Clonality and the Phenotype–Genotype Correlation of Antimicrobial Resistance in Acinetobacter baumannii Isolates: A Multicenter Study of Clinical Isolates from Romania
Abstract
1. Introduction
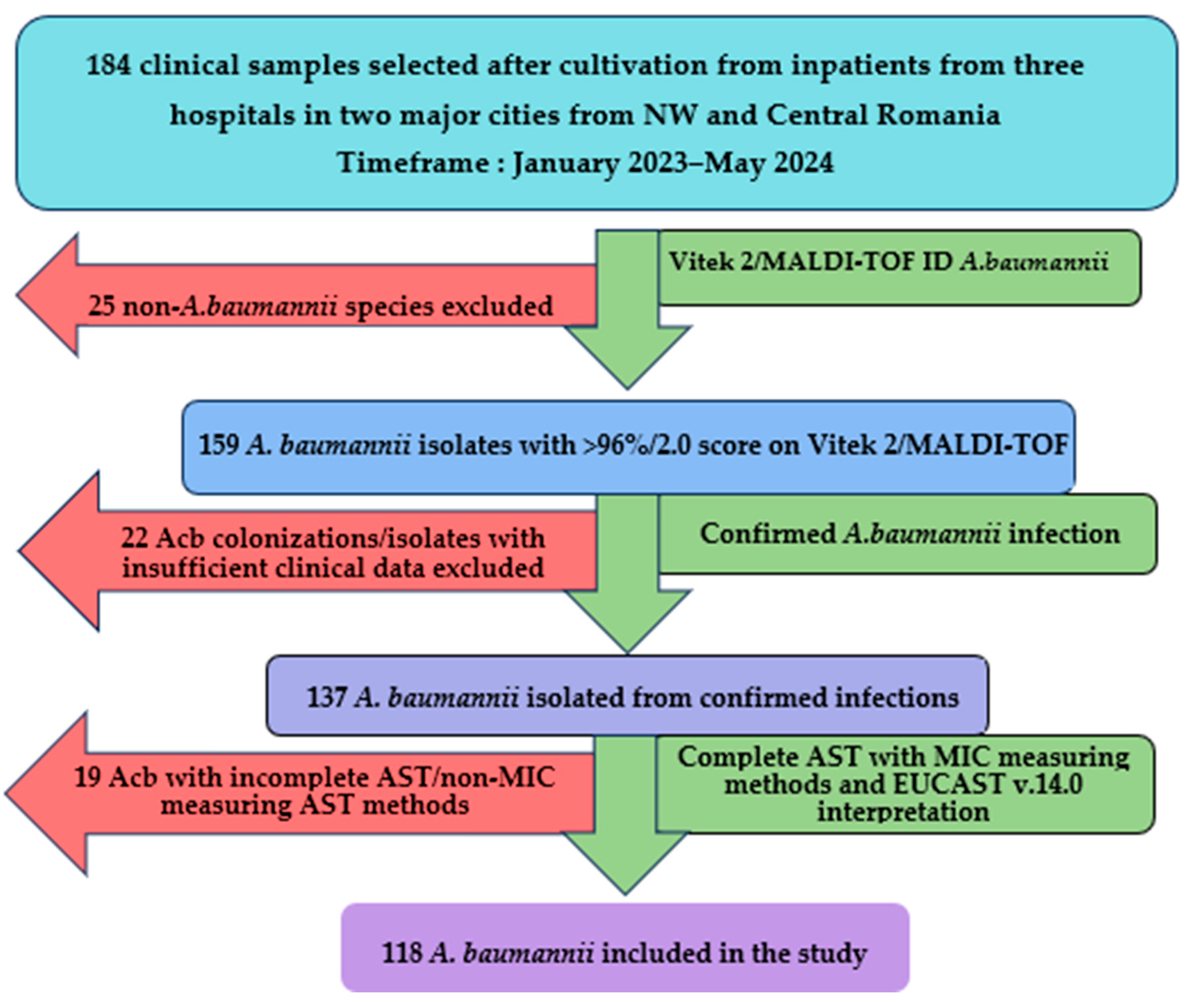
2. Materials and Methods
2.1. Study Design and Setting
2.2. Isolate Identification and Antibiotic Susceptibility Testing
2.3. Molecular Characterization of Antibiotic Resistance and Clonality
2.4. Data Analysis
3. Results
3.1. General Data
3.2. Resistance Phenotypes
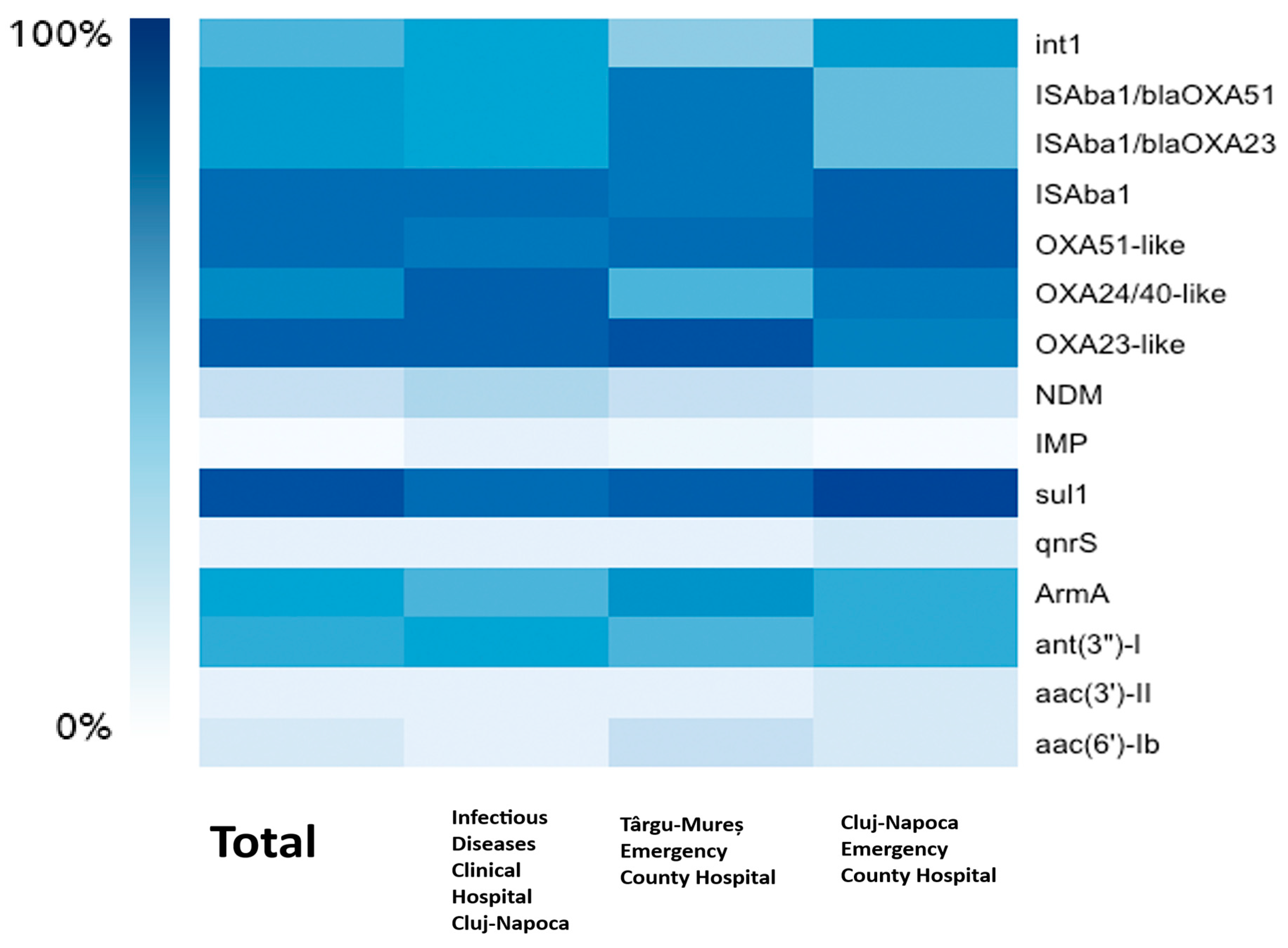
3.3. Resistance Genotypes
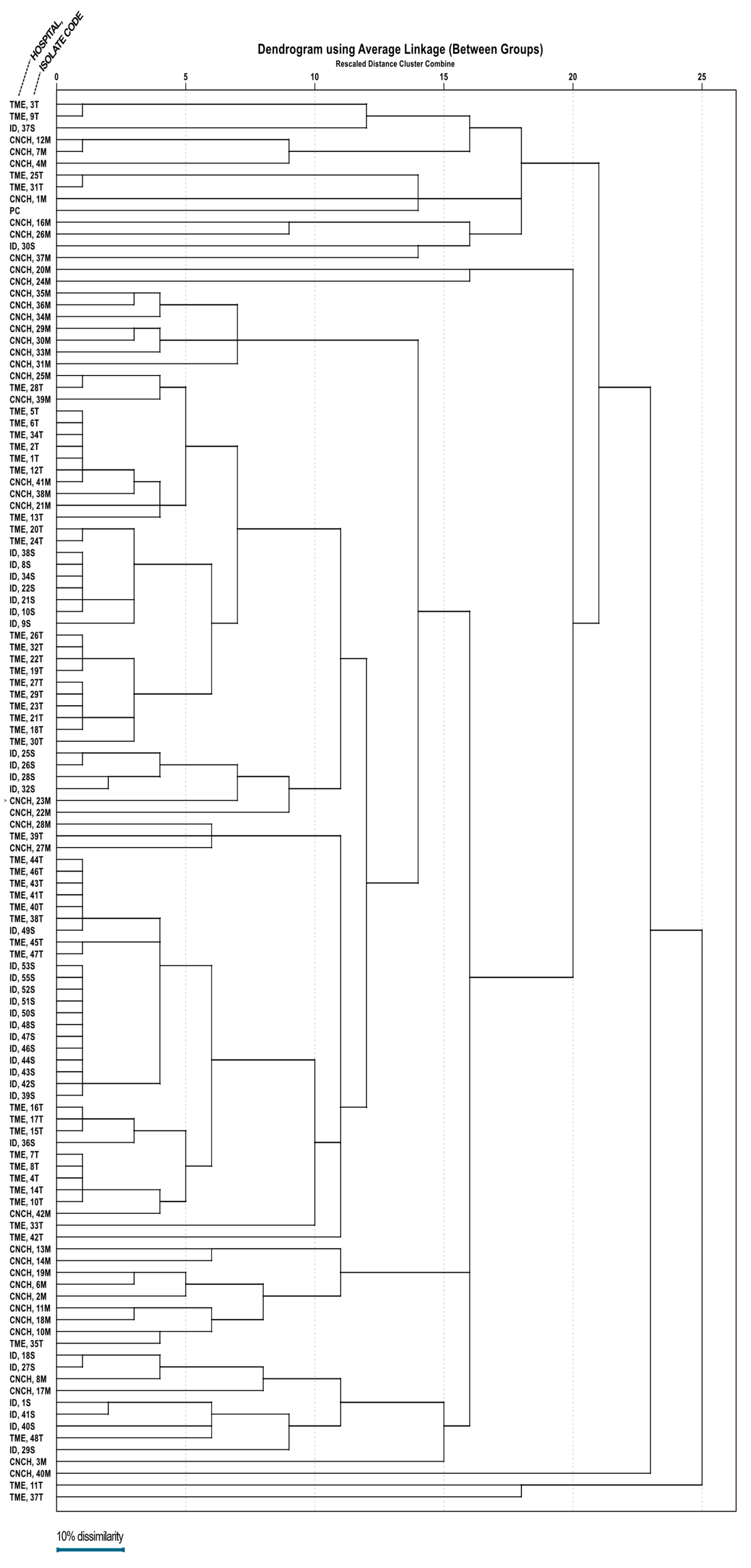
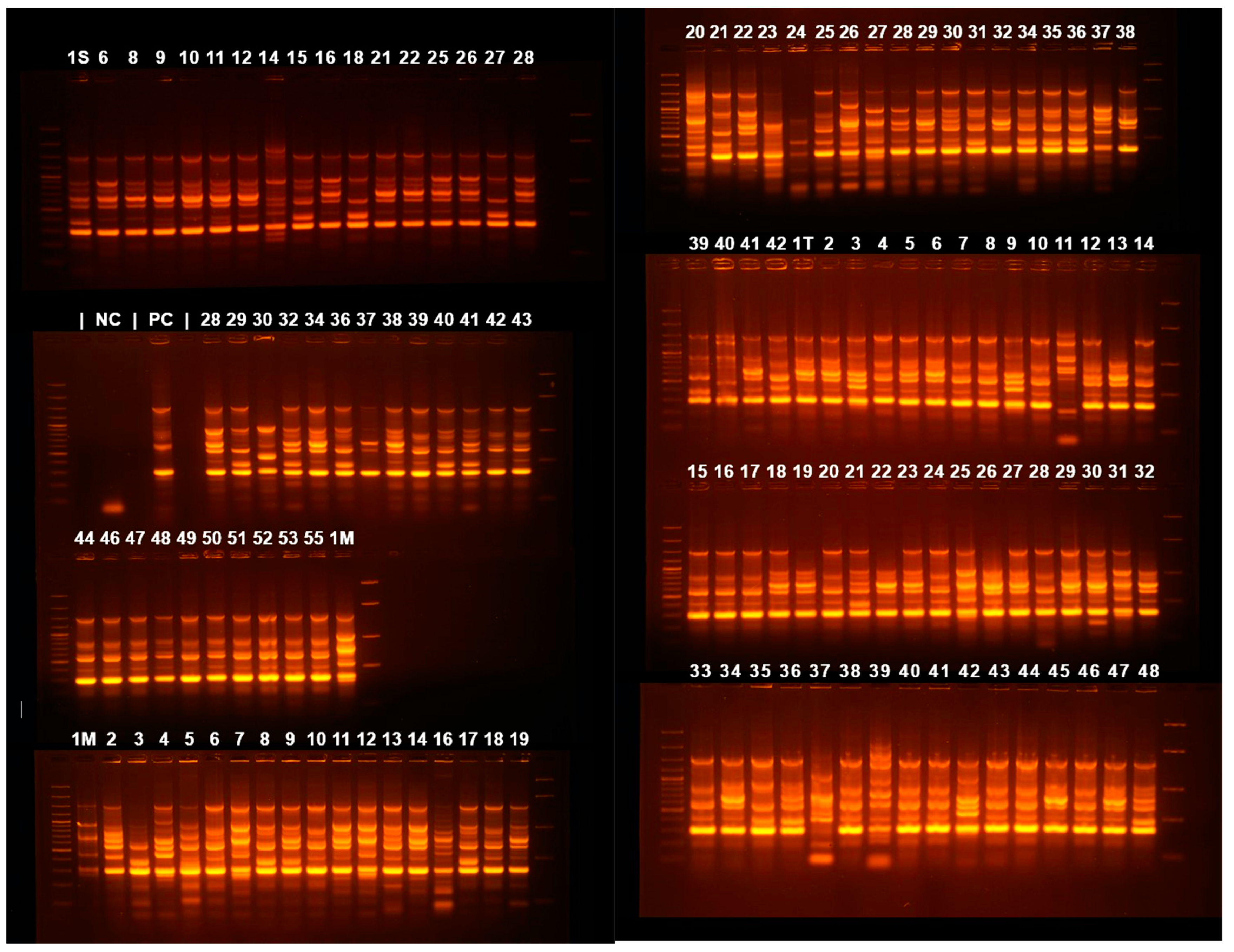
3.4. Clonality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 1 November 2022).
- Antunes, L.C.S.; Visca, P.; Towner, K.J. Acinetobacter baumannii: Evolution of a global pathogen. Pathog. Dis. 2014, 71, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Vrancianu, C.O.; Gheorghe, I.; Czobor, I.B.; Chifiriuc, M.C. Antibiotic