Effects of the Wheat Crab Model and the Pond Culture Model on the Growth, Metabolism and Intestinal Microbiota of the Chinese Mitten Crab (Eriocheir sinensis)
Abstract
1. Introduction
2. Materials and Methods
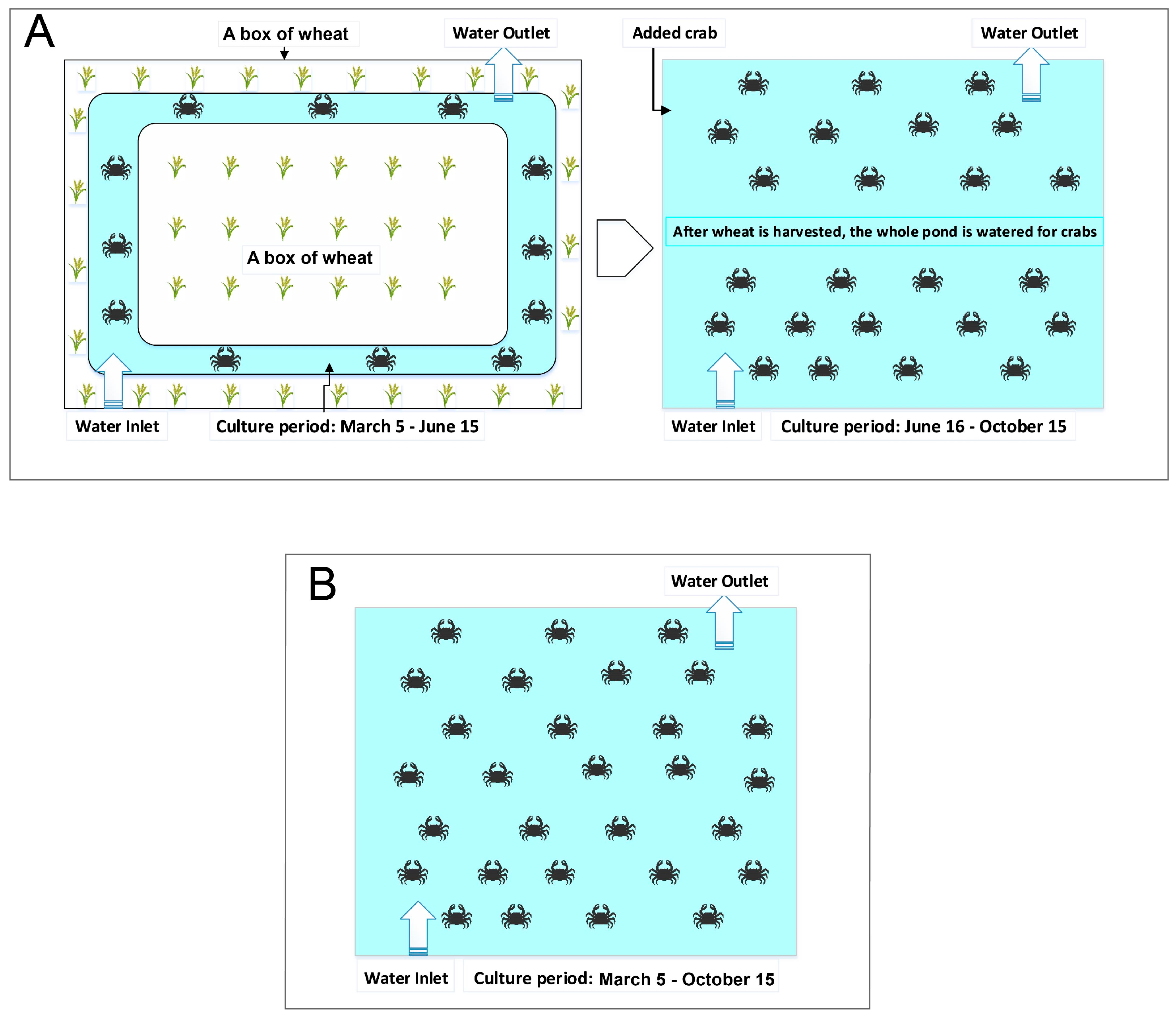
2.1. Sites and System Description
2.2. Sample Collection
2.3. Chemical Analysis
2.4. Gene Expression Analysis
2.5. Intestinal Microflora Identification
2.6. Non-Targeted Metabolomics Analysis
2.7. Statistical Analyses
3. Results
3.1. Performance Indexes
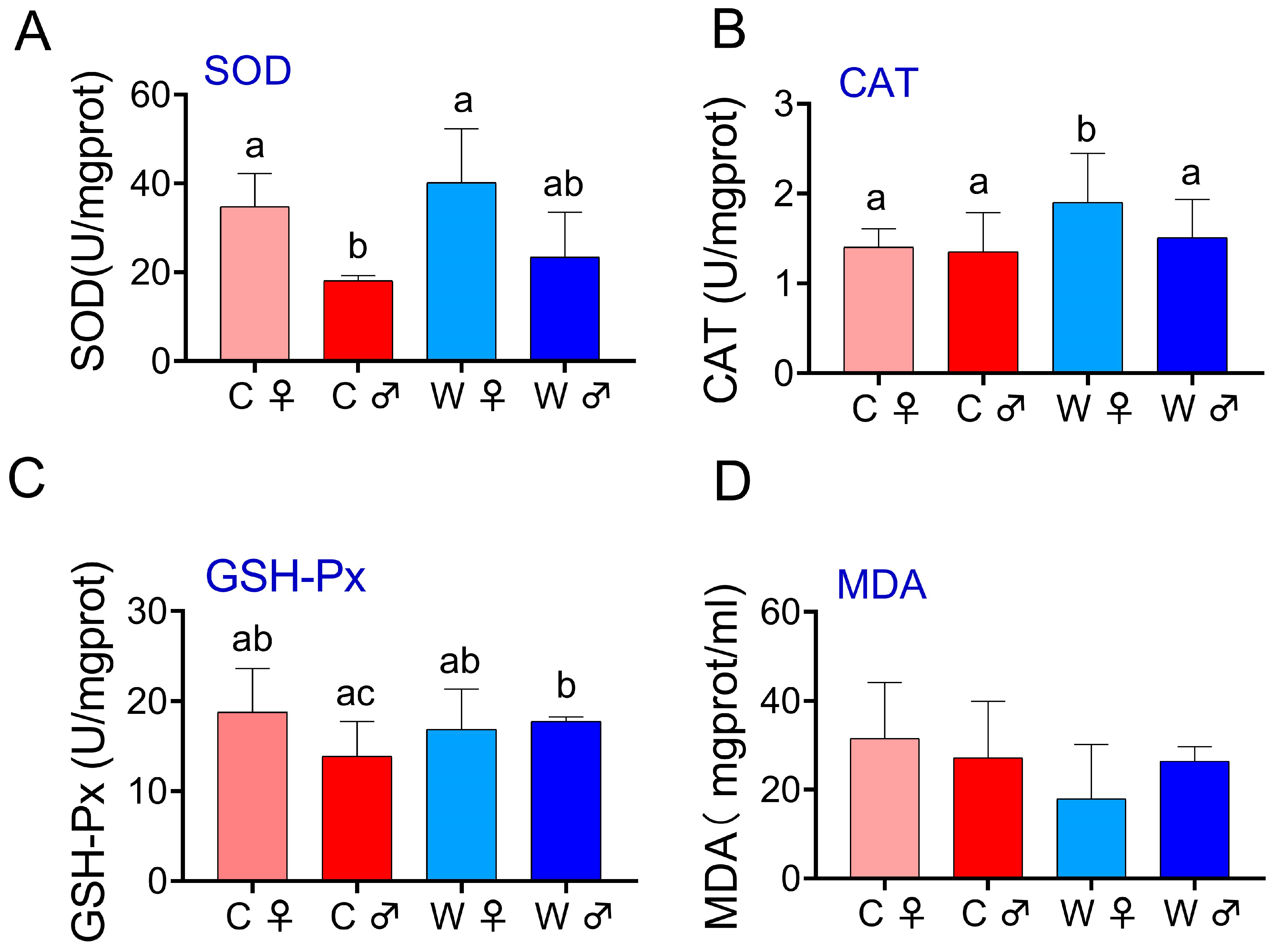
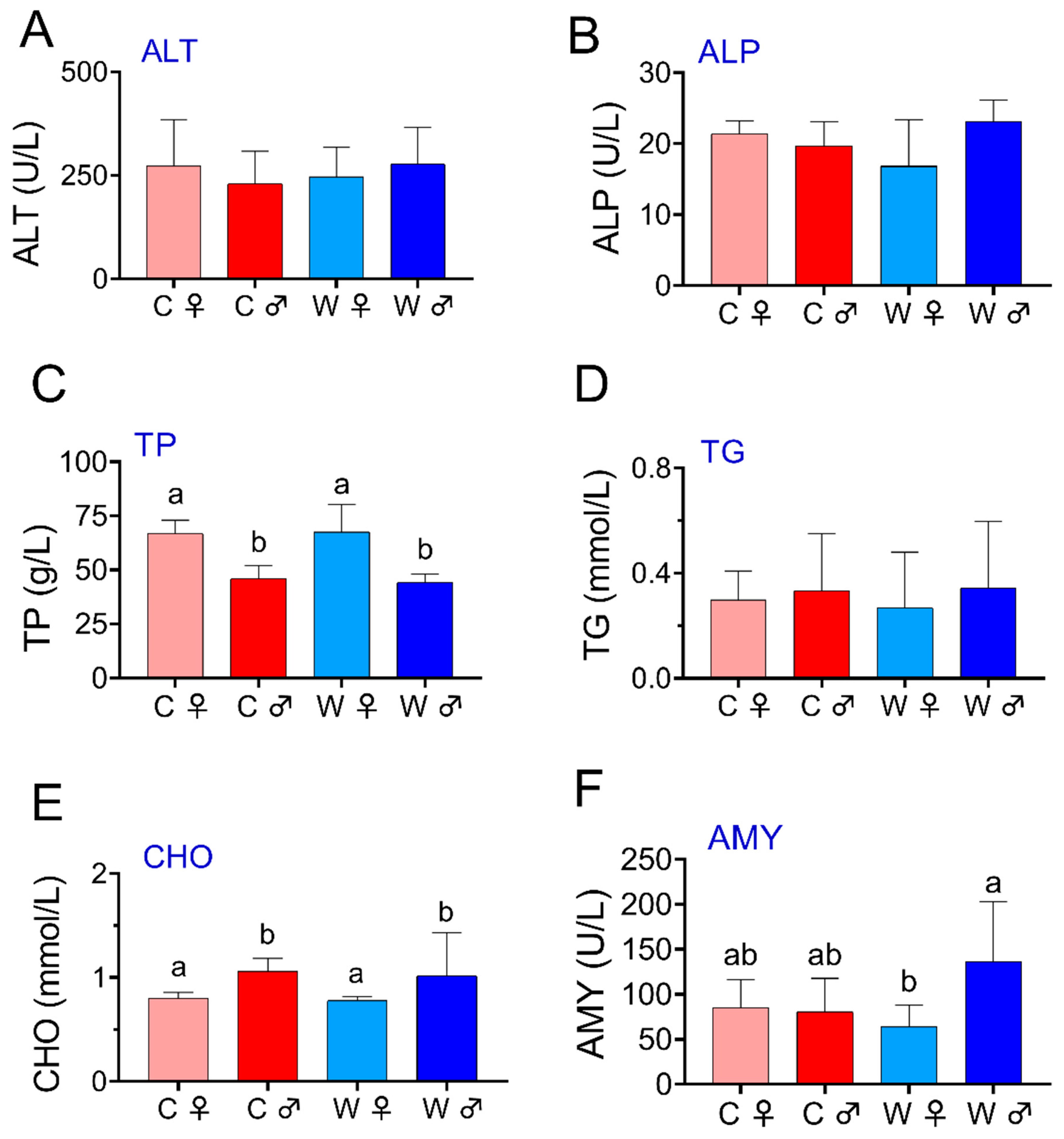
3.2. Biochemical Parameters
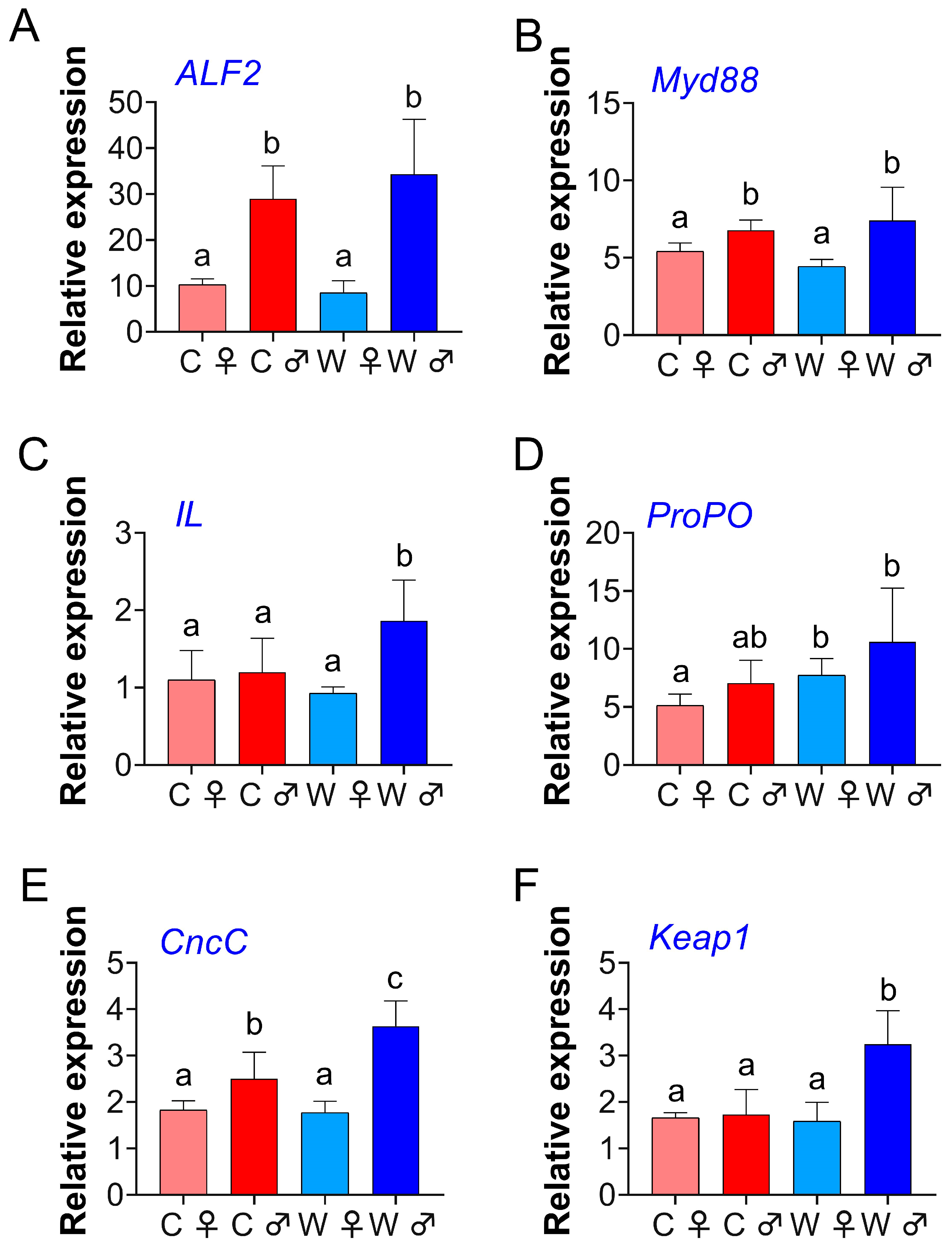
3.3. Gene Expression Analyses
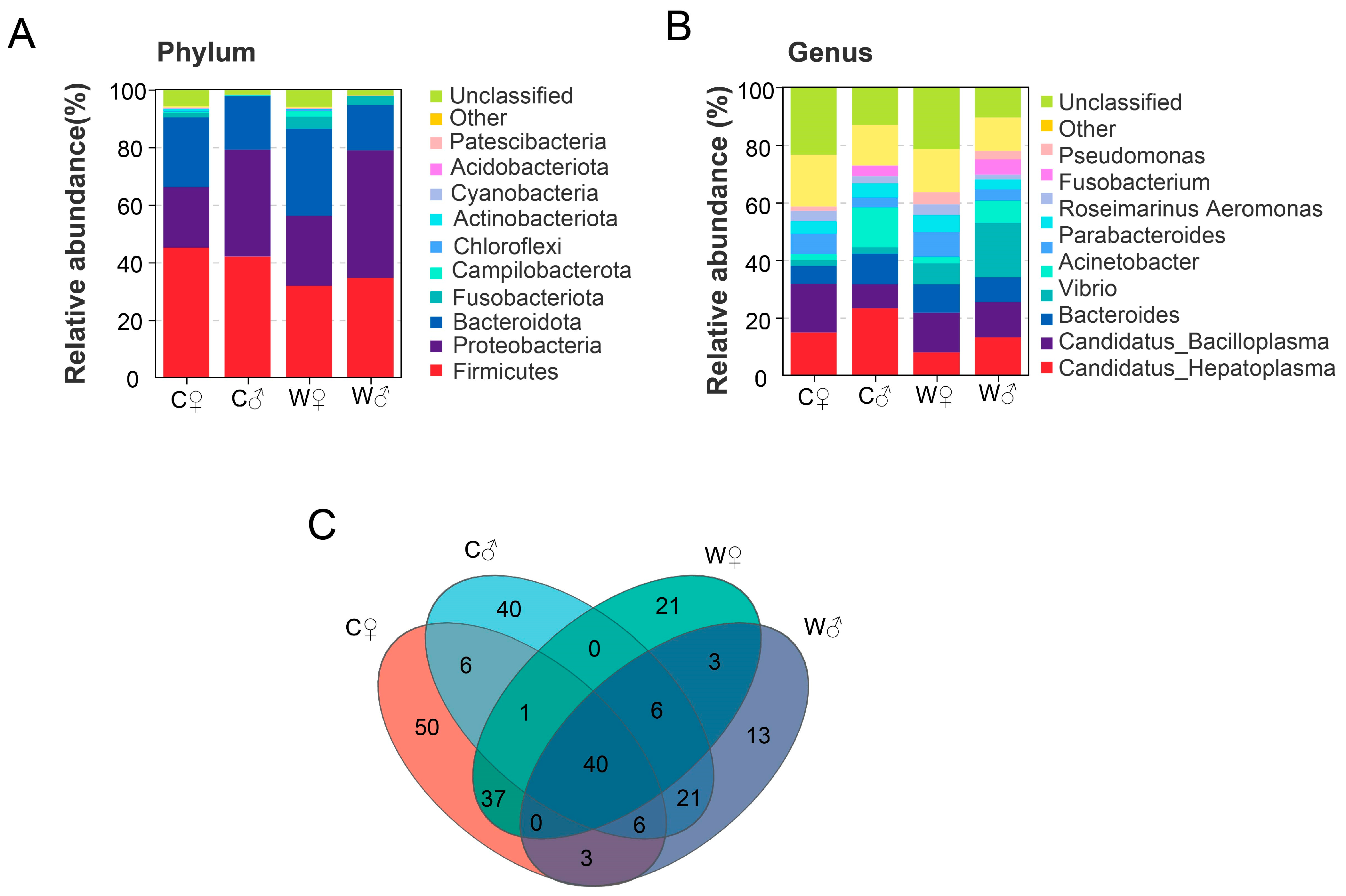
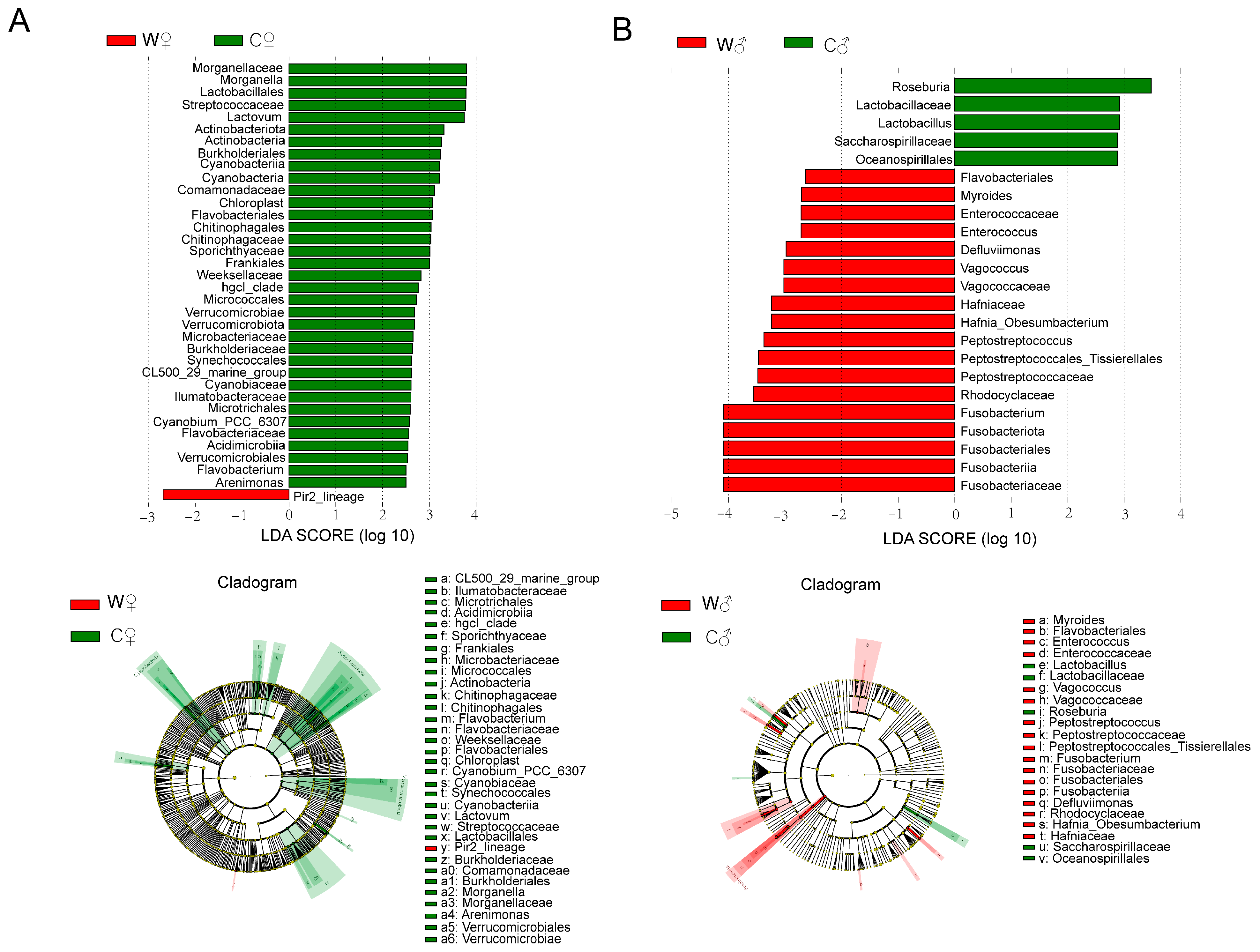
3.4. Intestinal Microbiota
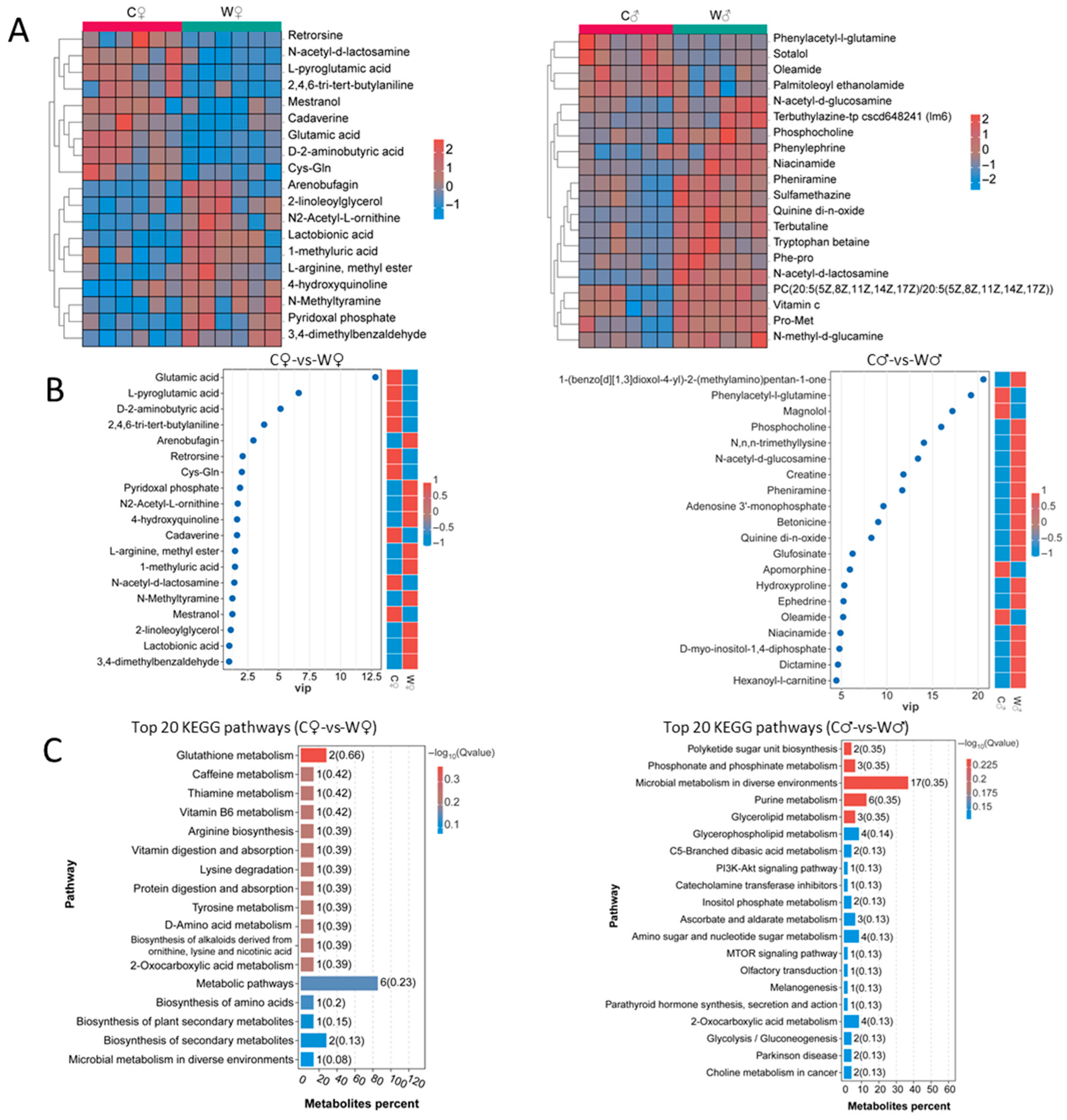
3.5. Non-Targeted Metabolomics
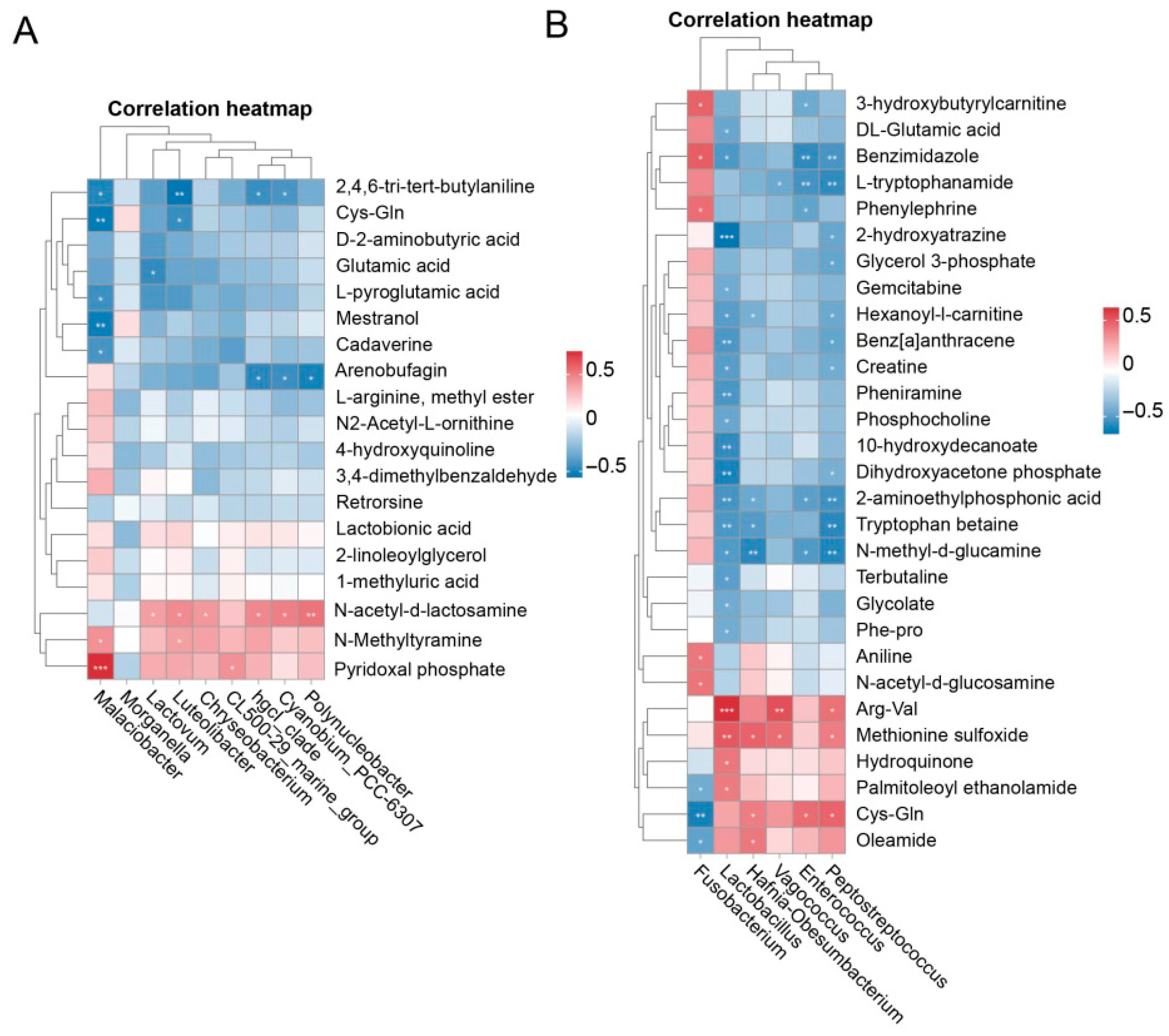
3.6. Correlation Analysis
4. Discussion
4.1. Performance Indexes
4.2. Biochemical Parameters
4.3. Gene Expression Analyses
4.4. Intestinal Microbiota
4.5. Non-Targeted Metabolomics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, Y.; Dong, J.; Wang, L.; Li, W.; Bao, J.; Jiang, H. Chronic chlorpyrifos exposure induces oxidative stress, neurological damage, and hepatopancreas enrichment in Chinese mitten crab (Eriocheir sinensis). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2025, 289, 110111. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.; Zhu, Y.; Li, Y.; Cui, J.; Yu, B. Multidimensional deconstruction and workable solutions for addressing China’s food security issues: From the perspective of sustainable diets. Land Use Policy 2025, 148, 107401. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Chen, J.; Jin, H.; Wang, T.; Zhu, W.; Li, L. Underestimated nutrient from aquaculture ponds to Lake Eutrophication: A case study on Taihu Lake Basin. J. Hydrol. 2024, 630, 130749. [Google Scholar] [CrossRef]
- Kong, L.; Cai, C.; Ye, Y.; Chen, D.; Wu, P.; Li, E.; Chen, L.; Song, L. Comparison of non-volatile compounds and sensory characteristics of Chinese mitten crabs (Eriocheir sinensis) reared in lakes and ponds: Potential environmental factors. Aquaculture 2012, 364–365, 96–102. [Google Scholar] [CrossRef]
