Comparative Genomics and Phylogenomics of Novel Radiation-Resistant Bacterium Paracoccus qomolangmaensis sp. nov. S3-43T, Showing Pyrethroid Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation, Identification, and Growth Conditions
2.2. Morphological, Physiological, Biochemical, and Chemotaxonomic Analysis
2.3. Whole Genome Sequencing, Assembly, and Annotation
2.4. Radiation Resistance Analysis
2.5. Antioxidant Analysis
2.6. Cyhalothrin Degradation Analysis
2.7. Comparative Genomic Analysis
2.8. Data Analysis and Statistics
3. Results and Discussion
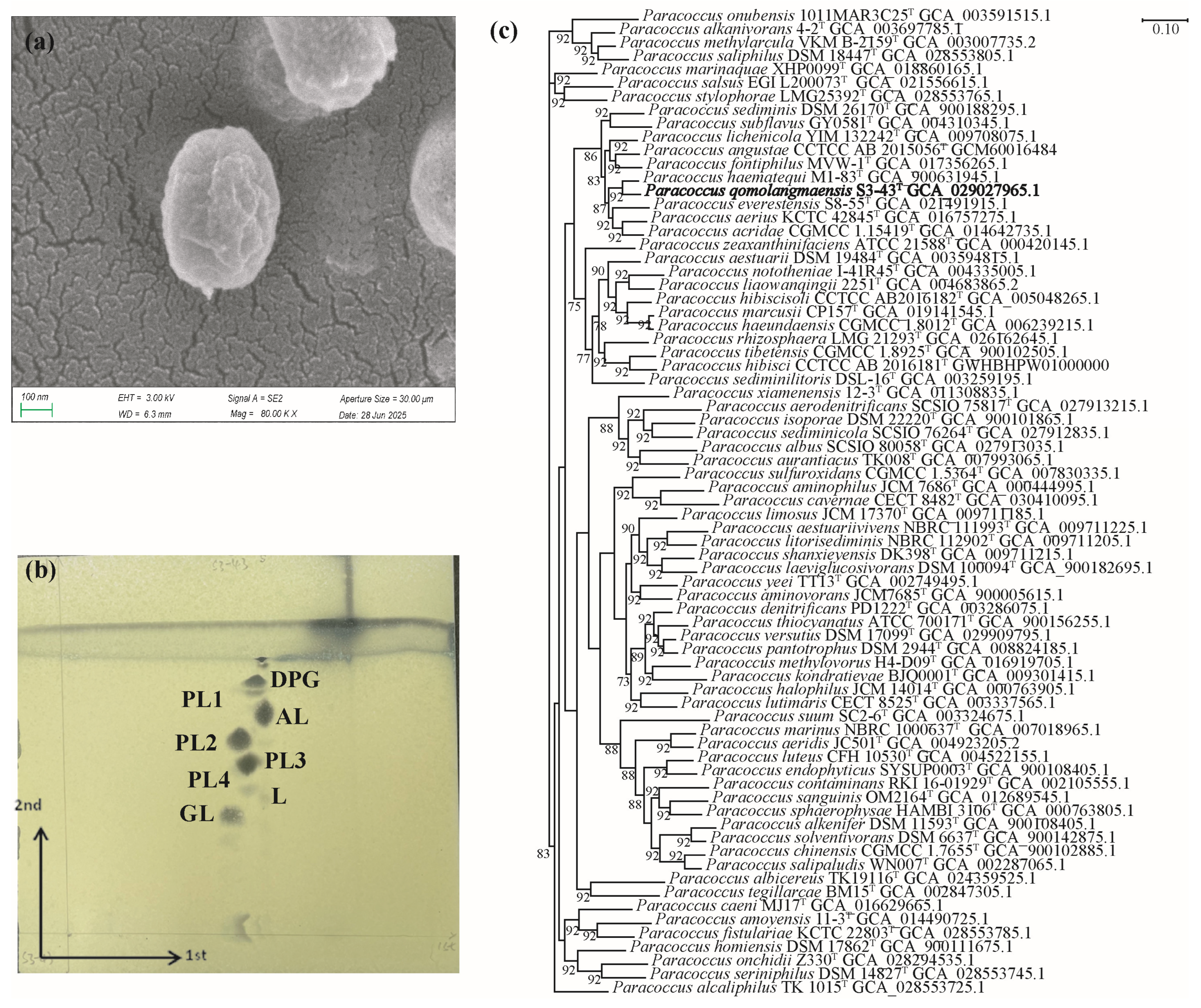
3.1. Phenotypic, Physiological, Phylogenetic, and Phylogenomic Characteristics
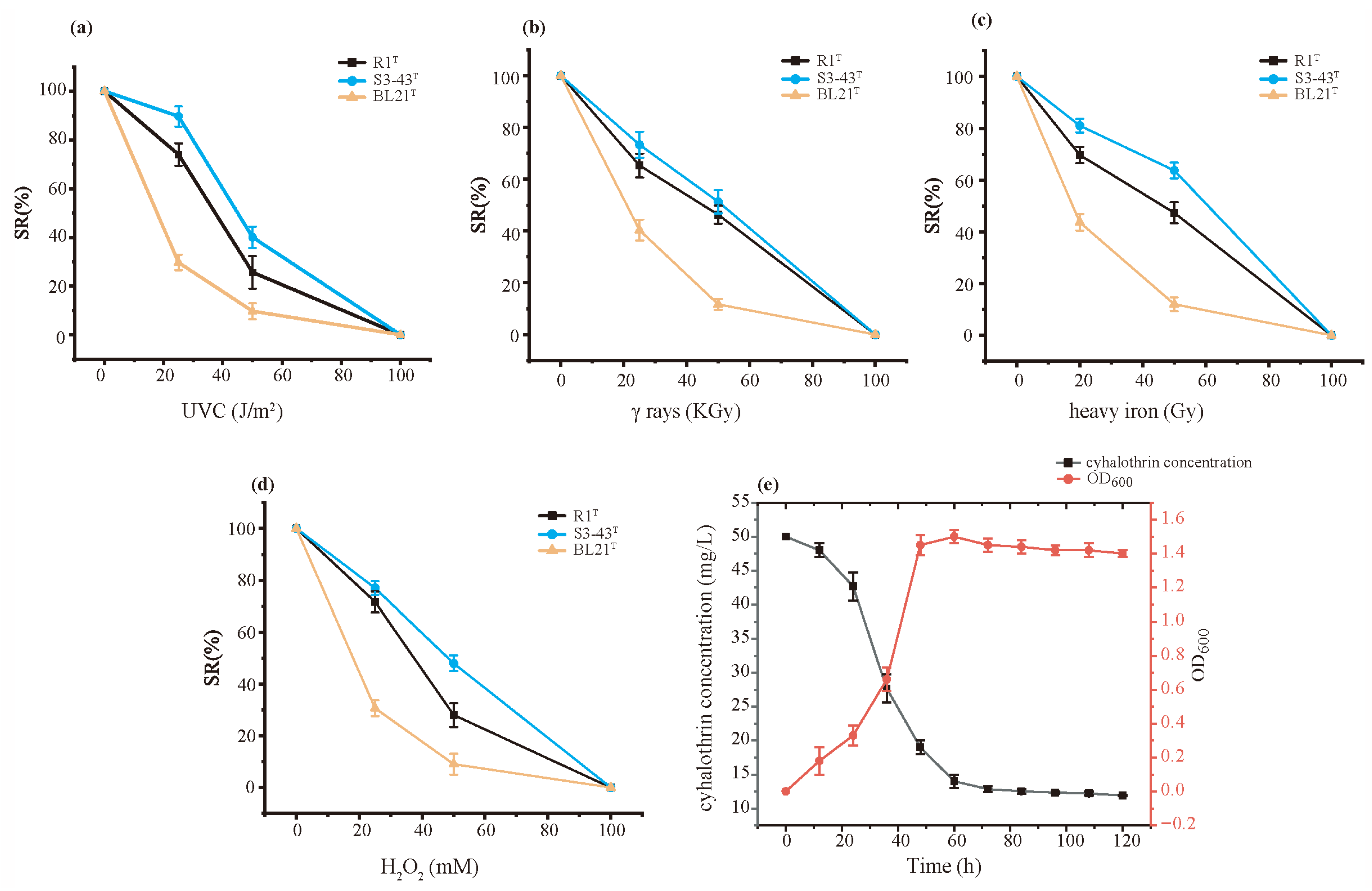
3.2. The Radiation Resistance and Antioxidant of S3-43T
3.3. Cyhalothrin Degradation Capability
3.4. Genomic Insights into a Novel Species Related to Biological Functions
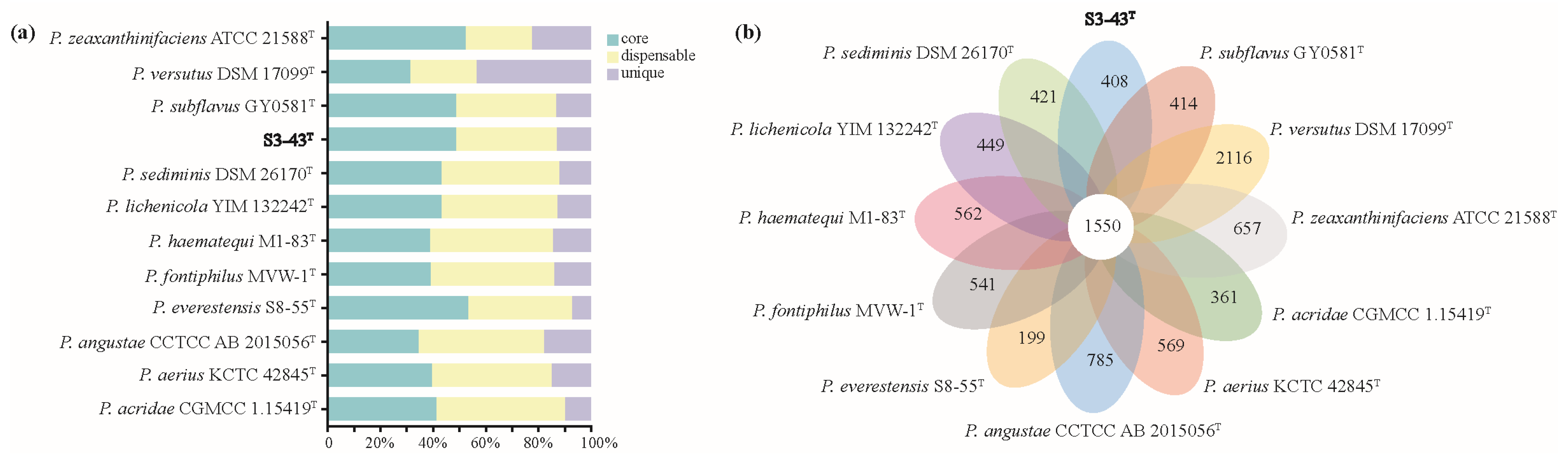
3.4.1. Pan-Genome Analysis
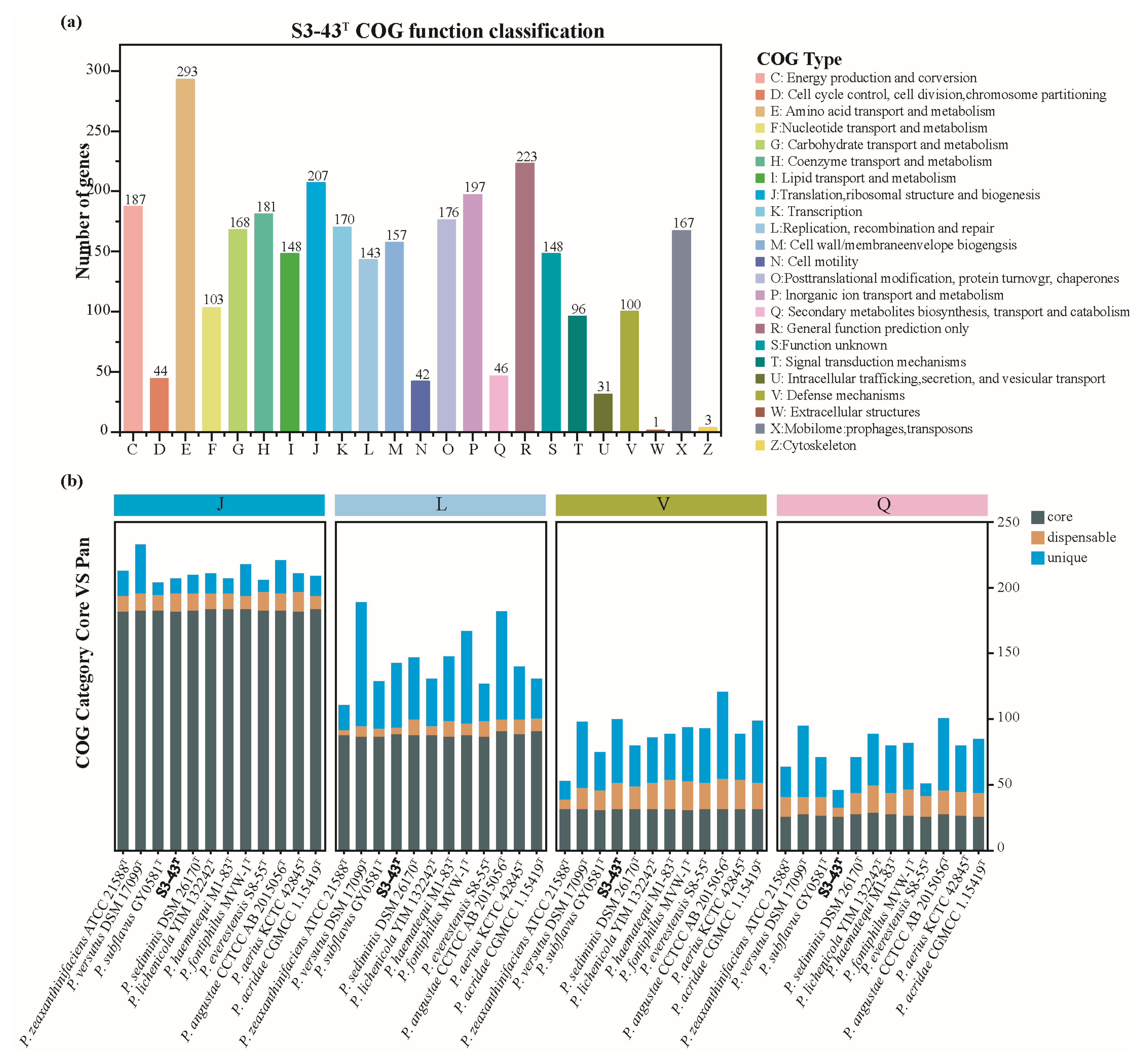
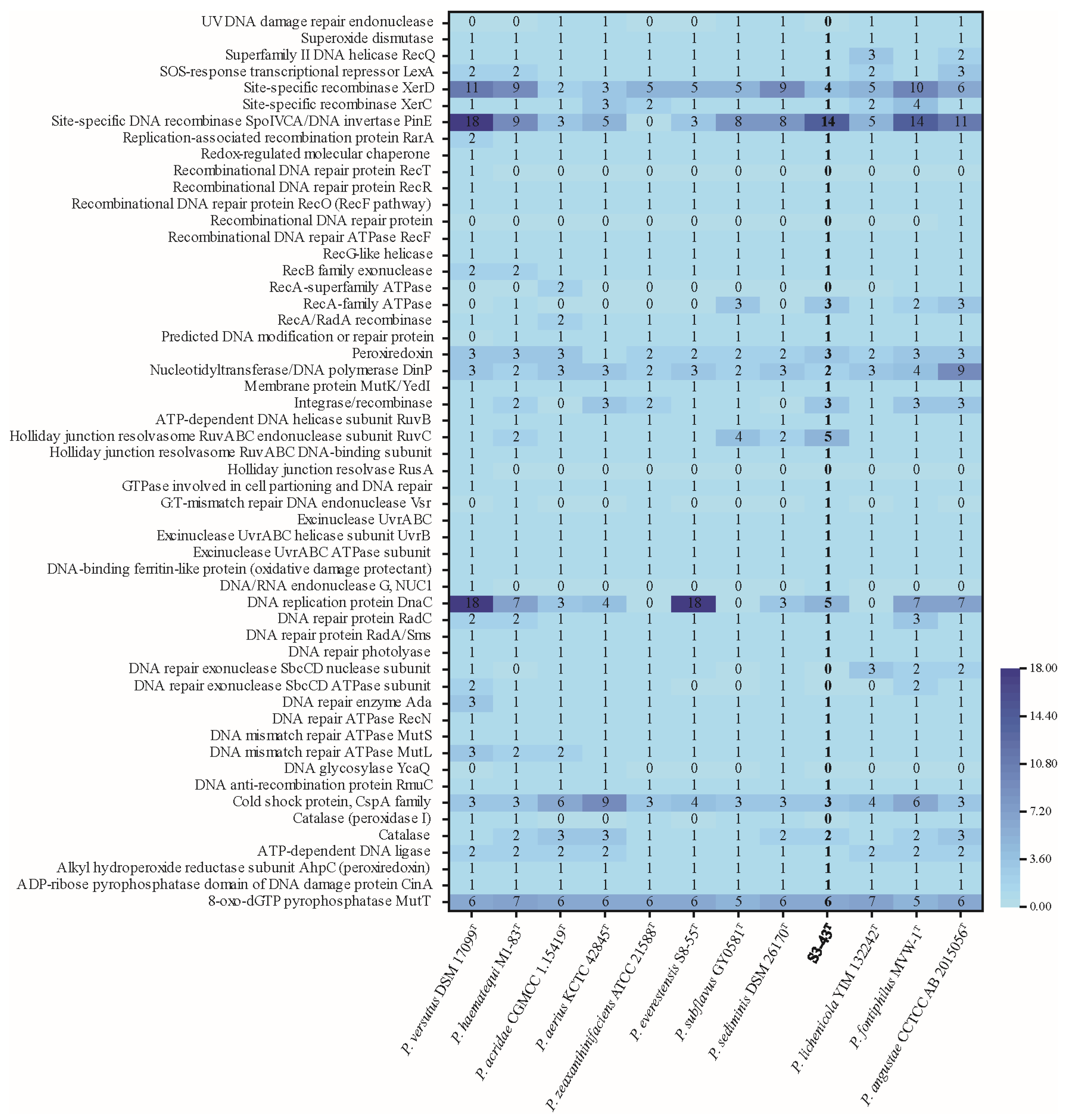
3.4.2. Genes Potentially Associated with Radiation Resistance and Antioxidant Capabilities
3.4.3. Gene Potentially Associated with Cyhalothrin Degradation Capability
3.4.4. Horizontal Gene Transfer Analysis
4. Conclusions
Description of Paracoccus qomolangaensis sp. nov.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sood, U.; Dhingra, G.G.; Anand, S.; Hira, P.; Kumar, R.; Kaur, J.; Verma, M.; Singhvi, N.; Lal, S.; Rawat, C.D.; et al. Microbial Journey: Mount Everest to Mars. Indian J. Microbiol. 2022, 62, 323–337. [Google Scholar] [CrossRef]
- Moore, G.W.; Semple, J.L. The impact of global warming on Mount Everest. High Alt. Med. Biol. 2009, 10, 383–385. [Google Scholar] [CrossRef]
- Lieberman, P.; Morey, A.; Hochstadt, J.; Larson, M.; Mather, S. Mount Everest: A space analogue for speech monitoring of cognitive deficits and stress. Aviat. Space Environ. Med. 2005, 76, B198–B207. [Google Scholar]
- Oren, A.; Garrity, G.M. Valid publication of the names of forty-two phyla of prokaryotes. Int. J. Syst. Evol. Microbiol. 2021, 71, 5056. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Hwang, K.; Lee, H.; Cho, A.; Davis, C.L.; Christner, B.C.; Priscu, J.C.; Kim, O.S. Genetic isolation and metabolic complexity of an Antarctic subglacial microbiome. Nat. Commun. 2025, 16, 7501. [Google Scholar] [CrossRef]
- Asaf, S.; Numan, M.; Khan, A.L.; Al-Harrasi, A. Sphingomonas: From diversity and genomics to functional role in environmental remediation and plant growth. Crit. Rev. Biotechnol. 2020, 40, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, T.; Cui, X.; Xu, Y.; Hu, S.; Zhao, Y.; Zhang, W.; Liu, G.; Zhang, G. Sphingomonas radiodurans sp. nov., a novel radiation-resistant bacterium isolated from the north slope of Mount Everest. Int. J. Syst. Evol. Microbiol. 2022, 72, 5312. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, R.A.; Aristilde, L. Degradation and metabolism of synthetic plastics and associated products by Pseudomonas sp.: Capabilities and challenges. J. Appl. Microbiol. 2017, 123, 582–593. [Google Scholar] [CrossRef]
- Kang, M.; Choi, T.R.; Ahn, S.; Heo, H.Y.; Kim, H.; Lee, H.S.; Lee, Y.K.; Joo, H.S.; Yune, P.S.; Kim, W.; et al. Arctic Psychrotolerant Pseudomonas sp. B14-6 Exhibits Temperature-Dependent Susceptibility to Aminoglycosides. Antibiotics 2022, 11, 1019. [Google Scholar] [CrossRef]