Resistance Profiles, Molecular Mechanisms and Innovative Treatment Strategies of Acinetobacter baumannii. Microorganisms 2020, 8, 935. [Google Scholar] [CrossRef] [PubMed]
- Colquhoun, J.M.; Rather, P.N. Insights Into Mechanisms of Biofilm Formation in Acinetobacter baumannii and Implications for Uropathogenesis. Front. Cell. Infect. Microbiol. 2020, 10, 253. [Google Scholar] [CrossRef]
- Gaddy, J.A.; Tomaras, A.P.; Actis, L.A. The Acinetobacter baumannii 19606 OmpA protein plays a role in biofilm formation on abiotic surfaces and in the interaction of this pathogen with eukaryotic cells. Infect. Immun. 2009, 77, 3150–3160. [Google Scholar] [CrossRef]
- A’shimi, M.H.N.; Alattraqchi, A.G.; Rani, F.M.; Rahman, N.I.A.; Ismail, S.; Abdullah, F.H.; Othman, N.; Cleary, D.W.; Clarke, S.C.; Yeo, C.C. Biocide susceptibilities and biofilm-forming capacities of Acinetobacter baumannii clinical isolates from Malaysia. J. Infect. Dev. Ctries. 2019, 13, 626–633. [Google Scholar] [CrossRef]
- Fux, C.A.; Costerton, J.W.; Stewart, P.S.; Stoodley, P. Survival strategies of infectious biofilms. Trends Microbiol. 2005, 13, 34–40. [Google Scholar] [CrossRef]
- Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report for 2019. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2019 (accessed on 16 November 2024).
- Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report for 2022. 2022. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2022 (accessed on 16 November 2024).
- Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report for 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-eueea-ears-net-annual-epidemiological-report-2020 (accessed on 16 November 2024).
- Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report for 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2021 (accessed on 16 November 2024).
