Microbial Dynamics: Assessing Skincare Regimens’ Impact on the Facial Skin Microbiome and Skin Health Parameters
Abstract
1. Introduction
2. Methods
2.1. Subject Population
2.2. Study Design
2.3. Composition of Test Products According to INCI
- -
- PFP Cleanser: Aqua, Cetearyl Alcohol, Stearic Acid, Glycerin, Kaolin, Coco-Betaine, Polyglyceryl-3 Dicitrate/Stearate, C10-18 Triglycerides, Lactobacillus Ferment, Avena Sativa Kernel Oil, Caprylic/Capric Triglyceride, Sodium Methyl Cocoyl Taurate, Squalane, Niacinamide, Dextrin, Polydextrose, Amylopectin, Sodium Cocoyl Isethionate, Salicylic Acid.
- -
- PFP Facial Cream: Aqua, Jojoba Esters, Squalane, Vegetable Oil, Glycerin, Hydrogenated Vegetable Oil, Stearyl Alcohol, Glyceryl Stearate, Lactobionic Acid, Urea, Phytosterol, Ceramide NP, Zinc PCA, Calcium PCA, Sodium Stearoyl Glutamate, Tocopherol, Sodium Polyacryloyldimethyl Taurate, Sodium Hydroxide.
- -
- CSP Cleanser: Aqua, Sodium Laureth Sulfate, Decyl Glucoside, Glycerine, Sodium Chloride, Coco-Betaine, Salicylic Acid, PEG-150 Pentaerythrityl Tetrastearate, PEG-6 Caprylic Glycerides, Zinc Gluconate, Sodium Hydroxide, Capryloyl Salicylic Acid, Tetrasodium EDTA, Citric Acid, Menthol, Polyquaterium-47, Hexylene Glycol, Sodium Benzoate.
- -
- CSP Facial Cream: Aqua, Caprylic/Capric Triglyceride, Propanediol, Glycerin, Silica, Cetearyl Alcohol, Dipentaerythrityl Tetrahydroxystearate/Tetraisostearate, Glyceryl Stearate, PEG-100 Stearate, Phenoxyethanol, Cetearyl Glucoside, Cetyl Alcohol, Perilla Ocymoides Seed Oil, Stearyl Alcohol, Dimethicone, Xylitylglucoside, Fragrance, Polyacrylamide, Anhydroxylitol, C13-14 Isoparaffin, Ethylhexylglycerin, Xylitol, Butylene Glycol, Dimethiconol, Tocopherol, Glyceryl Acrylate/Acrylic Acid Copolymer, Decylene Glycol, Disodium EDTA, Laureth-7, Diospyros Mespiliformis Leaf Extract, Glucose, Maltodextrin, Kalanchoe Pinnata Leaf Extract, Sanicula Europaea Extract, Tromethamine, Citric Acid, Lapsana Communis Flower/Leaf/Stem Extract, Furcellaria Lumbricalis Extract, Sodium Benzoate, Potassium Sorbate, Maris Sal/Sea Salt/Sel Marin, Callicarpa Japonica Fruit Extract.
