Evaluation of Biocontrol Efficacy of Bacillus velezensis HAB-2 Combined with Pseudomonas hunanensis and Enterobacter soli Against Cowpea Fusarium Wilt
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Plant Materials, and Culture Conditions
2.2. In Vitro Screening of Plant Growth-Promoting Traits
2.2.1. Nitrogen Fixation and Solubilization of Phosphorus and Potassium
2.2.2. Siderophore Production Assay
2.2.3. Indole-3-Acetic Acid Production Assay
2.3. Assessment of Extracellular Enzyme Activities of B. velezensis HAB-2
2.4. Inhibitory Effect of B. velezensis HAB-2 on F. oxysporum f. sp. tracheiphilum AIQBFO93
the treatment)/Colony diameter of the control] × 100%.
2.5. Colonization of B. velezensis HAB-2-GFP on Cowpea Roots
2.6. Evaluation of the Biocontrol Efficacy of HAB-2 Against Cowpea Fusarium Wilt
assessed plants × Maximum level) × 100%.
group)/Disease index in control group] × 100%.
2.7. Isolation and Identification of Rhizobacteria from HAB-2-Treated Soil
2.8. Evaluation of Biocontrol Efficacy of Microbial Combination Against Cowpea Fusarium Wilt
2.9. Analysis of Defense Gene Expression in Cowpea Leaves
2.10. Statistical Analysis
3. Results
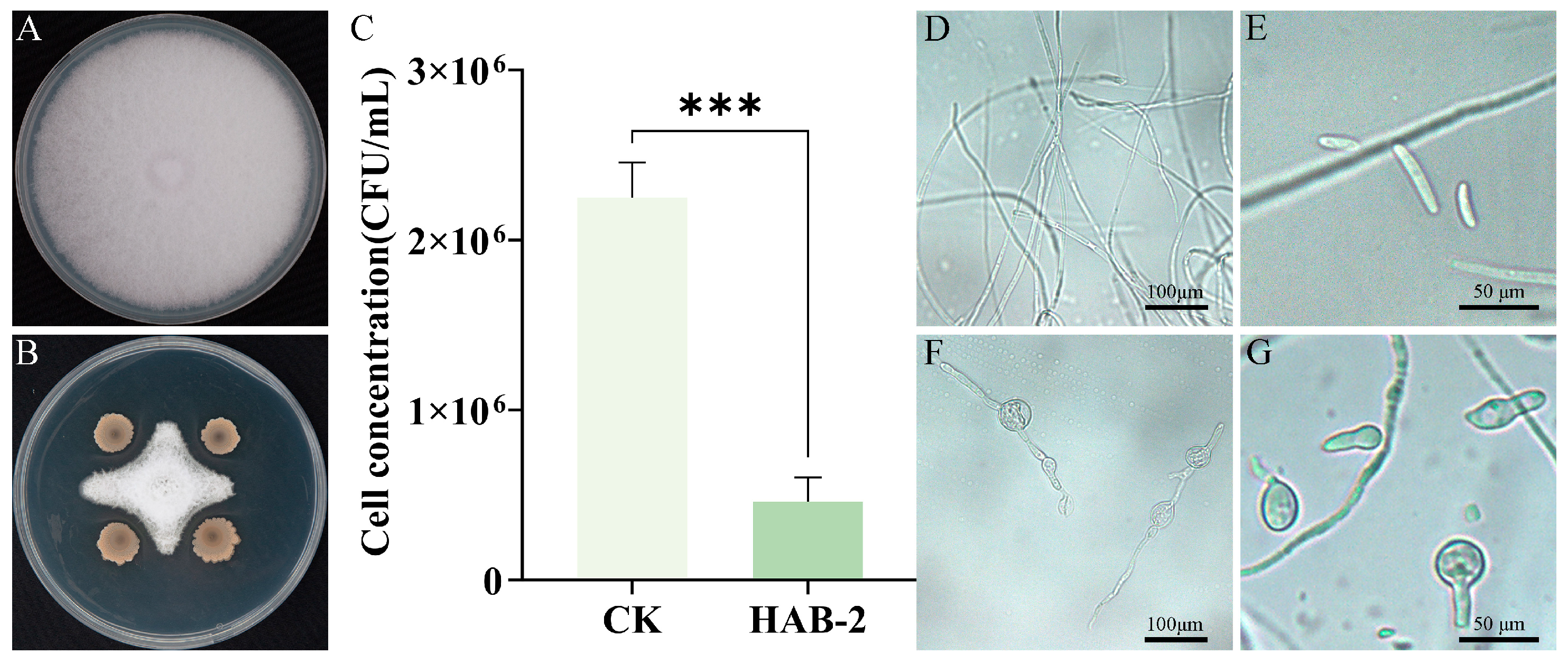
3.1. In Vitro Evaluation of Plant Growth-Promoting and Biocontrol Activities of B. velezensis HAB-2
3.2. Biocontrol Efficacy of B. velezensis HAB-2 Against Cowpea Fusarium Wilt
3.3. Isolation and Identification of Bacteria for Combination with B. velezensis HAB-2
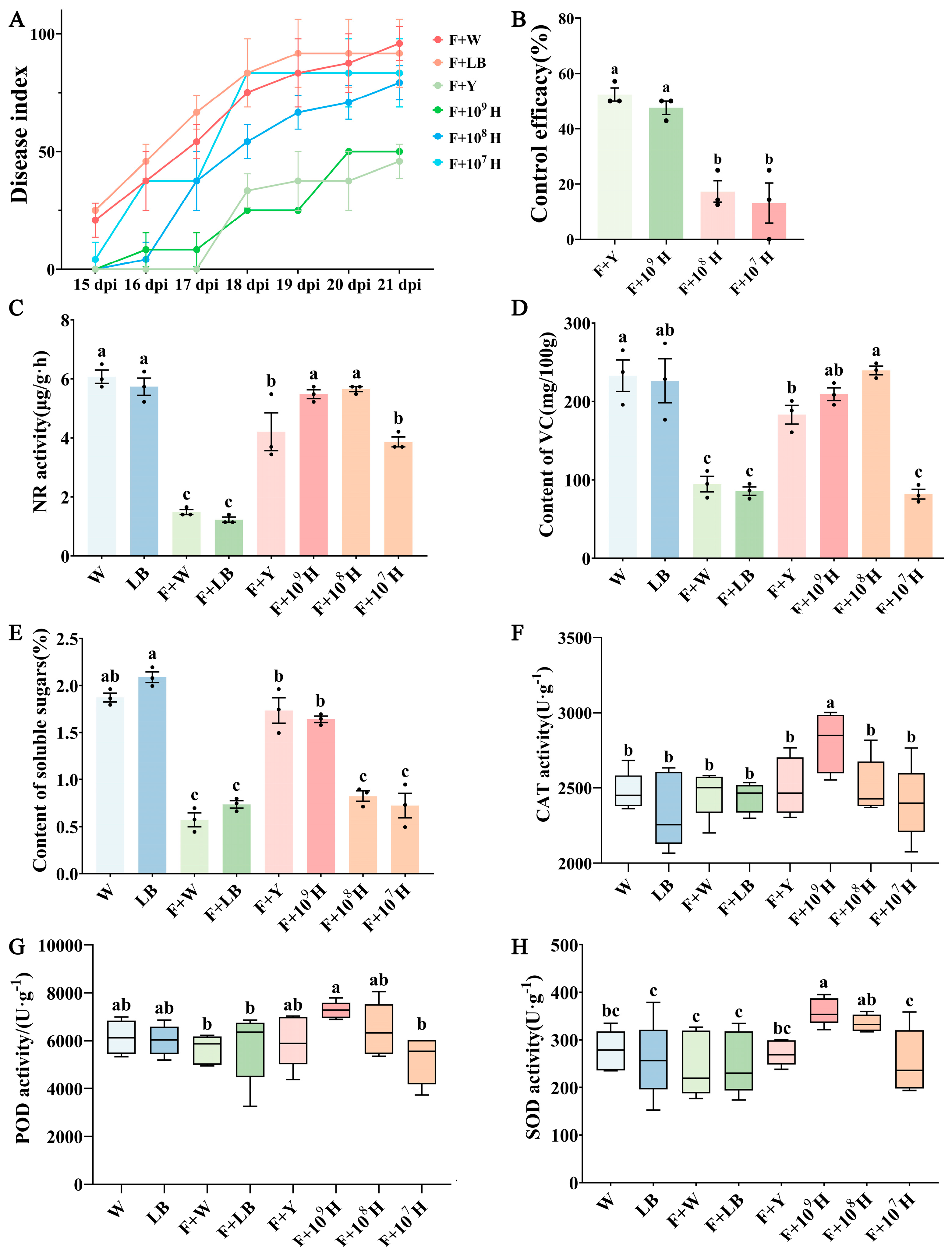
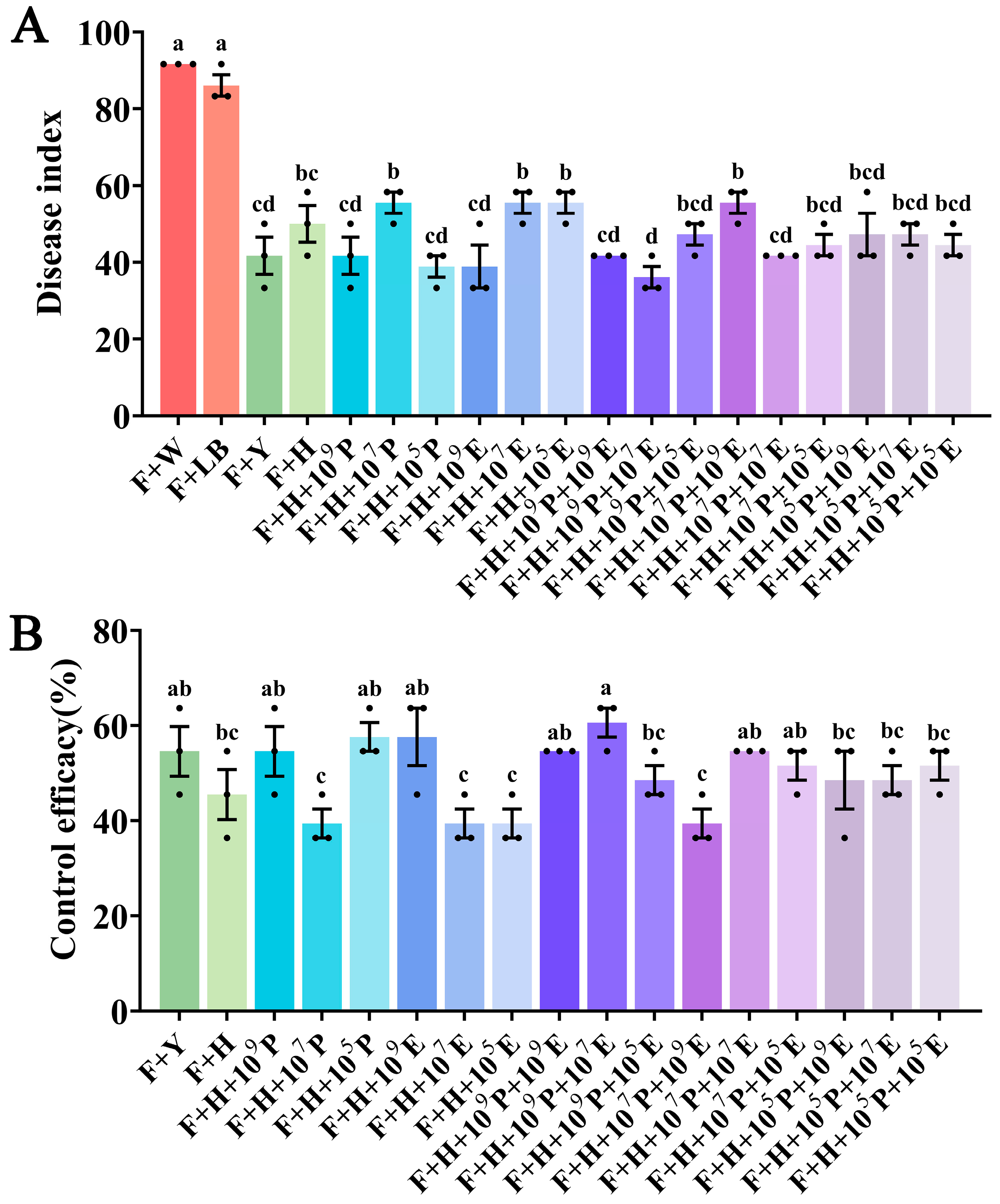
3.4. Control Efficacy of B. velezensis HAB-2 Combined with HD33 and HD42 Against Cowpea Fusarium Wilt
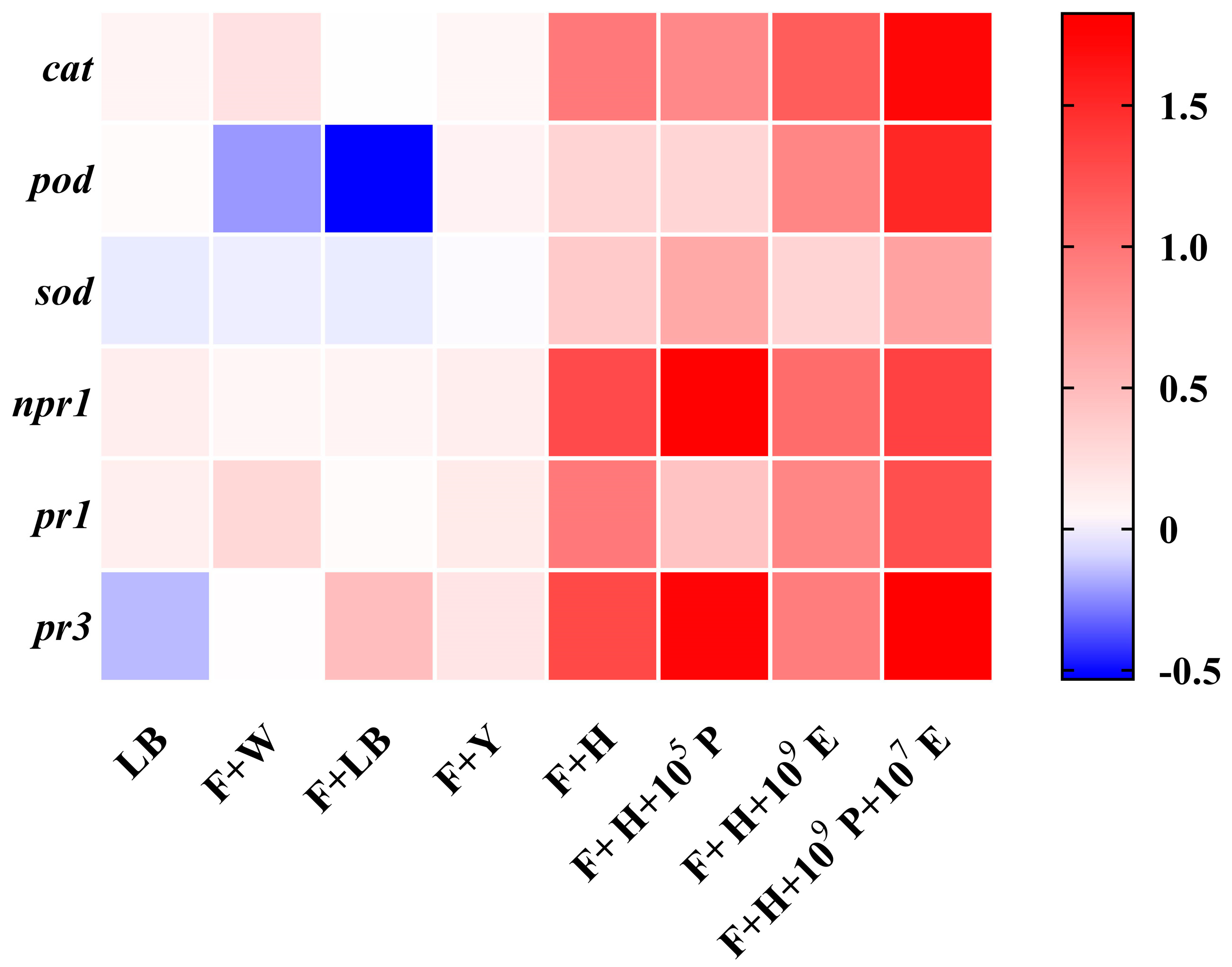
3.5. Analysis of Defense-Related Gene Expression in Cowpea Leaves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gomez, C. Cowpea Post-Harvest Operations in Developing Countries Cowpea Post-Harvest Operations in Developing Countries; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014. [Google Scholar]
- Boukar, O.; Fatokun, C.A.; Huynh, B.-L.; Roberts, P.A.; Close, T.J. Genomic Tools in Cowpea Breeding Programs: Status and Perspectives. Front. Plant Sci. 2016, 7, 757. [Google Scholar] [CrossRef]
- Phillips, R.D.; McWatters, K.H.; Chinnan, M.S.; Hung, Y.-C.; Beuchat, L.R.; Sefa-Dedeh, S.; Sakyi-Dawson, E.; Ngoddy, P.; Nnanyelugo, D.; Enwere, J.; et al. Utilization of cowpeas for human food. Field Crops Res. 2003, 82, 193–213. [Google Scholar] [CrossRef]
