Effects of Initiation Age of Starter Feeding on Growth Performance, Immunity and Antioxidant Capacity, Gastrointestinal Development, and Microbial Communities in Suckling Lambs
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Experimental Design and Feeding Management
2.3. Sample Collection
2.3.1. Nutrient Content of the Starter Diet
2.3.2. Serum Immunity and Antioxidant Capacity
2.3.3. Rumen Fermentation Parameters
2.3.4. Gastrointestinal Tissue Sectioning and Related Growth Genes
2.3.5. 16S rRNA Microbial Sequencing Analysis
2.4. Statistical Analysis
3. Results
3.1. Effects of Starter Feeding Initiation Age on Growth Performance of Suckling Lambs
3.2. Effects of Starter Feeding Initiation Age on Rumen Fermentation Parameters of Suckling Lambs
3.3. Effects of Starter Feeding Initiation Age on Serum Immunity and Antioxidant Capacity of Suckling Lambs
3.4. Effects of Starter Feeding Initiation Age on Gastrointestinal Tract Development of Suckling Lambs
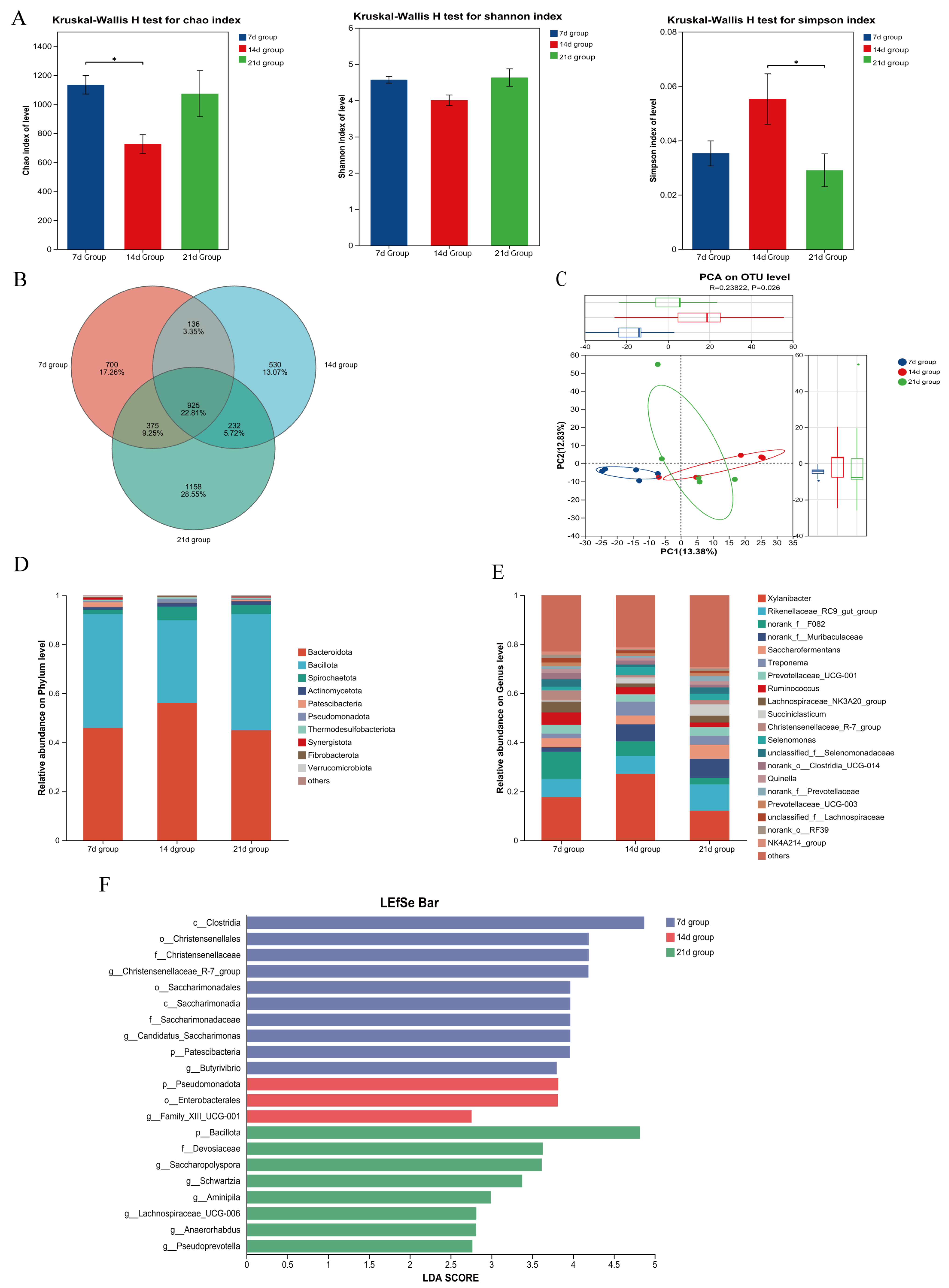
3.5. Effects of Starter Feeding Initiation Age on Rumen Microbial Communities of Suckling Lambs
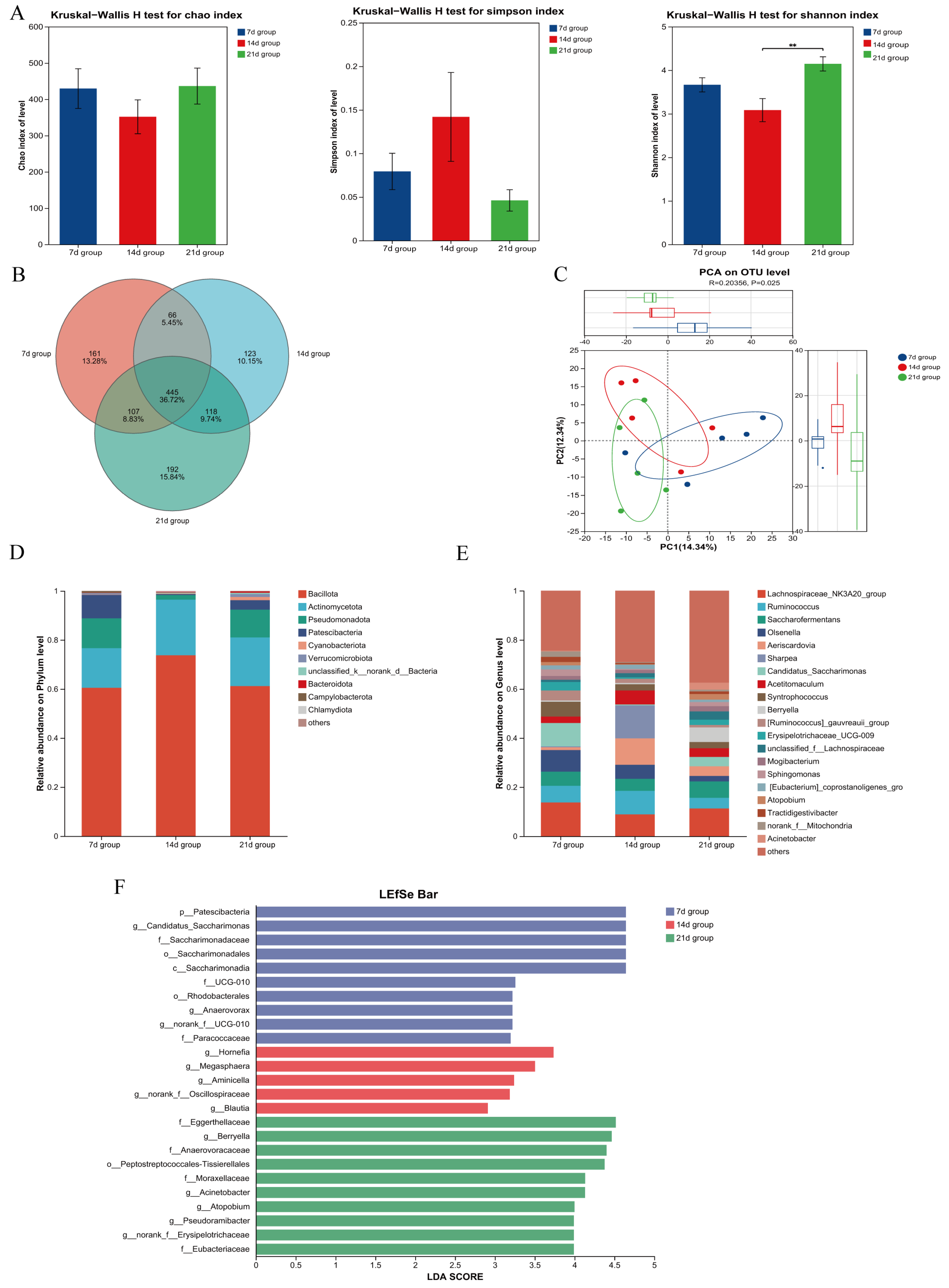
3.6. Effects of Starter Feeding Initiation Age on Small Intestinal Microbial Communities of Suckling Lambs
3.6.1. Effects on Duodenal Microbial Communities
3.6.2. Effects of Starter Feeding Initiation Age on Jejunal Microbial Communities of Suckling Lambs
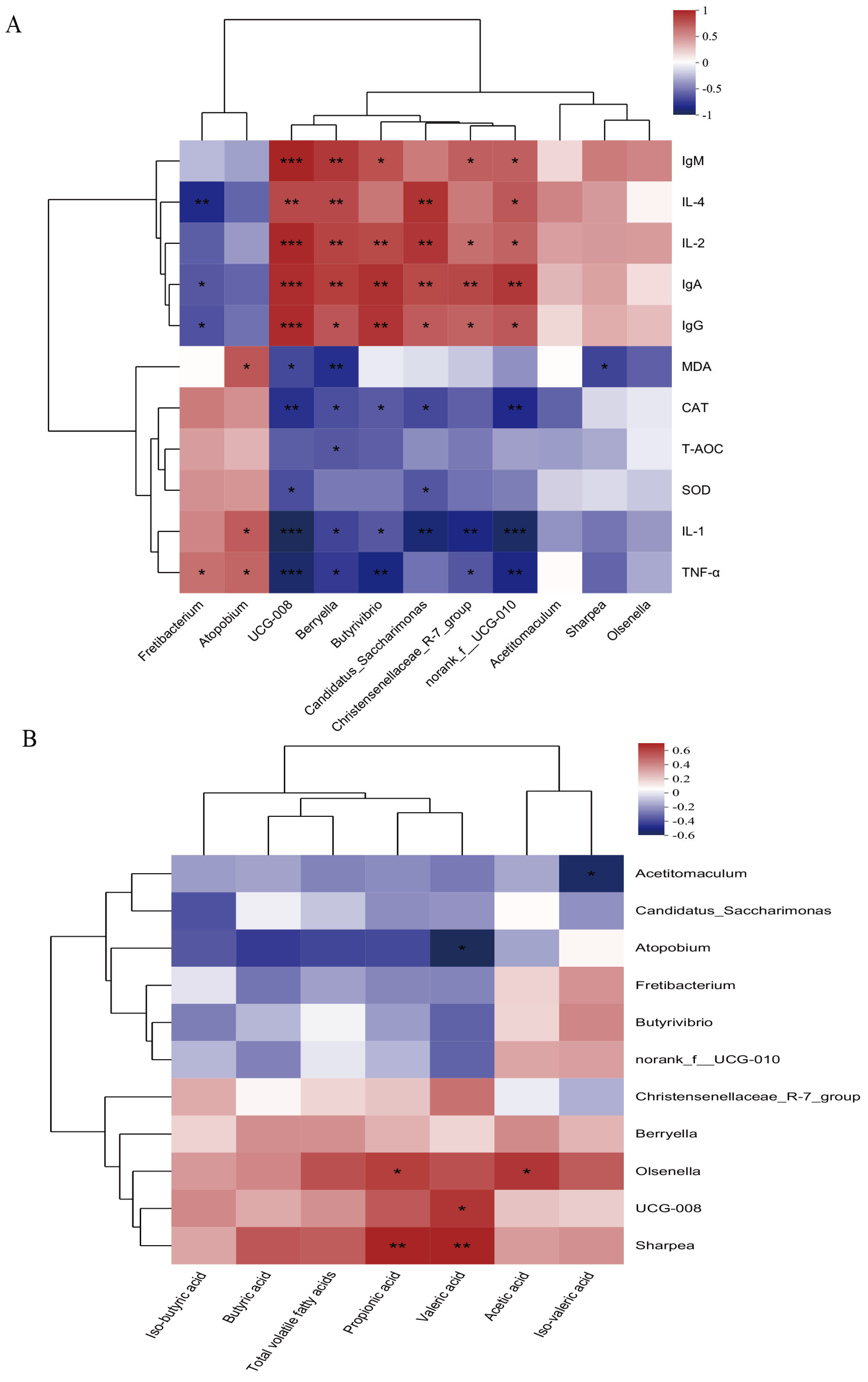
3.7. Correlation Analysis Between Rumen Microbiota and Rumen Fermentation Parameters/Immune Indicators
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, T.; Li, F.; Wang, W.; Wang, X.; Ma, Z.; Li, C.; Weng, X.; Zheng, C. Early Feeding Strategies in Lambs Affect Rumen Development and Growth Performance, with Advantages Persisting for Two Weeks after the Transition to Fattening Diets. Front. Vet. Sci. 2022, 9, 925649. [Google Scholar] [CrossRef] [PubMed]
- Sardinha, L.A.; Marques, R.S.; Miszura, A.A.; Barroso, J.P.R.; Oliveira, G.B.; Martins, A.S.; Limede, A.C.; Ferraz, M.V.C., Jr.; Ferreira, E.M.; Pires, A.V.; et al. Milk Yield and Composition from Ewes Fed Diets Containing Narasin and Their Lambs’ Performance. Transl. Anim. Sci. 2020, 4, txaa030. [Google Scholar] [CrossRef] [PubMed]
- Mazon, G.; Pereira, J.M.V.; Nishihara, K.; Steele, M.A.; Costa, J.H.C. Preweaning Megasphaera elsdenii Supplementation in Dairy-Beef Calves: Impact on Performance, Behavior, and Rumen Development. J. Dairy Sci. 2025, 108, 448–463. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, F.; Zhang, Z.; Zheng, C.; Shen, Z.; Ma, Z.; Wang, J.; Zhang, F.; Wang, J.; Ran, H.; et al. Early Supplementation with Starter Can Improve Production Performance of Lambs but This Growth Advantage Disappears after 154 Days of Age. Animals 2023, 13, 372. [Google Scholar] [CrossRef]