2.4. Sample Collection
2.5. Biophysical Measurement of Skin Quality
2.6. Extraction
2.7. Sequencing
2.8. Bioinformatic Analysis
- Quality control
- Taxonomic profile
2.9. Statistical Analysis
- Change in Microbiome
- Microbiome Profile-Based Subgrouping
- Alpha Diversity Comparison Between Treatments
- Antera Data Changes for Combined Samples and Clusters
- Significant Organismal Changes in Antera Variables with Treatment, Incorporating Bias Correction
- Spearman Analysis for Correlation Assessment
3. Results
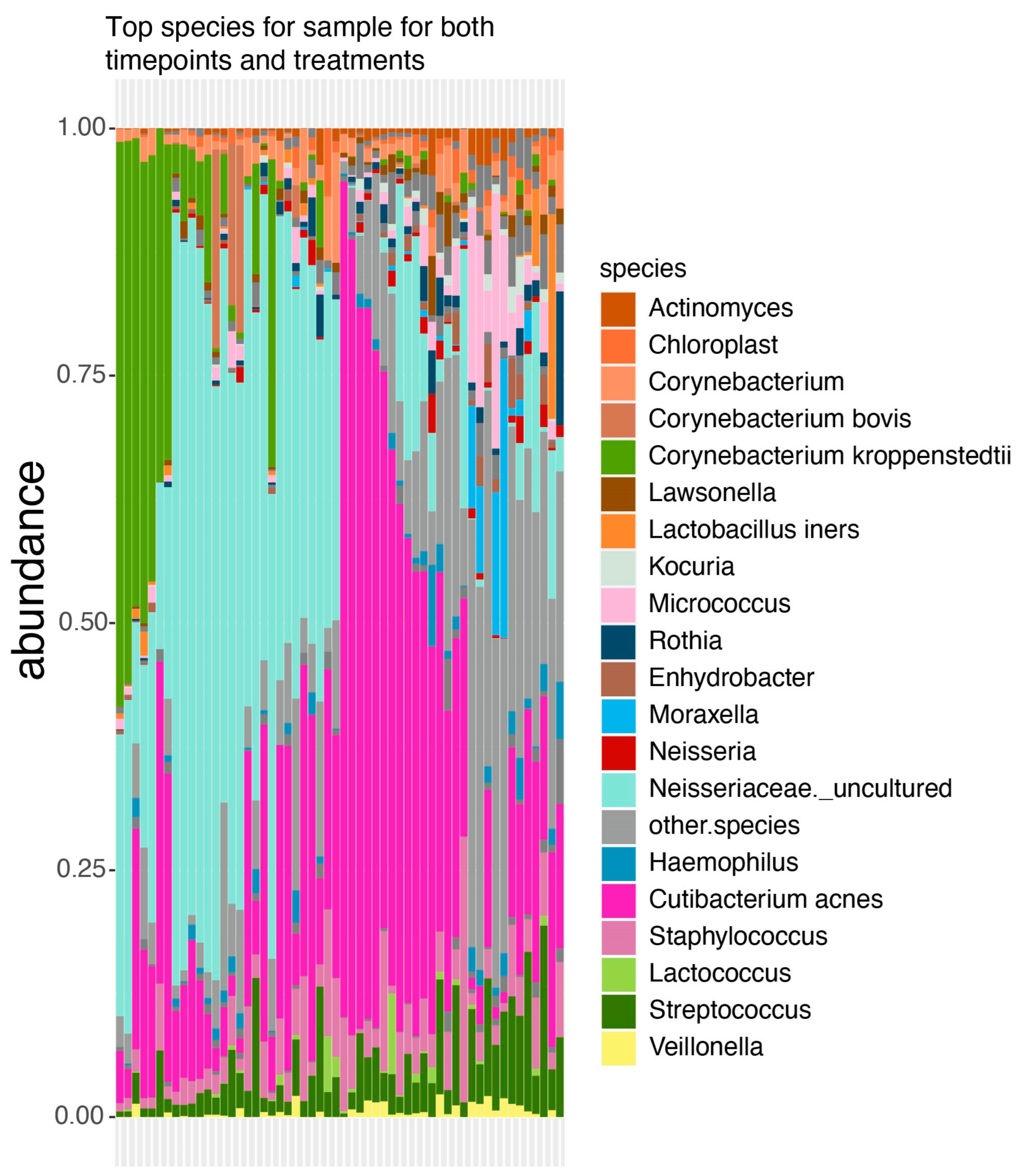
3.1. Taxonomic Profile
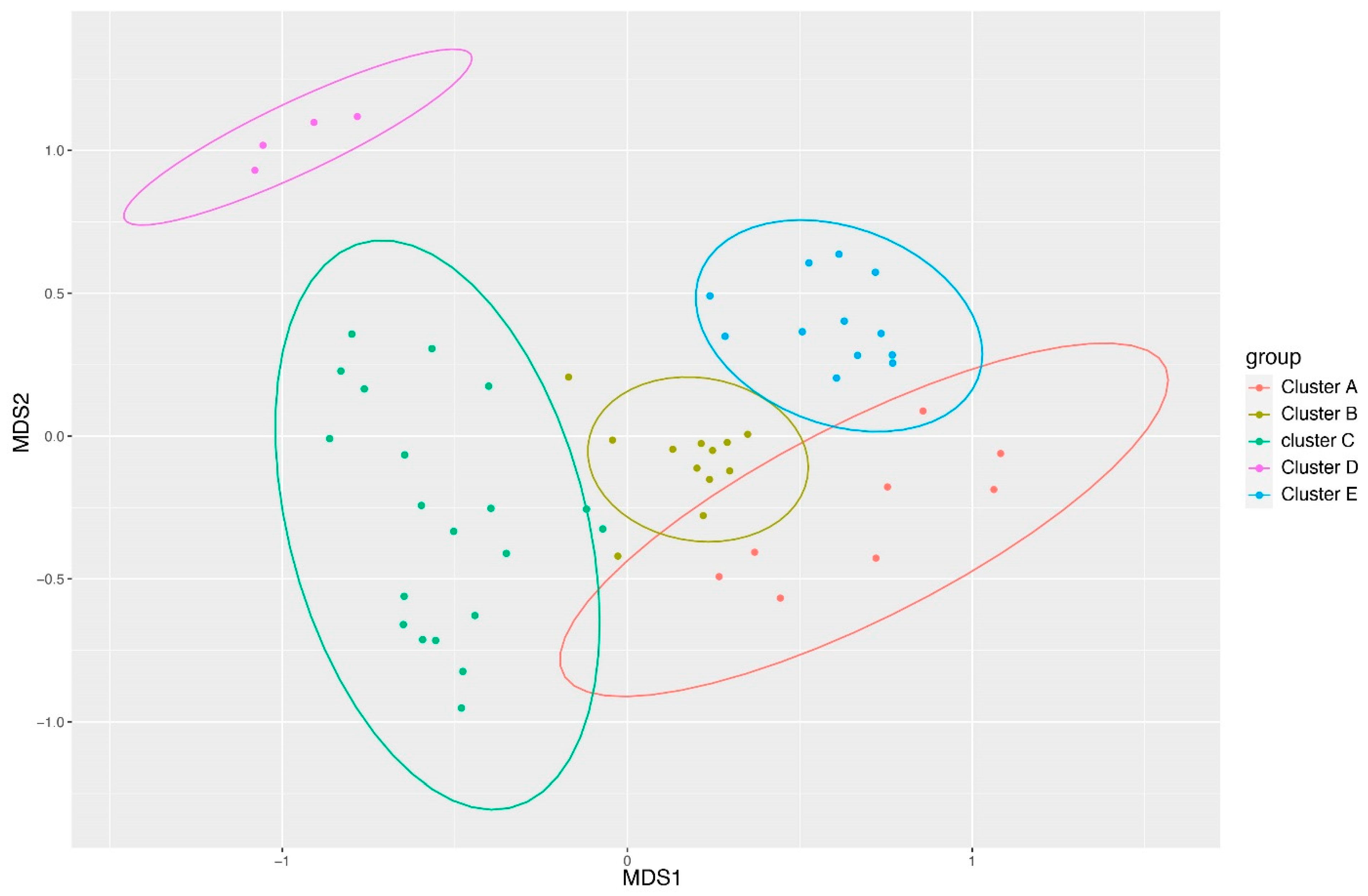
3.2. Clustering of Samples Based on Ward’s Method
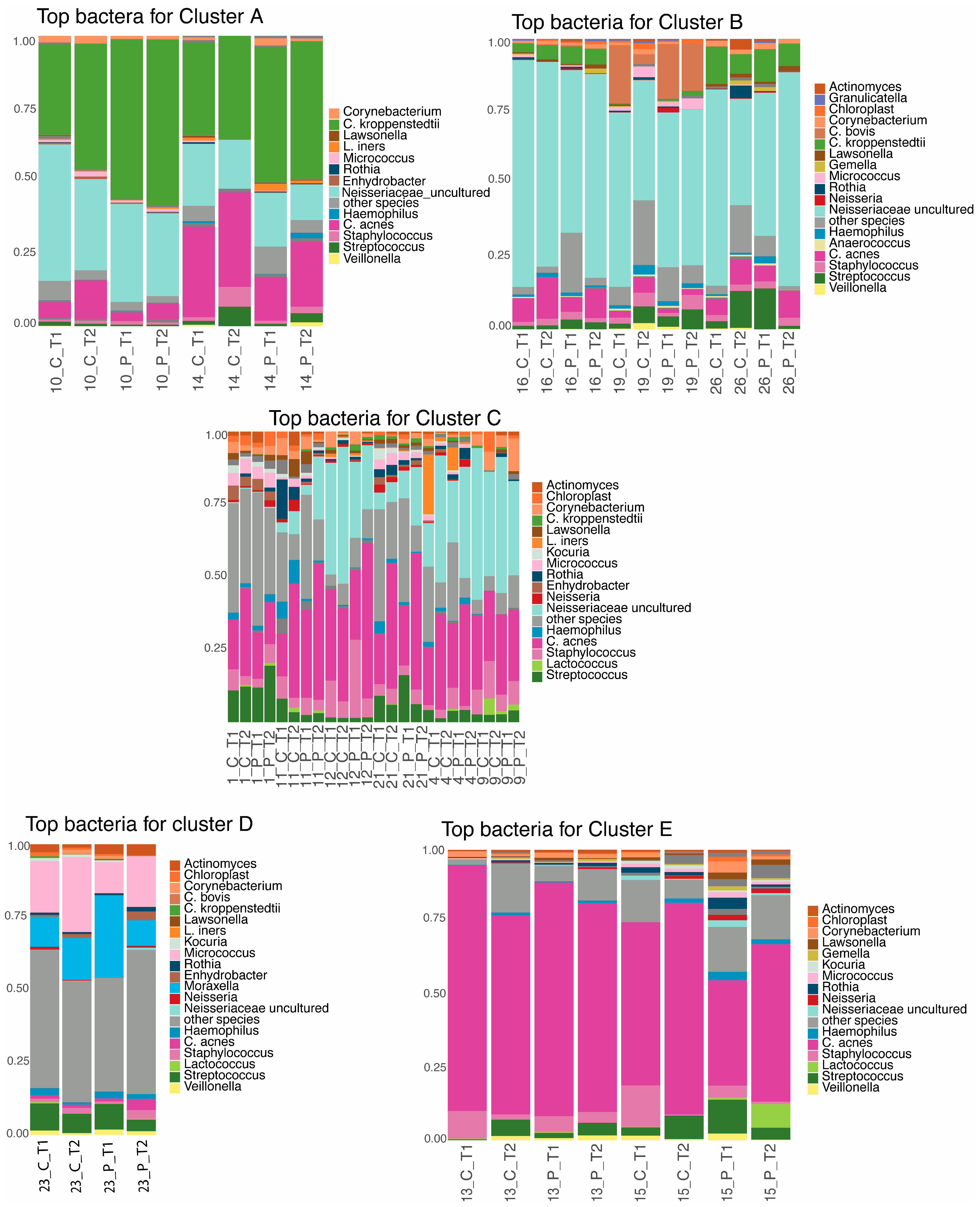
3.3. Taxonomic Profile per Cluster
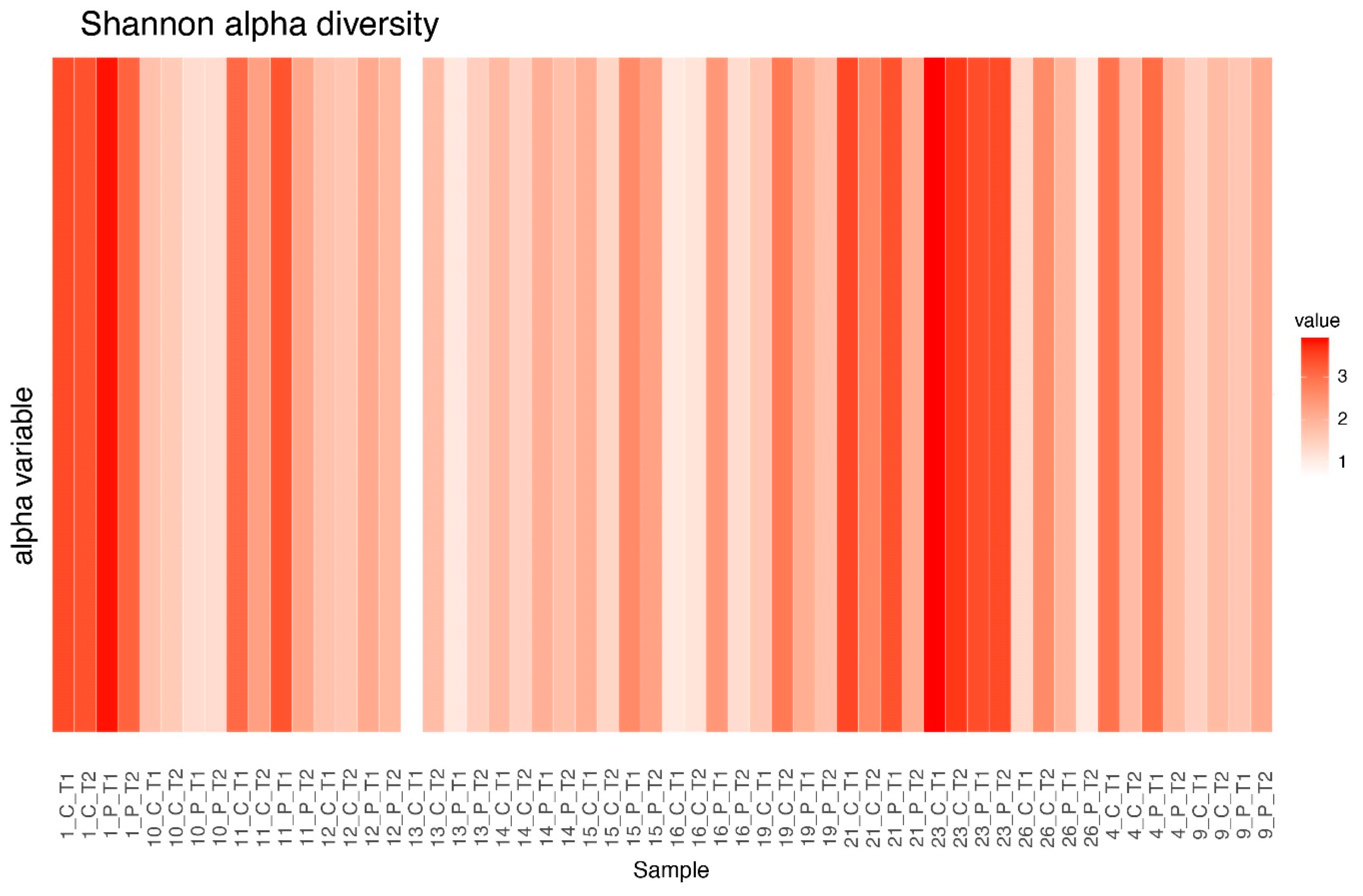
3.4. Alpha Diversity Before and After Treatment
3.5. Alpha Diversity Between Clusters
3.6. DeltaCLR of Species (For Top 10 Decreased and Top 10 Increased Species)
3.7. Jensen–Shannon Beta Diversity
3.8. Differential Abundance Analysis Using ANCOMBC2
3.9. Comparison of Change Between Treatments
3.10. Spearman Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santamaria, E.; Akerstrom, U.; Berger-Picard, N.; Lataste, S.; Gillbro, J.M. Randomized comparative double-blind study assessing the difference between topically applied microbiome supporting skincare versus conventional skincare on the facial microbiome in correlation to biophysical skin parameters. Int. J. Cosmet. Sci. 2023, 45, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Serra-Baldrich, E.; de Frutos, J.O.; Jauregui, I.; Armario-Hita, J.C.; Silvestre, J.F.; Herraez, L.; Martin-Santiago, A.; Valero, A.; Sastre, J. Changing perspectives in atopic dermatitis. Allergol. Immunopathol. 