- Hao, Y.; Liu, R.; Mao, Z.; Yang, Q.; Zheng, S.; Lu, X.; Yang, Y.; Xie, B.; Zhao, J.; Li, Y.; et al. Identification and Analysis of WRKY Transcription Factors in Response to Cowpea Fusarium Wilt in Cowpea. Plants 2024, 13, 2273. [Google Scholar] [CrossRef]
- Pottorff, M.; Wanamaker, S.; Ma, Y.Q.; Ehlers, J.D.; Roberts, P.A.; Close, T.J. Genetic and Physical Mapping of Candidate Genes for Resistance to Fusarium oxysporum f.sp. tracheiphilum Race 3 in Cowpea [Vigna unguiculata (L.) Walp]. PLoS ONE 2012, 7, e41600. [Google Scholar] [CrossRef]
- Dong, J.; Song, Y.; Wang, B.; Wu, X.; Wang, Y.; Wang, J.; Lu, Z.; Zhang, Y.; Li, G.; Wu, X.; et al. Identification of Genomic Regions Associated with Fusarium Wilt Resistance in Cowpea. Appl. Sci. 2022, 12, 6889. [Google Scholar] [CrossRef]
- Nelson, P.E. Life Cycle and Epidemiology of Fusarium oxysporum. In Fungal Wilt Diseases of Plants; Mace, M.E., Bell, A.A., Beckman, C.H., Eds.; Academic Press: Cambridge, MA, USA, 1981; pp. 51–80. ISBN 978-0-12-464450-2. [Google Scholar]
- Pottorff, M.O.; Li, G.; Ehlers, J.D.; Close, T.J.; Roberts, P.A. Genetic mapping, synteny, and physical location of two loci for Fusarium oxysporum f. sp. tracheiphilum race 4 resistance in cowpea [Vigna unguiculata (L.) Walp]. Mol. Breed. 2014, 33, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.-J.; Huang, Y.-Y.; Lin, X.-Y.; Feng, H.; Zhou, S.-X.; Xie, Y.; Liu, X.-X.; Liu, C.; Zhao, R.-M.; Zhao, W.-S.; et al. Loss and Natural Variations of Blast Fungal Avirulence Genes Breakdown Rice Resistance Genes in the Sichuan Basin of China. Front. Plant Sci. 2022, 13, 788876. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); Carrasco Cabrera, L.; Di Piazza, G.; Dujardin, B.; Medina Pastor, P. The 2021 European Union report on pesticide residues in food. EFSA J. 2023, 21, e07939. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); Carrasco Cabrera, L.; Medina Pastor, P. The 2020 European Union report on pesticide residues in food. EFSA J. 2022, 20, e07215. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); Carrasco Cabrera, L.; Medina Pastor, P. The 2019 European Union report on pesticide residues in food. EFSA J. 2021, 19, e06491. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Rahman, M.M.; Liu, Y.; Naidu, R. Nanoencapsulation, Nano-guard for Pesticides: A New Window for Safe Application. J. Agric. Food Chem. 2016, 64, 1447–1483. [Google Scholar] [CrossRef]