- Desriac, F.; Jégou, C.; Balnois, E.; Brillet, B.; Le Chevalier, P.; Fleury, Y. Antimicrobial peptides from marine proteobacteria. Mar. Drugs 2013, 11, 3632–3660. [Google Scholar] [CrossRef]
- Davis, D.H.; Doudoroff, M.; Stanier, R.Y.; Mandel, M. Proposal to reject the genus Hydrogenomonas: Taxonomic implications. Int. J. Syst. Bacteriol. 1969, 19, 375–390. [Google Scholar] [CrossRef]
- Steinrücke, P.; Ludwig, B. Genetics of Paracoccus denitrificans. FEMS Microbiol. Rev. 1993, 10, 83–117. [Google Scholar] [CrossRef]
- Puri, A.; Bajaj, A.; Singh, Y.; Lal, R. Harnessing taxonomically diverse and metabolically versatile genus Paracoccus for bioplastic synthesis and xenobiotic biodegradation. J. Appl. Microbiol. 2022, 132, 4208–4224. [Google Scholar] [CrossRef]
- Cui, X.; Liu, Y.; Xu, Y.; Chen, T.; Zhang, S.; Wang, J.; Yang, R.; Liu, G.; Zhang, W.; Zhang, G. Paracoccus everestensis sp. nov., a novel bacterium with great antioxidant capacity isolated from the north slope of Mount Everest. Int. J. Syst. Evol. Microbiol. 2022, 72, 5562. [Google Scholar] [CrossRef]
- Zhang, G.; Xian, W.; Yang, J.; Liu, W.; Jiang, H.; Li, W. Paracoccus gahaiensis sp. nov. isolated from sediment of Gahai Lake, Qinghai-Tibetan Plateau, China. Arch. Microbiol. 2016, 198, 227–232. [Google Scholar] [CrossRef]
- Li, J.; Lu, S.; Jin, D.; Yang, J.; Lai, X.H.; Huang, Y.; Tian, Z.; Dong, K.; Zhang, S.; Lei, W.; et al. Paracoccus liaowanqingii sp. nov., isolated from Tibetan antelope (Pantholops hodgsonii). Int. J. Syst. Evol. Microbiol. 2020, 70, 744–750. [Google Scholar] [CrossRef]
- Kampfer, P.; Irgang, R.; Poblete-Morales, M.; Fernandez-Negrete, G.; Glaeser, S.P.; Fuentes-Messina, D.; Avendano-Herrera, R. Paracoccus nototheniae sp. nov., isolated from a black rock cod fish (Notothenia coriiceps) from the Chilean Antarctic. Int. J. Syst. Evol. Microbiol. 2019, 69, 2794–2800. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhao, Q.; Zhang, G.; Jiang, Z.; Sheng, H.; Feng, H.; An, L. Paracoccus tibetensis sp. nov., isolated from Qinghai-Tibet Plateau permafrost. Int. J. Syst. Evol. Microbiol. 2013, 63, 1902–1905. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.S.; Choi, T.J.; Lee, W.J.; Kim, Y.T. Paracoccus haeundaensis sp. nov., a Gram-negative, halophilic, astaxanthin-producing bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 1699–1702. [Google Scholar] [CrossRef]
- Puri, A.; Bajaj, A.; Lal, S.; Singh, Y.; Lal, R. Phylogenomic Framework for Taxonomic Delineation of Paracoccus spp. and Exploration of Core-Pan Genome. Indian J. Microbiol. 2021, 61, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Ning, M.; Hao, W.; Cao, C.; Xie, X.; Fan, W.; Huang, H.; Yue, Y.; Tang, M.; Wang, W.; Gu, W.; et al. Toxicity of deltamethrin to Eriocheir sinensis and the isolation of a deltamethrin-degrading bacterium, Paracoccus sp. P-2. Chemosphere 2020, 257, 127162. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, B.; Ningthoujam, R.; Chaudhuri, S.J.I.M. Isolation and characterization of a lindane degrading bacteria Paracoccus sp. NITDBR1 and evaluation of its plant growth promoting traits. Int. Microbiol. 2018, 22, 155–167. