Unveiling the 2017 Karenia Bloom in NW Chilean Patagonia by Integrating Remote Sensing and Field Data
Abstract
1. Introduction
2. Materials and Methods
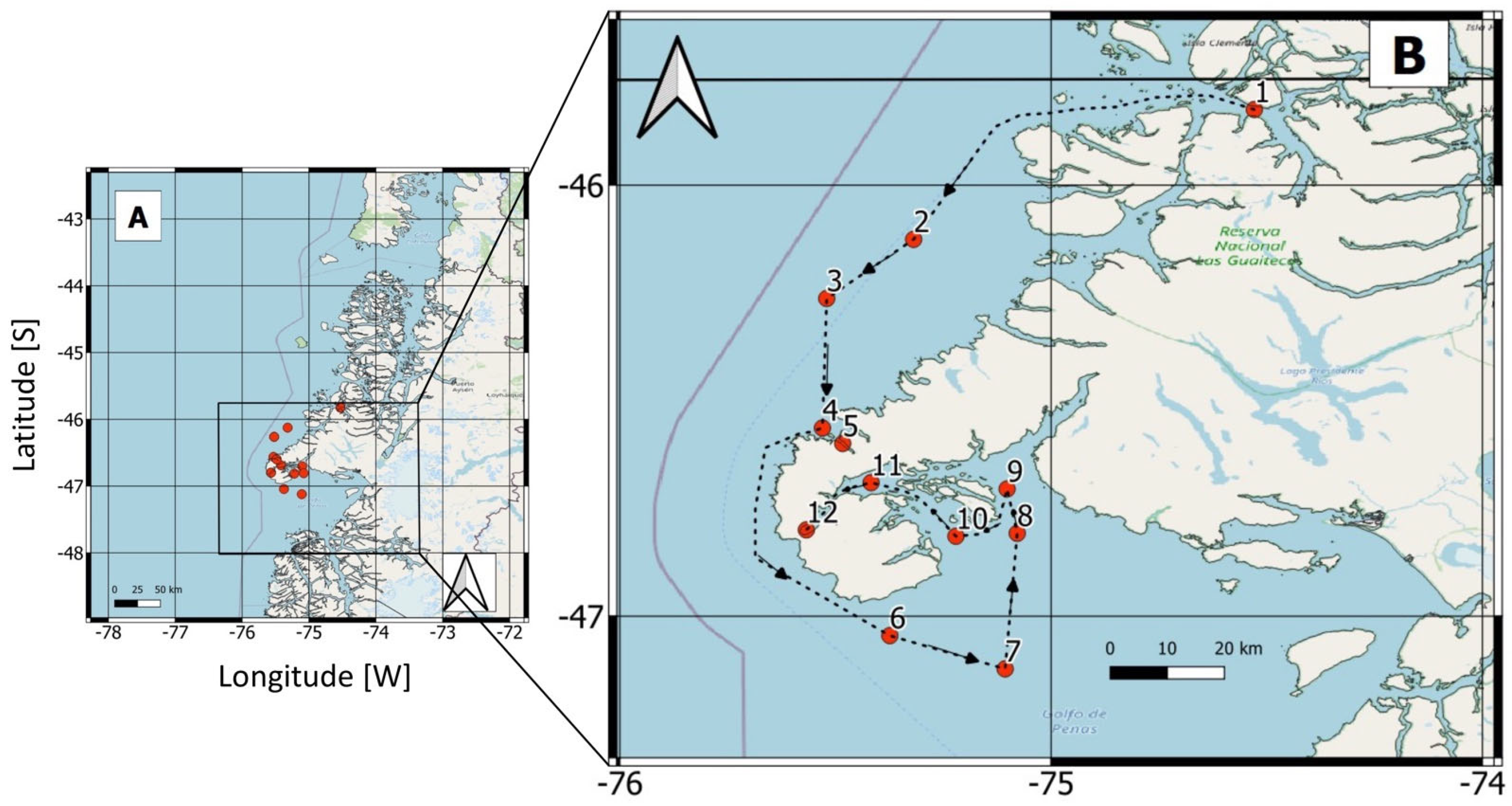
2.1. Study Area
2.2. Field Sampling
2.3. Phytoplankton Analysis
2.4. Satellite Data
2.5. Identification of Karenia Bloom from Satellite Images
2.6. Statistical Analysis
3. Results
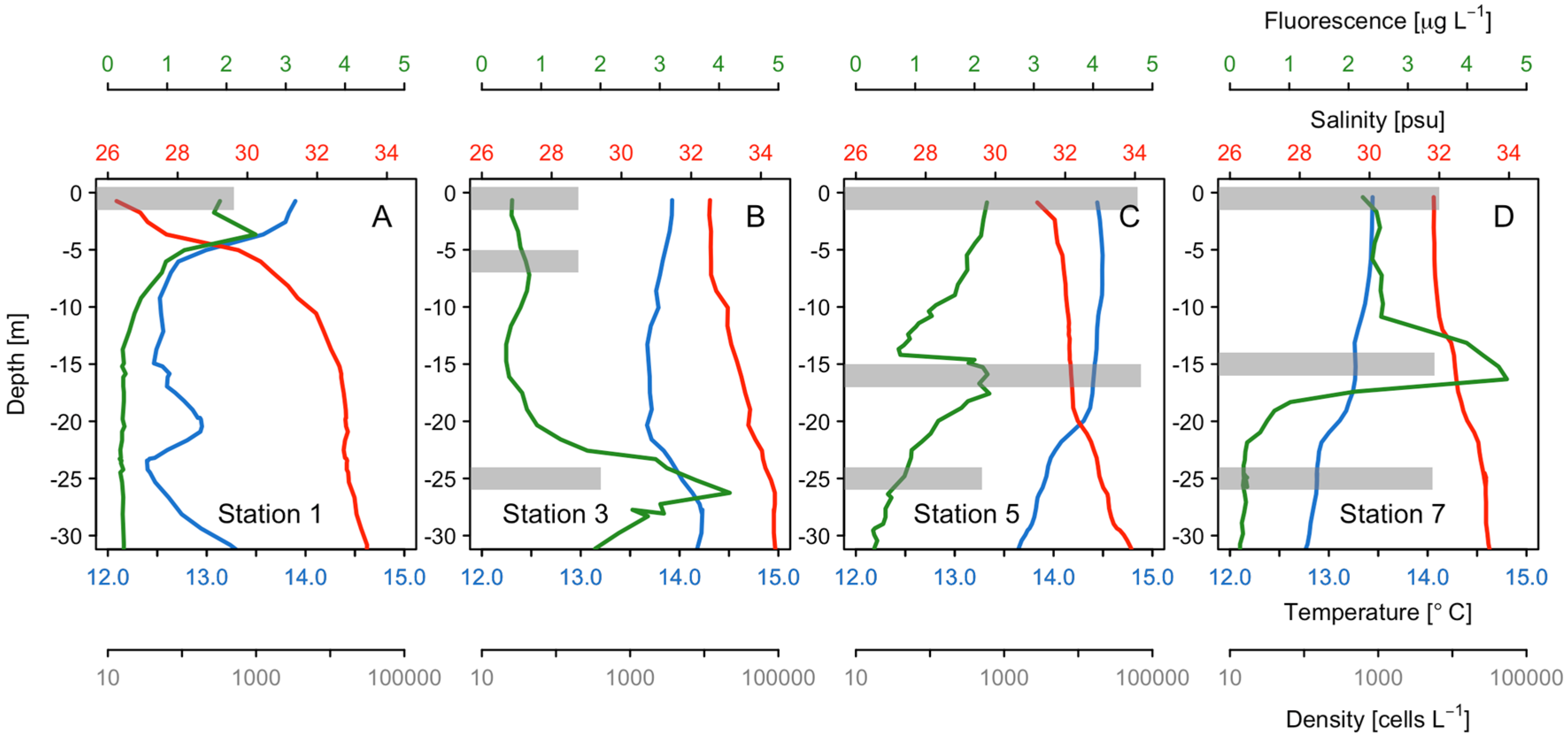
3.1. Hydrographic Characterization
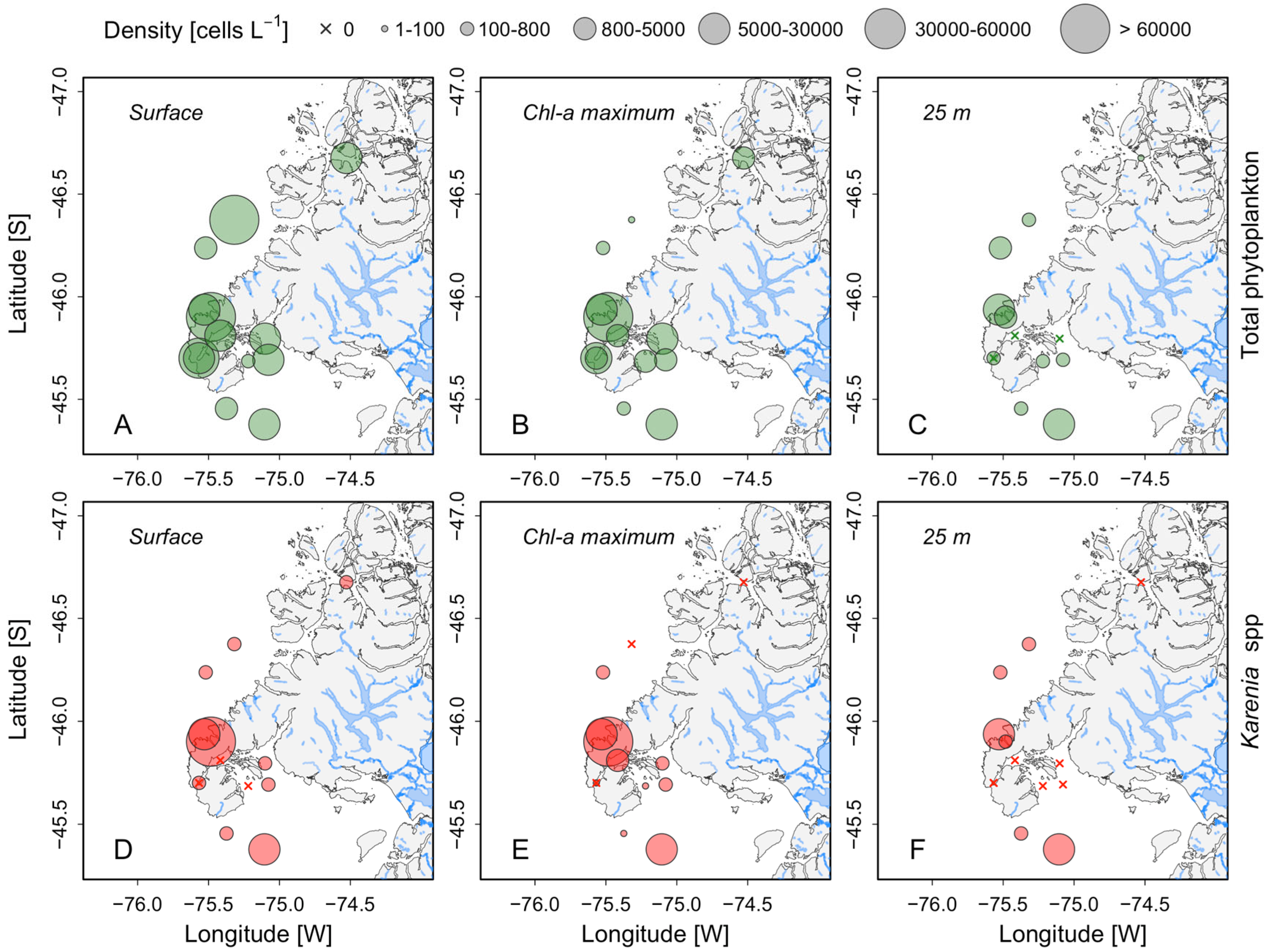
3.2. Distribution of Karenia Cells
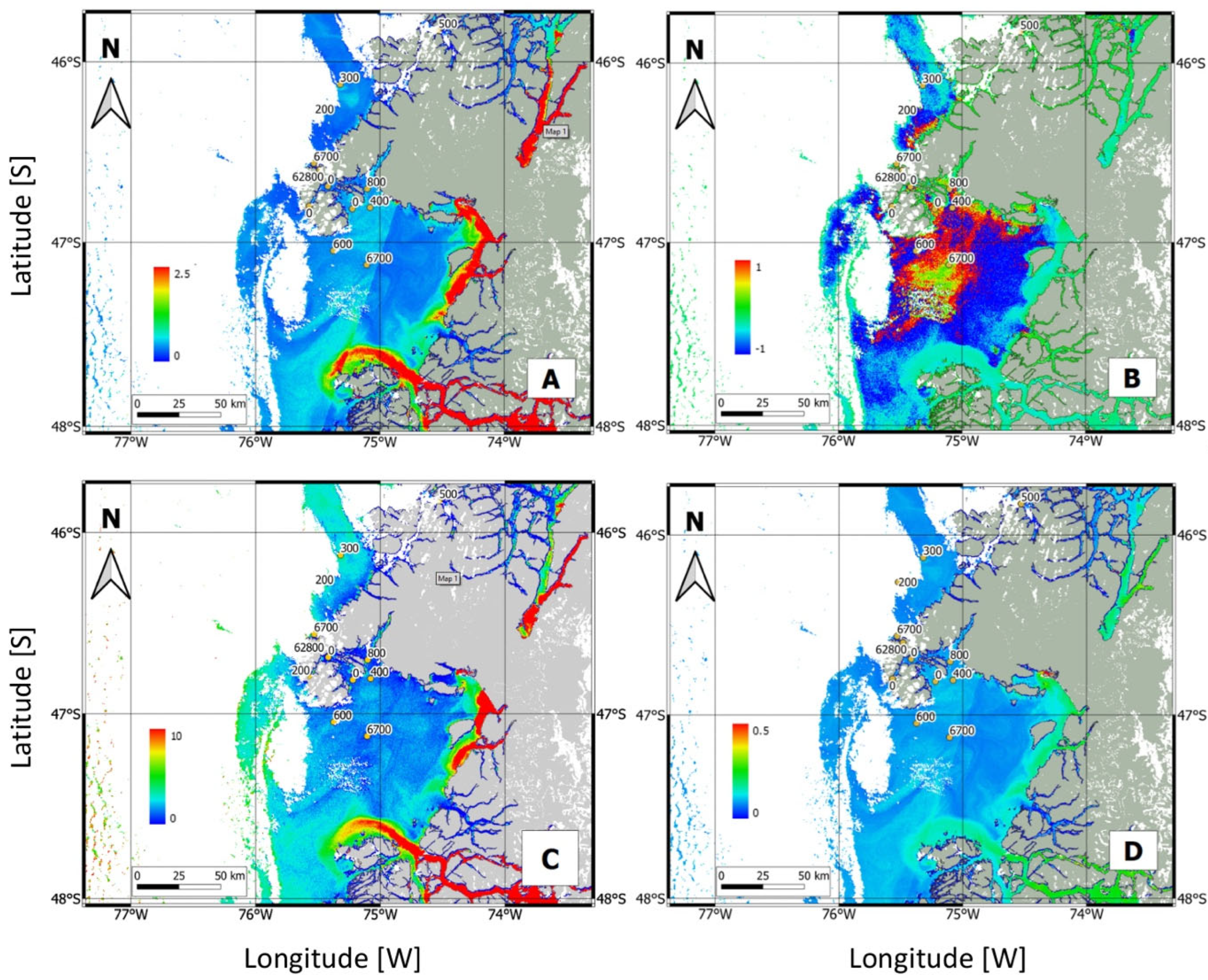
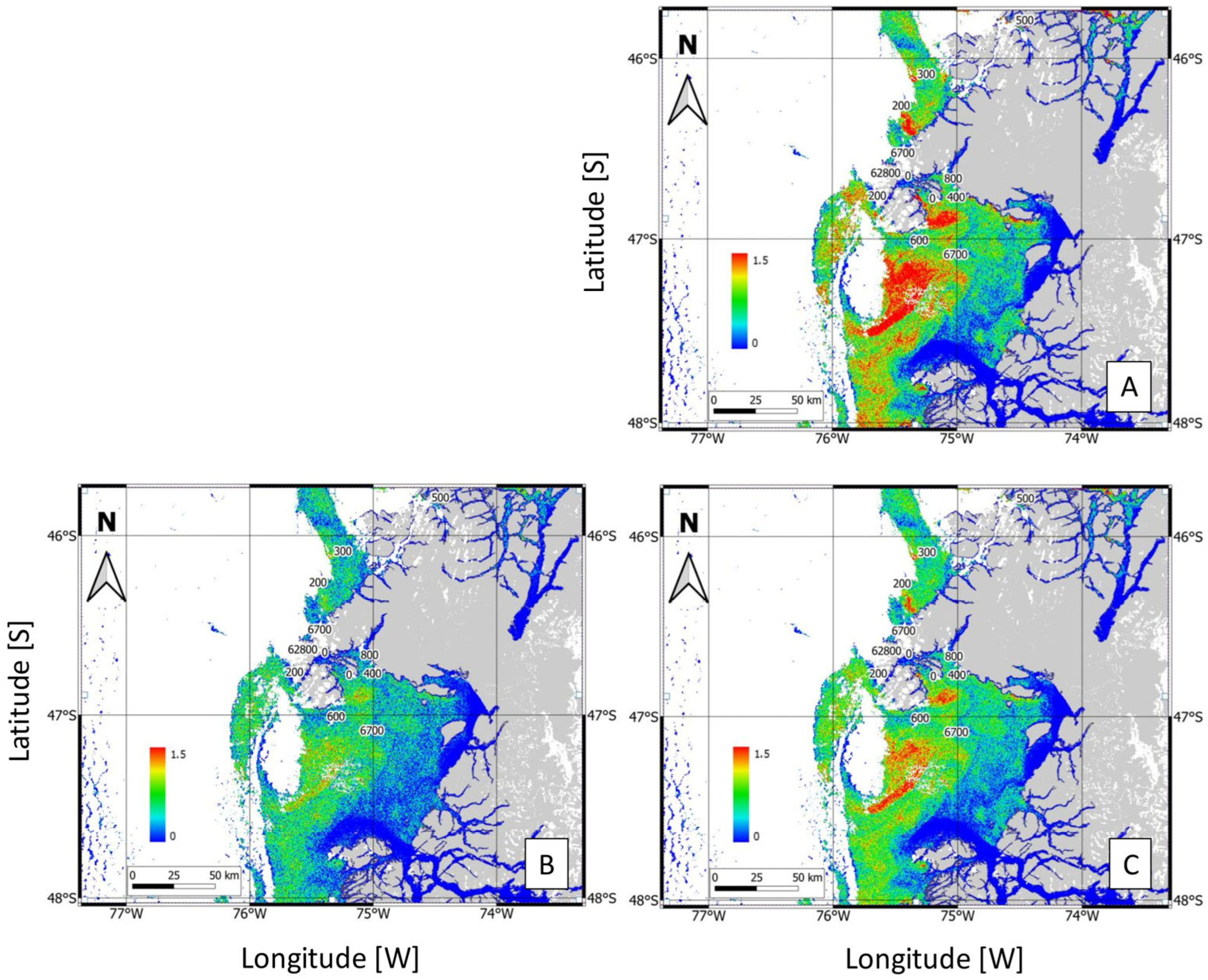
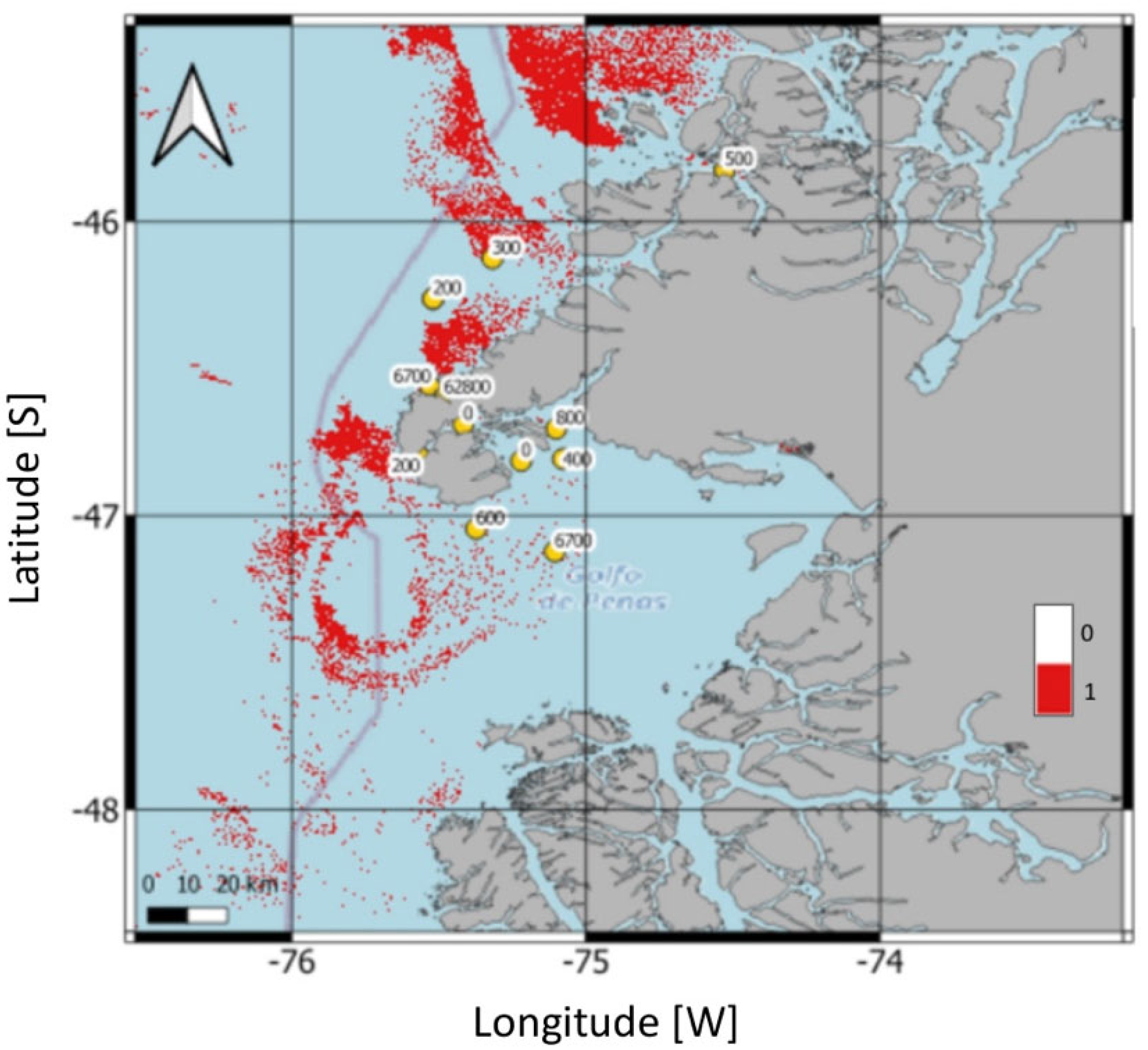
3.3. Satellite Bloom Detection
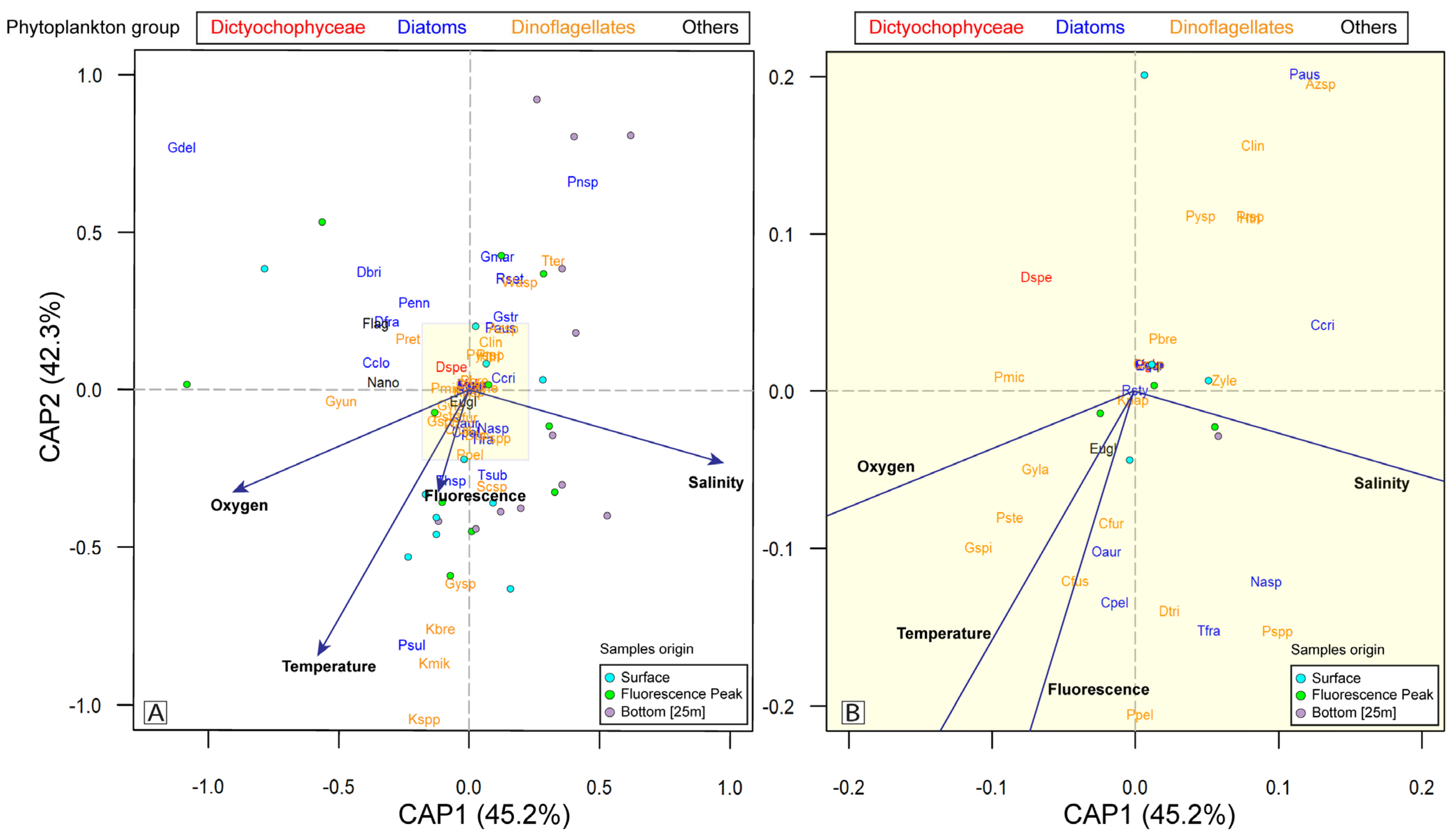
3.4. Statistical Analysis
4. Discussion
4.1. Satellite Tools and Classification Method for Bloom Detection
4.2. Implications for Monitoring Programs and Potential Impacts
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Spectral Band Characteristics
| Band | λ Center (nm) | Width (nm) | Function |
|---|---|---|---|
| Oa01 | 400 | 15 | Aerosol correction, improved water constituent retrieval |
| Oa02 | 412.5 | 10 | Yellow substances, detrital pigments (turbidity) |
| Oa03 | 442.5 | 10 | Chlorophyll absorption maximum, biogeochemistry, vegetation |
| Oa04 | 490 | 10 | High chlorophyll |
| Oa05 | 510 | 10 | Chlorophyll, sediment, turbidity, red tide |
| Oa06 | 560 | 10 | Chlorophyll reference (chlorophyll minimum) |
| Oa07 | 620 | 10 | Sediment loading |
| Oa08 | 665 | 10 | Chlorophyll (2nd chlorophyll absorption maximum), sediment, yellow substances, vegetation |
| Oa09 | 673.75 | 7.5 | Improved fluorescence retrieval and to account for smile together with the bands at 665 and 680 nm |
| Oa10 | 681.25 | 7.5 | Chlorophyll fluorescence peak, red edge |
| Oa11 | 708.75 | 10 | Chlorophyll fluorescence baseline, red edge transition |
| Oa12 | 753.75 | 7.5 | O2 absorption, clouds, vegetation |
| Oa13 | 761.25 | 2.5 | O2 absorption band, aerosol correction |
| Oa14 | 764.375 | 3.75 | Atmospheric correction |
| Oa15 | 767.5 | 2.5 | O2A, used for cloud top pressure, fluorescence over land |
| Oa16 | 778.75 | 15 | Atmospheric/aerosol correction |
| Oa17 | 865 | 20 | Atmospheric/aerosol correction, clouds, pixel co-registration |
| Oa18 | 885 | 10 | Water vapor absorption reference band, common reference band with the SLSTR instrument, vegetation monitoring |
| Oa19 | 900 | 10 | Water vapor absorption, vegetation monitoring (maximum reflectance) |
| Oa20 | 940 | 20 | Water vapor absorption; atmospheric/aerosol correction |
| Oa21 | 1020 | 40 | Atmospheric/aerosol correction |
| Band | Wavelength (1) | Bandwidth (nm) | Function |
|---|---|---|---|
