Respiratory Flora Intervention: A New Strategy for the Prevention and Treatment of Occupationally Related Respiratory Allergy in Healthcare Workers
Abstract
1. Introduction
2. Methods
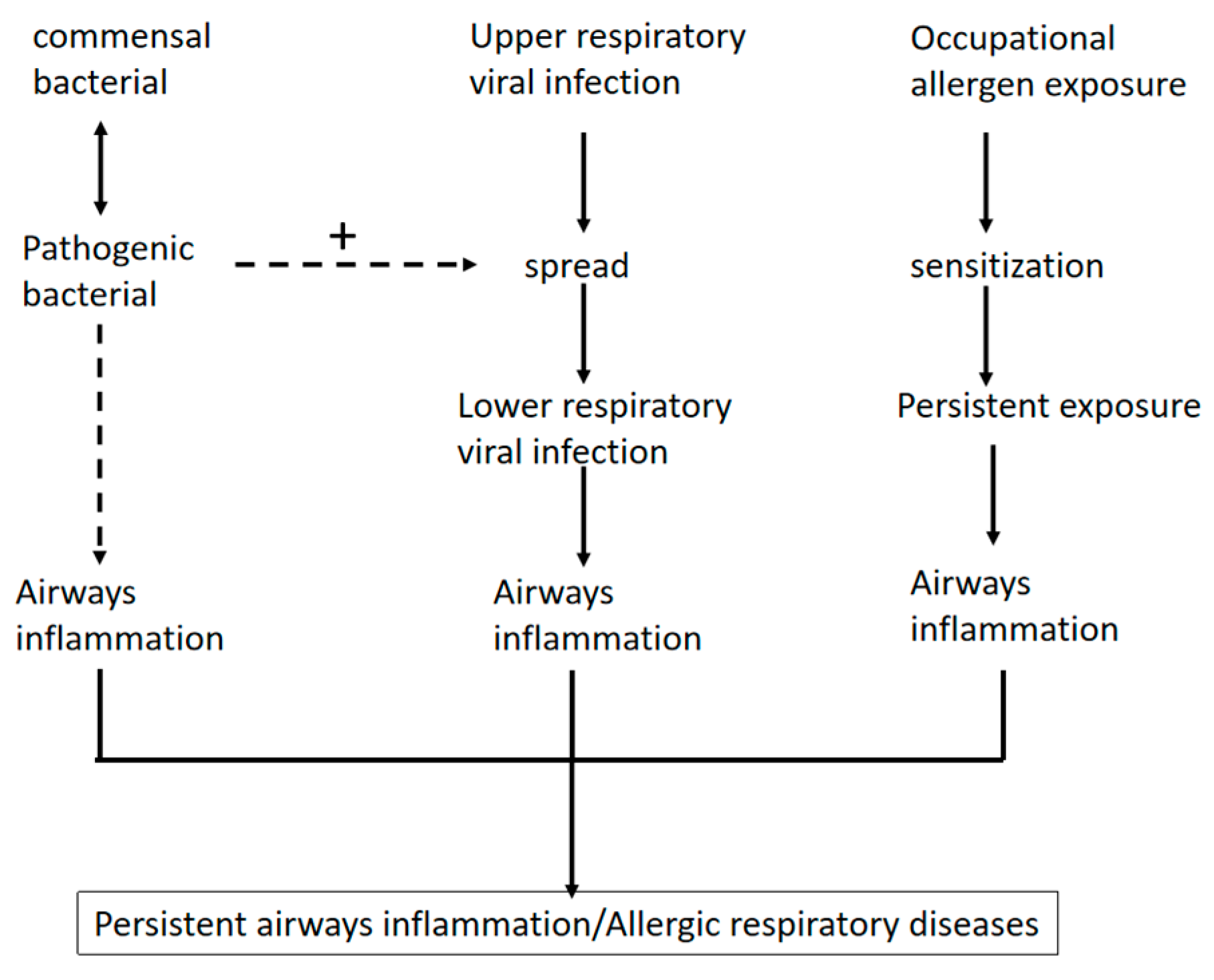
3. Burden of Respiratory Allergic Diseases Among Healthcare Workers
4. Changes in Respiratory Flora in People with Respiratory Allergic Diseases
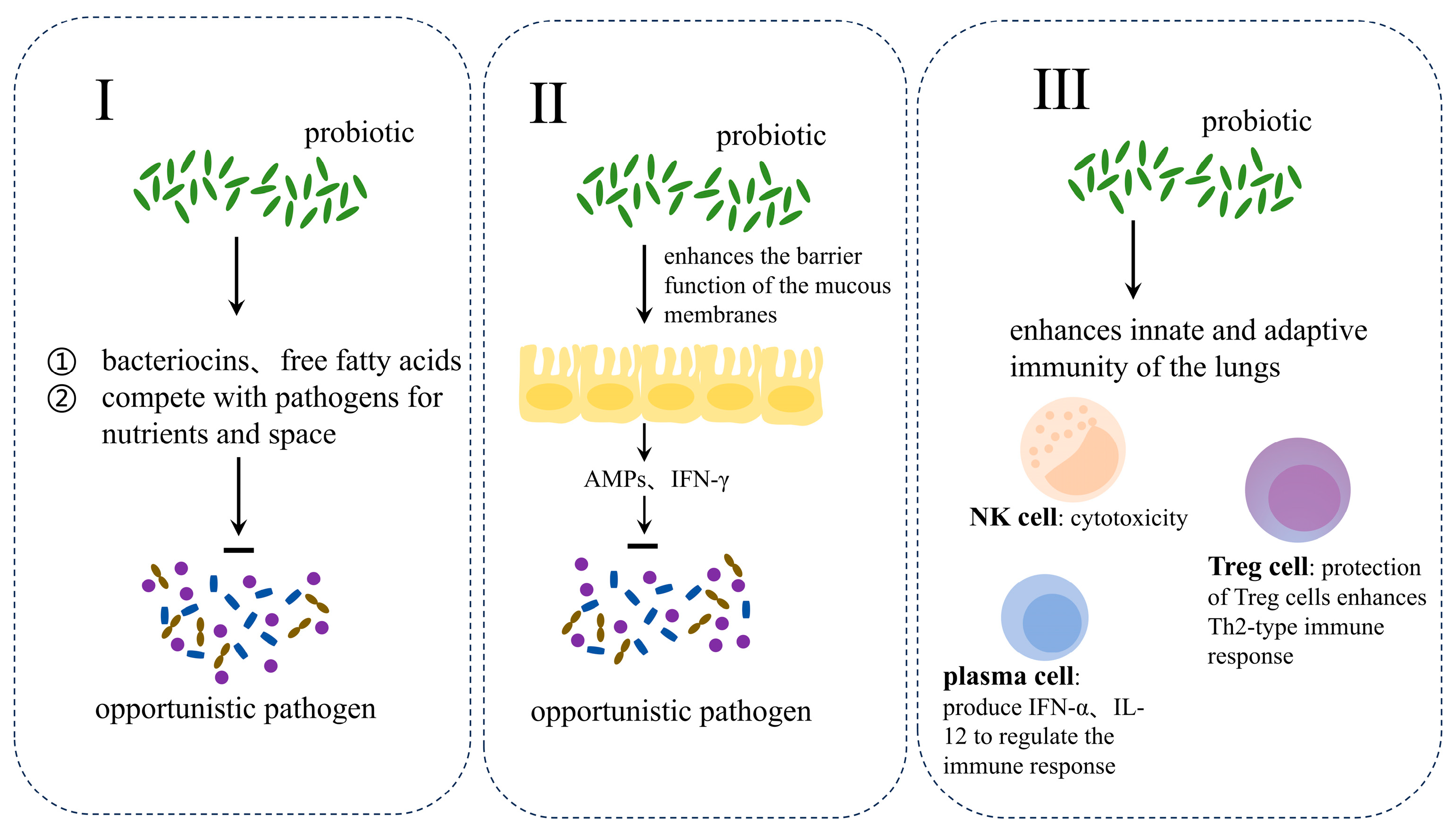
5. Flora Intervention to Assist in the Treatment of Respiratory Allergic Diseases
5.1. Asthma
5.2. Allergic Rhinitis
6. Safety and Efficacy of Probiotic-Assisted Treatment of Respiratory Allergic Diseases
7. Discussion
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nemat, A.; Asady, A.; Raufi, N.; Zaki, N.; Ehsan, E.; Noor, N.A.S.; Zeng, Q. A Survey of the Healthcare Workers in Afghanistan during the COVID-19 Pandemic. Am. J. Trop. Med. Hyg. 2020, 104, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Mandić-Rajčević, S.; Masci, F.; Crespi, E.; Franchetti, S.; Longo, A.; Bollina, I.; Velocci, S.; Amorosi, A.; Baldelli, R.; Boselli, L.; et al. Source and symptoms of COVID-19 among hospital workers in Milan. Occup. Med. 2020, 70, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Al-Tawfiq, J.A.; Temsah, M.H. Perspective on the challenges of COVID-19 facing healthcare workers. Infection 2023, 51, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ochoa, S.A.; Franco, O.H.; Rojas, L.Z.; Raguindin, P.F.; Roa-Díaz, Z.M.; Wyssmann, B.M.; Guevara, S.L.R.; Echeverría, L.E.; Glisic, M.; Muka, T. COVID-19 in Health-Care Workers: A Living Systematic Review and Meta-Analysis of Prevalence, Risk Factors, Clinical Characteristics, and Outcomes. Am. J. Epidemiol. 2021, 190, 161–175. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Bepko, J.; Mansalis, K. Common Occupational Disorders: Asthma, COPD, Dermatitis, and Musculoskeletal Disorders. Am. Fam. Physician 2016, 93, 1000–1006. [Google Scholar]
- Cohen, B. Allergic Rhinitis. Pediatr. Rev. 2023, 44, 537–550. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, C.; Zhang, L. Advances and novel developments in allergic rhinitis. Allergy 2020, 75, 3069–3076. [Google Scholar] [CrossRef]
- Bousquet, J.; Anto, J.M.; Bachert, C.; Baiardini, I.; Bosnic-Anticevich, S.; Walter Canonica, G.; Melén, E.; Palomares, O.; Scadding, G.K.; Togias, A.; et al. Allergic rhinitis. Nat. Rev. Dis. Primers 2020, 6, 95. [Google Scholar] [CrossRef]
- Chowdhury, N.U.; Guntur, V.P.; Newcomb, D.C.; Wechsler, M.E. Sex and gender in asthma. Eur. Respir. Rev. 2021, 30, 210067. [Google Scholar] [CrossRef]
- Logoń, K.; Świrkosz, G.; Nowak, M.; Wrześniewska, M.; Szczygieł, A.; Gomułka, K. The Role of the Microbiome in the Pathogenesis and Treatment of Asthma. Biomedicines 2023, 11, 1618. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Wang, J.B.; An, H.B.; Wen, M.Z.; You, J.X.; Yang, X.T. Effect of SARS-CoV-2 infection on asthma patients. Front. Med. 2022, 9, 928637. [Google Scholar] [CrossRef] [PubMed]
- Blanc, P.D.; Annesi-Maesano, I.; Balmes, J.R.; Cummings, K.J.; Fishwick, D.; Miedinger, D.; Murgia, N.; Naidoo, R.N.; Reynolds, C.J.; Sigsgaard, T.; et al. The Occupational Burden of Nonmalignant Respiratory Diseases. An Official American Thoracic Society and European Respiratory Society Statement. Am. J. Respir. Crit. Care Med. 2019, 199, 1312–1334. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Naskar, R.; Acharya, S.; Ba Vinh, L.; Kim, J.H.; Lee, J.Y.; Kim, Y.H.; Kang, J.S.; Hwang, I. Inotodiol, an antiasthmatic agent with efficacy and safety, preferentially impairs membrane-proximal signaling for mast cell activation. Int. Immunopharmacol. 2023, 117, 109854. [Google Scholar] [CrossRef]
- Singanayagam, A.; Johnston, S.L. Long-term impact of inhaled corticosteroid use in asthma and chronic obstructive pulmonary disease (COPD): Review of mechanisms that underlie risks. J. Allergy Clin. Immunol. 2020, 146, 1292–1294. [Google Scholar] [CrossRef]
- Lax, S.; Gilbert, J.A. Hospital-associated microbiota and implications for nosocomial infections. Trends Mol. Med. 2015, 21, 427–432. [Google Scholar] [CrossRef]
- Hufnagl, K.; Pali-Schöll, I.; Roth-Walter, F.; Jensen-Jarolim, E. Dysbiosis of the gut and lung microbiome has a role in asthma. Semin. Immunopathol. 2020, 42, 75–93. [Google Scholar] [CrossRef]
- Lena, P.; Karageorgos, S.A.; Loutsiou, P.; Poupazi, A.; Lamnisos, D.; Papageorgis, P.; Tsioutis, C. Multidrug-Resistant Bacteria on Healthcare Workers’ Uniforms in Hospitals and Long-Term Care Facilities in Cyprus. Antibiotics 2021, 11, 49. [Google Scholar] [CrossRef]
- Chen, M.; He, S.; Miles, P.; Li, C.; Ge, Y.; Yu, X.; Wang, L.; Huang, W.; Kong, X.; Ma, S.; et al. Nasal Bacterial Microbiome Differs Between Healthy Controls and Those With Asthma and Allergic Rhinitis. Front. Cell Infect. Microbiol. 2022, 12, 841995. [Google Scholar] [CrossRef]