- Blanco, M.; Ripoll, G.; Albertí, P.; Sanz, A.; Revilla, R.; Villalba, D.; Casasús, I. Effect of Early Weaning on Performance, Carcass and Meat Quality of Spring-Born Bull Calves Raised in Dry Mountain Areas. Livest. Sci. 2008, 115, 226–234. [Google Scholar] [CrossRef]
- Yang, B.; He, B.; Wang, S.S.; Liu, J.X.; Wang, J.K. Early Supplementation of Starter Pellets with Alfalfa Improves the Performance of Pre- and Postweaning Hu Lambs. J. Anim. Sci. 2015, 93, 4984–4994. [Google Scholar] [CrossRef]
- Wang, W.; Li, C.; Li, F.; Wang, X.; Zhang, X.; Liu, T.; Nian, F.; Yue, X.; Li, F.; Pan, X.; et al. Effects of Early Feeding on the Host Rumen Transcriptome and Bacterial Diversity in Lambs. Sci. Rep. 2016, 6, 32479. [Google Scholar] [CrossRef]
- Wang, S.; Ma, T.; Zhao, G.; Zhang, N.; Tu, Y.; Li, F.; Cui, K.; Bi, Y.; Ding, H.; Diao, Q. Effect of Age and Weaning on Growth Performance, Rumen Fermentation, and Serum Parameters in Lambs Fed Starter with Limited Ewe–Lamb Interaction. Animals 2019, 9, 825. [Google Scholar] [CrossRef]
- Eghtedari, M.; Khezri, A.; Kazemi-Bonchenari, M.; Mohammadabadi, M.; Mahani, S.E.; Aschenbach, J.R. Phosphorus Has a Crucial Role in Growth Performance of Calves Fed Starters with Incorporated Forage. Anim. Nutr. 2025, 22, 88–97. [Google Scholar] [CrossRef]
- Moreira, V.R.; Zeringue, L.K.; Williams, C.C.; Leonardi, C.; McCormick, M.E. Influence of Calcium and Phosphorus Feeding on Markers of Bone Metabolism in Transition Cows. J. Dairy Sci. 2009, 92, 5189–5198. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.K.; Trivedi, S.; Kolte, A.P.; Mohapatra, A.; Biswas, S.; Bhattar, A.V.K.; Bhatta, R.; Rahman, H. Comparative Rumen Metagenome and CAZyme Profiles in Cattle and Buffaloes: Implications for Methane Yield and Rumen Fermentation on a Common Diet. Microorganisms 2023, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, J.; Zhang, P.; Pang, S.; Ma, M.; Nie, Y.; Xu, Z.; Li, S.; Li, Y.; Zhang, W. Rumen-Protected Leucine Improved Growth Performance of Fattening Sheep by Changing Rumen Fermentation Patterns. Microorganisms 2025, 13, 2377. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, J.; Hao, Z.; Yin, P.; Wang, S.; Guo, Y.; Ren, C. Multi-Omics Reveals Effects of Diet FNDF/Starch Level on Growth Performance and Rumen Development of Hu Sheep. Front. Microbiol. 2025, 16, 1601950. [Google Scholar] [CrossRef]
- Cordatos, K. Theory and Practice of Histological Techniques: Fifth Edition. Pathology 2002, 34, 384. [Google Scholar] [CrossRef]
- Hayat, M.A.; Ding, J.; Zhang, X.; Liu, T.; Zhang, J.; Bokhari, S.G.; Akbar, H.; Wang, H. Enhanced Autophagy in Damaged Laminar Tissue of Acute Laminitis Induced by Oligofructose Overloading in Dairy Cows. Animals 2023, 13, 2478. [Google Scholar] [CrossRef]