- Koncz, M.; Stirling, T.; Mehdi, H.H.; Méhi, O.; Eszenyi, B.; Asbóth, A.; Apjok, G.; Tóth, Á.; Orosz, L.; Vásárhelyi, B.M.; et al. Genomic surveillance as a scalable framework for precision phage therapy against antibiotic-resistant pathogens. Cell 2024, 187, 5901–5918.e28. [Google Scholar] [CrossRef]
- Fernández-Vázquez, J.L.; Hernández-González, I.L.; Castillo-Ramírez, S.; Jarillo-Quijada, M.D.; Gayosso-Vázquez, C.; Mateo-Estrada, V.E.; Morfín-Otero, R.; Rodríguez-Noriega, E.; Santos-Preciado, J.I.; Alcántar-Curiel, M.D. Pandrug-resistant Acinetobacter baumannii from different clones and regions in Mexico have a similar plasmid carrying the blaOXA-72 gene. Front. Cell. Infect. Microbiol. 2023, 13, 1278819. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Ioannou, P.; Samonis, G.; Kofteridis, D.P. Systematic Review of Antimicrobial Combination Options for Pandrug-Resistant Acinetobacter baumannii. Antibiotics 2021, 10, 1344. [Google Scholar] [CrossRef]
- Papathanakos, G.; Andrianopoulos, I.; Papathanasiou, A.; Koulenti, D.; Gartzonika, K.; Koulouras, V. Pandrug-resistant Acinetobacter baumannii treatment: Still a debatable topic with no definite solutions. J. Antimicrob. Chemother. 2020, 75, 3081. [Google Scholar] [CrossRef]
- Kyriakidis, I.; Vasileiou, E.; Pana, Z.D.; Tragiannidis, A. Acinetobacter baumannii Antibiotic Resistance Mechanisms. Pathogens 2021, 10, 373. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Z.; Plésiat, P.; Nikaido, H. The Challenge of Efflux-Mediated Antibiotic Resistance in Gram-Negative Bacteria. Clin. Microbiol. Rev. 2015, 28, 337–418. [Google Scholar] [CrossRef] [PubMed]
- Warburton, P.J.; Amodeo, N.; Roberts, A.P. Mosaic tetracycline resistance genes encoding ribosomal protection proteins. J. Antimicrob. Chemother. 2016, 71, 3333–3339. [Google Scholar] [CrossRef] [PubMed]
- Aldred, K.J.; Kerns, R.J.; Osheroff, N. Mechanism of Quinolone Action and Resistance. Biochemistry 2014, 53, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Vashist, J.; Tiwari, V.; Das, R.; Kapil, A.; Rajeswari, M.R. Analysis of penicillin-binding proteins (PBPs) in carbapenem resistant Acinetobacter baumannii. Indian J. Med. Res. 2011, 133, 332–338. [Google Scholar] [PubMed]
- Tiwari, V. Post-translational modification of ESKAPE pathogens as a potential target in drug discovery. Drug Discov. Today 2019, 24, 814–822. [Google Scholar] [CrossRef]
- Ambler, R.P. The structure of beta-lactamases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1980, 289, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-López, R.; Solano-Gálvez, S.G.; Vignon-Whaley, J.J.J.; Vaamonde, J.A.A.; Alonzo, L.A.P.; Reséndiz, A.R.; Álvarez, M.M.; López, E.N.V.; Franyuti-Kelly, G.; Álvarez-Hernández, D.A.; et al. Acinetobacter baumannii Resistance: A Real Challenge for Clinicians. Antibiotics 2020, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Tooke, C.L.; Hinchliffe, P.; Bragginton, E.C.; Colenso, C.K.; Hirvonen, V.H.A.; Takebayashi, Y.; Spencer, J. β-Lactamases and β-Lactamase Inhibitors in the 21st Century. J. Mol. Biol. 2019, 431, 3472–3500. [Google Scholar] [CrossRef]
- Girija, A.S.S.; Vijayashree Priyadharsini, J.; Paramasivam, A. Plasmid-encoded resistance to trimethoprim/sulfamethoxazole mediated by dfrA1, dfrA5, sul1 and sul2 among Acinetobacter baumannii isolated from urine samples of patients with severe urinary tract infection. J. Glob. Antimicrob. Resist. 2019, 17, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile Genetic Elements Associated with Antimicrobial Resistance. Clin. Microbiol. Rev. 2018, 31, 10–1128. [Google Scholar] [CrossRef]
- Noel, H.R.; Petrey, J.R.; Palmer, L.D. Mobile genetic elements in Acinetobacter antibiotic-resistance acquisition and dissemination. Ann. N. Y. Acad. Sci. 2022, 1518, 166. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G.J.; Domingues, S. Insights on the Horizontal Gene Transfer of Carbapenemase Determinants in the Opportunistic Pathogen Acinetobacter baumannii. Microorganisms 2016, 4, 29. [Google Scholar] [CrossRef]
- eucast: Clinical Breakpoints and Dosing of Antibiotics. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 30 November 2024).
- Versalovic, J.; Koeuth, T.; Lupski, J.R. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991, 19, 6823–6831. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-M.; Ke, S.-C.; Li, C.-R.; Chang, C.-C. The comparison of genotyping, antibiogram, and antimicrobial resistance genes between carbapenem-susceptible and -resistant Acinetobacter baumannii. Comp. Immunol. Microbiol. Infect. Dis. 2014, 37, 339–346. [Google Scholar] [CrossRef]
- Higgins, P.G.; Lehmann, M.; Seifert, H. Inclusion of OXA-143 primers in a multiplex polymerase chain reaction (PCR) for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int. J. Antimicrob. Agents 2010, 35, 305. [Google Scholar] [CrossRef]
- Lukovic, B.; Gajic, I.; Dimkic, I.; Kekic, D.; Zornic, S.; Pozder, T.; Radisavljevic, S.; Opavski, N.; Kojic, M.; Ranin, L. The first nationwide multicenter study of Acinetobacter baumannii recovered in Serbia: Emergence of OXA-72, OXA-23 and NDM-1-producing isolates. Antimicrob. Resist. Infect. Control 2020, 9, 101. [Google Scholar] [CrossRef]
- Cicek, A.C.; Saral, A.; Iraz, M.; Ceylan, A.; Duzgun, A.O.; Peleg, A.Y.; Sandalli, C. OXA- and GES-type β-lactamases predominate in extensively drug-resistant Acinetobacter baumannii isolates from a Turkish University Hospital. Clin. Microbiol. Infect. 2014, 20, 410–415. [Google Scholar] [CrossRef]
- Dillon, B.; Thomas, L.; Mohmand, G.; Zelynski, A.; Iredell, J. Multiplex PCR for screening of integrons in bacterial lysates. J. Microbiol. Methods 2005, 62, 221–232. [Google Scholar] [CrossRef]
- Poirel, L.; Nordmann, P. Carbapenem resistance in Acinetobacter baumannii: Mechanisms and epidemiology. Clin. Microbiol. Infect. 2006, 12, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Lv, Y.; Yuan, M.; Hu, X.; Nie, T.; Yang, X.; Li, G.; Pang, J.; Zhang, J.; Li, C.; et al. Genetic basis of high level aminoglycoside resistance in Acinetobacter baumannii from Beijing, China. Acta Pharm. Sin. B 2014, 4, 295–300. [Google Scholar] [CrossRef]
- Farkas, A.; Tarco, E.; Butiuc-Keul, A. Antibiotic resistance profiling of pathogenic Enterobacteriaceae from Cluj-Napoca, Romania. Germs 2019, 9, 17–27. [Google Scholar] [CrossRef]
- Segal, H.; Garny, S.; Elisha, B.G. Is ISABA-1 customized for Acinetobacter? FEMS Microbiol. Lett. 2005, 243, 425–429. [Google Scholar] [CrossRef]
- Cheikh, H.B.; Domingues, S.; Silveira, E.; Kadri, Y.; Rosário, N.; Mastouri, M.; Da Silva, G.J. Molecular characterization of carbapenemases of clinical Acinetobacter baumannii–calcoaceticus complex isolates from a University Hospital in Tunisia. 3 Biotech. 2018, 8, 297. [Google Scholar] [CrossRef]
- Bhandari, S.; Adhikari, S.; Karki, D.; Chand, A.B.; Sapkota, S.; Dhungel, B.; Banjara, M.R.; Joshi, P.; Lekhak, B.; Rijal, K.R. Antibiotic Resistance, Biofilm Formation and Detection of mexA/mexB Efflux-Pump Genes Among Clinical Isolates of Pseudomonas aeruginosa in a Tertiary Care Hospital, Nepal. Front. Trop. Dis. 2022, 2, 810863. [Google Scholar] [CrossRef]
- Rebelo, A.R.; Bortolaia, V.; Kjeldgaard, J.S.; Pedersen, S.K.; Leekitcharoenphon, P.; Hansen, I.M.; Guerra, B.; Malorny, B.; Borowiak, M.; Hammerl, J.A.; et al. Multiplex PCR for detection of plasmid-mediated colistin resistance determinants, mcr-1, mcr-2, mcr-3, mcr-4 and mcr-5 for surveillance purposes. Euro Surveill. 2018, 23, 17–00672. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report 2023. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-eueea-ears-net-annual-epidemiological-report-2023 (accessed on 30 November 2024).