- Lynch, S.V.; Wood, R.A.; Boushey, H.; Bacharier, L.B.; Bloomberg, G.R.; Kattan, M.; O’Connor, G.T.; Sandel, M.T.; Calatroni, A.; Matsui, E.; et al. Effects of early-life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. J. Allergy Clin. Immunol. 2014, 134, 593–601.e12. [Google Scholar] [CrossRef]
- Díaz-Garrido, N.; Badia, J.; Baldomà, L. Microbiota-derived extracellular vesicles in interkingdom communication in the gut. J. Extracell. Vesicles 2021, 10, e12161. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.M.; Lefebvre, D.L.; et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 2015, 7, 307ra152. [Google Scholar] [CrossRef] [PubMed]
- Lins, M.; Puppin Zandonadi, R.; Raposo, A.; Ginani, V.C. Food Waste on Foodservice: An Overview through the Perspective of Sustainable Dimensions. Foods 2021, 10, 1175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L. Increasing Prevalence of Allergic Rhinitis in China. Allergy Asthma Immunol. Res. 2019, 11, 156–169. [Google Scholar] [CrossRef]
- Gao, W.X.; Ou, C.Q.; Fang, S.B.; Sun, Y.Q.; Zhang, H.; Cheng, L.; Wang, Y.J.; Zhu, D.D.; Lv, W.; Liu, S.X.; et al. Occupational and environmental risk factors for chronic rhinosinusitis in China: A multicentre cross-sectional study. Respir. Res. 2016, 17, 54. [Google Scholar] [CrossRef]
- Mazurek, J.M.; Weissman, D.N. Occupational Respiratory Allergic Diseases in Healthcare Workers. Curr. Allergy Asthma Rep. 2016, 16, 77. [Google Scholar] [CrossRef]
- Boonchai, W.; Sirikudta, W.; Iamtharachai, P.; Kasemsarn, P. Latex glove-related symptoms among health care workers: A self-report questionnaire-based survey. Dermatitis 2014, 25, 135–139. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, L.; Du, X.; Zhou, K.; Qin, L.; Wang, L.; Yang, M.; Wu, M.; Zheng, Z.; Xiang, Y.; et al. Involvement of epithelia-derived exosomes in chronic respiratory diseases. Biomed. Pharmacother. 2021, 143, 112189. [Google Scholar] [CrossRef]
- Su, F.C.; Friesen, M.C.; Humann, M.; Stefaniak, A.B.; Stanton, M.L.; Liang, X.; LeBouf, R.F.; Henneberger, P.K.; Virji, M.A. Clustering asthma symptoms and cleaning and disinfecting activities and evaluating their associations among healthcare workers. Int. J. Hyg. Environ. Health 2019, 222, 873–883. [Google Scholar] [CrossRef]
- Loftus, P.A.; Wise, S.K. Epidemiology and economic burden of asthma. Int. Forum Allergy Rhinol. 2015, 5 (Suppl. 1), S7–S10. [Google Scholar] [CrossRef]
- Buss, Z.S.; Fröde, T.S. Latex allergen sensitization and risk factors due to glove use by health care workers at public health units in Florianopolis, Brazil. J. Investig. Allergol. Clin. Immunol. 2007, 17, 27–33. [Google Scholar] [PubMed]
- Bilge, U.; Unluoglu, I.; Son, N.; Keskin, A.; Korkut, Y.; Unalacak, M. Occupational allergic diseases in kitchen and health care workers: An underestimated health issue. Biomed. Res. Int. 2013, 2013, 285420. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.L.; Torén, K.; Lohman, S.; Ekerljung, L.; Lötvall, J.; Lundbäck, B.; Andersson, E. Respiratory symptoms and respiratory-related absence from work among health care workers in Sweden. J. Asthma 2013, 50, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Kalm-Stephens, P.; Sterner, T.; Kronholm Diab, K.; Smedje, G. Hypersensitivity and the working environment for allergy nurses in sweden. J. Allergy 2014, 2014, 681934. [Google Scholar] [CrossRef]
- Dumas, O.; Gaskins, A.J.; Boggs, K.M.; Henn, S.A.; Le Moual, N.; Varraso, R.; Chavarro, J.E.; Camargo, C.A., Jr. Occupational use of high-level disinfectants and asthma incidence in early- to mid-career female nurses: A prospective cohort study. Occup. Environ. Med. 2021, 78, 244–247. [Google Scholar] [CrossRef]
- Dobashi, K.; Usami, A.; Yokozeki, H.; Tsurikisawa, N.; Nakamura, Y.; Sato, K.; Okumura, J.; Yamaguchi, M. Japanese guidelines for occupational allergic diseases 2020. Allergol. Int. 2020, 69, 387–404. [Google Scholar] [CrossRef]
- Siddiqui, M.I.; Dhanani, R.; Moiz, H. Prevalence of allergic rhinitis among healthcare workers and its impact on their work: A cross-sectional survey at a tertiary healthcare centre in Pakistan. J. Pak. Med. Assoc. 2020, 70, 1432–1435. [Google Scholar] [CrossRef]
- Mucci, N.; Tommasi, E.; Chiarelli, A.; Lulli, L.G.; Traversini, V.; Galea, R.P.; Arcangeli, G. WORKbiota: A Systematic Review about the Effects of Occupational Exposure on Microbiota and Workers’ Health. Int. J. Environ. Res. Public Health 2022, 19, 1043. [Google Scholar] [CrossRef]
- Ekezie, W.; Martin, C.A.; Baggaley, R.F.; Teece, L.; Nazareth, J.; Pan, D.; Sze, S.; Bryant, L.; Woolf, K.; Gray, L.J.; et al. Association between ethnicity and migration status with the prevalence of single and multiple long-term conditions in UK healthcare workers. BMC Med. 2023, 21, 433. [Google Scholar] [CrossRef]
- Choi, C.H.; Poroyko, V.; Watanabe, S.; Jiang, D.; Lane, J.; deTineo, M.; Baroody, F.M.; Naclerio, R.M.; Pinto, J.M. Seasonal allergic rhinitis affects sinonasal microbiota. Am. J. Rhinol. Allergy 2014, 28, 281–286. [Google Scholar] [CrossRef]