- Chen, S.; Liu, T.; Chen, J.; Shen, H.; Wang, J. Fecal Virome Transplantation Confirms Non-Bacterial Components (Virome and Metabolites) Participate in Fecal Microbiota Transplantation-Mediated Growth Performance Enhancement and Intestinal Development in Broilers with Spatial Heterogeneity. Microorganisms 2025, 13, 1795. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, C.; Wang, A.; Guo, Y.; Lee, D.-J. Denitrifying Sulfide Removal Process on High-Salinity Wastewaters. Appl. Microbiol. Biotechnol. 2015, 99, 6463–6469. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for Prediction of Metagenome Functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, G.; Chen, Z.; Jia, J.; Xu, H.; Xu, Y.; Liu, Z.; Liu, L.; Li, B.; Li, C. Impact of Crumbled Pelleted Starter Feed and Alfalfa Inclusion on Feed Intake, Growth, and Rumen Microbiota in Young Lambs. Anim. Biosci. 2025, 38, 2665–2678. [Google Scholar] [CrossRef]
- Baldwin, R.L.; McLeod, K.R.; Klotz, J.L.; Heitmann, R.N. Rumen Development, Intestinal Growth and Hepatic Metabolism In The Pre- and Postweaning Ruminant. J. Dairy Sci. 2004, 87, E55–E65. [Google Scholar] [CrossRef]
- Hill, T.M.; Bateman, H.G.; Aldrich, J.M.; Schlotterbeck, R.L. Effects of the Amount of Chopped Hay or Cottonseed Hulls in a Textured Calf Starter on Young Calf Performance. J. Dairy Sci. 2008, 91, 2684–2693. [Google Scholar] [CrossRef] [PubMed]
- Schwarzkopf, S.; Kinoshita, A.; Kluess, J.; Kersten, S.; Meyer, U.; Huber, K.; Dänicke, S.; Frahm, J. Weaning Holstein Calves at 17 Weeks of Age Enables Smooth Transition from Liquid to Solid Feed. Animals 2019, 9, 1132, Correction in Animals 2021, 11, 3044. https://doi.org/10.3390/ani11113044. [Google Scholar] [CrossRef] [PubMed]
- Pokhrel, B.; Jiang, H. Postnatal Growth and Development of the Rumen: Integrating Physiological and Molecular Insights. Biology 2024, 13, 269. [Google Scholar] [CrossRef]
- Boschiero, C.; Gao, Y.; Liu, M.; Baldwin, R.L.; Ma, L.; Li, C.-J.; Liu, G.E. The Dynamics of Chromatin Accessibility Prompted by Butyrate-Induced Chromatin Modification in Bovine Cells. Ruminants 2022, 2, 226–243. [Google Scholar] [CrossRef]
- Wang, D.; Chen, L.; Tang, G.; Yu, J.; Chen, J.; Li, Z.; Cao, Y.; Lei, X.; Deng, L.; Wu, S.; et al. Multi-Omics Revealed the Long-Term Effect of Ruminal Keystone Bacteria and the Microbial Metabolome on Lactation Performance in Adult Dairy Goats. Microbiome 2023, 11, 215. [Google Scholar] [CrossRef]
- Grummer, R.R.; Clark, J.H.; Davis, C.L.; Murphy, M.R. Effect of Ruminal Ammonia-Nitrogen Concentration on Protein Degradation in Situ1. J. Dairy Sci. 1984, 67, 2294–2301. [Google Scholar] [CrossRef]
- Lu, M.-L.; Yuan, G.-H.; Li, C.-C.; Hu, L.-H.; Feng, X.-W.; Jiang, H.; Liu, L.-L.; Rehemujiang, H.; Xu, G.-S. Effects of Spent Substrate of Oyster Mushroom (Pleurotus ostreatus) on Feed Utilization and Liver Serum Indices of Hu Sheep from the Perspective of Duodenal Microorganisms. Animals 2024, 14, 3416. [Google Scholar] [CrossRef]