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, J.S.; Li, Y.Y.; Li, W.; Xu, G.M.; Yan, Y.C. Biodegradation of insecticide carbofuran by Paracoccus sp. YM3. J. Environ. Sci. health. Part. B—Pestic. Food Contam. Agric. Wastes 2008, 43, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ge, W.; Zhang, X.; Chai, C.; Wu, J.; Xiang, D.; Chen, X. Biodegradation of aged polycyclic aromatic hydrocarbons in agricultural soil by Paracoccus sp. LXC combined with humic acid and spent mushroom substrate. J. Hazard. Mater. 2019, 379, 120820. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Lu, Y.; Li, X.; Su, J.; Zhao, P.; Zhang, Z.; Gu, Y.; Zhao, D.; Zheng, J. Paracoccus yibinensis sp. nov., a novel bacterium with astaxanthin producing isolated from the environment of Chinese distilled Baijiu. Antonie Leeuwenhoek 2025, 118, 74. [Google Scholar] [CrossRef]
- Katayama, Y.; Hiraishi, A.; Kuraishi, H. Paracoccus thiocyanatus sp. nov., a new species of thiocyanate-utilizing facultative chemolithotroph, and transfer of Thiobacillus versutus to the genus Paracoccus as Paracoccus versutus comb. nov. with emendation of the genus. Microbiology 1995, 141, 1469–1477. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Felsenstein, J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Logan, N.A.; Berge, O.; Bishop, A.H.; Busse, H.J.; De Vos, P.; Fritze, D.; Heyndrickx, M.; Kämpfer, P.; Rabinovitch, L.; Salkinoja-Salonen, M.S.; et al. Proposed minimal standards for describing new taxa of aerobic, endospore-forming bacteria. Int. J. Syst. Evol. Microbiol. 2009, 59, 2114–2121. [Google Scholar] [CrossRef]
- Shirling, E.B.; Gottlieb, D. Methods for characterization of Streptomyces species. Int. J. Syst. Evol. Microbiol. 1966, 16, 313–340. [Google Scholar] [CrossRef]
- Williams, S.T.; Goodfellow, M.; Alderson, G.; Wellington, E.M.; Sneath, P.H.; Sackin, M.J. Numerical classification of Streptomyces and related genera. J. Gen. Microbiol. 1983, 129, 1743–1813. [Google Scholar] [CrossRef] [PubMed]
- Kurup, P.V.; Schmitt, J.A. Numerical taxonomy of Nocardia. Can. J. Microbiol. 1973, 19, 1035–1048. [Google Scholar] [CrossRef] [PubMed]
- Staneck, J.L.; Roberts, G.D. Simplified approach to identification of aerobic actinomycetes by thin-layer chromatography. Appl. Microbiol. 1974, 28, 226–231. [Google Scholar] [CrossRef]
- Minnikin, D.E.; O’Donnell, A.G.; Goodfellow, M.; Alderson, G.; Athalye, M.; Schaal, A.; Parlett, J.H. An integrated procedure for the extraction of bacterial isoprenoid quinones and polar lipids. J. Microbiol. Methods 1984, 2, 233–241. [Google Scholar] [CrossRef]
- Sasser, M. Identification of Bacteria by Gas Chromatography of Cellular Fatty Acids; MIDI Technical Note 101; MIDI: Newark, NJ, USA, 1990. [Google Scholar]
- Collins, M.D.; Pirouz, T.; Goodfellow, M.; Minnikin, D.E. Distribution of menaquinones in actinomycetes and corynebacteria. J. Gen. Microbiol. 1977, 100, 221–230. [Google Scholar] [CrossRef]
- Luo, R.; Liu, B.; Xie, Y.; Li, Z.; Huang, W.; Yuan, J.; He, G.; Chen, Y.; Pan, Q.; Liu, Y.; et al. SOAPdenovo2: An empirically improved memory-efficient short-read de novo assembler. Gigascience 2012, 1, 18. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef]
- Overbeek, R.; Olson, R.; Pusch, G.D.; Olsen, G.J.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Parrello, B.; Shukla, M.; et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2014, 42, D206–D214. [Google Scholar] [CrossRef]