- Kavlock, R.J.; Daston, G.P.; DeRosa, C.; Fenner-Crisp, P.; Gray, L.E.; Kaattari, S.; Lucier, G.; Luster, M.; Mac, M.J.; Maczka, C.; et al. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: A report of the U.S. EPA-sponsored workshop. Environ. Health Perspect. 1996, 104, 715–740. [Google Scholar] [CrossRef]
- De Silva, N.I.; Brooks, S.; Lumyong, S.; Hyde, K.D. Use of endophytes as biocontrol agents. Fungal Biol. Rev. 2019, 33, 133–148. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, J.; Suo, M.; Wu, H.; Zhao, M.; Yang, H. Biocontrol mechanisms of Bacillus velezensis against Fusarium oxysporum from Panax ginseng. Biol. Control 2023, 182, 105222. [Google Scholar] [CrossRef]
- Fan, B.; Wang, C.; Song, X.; Ding, X.; Wu, L.; Wu, H.; Gao, X.; Borriss, R. Bacillus velezensis FZB42 in 2018: The Gram-Positive Model Strain for Plant Growth Promotion and Biocontrol. Front. Microbiol. 2018, 9, 2491. [Google Scholar] [CrossRef] [PubMed]
- Zaid, D.S.; Cai, S.; Hu, C.; Li, Z.; Li, Y. Comparative Genome Analysis Reveals Phylogenetic Identity of Bacillus velezensis HNA3 and Genomic Insights into Its Plant Growth Promotion and Biocontrol Effects. Microbiol. Spectr. 2022, 10, e0216921. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Gao, J.; Chen, X.; Zhang, M.; Yang, F.; Du, Y.; Moe, T.S.; Munir, I.; Xue, J.; Zhang, X. The Endophytic Bacteria Bacillus velezensis Lle-9, Isolated from Lilium leucanthum, Harbors Antifungal Activity and Plant Growth-Promoting Effects. J. Microbiol. Biotechnol. 2020, 30, 668–680. [Google Scholar] [CrossRef]
- Keshmirshekan, A.; Mesquita, L.M.d.S.; Ventura, S.P.M. Biocontrol manufacturing and agricultural applications of Bacillus velezensis. Trends Biotechnol. 2024, 42, 986–1001. [Google Scholar] [CrossRef] [PubMed]
- Chebotar, V.K.; Chizhevskaya, E.P.; Vorobyov, N.; Bobkova, V.V.; Pomyaksheva, L.; Khomyakov, Y.; Konovalov, S.N. The Quality and Productivity of Strawberry (Fragaria × ananassa Duch.) Improved by the Inoculation of PGPR Bacillus velezensis BS89 in Field Experiments. Agronomy 2022, 12, 2600. [Google Scholar] [CrossRef]
- Ta, Y.; Fu, S.; Liu, H.; Zhang, C.; He, M.; Yu, H.; Ren, Y.; Han, Y.; Hu, W.; Yan, Z.; et al. Evaluation of Bacillus velezensis F9 for Cucumber Growth Promotion and Suppression of Fusarium wilt Disease. Microorganisms 2024, 12, 1882. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, J.; Tian, G.; Liang, C.; Zhou, Y.; Guo, L.; Yang, Y.; Yang, L. Bacillus velezensis Isolate X5 Stimulates the Resistance of Resistant and Susceptible Banana Varieties to Foc Through Different Mechanisms. J. Fungi 2025, 11, 379. [Google Scholar] [CrossRef]