2018, 46, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.H.; Oh, J.; Deming, C.; Conlan, S.; Grice, E.A.; Beatson, M.A.; Nomicos, E.; Polley, E.C.; Komarow, H.D.; Program, N.C.S.; et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012, 22, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Whiting, C.; Abdel Azim, S.; Friedman, A. The Skin Microbiome and its Significance for Dermatologists. Am. J. Clin. Dermatol. 2024, 25, 169–177. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Cheng, J.Y.; Gallo, R.L. Mechanisms for control of skin immune function by the microbiome. Curr. Opin. Immunol. 2021, 72, 324–330. [Google Scholar] [CrossRef]
- Ersanli, C.; Tzora, A.; Voidarou, C.C.; Skoufos, S.; Zeugolis, D.I.; Skoufos, I. Biodiversity of Skin Microbiota as an Important Biomarker for Wound Healing. Biology 2023, 12, 1187. [Google Scholar] [CrossRef]
- Gonzalez, T.; Stevens, M.L.; Baatyrbek Kyzy, A.; Alarcon, R.; He, H.; Kroner, J.W.; Spagna, D.; Grashel, B.; Sidler, E.; Martin, L.J.; et al. Biofilm propensity of Staphylococcus aureus skin isolates is associated with increased atopic dermatitis severity and barrier dysfunction in the MPAACH pediatric cohort. Allergy 2021, 76, 302–313. [Google Scholar] [CrossRef]
- Glatthardt, T.; Lima, R.D.; de Mattos, R.M.; Ferreira, R.B.R. Microbe Interactions within the Skin Microbiome. Antibiotics 2024, 13, 49. [Google Scholar] [CrossRef]
- Salgaonkar, N.; Kadamkode, V.; Kumaran, S.; Mallemala, P.; Christy, E.; Appavoo, S.; Majumdar, A.; Mitra, R.; Dasgupta, A. Glycerol fermentation by skin bacteria generates lactic acid and upregulates the expression levels of genes associated with the skin barrier function. Exp. Dermatol. 2022, 31, 1364–1372. [Google Scholar] [CrossRef]
- Proksch, E. pH in nature, humans and skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Nodake, Y.; Matsumoto, S.; Miura, R.; Honda, H.; Ishibashi, G.; Matsumoto, S.; Dekio, I.; Sakakibara, R. Pilot study on novel skin care method by augmentation with Staphylococcus epidermidis, an autologous skin microbe—A blinded randomized