- Na, S.I.; Kim, Y.O.; Yoon, S.H.; Ha, S.M.; Baek, I.; Chun, J. UBCG: Up-to-date bacterial core gene set and pipeline for phylogenomic tree reconstruction. J. Microbiol. 2018, 56, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Wang, J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut Microbes 2020, 12, 1801944. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Ouk Kim, Y.; Park, S.C.; Chun, J. OrthoANI: An improved algorithm and software for calculating average nucleotide identity. Int. J. Syst. Evol. Microbiol. 2016, 66, 1100–1103. [Google Scholar] [CrossRef]
- Richter, M.; Rosselló-Móra, R.; Oliver Glöckner, F.; Peplies, J. JSpeciesWS: A web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics 2016, 32, 929–931. [Google Scholar] [CrossRef]
- Chun, J.; Oren, A.; Ventosa, A.; Christensen, H.; Arahal, D.R.; da Costa, M.S.; Rooney, A.P.; Yi, H.; Xu, X.W.; De Meyer, S.; et al. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int. J. Syst. Evol. Microbiol. 2018, 68, 461–466. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Carbasse, J.S.; Peinado-Olarte, R.L.; Göker, M. TYGS and LPSN: A database tandem for fast and reliable genome-based classification and nomenclature of prokaryotes. Nucleic Acids Res. 2022, 50, D801–D807. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-R, L.; Konstantinidis, K. Bypassing Cultivation To Identify Bacterial Species: Culture-independent genomic approaches identify credibly distinct clusters, avoid cultivation bias, and provide true insights into microbial species. Microbe Mag. 2014, 9, 111–118. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32, D277–D280. [Google Scholar] [CrossRef] [PubMed]
- Cantalapiedra, C.P.; Hernández-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. eggNOG-mapper v2: Functional Annotation, Orthology Assignments, and Domain Prediction at the Metagenomic Scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef]
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference sequence (RefSeq) database at NCBI: Current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A.; et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef]
- Bairoch, A.; Apweiler, R. The SWISS-PROT protein sequence database and its supplement TrEMBL in 2000. Nucleic Acids Res. 2000, 28, 45–48. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Augustijn, H.E.; Reitz, Z.L.; Biermann, F.; Alanjary, M.; Fetter, A.; Terlouw, B.R.; Metcalf, W.W.; Helfrich, E.J.N.; et al. antiSMASH 7.0: New and improved predictions for detection, regulation, chemical structures and visualisation. Nucleic Acids Res. 2023, 51, W46–W50. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Y.; Zhang, Z.; Zhao, Y.; Sun, C.; Yang, M.; Wang, J.; Liu, Q.; Zhang, B.; Chen, M.; et al. PGAweb: A Web Server for Bacterial Pan-Genome Analysis. Front. Microbiol. 2018, 9, 1910. [Google Scholar] [CrossRef]
- Kim, M.; Oh, H.S.; Park, S.C.; Chun, J. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int. J. Syst. Evol. Microbiol. 2014, 64, 346–351. [Google Scholar] [CrossRef]
- Wayne, L.G. International Committee on Systematic Bacteriology announcement of the report of the ad hoc Committee on Reconciliation of Approaches to Bacterial Systematics. J. Appl. Bacteriol. 1988, 64, 283–284. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, K.T.; Tiedje, J.M. Towards a genome-based taxonomy for prokaryotes. J. Bacteriol. 2005, 187, 6258–6264. [Google Scholar] [CrossRef]
- Galperin, M.Y.; Kristensen, D.M.; Makarova, K.S.; Wolf, Y.I.; Koonin, E.V. Microbial genome analysis: The COG approach. Brief. Bioinform. 2019, 20, 1063–1070. [Google Scholar] [CrossRef]
