Abstract
Antibiotic resistance is on the WHO’s top 10 list of global public health threats due to its rapid emergence and spread but also because of the high morbidity and mortality associated with it. Amongst the main species driving this phenomenon is A. baumannii, a member of the ESKAPE group of medical assistance-associated infections causing species famous for its extensively drug-resistant phenotypes. Our findings note a 91.52% frequency of extensively drug-resistant carbapenem-resistant A. baumannii (XDR CRAB) phenotype amongst clinical isolates from multiple hospitals in two major cities from northwestern and central Romania, harboring multiple antibiotic resistance genes such as blaOXA-23-like in 108 (91.5%) isolates, blaOXA-24/40-like in 88 (74.6%) isolates, blaNDM in 29 (25%) isolates, ArmA in 75 (63.6%) isolates, and ant(3″)-I in 69 (58.5%) isolates and sul1 in 113 (95.76%) isolates. The isolates, although nearly identical in phenotype, displayed different genotypical profiles, with varying degrees of similarity across hospitals and cities, raising the possibility of both local outbreaks of a single clone and widespread dissemination of resistant isolates.
1. Introduction
Antimicrobial resistance is rapidly emerging as one of the most critical public health challenges, approaching almost pandemic-level concerns. Acinetobacter baumannii, a Gram-negative, non-fermentative bacillus of the Moraxellaceae family, is among the species that most prominently exemplify this issue. In 2018, the World Health Organization (WHO) included A. baumannii in the ESKAPE group (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, A. baumannii, Pseudomonas aeruginosa, Enterobacter spp.), a collection of highly virulent bacterial pathogens. Furthermore, A. baumannii was designated as a top priority in research for antibiotic resistance patterns and novel drug development [1].
The medical importance of A. baumannii is determined by both its remarkable ability to survive in hospital environments and its implication in severe healthcare-associated infections with high mortality rates [2]. Multiple mechanisms may explain the environmental fitness and high virulence of A. baumannii: the ability to form biofilms on both epithelia and abiotic surfaces, metabolism modulatory capacity in adverse environments, and extensive drug resistance to most classes of antibiotics and antiseptics [3,4,5,6,7]. While some of its resistance is intrinsic (resistance to macrolides, fosfomycin, trimethoprim, penicillin, and cephalosporins), the concern mainly lies with acquired resistance, with three defined phenotypes.
The first phenotype of concern is multi-drug-resistant or MDR A. baumannii (resistant to at least one antibiotic from two separate classes). The second extensively drug-resistant carbapenem-resistant A. baumannii phenotype is XDR CRAB. Extensive drug resistance is defined as nonsusceptibility to at least one antibiotic from all but two or fewer antibiotic classes. This phenotype has been shown to be the most frequently encountered in clinical settings all across Eastern Europe ever since 2019 [8,9,10,11,12]. CRAB encompasses acquired resistance to carbapenems, aminoglycosides, quinolones, and sulfonamides. In some cases, CRAB can evolve into a third phenotype: pan-drug-resistant (PDR) isolate by acquiring resistance to colistin, a last-resort antibiotic frequently used in CRAB infections [13,14,15,16].
Antibiotic resistance mechanisms can be classified into three categories. Resistance may be attained by limiting access to the target through decreased membrane permeability or enhanced antibiotic efflux [17,18,19], by shielding the target through post-translational modifications or genetic mutations [18,20,21], or by directly inactivating the antibiotic molecule through structural alteration or complete hydrolysis [18,19,22,23,24,25]. The presence of these mechanisms in A. baumannii may be explained through its remarkable genetic flexibility. This adaptation allows for quick genetic changes and rearrangements as well as the acquisition of foreign determinants carried by mobile genetic elements (such as plasmids, transposons, integrons, and insertion sequences) [26,27]. By incorporating these elements, containing various types of antibiotic resistance genes (ARGs), A. baumannii gains the ability to protect itself from antibiotics from other bacterial species populating the hospital setting, a process known as HGT (horizontal gene transfer) [28].
While most studies that examine XDR CRAB isolates from clinical isolates seem to focus mostly on identifying carbapenemase-producing ARGs, it is equally important to assess the presence of ARGs associated with other classes of antibiotics that are monitored and utilized. The aim of this paper was to provide an extended description of the A. baumannii ARG resistome through phenotype–genotype correlation and to highlight the clonality relations between the isolates isolated from multiple hospital units across Romania, a country severely affected by CRAB and with scarce data regarding its isolation and its antimicrobial resistance patterns [10].
2. Materials and Methods
2.1. Study Design and Setting
A total of 118 clinical isolates of A. baumannii from confirmed infections have been collected between January 2023 and May 2024 from three major university hospitals (Cluj-Napoca Emergency County Hospital, Cluj-Napoca Infectious Diseases Clinical Hospital, and Târgu-Mureș Emergency County Hospital) in the largest two cities (Cluj-Napoca, Târgu-Mureș) in the northwestern and central development regions of Romania. For each, clinical isolate data were collected regarding gender, age, hospitalization period, the type of infection caused by A. baumannii, and the outcome of the patient (cured, improved, stationary, aggravated, and deceased). No personal or identifiable data were extracted regarding the patients. Furthermore, all patients signed informed consent forms upon admission in all three teaching hospitals. The distinction between colonization and infection was made based on clinical assessment, imaging results, and laboratory tests in accordance with national and international guidelines.
2.2. Isolate Identification and Antibiotic Susceptibility Testing
Identification of the isolates was achieved using matrix-assisted laser desorbtion ionization-time of flight mass spectrometry (MALDI-TOF MS) from Bruker (Billerica, MA, USA) with Bruker® flexControl and MBT Compass software (version 4.1.80) and VITEK 2 Compact with VITEK 2® GN identification cards (bioMérieux, Inc., Marcy l’Etoile, France). Antimicrobial resistance was determined using automated MIC determination techniques with VITEK 2® AST cards (AST-N222, N376, N397—bioMérieux, Inc., Marcy l’Etoile, France) and Bruker® Micronaut IVD System 96-well titer plates. Interpretation of the obtained resistance patterns was performed according to the v.14.0 EUCAST 2024 guidelines for the following antibiotics: meropenem, imipenem, amikacin, gentamicin, tobramycin, ciprofloxacin, trimethoprim-sulfamethoxazole, and colistin [29].
2.3. Molecular Characterization of Antibiotic Resistance and Clonality
Isolates were tested for 22 ARGs, mexA and mexB genes encoding the MexAB-OprM efflux pump, integrase genes for class I and II integrons, and insertion sequence ISAba1. For the insertion sequence, its presence upstream of blaOXA-23 and blaOXA-51 was also tested using the forward primer from ISAba1 and reverse primer from each specific gene.
Clonality was studied using the Enterobacterial Intergenic Consensus-PCR (ERIC-PCR) molecular typing method as previously described [30]. The list of the tested ARGs, integrons, and ERIC-PCR specific primers (Eurogentec, Liège, Belgium) and their respective amplicon size are found in Table 1, Table 2 and Table 3.

Table 1.
PCR primer gene names, sequences, annealing temperatures, and amplicon sizes for the detected antibiotic resistance genes and MexAB efflux pumps.

Table 2.
PCR primer gene names, sequences, annealing temperatures, and amplicon sizes for integrase genes amplifications.

Table 3.
PCR primer gene names and sequences for ERIC-PCR amplification.
Isolates were stored at −20 °C in a mixture of peptone water and Noble agar and subcultured on Columbia agar with 5% defibrinated sheep blood for genotypic analysis. A 0.5 McFarland bacterial suspension was prepared from each subculture, using distilled water as the suspension medium to facilitate cell wall lysis through hypoosmotic stress. This suspension was used as template for the PCR. An amount of 12.5 μL of DreamTaq Green PCR master mix (2×) (Thermo Fisher Scientific, Waltham, MA, USA), 10.25 μL of nuclease-free water (Lonza, Basel, Switzerland), 25 pmol of each primer (Eurogentec, Belgium), and 2 μL of bacterial suspension were mixed, yielding a 25 μL total reaction volume.
The general PCR program was 5 min at 94 °C initial denaturation stage followed by 35 cycles of 30 s at 94 °C denaturation, 45 s annealing with temperatures varying according to each primer pair (annealing temperatures listed in Table 1, Table 2 and Table 3), and 30 s at 72 °C elongation, with a final elongation of 8 min at 72 °C. For ERIC-PCR, the program had to be modified for the simultaneous accurate detection of multiple amplicons: 5 min at 94 °C initial denaturation stage followed by 5 cycles of 5 min at 94 °C denaturation, 5 min at 38 °C annealing, and 5 min at 72 °C elongation, followed by 30 cycles of 1 min at 94 °C denaturation, 1 min at 48 °C annealing, and 5 min at 72 °C elongation, with a final elongation of 10 min at 72 °C. Negative control was made using only primers, master mix, and nuclease-free water. For the positive control, a bacterial suspension of A. baumannii ATCC 19606 was used as template. TProfessional Trio (Analytik Jena, Jena, Germany) and Mastercycler Nexus (Eppendorf AG, Hamburg, Germany) thermocyclers were used for the PCR reaction. Agarose gel electrophoresis was used to separate the amplicons, using 1.5% w/v agarose (Cleaver Scientific, Rugby, UK) in 1 × TAE buffer (Lonza, Basel, Switzerland) stained with 0.5 μg/mL ethidium bromide (Thermo Fisher Scientific, Waltham, MA, USA) for the preparation of the gel. First well of each row was loaded with 10 μL of 100 bp GeneRuler DNA ladder (Thermo Fisher Scientific, Waltham, MA, USA). The gel was visualized under UV light, and data capture was conducted with the BDA Digital Compact System and BioDocAnalyze Software (version 2.64.11.20, Analytik Jena, Jena, Germany).