| 8 | 415 | 405–420 | Ocean color, phytoplankton, biogeochemistry |
| 9 | 443 | 438–448 | Ocean color, phytoplankton, biogeochemistry |
| 10 | 490 | 483–493 | Ocean color, phytoplankton, biogeochemistry |
| 11 | 531 | 526–536 | Ocean color, phytoplankton, biogeochemistry |
| 12 | 565 | 546–556 | Ocean color, phytoplankton, biogeochemistry |
| 13 | 653 | 662–672 | Ocean color, phytoplankton, biogeochemistry |
| 14 | 681 | 673–683 | Ocean color, phytoplankton, biogeochemistry |
| 15 | 750 | 743–753 | Ocean color, phytoplankton, biogeochemistry |
| 16 | 865 | 862–877 | Ocean color, phytoplankton, biogeochemistry |
| Band | Wavelength (1) | Bandwidth (nm) | Function |
|---|---|---|---|
| 1 | 412.5 | 10 | Yellow substances and detrital pigments |
| 2 | 442.5 | 10 | Chlorophyll absorption maximum |
| 3 | 490 | 10 | Chlorophyll and other pigments |
| 4 | 510 | 10 | Suspended sediment, red tides |
| 5 | 560 | 10 | Chlorophyll absorption minimum |
| 6 | 620 | 10 | Suspended sediment |
| 7 | 665 | 10 | Chlorophyll absorption and fluorescence reference |
| 8 | 681.25 | 7.5 | Chlorophyll fluorescence peak |
| 9 | 708.75 | 10 | Fluorescence reference, atmospheric corrections |
| 10 | 753.75 | 7.5 | Vegetation, cloud |
| 11 | 760.625 | 3.75 | Oxygen absorption R-branch |
| 12 | 778.75 | 15 | Atmosphere corrections |
Appendix B
Algorithms
Appendix C
Appendix C.1. Phytoplankton Species Key
| Scientific Name | Abbreviation |
|---|---|
| Dictyochophyceae | |
| Vicicitus globosus | Vglo |
| Dictyocha speculum | Dspe |
| Diatoms | |
| Cerataulina pelagica | Cpel |
| Eucampia cornuta | Ecor |
| Chaetoceros criophilus | Ccri |
| Chaetoceros spp. | Cspp |
| Dactyliosolen fragilissimus | Dfra |
| Ditylum brightwellii | Dbri |
| Guinardia delicatula | Gdel |
| Guinardia striata | Gstr |
| Lauderia spp. | Lspp |
| Leptocylindrus danicus | Ldan |
| Odontella aurita | Oaur |
| Paralia sulcata | Psul |
| Rhizosolenia aff. setígera | Rset |
| Rhizosolenia styliformis | Rsty |
| Skeletonema spp. | Sksp |
| Thalassiosira subtilis | Tsub |
| Thalassiosira spp. | Thsp |
| Cylindrotheca closterium | Cclo |
| Grammatophora marina | Gmar |
| Navicula spp. | Nasp |
| Pennadas | Penn |
| Pseudo-nitzschia cf. australis | Paus |
| Pseudo-nitzschia spp. | Pnsp |
| Thalassionema frauenfeldii | Tfra |
| Dinoflagellata (thecate) | |
| Azadinium spp. | Azsp |
| Ceratium furca | Cfur |
| Ceratium fusus | Cfus |
| Ceratium lineatum | Clin |
| Dinophysis acuminata | Dacu |
| Dinophysis acuta | Duta |
| Dinophysis tripos | Dtri |
| Heterocapsa triquetra | Htri |
| Prorocentrum micans | Pmic |
| Protoceratium reticulatum | Pret |
| Protoperidinium brevipes | Pbre |
| Protoperidinium pellucidum | Ppel |
| Protoperidinium steinii | Pste |
