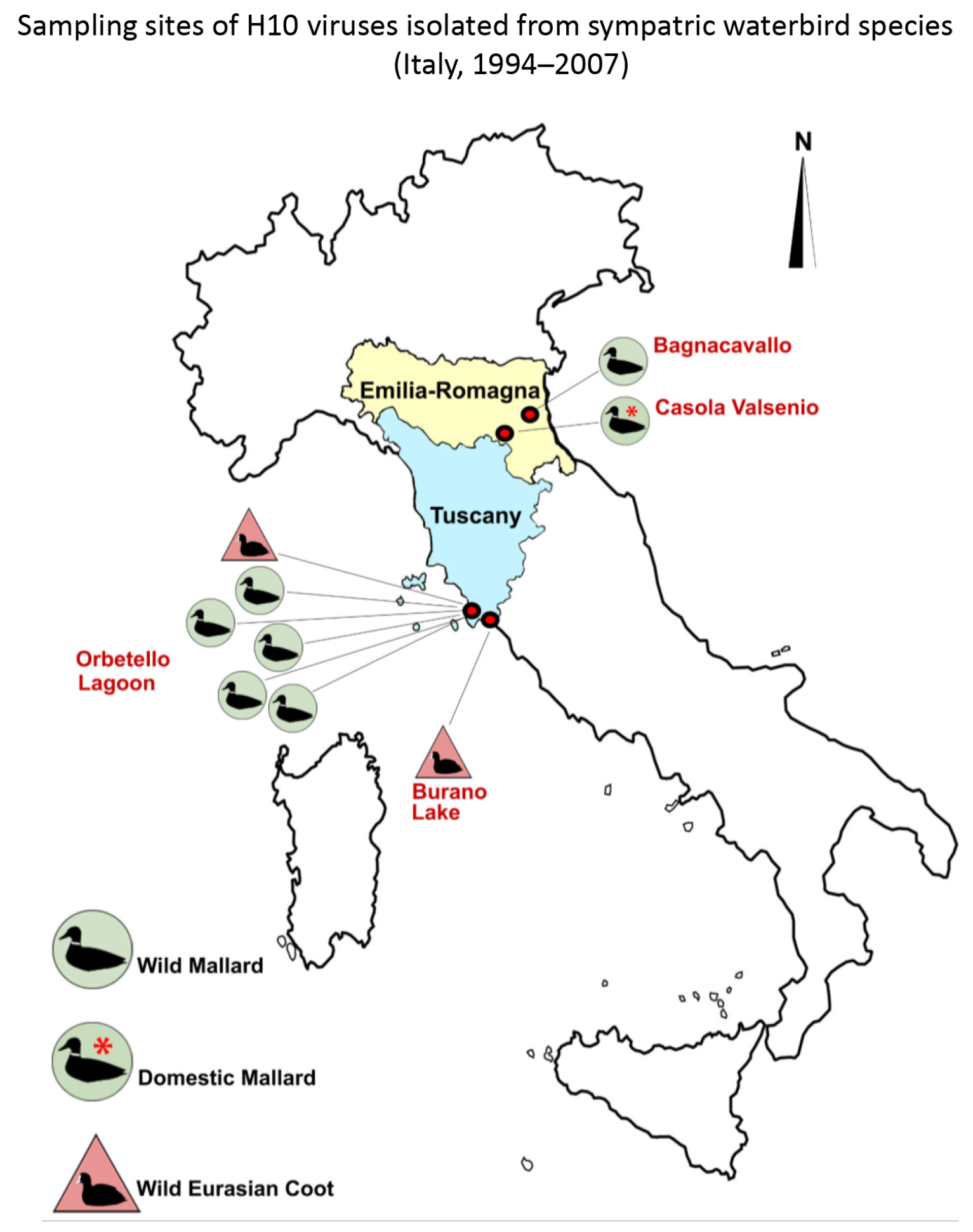
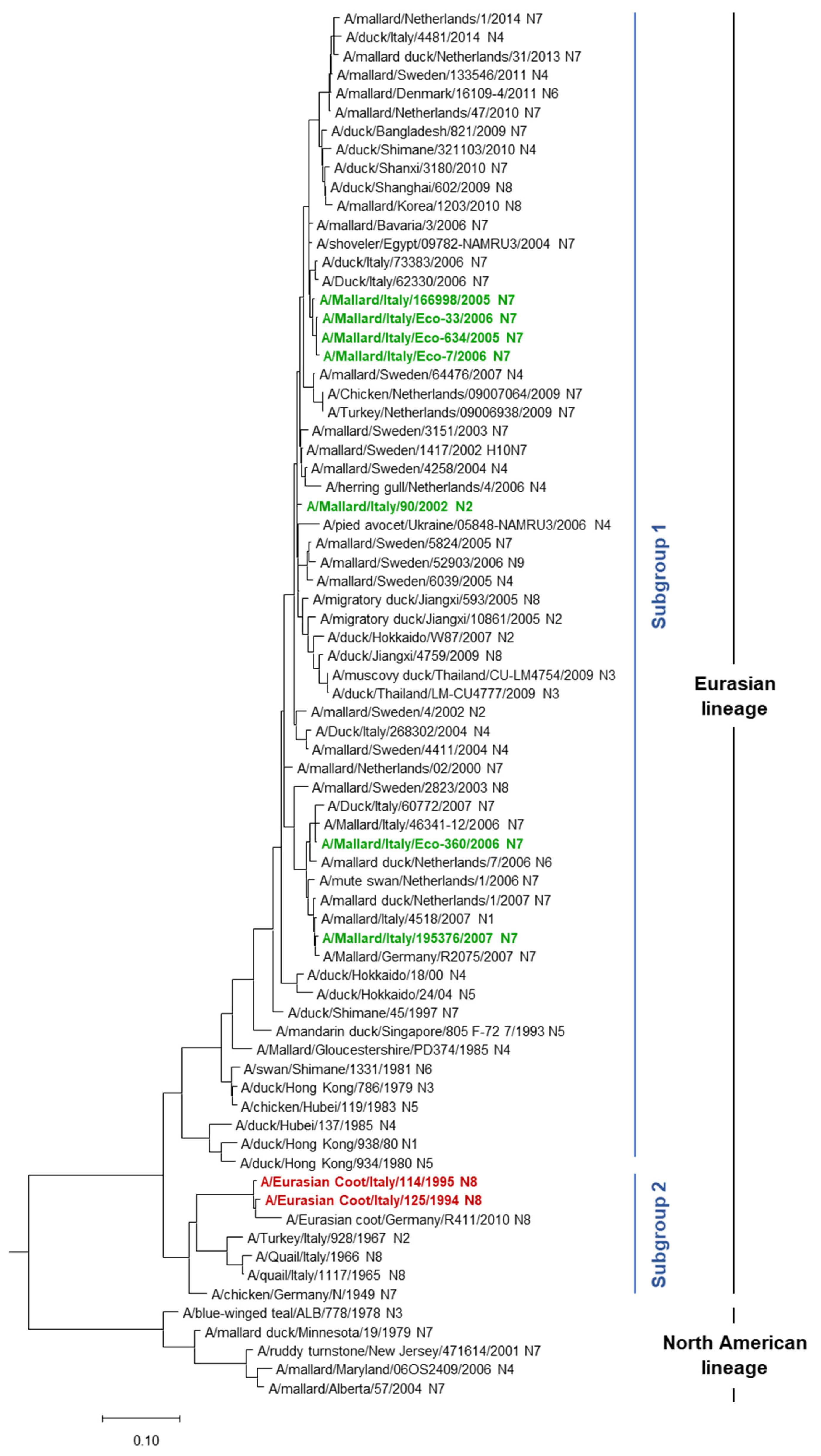
| AIV Isolate | HN Subtype | Sampling Date dd/mm/yy | Sampling Site | Bird | |||
|---|---|---|---|---|---|---|---|
| Species | Sex | Age | Origin | ||||
| A/Eurasian Coot/It/125/1994 | H10N8 | 11 January 1994 | Orbetello Lagoon ^ | Fulica atra | M | Ad | W |
| A/Eurasian Coot/It/114/1995 | H10N8 | 12 December 1995 | Burano Lake ^ | F. atra | F | Ad | W |
| A/Mallard/It/90/2002 | H10N2 | 30 October 2002 | Orbetello Lagoon | Anas platyrhynchos | M | Juv | W |
| A/Mallard/It/166998/2005 § | H10N7 | 21 July 2005 | Bagnacavallo * | A. platyrhynchos | Na | Na | D |
| A/Mallard/It/Eco-634/2005 | H10N7 | 28 December 2005 | Orbetello Lagoon | A. platyrhynchos | F | Juv | W |
| A/Mallard/It/Eco-7/2006 | H10N7 | 23 January 2006 | Orbetello Lagoon | A. platyrhynchos | F | Juv | W |
| A/Mallard/It/Eco-33/2006 | H10N7 | 24 January 2006 | Orbetello Lagoon | A. platyrhynchos | M | Juv | W |
| A/Mallard/It/Eco-360/2006 | H10N7 | 24 November 2006 | Orbetello Lagoon | A. platyrhynchos | M | Juv | W |
| A/Mallard/It/195376/2007 | H10N7 | 31 July 2007 | Casola Valsenio * | A. platyrhynchos | Na | Na | W |
| H10NX Strains | BLAST Results | Nt Identity |
|---|---|---|
| A/Eurasian Coot/It/125/1994 (H10N8) | A/Eurasian coot/Germany/R411/2010 (H10N8) | 96% |
| A/Eurasian Coot/It/114/1995 (H10N8) | A/Eurasian coot/Germany/R411/2010 (H10N8) | 96% |
| A/Mallard/It/90/2002 (H10N2) | A/mallard/Sweden/1417/2002 (H10N7) | 98% |
| A/Mallard/It/166998/2005 (H10N7) | A/shoveler/Egypt/09781-NAMRU3/2004 (H10N7) | 99% |
| A/Mallard/It/Eco-634/2005 (H10N7) | A/shoveler/Egypt/09781-NAMRU3/2004 (H10N7) | 98% |
| A/Mallard/It/Eco-7/2006 (H10N7) | A/shoveler/Egypt/09781-NAMRU3/2004 (H10N7) | 98% |