- Dijkstra, J.; Boer, H.; Bruchem, J.V.; Bruining, M.; Tamminga, S. Absorption of Volatile Fatty Acids from the Rumen of Lactating Dairy Cows as Influenced by Volatile Fatty Acid Concentration, pH and Rumen Liquid Volume. Br. J. Nutr. 1993, 69, 385–396. [Google Scholar] [CrossRef]
- da Rocha, L.T.; Del Valle, T.A.; Skonieski, F.R.; Pereira, S.N.; de Oliveira, P.S.; Facco, F.B.; Viégas, J. Exploratory Meta-Analysis of the Effect of Malic Acid or Malate Addition on Ruminal Parameters, Nutrient Digestibility, and Blood Characteristics of Cattle. Animals 2025, 15, 2177. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Meng, Z.; Tan, D.; Datsomor, O.; Zhan, K.; Lin, M.; Zhao, G. Effects of Supplementation of Sodium Acetate on Rumen Fermentation and Microbiota in Postpartum Dairy Cows. Front. Microbiol. 2022, 13, 1053503. [Google Scholar] [CrossRef] [PubMed]
- Urrutia, N.; Bomberger, R.; Matamoros, C.; Harvatine, K.J. Effect of Dietary Supplementation of Sodium Acetate and Calcium Butyrate on Milk Fat Synthesis in Lactating Dairy Cows. J. Dairy Sci. 2019, 102, 5172–5181. [Google Scholar] [CrossRef]
- Xiao, J.; Alugongo, G.M.; Li, J.; Wang, Y.; Li, S.; Cao, Z. Review: How Forage Feeding Early in Life Influences the Growth Rate, Ruminal Environment, and the Establishment of Feeding Behavior in Pre-Weaned Calves. Animals 2020, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, H.; Yue, Z.; Tan, P.; Sun, M.; Ji, L.; Bai, Y.; Ma, X. Wickerhamomyces anomalus Relieves Weaning Diarrhea via Improving Gut Microbiota and Redox Homeostasis Using a Piglet Model. Food Funct. 2022, 13, 11223–11235. [Google Scholar] [CrossRef]
- Wei, K.; Yang, X.; Zhao, H.; Chen, H.; Bei, W. Effects of Combined Application of Benzoic Acid and 1-Monolaurin on Growth Performance, Nutrient Digestibility, Gut Microbiome and Inflammatory Factor Levels in Weaned Piglets. Porc. Health Manag. 2023, 9, 46. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Boivin, M.; Ma, T. Mechanism of Cytokine Modulation of Epithelial Tight Junction Barrier. Front. Biosci. 2009, 14, 2765–2778. [Google Scholar] [CrossRef]
- Luzina, I.G.; Keegan, A.D.; Heller, N.M.; Rook, G.A.W.; Shea-Donohue, T.; Atamas, S.P. Regulation of Inflammation by Interleukin-4: A Review of “Alternatives”. J. Leukoc. Biol. 2012, 92, 753–764. [Google Scholar] [CrossRef]
- Guo, B.; Rothstein, T.L. IL-4 Upregulates Igα and Igβ Protein, Resulting in Augmented IgM Maturation and BCR-Triggered B Cell Activation. J. Immunol. 2013, 191, 670–677. [Google Scholar] [CrossRef]
- Du, S.; Bu, Z.; You, S.; Jiang, Z.; Su, W.; Wang, T.; Jia, Y. Integrated Rumen Microbiome and Serum Metabolome Analysis Responses to Feed Type That Contribution to Meat Quality in Lambs. Anim. microbiome 2023, 5, 65. [Google Scholar] [CrossRef]
