Towards Reliable Methodology: Microbiome Analysis of Fresh Frozen vs. Formalin-Fixed Paraffin-Embedded Bladder Tissue Samples: A Feasibility Study
Abstract
1. Introduction
2. Material and Methods
2.1. Sample Acquisition and Analysis
2.2. Patient Data Acquisition
2.3. Statistics
2.4. Ethics
3. Results
3.1. Patient Characteristics
3.2. DNA-Extraction
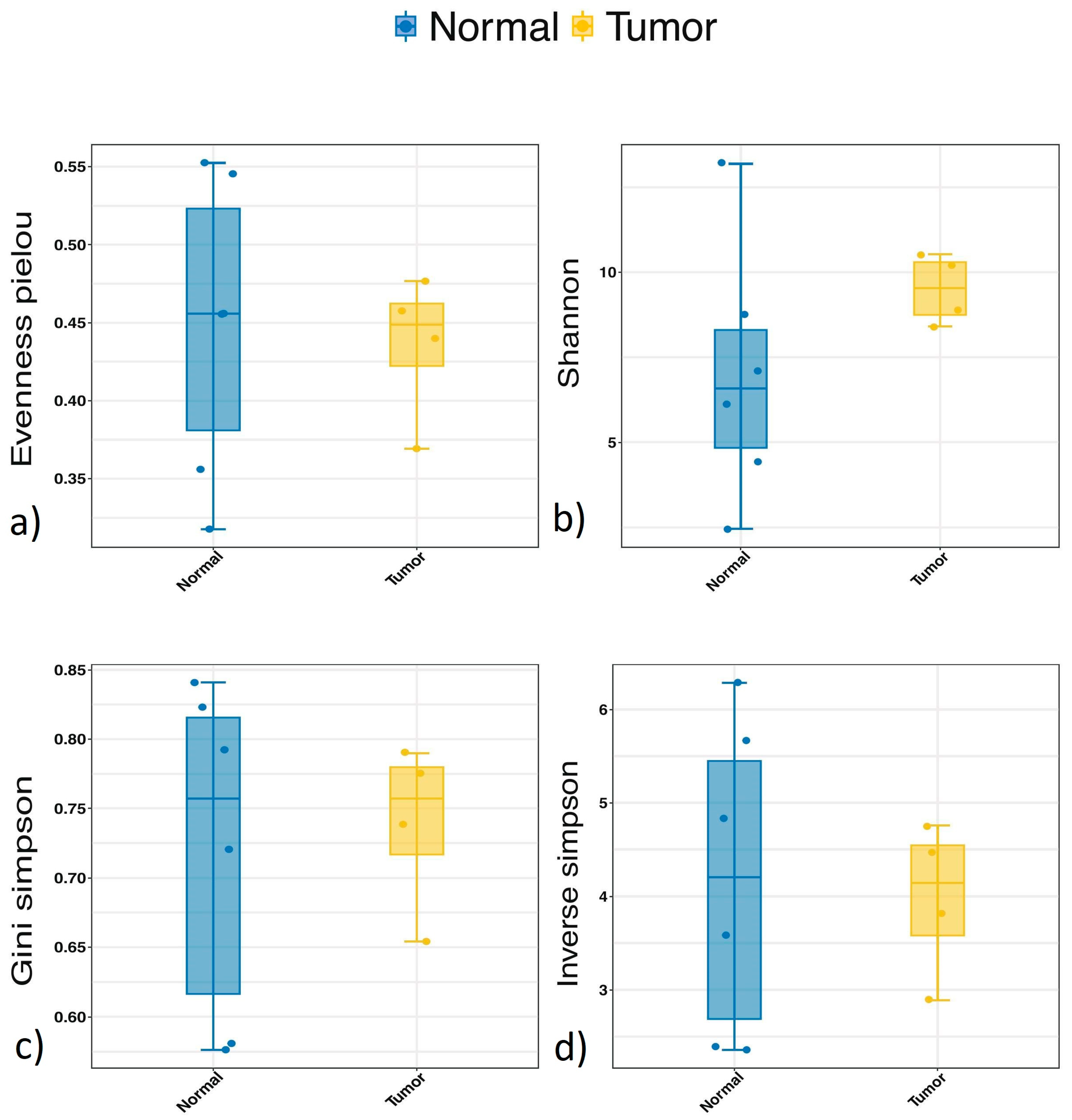
3.3. Alpha Diversity
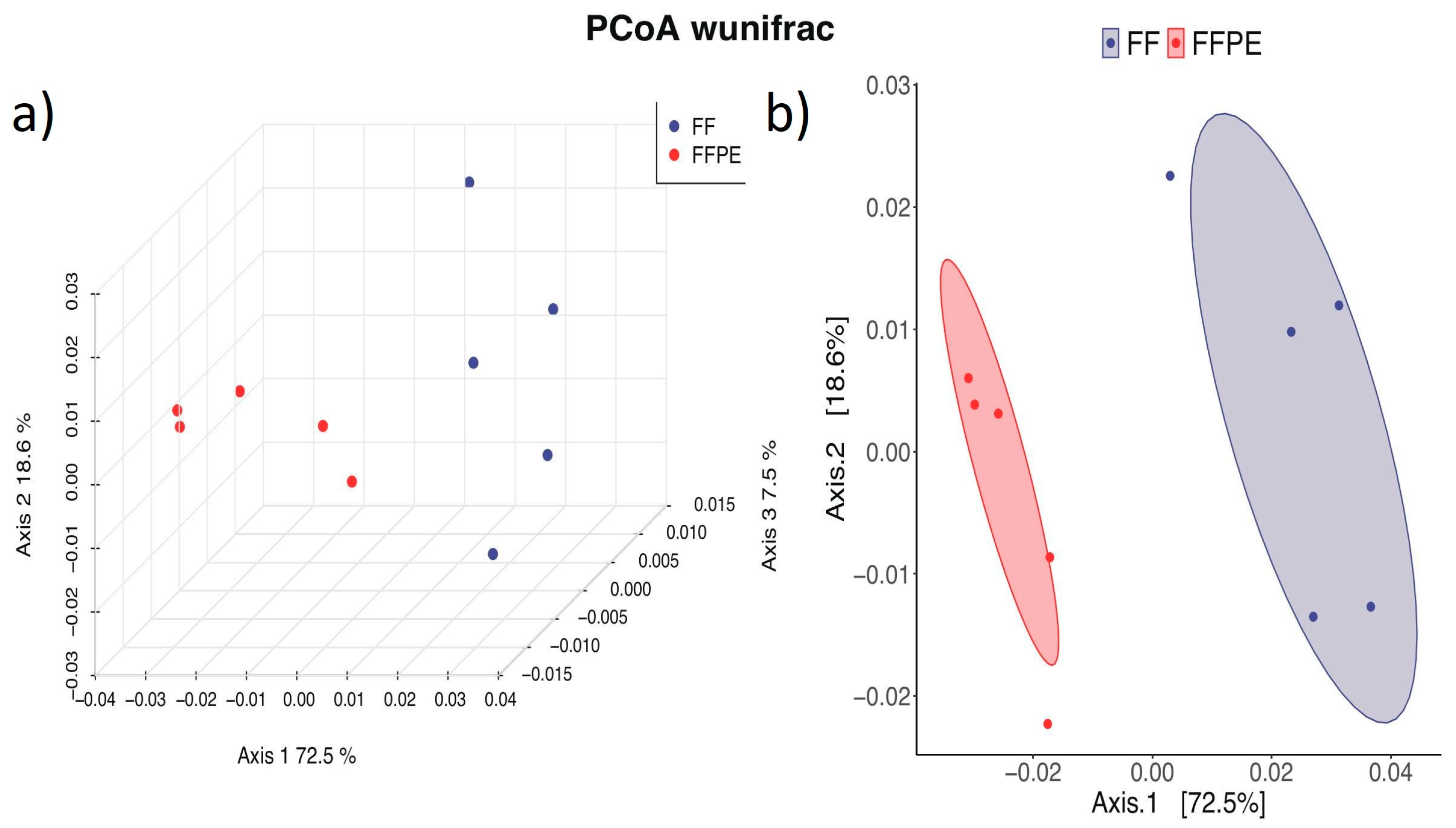
3.4. Beta Diversity
3.5. Taxonomy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Kirkali, Z.; Chan, T.; Manoharan, M.; Algaba, F.; Busch, C.; Cheng, L.; Kiemeney, L.; Kriegmair, M.; Montironi, R.; Murphy, W.M.; et al. Bladder cancer: Epidemiology, staging and grading, and diagnosis. Urology 2005, 66, 4–34. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Dominguez Escrig, J.L.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur. Urol. 2021, 81, 75–94. [Google Scholar] [CrossRef]
- Netto, G.J.; Amin, M.B.; Berney, D.M.; Compérat, E.M.; Gill, A.J.; Hartmann, A.; Menon, S.; Raspollini, M.R.; Rubin, M.A.; Srigley, J.R.; et al. The 2022 World Health Organization Classification of Tumors of the Urinary System and Male Genital Organs-Part B: Prostate and Urinary Tract Tumors. Eur. Urol. 2022, 82, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Inamura, K. Bladder cancer: New insights into its molecular pathology. Cancers 2018, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.Y.; Lee, E.S.; Lee, W.J.; Kim, H.H.; Min, K.J.; Lee, C. Analysis of the immunologic mechanism of intravesical bacillus Calmette-Guerin therapy for superficial bladder tumors: Distribution and function of immune cells. J. Korean Med. Sci. 1993, 8, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Burger, M.; Compérat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Rouprêt, M.; Shariat, S.F.; Sylvester, R.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ)—2019 Update. Eur. Urol. 2019, 76, 639–657. [Google Scholar] [CrossRef] [PubMed]
- Dobruch, J.; Oszczudłowski, M. Bladder Cancer: Current Challenges and Future Directions. Medicina 2021, 57, 749. [Google Scholar] [CrossRef] [PubMed]