- Tatusov, R.L.; Natale, D.A.; Garkavtsev, I.V.; Tatusova, T.A.; Shankavaram, U.T.; Rao, B.S.; Kiryutin, B.; Galperin, M.Y.; Fedorova, N.D.; Koonin, E.V. The COG database: New developments in phylogenetic classification of proteins from complete genomes. Nucleic Acids Res. 2001, 29, 22–28. [Google Scholar] [CrossRef]
- Ranawat, P.; Rawat, S. Radiation resistance in thermophiles: Mechanisms and applications. World J. Microbiol. Biotechnol. 2017, 33, 112. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Jung, K.W.; Lim, S.; Bahn, Y.S. Microbial radiation-resistance mechanisms. J. Microbiol. 2017, 55, 499–507. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant Properties of Probiotic Bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Hutchinson, F. Chemical changes induced in DNA by ionizing radiation. Prog. Nucleic Acid Res. Mol. Biol. 1985, 32, 115–154. [Google Scholar] [CrossRef]
- Slupphaug, G.; Kavli, B.; Krokan, H.E. The interacting pathways for prevention and repair of oxidative DNA damage. Mutat. Res. 2003, 531, 231–251. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Yadav, D.K.; Kumar, S.; Choi, E.H.; Chaudhary, S.; Kim, M.H. Molecular dynamic simulations of oxidized skin lipid bilayer and permeability of reactive oxygen species. Sci. Rep. 2019, 9, 4496. [Google Scholar] [CrossRef]
- Boiteux, S.; Coste, F.; Castaing, B. Repair of 8-oxo-7,8-dihydroguanine in prokaryotic and eukaryotic cells: Properties and biological roles of the Fpg and OGG1 DNA N-glycosylases. Free Radic. Biol. Med. 2017, 107, 179–201. [Google Scholar] [CrossRef]
- Tahara, Y.K.; Kietrys, A.M.; Hebenbrock, M.; Lee, Y.; Wilson, D.L.; Kool, E.T. Dual Inhibitors of 8-Oxoguanine Surveillance by OGG1 and NUDT1. ACS Chem. Biol. 2019, 14, 2606–2615. [Google Scholar] [CrossRef]
- Wagner, K.; Moolenaar, G.F.; Goosen, N. Role of the insertion domain and the zinc-finger motif of Escherichia coli UvrA in damage recognition and ATP hydrolysis. DNA Repair 2011, 10, 483–496. [Google Scholar] [CrossRef]
- Dubbs, J.M.; Mongkolsuk, S. Peroxiredoxins in bacterial antioxidant defense. In Peroxiredoxin Systems; Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2007; Volume 44, pp. 143–193. [Google Scholar] [CrossRef]
- Alharbi, A.; Rabadi, S.M.; Alqahtani, M.; Marghani, D.; Worden, M.; Ma, Z.; Malik, M.; Bakshi, C.S. Role of peroxiredoxin of the AhpC/TSA family in antioxidant defense mechanisms of Francisella tularensis. PLoS ONE 2019, 14, e0213699. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, F.; Wang, Q.; Rensing, C.; Yu, P.; Gong, J.; Wang, G. Disrupting ROS-protection mechanism allows hydrogen peroxide to accumulate and oxidize Sb(III) to Sb(V) in Pseudomonas stutzeri TS44. BMC Microbiol. 2016, 16, 279. [Google Scholar] [CrossRef]
- Tomášek, O.; Gabrielová, B.; Kačer, P.; Maršík, P.; Svobodová, J.; Syslová, K.; Vinkler, M.; Albrecht, T. Opposing effects of oxidative challenge and carotenoids on antioxidant status and condition-dependent sexual signalling. Sci. Rep. 2016, 6, 23546. [Google Scholar] [CrossRef]
- Saillenfait, A.M.; Ndiaye, D.; Sabaté, J.P. Pyrethroids: Exposure and health effects—An update. Int. J. Hyg. Environ. Health 2015, 218, 281–292. [Google Scholar] [CrossRef]
- Zhan, H.; Huang, Y.; Lin, Z.; Bhatt, P.; Chen, S. New insights into the microbial degradation and catalytic mechanism of synthetic pyrethroids. Environ. Res. 2020, 182, 109138. [Google Scholar] [CrossRef]