- Gebeyew, K.; Mi, H.; Liu, Y.; Liu, Y.; Wang, B.; Feyera, T.; Zhiliang, T.; He, Z. Differential Immunological Responses in Lamb Rumen and Colon to Alfalfa Hay and Wheat Straw in a Concentrate-Rich Diet: Insights into Microbe-Host Interactions. mSystems 2024, 9, e0048324. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chai, J.; Zhao, G.; Zhang, N.; Cui, K.; Bi, Y.; Ma, T.; Tu, Y.; Diao, Q. The Temporal Dynamics of Rumen Microbiota in Early Weaned Lambs. Microorganisms 2022, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Rabee, A.E.; Younan, B.R.; Kewan, K.Z.; Sabra, E.A.; Lamara, M. Modulation of Rumen Bacterial Community and Feed Utilization in Camel and Sheep Using Combined Supplementation of Live Yeast and Microalgae. Sci. Rep. 2022, 12, 12990. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qu, M.; Li, L.; Mei, W.; Zhang, F.; Hu, Z.; Li, G.; Xu, L.; Liang, H. Effects of Glycyrrhetinic Acid on Production Performance, Serum Biochemical Indexes, Ruminal Parameters, and Rumen Microflora of Beef Cattle. Front. Vet. Sci. 2025, 12, 1529383. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Z.; Zhou, Z.; Ma, Y.; Luo, D.; Zhang, S.; Yang, P.; An, T.; Sun, Q. Effect of Lactiplantibacillus Plantarum N-1 and Isomaltose-Oligosaccharide on Promoting Growth Performance and Modulating the Gastrointestinal Microbiota in Newborn Hu Sheep. Anim. Microbiome 2025, 7, 25. [Google Scholar] [CrossRef]
- Liu, H.; Chen, A.; Wang, W.; Peng, W.; Mao, K.; Yang, Y.; Wu, Q.; Zeng, M.; Wang, K.; Han, J.; et al. Analysis of Fecal Microbiome and Metabolome Changes in Goats When Consuming a Lower-Protein Diet with Varying Energy Levels. Microorganisms 2025, 13, 941. [Google Scholar] [CrossRef]
- Xie, Y.; Sun, J.; Hu, C.; Ruan, B.; Zhu, B. Oral Microbiota Is Associated With Immune Recovery in Human Immunodeficiency Virus-Infected Individuals. Front. Microbiol. 2021, 12, 794746. [Google Scholar] [CrossRef]
- Guo, Y.; Yin, G.; Hui, F.; Guo, X.; Shi, B.; Zhao, Y.; Yan, S. Effects of Dietary Energy Level on Antioxidant Capability, Immune Function and Rectal Microbiota in Late Gestation Donkeys. Front. Microbiol. 2024, 15, 1308171. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, W.; Cheng, Y.; Wang, X.; Zhao, Y.; Zhou, Q.; Hou, G.; Chen, T.; You, J.; Dong, W.; et al. Supplementing Sialyllactose to Colostrum Replacer Improved Intestinal Health and Blood Immunity by Affecting the Abundance of Intestinal Bacteria in Dairy Calves. J. Dairy Sci. 2025, 108, 7370–7386. [Google Scholar] [CrossRef]
- Luo, T.; Li, Y.; Zhang, W.; Liu, J.; Shi, H. Rumen and Fecal Microbiota Profiles Associated with Immunity of Young and Adult Goats. Front. Immunol. 2022, 13, 978402. [Google Scholar] [CrossRef]
- Shi, Z.; Lan, Y.; Qiao, Z.; Yan, X.; Wang, Y.; Zhang, B.; Ma, X.; Hassan, F.; Wang, W.; Deng, T. Changes in Fecal Microbiota of Dairy Cows with and without Endometritis. BMC Vet. Res. 2025, 21, 201. [Google Scholar] [CrossRef] [PubMed]
