Abstract
Alström (ALMS) and Bardet-Biedl syndromes (BBS) are rare ciliopathies characterized by obesity and hyperglycemia that lead to type 2 diabetes, but also other disorders, including neurodegeneration. However, isolated clinical manifestations can be observed in carriers of heterozygous mutations in the ALMS1 and BBS genes. Recently, the influence of oral bacteria on the presence of obesity, type 2 diabetes, and neurodegenerative processes has been widely discussed. The purpose of the research project was to analyze the profile of the microbiome in the oral cavity by sequencing the 16S rRNA gene in ALMS/BBS patients and carriers of causative variants in these genes. Oral mucosal swabs were taken from 8 ALMS/BBS patients, 24 family members, 20 obese patients, and 29 healthy individuals. Streptococcus (30.7%), Haemophilus (18.9%), and Prevotella (11%) were the most common bacteria in the study group. Comparison between groups showed a higher abundance of Prevotella, Enterococcus, Eikenella, Capnocytophaga, Parvimonas, Selenomonas, and Corynobacterium, and a lower abundance of Lactobacillus in the study group compared to other groups. The specific profile of the oral microbiome found in patients with variants in the ALMS1 and BBS genes may enable the identification of the modulatory role of the oral microbiome in these disorders and point to new directions for additional therapy for these patients and heterozygous family members in the future.
1. Introduction
Alström syndrome (ALMS) and Bardet-Biedl syndrome (BBS) represent rare diseases inherited in an autosomal recessive manner classified as ciliopathies, which underlie their common pathological mechanism and make them monogenic diabetic syndromes [1,2]. The estimated prevalence of BBS is approximately 1:160,000 in northern European populations, while ALMS is even rarer, occurring in 1:500,000 individuals [3,4,5]. In both syndromes, obesity appears as the first clinical symptom, which, along with the progression of insulin resistance and metabolic disorders, leads to the development of type 2 diabetes in most patients [6,7,8]. Another common early symptom for patients with both ALMS and BBS is visual impairment, ultimately defined as progressive retinal degeneration [9].
In addition, patients with ALMS and BBS may have other ophthalmologic, cognitive, and neurologic disorders of neurodegenerative origin, but also renal, pulmonary, and cardiac abnormalities, as well as bone defects, liver dysfunction, low stature and endocrine disorders [9,10,11,12,13]. Specifically, ALMS patients often exhibit short stature, skin changes such as acanthosis nigricans, hypertension, atherosclerosis, cataracts, cardiomyopathy in approximately 60% of patients, hearing impairment and deafness, bronchial asthma and pulmonary symptoms (50%), chronic hepatitis with fatty liver (50%), chronic renal failure, gynecomastia, alopecia, menstrual disorders and urological disorders in women, hypergonadotropic hypogonadism in men, diabetes insipidus, and neurological disorders [9,10,14].
The symptoms in patients with BBS are very similar. However, this syndrome also involves abnormalities in the long bones of the limbs, mainly in the form of polydactyly, brachydactyly, and syndactyly. Patients with BBS often suffer from cognitive impairment and/or intellectual disability [11,12,15]. Clinical observations suggest that isolated clinical manifestations may also occur in carriers of heterozygous mutations in the ALMS1 and BBS genes, i.e., in parents and siblings of patients with ALMS and BBS [2,16,17,18,19,20].
Although the genetic causes behind both syndromes have been extensively studied, evidence suggests that environmental factors may also influence the clinical manifestations and courses of the disease in individual patients. This may determine the diversity of clinical presentations among patients and different phenotypes present in members of the same families and in individuals who are carriers of the same causative genetic variant [21,22].
Among the potential environmental factors modulating the natural course of various chronic diseases is the contribution of oral bacteria, which has received considerable attention in recent years [23,24,25]. These articles described the importance of the protective tissue barrier in the oral cavity, as its disruption allows pathogenic microorganisms to migrate and influence the development of further disorders, including neurodegeneration [26,27]. Moreover, numerous findings highlight the combined influence of genetic and environmental factors, particularly specific bacterial species affecting the expression of specific genes, especially in conditions where periodontal disease is a contributing factor [24,28,29]. Finally, understanding the composition of the microbiome in patients with obesity and type 2 diabetes may translate into more effective treatment of these diseases [30,31].
Given the imprecise classification of some bacteria species in the microbiological studies used to date, it is reasonable to apply new methods to detect the microbial genome in the search for additional modulating mechanisms in patients with defined monogenic diabetes syndromes.
The goal of this study was to identify the microbial genome in buccal mucosal swabs from patients with Alström and Bardet-Biedl syndromes and their families, who are carriers of heterozygous mutations in the ALMS1 and BBS genes, in comparison to obese patients (comparison group) and healthy controls.
2. Patients and Methods
2.1. Characteristics of Patients and Examination
The study group included 8 participants with genetically confirmed ALMS (2) or BBS (6) syndromes from 8 families, as previously described [21,22]. All these participants were overweight or obese. Additionally, the group included 24 heterozygous carriers of causative variants in the ALMS1 and BBS genes from the same families (11 and 13 carriers, respectively), while the comparison group comprised 20 patients with simple obesity matched for BMI (Body Mass Index) (p = 0.989). The control group comprised 29 healthy individuals (BMI < 25 kg/m2 and without hyperglycemia, type 2 diabetes, retinal disorders, cardiomyopathy, renal dysfunction, or cirrhosis) matched to the other groups for age (p = 0.997) and gender (p = 0.507). Of the 24 heterozygous carriers of the causative variants, 14 individuals were overweight/obese and/or had type 2 diabetes. The exclusion criteria were the use of oral antibiotics and hormonal contraceptives within the last 2 months, as well as pregnancy.
Table 1 presents a detailed characterization of study participants.

Table 1.
Characteristics of the studied groups.
All studied individuals underwent a precise dental examination of the oral cavity performed by two experienced dentists. The Oral Hygiene Index (OHI), according to Greene and Vermillion, which assesses the state of oral hygiene, was recorded. The OHI is the sum of the plaque and calculus index. The plaque score ranged from 0 (no plaque), to 1 (soft plaque or supragingival calculus that covers a minimum of 1/3 of the tooth surface), 2 (soft plaque or supragingival calculus that covers 1/3 to 2/3 of the tooth surface or isolated subgingival calculus around the tooth neck), and 3 (soft plaque or supragingival calculus coating more than 2/3 of the tooth surface or dense accumulation of subgingival calculus encircling the tooth neck) [32]. No participant in the study, reference, or control groups was diagnosed with periodontal disease.