- Guo, Q.; Li, S.; Dong, L.; Su, Z.; Wang, P.; Liu, X.; Ma, P. Screening Biocontrol Agents for Cash Crop Fusarium Wilt Based on Fusaric Acid Tolerance and Antagonistic Activity against Fusarium oxysporum. Toxins 2023, 15, 381. [Google Scholar] [CrossRef]
- Kenfaoui, J.; Dutilloy, E.; Benchlih, S.; Lahlali, R.; Ait-Barka, E.; Esmaeel, Q. Bacillus velezensis: A versatile ally in the battle against phytopathogens—Insights and prospects. Appl. Microbiol. Biotechnol. 2024, 108, 439. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xu, Z.; Xie, J.; Hesselberg-Thomsen, V.; Tan, T.; Zheng, D.; Strube, M.L.; Dragoš, A.; Shen, Q.; Zhang, R.; et al. Bacillus velezensis stimulates resident rhizosphere Pseudomonas stutzeri for plant health through metabolic interactions. ISME J. 2022, 16, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Raupach, G.S.; Kloepper, J.W. Mixtures of Plant Growth-Promoting Rhizobacteria Enhance Biological Control of Multiple Cucumber Pathogens. Phytopathology 1998, 88, 1158–1164. [Google Scholar] [CrossRef]
- Xiang, N.; Lawrence, K.S.; Kloepper, J.W.; Donald, P.A.; McInroy, J.A. Biological control of Heterodera glycines by spore-forming plant growth-promoting rhizobacteria (PGPR) on soybean. PLoS ONE 2017, 12, e0181201. [Google Scholar] [CrossRef]
- Jetiyanon, K.; Kloepper, J.W. Mixtures of plant growth-promoting rhizobacteria for induction of systemic resistance against multiple plant diseases. Biol. Control 2002, 24, 285–291. [Google Scholar] [CrossRef]
- Hu, J.; Wei, Z.; Friman, V.-P.; Gu, S.; Wang, X.; Eisenhauer, N.; Yang, T.; Ma, J.; Shen, Q.; Xu, Y.; et al. Probiotic Diversity Enhances Rhizosphere Microbiome Function and Plant Disease Suppression. mBio 2016, 7, e01790-16. [Google Scholar] [CrossRef]
- Du, C.; Yang, D.; Ye, Y.; Pan, L.; Zhang, J.; Jiang, S.; Fu, G. Construction of a compound microbial agent for biocontrol against fusarium wilt of banana. Front. Microbiol. 2022, 13, 1066807. [Google Scholar] [CrossRef] [PubMed]
- Dahal, B.; NandaKafle, G.; Perkins, L.; Brözel, V.S. Diversity of free-Living nitrogen fixing Streptomyces in soils of the badlands of South Dakota. Microbiol. Res. 2017, 195, 31–39. [Google Scholar] [CrossRef]