- Tang, S.-Z.; Song, D.; Bai, S.-Y.; Huang, X.-L.; Chen, Z.-X.; Wang, P.; Qin, D.-L. Trace elements accumulation and health risk assessment of Chinese mitten crab (Eriocheir sinensis) from monoculture and rice-crab co-culture mode. J. Food Compos. Anal. 2023, 123, 105640. [Google Scholar] [CrossRef]
- Zhang, B.; Hou, Y.; Zhu, R.; Wang, W.; Liu, H.; Yang, Z.; Wu, M.; Wang, Q. Comparative study on growth performance and nutritional quality of Eriocheir sinensis between Liaohe River strain and Yangtze River strain under rice field culture environment. J. Food Compos. Anal. 2025, 137, 106942. [Google Scholar] [CrossRef]
- Wang, A.; Ma, X.; Xu, J.; Lu, W. Methane and nitrous oxide emissions in rice-crab culture systems of northeast China. Aquac. Fish. 2019, 4, 134–141, Erratum in Aquac. Fish. 2025, 10, 737–738. [Google Scholar] [CrossRef]
- Jiang, W.; Jia, X.; Zhang, M.; Qiang, W.; Shen, X.; Jiang, G.; Li, X.; Chi, C.; Liu, H.; Liu, W.; et al. The effect of dietary vitamin A supplementation on growth, gonadal development, molting, immunity, and ammonia nitrogen resistance in sub-adult male Chinese mitten crab, Eriocheir sinensis. Aquaculture 2024, 581, 740451. [Google Scholar] [CrossRef]
- Li, B.-W.; Xu, W.-B.; Dong, W.-R.; Zhang, Y.-M.; Cheng, Y.-X.; Chen, D.-Y.; Xiao, Y.; Chen, Y.-Y.; Shu, M.-A. Identification and function analysis of two fibroblast growth factor receptor (FGFR) from Scylla paramamosain: The evidence of FGFR involved in innate immunity in crustacean. Fish Shellfish Immunol. 2022, 131, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Resch-Sedlmeier, G.; Sedlmeier, D. Release of digestive enzymes from the crustacean hepatopancreas: Effect of vertebrate gastrointestinal hormones. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1999, 123, 187–192. [Google Scholar] [CrossRef]
- Yan, Z.; zhu, L.; Hou, C.; Zheng, Y.; Guo, H.; Shi, L.; Tan, B.; Zhang, S. The enhancement effect of low-dose dietary lipopolysaccharide on the growth and immunity of Litopenaeus vannamei, and transcriptome analysis. Fish Shellfish Immunol. 2023, 133, 108517. [Google Scholar] [CrossRef]
- Zheng, X.; Duan, Y.; Dong, H.; Zhang, J. Effects of dietary Lactobacillus plantarum in different treatments on growth performance and immune gene expression of white shrimp Litopenaeus vannamei under normal condition and stress of acute low salinity. Fish Shellfish Immunol. 2017, 62, 195–201. [Google Scholar] [CrossRef]