clinical trial. J. Dermatol. Sci. 2015, 79, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Halling, A.S.; Fritz, B.G.; Gerner, T.; Rinnov, M.R.; Bay, L.; Knudgaard, M.H.; Ravn, N.H.; Trautner, S.; Ruge, I.F.; Olesen, C.; et al. Reduced Skin Microbiome Diversity in Infancy Is Associated with Increased Risk of Atopic Dermatitis in High-Risk Children. J. Investg. Dermatol. 2023, 143, 2030–2038.e2036. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, X.; Li, X.; He, X.; Xiong, X.; Lai, J. Epidermal Barrier Integrity is Associated with Both Skin Microbiome Diversity and Composition in Patients with Acne Vulgaris. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2065–2075. [Google Scholar] [CrossRef]
- Boix-Amoros, A.; Badri, M.H.; Manasson, J.; Blank, R.B.; Haberman, R.H.; Neimann, A.L.; Girija, P.V.; Jimenez Hernandez, A.; Heguy, A.; Koralov, S.B.; et al. Alterations in the cutaneous microbiome of patients with psoriasis and psoriatic arthritis reveal similarities between non-lesional and lesional skin. Ann. Rheum. Dis. 2023, 82, 507–514. [Google Scholar] [CrossRef]
- Demessant-Flavigny, A.L.; Connetable, S.; Kerob, D.; Moreau, M.; Aguilar, L.; Wollenberg, A. Skin microbiome dysbiosis and the role of Staphylococcus aureus in atopic dermatitis in adults and children: A narrative review. J. Eur. Acad. Dermatol. Venereol. 2023, 37 (Suppl. S5), 3–17. [Google Scholar] [CrossRef]
- Veniaminova, N.A.; Jia, Y.Y.; Hartigan, A.M.; Huyge, T.J.; Tsai, S.Y.; Grachtchouk, M.; Nakagawa, S.; Dlugosz, A.A.; Atwood, S.X.; Wong, S.Y. Distinct mechanisms for sebaceous gland self-renewal and regeneration provide durability in response to injury. Cell Rep. 2023, 42, 113121. [Google Scholar] [CrossRef]
- Han, J.; Lin, K.; Choo, H.; Chen, Y.; Zhang, X.; Xu, R.H.; Wang, X.; Wu, Y. Distinct bulge stem cell populations maintain the pilosebaceous unit in a beta-catenin-dependent manner. iScience 2023, 26, 105805. [Google Scholar] [CrossRef]