- Cui, W.; Zhang, J.; Wang, W.; Wu, X.; Luo, X.; Zou, Y.; Chen, K.; He, P. Screening native Bacillus strains as potential biological control agents against ginger bacterial wilt and for promoting plant growth. Biol. Control 2024, 192, 105510. [Google Scholar] [CrossRef]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Ji, J.; Hugouvieux-Cotte-Pattat, N.; Robert-Baudouy, J. Use of Mu-lac Insertions to Study the Secretion of Pectate Lyases by Erwinia chrysanthemi. Microbiology 1987, 133, 793–802. [Google Scholar] [CrossRef][Green Version]
- Liu, F.; Chen, S.; Chen, X.; Yong, B.; He, B. Identification of chitinase from Bacillus velezensis strain S161 and its antifungal activity against Penicillium digitatum. Protein Expr. Purif. 2024, 223, 106562. [Google Scholar] [CrossRef] [PubMed]
- Devi, N.O.; Tombisana Devi, R.K.; Debbarma, M.; Hajong, M.; Thokchom, S. Effect of endophytic Bacillus and arbuscular mycorrhiza fungi (AMF) against Fusarium wilt of tomato caused by Fusarium oxysporum f. sp. lycopersici. Egypt. J. Biol. Pest Control 2022, 32, 1. [Google Scholar] [CrossRef]
- Hasan, N.; Farzand, A.; Heng, Z.; Khan, I.U.; Moosa, A.; Zubair, M.; Na, Y.; Ying, S.; Canming, T. Antagonistic Potential of Novel Endophytic Bacillus Strains and Mediation of Plant Defense against Verticillium Wilt in Upland Cotton. Plants 2020, 9, 1438. [Google Scholar] [CrossRef]
- Tang, Y.; Xue, Q.; Yu, J.; Zhang, Z.; Wang, Z.; Wang, L.; Feng, H. Biocontrol and Growth-Promoting Potential of Antagonistic Strain YL84 Against Verticillium dahliae. Agronomy 2025, 15, 1997. [Google Scholar] [CrossRef]
- Soni, R.; Keharia, H.; Bose, A.; Pandit, N.; Doshi, J.; Rao, S.V.R.; Paul, S.S.; Raju, M.V.L.N. Genome assisted probiotic characterization and application of Bacillus velezensis ZBG17 as an alternative to antibiotic growth promoters in broiler chickens. Genomics 2021, 113, 4061–4074. [Google Scholar] [CrossRef]
- Yi, L.; Qi, T.; Li, X.; Zeng, K. Controlling soft rot of green pepper by bacteriocin paracin wx3 and its effect on storage quality of green pepper. Food Chem. 2024, 447, 138962. [Google Scholar] [CrossRef]
- Gong, A.G.W.; Duan, R.; Wang, H.Y.; Dong, T.T.X.; Tsim, K.W.K. Calycosin Orchestrates Osteogenesis of Danggui Buxue Tang in Cultured Osteoblasts: Evaluating the Mechanism of Action by Omics and Chemical Knock-out Methodologies. Front. Pharmacol. 2018, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Sasaki, Y.; Ida, S.; Morikawa, H. Nitrite Reductase Gene Enrichment Improves Assimilation of NO2 in Arabidopsis. Plant Physiol. 2001, 126, 731–741. [Google Scholar] [CrossRef]