2.4. Data Analysis
The presence of ARGs amplicons and ERIC-PCR profiles were indicated by the absence (marked 0) or presence (marked 1) of bands in the expected position according to their molecular weight in comparison with the DNA ladder. Data analysis and dendrogram assembly for the ERIC-PCR patterns was conducted with DARwin (Dissimilarity Analysis and Representation for Windows) from Cirad (version 6, Montpellier, France) using a Dice coefficient for the calculus of dissimilarity and an UPGMA (unweighted pair group method with arithmetic mean) for hierarchical clustering for the dendrogram.
R (version 4.4.1, The R Foundation, Vienna, Austria), RStudio (version 2024.09.0+375, Posit Software, Boston, MA, USA), and IBM SPSS Statistics (version 27.0.1.0, Armonk, NY, USA) were used to conduct statistical tests. We used the Chi-square test (two-sided) and Fisher’s exact test (two-sided) on a case-by-case basis to assess the association between categorical variables, taking into account the sample size and distribution of data. Specifically, the Chi-square test was applied when the sample size was large, and the expected frequency in each cell of the contingency table met or exceeded the threshold of 5, ensuring the validity of the test assumptions. Fisher’s exact test was employed as an alternative to the Chi-square test in scenarios where the sample size was small or when the expected frequency in one or more cells of the contingency table fell below 5. Statistical significance was considered at p < 0.05.
3. Results
3.1. General Data
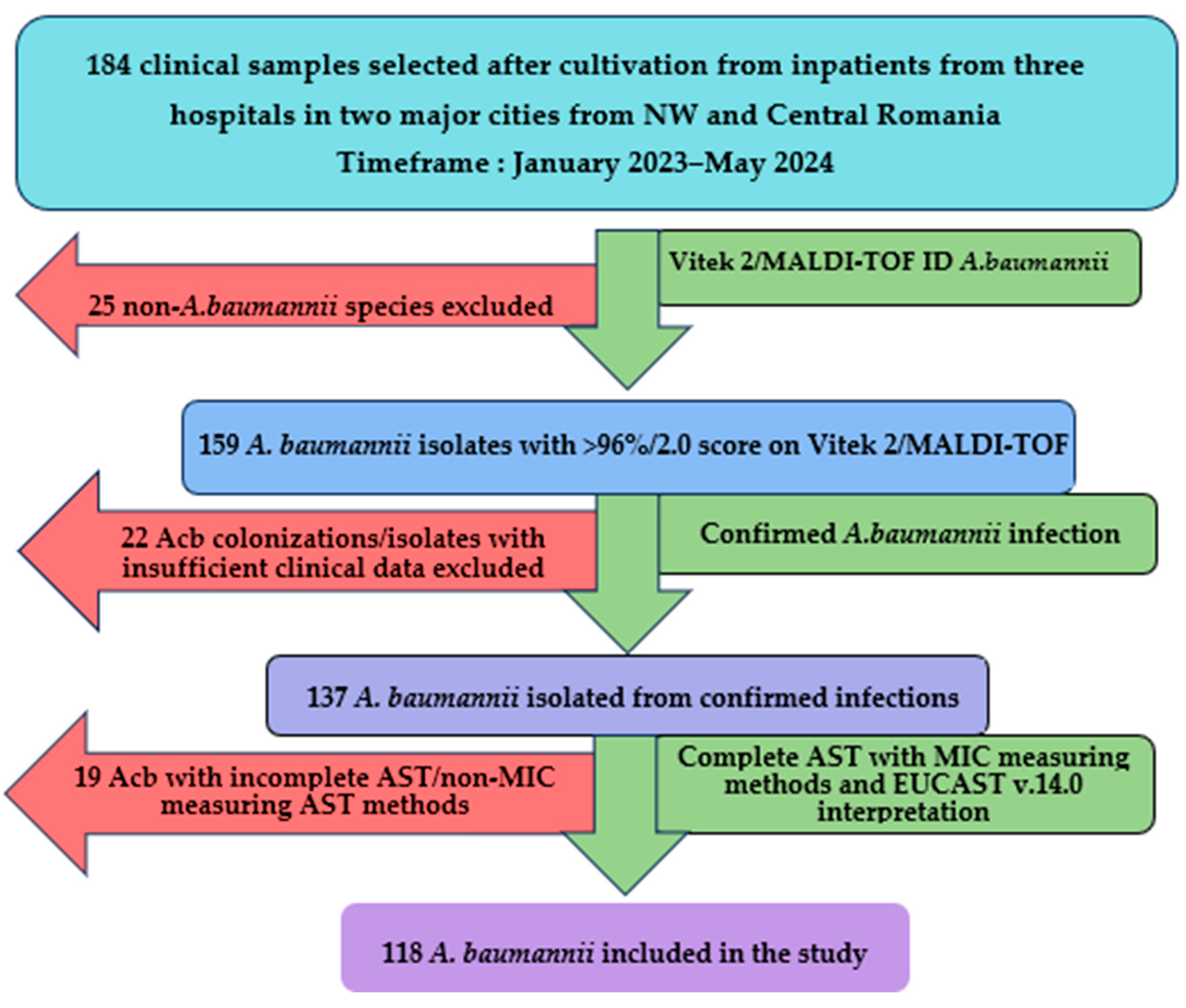
Between January 2023 and May 2024, a total of 184 clinical samples were analyzed following the culture of Gram-negative, lactose-negative colonies in aerobic conditions at 37 °C for at least 24 h incubation on multiple selective and differential culture media (MacConkey agar, CLED, URISelect by BioRad, Hercules, CA, USA). Following the exclusion of 66 isolates, a total of 118 isolates (64.13%) of A. baumannii were included in the study and validated for genotypical and molecular typing analysis, as shown in Figure 1.
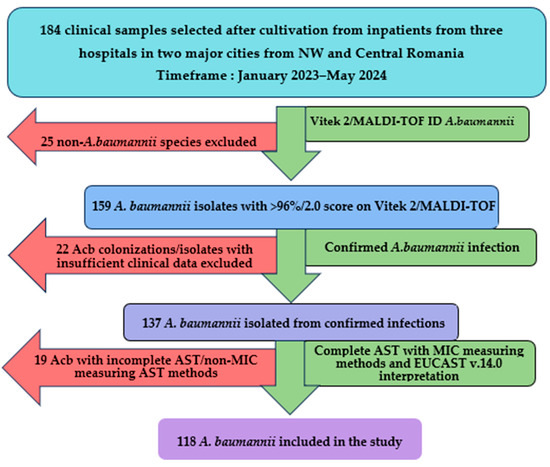
Figure 1.
A. baumannii isolates inclusion flowchart. Acb—A. baumannii, NW—northwestern, AST—antibiotic sensibility testing, MIC—minimum inhibitory concentration.
Out of the 118 A. baumannii isolates included in the study, the majority were isolated from respiratory tract infections (n = 54; 45.76%) and sepsis (n = 32; 27.11%) of male patients (n = 70; 59.32%) between 61 and 75 years (n = 45; 38.13%) or older (n = 37; 31.35%). Most of the infections were reported in the intensive care unit (ICU) (n = 50; 42.37%). An exceedingly high mortality rate was observed (n = 64/118; 54.23%), with 40 out of 118 patients (33.89%) being declared improved or cured. Antibiotic therapy was mostly performed with Colistin (n = 57, 48.3%) or antibiotic combinations with Colistin (Colistin + Meropenem and Colistin + Tigecycline being the most frequent options used in 21 (17.79%) and 8 cases (6.77%), respectively). More information regarding clinical data about the origin of the isolates is found in Table 4.

Table 4.
General and clinical data regarding inpatients with A. baumannii infections.
3.2. Resistance Phenotypes
Out of the 118 included isolates, the XDR CRAB phenotype was the most frequently encountered (n = 108; 91.52%). The remaining 10 isolates were classified as susceptible (n = 5; 4.23%), MDR (n = 1; 0.84%), or PDR (n = 4; 3.38%). All XDR isolates showed resistance to both imipenem and meropenem, ciprofloxacin, and trimethoprim-sulfamethoxazole and maintained sensitivity to colistin. Resistance to all three tested aminoglycosides was found in 96 out of 108 XDR isolates (88.88%), with the other 12 isolates (11.11%) being sensitive to one or two of the three aminoglycosides. Three of the PDR isolates were isolated from Târgu-Mureș Emergency County Hospital and the other one from Cluj-Napoca Emergency County Hospital. Three of the isolates were isolated from patients with respiratory tract infections and the other one from a postoperative wound infection. No statistical significance was observed between the resistance phenotype and the hospitals, wards, or type of infection the isolates were isolated from (p > 0.05).