To assess clinical gingival inflammation, all subjects were evaluated using the Löe and Silness gingival index (GI) and the bleeding on probing score (BOP%) [33]. The values did not exceed 0.2 and 10%, respectively, in any group. Moreover, there were no significant differences in GI (p = 0.407) and BOP% (p = 0.465) scores among the groups.
Next, non-invasive sampling of the buccal mucosa and buccal gingival margin in the region of the first lower permanent molar was performed in participants from the study, comparison, and control groups. We used a method of sampling the buccal mucosa and gingival area that, according to the literature, is optimal and recommended for assessing the microbiome, especially in the absence of periodontitis [34,35]. All study participants were asked to refrain from eating, smoking, and oral hygiene activities (brushing, flossing, and using mouthwash) for 12 h prior to sample collection, and from drinking for 1 h prior to the test.
The University Bioethics Committee at the Medical University in Lodz, Poland (RNN/216/23/KE) reviewed and approved the research protocol on 10 October 2023. Written informed consent for participation in the project was collected from patients and/or their parents. Collected samples intended for molecular analysis were transferred into sterile screw-cap vials and stored at −20 °C until further processing.
2.2. Molecular Analysis—DNA Isolation
Collected buccal swabs were first frozen. Bacterial DNA was then isolated from those samples using the Maxwell® RSC Pathogen Total Nucleic Acid Kit catalog number: AS1890 (Promega, Madison, WI, USA). The isolated DNA was suspended in TE buffer and stored at −20 °C until PCR amplification. DNA concentration and purity were determined spectrophotometrically using a NanoPhotometer® C40 (Implen, München, Germany).
2.3. Library Preparation and Sequencing
To assess the microbial community profiles, the 16S rRNA gene was sequenced. Initially, the V3/V4 variable regions of the genome were amplified following the protocol advised by Illumina (San Diego, CA, USA). PCR oligonucleotides with overhanging adapters compatible with Illumina Nextera indexes and sequencing adapters for paired-end sequencing were utilized (forward, TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG; reverse, GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC). The amplification of the gene fragments, averaging 464 base pairs in length, was performed using Kapa HiFi polymerase (Roche, Mannheim, Germany). The specificity of the PCR products was verified using agarose gel electrophoresis, followed by purification with AMPure XP magnetic beads (Beckman Coulter, CA, USA). Indexing reactions were also conducted using Kapa HiFi polymerase (Roche, Mannheim, Germany) with the Nextera XT dual-index set (Illumina, San Diego, CA, USA). Library concentrations were measured with the Qubit 2.0 system (Thermo Fisher Scientific, Waltham, MA, USA), and the libraries were pooled at equal concentrations. The prepared library was then sequenced on the MiSeq device (Illumina, San Diego, CA, USA) using the MiSeq Reagent Kit v3, 2x300 cycles.
2.4. NGS Data Processing
Sequencing data from the MiSeq platform were processed using a local instance of the Galaxy platform 20.01 [36]. The FASTQ Groomer tool was used to convert files to Sanger FASTQ encoding. Paired-end reads were then combined using the FLASH tool 1.2.11.4 [37], and the Trimmomatic 0.38.0 algorithm was applied to separate adapters and filter out low-quality reads (those below a quality score of Q20 and length of 220 bp) [38]. Operational Taxonomic Units (OTUs) were allocated using the Kraken 2 v.1.3.1 [39] algorithm with the Standard database, with filtering based on a classification confidence score threshold of 0.05.
Data were filtered to remove poor quality and non-informative data using a low count filter (minimum count of 4) and a low variance filter (inter-quartile range 10%). Data were then normalized using the total sum scaling (TSS) method. The OTU read counts for each taxonomic level were extracted and organized into tables. The relative abundance of each recognized bacterial taxon was then determined from these tables.
2.5. Data Analysis—Alpha and Beta Diversity
Microbiome composition diversity in the samples was assessed at the genus level using the MicrobiomeAnalyst 2.0 (update from 2 July 2024) [40]. To determine alpha diversity and statistical significance, Shannon algorithms, along with the ANOVA test, were employed. For beta diversity analysis, non-metric multidimensional scaling (NMDS) was used to visualize the data, with statistical significance assessed using the ANOSIM test.
2.6. Visualization of Data and Statistical Analysis
The normality distribution of the parameters was verified with the Shapiro–Wilk test. For comparisons of the BMI and OHI values, the Mann–Whitney U test was used. Gender distribution was compared using the chi-square test, and patients’ ages were compared using ANOVA. Correlations between parameters were calculated using Pearson’s correlation test. We conducted hierarchical clustering and heatmap analysis of the molecular data using Ward’s clustering algorithm and the Euclidean distance method. Differences in raw abundances between groups were visualized using stacked bar plots. The overall changes in the relative abundance of genera in single-factor analysis were assessed using the EdgeR method. Statistical significance was defined as a false discovery rate (FDR) below 0.05. The relevance or effect size of different genus abundance between groups was evaluated using the LEfSe (Linear discriminant analysis Effect Size) algorithm, with statistical significance defined as an FDR-adjusted p-value below 0.1.
3. Results
In the initial comparison, no statistically significant differences were observed between homozygous and heterozygous carriers of ALMS1 and BBS gene variants, both in relation to the OHI (p = 0.323) and alpha diversity (p = 0.424). This justified combining these patients into a single study group to increase the statistical power of the study, given the rarity of these genetic syndromes.
3.1. Oral Hygiene Index (OHI) Values
The Oral Hygiene Index (OHI) values differed between the groups. The highest OHI was observed in the study group (Me 1.25; IQR 0.62), which was significantly higher than in both the simple obesity group (Me 0.54; IQR 0.67; p < 0.001) and the healthy control group (Me 0.42; IQR 0.5; p < 0.00001). No significant difference in OHI values was found between the obese and healthy control groups (p = 0.490) (Figure 1).