- Bellaire, B.A.; Carmody, J.; Braud, J.; Gossett, D.R.; Banks, S.W.; Lucas, M.C.; Fowler, T.E. Involvement of abscisic acid-dependent and -independent pathways in the upregulation of antioxidant enzyme activity during NaCl stress in cotton callus tissue. Free Radic. Res. 2000, 33, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Rigert, K.S.; Foster, K.W. Inheritance of Resistance to Two Races of Fusarium Wilt in Three Cowpea Cultivars. Crop Sci. 1987, 27, 220–224. [Google Scholar] [CrossRef]
- Kumar, P.; Dubey, R.C.; Maheshwari, D.K. Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiol. Res. 2012, 167, 493–499. [Google Scholar] [CrossRef]
- Wakarera, P.W.; Ojola, P.; Njeru, E.M. Characterization and diversity of native Azotobacter spp. isolated from semi-arid agroecosystems of eastern Kenya. Biol. Lett. 2022, 18, 20210612. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Eljounaidi, K.; Lee, S.K.; Bae, H. Bacterial endophytes as potential biocontrol agents of vascular wilt diseases—Review and future prospects. Biol. Control 2016, 103, 62–68. [Google Scholar] [CrossRef]
- Su, T.; Shen, B.; Hu, X.; Teng, Y.; Weng, P.; Wu, Z.; Liu, L. Research advance of Bacillus velezensis: Bioinformatics, characteristics, and applications. Food Sci. Hum. Wellness 2024, 13, 1756–1766. [Google Scholar] [CrossRef]
- Kong, W.; Meldgin, D.R.; Collins, J.J.; Lu, T. Designing microbial consortia with defined social interactions. Nat. Chem. Biol. 2018, 14, 821–829. [Google Scholar] [CrossRef]
- Woo, S.L.; Pepe, O. Microbial consortia: Promising probiotics as plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1801. [Google Scholar] [CrossRef]
- Jin, P.; Wang, H.; Liu, W.; Fan, Y.; Miao, W. A new cyclic lipopeptide isolated from Bacillus amyloliquefaciens HAB-2 and safety evaluation. Pestic. Biochem. Physiol. 2018, 147, 40–45. [Google Scholar] [CrossRef]
- Xu, P.; Xie, S.; Liu, W.; Jin, P.; Wei, D.; Yaseen, D.G.; Wang, Y.; Miao, W. Comparative Genomics Analysis Provides New Strategies for Bacteriostatic Ability of Bacillus velezensis HAB-2. Front. Microbiol. 2020, 11, 594079. [Google Scholar] [CrossRef]
- Al-Ali, A.; Deravel, J.; Krier, F.; Béchet, M.; Ongena, M.; Jacques, P. Biofilm formation is determinant in tomato rhizosphere colonization by Bacillus velezensis FZB42. Environ. Sci. Pollut. Res. 2018, 25, 29910–29920. [Google Scholar] [CrossRef]
- Elbeltagy, A.; Nishioka, K.; Suzuki, H.; Sato, T.; Sato, Y.-I.; Morisaki, H.; Mitsui, H.; Minamisawa, K. Isolation and characterization of endophytic bacteria from wild and traditionally cultivated rice varieties. Soil. Sci. Plant Nutr. 2000, 46, 617–629. [Google Scholar] [CrossRef]