3.3. Resistance Genotypes
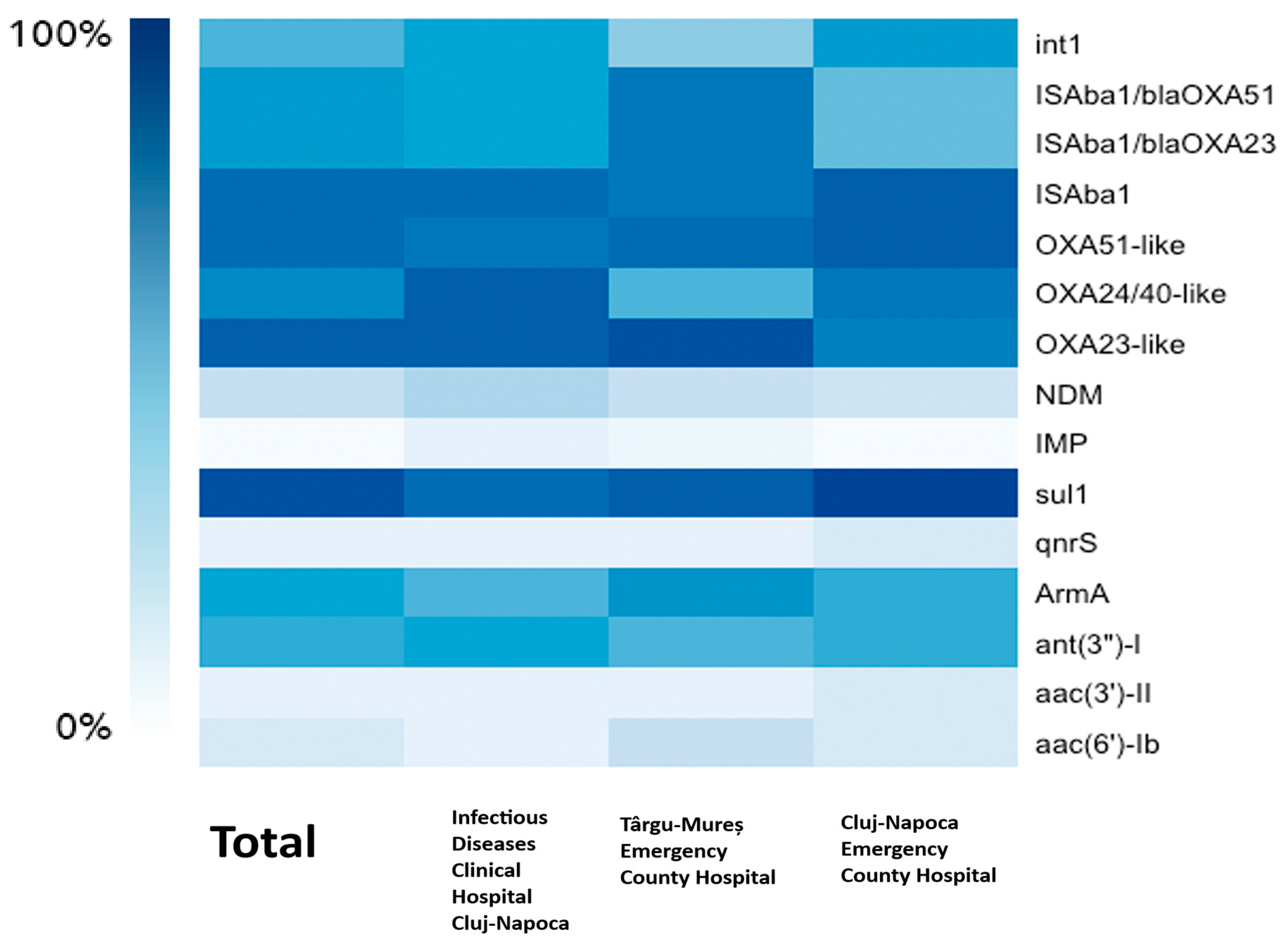
For resistance to beta-lactams, we have detected genes encoding Ambler class B MBL (metallo-beta-lactamase) and class D (oxacillinase) beta-lactamases. The analysis encompassed 118 A. baumannii isolates confirmed by MALDI-TOF or Vitek 2 analysis, with accuracy scores > 2.0 for MALDI-TOF and >96% identification probability (excellent identification) for Vitek. However, only 105 isolates (89%) were positive for the chromosomally encoded blaOXA-51-like gene. The blaOXA-23-like gene was found in 108 (91.5%) isolates and blaOXA-24/40-like in 88 (74.6%) isolates. No isolate has been found to harbor the blaOXA-143-like gene. For the metallo-beta-lactamase-encoding genes blaNDM was found in 29 (25%) isolates and blaIMP in only 1 isolate (0.84%) from Cluj-Napoca Emergency County Hospital. blaVIM, blaSIM, and blaSPM were not found in any of the 118 isolates. Insertion sequence Aba1 (ISAba1) was found in 104 (88%) isolates, while its placement upstream of blaOXA-23-like and blaOXA-51-like for the upregulation of the aforementioned gene’s expression was detected in 80 (68%) isolates. Aminoglycoside resistance was tested by searching for various classes of AMEs (aminoglycoside-modifying enzymes) and 16S rRNA methyltransferase. ArmA (75; 63.6%) and ant(3”)-I (69; 58.5%) were the most common genes found, and aac(6′)-Ib and aac(3′)-II were less prevalent, occurring in 15.3% and 6.8% of isolates, respectively. aph(3′)-IIb was not found in any isolate. For quinolone resistance, Qnr protein-encoding genes were screened for, with only 9 isolates (7.6%) exhibiting qnrS without statistical significance (p = 1) and none exhibiting qnrA and qnrB. With a prevalence of 113 out of 118 isolates (95.8%), the sulfonamide resistance gene sul1 is the most frequent ARG found in our study, being statistically significant as well (p = 0.02). No PDR isolate tested positive for MexA and MexB or mcr-1 genes. Last but not least, 64 isolates (54.2%) had the class 1 integron gene int1, a crucial indicator for gene acquisition and horizontal gene transfer. Details about the phenotype–genotype correlations and statistical significance for beta-lactams and aminoglycosides are displayed in Table 5 and Table 6. A heatmap of the frequency of the ARGs for each hospital can be found in Figure 2.

Table 5.
A contingency table of the frequency of beta-lactamase-encoding ARGs and resistance phenotype.

Table 6.
Contingency table of aminoglycoside ARGs and resistance phenotype frequency.
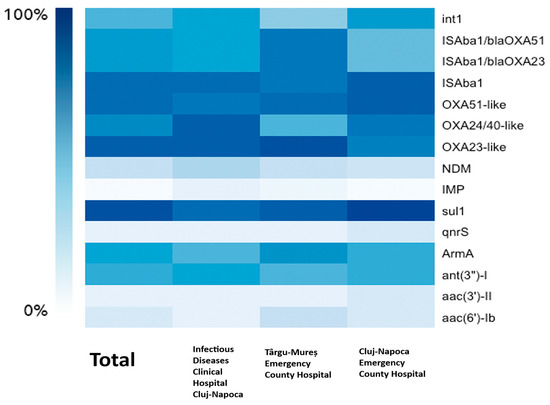
Figure 2.
The correlation between the hospitals and the frequency of the ARGs.