- Kolathingal-Thodika, N.; Elayadeth-Meethal, M.; Dunshea, F.R.; Eckard, R.; Flavel, M.; Chauhan, S.S. Is Early Life Programming a Promising Strategy for Methane Mitigation and Sustainable Intensification in Ruminants? Sci. Total Environ. 2025, 982, 179654. [Google Scholar] [CrossRef] [PubMed]
- Nehra, C.; Harshini, V.; Shukla, N.; Chavda, P.; Savaliya, K.; Patil, S.; Shah, T.; Pandit, R.; Patil, N.V.; Patel, A.K.; et al. Moringa Leaf Meal Exerts Growth Benefits in Small Ruminants through Modulating the Gastrointestinal Microbiome. Appl. Microbiol. Biotechnol. 2024, 108, 438. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wu, P.; Cai, Z.; Fang, Y.; Zhou, H.; Lasanajak, Y.; Tang, L.; Ye, L.; Hou, C.; Zhao, J. Puerariae Lobatae Radix with Chuanxiong Rhizoma for Treatment of Cerebral Ischemic Stroke by Remodeling Gut Microbiota to Regulate the Brain-Gut Barriers. J. Nutr. Biochem. 2019, 65, 101–114. [Google Scholar] [CrossRef]
- Yin, X.; Ji, S.; Duan, C.; Tian, P.; Ju, S.; Yan, H.; Zhang, Y.; Liu, Y. The Succession of Fecal Bacterial Community and Its Correlation with the Changes of Serum Immune Indicators in Lambs from Birth to 4 Months. J. Integr. Agric. 2023, 22, 537–550. [Google Scholar] [CrossRef]
- Qin, S.M.; Bai, W.Q.; Zhang, K.Y.; Ding, X.M.; Bai, S.P.; Wang, J.P.; Peng, H.W.; Yang, Y.F.; Chen, C.; Zeng, Q.F. Different Microbiomes Are Found in Healthy Breeder Ducks and Those with Foot Pad Dermatitis. Poult. Sci. 2019, 98, 6340–6348. [Google Scholar] [CrossRef]
- Wu, J.; Yu, G.; Zhang, X.; Staiger, M.P.; Gupta, T.B.; Yao, H.; Wu, X. A Fructan-Type Garlic Polysaccharide Upregulates Immune Responses in Macrophage Cells and in Immunosuppressive Mice. Carbohydr. Polym. 2024, 344, 122530. [Google Scholar] [CrossRef]
- Wylensek, D.; Hitch, T.C.A.; Riedel, T.; Afrizal, A.; Kumar, N.; Wortmann, E.; Liu, T.; Devendran, S.; Lesker, T.R.; Hernández, S.B.; et al. A Collection of Bacterial Isolates from the Pig Intestine Reveals Functional and Taxonomic Diversity. Nat. Commun. 2020, 11, 6389. [Google Scholar] [CrossRef]

| Feed Formula | Content | Nutrient Level | Content |
|---|---|---|---|
| Corn | 34.3% | Crude Protein | 19% |
| Soybean meal | 25% | Acid Detergent Fiber | 14.8% |
| Wheat bran | 5% | Neutral Detergent | 22.1% |
| Limestone | 2% | Calcium | 1.1% |
| Corn straw | 15% | Phosphorus | 0.5% |
| Cottonseed meal | 4% | Starch | 30% |
| Corn germ meal | 12.5% | Digestible Energy | 2980 kJ/kg |
| Salt | 0.5% | ||
| Soda ash | 0.2% | ||
| Premix 1 | 1% | ||
| Total | 100% |
| Gene | Primer Sequences (5′ to 3′) | Accession No. |
|---|---|---|
| TGFβ1 | F:TGACCCACAGAGAGGAAATAGA R:AACCCGTTGATGTCCACTTGAA | NM_001009400.2 |
| IGFBP3 | F:TCAGCCTTGCGGCGTCTA R:TGTGGGCGAGGTGGGATT | NM_001159276.1 |
| IGFBP5 | F:GCTGAAGGCTGAGGCTGTGAA R:TCCCATACTTGTCCACGCACC | NM_001129733.1 |
| IGFBP6 | F:AGAGTAAGCCCCAAGCAG R:CACGGAGTCCAGATGTTT | NM_001134308.1 |
