The Nitrogen Removal Characteristics of a Novel Salt-Tolerant Bacterium, Enterobacter quasihormaechei DGFC5, Isolated from Municipal Sludge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sediment Samples and Medium Preparation
2.2. Microbial Enrichment, Isolation, and Screening
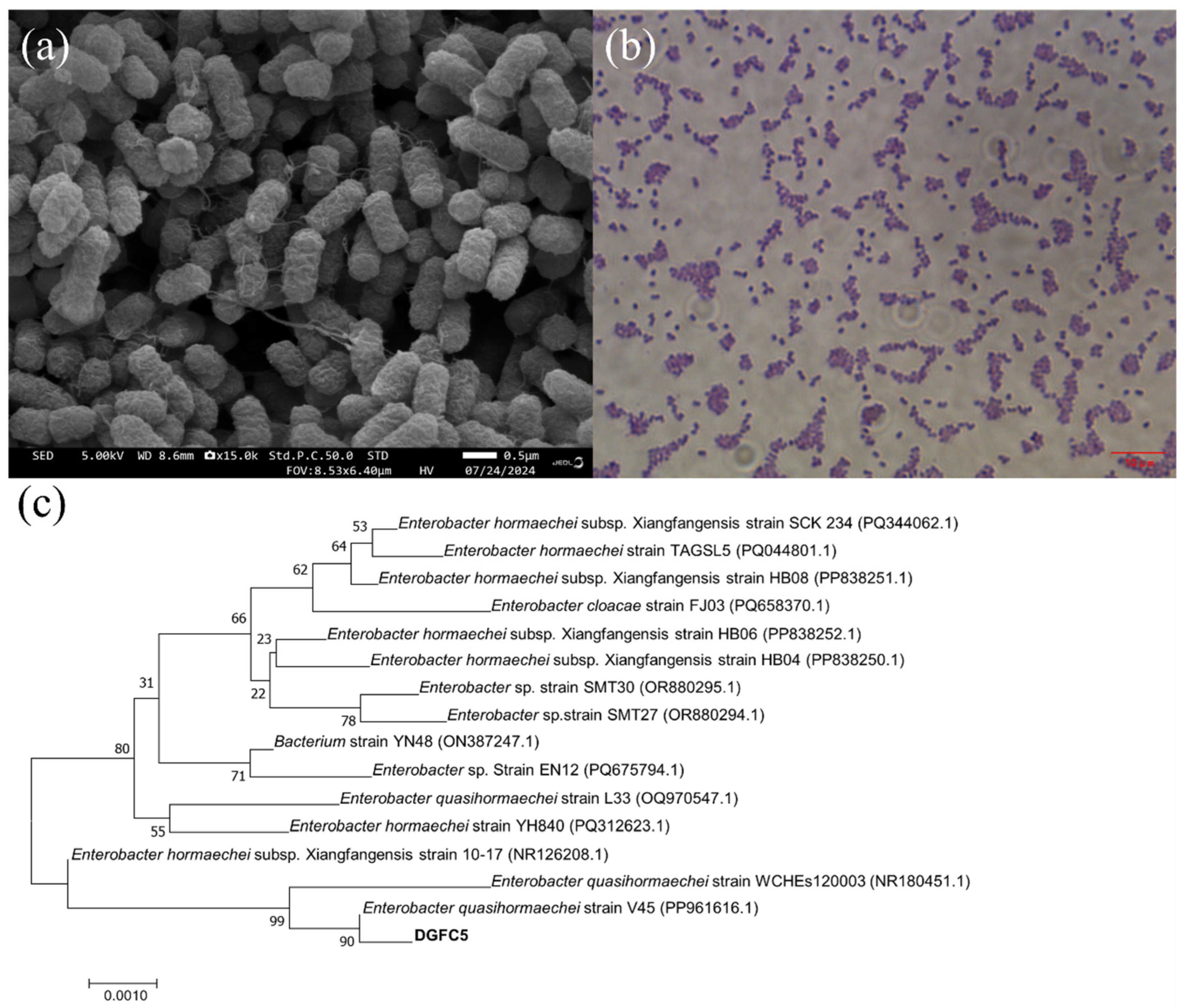