- Dreno, B.; Dekio, I.; Baldwin, H.; Demessant, A.L.; Dagnelie, M.A.; Khammari, A.; Corvec, S. Acne microbiome: From phyla to phylotypes. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 657–664. [Google Scholar] [CrossRef]
- Magin, P.; Adams, J.; Heading, G.; Pond, D. ‘Perfect skin’, the media and patients with skin disease: A qualitative study of patients with acne, psoriasis and atopic eczema. Aust. J. Prim. Health 2011, 17, 181–185. [Google Scholar] [CrossRef]
- Myers, T.; Bouslimani, A.; Huang, S.; Hansen, S.T.; Clavaud, C.; Azouaoui, A.; Ott, A.; Gueniche, A.; Bouez, C.; Zheng, Q.; et al. A multi-study analysis enables identification of potential microbial features associated with skin aging signs. Front. Aging 2023, 4, 1304705. [Google Scholar] [CrossRef] [PubMed]
- Hospodsky, D.; Pickering, A.J.; Julian, T.R.; Miller, D.; Gorthala, S.; Boehm, A.B.; Peccia, J. Hand bacterial communities vary across two different human populations. Microbiology 2014, 160, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Bouslimani, A.; da Silva, R.; Kosciolek, T.; Janssen, S.; Callewaert, C.; Amir, A.; Dorrestein, K.; Melnik, A.V.; Zaramela, L.S.; Kim, J.N.; et al. The impact of skin care products on skin chemistry and microbiome dynamics. BMC Biol. 2019, 17, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, X.; Zhao, L.; Gu, Y.; Chen, Y.; Liu, N.; An, L.; Lu, Y.; Cui, S. Effect of leave-on cosmetic antimicrobial preservatives on healthy skin resident Staphylococcus epidermidis. J. Cosmet. Dermatol. 2023, 22, 2115–2121. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, S.; Zhou, L.; He, K.; Song, L.; Liang, H.; He, C. Effect of cosmetic chemical preservatives on resident flora isolated from healthy facial skin. J. Cosmet. Dermatol. 2019, 18, 652–658. [Google Scholar] [CrossRef]
- Myles, I.A.; Castillo, C.R.; Barbian, K.D.; Kanakabandi, K.; Virtaneva, K.; Fitzmeyer, E.; Paneru, M.; Otaizo-Carrasquero, F.; Myers, T.G.; Markowitz, T.E.; et al. Therapeutic responses to Roseomonas mucosa in atopic dermatitis may involve lipid-mediated TNF-related epithelial repair. Sci. Transl. Med. 2020, 12, aaz8631. [Google Scholar] [CrossRef]