- Kim, J.-H.; Cho, J.-H.; Kim, S.-R.; Hur, Y.B. Toxic effects of waterborne ammonia exposure on hematological parameters, oxidative stress and stress indicators of juvenile hybrid grouper, Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀. Environ. Toxicol. Pharmacol. 2020, 80, 103453. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, H.; Yuan, H.; Hu, N.; Zheng, Y.; Tan, B.; Shi, L.; Zhang, S. Tapping Chlorella vulgaris potential for enhanced growth, immunity, digestion, microbiota, and immunometabolism in Litopenaeus vannamei feeding across varied salinities. Aquaculture 2024, 581, 740469. [Google Scholar] [CrossRef]
- Zhang, B.-Y.; Wang, W.-J.; Zhu, R.; Zhang, D.-M.; Wang, N.; Zheng, N.; Wang, S.; Liu, H.-J.; Wan, J.-W.; Chen, Y.-K.; et al. Comparative study on growth performance and edible portion nutritional composition of male Eriocheir sinensis at different growth stages in rice-crab culture systems. J. Food Compos. Anal. 2024, 130, 106156. [Google Scholar] [CrossRef]
- Yu, A.-Q.; Jin, X.-K.; Guo, X.-N.; Li, S.; Wu, M.-H.; Li, W.-W.; Wang, Q. Two novel Toll genes (EsToll1 and EsToll2) from Eriocheir sinensis are differentially induced by lipopolysaccharide, peptidoglycan and zymosan. Fish Shellfish Immunol. 2013, 35, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Xu, X.; Yu, D.; Long, M.; Xia, H.; Lu, Y.; Gan, Z. Evolutionary and functional conservation of myeloid differentiation factor 88 (MyD88) in amphibian Xenopus tropicalis. Gene 2023, 865, 147332. [Google Scholar] [CrossRef]
- Tiwari, N.; Suri, M.; Upadhyay, J.; Ansari, M.N.; Samad, A. Chapter 11—Interplay between gut microbiota in immune homeostasis and inflammatory diseases. In Recent Developments in Anti-Inflammatory Therapy; Prasher, P., Zacconi, F.C., Withey, J.H., Rathbone, M., Dua, K., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 143–154. [Google Scholar]
- Macpherson, A.J.; Pachnis, V.; Prinz, M. Boundaries and integration between microbiota, the nervous system, and immunity. Immunity 2023, 56, 1712–1726. [Google Scholar] [CrossRef]
- Shor, E.K.; Brown, S.P.; Freeman, D.A. A novel role for the pineal gland: Regulating seasonal shifts in the gut microbiota of Siberian hamsters. J. Pineal Res. 2020, 69, e12696. [Google Scholar] [CrossRef]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, F.; MacDonald, E.; Wishart, S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, Z.; Guo, K.; Zhao, X.; Deng, X.; Wang, S.; Luo, L. Effect of environmental factors on the control of milky disease in Chinese mitten crab (Eriocheir sinensis). Aquaculture 2026, 610, 742942. [Google Scholar] [CrossRef]