| Protoperidinium spp. | Pspp |
| Pyrocistis spp. | Pysp |
| Scrippsiella sp. | Scsp |
| Torodinium teredo | Tter |
| Zygabicodinium lenticulatum | Zyle |
| Dinoflagellata (athecate) | |
| Cochlodinium sp. | Cosp |
| Gymnodinials (unidentified) | Gyun |
| Gyrodinium sp. | Gysp |
| Gyrodinium lachryma | Gyla |
| Gyrodinium spirale | Gspi |
| Karenia cf. mikimotoi | Kmik |
| Karenia sp. 1 | Ksp1 |
| Karenia sp. 3 | Ksp3 |
| Karenia spp. | Kspp |
| Karenia brevisulcata/digitata | Kbre |
| Kaenia papilionacea/brevis | Kpap |
| Warnovia sp. | Wasp |
| Pronoctiluca sp. | Prsp |
| Others | |
| Ciliates | Cill |
| Euglenophyta | Eugl |
| Nanoflagellates | Nano |
| Flagellate | Flag |
References
- Wells, M.L.; Karlson, B.; Wulff, A.; Kudela, R.; Trick, C.; Asnaghi, V.; Berdalet, E.; Cochlan, W.; Davidson, K.; De Rijcke, M.; et al. Future HAB science: Directions and challenges in a changing climate. Harmful Algae 2020, 91, 101632. [Google Scholar] [CrossRef]
- Hallegraeff, G.; Enevoldsen, H.; Zingone, A. Global harmful algal bloom status reporting. Harmful Algae 2021, 102, 101992. [Google Scholar] [CrossRef]
- IOC-UNESCO. State of the Ocean Report. (IOC Technical Series, 190); IOC-UNESCO: Paris, France, 2024. [Google Scholar] [CrossRef]
- Hallegraeff, G. Ocean climate change, phytoplankton community responses, and harmful algal blooms: A formidable predictive challenge. J. Phycol. 2010, 46, 220–235. [Google Scholar] [CrossRef]
- Hallegraeff, G.M.; Anderson, D.M.; Belin, C.; Bottein, M.-Y.D.; Bresnan, E.; Chinain, M.; Enevoldsen, H.; Iwataki, M.; Karlson, B.; McKenzie, C.H.; et al. Perceived global increase in algal blooms is attributable to intensified monitoring and emerging bloom impacts. Commun. Earth Environ. 2021, 2, 117. [Google Scholar] [CrossRef]
- Díaz, P.A.; Pérez-Santos, I.; Basti, L.; Garreaud, R.; Pinilla, E.; Barrera, F.; Tello, A.; Schwerter, C.; Arenas-Uribe, S.; Soto-Riquelme, C.; et al. The impact of local and climate change drivers on the formation, dynamics, and potential recurrence of a massive fish-killing microalgal bloom in Patagonian fjord. Sci. Total Environ. 2023, 865, 161288. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, G.C.; Foord, C.J.; Macey, B.M.; Mansfield, L.; Mouton, A.; Smith, M.E.; Osmond, S.J.; van der Molen, L. Devastating farmed abalone mortalities attributed to yessotoxin-producing dinoflagellates. Harmful Algae 2019, 81, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Díaz, P.A.; Molinet, C.; Seguel, M.; Niklitschek, E.J.; Díaz, M.; Álvarez, G.; Pérez-Santos, I.; Varela, D.; Guzmán, L.; Rodríguez-Villegas, C.; et al. Modelling the spatial and temporal dynamics of paralytic shellfish toxins (PST) at different scales: Implications for research and management. Toxins 2022, 14, 786. [Google Scholar] [CrossRef]