- Lee, H.J.; Jeong, S.E.; Lee, S.; Kim, S.; Han, H.; Jeon, C.O. Effects of cosmetics on the skin microbiome of facial cheeks with different hydration levels. Microbiologyopen 2018, 7, e00557. [Google Scholar] [CrossRef]
- Jeppu, U.; Kaur, H.; Kotian, S. Comparison of normal resident flora on the face of medical students who use and who do not use cosmetics. J. Clin. Diagn. Res. 2017, 11, DC08–DC10. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Team, R.C. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org (accessed on 1 September 2022).
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- van den Boogaart, K.; Tolosana-Delgado, R.; Bren, M. Compositions: Compositional Data Analysis. R Package Version 2.0-8. 2024. Available online: https://CRAN.R-project.org/package=compositions (accessed on 1 September 2022).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. Ordination Methods, Diversity Analysis and Other Functions for Community and Vegetation Ecologists. 2022. Available online: https://www.cifor-icraf.org/knowledge/publication/30268/. (accessed on 1 September 2022).
- Lin, H.; Peddada, S.D. Multi-group Analysis of Compositions of Microbiomes with Covariate Adjustments and Repeated Measures. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Fourniere, M.; Latire, T.; Souak, D.; Feuilloley, M.G.J.; Bedoux, G. Staphylococcus epidermidis and Cutibacterium acnes: Two Major Sentinels of Skin Microbiota and the Influence of Cosmetics. Microorganisms 2020, 8, 1752. [Google Scholar] [CrossRef] [PubMed]
- Rozas, M.; Hart de Ruijter, A.; Fabrega, M.J.; Zorgani, A.; Guell, M.; Paetzold, B.; Brillet, F. From Dysbiosis to Healthy Skin: Major Contributions of Cutibacterium acnes to Skin Homeostasis. Microorganisms 2021, 9, 628. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.Y.; You, S.W.; Gu, K.N.; Kim, H.; Shin, J.G.; Leem, S.; Hwang, B.K.; Kim, Y.; Kang, N.G. Longitudinal study of the interplay between the skin barrier and facial microbiome over 1 year. Front. Microbiol. 2023, 14, 1298632. [Google Scholar] [CrossRef]