- Papadopoulou, M.; Deliolanis, I.; Polemis, M.; Vatopoulos, A.; Psichogiou, M.; Giakkoupi, P. Characteristics of the Genetic Spread of Carbapenem-Resistant Acinetobacter baumannii in a Tertiary Greek Hospital. Genes 2024, 15, 458. [Google Scholar] [CrossRef]
- Szczypta, A.; Talaga-Ćwiertnia, K.; Kielar, M.; Krzyściak, P.; Gajewska, A.; Szura, M.; Bulanda, M.; Chmielarczyk, A. Investigation of Acinetobacter baumannii Activity in Vascular Surgery Units through Epidemiological Management Based on the Analysis of Antimicrobial Resistance, Biofilm Formation and Genotyping. Int. J. Environ. Res. Public Health 2021, 18, 1563. [Google Scholar] [CrossRef] [PubMed]
- Jalali, Y.; Liptáková, A.; Jalali, M.; Payer, J. Moving toward Extensively Drug-Resistant: Four-Year Antimicrobial Resistance Trends of Acinetobacter baumannii from the Largest Department of Internal Medicine in Slovakia. Antibiotics 2023, 12, 1200. [Google Scholar] [CrossRef]
- Matsui, M.; Suzuki, M.; Suzuki, M.; Yatsuyanagi, J.; Watahiki, M.; Hiraki, Y.; Kawano, F.; Tsutsui, A.; Shibayama, K.; Suzuki, S. Distribution and Molecular Characterization of Acinetobacter baumannii International Clone II Lineage in Japan. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Thomsen, J.; Abdulrazzaq, N.M.; AlRand, H.; The UAE AMR Surveillance Consortium; Senok, A.; Alatoom, A.; Agnes-Sonnevend-Pal; Al Hammadi, A.A.; Ahmed, A.E.; Yousef, A.F.; et al. Epidemiology and antimicrobial resistance trends of Acinetobacter species in the United Arab Emirates: A retrospective analysis of 12 years of national AMR surveillance data. Front. Public Health 2024, 11, 1245131. [Google Scholar] [CrossRef] [PubMed]
- Leal, N.C.; Campos, T.L.; Rezende, A.M.; Docena, C.; Mendes-Marques, C.L.; de Sá Cavalcanti, F.L.; Wallau, G.L.; Rocha, I.V.; Cavalcanti, C.L.B.; Veras, D.L.; et al. Comparative Genomics of Acinetobacter baumannii Clinical Strains From Brazil Reveals Polyclonal Dissemination and Selective Exchange of Mobile Genetic Elements Associated With Resistance Genes. Front. Microbiol. 2020, 11, 1176. [Google Scholar] [CrossRef] [PubMed]
- Lob, S.H.; Hoban, D.J.; Sahm, D.F.; Badal, R.E. Regional differences and trends in antimicrobial susceptibility of Acinetobacter baumannii. Int. J. Antimicrob. Agents 2016, 47, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Arowolo, M.T.; Orababa, O.Q.; Olaitan, M.O.; Osibeluwo, B.V.; Essiet, U.U.; Batholomew, O.H.; Ogunrinde, O.G.; Lagoke, O.A.; Soriwei, J.D.; Ishola, O.D.; et al. Prevalence of carbapenem resistance in Acinetobacter baumannii and Pseudomonas aeruginosa in sub-Saharan Africa: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0287762. [Google Scholar] [CrossRef]
- Sanchez-Urtaza, S.; Ocampo-Sosa, A.; Molins-Bengoetxea, A.; El-Kholy, M.A.; Hernandez, M.; Abad, D.; Shawky, S.M.; Alkorta, I.; Gallego, L. Molecular Characterization of Multidrug Resistant Acinetobacter baumannii Clinical Isolates from Alexandria, Egypt. Front. Cell. Infect. Microbiol. 2023, 13, 1208046. [Google Scholar] [CrossRef]
- Beig, M.; Parvizi, E.; Navidifar, T.; Bostanghadiri, N.; Mofid, M.; Golab, N.; Sholeh, M. Geographical mapping and temporal trends of Acinetobacter baumannii carbapenem resistance: A comprehensive meta-analysis. PLoS ONE 2024, 19, e0311124. [Google Scholar] [CrossRef]
- Antibiotic-Resistant Bacteria Could Pose Major Health Threat Across Asia. Available online: https://www.birmingham.ac.uk/news/2024/antibiotic-resistant-bacteria-could-pose-major-health-threat-across-asia (accessed on 6 January 2025).