| A/Mallard/It/Eco-33/2006 (H10N7) | A/shoveler/Egypt/09781-NAMRU3/2004 (H10N7) | 98% |
| A/Mallard/It/Eco-360/2006 (H10N7) | A/Mallard/Italy/46341-12/2006 (H10N7) | 99% |
| A/Mallard/It/195376/2007 (H10N7) | A/Mallard/Germany/R2075/2007 (H10N7) | 99% |
| Viruses | Subtype | Hemagglutinin Cleavage Site | |
|---|---|---|---|
| Amino Acids | Nucleotides | ||
| A/Eurasian Coot/It/125/1994 | H10N8 | PEVVQGR/GLF | CCAGAAGTAGTGCAAGGAAGGGGTTTGTTT |
| A/Eurasian Coot/It/114/1995 | H10N8 | PEVVQGR/GLF | CCAGAAGTAGTGCAAGGAAGGGGTTTGTTT |
| A/Mallard/It/90/2002 | H10N2 | PEIMQGR/GLF | CCAGAAATAATGCAAGGGAGAGGTCTATTT |
| A/Mallard/It/166998/2005 | H10N7 | PEIMQGR/GLF | CCAGAAATAATGCAAGGGAGAGGTCTATTT |
| A/Mallard/It/Eco-634/2005 | H10N7 | PEIMQGR/GLF | CCAGAAATAATGCAAGGGAGAGGTCTATTT |
| A/Mallard/It/Eco-7/2006 | H10N7 | PEIMQGR/GLF | CCAGAAATAATGCAAGGGAGAGGTCTATTT |
| A/Mallard/It/Eco-33/2006 | H10N7 | PEIMQGR/GLF | CCAGAAATAATGCAAGGGAGAGGTCTATTT |
| A/Mallard/It/Eco-360/2006 | H10N7 | PEIMQGR/GLF | CCAGAAATAATGCAAGGGAGAGGTCTATTT |
| A/Mallard/It/195376/2007 | H10N7 | PEIMQGR/GLF | CCAGAAATAATGCAAGGGAGAGGTCTATTT |
| Viruses | Receptor-Binding Site (Amino Acidic Positions—H10 Numbering) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 105 | 161 | 163 | 193 | 200 | 204 | 205 | Left Edge 230–239 | Right Edge 142–146 | |
| A/Eurasian Coot/It/125/1994 | Y | W | V | H | E | L | Y | RPQVNGQSGR | GVTKA |
| A/Eurasian Coot/It/114/1995 | Y | W | V | H | E | L | Y | RPQVNGQSGR | GVTKA |
| A/Mallard/It/90/2002 | Y | W | V | H | E | L | Y | RPQVNGQSGR | GTTKA |
| A/Mallard/It/166998/2005 | Y | W | V | H | E | L | Y | RPQVNGQSGR | GTTKA |
| A/Mallard/It/Eco-634/2005 | Y | W | V | H | E | L | Y | RPQVNGQSGR | GTTKA |