- Hurek, T.; Reinhold-Hurek, B.; Van Montagu, M.; Kellenberger, E. Root colonization and systemic spreading of Azoarcus sp. strain BH72 in grasses. J. Bacteriol. 1994, 176, 1913–1923. [Google Scholar] [CrossRef] [PubMed]
- James, E.K.; Gyaneshwar, P.; Mathan, N.; Barraquio, W.L.; Reddy, P.M.; Iannetta, P.P.M.; Olivares, F.L.; Ladha, J.K. Infection and Colonization of Rice Seedlings by the Plant Growth-Promoting Bacterium Herbaspirillum seropedicae Z67. Mol. Plant-Microbe Interact. 2002, 15, 894–906. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-J.; Tang, S.-Y.; Gao, H.; Ren, J.-Y.; Xu, P.-L.; Dong, W.-P.; Zheng, Y.; Yang, W.; Yu, Y.-Y.; Guo, J.-H.; et al. Plant growth-promoting rhizobacterium Bacillus cereus AR156 induced systemic resistance against multiple pathogens by priming of camalexin synthesis. Plant Cell Environ. 2024, 47, 337–353. [Google Scholar] [CrossRef]
- Debray, R.; Herbert, R.A.; Jaffe, A.L.; Crits-Christoph, A.; Power, M.E.; Koskella, B. Priority effects in microbiome assembly. Nat. Rev. Microbiol. 2022, 20, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G.; Urtis-Flores, C.A.; Loeza-Lara, P.D.; Orozco-Mosqueda, M.d.C.; Glick, B.R. Rhizosphere colonization determinants by plant growth-promoting rhizobacteria (PGPR). Biology 2021, 10, 475. [Google Scholar] [CrossRef]
- Liang, F.; Yunxia, C.; Yuanyuan, L.; Shaowu, G.; Yunhong, B.; Yang, H.; Kebiao, A.; Jianfei, S.; Jiyou, D.; Maosong, Y.; et al. The effect of mixed microbial agents on tobacco black shank disease. Physiol. Mol. Plant Pathol. 2024, 134, 102442. [Google Scholar] [CrossRef]
- Prigigallo, M.I.; Gómez-Lama Cabanás, C.; Mercado-Blanco, J.; Bubici, G. Designing a synthetic microbial community devoted to biological control: The case study of Fusarium wilt of banana. Front. Microbiol. 2022, 13, 967885. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, X.; Jiang, H.; Chang, X.; Chen, W.; Shi, G.; Tian, B.; Yao, Q. Formation of a Novel Antagonistic Bacterial Combination to Enhance Biocontrol for Cucumber Fusarium Wilt. Microorganisms 2025, 13, 133. [Google Scholar] [CrossRef]
- Liu, K.; Garrett, C.; Fadamiro, H.; Kloepper, J.W. Antagonism of black rot in cabbage by mixtures of plant growth-promoting rhizobacteria (PGPR). Biocontrol 2016, 61, 605–613. [Google Scholar] [CrossRef]
- Du, C.; Yang, D.; Jiang, S.; Zhang, J.; Ye, Y.; Pan, L.; Fu, G. Biocontrol agents inhibit banana fusarium wilt and alter the rooted soil bacterial community in the field. J. Fungi 2024, 10, 771. [Google Scholar] [CrossRef] [PubMed]