3.4. Clonality
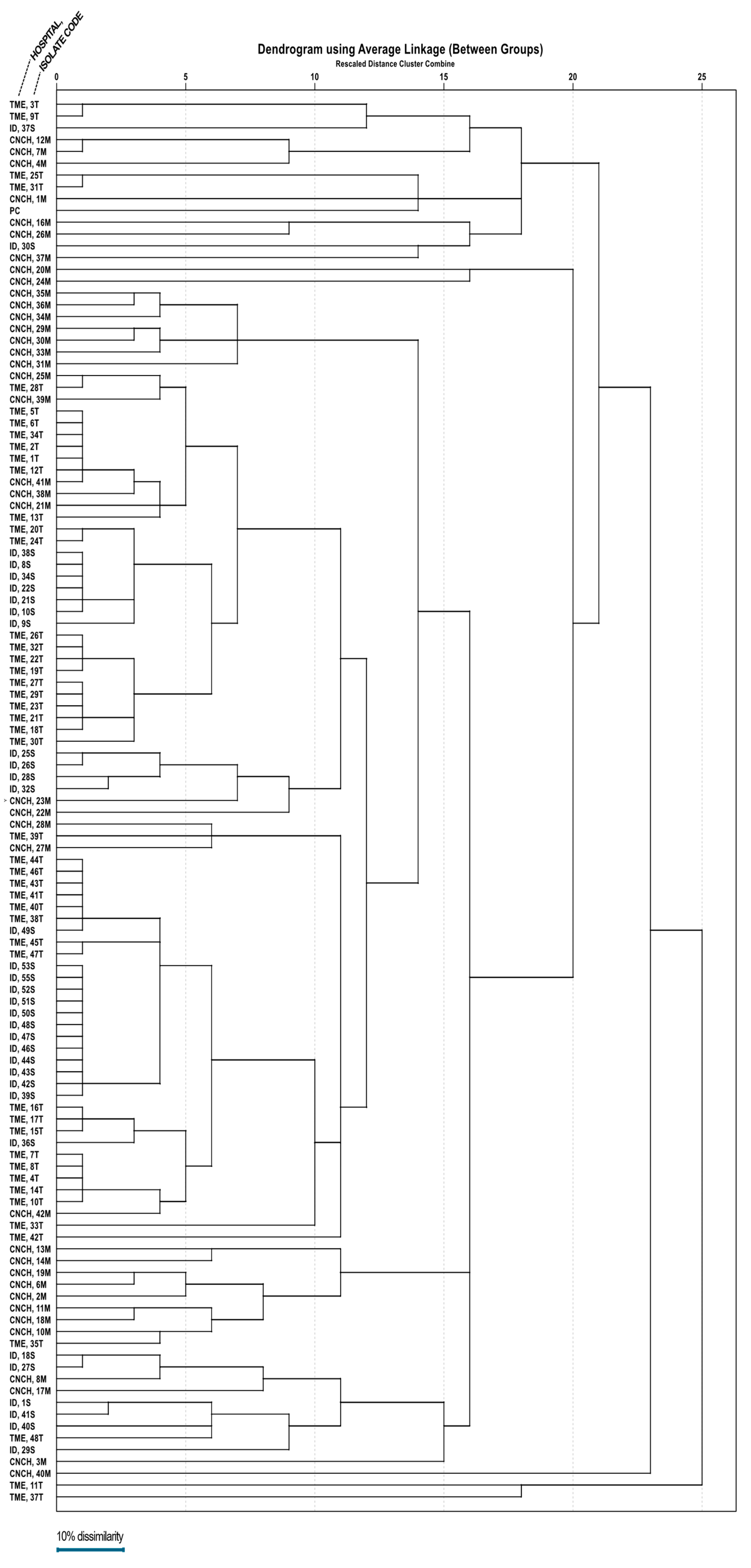
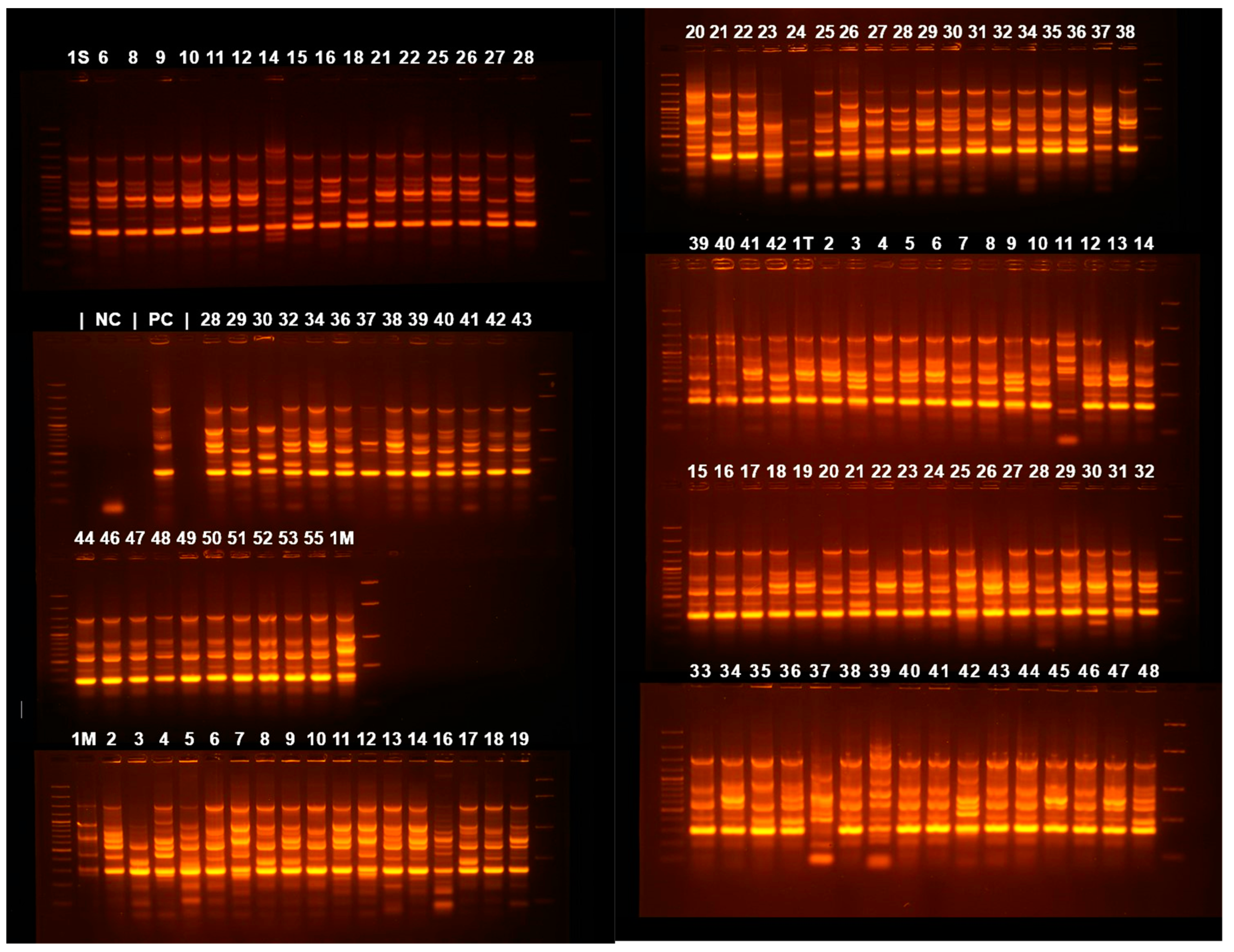
A dendrogram (Figure 3) was assembled based on ERIC-PCR electrophoresis patterns (Figure 4) of isolates obtained from all three hospitals. Mean dissimilarity was computed for each isolate group, and the total study population and clusters were designated following the establishment of a 0.1 threshold limit (90% similarity) as a reference point. The analysis showed that isolates from the Infectious Diseases Clinical Hospital had the highest degree of similarity (79.7%), isolates grouping into two main clusters under the 10% dissimilarity threshold. In comparison, the lowest degree of similarity belongs to the Cluj-Napoca Emergency County Hospital isolate group (57.35%) with five different clusters being established in order to encompass all isolates from this hospital. The total study population had a similarity percentage of 65.01%, being grouped into two main clusters, similar to the Infectious Diseases Clinical Hospital isolate group.
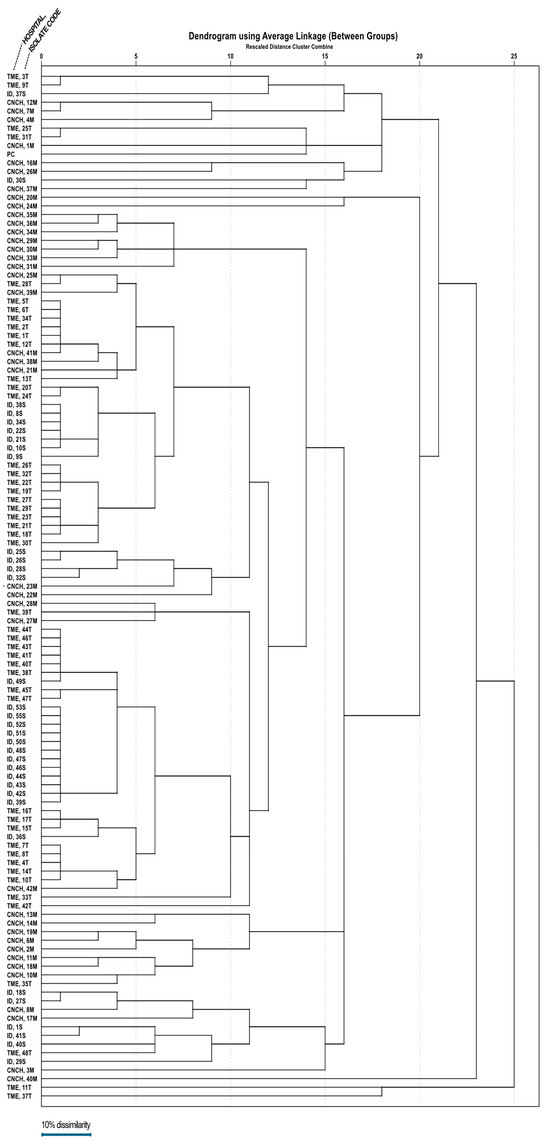
Figure 3.
The dendrogram representing clonality relations of 118 A. baumannii strains isolated from the three major hospitals in northwestern and central Romania.