- Challapa-Mamani, M.R.; Yareta, J.; Fajardo-Loyola, A.; Asmat Marrufo, P.; Siesquen, C.P.; Pino-Dueñas, J.; Meza-Fernández, H.; Cruz-Vargas, J.A.D.L.; Marcos-Carbajal, P. Acinetobacter baumannii Co-Resistant to Extended Spectrum Beta-Lactamases and Carbapenemases in Six Peruvian Hospital Centers. Microbiol. Res. 2024, 15, 2650–2660. [Google Scholar] [CrossRef]
- Turton, J.F.; Ward, M.E.; Woodford, N.; Kaufmann, M.E.; Pike, R.; Livermore, D.M.; Pitt, T.L. The role of ISAba1 in expression of OXA carbapenemase genes in Acinetobacter baumannii. FEMS Microbiol. Lett. 2006, 258, 72–77. [Google Scholar] [CrossRef]
- Su, P.-W.; Yang, E.C.; Moi, S.-H.; Yang, C.-H.; Chuang, L.-Y. Prevalence of Carbapenem Resistance Genes among Acinetobacter baumannii Isolated from a Teaching Hospital in Taiwan. Antibiotics 2023, 12, 1357. [Google Scholar] [CrossRef]
- Chagas, T.P.G.; Carvalho, K.R.; de Oliveira Santos, I.C.; Carvalho-Assef, A.P.D.; Asensi, M.D. Characterization of carbapenem-resistant Acinetobacter baumannii in Brazil (2008–2011): Countrywide spread of OXA-23–producing clones (CC15 and CC79). Diagn. Microbiol. Infect. Dis. 2014, 79, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.H.; Chan, B.K.; Chan, E.W.; Chen, S. Over-Expression of ISAba1-Linked Intrinsic and Exogenously Acquired OXA Type Carbapenem-Hydrolyzing-Class D-ß-Lactamase-Encoding Genes Is Key Mechanism Underlying Carbapenem Resistance in Acinetobacter baumannii. Front. Microbiol. 2019, 10, 2809. [Google Scholar] [CrossRef] [PubMed]
- Nigro, S.J.; Hall, R.M. Structure and context of Acinetobacter transposons carrying the oxa23 carbapenemase gene. J. Antimicrob. Chemother. 2016, 71, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Vila, J.; Martí, S.; Sánchez-Céspedes, J. Porins, Efflux Pumps and Multidrug Resistance in Acinetobacter Baumannii. J. Antimicrob. Chemother. 2007, 59, 1210–1215. Available online: https://academic.oup.com/jac/article-abstract/59/6/1210/711566?redirectedFrom=fulltext&login=false&utm_source=chatgpt.com (accessed on 1 December 2024). [CrossRef] [PubMed]
- Kherroubi, L.; Bacon, J.; Rahman, K.M. Navigating fluoroquinolone resistance in Gram-negative bacteria: A comprehensive evaluation. JAC-Antimicrob. Resist. 2024, 6, dlae127. [Google Scholar] [CrossRef]
- Siddhardha, B.; Dyavaiah, M.; Syed, A. (Eds.) Model Organisms for Microbial Pathogenesis, Biofilm Formation and Antimicrobial Drug Discovery; Springer: Singapore, 2020; ISBN 9789811516948. [Google Scholar]
- Hameed, F.; Khan, M.A.; Muhammad, H.; Sarwar, T.; Bilal, H.; Rehman, T.U. Plasmid-mediated mcr-1 gene in Acinetobacter baumannii and Pseudomonas aeruginosa: First report from Pakistan. Rev. Soc. Bras. Med. Trop. 2019, 52, e20190237. [Google Scholar] [CrossRef]
- Papazachariou, A.; Tziolos, R.-N.; Karakonstantis, S.; Ioannou, P.; Samonis, G.; Kofteridis, D.P. Treatment Strategies of Colistin Resistance Acinetobacter baumannii Infections. Antibiotics 2024, 13, 423. [Google Scholar] [CrossRef] [PubMed]
- Sorlí, L.; Luque, S.; Grau, S.; Berenguer, N.; Segura, C.; Montero, M.M.; Álvarez-Lerma, F.; Knobel, H.; Benito, N.; Horcajada, J.P. Trough colistin plasma level is an independent risk factor for nephrotoxicity: A prospective observational cohort study. BMC Infect. Dis. 2013, 13, 380. [Google Scholar] [CrossRef]
- Dalfino, L.; Puntillo, F.; Ondok, M.J.M.; Mosca, A.; Monno, R.; Coppolecchia, S.; Spada, M.L.; Bruno, F.; Brienza, N. Colistin-associated Acute Kidney Injury in Severely Ill Patients: A Step Toward a Better Renal Care? A Prospective Cohort Study. Clin. Infect. Dis. 2015, 61, 1771–1777. [Google Scholar] [CrossRef]
- Darwish, M.M.; Catalan, M.I.; Wilson, T.; McGlynn, C.C.; Suhd-Brondstatter, J.; Dow, A.L.; Kingsley, A.; Reilly, M.E.; Cohen, S.H.; Desai, A.N. Hospital outbreak of Carbapenem-resistant acinetobacter baumannii in the context of local facility transmission. Am. J. Infect. Control 2024, 52, 739–741. [Google Scholar] [CrossRef]
- McDonald, L.C.; Banerjee, S.N.; Jarvis, W.R. Seasonal variation of Acinetobacter infections: 1987–1996. Nosocomial Infections Surveillance System. Clin. Infect. Dis. 1999, 29, 1133–1137. [Google Scholar] [CrossRef]