- Sweis, R.; Golan, S.; Barashi, N.; Hill, E.; Andolfi, C.; Bloodworth, J.; Werntz, R.; Steinberg, G. Characterization of the urinary microbiome in patients with non-muscle invasive bladder cancer treated with bacillus Calmette-Guérin immunotherapy. J. Urol. 2019, 201, e621. [Google Scholar] [CrossRef]
- Aragón, I.M.; Herrera-Imbroda, B.; Queipo-Ortuño, M.I.; Castillo, E.; Del Moral, J.S.G.; Gómez-Millán, J.; Yucel, G.; Lara, M.F. The Urinary Tract Microbiome in Health and Disease. Eur. Urol. Focus 2018, 4, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Yacouba, A.; Tidjani Alou, M.; Lagier, J.-C.; Dubourg, G.; Raoult, D. Urinary microbiota and bladder cancer: A systematic review and a focus on uropathogens. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar] [CrossRef]
- Chipollini, J.; Wright, J.R.; Nwanosike, H.; Kepler, C.Y.; Batai, K.; Lee, B.R.; Spiess, P.E.; Stewart, D.B.; Lamendella, R. Characterization of urinary microbiome in patients with bladder cancer: Results from a single-institution, feasibility study. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 615–621. [Google Scholar] [CrossRef]
- Mansour, B.; Monyók, Á.; Gajdács, M.; Stercz, B.; Makra, N.; Pénzes, K.; Vadnay, I.; Szabó, D.; Ostorházi, E. Bladder Tissue Microbiome Composition in Patients of Bladder Cancer or Benign Prostatic Hyperplasia and Related Human Beta Defensin Levels. Biomedicines 2022, 10, 1758. [Google Scholar] [CrossRef]
- Oláh, C.; Váradi, M.; Horváth, O.; Nyirády, P.; Szarvas, T. Oncological relevance of gut and urine microbiomes. Orv. Hetil. 2021, 162, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-K.; Xie, R.-L.; You, R.; Liu, Y.-P.; Chen, X.-Y.; Chen, M.-Y.; Huang, P.-Y. The role of the bacterial microbiome in the treatment of cancer. BMC Cancer 2021, 21, 934. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.; Kandalgaonkar, M.R.; Golonka, R.M.; Yeoh, B.S.; Vijay-Kumar, M.; Saha, P. Crosstalk between Gut Microbiota and Host Immunity: Impact on Inflammation and Immunotherapy. Biomedicines 2023, 11, 294. [Google Scholar] [CrossRef]
- Bieri, U.; Scharl, M.; Sigg, S.; Szczerba, B.M.; Morsy, Y.; Rüschoff, J.H.; Schraml, P.H.; Krauthammer, M.; Hefermehl, L.J.; Eberli, D.; et al. Prospective observational study of the role of the microbiome in BCG responsiveness prediction (SILENT-EMPIRE): A study protocol. BMJ Open 2022, 12, e061421. [Google Scholar] [CrossRef]