- Kang, H.M.; Kang, J.H. Effects of nasopharyngeal microbiota in respiratory infections and allergies. Clin. Exp. Pediatr. 2021, 64, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Rubin, K.; Glazer, S. The pertussis hypothesis: Bordetella pertussis colonization in the etiology of asthma and diseases of allergic sensitization. Med. Hypotheses 2018, 120, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jackson, D.; Bacharier, L.B.; Mauger, D.; Boushey, H.; Castro, M.; Durack, J.; Huang, Y.; Lemanske, R.F., Jr.; Storch, G.A.; et al. The upper-airway microbiota and loss of asthma control among asthmatic children. Nat. Commun. 2019, 10, 5714. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.D.; Xepapadaki, P.; Cui, Y.W.; Stanic, B.; Maurer, D.J.; Bachert, C.; Zhang, N.; Finotto, S.; Chalubinski, M.; Lukkarinen, H.; et al. Effect of Haemophilus influenzae, Streptococcus pneumoniae and influenza vaccinations on infections, immune response and asthma control in preschool children with asthma. Allergy 2023, 78, 1473–1488. [Google Scholar] [CrossRef]
- Jorde, I.; Schreiber, J.; Stegemann-Koniszewski, S. The Role of Staphylococcus aureus and Its Toxins in the Pathogenesis of Allergic Asthma. Int. J. Mol. Sci. 2022, 24, 654. [Google Scholar] [CrossRef]
- Fazlollahi, M.; Lee, T.D.; Andrade, J.; Oguntuyo, K.; Chun, Y.; Grishina, G.; Grishin, A.; Bunyavanich, S. The nasal microbiome in asthma. J. Allergy Clin. Immunol. 2018, 142, 834–843.e2. [Google Scholar] [CrossRef]
- Miao, P.; Jiang, Y.; Jian, Y.; Shi, J.; Liu, Y.; Piewngam, P.; Zheng, Y.; Cheung, G.Y.C.; Liu, Q.; Otto, M.; et al. Exacerbation of allergic rhinitis by the commensal bacterium Streptococcus salivarius. Nat. Microbiol. 2023, 8, 218–230. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Gil, C.H.; Won, J.; Jo, A.; Kim, H.J. Symbiotic microbiome Staphylococcus aureus from human nasal mucus modulates IL-33-mediated type 2 immune responses in allergic nasal mucosa. BMC Microbiol. 2020, 20, 301. [Google Scholar] [CrossRef]
- Minoretti, P.; Sigurtà, C.; Fachinetti, A.; Cerone, E.; Rotta, F.; Emanuele, E. A Preliminary Study of Gut Microbiota in Airline Pilots: Comparison With Construction Workers and Fitness Instructors. Cureus 2023, 15, e39841. [Google Scholar] [CrossRef]
- Lopez-Santamarina, A.; Mondragon, A.D.C.; Cardelle-Cobas, A.; Santos, E.M.; Porto-Arias, J.J.; Cepeda, A.; Miranda, J.M. Effects of Unconventional Work and Shift Work on the Human Gut Microbiota and the Potential of Probiotics to Restore Dysbiosis. Nutrients 2023, 15, 3070. [Google Scholar] [CrossRef]
- Amritha, G.N.; Meenakshi, N.; Alice Peace Selvabai, R.; Shanmugam, P.; Jayaraman, P. A comparative profile of oropharyngeal colonization of Streptococcus pneumoniae and Hemophilus influenzae among HealthCare Workers (HCW) in a tertiary care hospital and non-healthcare individuals. J. Prev. Med. Hyg. 2020, 61, E379–E385. [Google Scholar] [CrossRef] [PubMed]
- Hosuru Subramanya, S.; Thapa, S.; Dwedi, S.K.; Gokhale, S.; Sathian, B.; Nayak, N.; Bairy, I. Streptococcus pneumoniae and Haemophilus species colonization in health care workers: The launch of invasive infections? BMC Res. Notes 2016, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Dai, X.; Ni, L.; Chen, B.; Luo, Z.; Yao, Y.; Wu, X.; Li, H.; Huang, S. Molecular epidemiology and virulence characteristics of Staphylococcus aureus nasal colonization in medical laboratory staff: Comparison between microbiological and non-microbiological laboratories. BMC Infect. Dis. 2018, 18, 122. [Google Scholar] [CrossRef] [PubMed]
- Ohadian Moghadam, S.; Modoodi Yaghooti, M.; Pourramezan, N.; Pourmand, M.R. Molecular characterization and antimicrobial susceptibility of the CA-MRSA isolated from healthcare workers, Tehran, Iran. Microb. Pathog. 2017, 107, 409–412. [Google Scholar] [CrossRef]
- Lena, P.; Ishak, A.; Karageorgos, S.A.; Tsioutis, C. Presence of Methicillin-Resistant Staphylococcus aureus (MRSA) on Healthcare Workers’ Attire: A Systematic Review. Trop. Med. Infect. Dis. 2021, 6, 42. [Google Scholar] [CrossRef]
- Slingerland, B.; Verkaik, N.J.; Klaassen, C.H.W.; Zandijk, W.H.A.; Reiss, I.K.M.; Vos, M.C. Neonatal Staphylococcus aureus acquisition at a tertiary intensive care unit. Am. J. Infect. Control 2020, 48, 1023–1027. [Google Scholar] [CrossRef]
- Bayraktar, M.; Kaya, E.; Ozturk, A.; İbahim, B.M.S. Antimicrobial susceptibility of bacterial pathogens isolated from healthcare workers’ cellphones. Infect. Dis. Now. 2021, 51, 596–602. [Google Scholar] [CrossRef]
- Habibi, N.; Mustafa, A.S.; Khan, M.W. Composition of nasal bacterial community and its seasonal variation in health care workers stationed in a clinical research laboratory. PLoS ONE 2021, 16, e0260314. [Google Scholar] [CrossRef]
- Conceição, T.; Santos Silva, I.; de Lencastre, H.; Aires-de-Sousa, M. Staphylococcus aureus nasal carriage among patients and health care workers in São Tomé and Príncipe. Microb. Drug Resist. 2014, 20, 57–66. [Google Scholar] [CrossRef]