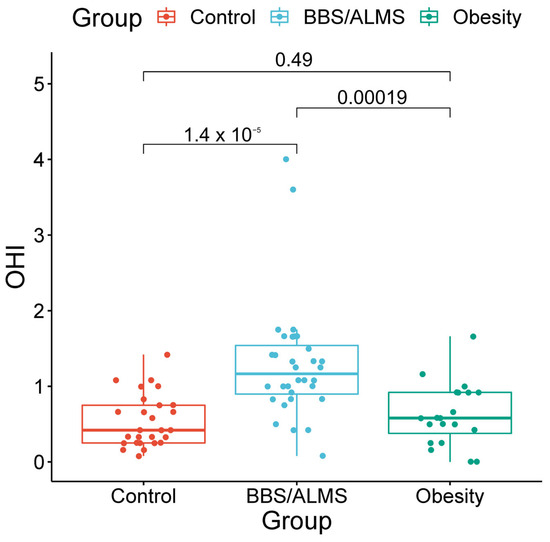
Figure 1.
Box plots of the Oral Hygiene Index (OHI) with marked p-values for the t-test.
In both the study group and the comparison and control groups, there was no significant correlation between the OHI and patient age (p respectively: 0.327; 0.229 and 0.190).
3.2. Oral Microbiome RNA Sequencing
Following the 16S rRNA gene sequencing, a total of 6,721,299 reads classified as bacteria were obtained. Each sample had an average of 83,124 reads, and, on average, 10.7% of the reads could not be assigned to any bacterial genus. Next-generation sequencing (NGS) identified Streptococcus (30.7%), Haemophilus (18.9%) and Prevotella (11%) as the most prevalent genera in the study group. In the obesity group, Streptococcus was the most common, accounting for 33.3%, followed by Haemophilus (18%) and Veillonella (8.3%). The control group was dominated by Streptococcus (33.1%), Haemophilus (14.1%), and Veillonella (8.7%). Figure 2 presents a detailed distinction of the types of bacteria found in all the studied groups. An overall analysis of the core microbiome is shown in Figure 3.
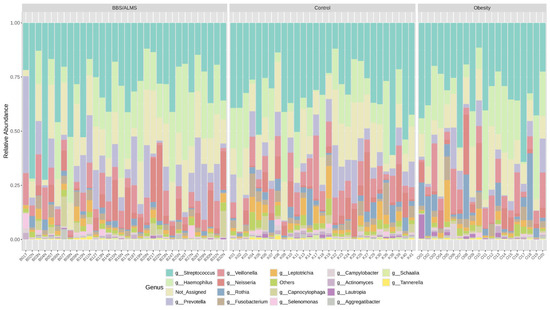
Figure 2.
Taxonomic composition of the bacterial community at the genus level in the studied groups.
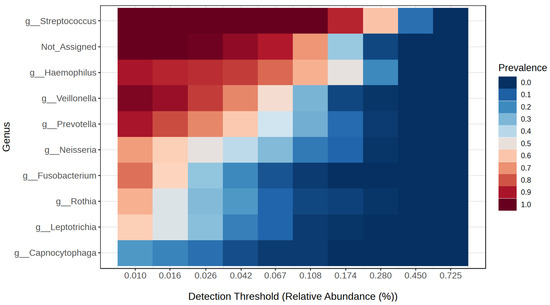
Figure 3.
Core microbiome genera of bacteria in the studied groups.
3.3. Diversity and Discriminant Analyses
Analysis of alpha diversity using Shannon’s method indicated no statistically significant differences in diversity between the studied groups. However, there was a trend suggesting that the control group had the highest average alpha diversity compared to the other two studied groups, p = 0.133 (Figure 4). However, beta diversity, an analysis that indicates differences in the composition of bacterial genera, suggests that the BBS/ALMS group stands out from the other studied groups, p < 0.031 (Figure 5).
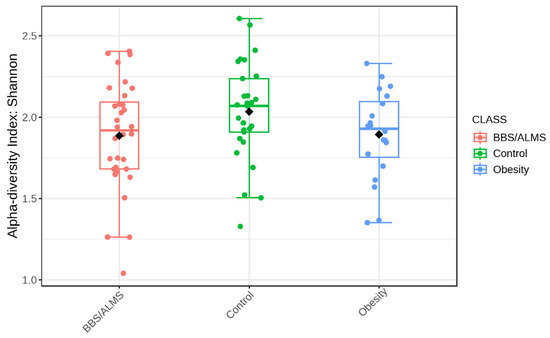
Figure 4.
Box plot illustrating the overall measure of alpha-diversity across the studied groups based on the Shannon method at the bacterial genus level. The black dot indicates the average value. Statistical significance was evaluated by ANOVA, with an F-value of 2.0673 and a p-value of 0.1334.
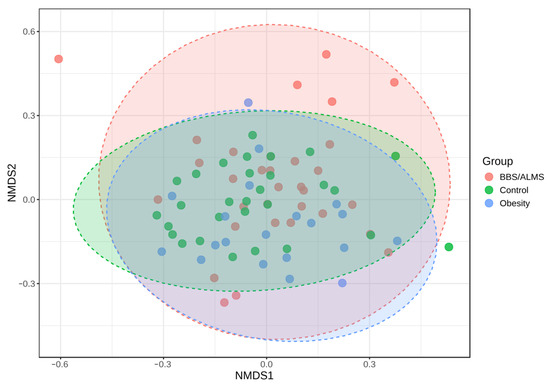
Figure 5.
Non-metric multidimensional scaling (NMDS) analysis diagram based on the Bray-Curtis distance. Statistical significance was evaluated by ANOSIM, with a p-value < 0.031; [NMDS] Stress = 0.2384.
Interestingly, the LEfSe analysis identified the seven most differentiating bacterial genera, including the three main ones: Rothia, Shaalia and Lactobacillus, for which the Linear Discriminant Analysis (LDA) score was above 3 (p < 0.015) (Figure 6).
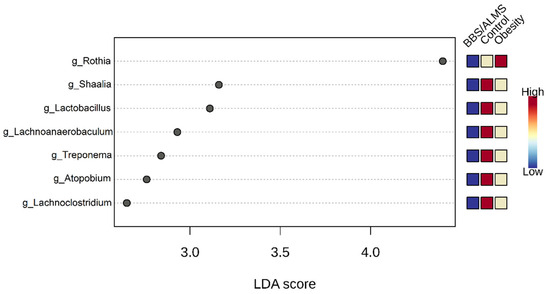
Figure 6.