- Gurina, T.S.; Simms, L. Histology, Staining; StatPearls Publ. LLC: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Vojtechova, Z.; Zavadil, J.; Klozar, J.; Grega, M.; Tachezy, R. Comparison of the miRNA expression profiles in fresh frozen and formalin-fixed paraffin-embedded tonsillar tumors. PLoS ONE 2017, 12, e0179645. [Google Scholar] [CrossRef] [PubMed]
- Hewitson, T.D.; Wigg, B.; Becker, G.J. Tissue preparation for histochemistry: Fixation, embedding, and antigen retrieval for light microscopy. Methods Mol. Biol. 2010, 611, 3–18. [Google Scholar] [CrossRef]
- Schoffman, H.; Levin, Y.; Itzhaki-Alfia, A.; Tselekovits, L.; Gonen, L.; Vainer, G.W.; Hout-Siloni, G.; Barshack, I.; Cohen, Z.R.; Margalit, N.; et al. Comparison of matched formalin-fixed paraffin embedded and fresh frozen meningioma tissue reveals bias in proteomic profiles. Proteomics 2022, 22, 2200085. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Gouda-Vossos, A.; Dzamko, N.; Halliday, G.; Huang, Y. DNA extraction from fresh-frozen and formalin-fixed, paraffin-embedded human brain tissue. Neurosci. Bull. 2013, 29, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Qiagen. Purification of genomic DNA from FFPE tissue using the QIAamp® DNA FFPE Tissue Kit and Deparaffinization Solution; Qiagen: Hilden, Germany, 2011; pp. 1–3. [Google Scholar]
- Research, Z. ZymoBIOMICS TM DNA Miniprep Kit Instruction Manual ver.1.5.3; ZymoResearch: Irvine, CA, USA, 2022; pp. 1–28. [Google Scholar]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Mcdonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; Desantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012, 6, 610–618. [Google Scholar] [CrossRef] [PubMed]
- WE, G. Special symposium: Fixation and tissue processing models. Biotech. Histochem. 2009, 84, 185–193. [Google Scholar] [CrossRef]
- Bøttcher, S.; Id, J.; Tfelt-Hansen, J.; Smerup, M.H.; Andersen, J.D.; Morlingid, N. Comparison of whole transcriptome sequencing of fresh, frozen, and formalin-fixed, paraffin-embedded cardiac tissue. PLoS ONE 2023, 18, e0283159. [Google Scholar] [CrossRef]