- Spellberg, B.; Bonomo, R.A. “Airborne assault”: A new dimension in Acinetobacter baumannii transmission*. Crit. Care Med. 2013, 41, 2042–2044. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, M.D.; Voor in ’t holt, A.F.; Alp, E.; Hell, M.; Petrosillo, N.; Presterl, E.; Tsakris, A.; Severin, J.A.; Vos, M.C.; on behalf of the ESCMID Study Group for Nosocomial Infections (ESGNI). Infection prevention and control policies in hospitals and prevalence of highly resistant microorganisms: An international comparative study. Antimicrob. Resist. Infect. Control 2022, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Cataldo, M.A.; Dancer, S.J.; Angelis, G.D.; Falcone, M.; Frank, U.; Kahlmeter, G.; Pan, A.; Petrosillo, N.; Rodríguez-Baño, J.; et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin. Microbiol. Infect. 2014, 20, 1–55. [Google Scholar] [CrossRef]
- Wong, V.W.Y.; Huang, Y.; Wei, W.I.; Wong, S.Y.S.; Kwok, K.O. Approaches to multidrug-resistant organism prevention and control in long-term care facilities for older people: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2022, 11, 7. [Google Scholar] [CrossRef]
| Gene Name | Primer Sequence (5′-3′) | Annealing t °C | Amplicon Size | Reference |
|---|---|---|---|---|
| blaOXA-23-like | Fw-GATCGGATTGGAGAACCAGA | 58 °C | 501 bp | [31] |
| Rev-ATTTCTGACCGCATTTCCAT | ||||
| blaOXA-24/40-like | Fw-GGTTAGTTGGCCCCCTTAAA | 59 °C | 246 bp | [31] |
| Rev-AGTTGAGCGAAAAGGGGATT | ||||
| blaOXA-51-like | Fw-TAATGCTTTGATCGGCCTTG | 58 °C | 353 bp | [31] |
| Rev-TGGATTGCACTTCATCTTGG | ||||
| blaOXA-143-like | Fw-TGGCACTTTCAGCAGTTCCT | 61 °C | 149 bp | [32] |
| Rev-TAATCTTGAGGGGGCCAACC | ||||
| blaNDM | Fw-GGTTTGGCGATCTGGTTTTC | 61 °C | 621 bp | [33] |
| Rev-CGGAATGGCTCATCACGATC | ||||
| blaVIM | Fw-ATTGGTCTATTTGACCGCGTC | 58 °C | 780 bp | [34] |
| Rev-TGCTACTCAACGACTGAGCG | ||||
| blaIMP | Fw-GGAATAGAGTGGCTTAATTC | 52 °C | 232 bp | [31] |
| Rev-TCGGTTTAATAAAACAACCACC | ||||
| blaSIM | Fw-TACAAGGGATTCGGCATCG | 59 °C | 570 bp | [35] |
| Rev-TAATGGCCTGTTCCCATGTG | ||||
| blaSPM | Fw-AAAATCTGGGTACGCAAACG | 57 °C | 271 bp | [36] |
| Rev-ACATTATCCGCTGGAACAGG | ||||
| aac(6′)-Ib | Fw-CATGACCTTGCGATGCTCTA | 62 °C | 490 bp | [37] |
| Rev – GCTCGAATGCCTGGCGTCTT | ||||
| aac(3′)-IIc | Fw-ACGCGGAAGGCAATAACGGA | 61 °C | 854 bp | [37] |
| Rev-TAACCTGAAGGCTCGCAAGA | ||||
| ant(3”)-I | Fw-TGATTTGCTGGTTACGGTGAC | 46 °C | 284 bp | [37] |
| Rev-CGCTATGTTCTCTTGCTTTTG | ||||
| aph(3′)-IIb | Fw-ATGCATGATGCAGCCACCTCC | 51 °C | 804 bp | [37] |
| Rev-CTAGAAGAACTCGTCCAATAGCCT | ||||
| ArmA | Fw-TGGGAAGTTAAAGACGACGA | 56 °C | 315 bp | [37] |
| Rev-CCATTCCCTTCTCCTTTCCA | ||||
| sul1 | Fw-AGGCATGATCTAACCCTCGG | 62 °C | 665 bp | [38] |
| Rev-GGCCGATGAGATCAGACGTA | ||||
| qnrA | Fw-AGTTTGATGGTTGCCGCTTT | 59 °C | 541 bp | [38] |
| Rev-TCTTCATTGATCTGCACGCC | ||||
| qnrB | Fw-TCGTGCGATGCTGAAAGATG | 60 °C | 368 bp | [38] |
| Rev-CCGAATTGGTCAGATCGCAA | ||||
| qnrS | Fw-TGATCTCACCTTCACCGCTT | 60 °C | 496 bp | [38] |
| Rev-GAGTTCGGCGTGGCATAAAT | ||||
| ISAba1 | Fw-CACGAATGCAGAAGTTG | 51 °C | 550 bp | [39] |
| Rev-CGACGAATACTATGACAC | ||||
| ISAba1/blaOXA-23-like | Fw- AATGATTGGTGACAATGAAG | 55 °C | 1433 bp | [40] |
| Rev- ATTTCTGACCGCATTTCCAT | ||||
| ISAba1/blaOXA-51-like | Fw- AATGATTGGTGACAATGAAG | 56 °C | 1252 bp | [40] |
| Rev- TGGATTGCACTTCATCTTGG | ||||
| MexA | Fw-ATCAACCTGCGCTACACCAAG | 61 °C | 291 bp | [41] |
| Rev-AGGCCTTCGGTAATGATCTTGT | ||||
| MexB | Fw-TTTCATTGATAGGCCCATTTTC | 57 °C | 353 bp | [41] |
| Rev-AGGGTCTTCACTACCTCATGGA | ||||
| mcr-1 | Fw-CGGTCAGTCCGTTTGTTC | 58 °C | 320 bp | [42] |
| Rev-CTTGGTCGGTCTGTAGGG |
| Integron Class | Targeted Gene | Primer Sequence (5′-3′) | Annealing t °C | Amplicon Size | Reference |
|---|---|---|---|---|---|
| Class I | int1 | Fw-CAGTGGACATAAGCCTGTTC | 58 °C | 160 bp | [38] |
| Rev-CCCGACGCATAGACTGTA | |||||
| Class II | int2 | Fw-TTGCGAGTATCCATAACCTG | 788 bp | [38] | |
| Rev-TTACCTGCACTGGATTAAGC |
| ERIC-PCR Primer | Primer Sequence (5′-3′) | Reference |
|---|---|---|
| ERIC1R | TGTAAGCTCCTGGGGATTCAC | [30] |
| ERIC2 | AAGTAAGTGACTGGGGTGAGCG |
| Data | Nr. and % of Inpatients |
|---|---|
| Sex | |
| Male | 70 (59.32%) |
| Female | 48 (40.67%) |
| Age group (years old) | |
| <18 | 4 (3.38%) |
| 18–30 | 3 (2.54%) |
| 31–45 | 5 (4.23%) |
| 46–60 | 24 (20.33%) |
| 61–75 | 45 (38.13%) |
| >76 | 37 (31.35%) |
| Admission ward | |