- Álvarez-Salgado, X.A.; Labarta, U.; Fernández-Reiriz, M.J.; Figueiras, F.G.; Rosón, G.; Piedracoba, S.; Filgueira, R.; Cabanas, J.M. Renewal time and the impact of harmful algal blooms on the extensive mussel raft culture of the Iberian coastal upwelling system (SW Europe). Harmful Algae 2008, 7, 849–855. [Google Scholar] [CrossRef]
- Blanco, J.; Correa, J.; Muñíz, S.; Mariño, C.; Martín, H.; Arévalo, F. Evaluación del impacto de los métodos y niveles utilizados para el control de toxinas en el mejillón. Rev. Galega Dos Recur. Mariños 2013, 3, 1–55. [Google Scholar]
- Díaz, P.A.; Álvarez, A.; Varela, D.; Pérez-Santos, I.; Díaz, M.; Molinet, C.; Seguel, M.; Aguilera-Belmonte, A.; Guzmán, L.; Uribe, E.; et al. Impacts of harmful algal blooms on the aquaculture industry: Chile as a case study. Perspect. Phycol. 2019, 6, 39–50. [Google Scholar] [CrossRef]
- Clément, A.; Lincoqueo, L.; Saldivia, M.; Brito, C.G.; Muñoz, F.; Fernández, C.; Pérez, F.; Maluje, C.P.; Correa, N.; Mondaca, V.; et al. Exceptional summer conditions and HABs of Pseudochattonella in southern Chile create record impacts on salmon farm. Harmful Algae News 2016, 53, 1–3. [Google Scholar]
- León-Muñoz, J.; Urbina, M.A.; Garreaud, R.; Iriarte, J.L. Hydroclimatic conditions trigger record harmful algal bloom in western Patagonia (summer 2016). Sci. Rep. 2018, 8, 1330. [Google Scholar] [CrossRef] [PubMed]
- Mardones, J.I.; Paredes-Mella, J.; Flores-Leñero, A.; Yarimuzu, K.; Godoy, M.; Cascales, E.; Espinoza, J.P.; Norambuena, L.; Garreaud, R.; Gonzáles, H.E.; et al. Extreme harmful algal blooms, climate change, and potential risk of eutrophication in Patagonian fjords: Insights from an exceptional Heterosigma akashiwo fish-killing event. Prog. Oceanogr. 2023, 210, 102921. [Google Scholar] [CrossRef]
- Villanueva, F.; Cortez, H.; Uribe, C.; Peña, P.; Cassis, D. Mortality of Chilean farmed salmon in wellboats in transit through a Karenia bloom. Harmful Algae News 2017, 57, 4–5. [Google Scholar]
- Toro, C.; Alarcón, C.; Pacheco, H.; Salgado, P.; Frangopulos, M.; Rodríguez, F.; Fuenzalida, G.; Raimapo, R.; Pizarro, G.; Guzmán, L. Harmful Algal bloom species associated with massive Atlantic salmon mortalities while transported through the Gulf of Penas, southern Chile. In Harmful Algae 2018—From Ecosystems to Socioecosystems, Proceedings of the 18th International Conference on Harmful Algae, Nantes, France, 21–26 October 2018; Hess, P., Ed.; International Society for the Study of Harmful Algae: Punta Arenas, Chile, 2020; pp. 154–157. [Google Scholar]