- AlAli, H.A.; Khalifa, A.; Almalki, M. Plant Growth-Promoting Rhizobacteria from Ocimum basilicum Improve Growth of Phaseolus vulgaris and Abelmoschus esculentu. S. Afr. J. Bot. 2021, 139, 200–209. [Google Scholar] [CrossRef]


| Gene Name | Accession | Forward Primer | Reverse Primer |
|---|---|---|---|
| cat | XM_028047190.1 | GTCTCAGGCTGACCGTTCTC | CTAACACACAAACGGCATCG |
| pod | XM_028078850.1 | TGTCTGGAGGTCCTTCGTG | AGGTTGAGTTCAGGGTTGG |
| sod | XM_028076317.1 | CAATGGTTGCCTGTCAACTG | AACAGCTCTCCCAATGATGG |
| npr1 | XM_028075607.1 | TTGGACTCCGATGATGTTGA | GCAGCAACATGAAGCACTGT |
| pr1 | QCD76639.1 | TGCTTTCGCACAAAACTACG | GTCTGCACTCTCCACCAACA |
| pr3 | BAA77691.1 | TGAGGCAAACTTGGGATACAG | CTTCGTTGGGTGGAGCA |
| EF1-α | XM_007151727.1 | GGTCATTGGTCATGTCGACTCTG | GCACCCAGGCATACTTGAATGAC |
| Strains | Nitrogen Fixation | Phosphate Solubilization (Inorganic, Organic Forms) | Solubilization of Potassium | Siderophores | IAA |
|---|---|---|---|---|---|
| HD31 | + | −,− | − | − | − |
| HD33 | + | −,− | − | + | − |
| HD36 | + | −,− | − | − | − |
| HD41 | + | −,− | − | − | + |
| HD42 | + | +,− | − | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, W.; Qi, T.; Lu, J.; Wei, X.; Wu, P.; Norvienyeku, J.; Miao, W.; Liu, W. Evaluation of Biocontrol Efficacy of Bacillus velezensis HAB-2 Combined with Pseudomonas hunanensis and Enterobacter soli Against Cowpea Fusarium Wilt. Microorganisms 2025, 13, 2578. https://doi.org/10.3390/microorganisms13112578
Wei W, Qi T, Lu J, Wei X, Wu P, Norvienyeku J, Miao W, Liu W. Evaluation of Biocontrol Efficacy of Bacillus velezensis HAB-2 Combined with Pseudomonas hunanensis and Enterobacter soli Against Cowpea Fusarium Wilt. Microorganisms. 2025; 13(11):2578. https://doi.org/10.3390/microorganisms13112578
Chicago/Turabian StyleWei, Wei, Tianlong Qi, Jinpeng Lu, Xi Wei, Peilin Wu, Justice Norvienyeku, Weiguo Miao, and Wenbo Liu. 2025. "Evaluation of Biocontrol Efficacy of Bacillus velezensis HAB-2 Combined with Pseudomonas hunanensis and Enterobacter soli Against Cowpea Fusarium Wilt" Microorganisms 13, no. 11: 2578. https://doi.org/10.3390/microorganisms13112578
APA StyleWei, W., Qi, T., Lu, J., Wei, X., Wu, P., Norvienyeku, J., Miao, W., & Liu, W. (2025). Evaluation of Biocontrol Efficacy of Bacillus velezensis HAB-2 Combined with Pseudomonas hunanensis and Enterobacter soli Against Cowpea Fusarium Wilt. Microorganisms, 13(11), 2578. https://doi.org/10.3390/microorganisms13112578