- Han, C.; Xiao, Y.; Dai, Z.; Feng, L.; Shi, Y.; Liu, X.; Ge, J.; Yang, J. Use of stable isotopic signatures and fatty acid profiles to authenticate Chinese mitten crab (Eriocheir sinensis) reared in ponds and rice fields. Food Control 2025, 174, 111253. [Google Scholar] [CrossRef]
- Fang, L.; Chen, X.; Fan, L.; Hu, G.; Qiu, L.; Song, C.; Xie, Y.; Giesy, J.P.; Wang, C.; Meng, S. Environment consistently impact on aquaculture: The predominant source of residual pollutants in cultured Chinese mitten crab (Eriocheir sinensis) across China. Heliyon 2024, 10, e32418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-Y.; Yao, Q.; Zhang, D.-M.; Wang, N.; Liu, H.-J.; Wan, J.-W.; Chen, Y.-K.; Wang, Q.-J.; Guo, Z.-X. Comparative study on growth, digestive function and intestinal microbial composition of female Chinese mitten crab Eriocheir sinensis selected at different growth stages in rice-crab culture systems. Aquaculture 2022, 554, 738120. [Google Scholar] [CrossRef]
- Long, X.; Guo, Q.; Wang, X.; Francis, D.S.; Cheng, Y.; Wu, X. Effects of fattening period on ovarian development and nutritional quality of adult female Chinese mitten crab Eriocheir sinensis. Aquaculture 2020, 519, 734748. [Google Scholar] [CrossRef]
- Guo, J.; Chen, S.; Zhang, Y.; Liu, J.; Jiang, L.; Hu, L.; Yao, K.; Yu, Y.; Chen, X. Cholesterol metabolism: Physiological regulation and diseases. MedComm 2024, 5, e476. [Google Scholar] [CrossRef] [PubMed]
- Semova, I.; Biddinger, S.B. Triglycerides in Nonalcoholic Fatty Liver Disease: Guilty Until Proven Innocent. Trends Pharmacol. Sci. 2021, 42, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Wirkner, C.S.; Richter, S. Circulatory System and Respiration. In Functional Morphology and Diversity; Watling, L., Thiel, M., Eds.; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Tian, Y.; Guo, C.; Zhang, X.; Xie, S.; Zhou, Q.; Luo, J.; Zhu, T.; Yang, Y.; Li, X.; Jin, M. Effects of dietary histidine level on growth, antioxidant capacity and TOR signaling pathway in juvenile swimming crabs, Portunus trituberculatus. Aquac. Rep. 2023, 33, 101869. [Google Scholar] [CrossRef]
- Suri, A.; Singh, N.; Bansal, S.K. A Study on the Serum γ-Glutamyltranspeptidase and Plasma Osteopontin in Alcoholic Liver Disease. J. Lab. Physicians 2022, 14, 101–108. [Google Scholar] [CrossRef]