- Baldrich, A.M.; Díaz, P.A.; Rosales, S.A.; Rodríguez-Villegas, C.; Álvarez, G.; Pérez-Santos, I.; Díaz, M.; Schwerter, C.; Araya, M.; Reguera, B. An unprecedented bloom of oceanic dinoflagellates (Karenia spp.) inside a fjord within a highly dynamic multifrontal ecosystem in Chilean Patagonia. Toxins 2024, 16, 77. [Google Scholar] [CrossRef]
- Mardones, J.; Norambuena, L.; Paredes-Mella, J.; Fuenzalida, G.; Dorantes-Aranda, J.J.; Lee Chang, K.L.; Guzmán, L.; Krock, B.; Hallegraeff, G. Unraveling the Karenia selliformis complex with the description of a non-gymnodimine producing Patagonian phylotype. Harmful Algae 2020, 98, 101892. [Google Scholar] [CrossRef]
- Mardones, J.I.; Paredes, J.; Godoy, M.; Suarez, R.; Norambuena, L.; Vargas, V.; Fuenzalida, G.; Pinilla, E.; Artal, O.; Rojas, X.; et al. Disentangling the environmental processes responsible for the world’s largest farmed fish-killing harmful algal bloom: Chile, 2016. Sci. Total Environ. 2021, 766, 144383. [Google Scholar] [CrossRef]
- Montes, R.M.; Rojas, X.; Artacho, P.; Tello, A.; Quiñones, R. Quantifying harmful algal bloom thresholds for farmed salmon in southern Chile. Harmful Algae 2018, 77, 55–65. [Google Scholar] [CrossRef]
- Molinet, C.; Lafón, A.; Lembeye, G.; Moreno, C.A. Patrones de distribución espacial y temporal de floraciones de Alexandrium catenella (Whedon & Kofoid) Balech 1985, en aguas interiores de la Patagonia noroccidental de Chile. Rev. Chil. Hist. Nat. 2003, 76, 681–698. [Google Scholar]
- Díaz, P.A.; Rosales, S.A.; Molinet, C.; Niklitschek, E.J.; Marín, A.; Varela, D.; Seguel, M.; Díaz, M.; Figueroa, R.I.; Basti, L.; et al. Are Alexandrium catenella blooms spreading offshore in Southern Chile? An in-depth analysis of the first PSP outbreak in the oceanic coast. Fishes 2024, 9, 340. [Google Scholar] [CrossRef]
- Díaz, P.A.; Álvarez, G.; Pizarro, G.; Blanco, J.; Reguera, B. Lipophilic toxins in Chile: History, producers and impacts. Mar. Drugs 2022, 20, 122. [Google Scholar] [CrossRef]
- Baldrich, A.; Pérez-Santos, I.; Álvarez, G.; Reguera, B.; Fernández-Pena, C.; Rodríguez-Villegas, C.; Araya, M.; Álvarez, F.; Barrera, F.; Karasiewicz, S.; et al. Niche differentiation of Dinophysis acuta and D. acuminata in a stratified fjord. Harmful Algae 2021, 103, 102010. [Google Scholar] [CrossRef]
- Suárez-Isla, B.A.; Barrera, F.; Carrasco, D.; Cigarra, L.; López-Rivera, A.M.; Rubilar, I.; Alcayaga, C.; Contreras, V.; Seguel, M. Comprehensive study of the occurrence and distribution of lipophilic marine toxins in shellfish from production areas in Chile. In Harmful Algae 2018—From Ecosystems to Socioecosystems, Proceedings of the 18th International Conference on Harmful Algae, Nantes, France, 21–26 October 2018; Hess, P., Ed.; International Society for the Study of Harmful Algae: Punta Arenas, Chile, 2020; pp. 163–166. ISBN 978-87-990827-7-3. [Google Scholar]