- Snyder, G.M.; Thom, K.A.; Furuno, J.P.; Perencevich, E.N.; Roghmann, M.C.; Strauss, S.M.; Netzer, G.; Harris, A.D. Detection of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci on the gowns and gloves of healthcare workers. Infect. Control Hosp. Epidemiol. 2008, 29, 583–589. [Google Scholar] [CrossRef]
- Tselebonis, A.; Nena, E.; Nikolaidis, C.; Konstantinidis, T.; Kontogiorgis, C.; Panopoulou, M.; Constantinidis, T.C. Monitoring of Frequency and Antimicrobial Susceptibility of Pathogens on the Hands of Healthcare Workers in a Tertiary Hospital. Folia Med. 2016, 58, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Ye, J.; Zhou, W.; Jiang, Y.; Lin, H.; Zhang, X.; Qian, J.; Zhang, Y.; Ge, H.; Li, Y. Prevalence of methicillin-resistant Staphylococcus aureus colonisation among healthcare workers at a tertiary care hospital in southeastern China. J. Glob. Antimicrob. Resist. 2018, 15, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Kinnevey, P.M.; Kearney, A.; Shore, A.C.; Earls, M.R.; Brennan, G.; Poovelikunnel, T.T.; Humphreys, H.; Coleman, D.C. Meticillin-resistant Staphylococcus aureus transmission among healthcare workers, patients and the environment in a large acute hospital under non-outbreak conditions investigated using whole-genome sequencing. J. Hosp. Infect. 2021, 118, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Qadi, M.; Khayyat, R.; AlHajhamad, M.A.; Naji, Y.I.; Maraqa, B.; Abuzaitoun, K.; Mousa, A.; Daqqa, M. Microbes on the Mobile Phones of Healthcare Workers in Palestine: Identification, Characterization, and Comparison. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 8845879. [Google Scholar] [CrossRef]
- Kinnevey, P.M.; Kearney, A.; Shore, A.C.; Earls, M.R.; Brennan, G.I.; Poovelikunnel, T.T.; Humphreys, H.; Coleman, D.C. Meticillin-susceptible Staphylococcus aureus transmission among healthcare workers, patients and the environment in a large acute hospital under non-outbreak conditions investigated using whole-genome sequencing. J. Hosp. Infect. 2022, 127, 15–25. [Google Scholar] [CrossRef]
- Ymaña, B.; Luque, N.; Ruiz, J.; Pons, M.J. Worrying levels of antimicrobial resistance in Gram-negative bacteria isolated from cell phones and uniforms of Peruvian intensive care unit workers. Trans. R. Soc. Trop. Med. Hyg. 2022, 116, 676–678. [Google Scholar] [CrossRef]
- Weiss, A.; Kramer, A.; Taube, R.; Mattner, F.; Premke, K. Prevalence of methicillin sensitive and resistant Staphylococcus aureus carriage among German emergency medical providers. GMS Hyg. Infect. Control 2024, 19, Doc35. [Google Scholar] [CrossRef]
- Ballarini, S.; Rossi, G.A.; Principi, N.; Esposito, S. Dysbiosis in Pediatrics Is Associated with Respiratory Infections: Is There a Place for Bacterial-Derived Products? Microorganisms 2021, 9, 448. [Google Scholar] [CrossRef]
- Lunjani, N.; Satitsuksanoa, P.; Lukasik, Z.; Sokolowska, M.; Eiwegger, T.; O’Mahony, L. Recent developments and highlights in mechanisms of allergic diseases: Microbiome. Allergy 2018, 73, 2314–2327. [Google Scholar] [CrossRef]
- Kozik, A.J.; Huang, Y.J. The microbiome in asthma: Role in pathogenesis, phenotype, and response to treatment. Ann. Allergy Asthma Immunol. 2019, 122, 270–275. [Google Scholar] [CrossRef]
- Li, L.; Liu, R.; Peng, C.; Chen, X.; Li, J. Pharmacogenomics for the efficacy and side effects of antihistamines. Exp. Dermatol. 2022, 31, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, L.; Wang, Y.; Dai, T.; Qin, Z.; Zhou, F.; Zhang, L. Alterations in microbiota of patients with COVID-19: Potential mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Cui, J.; Liu, S.; Wei, S.; Wu, Q.; You, Y. Synbiotic regulates gut microbiota in patients with lupus nephritis: An analysis using metagenomic and metabolome sequencing. Front. Microbiol. 2024, 15, 1295378. [Google Scholar] [CrossRef] [PubMed]
- Snaidr, L.; Mühlhahn, P.; Beimfohr, C.; Kreuzer, C.; Richly, C.; Snaidr, J. Specific cultivation-independent enumeration of viable cells in probiotic products using a combination of fluorescence in situ hybridization and flow cytometry. Front. Microbiol. 2024, 15, 1410709. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef]
- Wu, C.T.; Lin, F.H.; Lee, Y.T.; Ku, M.S.; Lue, K.H. Effect of Lactobacillus rhamnosus GG immunopathologic changes in chronic mouse asthma model. J. Microbiol. Immunol. Infect. 2019, 52, 911–919. [Google Scholar] [CrossRef]
- Lin, C.H.; Tseng, C.Y.; Chao, M.W. Administration of Lactobacillus paracasei HB89 mitigates PM2.5-induced enhancement of inflammation and allergic airway response in murine asthma model. PLoS ONE 2020, 15, e0243062. [Google Scholar] [CrossRef]
- Yun, X.; Shang, Y.; Li, M. Effect of Lactobacillus salivarius on Th1/Th2 cytokines and the number of spleen CD4⁺ CD25⁺ Foxp3⁺ Treg in asthma Balb/c mouse. Int. J. Clin. Exp. Pathol. 2015, 8, 7661–7674. [Google Scholar]