- Oh, E.; La Choi, Y.; Kwon, M.J.; Kim, R.N.; Kim, Y.J.; Song, J.Y.; Jung, K.S.; Shin, Y.K. Comparison of Accuracy of Whole-Exome Sequencing with Formalin-Fixed Paraffin-Embedded and Fresh Frozen Tissue Samples. PLoS ONE 2015, 10, e0144162. [Google Scholar] [CrossRef] [PubMed]
- Pederzoli, F.; Ferrarese, R.; Amato, V.; Locatelli, I.; Alchera, E.; Lucianò, R.; Nebuloni, M.; Briganti, A.; Gallina, A.; Colombo, R.; et al. Sex-specific Alterations in the Urinary and Tissue Microbiome in Therapy-naïve Urothelial Bladder Cancer Patients. Eur. Urol. Oncol. 2020, 3, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Mansour, B.; Monyók, Á.; Makra, N.; Gajdács, M.; Vadnay, I.; Ligeti, B.; Juhász, J.; Szabó, D.; Ostorházi, E. Bladder cancer-related microbiota: Examining differences in urine and tissue samples. Sci. Rep. 2020, 10, 11042. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, A.; Lu, X.; Zhang, Z.; Xue, Y.; Xu, J.; Zeng, S.; Xiong, Q.; Tan, H.; He, X.; et al. Dysbiosis signatures of the microbial profile in tissue from bladder cancer. Cancer Med. 2019, 8, 6904–6914. [Google Scholar] [CrossRef]
- Parra-Grande, M.; Ore-Arce, M.; Martinez-Priego, L.; D’Auria, G.; Rossello-Mora, R.; Lillo, M.; Sempere, A.; Lumbreras, B.; Sanchez-Hellin, V. Profiling the Bladder Microbiota in Patients With Bladder Cancer. Front. Microbiol. 2022, 12, 718776. [Google Scholar] [CrossRef]
- Ahn, H.K.; Kim, K.; Park, J.; Kim, K.H. Urinary microbiome profile in men with genitourinary malignancies. Investig. Clin. Urol. 2022, 63, 569–576. [Google Scholar] [CrossRef]
- Bučević Popović, V.; Šitum, M.; Chow, C.E.T.; Chan, L.S.; Roje, B.; Terzić, J. The urinary microbiome associated with bladder cancer. Sci. Rep. 2018, 8, 12157. [Google Scholar] [CrossRef] [PubMed]
- Oresta, B.; Braga, D.; Lazzeri, M.; Frego, N.; Saita, A.; Faccani, C.; Fasulo, V.; Colombo, P.; Guazzoni, G.; Hurle, R.; et al. The Microbiome of Catheter Collected Urine in Males with Bladder Cancer According to Disease Stage. J. Urol. 2021, 205, 86–93. [Google Scholar] [CrossRef]