- Khan, K.; Praba, L.K.; Ali, H.A.J. Ascidians as Bioresources: An Anti-inflammatory Activity of Colonial Ascidians Eudistoma ovatum and Didemnum perlucidum. J. Biol. Act. Prod. Nat. 2021, 11, 254–268. [Google Scholar] [CrossRef]
- Männistö, V.; Salomaa, V.; Jula, A.; Lundqvist, A.; Männistö, S.; Perola, M.; Åberg, F. ALT levels, alcohol use, and metabolic risk factors have prognostic relevance for liver-related outcomes in the general population. JHEP Rep. 2024, 6, 101172. [Google Scholar] [CrossRef]
- Sigurdarson, S.S.; Bjornsson, H.K.; Bjornsson, E.S. THU-151 A prospective study on the causes of notable elevation of alanine aminotransferase (ALT) as well as elevation of both ALT and alkaline phosphatase (ALP) with special emphasis on drug-induced liver injury (DILI). J. Hepatol. 2024, 80, S328. [Google Scholar] [CrossRef]
- Feng, M.; Liu, K.; Zhao, G.; Lou, S.; An, B.; Lin, L.; Ding, Y.; Bao, S.; Wang, H. A novel model based on qAnti-HBc and conventional biomarkers for identifying significant liver injury among CHB patients with ALT ≤ ULN. Antivir. Res. 2022, 202, 105315. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Kwon, T.W.; Choi, J.H.; Kim, Y.; Moon, S.-K.; Nah, S.-Y.; Cho, I.-H. Ginsenoside-Re inhibits experimental autoimmune encephalomyelitis as a mouse model of multiple sclerosis by downregulating TLR4/MyD88/NF-κB signaling pathways. Phytomedicine 2024, 122, 155065. [Google Scholar] [CrossRef] [PubMed]
- Imjongjirak, C.; Amparyup, P.; Tassanakajon, A.; Sittipraneed, S. Antilipopolysaccharide factor (ALF) of mud crab Scylla paramamosain: Molecular cloning, genomic organization and the antimicrobial activity of its synthetic LPS binding domain. Mol. Immunol. 2007, 44, 3195–3203. [Google Scholar] [CrossRef]
- Amezian, D.; Fricaux, T.; de Sousa, G.; Maiwald, F.; Huditz, H.-I.; Nauen, R.; Le Goff, G. Investigating the role of the ROS/CncC signaling pathway in the response to xenobiotics in Spodoptera frugiperda using Sf9 cells. Pestic. Biochem. Physiol. 2023, 195, 105563. [Google Scholar] [CrossRef]
- Zhao, L.; Tao, X.; Wang, Q.; Yu, X.; Dong, D. Diosmetin alleviates neuropathic pain by regulating the Keap1/Nrf2/NF-κB signaling pathway. Biomed. Pharmacother. 2024, 170, 116067. [Google Scholar] [CrossRef] [PubMed]