| A/Mallard/It/Eco-7/2006 | Y | W | V | H | E | L | Y | RPQVNGQSGR | GTTKA |
| A/Mallard/It/Eco-33/2006 | Y | W | V | H | E | L | Y | RPQVNGQSGR | GTTKA |
| A/Mallard/It/Eco-360/2006 | Y | W | V | H | E | L | Y | RPQVNGQSGR | GTTKA |
| A/Mallard/It/195376/2007 | Y | W | V | H | E | L | Y | RPQVNGQSGR | GTTKA |
| Genes | No. of Mutation/Motif | Mutation/Motif Grouped by Bird Species, and Sampling dd/mm/yy of Nine H10 Isolates | ||||||||
| Eurasian Coot | Mallard | |||||||||
| 11 January 1994 ^ H10N8 (1) | 12 December 1995 H10N8 (2) | 30 October 2002 H10N2 (3) | 21 July 2005 H10N7 *,(4) | 28 December 2005 H10N7 (5) | 23 January 2006 H10N7 (6) | 24 January 2006 H10N7 (7) | 24 November 2006 H10N7 (8) | 31 July 2007 H10N7 (9) | ||
| PB2 | 4/2 | 4/2 | 4/2 | 2/2 | 2/2 | 3/2 | 3/2 | 3/2 | 2/2 | 2/2 |
| PB1 | 2/0 | 2/0 | 2/0 | 2/0 | 2/0 | 2/0 | 2/0 | 2/0 | 2/0 | 2/0 |
| PA | 3/0 | 3/0 | 3/0 | 3/0 | 3/0 | 3/0 | 3/0 | 3/0 | 3/0 | 3/0 |
| NP | 3/0 | 1/0 | 1/0 | 1/0 | 2/0 | 2/0 | 2/0 | 3/0 | 2/0 | 2/0 |
| M1 | 3/0 | 3/0 | 3/0 | 3/0 | 3/0 | 3/0 | 3/0 | 3/0 | 3/0 | 3/0 |
| NS1 | 3/2 | 3/2 | 3/2 | 3/2 | 3/2 | 3/2 | 3/2 | 3/2 | 3/2 | 2/0 |
| Isolate | Subtype | Mean IC50 ± SD (nM) * | |
|---|---|---|---|
| Oseltamivir | Zanamivir | ||
| A/Eurasian Coot/It/125/1994 | H10N8 | 3.4 ± 0.07 | 1.3 ± 0.02 |
| A/Eurasian Coot/It/114/1995 | H10N8 | 3.2 ± 0.07 | 1.1 ± 0.01 |
| A/Mallard/It/90/2002 | H10N2 | 0.3 ± 0.001 | 0.7 ± 0.04 |
| A/Mallard/It/Eco-634/2005 | H10N7 | 0.7 ± 0.03 | 1 ± 0.06 |
| A/Mallard/It/Eco-7/2006 | H10N7 | 0.8 ± 0.07 | 1 ± 0.002 |
| A/Mallard/It/Eco-33/2006 | H10N7 | 1.1 ± 0.07 | 1.3 ± 0.02 |
| A/Mallard/It/Eco-360/2006 | H10N7 | 1.2 ± 0.07 | 1.2 ± 0.06 |
| A/Mallard/It/195376/2007 | H10N7 | 0.7 ± 0.09 | 0.9 ± 0.02 |
| A/Victoria/4897/2022 (Wt) | H1N1pdm09 | 1.1 ± 0.01 | 0.3 ± 0.006 |