Linear discriminant analysis Effect Size (LEfSe) analysis of taxonomic biomarkers of oral microbiota. The analysis determined the bacterial genera with the most differential abundance. The top seven genera are shown with p-value < 0.015. Statistical values were evaluated by the Kruskal-Wallis rank sum test.
3.4. Univariate Analysis
Univariate analysis showed that eight bacterial genera were significantly different in the study group compared to both the obese and healthy groups (Figure 7). The genera Prevotella and Enterococcus were considerably more abundant in the BBS/ALMS group than in the group with obesity (p = 0.00006 and 0.00007, respectively) and the control group (p = 0.0034 and 0.0088, respectively) (Figure 7A,D). In contrast, Eikenella, Capnocytophaga, Parvimonas, and Selenomonas were more abundant in the study group than in the obese group (p = 0.000006, 0.00004, 0.0044, and 0.000002, respectively) but less abundant than in the healthy group (p = 0.00001, 0.0001, 0.00016, and 0.0000015, respectively) (Figure 7B,C,F,G). Furthermore, Corynobacterium was more abundant in the BBS/ALMS group than in the healthy group (p = 0.00824), but less abundant than in the obese group (p = 0.00031) (Figure 7H). Lactobacillus abundance was lowest in the study group compared to both other groups (p = 0.00087 and 0.0000003, respectively) (Figure 7E).
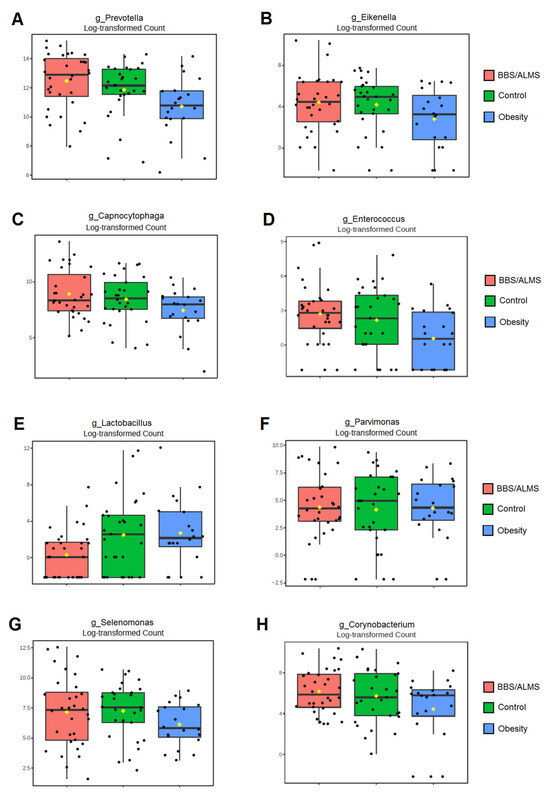
Figure 7.
Box plots of univariate analysis for: (A)—Prevotella; (B)—Eikenella; (C)—Capnocytophaga; (D)—Enterococcus; (E)—Lactobacillus; (F)—Parvimonas; (G)—Selenomonas; (H)—Corynobacterium; horizontal line inside each box represents the median; yellow dot indicates the mean; black dots represent individual data points.
4. Discussion
This study represents the first evaluation of the oral microbiome in patients with Alström and Bardet-Biedl syndromes and heterozygous carriers of causative variants in the ALMS1 and BBS genes. Importantly, although none of the subjects had periodontitis, patients in the study group had the highest OHI scores, indicating inadequate oral hygiene. Due to neurodevelopmental and intellectual disorders, our patients have and, unfortunately, will continue to have poorer hygiene, as described in many genetic syndromes, including ciliopathies [41,42,43,44,45]. Recently, higher OHI values were described in patients with poor metabolic control of type 2 diabetes and in patients with type 2 diabetes and obesity [46,47].
In the study group, the most prevalent oral bacteria were Streptococcus, Haemophilus and Prevotella. An increased abundance of the genus Prevotella has previously been reported in studies investigating changes in the oral microbiome of patients with type 2 diabetes [48] and overweight or obese individuals [49]. While no statistically significant distinctions in alpha diversity were found between groups, beta diversity analysis showed differences in the composition of bacterial types between groups. Almeida-Santos et al. reported no differences in alpha and beta diversity between healthy individuals and patients with type 2 diabetes [50].
However, further analysis identified eight bacterial genera that differentiate patients carrying at least 1 causative variant in the ALMS and BBS genes from the comparison and control groups. The higher abundances of Eikenella, Capnocytophaga and Selenomonas were reported in the BBS/ALMS group compared to patients with simple obesity, which may correspond with the reported increase in these bacteria in patients with diabetes [51,52,53]. A diversity of bacterial types has already been observed in patients with type 2 diabetes compared to normoglycemic individuals [54,55].
The relationships between diabetes and oral microbiota are complex. Periodontal disease is known to be influenced by type 2 diabetes [56,57,58]. On the other hand, it may worsen glycemic control [57]. Moreover, high levels of certain bacteria, such as Porphyromonas, can exacerbate insulin resistance [58]. Alterations in the oral microbiome have also been found to affect metabolic control of diabetes as measured by HbA1c levels in patients with type 1 diabetes [59,60].
Goodson et al. associated an increased presence of Selenomonas sp. and Prevotella sp. with being overweight, while our study reported an even higher abundance of Selenomonas and Prevotella genera in the ALMS/BBS group compared to participants with simple obesity [49]. Other studies have linked the genera Veillonella, Oribacterium, and Soonwooa [61] as well as Firmicutes and Actinobacteria [62] with obesity. Changes in oral microbiome composition appear to be associated with obesity development in multiple mechanisms [63], as well as different taste perceptions [64,65]. Other systemic mechanisms may involve changes in the composition of gut bacteria. This may lead to changes in adipose tissue function, inflammation along the oral–blood axis, and feeding behavior via the gut–brain axis [63,66].