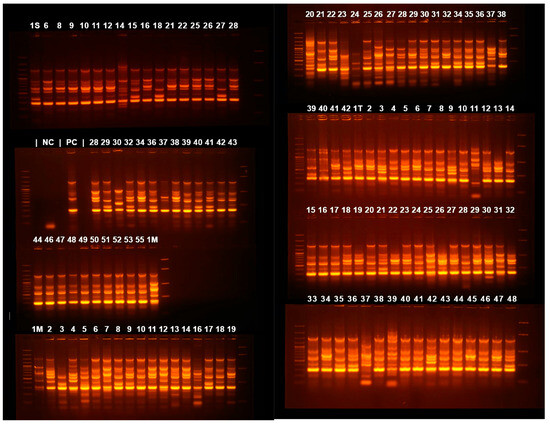
Figure 4.
Agarose gel electrophoresis of ERIC-PCR amplification products. 1S—55 are samples from the Cluj-Napoca Infectious Diseases Clinical Hospital; 1M—55 from Cluj-Napoca Emergency County Hospital; 1T—42 from Târgu-Mureș Emergency County Hospital.
4. Discussion
The importance of A. baumannii as a causative agent of medical assistance-associated infections has been in the spotlight of microbiological and epidemiological research ever since its discovery in the late 1980s, gaining the top spot since its introduction into the ESKAPE list in 2017 by the WHO [1]. Although this species’ XDR CRAB phenotype represents a global concern nowadays, its presence in Southern and Eastern Europe is especially concerning due to its frequency of >50% of all reported isolates as of 2023 [12,43]. For a better understanding of the environmental resilience and spread of this phenotype, a characterization of its underlying genotypic profile and molecular fingerprinting studies are needed. To the best of our knowledge and database searches (PubMed, Web of Science), this is the first study on clinical isolates from confirmed infections obtained from different hospital settings in multiple cities across Romania.
Universal resistance to carbapenems (imipenem and meropenem), ciprofloxacin, and trimethoprim-sulfamethoxazole in XDR isolates confirms the limited efficacy of these antibiotics in treating various types of A. baumannii infections. In our study, the overwhelming majority of isolates (91.52%) were XDR, emphasizing the widespread presence of highly resistant bacteria in clinical environments. A study conducted in Serbia, a neighboring country in Eastern Europe, showed similar results in the predominant types of infection caused (respiratory infections, sepsis, and wound infections) and in the XDR CRAB phenotype isolation frequency (222 out of 237 isolates, 93.7%) [33]. Studies on isolates from Greece, Poland, and Slovakia report similarly high XDR phenotype isolation frequencies as well [44,45,46].
International reports show a variable but relatively less frequent presence of this phenotype [47,48,49], finding confirmed by a study on regional differences [50]. Recent studies from African countries provide updated data on the prevalence and resistance phenotypes of A. baumannii, with a meta-analysis covering data from sub-Saharan Africa between 2012 and 2022 finding a pooled prevalence of carbapenem-resistant A. baumannii (CRAB) at 8% (95% CI: 2–17%). Although this prevalence is relatively low compared to other regions, it underscores the impact CRAB phenotype has on African healthcare settings, particularly in intensive care units (ICUs) during this period [51]. A more recent study from Egypt in 2023 highlights a significant shift in resistance patterns. Analyzing 36 clinical isolates of A. baumannii, researchers reported markedly higher resistance rates compared to earlier studies (>90% of strains resistant to β-lactams (including carbapenems), 85–95% of strains resistant to fluoroquinolones and aminoglycosides, and 80% of strains resistant to trimethoprim-sulfamethoxazole) [52]. A comprehensive meta-analysis of A. baumannii isolates from six Asian countries, including China, India, Iran, Japan, South Korea, and Thailand, revealed high carbapenem resistance rates, with a pooled prevalence of resistance of 76.1% for imipenem, 73.5% for meropenem, and 74.3% for carbapenems overall. Resistance to meropenem peaked at 80.7% during the period from 2020 to 2023, indicating an upward trend in carbapenem resistance across the region [53]. In China, a study conducted in an intensive care unit (ICU) in Hangzhou over a three-month period revealed that 80.9% of A. baumannii isolates were carbapenem resistant (CRAB), with a newly emerging strain, ST164, accounting for 40.2% of the samples. This strain exhibited higher levels of antibiotic resistance compared to previously dominant strains, reflecting a significant shift in the resistance phenotype landscape [54]. In Latin America, the situation is equally concerning. A study conducted across six public hospital centers in Peru found that 85% of isolates were CRAB phenotypes, with a significant proportion harboring OXA-type carbapenemase genes, such as blaOXA-23 and blaOXA-24/40 [55].
The high frequency of an oxacillinase-encoding blaOXA-23-like gene (91.5% of isolates) and blaOXA-24/40-like gene (74.6% of isolates) highlights their involvement in the resistance phenotype. Alongside them, the blaNDM gene (25% of isolates) and blaIMP gene (0.84% of isolates) contribute to the carbapenem-resistant phenotype. In contrast, the blaOXA-51-like gene, a chromosomally encoded species marker, was only identified in 89% of isolates in spite of the very accurate identification using MALDI-TOF-MS and Vitek 2. This finding possibly reflects mutations in the primer binding sites or the involvement of ISAba1 or another insertion sequence being present upstream of the blaOXA-51-like gene, which could alter the sequence near the primer binding sites, thus preventing the primers from annealing effectively [56]. However, technical PCR limitations cannot be entirely ruled out. This finding was similar to another study that found 87.6% of isolates positive for this gene [57]. We have also identified the insertion sequence ISAba1 in 104 isolates (88%), and its upstream placement relative to the blaOXA-23-like and blaOXA-51-like genes was observed in 80 isolates (68%). When ISAba1 is located upstream of an oxacillinase-encoding gene, it can lead to higher expression levels of the oxacillinase enzyme, enhancing resistance to carbapenems like imipenem or meropenem [31,44,56,58]. Overexpressed oxacillinases (due to ISAba1) work synergistically with MBLs, leading to higher-level resistance in comparison with the effect of either enzyme alone. This combination, along with A. baumannii-derived cephalosporinase (an Ambler C class chromosomally encoded beta-lactamase), makes infections very difficult to treat, as these enzymes target different categories of beta-lactam antibiotics, leaving few effective therapeutic options [59]. One possible explanation for the presence of ISAba1 upstream of the blaOXA-23 gene is that the isolates may have acquired it by means of one of the four transposons containing this sequence [60].
Resistance to all three tested aminoglycosides was found in 88.88% of XDR isolates, with 11.11% retaining partial sensitivity, which might provide limited therapeutic options in combination regimens. The large proportion of ArmA (63.6%) and ant(3”)-I (58.5%) genes, encoding a 16S rRNA methyltransferase and an aminoglycoside-modifying enzyme, respectively, reinforces their role in mediating high-level aminoglycoside resistance. Less prevalent genes such as aac(6′)-Ib (15.3%) and aac(3′)-II (6.8%) also contribute to the resistance landscape to a lesser extent. In total, all isolates displaying one of the aforementioned genes were resistant to at least one aminoglycoside. By contrast, there were 11 XDR isolates (9.32%) with resistance to two or all three aminoglycosides and none of the tested AME or 16S rRNA methyltransferase genes present, implying that there are other possible mechanisms responsible for aminoglycoside resistance, such as efflux pumps and modification of membrane permeability [61].
Quinolone resistance was identified in all MDR and XDR isolates, with only nine isolates (7.62%) testing positive for qnrS protein protecting DNA gyrase and topoisomerase IV from the effect of quinolones. This finding suggests that the main driver of quinolone resistance in A. baumannii hospital isolates lies in other mechanisms, point mutations in gyrA and parC genes encoding DNA gyrase, and topoisomerase IV having the highest impact alongside efflux pumps [62].
For trimethoprim-sulfamethoxazole resistance, the focus is on acquiring mechanisms generating sulfonamide resistance since A. baumannii is intrinsically resistant to trimethoprim due to its lack of effect on this species’ dihydrofolate reductase (DHFR) enzyme. The main mechanism of resistance is the production of modified dihydropteroate synthase (DHPS) enzymes that cannot be inhibited by sulfonamides, production mediated by sul1, sul2, and sul3 genes. This process is confirmed by our study findings, sul1 genes being present in 113 isolates (95.76%). However, we also discovered three resistant isolates (2.54%) with no sul1 gene, where resistance to sulfonamides may be due to other structures [63].
Colistin was the primary antibiotic used to treat A. baumannii infections, administered as monotherapy in 48.3% of cases and in combination regimens, most commonly with meropenem (17.79%) or tigecycline (6.77%). Sensitivity to colistin remains generally intact, consistent with its status as a last-resort treatment, though concerns about emerging colistin resistance remain (3.38% of the isolates in these studies were PDR). For the colistin-resistant isolates, we tested for the acquisition of mcr-1 gene encoding a phosphoethanolamine transferase, which modifies the lipid A in the outer membrane and mexA and mexB genes encoding proteins of the MexAB-OprM efflux pump, with none of the isolates testing positive. The literature describes the aforementioned mechanisms of colistin resistance predominantly in Escherichia coli and Pseudomonas aeruginosa, but they can be present in A. baumannii through HGT [64]. The continued reliance on colistin, despite its well-documented nephrotoxicity and neurotoxicity [65,66,67], underscores its critical role in managing extensively drug-resistant infections. Combination therapies are often employed to enhance its efficacy and mitigate resistance. These findings highlight the limited treatment options available and the significant challenges in managing A. baumannii infections.