| A/Darwin/9/2021 (Wt) | H3N2 | 0.3 ± 0.06 | 0.3 ± 0.002 |
| H1-H275Y ^ | H1N1pdm09 | 492.9 ± 0.07 | 0.7 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Facchini, M.; De Marco, M.A.; Piacentini, S.; Di Martino, A.; Gruber, C.E.M.; Cotti, C.; Di Mario, G.; Calzoletti, L.; Fabiani, C.; Delogu, M.; et al. Divergent Avian Influenza H10 Viruses from Sympatric Waterbird Species in Italy: Zoonotic Potential Assessment by Molecular Markers. Microorganisms 2025, 13, 2575. https://doi.org/10.3390/microorganisms13112575
Facchini M, De Marco MA, Piacentini S, Di Martino A, Gruber CEM, Cotti C, Di Mario G, Calzoletti L, Fabiani C, Delogu M, et al. Divergent Avian Influenza H10 Viruses from Sympatric Waterbird Species in Italy: Zoonotic Potential Assessment by Molecular Markers. Microorganisms. 2025; 13(11):2575. https://doi.org/10.3390/microorganisms13112575
Chicago/Turabian StyleFacchini, Marzia, Maria Alessandra De Marco, Sara Piacentini, Angela Di Martino, Cesare Ernesto Maria Gruber, Claudia Cotti, Giuseppina Di Mario, Laura Calzoletti, Concetta Fabiani, Mauro Delogu, and et al. 2025. "Divergent Avian Influenza H10 Viruses from Sympatric Waterbird Species in Italy: Zoonotic Potential Assessment by Molecular Markers" Microorganisms 13, no. 11: 2575. https://doi.org/10.3390/microorganisms13112575
APA StyleFacchini, M., De Marco, M. A., Piacentini, S., Di Martino, A., Gruber, C. E. M., Cotti, C., Di Mario, G., Calzoletti, L., Fabiani, C., Delogu, M., Palamara, A. T., Stefanelli, P., & Puzelli, S. (2025). Divergent Avian Influenza H10 Viruses from Sympatric Waterbird Species in Italy: Zoonotic Potential Assessment by Molecular Markers. Microorganisms, 13(11), 2575. https://doi.org/10.3390/microorganisms13112575