| IGF-1 | F:AAGATGCCAGTCACATCCTCC R:ATAAAAGCCCCTGTCTCCAC | NM_001009774.3 |
| IGF-1R | F:AGCAAAGGCGACATAAACAC R:GGCTTCCTTGTAGTAGACGGT | XM_027957015.2 |
| β-actin | F:GCTCTTCCAGCCGTCCTT R:TGAAGGTGGTCTCGTGAATGC | NM_205518.2 |
| Days of Age | Treatment 1 | SEM 3 | p-Value | ||||
|---|---|---|---|---|---|---|---|
| 7 d Group | 14 d Group | 21 d Group | Treatment | Time | T × T 2 | ||
| BW 3, kg | |||||||
| 7 | 4.92 | 5.51 | 5.32 | 0.72 | 0.14 | 0.98 | 0.991 |
| 14 | 5.13 | 7.25 | 7.27 | 1.02 | 0.07 | <0.01 | <0.01 |
| 21 | 6.43 | 8.77 | 9.05 | 0.35 | 0.06 | <0.01 | <0.01 |
| 28 | 8.29 | 10.58 | 10.68 | 0.96 | 0.06 | <0.01 | <0.01 |
| 35 | 10.69 | 12.79 | 12.32 | 1.73 | 0.08 | <0.01 | <0.01 |
| 42 | 12.07 | 14.78 | 13.64 | 1.63 | 0.16 | <0.01 | 0.01 |
| 49 | 12.78 b | 16.48 a | 15.12 a | 2.03 | 0.02 | <0.01 | 0.02 |
| 60 | 14.72 b | 18.75 a | 16.70 a | 1.95 | 0.01 | <0.01 | 0.01 |
| ADG 3, kg/d | |||||||
| 7–14 | 0.07 | 0.08 | 0.28 | 0.02 | 0.35 | 0.61 | 0.59 |
| 14–21 | 0.19 | 0.27 | 0.25 | 0.03 | 0.52 | 0.73 | 0.71 |
| 21–28 | 0.27 | 0.26 | 0.23 | 0.02 | 0.49 | 0.68 | 0.66 |
| 28–35 | 0.34 a | 0.32 a | 0.23 b | 0.03 | 0.02 | <0.01 | 0.02 |
| 35–42 | 0.20 | 0.28 | 0.19 | 0.02 | 0.06 | <0.01 | 0.03 |
| 42–49 | 0.25 | 0.24 | 0.21 | 0.04 | 0.68 | 0.82 | 0.79 |
| 49–60 | 0.28 | 0.32 | 0.23 | 0.05 | 0.13 | 0.22 | 0.21 |
| Item | Treatment 1 | SEM 3 | p-Value | ||
|---|---|---|---|---|---|
| 7 d Group | 14 d Group | 21 d Group | |||
| pH | 6.59 | 6.71 | 6.68 | 0.08 | 0.68 |
| NH3-N 2, mg/dL | 7.20 a | 9.09 a | 5.34 b | 0.56 | 0.01 |
| Acetic acid, mmol/L | 267.21 a | 216.21 a | 128.03 b | 29.35 | 0.01 |
| Propionic acid, mmol/L | 13.63 a | 15.89 a | 8.22 b | 1.97 | 0.02 |
| Isobutyric acid, mmol/L | 2.42 | 2.71 | 1.98 | 0.16 | 0.22 |
| Butyric acid, mmol/L | 11.78 | 10.53 | 10.41 | 1.47 | 0.35 |
| Isovaleric acid, mmol/L | 5.71 | 6.58 | 4.12 | 0.71 | 0.41 |
| Valeric acid, mmol/L | 4.18 ab | 6.85 a | 3.71 b | 0.69 | 0.01 |
| TVFA 2, mmol/L | 314.93 a | 258.77 a | 156.47 b | 9.42 | 0.01 |
| Item | Treatment 1 | SEM 5 | p-Value | ||
|---|---|---|---|---|---|
| 7 d Group | 14 d Group | 21 d Group | |||
| CAT 2, μmol/mL | 50.62 | 47.58 | 55.37 | 5.88 | 0.26 |
| T-AOC 2, μmol/mL | 0.15 | 0.11 | 0.11 | 0.01 | 0.26 |
| SOD 2, U/mL | 6.11 | 3.70 | 5.16 | 0.66 | 0.37 |
| MDA 2, nmol/mL | 0.17 a | 0.11 b | 0.12 b | 0.01 | 0.02 |
| IL-1β 3, pg/mL | 169.94 a | 93.41 b | 140.91 ab | 6.64 | <0.01 |
| IL-2 3, pg/mL | 649.02 b | 1241.29 a | 1017.77 ab | 49.77 | <0.01 |
| IL-4 3, pg/mL | 77.65 b | 128.03 a | 105.47 ab | 4.55 | <0.01 |
| TNF-α 3, pg/mL | 67.63 a | 47.92 b | 68.99 a | 2.27 | <0.01 |
| IgA 4, μg/mL | 0.63 b | 1.02 a | 0.76 ab | 0.04 | <0.01 |
| IgG 4, μg/mL | 1.06 b | 2.21 a | 1.52 ab | 0.10 | <0.01 |
| IgM 4, μg/mL | 1.43 b | 2.29 a | 1.80 ab | 0.08 | <0.01 |
| Site | Item | Treatment 1 | SEM 3 | p-Value | ||
|---|---|---|---|---|---|---|