The in-depth association between resistance phenotypes and resistance genotype patterns could have multiple implications for diagnosis and future antibiotic usage optimization. Screening for ISAba1 upstream of resistance genes, especially OXA-type carbapenemase genes, could predict upregulated resistance phenotypes, supporting early and targeted therapeutic interventions. Screening for molecular markers that can be integrated into diagnostic tools will enable a rapid detection of resistant strains, even before conventional culture and susceptibility tests are completed. Furthermore, understanding the resistome highlights potential targets for new antimicrobials or adjuvants. For example, inhibitors of efflux pumps or enzymes encoded by genes like blaOXA-23-like could restore the efficacy of existing antibiotics.
A high similarity between isolates might be indicative of a cluster of carbapenem-resistant A. baumannii involved in a hospital outbreak rather than being community acquired since this species has been previously implicated in such outbreaks [68]. Local transmission of Acinetobacter spp. is facilitated by its persistence on abiotic surfaces and colonization of the hands of healthcare workers [69,70]. We found differences in isolate similarity between hospitals, suggesting a variability in transmission. However, the lack of statistical association between resistance phenotypes and hospitals, wards, or infection types suggests a widespread dissemination of resistant isolates rather than localized outbreaks.
Differences in infection prevention and control (IPC) measures can greatly impact the clonal similarity of bacteria between hospitals. This hypothesis is confirmed by studies regarding the correlation between IPCs and the frequency of multi-drug-resistant species isolation from clinical settings. Fewer IPC measures were generally implemented by hospitals with the highest prevalence of multi-drug-resistant species. Those who have the lowest multi-drug-resistant species prevalence adopt more IPC measures. They also better adhere to their policies. Multi-drug-resistance endemicity or resource limitations may cause differences in IPC strategies. Increased adherence to IPC policies could lead to a large reduction in the prevalence of such isolates, thereby benefiting overall public health and safety [71,72,73].
5. Conclusions
A. baumannii is a notoriously efficient species when it comes to survivability in hospital settings and medical assistance-associated infections. This is in part due to the high prevalence of XDR isolates with limited therapeutic options to counter them, with colistin often being the only viable antibiotic. However, reliance on colistin heightens the risk of resistance development through selective pressure, emphasizing the urgent need for novel antimicrobial agents and combination therapies. The widespread distribution of XDR isolates across hospitals and infection types calls for enhanced infection prevention measures, particularly in high-risk areas such as ICUs. Last but not least, the genetic diversity of resistance mechanisms and acquisition methods highlights the importance of continuous molecular surveillance in addition to the phenotype monitorization in order to precisely track resistance trends and devise new and improved infection control policies.
Author Contributions
Conceptualization, A.-G.P., P.Ș. and D.A.Ț.; methodology, A.-G.P., P.Ș., D.A.Ț., A.F., A.B.-K., M.-Ș.D. and M.F.; software, A.-G.P., P.Ș., A.F. and M.-Ș.D.; validation, A.F., A.B.-K., C.C., L.-M.J.; investigation, A.-G.P.; resources, A.-G.P., C.C., A.F., A.B.-K., M.-Ș.D., I.É.S. and L.-M.J.; data curation, A.-G.P., P.Ș., V.S.N., A.F., D.A.Ț. and M.-Ș.D.; writing—original draft preparation, A.-G.P.; writing—review and editing, A.-G.P., P.Ș., V.S.N. and D.A.Ț.; supervision C.C., A.F., A.B.-K., M.F., I.É.S. and L.-M.J.; project administration, A.-G.P.; funding acquisition, A.-G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Iuliu Hațieganu University of Medicine and Pharmacy and the materials were funded from the doctoral grant of Adrian-Gabriel Pană.
Institutional Review Board Statement
This study was approved by the ethical committee of Iuliu Hațieganu University of Medicine and Pharmacy (no. AVZ161/10.07.2023) and the ethical committees of Cluj-Napoca Emergency Clinical Hospital (no.48326/31.10.2022), Infectious Diseases Clinical Hospital Cluj-Napoca (no. 8268/11.04.2023), and Târgu-Mureș Emergency Clinical Hospital (no. 12113/28.05.2024).
Informed Consent Statement
Not applicable for this study.
Data Availability Statement
Information about antibiotic resistance genes were curated from The Comprehensive Antibiotic Resistance Database (https://card.mcmaster.ca, accessed on 3 December 2024). All data generated or analyzed during this study are included in this published article.
Acknowledgments
The collective of Cluj-Napoca Emergency County Hospital, Cluj-Napoca Infectious Diseases Clinical Hospital and Târgu-Mureș Emergency County Hospital for help in isolating the isolates and collecting clinical data. Avram Patricia, Bilț Daria, Brudan Alexandra, Todoruț Larisa, and Șoimu Gabriela for laboratory work. Kati Ilonka Salamon for help in data organization and logistical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 1 November 2022).
- Antunes, L.C.S.; Visca, P.; Towner, K.J. Acinetobacter baumannii: Evolution of a global pathogen. Pathog. Dis. 2014, 71, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Vrancianu, C.O.; Gheorghe, I.; Czobor, I.B.; Chifiriuc, M.C. Antibiotic Resistance Profiles, Molecular Mechanisms and Innovative Treatment Strategies of Acinetobacter baumannii. Microorganisms 2020, 8, 935. [Google Scholar] [CrossRef] [PubMed]
- Colquhoun, J.M.; Rather, P.N. Insights Into Mechanisms of Biofilm Formation in Acinetobacter baumannii and Implications for Uropathogenesis. Front. Cell. Infect. Microbiol. 2020, 10, 253. [Google Scholar] [CrossRef]
- Gaddy, J.A.; Tomaras, A.P.; Actis, L.A. The Acinetobacter baumannii 19606 OmpA protein plays a role in biofilm formation on abiotic surfaces and in the interaction of this pathogen with eukaryotic cells. Infect. Immun. 2009, 77, 3150–3160. [Google Scholar] [CrossRef]
- A’shimi, M.H.N.; Alattraqchi, A.G.; Rani, F.M.; Rahman, N.I.A.; Ismail, S.; Abdullah, F.H.; Othman, N.; Cleary, D.W.; Clarke, S.C.; Yeo, C.C. Biocide susceptibilities and biofilm-forming capacities of Acinetobacter baumannii clinical isolates from Malaysia. J. Infect. Dev. Ctries. 2019, 13, 626–633. [Google Scholar] [CrossRef]
- Fux, C.A.; Costerton, J.W.; Stewart, P.S.; Stoodley, P. Survival strategies of infectious biofilms. Trends Microbiol. 2005, 13, 34–40. [Google Scholar] [CrossRef]
- Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report for 2019. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2019 (accessed on 16 November 2024).
- Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report for 2022. 2022. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2022 (accessed on 16 November 2024).
- Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report for 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-eueea-ears-net-annual-epidemiological-report-2020 (accessed on 16 November 2024).
- Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report for 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2021 (accessed on 16 November 2024).