- Zhan, H.; Wang, H.; Liao, L.; Feng, Y.; Fan, X.; Zhang, L.; Chen, S. Kinetics and Novel Degradation Pathway of Permethrin in Acinetobacter baumannii ZH-14. Front. Microbiol. 2018, 9, 98. [Google Scholar] [CrossRef]
- Cai, X.; Wang, W.; Lin, L.; He, D.; Huang, G.; Shen, Y.; Wei, W.; Wei, D. Autotransporter domain-dependent enzymatic analysis of a novel extremely thermostable carboxylesterase with high biodegradability towards pyrethroid pesticides. Sci. Rep. 2017, 7, 3461. [Google Scholar] [CrossRef]
- Chen, S.; Lin, Q.; Xiao, Y.; Deng, Y.; Chang, C.; Zhong, G.; Hu, M.; Zhang, L.H. Monooxygenase, a novel beta-cypermethrin degrading enzyme from Streptomyces sp. PLoS ONE 2013, 8, e75450. PLoS ONE 2013, 8, e75450. [Google Scholar] [CrossRef]
- Palmer-Brown, W.; de Melo Souza, P.L.; Murphy, C.D. Cyhalothrin biodegradation in Cunninghamella elegans. Environ. Sci. Pollut. Res. Int. 2019, 26, 1414–1421. [Google Scholar] [CrossRef]
- Tang, A.; Wang, B.; Liu, Y.; Li, Q.; Tong, Z.; Wei, Y. Biodegradation and extracellular enzymatic activities of Pseudomonas aeruginosa strain GF31 on β-cypermethrin. Environ. Sci. Pollut. Res. Int. 2015, 22, 13049–13057. [Google Scholar] [CrossRef]
- Tang, A.X.; Liu, H.; Liu, Y.Y.; Li, Q.Y.; Qing, Y.M. Purification and Characterization of a Novel β-Cypermethrin-Degrading Aminopeptidase from Pseudomonas aeruginosa GF31. J. Agric. Food Chem. 2017, 65, 9412–9418. [Google Scholar] [CrossRef]
- Yang, X.Q.; Wang, W.; Tan, X.L.; Wang, X.Q.; Dong, H. Comparative Analysis of Recombinant Cytochrome P450 CYP9A61 from Cydia pomonella Expressed in Escherichia coli and Pichia pastoris. J. Agric. Food Chem. 2017, 65, 2337–2344. [Google Scholar] [CrossRef]
- Wheelock, C.E.; Shan, G.; Ottea, J. Overview of Carboxylesterases and Their Role in the Metabolism of Insecticides. J. Pestic. Sci. 2005, 30, 75–83. [Google Scholar] [CrossRef]
- Wang, Z.G.; Jiang, S.S.; Mota-Sanchez, D.; Wang, W.; Li, X.R.; Gao, Y.L.; Lu, X.P.; Yang, X.Q. Cytochrome P450-Mediated λ-Cyhalothrin-Resistance in a Field Strain of Helicoverpa armigera from Northeast China. J. Agric. Food Chem. 2019, 67, 3546–3553. [Google Scholar] [CrossRef]
- Yang, Y.; Yue, L.; Chen, S.; Wu, Y. Functional expression of Helicoverpa armigera CYP9A12 and CYP9A14 in Saccharomyces cerevisiae. Pestic. Biochem. Physiol. 2008, 92, 101–105. [Google Scholar] [CrossRef]
- Sharples, G.J.; Benson, F.E.; Illing, G.T.; Lloyd, R.G. Molecular and functional analysis of the ruv region of Escherichia coli K-12 reveals three genes involved in DNA repair and recombination. Mol. Gen. Genet.—MGG 1990, 221, 219–226. [Google Scholar] [CrossRef]
- West, S.C.; Connolly, B. Biological roles of the Escherichia coli RuvA, RuvB and RuvC proteins revealed. Mol. Microbiol. 1992, 6, 2755–2759. [Google Scholar] [CrossRef]
- Newton, K.N.; Courcelle, C.T.; Courcelle, J. UvrD Participation in Nucleotide Excision Repair Is Required for the Recovery of DNA Synthesis following UV-Induced Damage in Escherichia coli. J. Nucleic Acids 2012, 2012, 271453. [Google Scholar] [CrossRef]
- Lage, C.; Gonçalves, S.R.F.; Souza, L.L.; Pádula, M.d.; Leitão, A.C. Differential survival of Escherichia coli uvrA, uvrB, and uvrC mutants to psoralen plus UV-A (PUVA): Evidence for uncoupled action of nucleotide excision repair to process DNA adducts. J. Photochem. Photobiol. B—Biol. 2010, 98, 40–47. [Google Scholar] [CrossRef]