- Díaz, P.A.; Álvarez, G.; Figueroa, R.I.; Garreaud, R.; Pérez-Santos, I.; Schwerter, C.; Díaz, M.; López, L.; Pinto-Torres, M.; Krock, B. From lipophilic to hydrophilic toxin producers: Phytoplankton succession driven by an atmospheric river in western Patagonia. Mar. Pollut. Bull. 2023, 193, 115214. [Google Scholar] [CrossRef]
- Pantoja, S.; Iriarte, J.L.; Daneri, G. Oceanography of the Chilean Patagonia. Cont. Shelf. Res. 2011, 31, 149–153. [Google Scholar]
- Brand, L.E.; Campbell, L.; Bresnan, E. Karenia: The biology and ecology of a toxic genus. Harmful Algae 2012, 14, 156–178. [Google Scholar] [CrossRef] [PubMed]
- Magaña, H.A.; Villareal, T.A. The effects of environmental factors on the growth rate of Karenia brevis (Davis) G. Hansen and Moestrup. Harmful Algae 2006, 5, 192–198. [Google Scholar] [CrossRef]
- Brown, A.F.M.; Dortch, Q.; Van Dolah, F.M.; Leighfield, T.A.; Morrison, W.; Thessen, A.E.; Steidinger, K.; Richardson, B.; Moncreiff, C.A.; Pennock, J.R. Effect of salinity on the distribution, growth, and toxicity of Karenia spp. Harmful Algae 2006, 5, 199–212. Harmful Algae 2006, 5, 199–212. [Google Scholar]
- Liu, Y.; Hu, Z.; Deng, Y.; Tang, Y.Z. Evidence for production of sexual resting cysts by the toxic dinoflagellate Karenia mikimotoi in clonal cultures and marine sediments. J. Phycol. 2020, 56, 121–134. [Google Scholar] [CrossRef]
- Díaz, P.A.; Ruiz-Villarreal, M.; Velo-Suárez, L.; Ramilo, I.; Gentien, P.; Lunven, M.; Fernand, L.; Raine, R.; Reguera, B. Tidal and wind-event variability and the distribution of two groups of Pseudo-nitzschia species in an upwelling-influenced Ría. Deep Sea Res. II 2014, 101, 163–179. [Google Scholar] [CrossRef]
- Soto, I.M.; Cannizzaro, J.; Muller-Karger, F.E.; Hu, C.; Wolny, J.; Goldgof, D. Evaluation and optimization of remote sensing techniques for detection of Karenia brevis blooms on the West Florida Shelf. Remote Sens. Environ. 2015, 170, 239–254. [Google Scholar] [CrossRef]
- Torres-Palenzuela, J.M.; González-Vilas, L.; Mosquera Giménez, Á. Detection of Pseudo-nitzschia spp. Toxic Blooms Using MERIS Images on the Galician Coast; European Space Agency (Special Publication): Paris, France, 2005; pp. 1915–1919. [Google Scholar]
- Rast, M.; Bezy, J.L.; Bruzzi, S. The ESA Medium Resolution Imaging Spectrometer (MERIS): A review of the instrument and its mission. Int. J. Remote Sens. 1999, 20, 1681–1702. [Google Scholar] [CrossRef]
- ESA. Sentinel-3 OLCI Technical Guide, European Space Agency. 2019. Available online: https://sentiwiki.copernicus.eu/web/document-library#DocumentLibrary-SENTINEL-3Documents (accessed on 25 April 2023).