Patients with ALMS and BBS may also present neurologic disorders of neurodegenerative origin [9,10,11,12,13]. Changes in gut and oral microbiome were reported to have a significant role in the development of psychiatric and neurodegenerative disorders [26,27,67]. The changes in the oral microbiome may lead to periodontitis, a known severe risk factor for Alzheimer’s disease. Alzheimer’s-related brain immunity and neuroinflammation are thought to be caused by prolonged exposure to various microorganisms and/or their toxins, rather than by any specific bacteria [67].
Additionally, patients with ALMS and BBS were observed to have dental anomalies such as abnormalities in tooth number, shape, and size [68,69,70,71]. Patients with BBS are also more prone to oral infections, such as periodontal disease and dental caries, and they may experience drug-induced gingival hyperplasia [68,70]. Meanwhile, gingivitis and discolored enamel were reported in ALMS patients [72].
Moreover, in our study, the abundance of Lactobacillus genera was the lowest in the study group. Interestingly, the association between dysbiosis and periodontal disease has already been found in patients with diabetes [24,29]. Regarding specific beneficial genera, the therapeutic use of Lactobacillus sp. in periodontitis associated with type 2 diabetes is established, operating through various mechanisms [73]. Furthermore, Lactobacillus sp. may also be helpful in reducing body weight in overweight and obese patients [74].
The relatively small sample size represents a limitation of our study, reflecting the rarity of ALMS and BBS [9,12]. Consequently, the data should be considered preliminary. Furthermore, the molecular analysis of ALMS1 and BBS genes was not performed in the comparison and control groups. However, clinical examination and family histories of overweight, obesity, type 2 diabetes, and additional disorders (no ophthalmic, hepatic, or renal abnormalities, or cardiomyopathy) conducted by an experienced clinical geneticist did not indicate suspected monogenic diabetes syndromes in these individuals.
In conclusion, evaluating the oral microbiome of patients with Alström and Bardet-Biedl syndromes and heterozygous carriers of causative variants in the ALMS1 and BBS genes compared to patients with simple obesity and healthy subjects revealed a distinctive oral bacterial profile in ALMS/BBS patients. Further research is needed to link the oral bacterial profile with the prevalence and severity of clinical symptoms in both ALMS/BBS patients and symptomatic members of their families carrying the genetic defect. Such studies will enable future evaluation of the modulating role of oral microflora in these disorders and may inform therapeutic strategies for these patients and their heterozygous family members.
Author Contributions
Conceptualization, E.Z.-P.; investigation, E.Z.-P., J.G.-A., A.P.-U. and A.Z.; formal analysis, T.P. and S.S.; data curation, M.Ł.-S.; writing—original draft preparation, E.Z.-P.; writing—review and editing, A.Z.; supervision, A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of Medical University in Lodz, Poland (RNN/216/23/KE) on 10 October 2023.
Informed Consent Statement
Informed consent was obtained from all patients and/or their parents involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank all patients and their family members for participating in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ALMS | Alström syndrome |
| ANOVA | Analysis of Variance |
| BBS | Bardet-Biedl syndrome |
| BMI | Body Mass Index |
| DNA | deoxyribonucleic acid |
| FDR | false discovery rate |
| F | female |
| HbA1c | glycated hemoglobin |
| IQR | interquartile range |
| LEfSe | Linear discriminant analysis Effect Size |
| LDA | Linear Discriminant Analysis |
| M | male |
| Me | median |
| NGS | Next-generation sequencing |
| NMDS | Non-metric multidimensional scaling |
| OHI | Oral Hygiene Index |
| OTUs | Operational Taxonomic Units |
| PCR | Polymerase Chain Reaction |
| rRNA | ribosomal ribonucleic acid |
| SD | standard deviation |
References
- Álvarez-Satta, M.; Castro-Sánchez, S.; Valverde, D. Bardet-Biedl Syndrome as a Chaperonopathy: Dissecting the Major Role of Chaperonin-Like BBS Proteins (BBS6-BBS10-BBS12). Front. Mol. Biosci. 2017, 4, 55. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.D.; Muller, J.; Collin, G.B.; Milan, G.; Kingsmore, S.F.; Dinwiddie, D.; Farrow, E.G.; Miller, N.A.; Favaretto, F.; Maffei, P.; et al. Alström Syndrome: Mutation Spectrum of ALMS1. Hum. Mutat. 2015, 36, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, E.; Beales, P.L. Bardet-Biedl syndrome. Eur. J. Hum. Genet. 2013, 21, 8–13. [Google Scholar] [CrossRef]
- Girard, D.; Petrovsky, N. Alström syndrome: Insights into the pathogenesis of metabolic disorders. Nat. Rev. Endocrinol. 2011, 7, 77–88. [Google Scholar] [CrossRef]
- Farmer, A.; Aymé, S.; de Heredia, M.L.; Maffei, P.; McCafferty, S.; Młynarski, W.; Nunes, V.; Parkinson, K.; Paquis-Flucklinger, V.; Rohayem, J.; et al. EURO-WABB: An EU rare diseases registry for Wolfram syndrome, Alström syndrome and Bardet-Biedl syndrome. BMC Pediatr. 2013, 13, 130. [Google Scholar] [CrossRef]