- Thakur, M.; Agarwal, A.; Muniyappa, K. The intrinsic ATPase activity of Mycobacterium tuberculosis UvrC is crucial for its damage-specific DNA incision function. FEBS J. 2021, 288, 1179–1200. [Google Scholar] [CrossRef]
- Hennecke, F.; Kolmar, H.; Bründl, K.; Fritz, H.J. The vsr gene product of E. coli K-12 is a strand- and sequence-specific DNA mismatch endonuclease. Nature 1991, 353, 776–778. [Google Scholar] [CrossRef]
- Dar, M.E.; Bhagwat, A.S. Mechanism of expression of DNA repair gene vsr, an Escherichia coli gene that overlaps the DNA cytosine methylase gene, dcm. Mol. Microbiol. 1993, 9, 823–833. [Google Scholar] [CrossRef]
- Saikolappan, S.; Das, K.; Sasindran, S.J.; Jagannath, C.; Dhandayuthapani, S. OsmC proteins of Mycobacterium tuberculosis and Mycobacterium smegmatis protect against organic hydroperoxide stress. Tuberculosis 2011, 91 (Suppl. S1), S119–S127. [Google Scholar] [CrossRef]
- Sheu, S.Y.; Hsieh, T.Y.; Young, C.C.; Chen, W.M. Paracoccus fontiphilus sp. nov., isolated from a freshwater spring. Int. J. Syst. Evol. Microbiol. 2018, 68, 2054–2060. [Google Scholar] [CrossRef]
- Berry, A.; Janssens, D.; Humbelin, M.; Jore, J.P.M.; Hoste, B.; Cleenwerck, I.; Vancanneyt, M.; Bretzel, W.; Mayer, A.F.; Lopez-Ulibarri, R.; et al. Paracoccus zeaxanthinifaciens sp. nov., a zeaxanthin-producing bacterium. Int. J. Syst. Evol. Microbiol. 2003, 53, 231–238. [Google Scholar] [CrossRef]
- Lang, L.; An, D.F.; Jiang, L.Q.; Li, G.D.; Wang, L.S.; Wang, X.Y.; Li, Q.Y.; Jiang, C.L.; Jiang, Y. Paracoccus lichenicola sp. nov., Isolated from Lichen. Curr. Microbiol. 2021, 78, 816–821. [Google Scholar] [CrossRef]
- Pan, J.; Sun, C.; Zhang, X.Q.; Huo, Y.Y.; Zhu, X.F.; Wu, M. Paracoccus sediminis sp. nov., isolated from Pacific Ocean marine sediment. Int. J. Syst. Evol. Microbiol. 2014, 64, 2512–2516. [Google Scholar] [CrossRef]
- Zhang, G.; Jiao, K.; Xie, F.; Pei, S.; Jiang, L. Paracoccus subflavus sp. nov., isolated from Pacific Ocean sediment. Int. J. Syst. Evol. Microbiol. 2019, 69, 1472–1476. [Google Scholar] [CrossRef] [PubMed]
| GenBank ID | Symbol | Protein Name | GenBank ID | Symbol | Protein Name |
|---|---|---|---|---|---|
| RS08485 | - | cold-shock protein | RS02950 | yghU | glutathione-dependent disulfide-bond oxidoreductase |
| RS14830 | - | cold-shock protein | RS13140 | ruvB | Holliday junction branch migration DNA helicase RuvB |
| RS13155 | ruvC | crossover junction endodeoxyribonuclease RuvC | RS13150 | ruvA | Holliday junction branch migration protein RuvA |
| RS06430 | - | DNA alkylation repair protein | RS15020 | ruvX | Holliday junction resolvase RuvX |
| RS01405 | recQ | DNA helicase RecQ | RS00030 | - | ligase-associated DNA damage response DEXH box helicase |
| RS10405 | vsr | DNA mismatch endonuclease Vsr | RS00025 | pdeM | ligase-associated DNA damage response endonuclease PdeM |
| RS03235 | mutL | DNA mismatch repair endonuclease MutL | RS00040 | - | ligase-associated DNA damage response exonuclease |
| RS01015 | mutS | DNA mismatch repair protein MutS | RS15440 | msrB | peptide-methionine (R)-S-oxide reductase MsrB |
| RS10955 | - | DNA mismatch repair protein MutT | RS14820 | msrA | peptide-methionine (S)-S-oxide reductase MsrA |
| RS07735 | rmuC | DNA recombination protein RmuC | RS15445 | msrA | peptide-methionine (S)-S-oxide reductase MsrA |