- Murray, S.; Hallegraeff, G. Harmful Algae Introductions: Vectors of Transfer, Mitigation, and Management. In Harmful Algal Blooms: A Compendium Desk Reference; Wiley: Hoboken, NJ, USA, 2018; pp. 493–506. [Google Scholar]
- Judice, T.J.; Widder, E.A.; Falls, W.H.; Avouris, D.M.; Cristiano, D.J.; Ortiz, J.D. Field-validated detection of Aureoumbra lagunensis brown tide blooms in the Indian River Lagoon, Florida using Sentinel-3A OLCI and ground-based hyperspectral spectroradiometers. GeoHealth 2020, 4, e2019GH000238. [Google Scholar] [CrossRef]
- Ogashawara, I. The use of Sentinel-3 Imagery to monitor cyanobacterial blooms. Environments 2019, 6, 60. [Google Scholar] [CrossRef]
- Rodríguez-Benito, C.; Navarro, C.; Caballero, I. Using Copernicus Sentinel-2 and Sentinel-3 data to monitor harmful algal blooms in Southern Chile during the COVID-19 lockdown. Mar. Pollut. Bull. 2020, 161, 111722. [Google Scholar] [CrossRef]
- Smith, M.E.; Bernard, S. Satellite ocean color based harmful algal bloom indicators for aquaculture decision support in the Southern Benguela. Front. Mar. Sci. 2020, 7, 61. [Google Scholar] [CrossRef]
- Torres-Palenzuela, J.M.T.; González-Vilas, L.; Aláez, F.M.B.; Pazos, Y. Potential application of the new sentinel satellites for monitoring of harmful algal blooms in the Galician aquaculture. Thalassas 2019, 36, 85–93. [Google Scholar] [CrossRef]
- Bedington, M.; García-García, L.M.; Sourisseau, M.; Ruiz-Villareal, M. Assessing the performance and application of operational lagrangian transport HAB forecasting systems. Front. Mar. Sci. 2022, 9, 749071. [Google Scholar] [CrossRef]
- Davidson, K.; Andersen, D.M.; Mateus, M.; Reguera, B.; Silke, J.; Sourisseau, M.; Maguire, J. Forecasting the risk of harmful algal blooms. Harmful Algae 2016, 53, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pickard, G.L. Some physical oceanographic features of inlets of Chile. J. Fish. Res. Board Can. 1971, 28, 1077–1106. [Google Scholar] [CrossRef]
- Aracena, C.; Lange, C.; Iriarte, J.L.; Rebolledo, L.; Pantoja, S. Latitudinal patterns of export production recorded in surface sediments of the Chilean Patagonian fjords (41–551S) as a response to water column productivity. Cont. Shelf. Res. 2011, 31, 340–355. [Google Scholar] [CrossRef]
- Silva, N.; Calvete, C. Características oceanográficas físicas y químicas de canales australes chilenos entre el golfo de Penas y el estrecho de Magallanes (crucero Cimar Fiordo-2). Cienc. Tecnol. Mar. 2002, 25, 23–88. [Google Scholar]
- Rodríguez-Villegas, C.; Figueroa, R.I.; Baldrich, A.; Pérez-Santos, I.; Díaz, M.; Tomasetti, S.J.; Seguel, M.; Álvarez, G.; Salgado, P.; Díaz, P.A. Small and patchy is enough: An example about how toxic HAB events can spread through low resting cyst loads. Harmful Algae 2023, 129, 102495. [Google Scholar] [CrossRef]
- Narváez, D.; Vargas, C.; Cuevas, A.; García-Loyola, S.; Lara, C.; Segura, C.; Tapia, F.; Broitman, B. Dominant scales of subtidal variability in coastal hydrography of the Northern Chilean Patagonia. J. Mar. Syst. 2019, 193, 59–73. [Google Scholar] [CrossRef]
- Pérez-Santos, I.; Seguel, R.; Schneider, W.; Linford, P.; Donoso, D.; Navarro, E.; Amaya-Cárcamo, C.; Pinilla, E.; Daneri, G. Synoptic-scale variability of surface winds and ocean response to atmospheric forcing in the eastern austral Pacific Ocean. Ocean Sci. Discuss. 2019, 15, 1247–1266. [Google Scholar] [CrossRef]
- Schlitzer, R. Data analysis and visualization with Ocean Data View. CMOS Bull. SCMO 2015, 41, 9–13. [Google Scholar]
- Lovegrove, T. An improved form of sedimentation apparatus for use with an inverted microscope. J. Cons. Int. Explor. Mer. 1960, 25, 279–284. [Google Scholar] [CrossRef]
- Utermöhl, H. Zur Vervollkomnung der quantitativen phytoplankton-Methodik. Mitt. Int. Ver. Limnol. 1958, 9, 1–38. [Google Scholar]
- De Keukelaere, L.; Sterckx, S.; Adriaensen, S.; Knaeps, E.; Reusen, I.; Giardino, C.; Bresciani, M.; Hunter, P.; Neil, C.; Van der Zande, D.; et al. Atmospheric correction of Landsat-8/OLI and Sentinel-2/MSI data using iCOR algorithm: Validation for coastal and inland waters. Eur. J. Remote Sens. 2018, 51, 525–542. [Google Scholar] [CrossRef]
- Berk, A.; Anderson, G.; Acharya, P.; Bernstein, L.; Muratov, L.; Lee, J.; Fox, M.; Adler-Golden, S.; Chetwynd, J.; Hoke, M. MODTRAN: 2006 update. In Proceedings of the SPIE—The International Society for Optical Engineering, Orlando, FL, USA, 13–17 August 2006; Volume 6233. [Google Scholar]
- Morel, A.; Antoine, D. Pigment Index Retrieval in Case 1 Waters; MERIS ESL ATBD 2.9, Doc: PO-TN-MEL-GS-0005; European Space Agency: Paris, France, 2011. [Google Scholar]
- Mishra, S.; Mishra, D.R. Normalized difference chlorophyll index: A novel model for remote estimation of chlorophyll-a concentration in turbid productive waters. Remote Sens. Environ. 2012, 117, 394–406. [Google Scholar] [CrossRef]
- Letelier, R.M.; Abbott, M.R. An analysis of chlorophyll fluorescence algorithms for the Moderate Resolution Imaging Spectrometer (MODIS). Remote Sens. Environ. 1996, 58, 215–223. [Google Scholar] [CrossRef]
- Chang, C.-C.; Lin, C.-J. LIBSVM: A Library for Support Vector Machines. ACM Trans. Intell. Syst. Technol. 2011, 2, 1–40. [Google Scholar] [CrossRef]
- Gitelson, A.A. The peak near 700 nm on radiance spectra of algae and water: Relationships of its magnitude and position with chlorophyll concentration. Int. J. Remote Sens. 1992, 13, 3367–3373. [Google Scholar] [CrossRef]
- Hu, C.; Lee, Z.; Franz, B. Chlorophyll a algorithms for oligotrophic oceans: A novel approach based on three-band reflectance. J. Geophys. Res. Ocean. 2012, 117, C01011. [Google Scholar] [CrossRef]
- Toming, K.; Kutser, T.; Laas, A.; Sepp, M.; Paavel, B.; Nõges, T. First experiences in mapping lake water quality parameters with Sentinel-3 OLCI data. Remote Sens. 2016, 8, 640. [Google Scholar] [CrossRef]
- Anderson, M.J.; Willis, T.J. Canonical analysis of principal coordinates: A useful method of constrained ordination for ecology. Ecology 2003, 84, 511–525. [Google Scholar] [CrossRef]
- Legendre, P.; Anderson, M.J. Distance-based redundancy analysis: Testing multispecies responses in multifactorial ecological experiments. Ecol. Monogr. 1999, 69, 1–24. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutational multivariate analysis of variance (PERMANOVA). In Wiley StatsRef: Statistics Reference Online; Wiley: Hoboken, NJ, USA, 2014; pp. 1–15. [Google Scholar]
- Paliy, O.; Shankar, V. Application of multivariate statistical techniques in microbial ecology. Mol. Ecol. 2016, 25, 1032–1057. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P.; Stevens, M.; Wagner, H. Package ‘Vegan’—Community Ecology Package. 2019. Available online: http://CRAN.R-project.org/package=vegan (accessed on 10 March 2025).