- Starke, S.; Harris, D.M.M.; Paulay, A.; Aden, K.; Waschina, S. Comparative analysis of amino acid auxotrophies and peptidase profiles in non-dysbiotic and dysbiotic small intestinal microbiomes. Comput. Struct. Biotechnol. J. 2025, 27, 821–831. [Google Scholar] [CrossRef]
- Foysal, M.J. Host habitat shapes the core gut bacteria of decapod crustaceans: A meta-analysis. Heliyon 2023, 9, e16511. [Google Scholar] [CrossRef]
- van de Guchte, M.; Mondot, S.; Doré, J. Dynamic Properties of the Intestinal Ecosystem Call for Combination Therapies, Targeting Inflammation and Microbiota, in Ulcerative Colitis. Gastroenterology 2021, 161, 1969–1981.e12. [Google Scholar] [CrossRef]
- Yonoichi, S.; Hirano, T.; Hara, Y.; Ishida, Y.; Shoda, A.; Kimura, M.; Murata, M.; Mantani, Y.; Yokoyama, T.; Ikenaka, Y.; et al. Effects of exposure to the neonicotinoid pesticide clothianidin on mouse intestinal microbiota under unpredictable environmental stress. Toxicol. Appl. Pharmacol. 2024, 482, 116795. [Google Scholar] [CrossRef]
- Baldeon, M.E.; Cardenas, P.; Carpio, V.; Chavez, M.; Benitez, D.; Robles, J.; Loza, G.; Alvarado, R.; Rios, G.; Pazmiño, K.; et al. Nutritional Status and Intestinal Microbiota in Children with High Prevalence of Malnutrition Supplemented with a Snack Based on Lupinus mutabilis Sweet—2022–2023. Curr. Dev. Nutr. 2025, 9, 106245. [Google Scholar] [CrossRef]
- Fu, C.; Cui, Z.; Shi, X.; Liu, J.; Jiang, Y.; Zhang, R. Effects of dietary glyceryl monolaurate supplementation on growth performance, non-specific immunity, antioxidant status and intestinal microflora of Chinese mitten crabs. Fish Shellfish Immunol. 2022, 125, 65–73. [Google Scholar] [CrossRef]
- Yu, C.; Li, L.; Jin, J.; Zhang, B.; Wei, H.; Zhao, Y.; Li, X.; Li, Y. Comparative analysis of gut bacterial community composition during a single day cycle in Chinese mitten crab (Eriocheir sinensis). Aquac. Rep. 2021, 21, 100907. [Google Scholar] [CrossRef]
- Shastry, R.P.; Ghate, S.D.; Hameed, A.; Prasad Rao, R.S.; Bhandary, Y.P.; Shetty, R. Emergence of rare and low abundant anaerobic gut Firmicutes is associated with a significant downfall of Klebsiella in human colon cancer. Microb. Pathog. 2024, 193, 106726. [Google Scholar] [CrossRef] [PubMed]
- Fadare, O.S.; Enabulele, O.I.; Singh, V.; Pradhan, D. GC-MS based metabolomic profiling of synbiotic action of probiotic lactobacilli with aqueous garlic extract against Salmonella spp. Microbe 2025, 8, 100494. Microbe 2025, 8, 100494. [Google Scholar] [CrossRef]