- Pohl, H.G.; Groah, S.L.; Pérez-Losada, M.; Ljungberg, I.; Sprague, B.M.; Chandal, N.; Caldovic, L.; Hsieh, M. The Urine Microbiome of Healthy Men and Women Differs by Urine Collection Method. Int. Neurourol. J. 2020, 24, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acid Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]

| Patient | Age | Stage | Grade | Prior AB 1 | Smoke Status | Primary/Recurrence |
|---|---|---|---|---|---|---|
| 1 | 71 | pTa | Low grade | None | ca. 10PY 2 | Recurrence |
| 2 | 68 | pTa | High grade | None | ca. 20PY 2 | Primary |
| 3 | 78 | pTa | High grade | None | ca. 40PY 2 | Primary |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enderlin, D.; Bieri, U.; Gadient, J.; Morsy, Y.; Scharl, M.; Rüschoff, J.H.; Hefermehl, L.J.; Nikitin, A.; Langenauer, J.; Engeler, D.S.; et al. Towards Reliable Methodology: Microbiome Analysis of Fresh Frozen vs. Formalin-Fixed Paraffin-Embedded Bladder Tissue Samples: A Feasibility Study. Microorganisms 2024, 12, 2594. https://doi.org/10.3390/microorganisms12122594
Enderlin D, Bieri U, Gadient J, Morsy Y, Scharl M, Rüschoff JH, Hefermehl LJ, Nikitin A, Langenauer J, Engeler DS, et al. Towards Reliable Methodology: Microbiome Analysis of Fresh Frozen vs. Formalin-Fixed Paraffin-Embedded Bladder Tissue Samples: A Feasibility Study. Microorganisms. 2024; 12(12):2594. https://doi.org/10.3390/microorganisms12122594
Chicago/Turabian StyleEnderlin, Dominik, Uwe Bieri, Jana Gadient, Yasser Morsy, Michael Scharl, Jan Hendrik Rüschoff, Lukas John Hefermehl, Anna Nikitin, Janine Langenauer, Daniel Stephan Engeler, and et al. 2024. "Towards Reliable Methodology: Microbiome Analysis of Fresh Frozen vs. Formalin-Fixed Paraffin-Embedded Bladder Tissue Samples: A Feasibility Study" Microorganisms 12, no. 12: 2594. https://doi.org/10.3390/microorganisms12122594
APA StyleEnderlin, D., Bieri, U., Gadient, J., Morsy, Y., Scharl, M., Rüschoff, J. H., Hefermehl, L. J., Nikitin, A., Langenauer, J., Engeler, D. S., Förster, B., Obrecht, F., Surber, J., Scherer, T. P., Eberli, D., & Poyet, C. (2024). Towards Reliable Methodology: Microbiome Analysis of Fresh Frozen vs. Formalin-Fixed Paraffin-Embedded Bladder Tissue Samples: A Feasibility Study. Microorganisms, 12(12), 2594. https://doi.org/10.3390/microorganisms12122594