- Wasserstein, R.L.; Lazar, N.A. The ASA statement on p-values: Context, process, and purpose. Am. Stat. 2016, 70, 129–133. [Google Scholar] [CrossRef]
- Amrhein, V.; Greenland, S.; McShane, B. Scientists rise up against statistical significance. Nature 2019, 567, 305–307. [Google Scholar] [CrossRef]
- Baldrich, Á.M.; Molinet, C.; Reguera, B.; Espinoza-González, O.; Pizarro, G.; Rodríguez-Villegas, C.; Opazo, D.; Mejías, P.; Díaz, P.A. Interannual variability in mesoscale distribution of Dinophysis acuminata and D. acuta in Northwestern Patagonian fjords. Harmful Algae 2022, 115, 102228. [Google Scholar] [CrossRef]
- Garreaud, R. Record-breaking climate anomalies lead to severe drought and environmental disruption in western Patagonia in 2016. Clim. Res. 2018, 74, 217–229. [Google Scholar] [CrossRef]
- Díaz, P.A.; Figueroa, R.I. Toxic Algal Bloom Recurrence in the Era of Global Change: Lessons from the Chilean Patagonian Fjords. Microorganisms 2023, 11, 1874. [Google Scholar] [CrossRef]
- Baldrich, Á.; Díaz, M.; Rodríguez-Villegas, C.; Garreaud, R.; Ross, L.; Pérez-Santos, I.; Schwerter, C.; Carbonell, P.; Díaz, P. Drivers of a window of opportunity for Dinophysis acuminata in a mussel seed-bank hotspot in Northwestern Patagonia. Harmful Algae 2025, 144, 102830. [Google Scholar] [CrossRef]
- Steidinger, K.A. Historical perspective on Karenia brevis red tide research in the Gulf of Mexico. Harmful Algae 2009, 8, 549–561. [Google Scholar] [CrossRef]
- Oh, J.-W.; Pushparaj, S.S.C.; Muthu, M.; Gopal, J. Review of Harmful Algal Blooms (HABs) Causing Marine Fish Kills: Toxicity and Mitigation. Plants 2023, 12, 3936. [Google Scholar] [CrossRef]
- Tomlinson, M.C.; Wynne, T.T.; Stumpf, R.P. An evaluation of remote sensing techniques for enhanced detection of the toxic dinoflagellate, Karenia brevis. Remote Sens. Environ. 2009, 113, 598–609. [Google Scholar] [CrossRef]
- Li, X.; Yan, T.; Yu, R.; Zhou, M. A review of Karenia mikimotoi: Bloom events, physiology, toxicity and toxic mechanism. Harmful Algae 2019, 90, 101702. [Google Scholar] [CrossRef]
- Mitra, A.; Flynn, K.J.; Tillmann, U.; Raven, J.A.; Caron, D.; Stoecker, D.K.; Not, F.; Hansen, P.J.; Hallegraeff, G.; Sanders, R. Defining planktonic protist functional groups on mechanisms for energy and nutrient acquisition: Incorporation of diverse mixotrophic strategies. Protist 2016, 167, 106–120. [Google Scholar] [CrossRef]
- Lara, C.; Saldías, G.A.; Westberry, T.K.; Beherenfeld, M.J.; Broitman, B.R. First assessment of MODIS satellite ocean color products (OC3 and nFLH) in the Inner Sea of Chiloé, northern Patagonia. Lat. Am. J. Aquat. Res. 2017, 45, 822–827. [Google Scholar] [CrossRef]
- Silva, S.; Palma, S. Progress in the Oceanographic Knowledge of Chilean Interior Water from Puerto Montt to Cape Horn; Comité Oceanográfico Nacional—Pontificia Universidad Católica de Valparaíso: Valparaíso, Chile, 2006; p. 176. [Google Scholar]
- Hu, C.; Muller-Karger, F.E.; Taylor, C.J.; Carder, K.L.; Kelble, C.; Johns, E.; Heil, C.A. Red tide detection and tracing using MODIS fluorescence data: A regional example in SW Florida coastal waters. Remote Sens. Environ. 2005, 97, 311–321. [Google Scholar] [CrossRef]
- ESA. Sentinel Online User Guide Sentinel-3 OLCI Radiometric Resolution—21 Bands in VIS/SWIR. 2021. Available online: https://sentiwiki.copernicus.eu/web/document-library#DocumentLibrary-SENTINEL-3Documents (accessed on 10 January 2025).
- Ackerman, S.A.; Strabala, K.I.; Menzel, W.P.; Frey, R.A.; Moeller, C.C.; Gumley, L.E. Discriminating clear sky from clouds with MODIS. J. Geophys. Res. Atmos. 1998, 103, 32141–32157. [Google Scholar] [CrossRef]
- NASA. MODIS Moderate Resolution Imaging Spectroradiometer Specifications. 2021. Available online: https://modis.gsfc.nasa.gov/about/specifications.php (accessed on 12 March 2024).
- NASA. Envisat MEdium Resolution Imaging Spectrometer (MERIS). 2021. Available online: https://ladsweb.modaps.eosdis.nasa.gov/missions-and-measurements/meris/ (accessed on 12 March 2024).
- Morel, A.; Gentili, B.; Claustre, H.; Babin, M.; Bricaud, A.; Ras, J.; Tièche, F. Optical properties of the “clearest” natural waters. Limnol. Oceanogr. 2007, 52, 217–229. [Google Scholar] [CrossRef]
- Morel, A.; Huot, Y.; Gentili, B.; Werdell, P.J.; Hooker, S.B.; Franz, B.A. Examining the consistency of products derived from various ocean color sensors in open ocean (Case 1) waters in the perspective of a multi-sensor approach. Remote Sens. Environ. 2007, 111, 69–88. [Google Scholar] [CrossRef]

| Station | Bloom | Computed RBD | Predicted Bloom | Coincidence |
|---|---|---|---|---|
| 1 | 0 | 1.088 | 1 | 0 |
| 2 | 0 | 0.925 | 1 | 0 |
| 3 | 0 | 0.552 | 1 | 0 |
| 4 | 1 | 0.173 | 1 | 1 |
| 5 | 1 | −0.698 | 0 | 0 |
| 6 | 0 | 0.015 | 0 | 1 |
| 7 | 1 | 1.328 | 1 | 1 |
| 8 | 0 | 0.691 | 1 | 0 |
| 9 | 1 | 0.742 | 1 | 1 |
| 10 | 0 | 0.515 | 1 | 0 |
| 11 | 0 | −0.111 | 0 | 1 |
| 12 | 0 | −1.306 | 0 | 1 |
| Total matches | 6 |
| Station | Bloom | Computed V | Predicted Bloom | Coincidence |
|---|---|---|---|---|
| 1 | 0 | −1.000 | 0 | 1 |
| 2 | 0 | −1.220 | 0 | 1 |
| 3 | 0 | −1.001 | 0 | 1 |
| 4 | 1 | 1.001 | 1 | 1 |
| 5 | 1 | −3.522 | 0 | 0 |
| 6 | 0 | −2.029 | 0 | 1 |
| 7 | 1 | 0.079 | 1 | 1 |
| 8 | 0 | −1.092 | 0 | 1 |
| 9 | 1 | −2.160 | 0 | 0 |
| 10 | 0 | −1.000 | 0 | 1 |
| 11 | 0 | −0.777 | 0 | 1 |
| 12 | 0 | −2.734 | 0 | 1 |
| Total matches | 10 |
| Predictive Variables | DF | Sum of Squares | R2 | Pseudo—F | Pr > F |
|---|---|---|---|---|---|
| Temperature | 1 | 0.625 | 0.039 | 1.418 | 0.056 |
| Salinity | 1 | 0.638 | 0.040 | 1.448 | 0.049 |
| Oxygen | 1 | 0.617 | 0.038 | 1.401 | 0.069 |
| Fluorescence | 1 | 0.255 | 0.016 | 0.579 | 0.984 |
| Residuals | 31 | 13.54 | 0.862 | ||
| Total | 35 | 15.84 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, P.A.; Gormaz, R.; Aguayo, P.; Pérez-Santos, I.; Saldías, G.S.; Figueroa, R.I.; Fernández, P.A.; Álvarez, G.; Rodríguez-Villegas, C.; Schwerter, C.; et al. Unveiling the 2017 Karenia Bloom in NW Chilean Patagonia by Integrating Remote Sensing and Field Data. Microorganisms 2025, 13, 2440. https://doi.org/10.3390/microorganisms13112440
Díaz PA, Gormaz R, Aguayo P, Pérez-Santos I, Saldías GS, Figueroa RI, Fernández PA, Álvarez G, Rodríguez-Villegas C, Schwerter C, et al. Unveiling the 2017 Karenia Bloom in NW Chilean Patagonia by Integrating Remote Sensing and Field Data. Microorganisms. 2025; 13(11):2440. https://doi.org/10.3390/microorganisms13112440
Chicago/Turabian StyleDíaz, Patricio A., Raúl Gormaz, Paula Aguayo, Iván Pérez-Santos, Gonzalo S. Saldías, Rosa I. Figueroa, Pamela A. Fernández, Gonzalo Álvarez, Camilo Rodríguez-Villegas, Camila Schwerter, and et al. 2025. "Unveiling the 2017 Karenia Bloom in NW Chilean Patagonia by Integrating Remote Sensing and Field Data" Microorganisms 13, no. 11: 2440. https://doi.org/10.3390/microorganisms13112440
APA StyleDíaz, P. A., Gormaz, R., Aguayo, P., Pérez-Santos, I., Saldías, G. S., Figueroa, R. I., Fernández, P. A., Álvarez, G., Rodríguez-Villegas, C., Schwerter, C., Cassis, D., Vera, R., & Conca, C. (2025). Unveiling the 2017 Karenia Bloom in NW Chilean Patagonia by Integrating Remote Sensing and Field Data. Microorganisms, 13(11), 2440. https://doi.org/10.3390/microorganisms13112440