- Du, X.; Wang, L.; Wu, S.; Yuan, L.; Tang, S.; Xiang, Y.; Qu, X.; Liu, H.; Qin, X.; Liu, C. Efficacy of probiotic supplementary therapy for asthma, allergic rhinitis, and wheeze: A meta-analysis of randomized controlled trials. Allergy Asthma Proc. 2019, 40, 250–260. [Google Scholar] [CrossRef]
- Huang, C.F.; Chie, W.C.; Wang, I.J. Efficacy of Lactobacillus Administration in School-Age Children with Asthma: A Randomized, Placebo-Controlled Trial. Nutrients 2018, 10, 1678. [Google Scholar] [CrossRef]
- Dargahi, N.; Johnson, J.; Donkor, O.; Vasiljevic, T.; Apostolopoulos, V. Immunomodulatory effects of probiotics: Can they be used to treat allergies and autoimmune diseases? Maturitas 2019, 119, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Lei, A.; Zhang, N.; Zhu, C. The Beneficial Role of Probiotic Lactobacillus in Respiratory Diseases. Front. Immunol. 2022, 13, 908010. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Cheng, S.H.; Chen, Y.H.; Wu, C.C.; Hsu, C.C.; Lin, C.T.; Tsai, Y.C. Supplementation with heat-inactivated Lacticaseibacillus paracasei K47 ameliorates allergic asthma in mice by regulating the Th1/Th2 balance. Benef. Microbes 2022, 13, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Mehrabi Nasab, E.; Arora, P.; Athari, S.S. Study effect of probiotics and prebiotics on treatment of OVA-LPS-induced of allergic asthma inflammation and pneumonia by regulating the TLR4/NF-kB signaling pathway. J. Transl. Med. 2022, 20, 130. [Google Scholar] [CrossRef]
- McLoughlin, R.; Berthon, B.S.; Rogers, G.B.; Baines, K.J.; Leong, L.E.X.; Gibson, P.G.; Williams, E.J.; Wood, L.G. Soluble fibre supplementation with and without a probiotic in adults with asthma: A 7-day randomised, double blind, three way cross-over trial. EBioMedicine 2019, 46, 473–485. [Google Scholar] [CrossRef]
- Rao, R.K.; Samak, G. Protection and Restitution of Gut Barrier by Probiotics: Nutritional and Clinical Implications. Curr. Nutr. Food Sci. 2013, 9, 99–107. [Google Scholar] [CrossRef]
- Zwicker, C.; Sarate, P.; Drinić, M.; Ambroz, K.; Korb, E.; Smole, U.; Köhler, C.; Wilson, M.S.; Kozakova, H.; Sebo, P.; et al. Prophylactic and therapeutic inhibition of allergic airway inflammation by probiotic Escherichia coli O83. J. Allergy Clin. Immunol. 2018, 142, 1987–1990.e7. [Google Scholar] [CrossRef]
- Schmid, A.M.; Razim, A.; Wysmołek, M.; Kerekes, D.; Haunstetter, M.; Kohl, P.; Brazhnikov, G.; Geissler, N.; Thaler, M.; Krčmářová, E.; et al. Extracellular vesicles of the probiotic bacteria E. coli O83 activate innate immunity and prevent allergy in mice. Cell Commun. Signal 2023, 21, 297. [Google Scholar] [CrossRef]
- Sim, S.; Park, H.J.; Kim, Y.K.; Choi, Y.; Park, H.S. Lactobacillus paracasei-derived extracellular vesicles alleviate neutrophilic asthma by inhibiting the JNK pathway in airway epithelium. Allergol. Int. 2024, 73, 302–312. [Google Scholar] [CrossRef]
- Kawase, M.; He, F.; Kubota, A.; Hata, J.Y.; Kobayakawa, S.; Hiramatsu, M. Inhibitory effect of Lactobacillus gasseri TMC0356 and Lactobacillus GG on enhanced vascular permeability of nasal mucosa in experimental allergic rhinitis of rats. Biosci. Biotechnol. Biochem. 2006, 70, 3025–3030. [Google Scholar] [CrossRef]
- Choi, S.P.; Oh, H.N.; Choi, C.Y.; Ahn, H.; Yun, H.S.; Chung, Y.M.; Kim, B.; Lee, S.J.; Chun, T. Oral administration of Lactobacillus plantarum CJLP133 and CJLP243 alleviates birch pollen-induced allergic rhinitis in mice. J. Appl. Microbiol. 2018, 124, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Dennis-Wall, J.C.; Culpepper, T.; Nieves, C., Jr.; Rowe, C.C.; Burns, A.M.; Rusch, C.T.; Federico, A.; Ukhanova, M.; Waugh, S.; Mai, V.; et al. Probiotics (Lactobacillus gasseri KS-13, Bifidobacterium bifidum G9-1, and Bifidobacterium longum MM-2) improve rhinoconjunctivitis-specific quality of life in individuals with seasonal allergies: A double-blind, placebo-controlled, randomized trial. Am. J. Clin. Nutr. 2017, 105, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Ivory, K.; Wilson, A.M.; Sankaran, P.; Westwood, M.; McCarville, J.; Brockwell, C.; Clark, A.; Dainty, J.R.; Zuidmeer-Jongejan, L.; Nicoletti, C. Oral delivery of a probiotic induced changes at the nasal mucosa of seasonal allergic rhinitis subjects after local allergen challenge: A randomised clinical trial. PLoS ONE 2013, 8, e78650. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Nakamura, F.; Kanzato, H.; Sawada, D.; Hirata, H.; Nishimura, A.; Kajimoto, O.; Fujiwara, S. Clinical effects of Lactobacillus acidophilus strain L-92 on perennial allergic rhinitis: A double-blind, placebo-controlled study. J. Dairy. Sci. 2005, 88, 527–533. [Google Scholar] [CrossRef]
- Li, L.; Wen, X.; Gong, Y.; Chen, Y.; Xu, J.; Sun, J.; Deng, H.; Guan, K. HMGN2 and Histone H1.2: Potential targets of a novel probiotic mixture for seasonal allergic rhinitis. Front. Microbiol. 2023, 14, 1202858. [Google Scholar] [CrossRef]