- Milani, D.; Cerutti, M.; Pezzani, L.; Maffei, P.; Milan, G.; Esposito, S. Syndromic obesity: Clinical implications of a correct diagnosis. Ital. J. Pediatr. 2014, 40, 33. [Google Scholar] [CrossRef]
- Mokashi, A.; Cummings, E.A. Presentation and course of diabetes in children and adolescents with Alstrom syndrome. Pediatr. Diabetes 2011, 12, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Jeziorny, K.; Zmyslowska-Polakowska, E.; Wyka, K.; Pyziak-Skupień, A.; Borowiec, M.; Szadkowska, A.; Zmysłowska, A. Identification of bone metabolism disorders in patients with Alström and Bardet-Biedl syndromes based on markers of bone turnover and mandibular atrophy. Bone Rep. 2022, 17, 101600. [Google Scholar] [CrossRef]
- Marshall, J.D.; Beck, S.; Maffei, P.; Naggert, J.K. Alström syndrome. Eur. J. Hum. Genet. 2007, 15, 1193–1202. [Google Scholar] [CrossRef]
- Marshall, J.D.; Bronson, R.T.; Collin, G.B.; Nordstrom, A.D.; Maffei, P.; Paisey, R.B.; Carey, C.; Macdermott, S.; Russell-Eggitt, I.; Shea, S.E.; et al. New Alström Syndrome Phenotypes Based on the Evaluation of 182 Cases. Arch. Intern. Med. 2005, 165, 675. [Google Scholar] [CrossRef]
- Castro-Sánchez, S.; Álvarez-Satta, M.; Cortón, M.; Guillén, E.; Ayuso, C.; Valverde, D. Exploring genotype-phenotype relationships in Bardet-Biedl syndrome families. J. Med. Genet. 2015, 52, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, S.; Hunt, K.F.; Cheah, Y.S.; Forsythe, E.; Hazlehurst, J.M.; Sparks, K.; Mohammed, S.; Tomlinson, J.W.; Amiel, S.A.; Carroll, P.V.; et al. The endocrine and metabolic characteristics of a large Bardet-Biedl Syndrome clinic population. J. Clin. Endocrinol. Metab. 2018, 103, 1834–1841. [Google Scholar] [CrossRef] [PubMed]
- Waszczykowska, A.; Jeziorny, K.; Barańska, D.; Matera, K.; Pyziak-Skupien, A.; Ciborowski, M.; Zmysłowska, A. Searching for Effective Methods of Diagnosing Nervous System Lesions in Patients with Alström and Bardet-Biedl Syndromes. Genes 2023, 14, 1784. [Google Scholar] [CrossRef] [PubMed]
- Citton, V.; Favaro, A.; Bettini, V.; Gabrieli, J.; Milan, G.; Greggio, N.A.; Marshall, J.D.; Naggert, J.K.; Manara, R.; Maffei, P. Brain involvement in Alström syndrome. Orphanet J. Rare Dis. 2013, 8, 24. [Google Scholar] [CrossRef]
- Priya, S.; Nampoothiri, S.; Sen, P.; Sripriya, S. Bardet-Biedl syndrome: Genetics, molecular pathophysiology, and disease management. Indian J. Ophthalmol. 2016, 64, 620–627. [Google Scholar] [CrossRef]
- Marshall, J.D.; Maffei, P.; Collin, G.B.; Naggert, J.K. Alström syndrome: Genetics and clinical overview. Curr. Genom. 2011, 12, 225–235. [Google Scholar] [CrossRef]
- Croft, J.B.; Morrell, D.; Chase, C.L.; Swift, M. Obesity in heterozygous carriers of the gene for the Bardet-Biedl syndrome. Am. J. Med. Genet. 1995, 55, 12–15. [Google Scholar] [CrossRef]
- Beales, P.L.; Reid, H.A.; Griffiths, M.H.; Maher, E.R.; Flinter, F.A.; Woolf, A.S. Renal cancer and malformations in relatives of patients with Bardet-Biedl syndrome. Nephrol. Dial. Transplant. 2000, 15, 1977–1985. [Google Scholar] [CrossRef]
- Li, M.H.; Chen, I.C.; Yang, H.W.; Yen, H.C.; Huang, Y.C.; Hsu, C.C.; Chen, Y.M.; Ke, Y.Y. The characterization and comorbidities of heterozygous Bardet-Biedl syndrome carriers. Int. J. Med. Sci. 2024, 21, 784–794. [Google Scholar] [CrossRef]
- Roberts, K.J.; Ariza, A.J.; Selvaraj, K.; Quadri, M.; Mangarelli, C.; Neault, S.; Davis, E.E.; Binns, H.J. Testing for rare genetic causes of obesity: Findings and experiences from a pediatric weight management program. Int. J. Obes. 2022, 46, 1493–1501. [Google Scholar] [CrossRef]
- Zmyslowska, A.; Borowiec, M.; Antosik, K.; Ploski, R.; Ciechanowska, M.; Iwaniszewska, B.; Jakubiuk-Tomaszuk, A.; Janczyk, W.; Krawczynski, M.; Salmonowicz, B.; et al. Genetic evaluation of patients with Alström syndrome in the Polish population. Clin. Genet. 2016, 89, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Jeziorny, K.; Antosik, K.; Jakiel, P.; Młynarski, W.; Borowiec, M.; Zmysłowska, A. Next-generation sequencing in the diagnosis of patients with Bardet-Biedl syndrome—New variants and relationship with hyperglycemia and insulin resistance. Genes 2020, 11, 1283. [Google Scholar] [CrossRef]
- Kilian, M.; Chapple, I.L.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The oral microbiome—An update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef]
- Carter, C.J.; France, J.; Crean, S.; Singhrao, S.K. The Porphyromonas gingivalis/host interactome shows enrichment in GWASdb genes related to Alzheimer’s disease, diabetes, and cardiovascular diseases. Front. Aging Neurosci. 2017, 9, 408. [Google Scholar] [CrossRef]
- Zmysłowska-Polakowska, E.; Płoszaj, T.; Skoczylas, S.; Mojsak, P.; Ciborowski, M.; Kretowski, A.; Lukomska-Szymanska, M.; Szadkowska, A.; Mlynarski, W.; Zmysłowska, A. Evaluation of the Oral Bacterial Genome and Metabolites in Patients with Wolfram Syndrome. Int. J. Mol. Sci. 2023, 24, 5596. [Google Scholar] [CrossRef]
- Ilievski, V.; Zuchowska, P.K.; Green, S.J.; Toth, P.T.; Ragozzino, M.E.; Le, K.; Aljewari, H.W.; O’Brien-Simpson, N.M.; Reynolds, E.C.; Watanabe, K. Chronic oral application of a periodontal pathogen results in brain inflammation, neurodegeneration, and amyloid beta production in wild-type mice. PLoS ONE 2018, 13, e0204941. [Google Scholar] [CrossRef] [PubMed]