| 7 d Group | 14 d Group | 21 d Group | ||||
| Rumen | TGFβ1 2 | 1.01 b | 3.11 a | 2.78 ab | 0.21 | <0.05 |
| IGFBP3 2 | 1.01 | 1.67 | 1.62 | 0.43 | 0.20 | |
| IGFBP5 2 | 1.05 | 1.21 | 0.91 | 0.09 | 0.48 | |
| IGFBP6 2 | 1.00 | 1.54 | 1.46 | 0.15 | 0.35 | |
| Duodenum | IGF-1 2 | 1.00 | 1.54 | 1.32 | 0.36 | 0.12 |
| IGF-1R 2 | 1.01 | 1.38 | 1.18 | 0.10 | 0.09 | |
| Site | Item | Treatment 1 | SEM 2 | p-Value | ||
|---|---|---|---|---|---|---|
| 7 d Group | 14 d Group | 21 d Group | ||||
| Rumen | Papilla length, μm | 1750.61 | 1573.16 | 1687.21 | 96.78 | 0.43 |
| Papilla width, μm | 697.33 a | 714.15 a | 598.33 b | 23.23 | 0.03 | |
| Muscle thickness, μm | 1108.33 | 1005.16 | 932.78 | 55.37 | 0.29 | |
| Duodenum | Villus height, μm | 832.15 | 833.18 | 731.88 | 69.34 | 0.80 |
| Villus width, μm | 163.22 | 150.31 | 147.19 | 28.31 | 0.15 | |
| Crypt depth, μm | 492.36 | 501.33 | 430.79 | 51.33 | 0.67 | |
| Muscularis thickness, μm | 337.11 | 381.72 | 360.72 | 28.14 | 0.24 | |
| Villus height/Crypt depth ratio (V/C) | 1.69 | 1.66 | 1.70 | 0.03 | 0.87 | |
| Jejunum | Villus height, μm | 761.33 a | 612.56 b | 599.36 b | 47.13 | <0.01 |
| Villus width, μm | 143.51 a | 121.46 b | 117.75 b | 12.08 | <0.01 | |
| Crypt depth, μm | 769.30 a | 551.65 b | 491.89 b | 37.97 | <0.01 | |
| Muscularis thickness, μm | 201.49 | 198.17 | 203.33 | 21.28 | 0.35 | |
| Villus height/Crypt depth ratio (V/C) | 0.99 | 1.11 | 1.22 | 0.01 | 0.65 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, S.; Peng, X.; Li, S.; Niu, J.; Wang, W.; Liu, Y.; Nie, C.; Zhang, W. Effects of Initiation Age of Starter Feeding on Growth Performance, Immunity and Antioxidant Capacity, Gastrointestinal Development, and Microbial Communities in Suckling Lambs. Microorganisms 2025, 13, 2490. https://doi.org/10.3390/microorganisms13112490
Pang S, Peng X, Li S, Niu J, Wang W, Liu Y, Nie C, Zhang W. Effects of Initiation Age of Starter Feeding on Growth Performance, Immunity and Antioxidant Capacity, Gastrointestinal Development, and Microbial Communities in Suckling Lambs. Microorganisms. 2025; 13(11):2490. https://doi.org/10.3390/microorganisms13112490
Chicago/Turabian StylePang, Shaoyang, Xiangjian Peng, Shu Li, Junli Niu, Wenqi Wang, Yanfeng Liu, Cunxi Nie, and Wenju Zhang. 2025. "Effects of Initiation Age of Starter Feeding on Growth Performance, Immunity and Antioxidant Capacity, Gastrointestinal Development, and Microbial Communities in Suckling Lambs" Microorganisms 13, no. 11: 2490. https://doi.org/10.3390/microorganisms13112490
APA StylePang, S., Peng, X., Li, S., Niu, J., Wang, W., Liu, Y., Nie, C., & Zhang, W. (2025). Effects of Initiation Age of Starter Feeding on Growth Performance, Immunity and Antioxidant Capacity, Gastrointestinal Development, and Microbial Communities in Suckling Lambs. Microorganisms, 13(11), 2490. https://doi.org/10.3390/microorganisms13112490