- Kang, M.G.; Han, S.W.; Kang, H.R.; Hong, S.J.; Kim, D.H.; Choi, J.H. Probiotic NVP-1703 Alleviates Allergic Rhinitis by Inducing IL-10 Expression: A Four-week Clinical Trial. Nutrients 2020, 12, 51427. [Google Scholar] [CrossRef]
- Lin, E.K.; Chang, W.W.; Jhong, J.H.; Tsai, W.H.; Chou, C.H.; Wang, I.J. Lacticaseibacillus paracasei GM-080 Ameliorates Allergic Airway Inflammation in Children with Allergic Rhinitis: From an Animal Model to a Double-Blind, Randomized, Placebo-Controlled Trial. Cells 2023, 12, 768. [Google Scholar] [CrossRef]
- Montamat, G.; Leonard, C.; Poli, A.; Klimek, L.; Ollert, M. CpG Adjuvant in Allergen-Specific Immunotherapy: Finding the Sweet Spot for the Induction of Immune Tolerance. Front. Immunol. 2021, 12, 590054. [Google Scholar] [CrossRef]
- Han, H.; Chen, G.; Zhang, B.; Zhang, X.; He, J.; Du, W.; Li, M.D. Probiotic Lactobacillus plantarum GUANKE effectively alleviates allergic rhinitis symptoms by modulating functions of various cytokines and chemokines. Front. Nutr. 2023, 10, 1291100. [Google Scholar] [CrossRef]
- Kim, W.G.; Kang, G.D.; Kim, H.I.; Han, M.J.; Kim, D.H. Bifidobacterium longum IM55 and Lactobacillus plantarum IM76 alleviate allergic rhinitis in mice by restoring Th2/Treg imbalance and gut microbiota disturbance. Benef. Microbes 2019, 10, 55–67. [Google Scholar] [CrossRef]
- Ragan, M.V.; Wala, S.J.; Goodman, S.D.; Bailey, M.T.; Besner, G.E. Next-Generation Probiotic Therapy to Protect the Intestines From Injury. Front. Cell Infect. Microbiol. 2022, 12, 863949. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Xiang, X.; Lin, X.; Wang, Q.; Qin, S.; Lu, X.; Xu, J.; Fang, Y.; Liu, Y.; Cui, J.; et al. Oropharyngeal Probiotic ENT-K12 as an Effective Dietary Intervention for Children With Recurrent Respiratory Tract Infections During Cold Season. Front. Nutr. 2022, 9, 900448. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lin, X.; Xiang, X.; Liu, W.; Fang, Y.; Chen, H.; Tang, F.; Guo, H.; Chen, D.; Hu, X.; et al. Oropharyngeal Probiotic ENT-K12 Prevents Respiratory Tract Infections Among Frontline Medical Staff Fighting Against COVID-19: A Pilot Study. Front. Bioeng. Biotechnol. 2021, 9, 646184. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.A.; Stieglitz, F.; Köksal, A.; Schubert, R.; Schulze, J.; Zielen, S. Efficacy of probiotic Lactobacillus GG on allergic sensitization and asthma in infants at risk. Clin. Exp. Allergy 2010, 40, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Cabana, M.D.; McKean, M.; Caughey, A.B.; Fong, L.; Lynch, S.; Wong, A.; Leong, R.; Boushey, H.A.; Hilton, J.F. Early Probiotic Supplementation for Eczema and Asthma Prevention: A Randomized Controlled Trial. Pediatrics 2017, 140, e20163000. [Google Scholar] [CrossRef]
- Zhao, Y.; Dong, B.R.; Hao, Q. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst. Rev. 2022, 8, Cd006895. [Google Scholar] [CrossRef]
- Wang, X.; Tan, X.; Zhou, J. Effectiveness and safety of probiotic therapy for pediatric allergic rhinitis management: A systematic review and meta-analysis. Int. J. Pediatr. Otorhinolaryngol. 2022, 162, 111300. [Google Scholar] [CrossRef]
- Liu, D.; Wang, X.; Zhang, H. Efficacy and safety of gastrointestinal microbiome supplementation for allergic rhinitis: A systematic review and meta-analysis with trial sequential analysis. Phytomedicine 2023, 118, 154948. [Google Scholar] [CrossRef]
- Zhu, J.; Pitre, T.; Ching, C.; Zeraatkar, D.; Gruchy, S. Safety and efficacy of probiotic supplements as adjunctive therapies in patients with COVID-19: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0278356. [Google Scholar] [CrossRef]
- Chen, X.; Yong, S.B.; Yii, C.Y.; Feng, B.; Hsieh, K.S.; Li, Q. Intestinal microbiota and probiotic intervention in children with bronchial asthma. Heliyon 2024, 10, e34916. [Google Scholar] [CrossRef]
- Kallio, S.; Jian, C.; Korpela, K.; Kukkonen, A.K.; Salonen, A.; Savilahti, E.; Kuitunen, M.; de Vos, W.M. Early-life gut microbiota associates with allergic rhinitis during 13-year follow-up in a Finnish probiotic intervention cohort. Microbiol. Spectr. 2024, 12, e0413523. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Peng, S.; Li, M.; Ao, X.; Liu, Z. The Efficacy and Safety of Probiotics for Allergic Rhinitis: A Systematic Review and Meta-Analysis. Front. Immunol. 2022, 13, 848279. [Google Scholar] [CrossRef] [PubMed]
- Zubeldia-Varela, E.; Barker-Tejeda, T.C.; Obeso, D.; Villaseñor, A.; Barber, D.; Pérez-Gordo, M. Microbiome and Allergy: New Insights and Perspectives. J. Investig. Allergol. Clin. Immunol. 2022, 32, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Boggio Marzet, C.; Burgos, F.; Del Compare, M.; Gerold, I.; Tabacco, O.; Vinderola, G. Approach to probiotics in pediatrics: The role of Lactobacillus rhamnosus GG. Arch. Argent. Pediatr. 2022, 120, e1–e7. [Google Scholar] [CrossRef]