- Shinjyo, N.; Kagaya, W.; Pekna, M. Interaction Between the Complement System and Infectious Agents—A Potential Mechanistic Link to Neurodegeneration and Dementia. Front. Cell. Neurosci. 2021, 15, 710390. [Google Scholar] [CrossRef]
- Tran, V.T.A.; Kang, Y.J.; Kim, H.K.; Kim, H.R.; Cho, H. Oral pathogenic bacteria-inducing neurodegenerative microgliosis in human neural cell platform. Int. J. Mol. Sci. 2021, 22, 6925. [Google Scholar] [CrossRef]
- Vázquez-Ramos, V.R.; Pérez-Serrano, R.M.; García-Solís, P.; Solís-Sainz, J.C.; Espinosa-Cristóbal, L.F.; Castro-Ruíz, J.E.; Domínguez-Pérez, R.A. Root canal microbiota as an augmented reservoir of antimicrobial resistance genes in type 2 diabetes mellitus patients. J. Appl. Oral Sci. 2023, 30, e20220362. [Google Scholar] [CrossRef]
- Lin, W.S.; Hwang, S.E.; Koh, Y.C.; Ho, P.Y.; Pan, M.H. Modulatory Effects of Lactobacillus Paracasei-Fermented Turmeric on Metabolic Dysregulation and Gut Microbiota in High-Fat Diet-Induced Obesity in Mice. J. Agric. Food Chem. 2024, 72, 17924–17937. [Google Scholar] [CrossRef] [PubMed]
- Romaní-Pérez, M.; López-Almela, I.; Bullich-Vilarrubias, C.; Evtoski, Z.; Benítez-Páez, A.; Sanz, Y. Bacteroides uniformis CECT 7771 requires adaptive immunity to improve glucose tolerance but not to prevent body weight gain in diet-induced obese mice. Microbiome 2024, 12, 103. [Google Scholar] [CrossRef]
- Knychalska-Karwan, Z. Zbiór wskaźników stomatologicznych i niektórych testów oraz klasyfikacji; Wydawnictwo CZELEJ: Lublin, Poland, 2006. [Google Scholar]
- Trombelli, L.; Farina, R.; Silva, C.O.; Tatakis, D.N. Plaque-induced gingivitis: Case definition and diagnostic considerations. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S44–S67. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qiao, F.; Wang, Z.; Meng, G.; Gu, Y.; Wu, H.; Liu, D.; Niu, K. Analysis of the microbial community diversity in various regions of the healthy oral cavity. BMC Oral Health 2024, 24, 978. [Google Scholar] [CrossRef]
- Gregorczyk-Maga, I.; Fiema, M.; Kania, M.; Jachowicz-Matczak, E.; Romaniszyn, D.; Gerreth, K.; Klupa, T.; Wójkowska-Mach, J. Oral Microbiota-One Habitat or Diverse Niches? A Pilot Study of Sampling and Identification of Oral Bacterial and Fungal Biota in Patients with Type I Diabetes Mellitus Treated with Insulin Pump. Int. J. Environ. Res. Public Health 2023, 20, 2252. [Google Scholar] [CrossRef] [PubMed]
- Afgan, E.; Baker, D.; Batut, B.; van den Beek, M.; Bouvier, D.; Cech, M.; Chilton, J.; Clements, D.; Coraor, N.; Grüning, B.A.; et al. The Galaxy platform for accessible, reproducible, and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Wood, D.E.; Lu, J.; Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef]
- Bantim, Y.C.V.; Kussaba, S.T.; de Carvalho, G.P.; Garcia-Junior, I.R.; Roman-Torres, C.V.G. Oral health in patients with Prader-Willi syndrome: Current perspectives. Clin. Cosmet. Investig. Dent. 2019, 11, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Rafatjou, R.; Torkaman, S.; Daneshyar, F. Dental Management of a Child with Joubert Syndrome. Iran. J. Child Neurol. 2022, 16, 137–142. [Google Scholar] [CrossRef]
- Aung, S.W.K.H.; Orack, J.; Doherty, D.; Nelson, T.; Dempsey, J.C.; Li, S.R.; Chi, D.L. Behavioral Correlates of Caregiver-Reported Oral Health of Individuals with Joubert Syndrome: A Cross-Sectional Observational Study. Spec. Care Dentist. 2025, 45, e70050. [Google Scholar] [CrossRef] [PubMed]
- Anders, P.L.; Davis, E.L. Oral health of patients with intellectual disabilities: A systematic review. Spec. Care Dentist. 2010, 30, 110–117. [Google Scholar] [CrossRef]
- Abed, H.; Gogandi, H.; Almutawwif, M.; Aloufi, A.; Tashkandi, M.; Alqarni, A.; Aladwani, F.; Sadek, H.S. Dental management of Kartagener syndrome: A case report. Spec. Care Dentist. 2024, 44, 729–736. [Google Scholar] [CrossRef]
- Asthana, G.; Palwankar, P.; Pandey, R. Association of obesity and type 2 diabetes mellitus with periodontitis: A cross-sectional study. Cureus 2024, 16, e68779. [Google Scholar] [CrossRef] [PubMed]
- Rahim, A.; Hassan, S.; Ullah, N.; Noor, N.; Ahmed Rafique, R.; Khattak, F.A.; Afaq, S. Association and comparison of periodontal and oral hygiene status with serum HbA1c levels: A cross-sectional study. BMC Oral Health 2023, 23, 442. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qian, F.; Cheng, X.; Wang, D.; Wang, Y.; Pan, Y.; Chen, L.; Wang, W.; Tian, Y. Dysbiosis of oral microbiota and metabolite profiles associated with type 2 diabetes mellitus. Microbiol. Spectr. 2023, 11, e0379622. [Google Scholar] [CrossRef] [PubMed]
- Goodson, J.M.; Groppo, D.; Halem, S.; Carpino, E. Is obesity an oral bacterial disease? J. Dent. Res. 2009, 88, 519–523. [Google Scholar] [CrossRef]
- Almeida-Santos, A.; Martins-Mendes, D.; Gayà-Vidal, M.; Pérez-Pardal, L.; Beja-Pereira, A. Characterization of the oral microbiome of medicated type-2 diabetes patients. Front. Microbiol. 2021, 12, 610370. [Google Scholar] [CrossRef]
- Graves, D.T.; Corrêa, J.D.; Silva, T.A. The oral microbiota is modified by systemic diseases. J. Dent. Res. 2019, 98, 148–156. [Google Scholar] [CrossRef]