- Koncz, M.; Stirling, T.; Mehdi, H.H.; Méhi, O.; Eszenyi, B.; Asbóth, A.; Apjok, G.; Tóth, Á.; Orosz, L.; Vásárhelyi, B.M.; et al. Genomic surveillance as a scalable framework for precision phage therapy against antibiotic-resistant pathogens. Cell 2024, 187, 5901–5918.e28. [Google Scholar] [CrossRef]
- Fernández-Vázquez, J.L.; Hernández-González, I.L.; Castillo-Ramírez, S.; Jarillo-Quijada, M.D.; Gayosso-Vázquez, C.; Mateo-Estrada, V.E.; Morfín-Otero, R.; Rodríguez-Noriega, E.; Santos-Preciado, J.I.; Alcántar-Curiel, M.D. Pandrug-resistant Acinetobacter baumannii from different clones and regions in Mexico have a similar plasmid carrying the blaOXA-72 gene. Front. Cell. Infect. Microbiol. 2023, 13, 1278819. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Ioannou, P.; Samonis, G.; Kofteridis, D.P. Systematic Review of Antimicrobial Combination Options for Pandrug-Resistant Acinetobacter baumannii. Antibiotics 2021, 10, 1344. [Google Scholar] [CrossRef]
- Papathanakos, G.; Andrianopoulos, I.; Papathanasiou, A.; Koulenti, D.; Gartzonika, K.; Koulouras, V. Pandrug-resistant Acinetobacter baumannii treatment: Still a debatable topic with no definite solutions. J. Antimicrob. Chemother. 2020, 75, 3081. [Google Scholar] [CrossRef]
- Kyriakidis, I.; Vasileiou, E.; Pana, Z.D.; Tragiannidis, A. Acinetobacter baumannii Antibiotic Resistance Mechanisms. Pathogens 2021, 10, 373. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Z.; Plésiat, P.; Nikaido, H. The Challenge of Efflux-Mediated Antibiotic Resistance in Gram-Negative Bacteria. Clin. Microbiol. Rev. 2015, 28, 337–418. [Google Scholar] [CrossRef] [PubMed]
- Warburton, P.J.; Amodeo, N.; Roberts, A.P. Mosaic tetracycline resistance genes encoding ribosomal protection proteins. J. Antimicrob. Chemother. 2016, 71, 3333–3339. [Google Scholar] [CrossRef] [PubMed]
- Aldred, K.J.; Kerns, R.J.; Osheroff, N. Mechanism of Quinolone Action and Resistance. Biochemistry 2014, 53, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Vashist, J.; Tiwari, V.; Das, R.; Kapil, A.; Rajeswari, M.R. Analysis of penicillin-binding proteins (PBPs) in carbapenem resistant Acinetobacter baumannii. Indian J. Med. Res. 2011, 133, 332–338. [Google Scholar] [PubMed]
- Tiwari, V. Post-translational modification of ESKAPE pathogens as a potential target in drug discovery. Drug Discov. Today 2019, 24, 814–822. [Google Scholar] [CrossRef]
- Ambler, R.P. The structure of beta-lactamases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1980, 289, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-López, R.; Solano-Gálvez, S.G.; Vignon-Whaley, J.J.J.; Vaamonde, J.A.A.; Alonzo, L.A.P.; Reséndiz, A.R.; Álvarez, M.M.; López, E.N.V.; Franyuti-Kelly, G.; Álvarez-Hernández, D.A.; et al. Acinetobacter baumannii Resistance: A Real Challenge for Clinicians. Antibiotics 2020, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Tooke, C.L.; Hinchliffe, P.; Bragginton, E.C.; Colenso, C.K.; Hirvonen, V.H.A.; Takebayashi, Y.; Spencer, J. β-Lactamases and β-Lactamase Inhibitors in the 21st Century. J. Mol. Biol. 2019, 431, 3472–3500. [Google Scholar] [CrossRef]
- Girija, A.S.S.; Vijayashree Priyadharsini, J.; Paramasivam, A. Plasmid-encoded resistance to trimethoprim/sulfamethoxazole mediated by dfrA1, dfrA5, sul1 and sul2 among Acinetobacter baumannii isolated from urine samples of patients with severe urinary tract infection. J. Glob. Antimicrob. Resist. 2019, 17, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile Genetic Elements Associated with Antimicrobial Resistance. Clin. Microbiol. Rev. 2018, 31, 10–1128. [Google Scholar] [CrossRef]
- Noel, H.R.; Petrey, J.R.; Palmer, L.D. Mobile genetic elements in Acinetobacter antibiotic-resistance acquisition and dissemination. Ann. N. Y. Acad. Sci. 2022, 1518, 166. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G.J.; Domingues, S. Insights on the Horizontal Gene Transfer of Carbapenemase Determinants in the Opportunistic Pathogen Acinetobacter baumannii. Microorganisms 2016, 4, 29. [Google Scholar] [CrossRef]
- eucast: Clinical Breakpoints and Dosing of Antibiotics. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 30 November 2024).
- Versalovic, J.; Koeuth, T.; Lupski, J.R. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991, 19, 6823–6831. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-M.; Ke, S.-C.; Li, C.-R.; Chang, C.-C. The comparison of genotyping, antibiogram, and antimicrobial resistance genes between carbapenem-susceptible and -resistant Acinetobacter baumannii. Comp. Immunol. Microbiol. Infect. Dis. 2014, 37, 339–346. [Google Scholar] [CrossRef]
- Higgins, P.G.; Lehmann, M.; Seifert, H. Inclusion of OXA-143 primers in a multiplex polymerase chain reaction (PCR) for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int. J. Antimicrob. Agents 2010, 35, 305. [Google Scholar] [CrossRef]
- Lukovic, B.; Gajic, I.; Dimkic, I.; Kekic, D.; Zornic, S.; Pozder, T.; Radisavljevic, S.; Opavski, N.; Kojic, M.; Ranin, L. The first nationwide multicenter study of Acinetobacter baumannii recovered in Serbia: Emergence of OXA-72, OXA-23 and NDM-1-producing isolates. Antimicrob. Resist. Infect. Control 2020, 9, 101. [Google Scholar] [CrossRef]
- Cicek, A.C.; Saral, A.; Iraz, M.; Ceylan, A.; Duzgun, A.O.; Peleg, A.Y.; Sandalli, C. OXA- and GES-type β-lactamases predominate in extensively drug-resistant Acinetobacter baumannii isolates from a Turkish University Hospital. Clin. Microbiol. Infect. 2014, 20, 410–415. [Google Scholar] [CrossRef]
- Dillon, B.; Thomas, L.; Mohmand, G.; Zelynski, A.; Iredell, J. Multiplex PCR for screening of integrons in bacterial lysates. J. Microbiol. Methods 2005, 62, 221–232. [Google Scholar] [CrossRef]
- Poirel, L.; Nordmann, P. Carbapenem resistance in Acinetobacter baumannii: Mechanisms and epidemiology. Clin. Microbiol. Infect. 2006, 12, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Lv, Y.; Yuan, M.; Hu, X.; Nie, T.; Yang, X.; Li, G.; Pang, J.; Zhang, J.; Li, C.; et al. Genetic basis of high level aminoglycoside resistance in Acinetobacter baumannii from Beijing, China. Acta Pharm. Sin. B 2014, 4, 295–300. [Google Scholar] [CrossRef]
- Farkas, A.; Tarco, E.; Butiuc-Keul, A. Antibiotic resistance profiling of pathogenic Enterobacteriaceae from Cluj-Napoca, Romania. Germs 2019, 9, 17–27. [Google Scholar] [CrossRef]
- Segal, H.; Garny, S.; Elisha, B.G. Is ISABA-1 customized for Acinetobacter? FEMS Microbiol. Lett. 2005, 243, 425–429. [Google Scholar] [CrossRef]
- Cheikh, H.B.; Domingues, S.; Silveira, E.; Kadri, Y.; Rosário, N.; Mastouri, M.; Da Silva, G.J. Molecular characterization of carbapenemases of clinical Acinetobacter baumannii–calcoaceticus complex isolates from a University Hospital in Tunisia. 3 Biotech. 2018, 8, 297. [Google Scholar] [CrossRef]
- Bhandari, S.; Adhikari, S.; Karki, D.; Chand, A.B.; Sapkota, S.; Dhungel, B.; Banjara, M.R.; Joshi, P.; Lekhak, B.; Rijal, K.R. Antibiotic Resistance, Biofilm Formation and Detection of mexA/mexB Efflux-Pump Genes Among Clinical Isolates of Pseudomonas aeruginosa in a Tertiary Care Hospital, Nepal. Front. Trop. Dis. 2022, 2, 810863. [Google Scholar] [CrossRef]
- Rebelo, A.R.; Bortolaia, V.; Kjeldgaard, J.S.; Pedersen, S.K.; Leekitcharoenphon, P.; Hansen, I.M.; Guerra, B.; Malorny, B.; Borowiak, M.; Hammerl, J.A.; et al. Multiplex PCR for detection of plasmid-mediated colistin resistance determinants, mcr-1, mcr-2, mcr-3, mcr-4 and mcr-5 for surveillance purposes. Euro Surveill. 2018, 23, 17–00672. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report 2023. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-eueea-ears-net-annual-epidemiological-report-2023 (accessed on 30 November 2024).