| ICU | 50 (42.37%) |
| Medical wards | 20 (16.94%) |
| General surgery | 17 (14.4%) |
| Neurology | 10 (8.47%) |
| Neurosurgery | 8 (6.77%) |
| Orthopedics | 3 (2.54%) |
| Nephrology | 3 (2.54%) |
| Cardiology | 2 (1.69%) |
| Cardiovascular surgery | 2 (1.69%) |
| Obstetrics–Gynecology | 1 (0.84%) |
| Immunosuppressed patients ward | 1 (0.84%) |
| Neonatology | 1 (0.84%) |
| Type of infection | |
| Respiratory tract infections | 54 (45.76%) |
| Sepsis | 32 (27.11%) |
| Wound infections | 20 (16.94%) |
| Urinary tract infections | 6 (5.08%) |
| Abscess | 3 (2.54%) |
| Peritonitis | 2 (1.69%) |
| Meningitis | 1 (0.84%) |
| Collected specimen | |
| Tracheal aspirate | 52 (44.06%) |
| Blood | 22 (18.64%) |
| Wound exudate | 22 (18.64%) |
| Urine | 6 (5.08%) |
| Bronchoalveolar lavage fluid | 5 (4.23%) |
| Sputum | 4 (3.38%) |
| Pus | 2 (1.69%) |
| Central venous catheter tip | 2 (1.69%) |
| Peritoneal fluid | 2 (1.69%) |
| Cerebrospinal fluid | 1 (0.84%) |
| Outcome | |
| Deceased | 64 (54.23%) |
| Worsened | 1 (0.84%) |
| Stationary | 13 (11.01%) |
| Improved | 34 (28.81%) |
| Cured | 6 (5.08%) |
| Negative/Positive (0/1) | Meropenem | Imipenem | p-Value Meropenem | p-Value Imipenem | |||
|---|---|---|---|---|---|---|---|
| S | R | S | R | ||||
| blaOXA-23-like | 0 | 2 | 8 | 2 | 8 | NS | NS |
| 1 | 3 | 105 | 3 | 105 | |||
| blaOXA-24/40-like | 0 | 1 | 29 | 1 | 29 | NS | NS |
| 1 | 4 | 84 | 4 | 84 | |||
| blaOXA-51-like | 0 | 3 | 10 | 3 | 10 | p < 0.01 | p < 0.01 |
| 1 | 2 | 103 | 2 | 103 | |||
| ISAba1 | 0 | 2 | 12 | 2 | 12 | NS | NS |
| 1 | 3 | 101 | 3 | 101 | |||
| ISAba1/blaOXA-23-like | 0 | 5 | 33 | 5 | 33 | p < 0.01 | p < 0.01 |
| 1 | 0 | 80 | 0 | 80 | |||
| ISAba1/blaOXA-51-like | 0 | 5 | 33 | 5 | 33 | p < 0.01 | p < 0.01 |
| 1 | 0 | 80 | 0 | 80 | |||
| blaNDM | 0 | 5 | 84 | 5 | 84 | NS | NS |
| 1 | 0 | 29 | 0 | 29 | |||
| blaIMP | 0 | 5 | 112 | 5 | 112 | NS | NS |
| 1 | 0 | 1 | 0 | 1 | |||
| Negative/Positive (0/1) | Amikacin | Gentamycin | Tobramycin | p-Value Amikacin | p-Value Gentamycin | p-Value Tobramycin | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| S | R | S | R | S | R | |||||
| aac(6′)-Ib | 0 | 10 | 90 | 8 | 92 | 12 | 88 | NS | NS | NS |
| 1 | 0 | 18 | 1 | 17 | 1 | 17 | ||||
| aac(3′)-II | 0 | 9 | 101 | 9 | 101 | 12 | 98 | NS | NS | NS |
| 1 | 1 | 7 | 0 | 8 | 1 | 7 | ||||
| ant(3”)-I | 0 | 7 | 42 | 5 | 44 | 8 | 41 | NS | NS | NS |
| 1 | 3 | 66 | 4 | 65 | 5 | 64 | ||||
| ArmA | 0 | 8 | 35 | 8 | 35 | 9 | 34 | p < 0.01 | p < 0.01 | p < 0.05 |
| 1 | 2 | 73 | 1 | 74 | 4 | 71 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pană, A.-G.; Șchiopu, P.; Țoc, D.A.; Neculicioiu, V.S.; Butiuc-Keul, A.; Farkas, A.; Dobrescu, M.-Ș.; Flonta, M.; Costache, C.; Szász, I.É.; et al. Clonality and the Phenotype–Genotype Correlation of Antimicrobial Resistance in Acinetobacter baumannii Isolates: A Multicenter Study of Clinical Isolates from Romania. Microorganisms 2025, 13, 176. https://doi.org/10.3390/microorganisms13010176
Pană A-G, Șchiopu P, Țoc DA, Neculicioiu VS, Butiuc-Keul A, Farkas A, Dobrescu M-Ș, Flonta M, Costache C, Szász IÉ, et al. Clonality and the Phenotype–Genotype Correlation of Antimicrobial Resistance in Acinetobacter baumannii Isolates: A Multicenter Study of Clinical Isolates from Romania. Microorganisms. 2025; 13(1):176. https://doi.org/10.3390/microorganisms13010176
Chicago/Turabian StylePană, Adrian-Gabriel, Pavel Șchiopu, Dan Alexandru Țoc, Vlad Sever Neculicioiu, Anca Butiuc-Keul, Anca Farkas, Matei-Ștefan Dobrescu, Mirela Flonta, Carmen Costache, Izabella Éva Szász, and et al. 2025. "Clonality and the Phenotype–Genotype Correlation of Antimicrobial Resistance in Acinetobacter baumannii Isolates: A Multicenter Study of Clinical Isolates from Romania" Microorganisms 13, no. 1: 176. https://doi.org/10.3390/microorganisms13010176
APA StylePană, A.-G., Șchiopu, P., Țoc, D. A., Neculicioiu, V. S., Butiuc-Keul, A., Farkas, A., Dobrescu, M.-Ș., Flonta, M., Costache, C., Szász, I. É., & Junie, L.-M. (2025). Clonality and the Phenotype–Genotype Correlation of Antimicrobial Resistance in Acinetobacter baumannii Isolates: A Multicenter Study of Clinical Isolates from Romania. Microorganisms, 13(1), 176. https://doi.org/10.3390/microorganisms13010176