| RS12135 | radA | DNA repair protein RadA | RS12420 | recA | recombinase RecA |
| RS01075 | radC | DNA repair protein RadC | RS08935 | recR | recombination mediator RecR |
| RS04590 | recN | DNA repair protein RecN | RS15945 | recJ | single-stranded-DNA-specific exonuclease RecJ |
| RS13770 | recO | DNA repair protein RecO | RS01565 | - | UvrD-helicase domain-containing protein |
| RS00015 | recF | DNA replication/repair protein RecF | RS08415 | - | UvrD-helicase domain-containing protein |
| RS00950 | addA | double-strand break repair helicase AddA | RS13235 | - | UvrD-helicase domain-containing protein |
| RS00955 | addB | double-strand break repair protein AddB | RS16030 | - | NAD(P)H-quinone oxidoreductase |
| RS08490 | - | DsbA family oxidoreductase | RS00945 | trxA | thioredoxin |
| RS05580 | uvrA | excinuclease ABC subunit UvrA | RS16155 | trxA | thioredoxin |
| RS05645 | uvrB | excinuclease ABC subunit UvrB | RS12075 | trxB | thioredoxin-disulfide reductase |
| RS15085 | uvrC | excinuclease ABC subunit UvrC |
| Gene Description | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 | 4 | 3 | 4 | 3 | 4 | 3 | 2 | 2 | 2 | 4 | 3 | 4 |
| Heme-degrading monooxygenase HmoA and related ABM domain proteins | 2 | 1 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 |
| Quinol monooxygenase YgiN | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 1 | 2 | 0 | 2 |
| Aminopeptidase N, contains DUF3458 domain | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| D-aminopeptidase | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| L-aminopeptidase/D-esterase | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Leucyl aminopeptidase | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 2 | 2 |
| Methionine aminopeptidase | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 1 |
| Putative aminopeptidase FrvX | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Xaa-Pro aminopeptidase | 2 | 4 | 4 | 3 | 2 | 2 | 2 | 1 | 3 | 2 | 3 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Chen, T.; Zhang, Y.; Zhang, L.; Cui, X.; Cheng, T.; Liu, G.; Zhang, W.; Zhang, G. Comparative Genomics and Phylogenomics of Novel Radiation-Resistant Bacterium Paracoccus qomolangmaensis sp. nov. S3-43T, Showing Pyrethroid Degradation. Microorganisms 2025, 13, 2441. https://doi.org/10.3390/microorganisms13112441
Liu Y, Chen T, Zhang Y, Zhang L, Cui X, Cheng T, Liu G, Zhang W, Zhang G. Comparative Genomics and Phylogenomics of Novel Radiation-Resistant Bacterium Paracoccus qomolangmaensis sp. nov. S3-43T, Showing Pyrethroid Degradation. Microorganisms. 2025; 13(11):2441. https://doi.org/10.3390/microorganisms13112441
Chicago/Turabian StyleLiu, Yang, Tuo Chen, Yiyang Zhang, Lu Zhang, Xiaowen Cui, Tian Cheng, Guangxiu Liu, Wei Zhang, and Gaosen Zhang. 2025. "Comparative Genomics and Phylogenomics of Novel Radiation-Resistant Bacterium Paracoccus qomolangmaensis sp. nov. S3-43T, Showing Pyrethroid Degradation" Microorganisms 13, no. 11: 2441. https://doi.org/10.3390/microorganisms13112441
APA StyleLiu, Y., Chen, T., Zhang, Y., Zhang, L., Cui, X., Cheng, T., Liu, G., Zhang, W., & Zhang, G. (2025). Comparative Genomics and Phylogenomics of Novel Radiation-Resistant Bacterium Paracoccus qomolangmaensis sp. nov. S3-43T, Showing Pyrethroid Degradation. Microorganisms, 13(11), 2441. https://doi.org/10.3390/microorganisms13112441