- Qin, H.; Li, G.; Xu, X.; Zhang, C.; Zhong, W.; Xu, S.; Yin, Y.; Song, J. The role of oral microbiome in periodontitis under diabetes mellitus. J. Oral Microbiol. 2022, 14, 2078031. [Google Scholar] [CrossRef]
- Ogawa, T.; Honda-Ogawa, M.; Ikebe, K.; Notomi, Y.; Iwamoto, Y.; Shirobayashi, I.; Hata, S.; Kibi, M.; Masayasu, S.; Sasaki, S.; et al. Characterizations of oral microbiota in elderly nursing home residents with diabetes. J. Oral Sci. 2017, 59, 549–555. [Google Scholar] [CrossRef]
- Saeb, A.T.M.; Al-Rubeaan, K.A.; Aldosary, K.; Udaya Raja, G.K.; Mani, B.; Abouelhoda, M.; Tayeb, H.T. Relative reduction of biological and phylogenetic diversity of the oral microbiota of diabetes and pre-diabetes patients. Microb. Pathog. 2019, 128, 215–229. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, S.; Wang, Y.; Wang, Z.; Ding, W.; Sun, X.; He, K.; Feng, Q.; Zhang, X. Changes of saliva microbiota in the onset and after the treatment of diabetes in patients with periodontitis. Aging 2020, 12, 13090–13114. [Google Scholar] [CrossRef] [PubMed]
- Genco, R.J.; Borgnakke, W.S. Diabetes as a potential risk for periodontitis: Association studies. Periodontol. 2000 2020, 83, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef]
- Minty, M.; Canceil, T.; Serino, M.; Burcelin, R.; Tercé, F.; Blasco-Baque, V. Oral microbiota-induced periodontitis: A new risk factor of metabolic diseases. Rev. Endocr. Metab. Disord. 2019, 20, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Babatzia, A.; Papaioannou, W.; Stavropoulou, A.; Pandis, N.; Kanaka-Gantenbein, C.; Papagiannoulis, L.; Gizani, S. Clinical and microbial oral health status in children and adolescents with type 1 diabetes mellitus. Int. Dent. J. 2020, 70, 136–144. [Google Scholar] [CrossRef]
- Jensen, E.D.; Selway, C.A.; Allen, G.; Bednarz, J.; Weyrich, L.S.; Gue, S.; Peña, A.S.; Couper, J. Early markers of periodontal disease and altered oral microbiota are associated with glycemic control in children with type 1 diabetes. Pediatr. Diabetes 2021, 22, 474–481. [Google Scholar] [CrossRef]
- Stefura, T.; Zapała, B.; Gosiewski, T.; Skomarovska, O.; Dudek, A.; Pędziwiatr, M.; Major, P. Differences in compositions of oral and fecal microbiota between patients with obesity and controls. Medicina 2021, 57, 678. [Google Scholar] [CrossRef]
- Zeigler, C.C.; Persson, G.R.; Wondimu, B.; Marcus, C.; Sobko, T.; Modéer, T. Microbiota in the oral subgingival biofilm is associated with obesity in adolescence. Obesity 2012, 20, 157–164. [Google Scholar] [CrossRef]
- Schamarek, I.; Anders, L.; Chakaroun, R.M.; Kovacs, P.; Rohde-Zimmermann, K. The role of the oral microbiome in obesity and metabolic disease: Potential systemic implications and effects on taste perception. Nutr. J. 2023, 22, 28. [Google Scholar] [CrossRef]
- Ballini, A.; Scacco, S.; Boccellino, M.; Santacroce, L.; Arrigoni, R. Microbiota and obesity: Where are we now? Biology 2020, 9, 415. [Google Scholar] [CrossRef]
- Mameli, C.; Cattaneo, C.; Panelli, S.; Comandatore, F.; Sangiorgio, A.; Bedogni, G.; Bandi, C.; Zuccotti, G.; Pagliarini, E. Taste perception and oral microbiota are associated with obesity in children and adolescents. PLoS ONE 2019, 14, e0221656. [Google Scholar] [CrossRef] [PubMed]
- Gasmi Benahmed, A.; Gasmi, A.; Doşa, A.; Chirumbolo, S.; Mujawdiya, P.K.; Aaseth, J.; Dadar, M.; Bjørklund, G. Association between the gut and oral microbiome with obesity. Anaerobe 2021, 70, 102248. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.; Xiong, W.; Luo, Y.; Feng, H.; Zhou, H.; Peng, Y.; He, Y.; Ye, Q. The oral-brain axis: Can periodontal pathogens trigger the onset and progression of Alzheimer’s disease? Front. Microbiol. 2024, 15, 1358179. [Google Scholar] [CrossRef] [PubMed]
- Panny, A.; Glurich, I.; Haws, R.M.; Acharya, A. Oral and Craniofacial Anomalies of Bardet-Biedl Syndrome: Dental Management in the Context of a Rare Disease. J. Dent. Res. 2017, 96, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Rustad, C.F.; Bragadottir, R.; Tveten, K.; Nordgarden, H.; Miller, J.U.; Åsten, P.M.; Vasconcelos, G.; Kulseth, M.A.; Holla, Ø.L.; Olsen, H.G.; et al. Clinical and genetic aspects of Bardet-Biedl syndrome in adults in Norway. Orphanet J. Rare Dis. 2025, 20, 127. [Google Scholar] [CrossRef]
- Forsyth, R.; Gunay-Aygun, M. Bardet-Biedl Syndrome Overview. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2003. [Google Scholar]
- Paisey, R.B.; Steeds, R.; Barrett, T.; Williams, D.; Geberhiwot, T.; Gunay-Aygun, M. Alström Syndrome. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2003. [Google Scholar]
- Koray, F.; Dorter, C.; Benderli, Y.; Satman, I.; Yilmaz, T.; Dinccag, N.; Karsidag, K. Alstrom syndrome: A case report. J. Oral Sci. 2001, 43, 221–224. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Y. Mechanism and application of Lactobacillus in type 2 diabetes-associated periodontitis. Front. Public Health 2023, 11, 1248518. [Google Scholar] [CrossRef]
- Álvarez-Arraño, V.; Martín-Peláez, S. Effects of probiotics and synbiotics on weight loss in subjects with overweight or obesity: A systematic review. Nutrients 2021, 13, 3627. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).