- Saturio, S.; Nogacka, A.M.; Alvarado-Jasso, G.M.; Salazar, N.; de Los Reyes-Gavilán, C.G.; Gueimonde, M.; Arboleya, S. Role of Bifidobacteria on Infant Health. Microorganisms 2021, 9, 2415. [Google Scholar] [CrossRef]
- Legesse Bedada, T.; Feto, T.K.; Awoke, K.S.; Garedew, A.D.; Yifat, F.T.; Birri, D.J. Probiotics for cancer alternative prevention and treatment. Biomed. Pharmacother. 2020, 129, 110409. [Google Scholar] [CrossRef]
- Dou, W.; Abdalla, H.B.; Chen, X.; Sun, C.; Chen, X.; Tian, Q.; Wang, J.; Zhou, W.; Chi, W.; Zhou, X.; et al. ProbResist: A database for drug-resistant probiotic bacteria. Database 2022, 2022, baac064. [Google Scholar] [CrossRef]

| Year | Regions | Conclusions | References |
|---|---|---|---|
| 2007 | Brazil | Healthcare workers are more likely to experience asthma or allergic rhinitis symptoms from exposure to latex gloves than non-healthcare workers. | [31] |
| 2013 | European | Healthcare workers have 2 times the risk of developing asthma and 1.97 times the risk of developing non-infectious rhinitis than the general population. | [32] |
| 2013 | Sweden | The prevalence of asthma among healthcare workers was 4.3%, which was 1.3% higher than among non-healthcare workers and represented an increase in the number of healthcare workers with asthma symptoms compared to the previous year. | [33] |
| 2014 | Sweden | Nurses with allergies have an increased prevalence of lower respiratory symptoms, asthma, and allergic rhinitis. | [34] |
| 2016 | China | The prevalence of chronic sinusitis in Chinese adults is 8% and is strongly associated with occupational and environmental exposures. | [25] |
| 2016 | America | Occupational allergic rhinitis affects 10–60% of healthcare workers. | [26] |
| 2016 | America | Chronic respiratory diseases are the most common occupational diseases. | [6] |
| 2020 | America Canada | Occupational use of high levels of disinfectants increases asthma rates among nurses. | [35] |
| 2020 | Japan | The prevalence of occupational asthma among nurses in Japan is as high as 10.7%. | [36] |
| 2020 | Pakistan | The prevalence of allergic rhinitis among healthcare workers was as high as 19.2%, of which 35.9% of working hours were affected by allergic rhinitis. | [37] |
| 2022 | European | Occupational factors can interact with bacterial biorhythms and may contribute to the development of disease, leading to the concept of “WORKbiota”. | [38] |
| 2023 | England | In total, 12.2% of healthcare workers have chronic asthma. | [39] |
| Year | Regions | Conclusions | References |
|---|---|---|---|
| 2014 | Sao Tome and Principe | S. aureus colonization in the nasopharynx of healthcare workers was 20.5%, more frequent than in patients. | [59] |
| 2015 | America | Healthcare workers’ gloves and overalls are often contaminated with MRSA and vancomycin-resistant enterococci (VRE). | [60] |
| 2016 | Greece | S. aureus is six times more likely to be detected on the hands of medical and surgical staff than in neonatal units, and many of these strains are multi-drug resistant. | [61] |
| 2017 | Iran | MRSA detection rate in nasopharyngeal swabs was higher among healthcare workers without any history of risk factors for MRSA acquisition. | [54] |
| 2018 | China | Nasal flora of those who perform microbiology experiments have a high detection rate of S. aureus and high virulence, which may be transmitted to cohabiting family members or partners; family members of healthcare workers are at increased risk of detection. | [53] |
| 2018 | China | The detection rate of S. aureus in the pharyngeal swabs of healthcare workers was highest among surgeons (32.4%), followed by nurses (30.8%), and the highest percentage of MRSA was detected in the pharyngeal swabs of nurses. | [62] |
| 2020 | India | Oropharyngeal flora of healthcare workers can detect up to twice as many S. pneumoniae and H. influenzae compared to non-healthcare workers. | [51] |
| 2021 | Ireland | Colonization of 4.6% of the nasal and oral cavity by MRSA among healthcare workers. | [63] |
| 2021 | Palestine | Cell phones of healthcare workers carry more bacterial species and more drug-resistant bacteria than cell phones of non-healthcare workers. | [64] |
| 2022 | Ireland | Methicillin-susceptible Staphylococcus aureus (MSSA) carriage was higher among healthcare workers, with 21.5% of them showing both nasal and oral carriage. | [65] |
| 2022 | Peru | High levels of resistance to cephalosporins, quinolones, co-trimoxazole, and colistin in E. coli detected in healthcare workers’ work clothes and cell phones. | [66] |
| 2022 | Cyprus | Multiple multidrug-resistant bacteria, including VRE, MRSA, extended spectrum β-lactamase (ESBL)-producing bacteria, and carbapenem-resistant Acinetobacter baumannii, are present in healthcare workers’ work clothes. | [18] |
| 2024 | Germany | The prevalence of MRSA among healthcare workers is 1%, while the prevalence of MSSA is as high as 43.7%. | [67] |
| Year | Regions | Subject | Probiotics | Results of the Study | References |