- Infante-Villamil, S.; Huerlimann, R.; Condon, K.; Maes, G.E.; Jerry, D.R. Bacterial signatures of productivity decay in Penaeus monodon ponds infected with PirA toxin. Aquaculture 2019, 511, 734202. [Google Scholar] [CrossRef]
- Liu, J.D.; Liu, W.B.; Zhang, C.Y.; Xu, C.Y.; Zheng, X.C.; Zhang, D.D.; Chi, C. Dietary glutathione supplementation enhances antioxidant activity and protects against lipopolysaccharide-induced acute hepatopancreatic injury and cell apoptosis in Chinese mitten crab, Eriocheir sinensis. Fish Shellfish Immunol. 2020, 97, 440–454, Erratum in Fish Shellfish Immunol. 2020, 100, 496–497. [Google Scholar] [CrossRef]
- Zhang, C.; Chi, C.; Liu, J.; Ye, M.; Zheng, X.; Zhang, D.; Liu, W. Protective effects of dietary arginine against oxidative damage and hepatopancreas immune responses induced by T-2 toxin in Chinese mitten crab (Eriocheir sinensis). Fish Shellfish Immunol. 2020, 104, 447–456. [Google Scholar] [CrossRef]
- Gonzalez-Baro, M.R.; Coleman, R.A. Mitochondrial acyltransferases and glycerophospholipid metabolism. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2017, 1862, 49–55. [Google Scholar] [CrossRef]
- Zamer, B.A.; Shafarin, J.; Sharaf, B.; Hroub, H.A.; Soares, N.C.; Semreen, M.H.; Hamad, M.; Muhammad, J.S. Estrogen-mediated inhibition of purine metabolism and cell cycle arrest as a novel therapeutic approach in colorectal cancer. Mol. Cell. Endocrinol. 2025, 596, 112414. [Google Scholar] [CrossRef] [PubMed]
- Kennelly, C.; Tran, P.; Prindle, A. Environmental purines decrease Pseudomonas aeruginosa biofilm formation by disrupting c-di-GMP metabolism. Cell Rep. 2024, 43, 114154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ge, H.M. Recent progresses in the cyclization and oxidation of polyketide biosynthesis. Curr. Opin. Chem. Biol. 2024, 81, 102507. [Google Scholar] [CrossRef]
- Guth, F.M.; Lindner, F.; Rydzek, S.; Peil, A.; Friedrich, S.; Hauer, B.; Hahn, F. Rieske Oxygenase-Catalyzed Oxidative Late-Stage Functionalization during Complex Antifungal Polyketide Biosynthesis. ACS Chem. Biol. 2023, 18, 2450–2456. [Google Scholar] [CrossRef]
- Cheng, X.; Chen, J.; Guo, X.; Cao, H.; Zhang, C.; Hu, G.; Zhuang, Y. Disrupting the gut microbiota/metabolites axis by Di-(2-ethylhexyl) phthalate drives intestinal inflammation via AhR/NF-κB pathway in mice. Environ. Pollut. 2024, 343, 123232. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer Sequence Forward (5′-3′) | Primer Sequence Reverse (5′-3′) | Tm (°C) | Length (bp) |