- Li, M.; Budding, A.E.; van der Lugt-Degen, M.; Du-Thumm, L.; Vandeven, M.; Fan, A. The influence of age, gender and race/ethnicity on the composition of the human axillary microbiome. Int. J. Cosmet. Sci. 2019, 41, 371–377. [Google Scholar] [CrossRef]
- Harel, N.; Ogen-Shtern, N.; Reshef, L.; Biran, D.; Ron, E.Z.; Gophna, U. Skin microbiome bacteria enriched following long sun exposure can reduce oxidative damage. Res. Microbiol. 2023, 174, 104138. [Google Scholar] [CrossRef]
- Gervason, S.; Napoli, M.; Dreux-Zhiga, A.; Lazzarelli, C.; Garcier, S.; Briand, A.; Albouy, M.; Thepot, A.; Berthon, J.Y.; Filaire, E. Attenuation of negative effects of senescence in human skin using an extract from Sphingomonas hydrophobicum: Development of new skin care solution. Int. J. Cosmet. Sci. 2019, 41, 391–397. [Google Scholar] [CrossRef]
- Tamas, B.; Gabriella, K.; Kristof, A.; Anett, I.; Janos Pal, K.; Balint, T.; Peter, L.; Marton, P.; Katalin, N. The Effects of Lakitelek Thermal Water and Tap Water on Skin Microbiome, a Randomized Control Pilot Study. Life 2023, 13, 746. [Google Scholar] [CrossRef]
- Shao, L.; Jiang, S.; Li, Y.; Shi, Y.; Wang, M.; Liu, T.; Yang, S.; Ma, L. Regular Late Bedtime Significantly Affects the Skin Physiological Characteristics and Skin Bacterial Microbiome. Clin. Cosmet. Investig. Dermatol. 2022, 15, 1051–1063. [Google Scholar] [CrossRef]
- Salem, I.; Ramser, A.; Isham, N.; Ghannoum, M.A. The Gut Microbiome as a Major Regulator of the Gut-Skin Axis. Front. Microbiol. 2018, 9, 1459. [Google Scholar] [CrossRef]



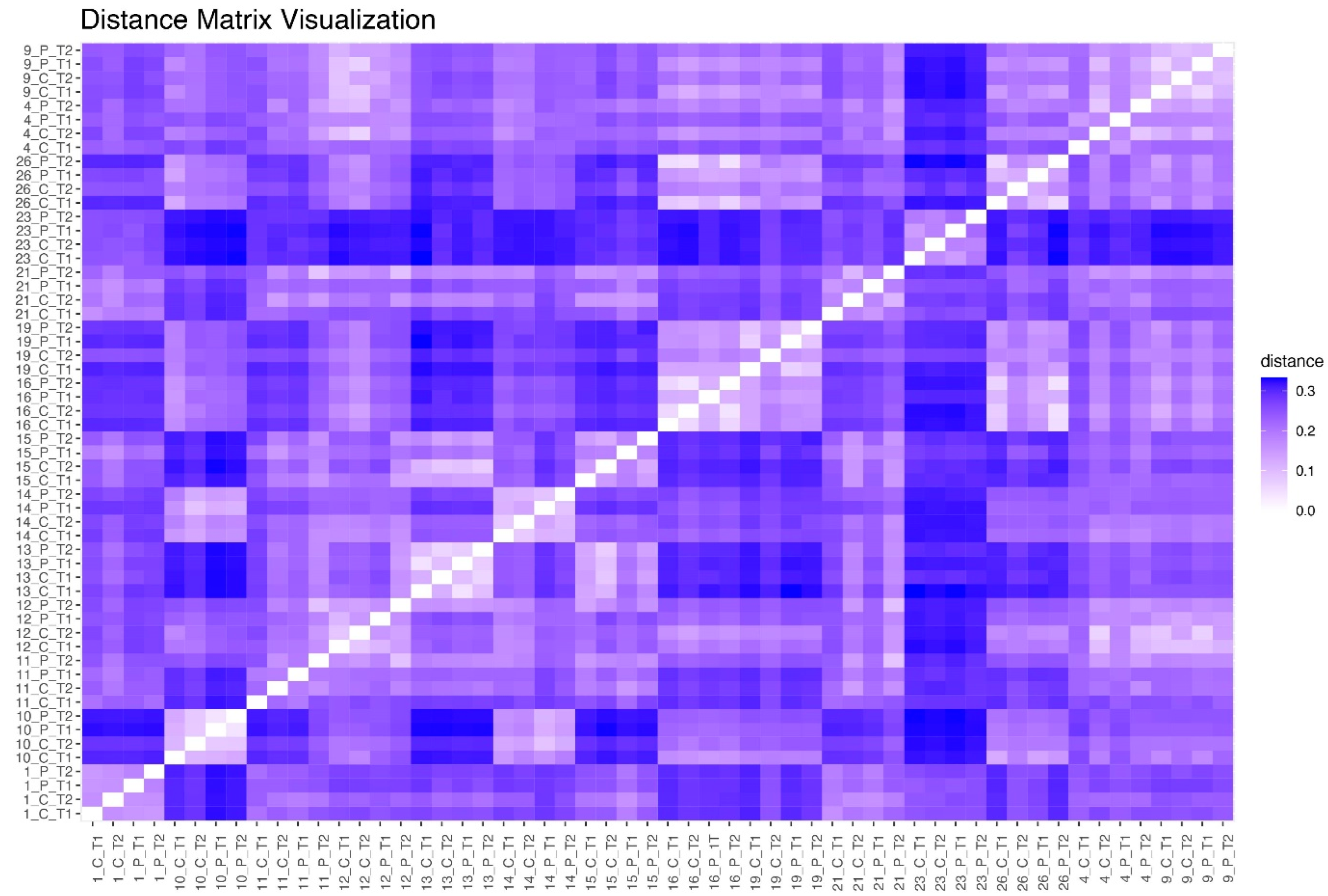
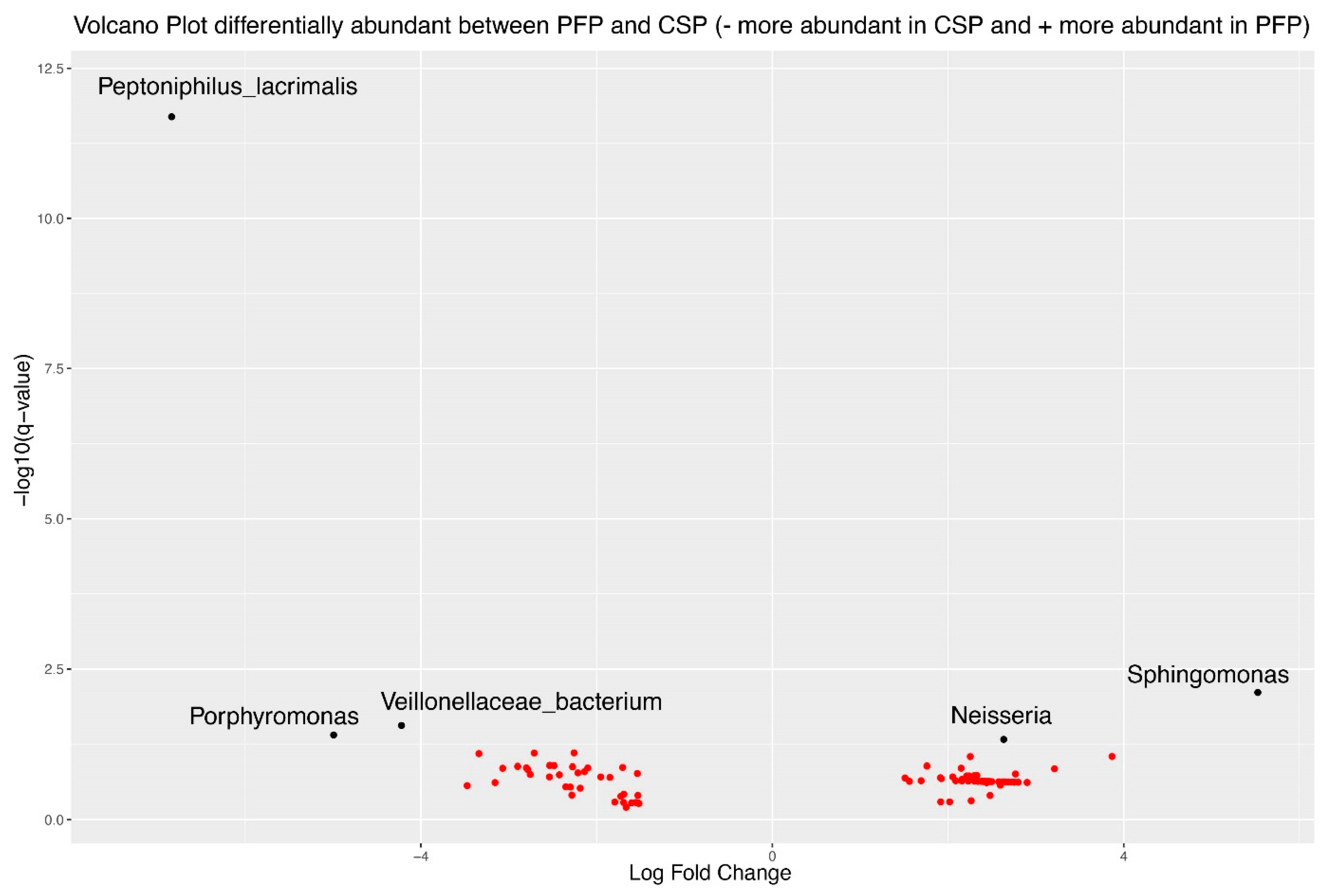

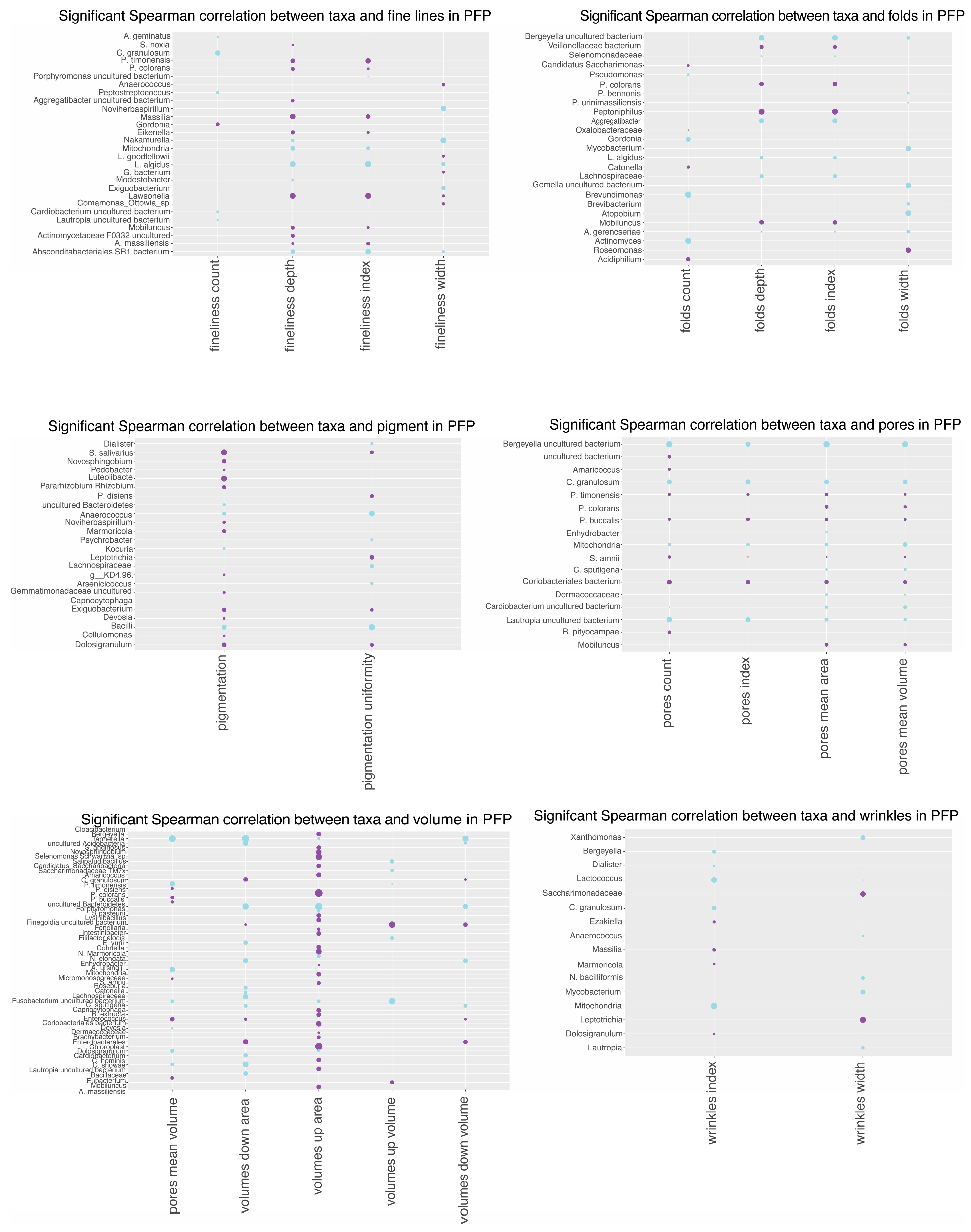
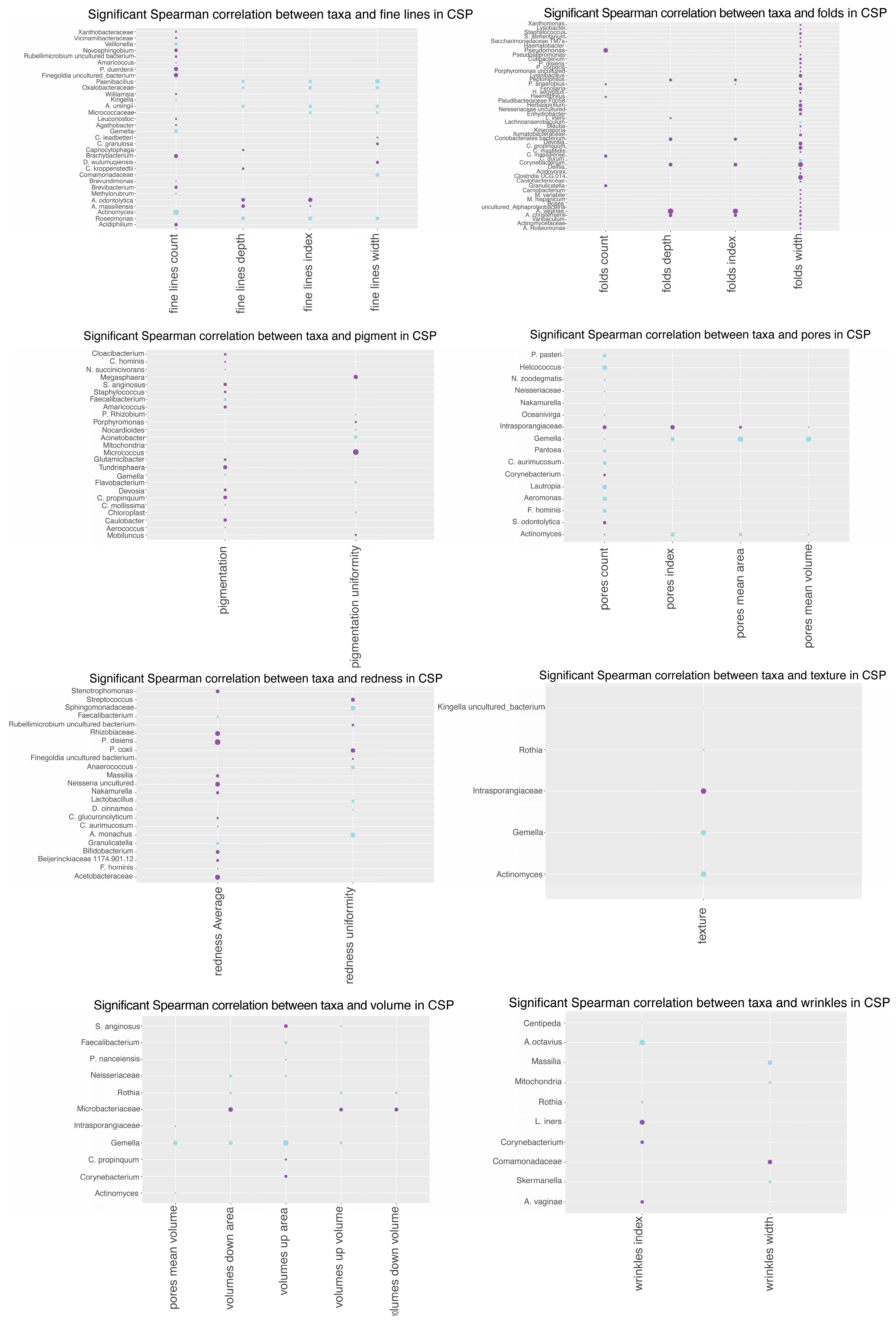
| CSP T1 | A | B | C | E |