|---|---|---|---|---|---|
| 2010 | Germany | Children aged 6–24 months with at least two wheezing episodes and a family history of first-degree atopic disease (n = 131), they were randomized into intervention and control groups | LGG | After 6 months of LGG treatment and 6 months of follow-up, sensitization of infants to airborne allergens was reduced and well tolerated without serious adverse events. | [104] |
| 2017 | America | Newborns with at least one parent with a history of asthma (n = 184), they were randomized into intervention and control groups | LGG | The cumulative prevalence of asthma at age 5 years was 9.7% in the intervention group using LGG in the first 6 months of life, compared with 17.4% in the control group. | [105] |
| 2018 | China | Adolescents aged 6–18 years with a history of intermittent to moderate persistent asthma for at least one year (n = 160), they were randomized into LP group (n = 40), LF group (n = 40), LP + LF (n = 40), placebo group (n = 40) | L. paracasei GMNL-133 (LP), L. fermentum GM-090 (LF) | Significant improvement in asthma severity and Childhood Asthma Control Test (C-ACT) scores in the LP, LF, and LP + LF groups compared to the placebo group. | [80] |
| 2022 | Meta-Analysis | Children, adults, or the elderly in the community, care facilities, schools, or hospitals (n = 6950) | L. plantarum HEAL9, L. paracasei | Probiotics may reduce the incidence rate of acute URTIs by about 18%; may reduce the mean duration of an episode of acute URTIs by about 1.22 days. Adverse events from probiotics were minor, and most commonly gastrointestinal symptoms, such as vomiting, flatulence, diarrhea, and bowel pain. | [106] |
| 2022 | Meta-Analysis | Pediatric patients with allergic rhinitis (n = 2644), including intervention groups (n = 1362) and control groups (n = 1282) | Bifidobacterium, Saccharomyces boulardii, Lactobacillus, S. thermophilus, Bacillus and Enterococcus faecium | Probiotics improved the remission rate of nasal symptoms, and reduced the serum levels of interleukin-4 (IL-4), IL-6, and IL-17, and significantly elevated the serum levels of interferon -γ and IL-10. Probiotics also reduced the duration of cetirizine use in pediatric AR. | [107] |
| 2023 | Meta-Analysis | Patients with allergic rhinitis (n = 3634) | Probiotics, prebiotics, and synbiotics | Gastrointestinal microbiome supplementation (GMS) yielded acceptable benefits for patients with AR compared with controls with sound certainties, after balancing the benefits and harms. | [108] |
| 2023 | America Italy Russia | COVID-19 infected individuals who are symptomatic and test positive by COVID-19 (n = 1027) | LGG, S. thermophilus DSM 32345, Bifidobacterium Lactis LA 304, L. salivarius LA 302, etc. | Probiotics supplements probably reduce cough or dyspnea compared to standard care; The probiotic supplement is associated with reduced adverse events. | [109] |
| 2024 | China | 66 children aged 3–6 years with bronchial asthma (asthma group, n = 66), 35 healthy children undergoing physical examination | Lactobacillus reuteri GL-104, L. paracasei, L. rhamnosus, Lactobacillus acidophilus GL-206, and Bifidobacterium longum | After probiotic intervention, the abundance of Bacteroides, Clostridium genera, Faecalibacterium, and Veillonella in the asthma group approached that of the healthy group. | [110] |
| 2024 | Finland | Pregnant women with babies at high risk of allergic diseases (n = 1223), randomized into a probiotic intervention group and a placebo group | LGG, L. rhamnosus LC705, Bifidobacterium breve Bb99 | Probiotic interventions during pregnancy and lactation and altered gut flora in infants and children have a greater impact on the development of allergic rhinitis. | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Chen, X.; Jiang, Z.; Zhu, J.; Wang, Q. Respiratory Flora Intervention: A New Strategy for the Prevention and Treatment of Occupationally Related Respiratory Allergy in Healthcare Workers. Microorganisms 2024, 12, 2653. https://doi.org/10.3390/microorganisms12122653
Gao L, Chen X, Jiang Z, Zhu J, Wang Q. Respiratory Flora Intervention: A New Strategy for the Prevention and Treatment of Occupationally Related Respiratory Allergy in Healthcare Workers. Microorganisms. 2024; 12(12):2653. https://doi.org/10.3390/microorganisms12122653
Chicago/Turabian StyleGao, Linglin, Xi Chen, Ziyi Jiang, Jie Zhu, and Qiang Wang. 2024. "Respiratory Flora Intervention: A New Strategy for the Prevention and Treatment of Occupationally Related Respiratory Allergy in Healthcare Workers" Microorganisms 12, no. 12: 2653. https://doi.org/10.3390/microorganisms12122653
APA StyleGao, L., Chen, X., Jiang, Z., Zhu, J., & Wang, Q. (2024). Respiratory Flora Intervention: A New Strategy for the Prevention and Treatment of Occupationally Related Respiratory Allergy in Healthcare Workers. Microorganisms, 12(12), 2653. https://doi.org/10.3390/microorganisms12122653