|---|---|---|---|---|
| ALF2 | TCGGCAAAGTAACTGAAACTCTG | TAATGAGGCGGGGTGACAAG | 60.5 | 90 |
| Myd88 | GTGGGTCAGCTGTGGAACTAT | TCCCTGGCTTACAACCTTGTC | 59.7 | 105 |
| ProPO | GTGACCGTCCGCATCTTCAT | TGTGCCTGGCTTCAATGTGT | 60.5 | 123 |
| IL | CGATCCTACGAGTTCTTCA | GCACTTGGTGTTGTCATC | 59.5 | 125 |
| Keap1 | CTTCATGTACACTGGCGAGA | GTACAGCAAGCGTCAATCACA | 61.0 | 105 |
| CncC | GCATCCTTCTGGTACCTCGTT | CACTGCTTTGGCTCATCCTTG | 58.5 | 91 |
| β-actin | TCGTGCGAGACATCAAGGAAA | AGGAAGGAAGGCTGGAAGAGTG | 59.5 | 178 |
| Groups | Weight (g) | HIS (%) | GSI (%) | MYI (%) | FNS (%) |
|---|---|---|---|---|---|
| C♀ | 166.92 ± 24.11 a | 8.43 ± 1.02 a | 4.45 ± 1.09 b | 26.53 ± 2.31 a | 0.43 ± 0.01 a |
| C♂ | 220.75 ± 43.97 b | 8.67 ± 1.35 a | 2.86 ± 0.41 a | 29.41 ± 1.68 b | 0.46 ± 0.04 a |
| W♀ | 167.30 ± 25.06 a | 7.68 ± 0.81 b | 5.59 ± 2.07 b | 26.63 ± 1.45 a | 0.43 ± 0.02 a |
| W♂ | 221.43 ± 38.49 b | 8.01 ± 1.07 a | 3.37 ± 0.53 a | 29.08 ± 2.04 b | 0.47 ± 0.03 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Ling, J.; Li, T.; Yu, C.; Jiang, H.; Pan, T. Effects of the Wheat Crab Model and the Pond Culture Model on the Growth, Metabolism and Intestinal Microbiota of the Chinese Mitten Crab (Eriocheir sinensis). Microorganisms 2025, 13, 2396. https://doi.org/10.3390/microorganisms13102396
Yang M, Ling J, Li T, Yu C, Jiang H, Pan T. Effects of the Wheat Crab Model and the Pond Culture Model on the Growth, Metabolism and Intestinal Microbiota of the Chinese Mitten Crab (Eriocheir sinensis). Microorganisms. 2025; 13(10):2396. https://doi.org/10.3390/microorganisms13102396
Chicago/Turabian StyleYang, Min, Jun Ling, Tong Li, Chengchen Yu, He Jiang, and Tingshuang Pan. 2025. "Effects of the Wheat Crab Model and the Pond Culture Model on the Growth, Metabolism and Intestinal Microbiota of the Chinese Mitten Crab (Eriocheir sinensis)" Microorganisms 13, no. 10: 2396. https://doi.org/10.3390/microorganisms13102396
APA StyleYang, M., Ling, J., Li, T., Yu, C., Jiang, H., & Pan, T. (2025). Effects of the Wheat Crab Model and the Pond Culture Model on the Growth, Metabolism and Intestinal Microbiota of the Chinese Mitten Crab (Eriocheir sinensis). Microorganisms, 13(10), 2396. https://doi.org/10.3390/microorganisms13102396