- Papadopoulou, M.; Deliolanis, I.; Polemis, M.; Vatopoulos, A.; Psichogiou, M.; Giakkoupi, P. Characteristics of the Genetic Spread of Carbapenem-Resistant Acinetobacter baumannii in a Tertiary Greek Hospital. Genes 2024, 15, 458. [Google Scholar] [CrossRef]
- Szczypta, A.; Talaga-Ćwiertnia, K.; Kielar, M.; Krzyściak, P.; Gajewska, A.; Szura, M.; Bulanda, M.; Chmielarczyk, A. Investigation of Acinetobacter baumannii Activity in Vascular Surgery Units through Epidemiological Management Based on the Analysis of Antimicrobial Resistance, Biofilm Formation and Genotyping. Int. J. Environ. Res. Public Health 2021, 18, 1563. [Google Scholar] [CrossRef] [PubMed]
- Jalali, Y.; Liptáková, A.; Jalali, M.; Payer, J. Moving toward Extensively Drug-Resistant: Four-Year Antimicrobial Resistance Trends of Acinetobacter baumannii from the Largest Department of Internal Medicine in Slovakia. Antibiotics 2023, 12, 1200. [Google Scholar] [CrossRef]
- Matsui, M.; Suzuki, M.; Suzuki, M.; Yatsuyanagi, J.; Watahiki, M.; Hiraki, Y.; Kawano, F.; Tsutsui, A.; Shibayama, K.; Suzuki, S. Distribution and Molecular Characterization of Acinetobacter baumannii International Clone II Lineage in Japan. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Thomsen, J.; Abdulrazzaq, N.M.; AlRand, H.; The UAE AMR Surveillance Consortium; Senok, A.; Alatoom, A.; Agnes-Sonnevend-Pal; Al Hammadi, A.A.; Ahmed, A.E.; Yousef, A.F.; et al. Epidemiology and antimicrobial resistance trends of Acinetobacter species in the United Arab Emirates: A retrospective analysis of 12 years of national AMR surveillance data. Front. Public Health 2024, 11, 1245131. [Google Scholar] [CrossRef] [PubMed]
- Leal, N.C.; Campos, T.L.; Rezende, A.M.; Docena, C.; Mendes-Marques, C.L.; de Sá Cavalcanti, F.L.; Wallau, G.L.; Rocha, I.V.; Cavalcanti, C.L.B.; Veras, D.L.; et al. Comparative Genomics of Acinetobacter baumannii Clinical Strains From Brazil Reveals Polyclonal Dissemination and Selective Exchange of Mobile Genetic Elements Associated With Resistance Genes. Front. Microbiol. 2020, 11, 1176. [Google Scholar] [CrossRef] [PubMed]
- Lob, S.H.; Hoban, D.J.; Sahm, D.F.; Badal, R.E. Regional differences and trends in antimicrobial susceptibility of Acinetobacter baumannii. Int. J. Antimicrob. Agents 2016, 47, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Arowolo, M.T.; Orababa, O.Q.; Olaitan, M.O.; Osibeluwo, B.V.; Essiet, U.U.; Batholomew, O.H.; Ogunrinde, O.G.; Lagoke, O.A.; Soriwei, J.D.; Ishola, O.D.; et al. Prevalence of carbapenem resistance in Acinetobacter baumannii and Pseudomonas aeruginosa in sub-Saharan Africa: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0287762. [Google Scholar] [CrossRef]
- Sanchez-Urtaza, S.; Ocampo-Sosa, A.; Molins-Bengoetxea, A.; El-Kholy, M.A.; Hernandez, M.; Abad, D.; Shawky, S.M.; Alkorta, I.; Gallego, L. Molecular Characterization of Multidrug Resistant Acinetobacter baumannii Clinical Isolates from Alexandria, Egypt. Front. Cell. Infect. Microbiol. 2023, 13, 1208046. [Google Scholar] [CrossRef]
- Beig, M.; Parvizi, E.; Navidifar, T.; Bostanghadiri, N.; Mofid, M.; Golab, N.; Sholeh, M. Geographical mapping and temporal trends of Acinetobacter baumannii carbapenem resistance: A comprehensive meta-analysis. PLoS ONE 2024, 19, e0311124. [Google Scholar] [CrossRef]
- Antibiotic-Resistant Bacteria Could Pose Major Health Threat Across Asia. Available online: https://www.birmingham.ac.uk/news/2024/antibiotic-resistant-bacteria-could-pose-major-health-threat-across-asia (accessed on 6 January 2025).
- Challapa-Mamani, M.R.; Yareta, J.; Fajardo-Loyola, A.; Asmat Marrufo, P.; Siesquen, C.P.; Pino-Dueñas, J.; Meza-Fernández, H.; Cruz-Vargas, J.A.D.L.; Marcos-Carbajal, P. Acinetobacter baumannii Co-Resistant to Extended Spectrum Beta-Lactamases and Carbapenemases in Six Peruvian Hospital Centers. Microbiol. Res. 2024, 15, 2650–2660. [Google Scholar] [CrossRef]
- Turton, J.F.; Ward, M.E.; Woodford, N.; Kaufmann, M.E.; Pike, R.; Livermore, D.M.; Pitt, T.L. The role of ISAba1 in expression of OXA carbapenemase genes in Acinetobacter baumannii. FEMS Microbiol. Lett. 2006, 258, 72–77. [Google Scholar] [CrossRef]
- Su, P.-W.; Yang, E.C.; Moi, S.-H.; Yang, C.-H.; Chuang, L.-Y. Prevalence of Carbapenem Resistance Genes among Acinetobacter baumannii Isolated from a Teaching Hospital in Taiwan. Antibiotics 2023, 12, 1357. [Google Scholar] [CrossRef]
- Chagas, T.P.G.; Carvalho, K.R.; de Oliveira Santos, I.C.; Carvalho-Assef, A.P.D.; Asensi, M.D. Characterization of carbapenem-resistant Acinetobacter baumannii in Brazil (2008–2011): Countrywide spread of OXA-23–producing clones (CC15 and CC79). Diagn. Microbiol. Infect. Dis. 2014, 79, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.H.; Chan, B.K.; Chan, E.W.; Chen, S. Over-Expression of ISAba1-Linked Intrinsic and Exogenously Acquired OXA Type Carbapenem-Hydrolyzing-Class D-ß-Lactamase-Encoding Genes Is Key Mechanism Underlying Carbapenem Resistance in Acinetobacter baumannii. Front. Microbiol. 2019, 10, 2809. [Google Scholar] [CrossRef] [PubMed]
- Nigro, S.J.; Hall, R.M. Structure and context of Acinetobacter transposons carrying the oxa23 carbapenemase gene. J. Antimicrob. Chemother. 2016, 71, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Vila, J.; Martí, S.; Sánchez-Céspedes, J. Porins, Efflux Pumps and Multidrug Resistance in Acinetobacter Baumannii. J. Antimicrob. Chemother. 2007, 59, 1210–1215. Available online: https://academic.oup.com/jac/article-abstract/59/6/1210/711566?redirectedFrom=fulltext&login=false&utm_source=chatgpt.com (accessed on 1 December 2024). [CrossRef] [PubMed]
- Kherroubi, L.; Bacon, J.; Rahman, K.M. Navigating fluoroquinolone resistance in Gram-negative bacteria: A comprehensive evaluation. JAC-Antimicrob. Resist. 2024, 6, dlae127. [Google Scholar] [CrossRef]
- Siddhardha, B.; Dyavaiah, M.; Syed, A. (Eds.) Model Organisms for Microbial Pathogenesis, Biofilm Formation and Antimicrobial Drug Discovery; Springer: Singapore, 2020; ISBN 9789811516948. [Google Scholar]
- Hameed, F.; Khan, M.A.; Muhammad, H.; Sarwar, T.; Bilal, H.; Rehman, T.U. Plasmid-mediated mcr-1 gene in Acinetobacter baumannii and Pseudomonas aeruginosa: First report from Pakistan. Rev. Soc. Bras. Med. Trop. 2019, 52, e20190237. [Google Scholar] [CrossRef]
- Papazachariou, A.; Tziolos, R.-N.; Karakonstantis, S.; Ioannou, P.; Samonis, G.; Kofteridis, D.P. Treatment Strategies of Colistin Resistance Acinetobacter baumannii Infections. Antibiotics 2024, 13, 423. [Google Scholar] [CrossRef] [PubMed]
- Sorlí, L.; Luque, S.; Grau, S.; Berenguer, N.; Segura, C.; Montero, M.M.; Álvarez-Lerma, F.; Knobel, H.; Benito, N.; Horcajada, J.P. Trough colistin plasma level is an independent risk factor for nephrotoxicity: A prospective observational cohort study. BMC Infect. Dis. 2013, 13, 380. [Google Scholar] [CrossRef]
- Dalfino, L.; Puntillo, F.; Ondok, M.J.M.; Mosca, A.; Monno, R.; Coppolecchia, S.; Spada, M.L.; Bruno, F.; Brienza, N. Colistin-associated Acute Kidney Injury in Severely Ill Patients: A Step Toward a Better Renal Care? A Prospective Cohort Study. Clin. Infect. Dis. 2015, 61, 1771–1777. [Google Scholar] [CrossRef]
- Darwish, M.M.; Catalan, M.I.; Wilson, T.; McGlynn, C.C.; Suhd-Brondstatter, J.; Dow, A.L.; Kingsley, A.; Reilly, M.E.; Cohen, S.H.; Desai, A.N. Hospital outbreak of Carbapenem-resistant acinetobacter baumannii in the context of local facility transmission. Am. J. Infect. Control 2024, 52, 739–741. [Google Scholar] [CrossRef]
- McDonald, L.C.; Banerjee, S.N.; Jarvis, W.R. Seasonal variation of Acinetobacter infections: 1987–1996. Nosocomial Infections Surveillance System. Clin. Infect. Dis. 1999, 29, 1133–1137. [Google Scholar] [CrossRef]
- Spellberg, B.; Bonomo, R.A. “Airborne assault”: A new dimension in Acinetobacter baumannii transmission*. Crit. Care Med. 2013, 41, 2042–2044. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, M.D.; Voor in ’t holt, A.F.; Alp, E.; Hell, M.; Petrosillo, N.; Presterl, E.; Tsakris, A.; Severin, J.A.; Vos, M.C.; on behalf of the ESCMID Study Group for Nosocomial Infections (ESGNI). Infection prevention and control policies in hospitals and prevalence of highly resistant microorganisms: An international comparative study. Antimicrob. Resist. Infect. Control 2022, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Cataldo, M.A.; Dancer, S.J.; Angelis, G.D.; Falcone, M.; Frank, U.; Kahlmeter, G.; Pan, A.; Petrosillo, N.; Rodríguez-Baño, J.; et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin. Microbiol. Infect. 2014, 20, 1–55. [Google Scholar] [CrossRef]
- Wong, V.W.Y.; Huang, Y.; Wei, W.I.; Wong, S.Y.S.; Kwok, K.O. Approaches to multidrug-resistant organism prevention and control in long-term care facilities for older people: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2022, 11, 7. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).