|---|---|---|---|---|
| A | - | No | Yes * | No |
| B | No | - | Yes * | No |
| C | Yes * | Yes * | - | Yes * |
| E | No | No | Yes * | - |
| CSP T2 | A | B | C | E |
|---|---|---|---|---|
| A | - | No | No | Yes * |
| B | No | - | No | No |
| C | No | No | - | Yes * |
| E | Yes * | No | Yes * | - |
| PFP T1 | A | B | C | E |
|---|---|---|---|---|
| A | - | No | Yes * | No |
| B | No | - | Yes * | No |
| C | Yes * | Yes * | - | No |
| E | No | No | No | - |
| PFP T2 | A | B | C | E |
| A | - | No | No | No |
| B | No | - | No | No |
| C | No | No | - | No |
| E | No | No | No | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagner, N.; Valeriano, V.D.; Diou-Hirtz, S.; Björninen, E.; Åkerström, U.; Engstrand, L.; Schuppe-Koistinen, I.; Gillbro, J.M. Microbial Dynamics: Assessing Skincare Regimens’ Impact on the Facial Skin Microbiome and Skin Health Parameters. Microorganisms 2024, 12, 2655. https://doi.org/10.3390/microorganisms12122655
Wagner N, Valeriano VD, Diou-Hirtz S, Björninen E, Åkerström U, Engstrand L, Schuppe-Koistinen I, Gillbro JM. Microbial Dynamics: Assessing Skincare Regimens’ Impact on the Facial Skin Microbiome and Skin Health Parameters. Microorganisms. 2024; 12(12):2655. https://doi.org/10.3390/microorganisms12122655
Chicago/Turabian StyleWagner, Nicole, Valerie Diane Valeriano, Samuel Diou-Hirtz, Evelina Björninen, Ulf Åkerström, Lars Engstrand, Ina Schuppe-Koistinen, and Johanna Maria Gillbro. 2024. "Microbial Dynamics: Assessing Skincare Regimens’ Impact on the Facial Skin Microbiome and Skin Health Parameters" Microorganisms 12, no. 12: 2655. https://doi.org/10.3390/microorganisms12122655
APA StyleWagner, N., Valeriano, V. D., Diou-Hirtz, S., Björninen, E., Åkerström, U., Engstrand, L., Schuppe-Koistinen, I., & Gillbro, J. M. (2024). Microbial Dynamics: Assessing Skincare Regimens’ Impact on the Facial Skin Microbiome and Skin Health Parameters. Microorganisms, 12(12), 2655. https://doi.org/10.3390/microorganisms12122655