2.3. Strain Identification
2.4. Nitrogen Removal Capacity and Nitrogen Balance
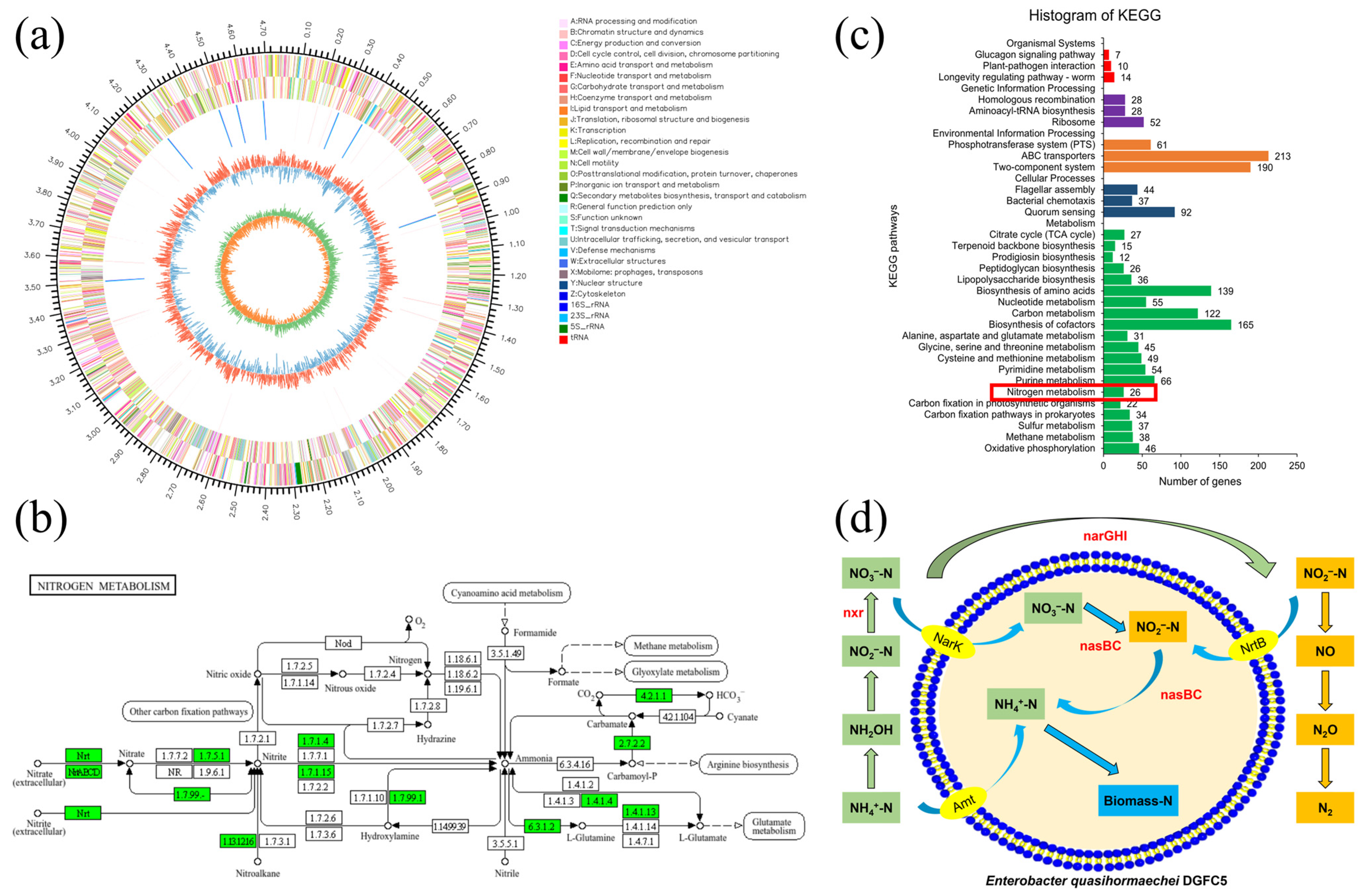
2.5. Genomic Analysis
2.6. Effects of Environmental Factors on Denitrification Efficiency of DGFC5
2.7. Analytical Methods
3. Results and Discussion
3.1. Isolation and Identification of DGFC5
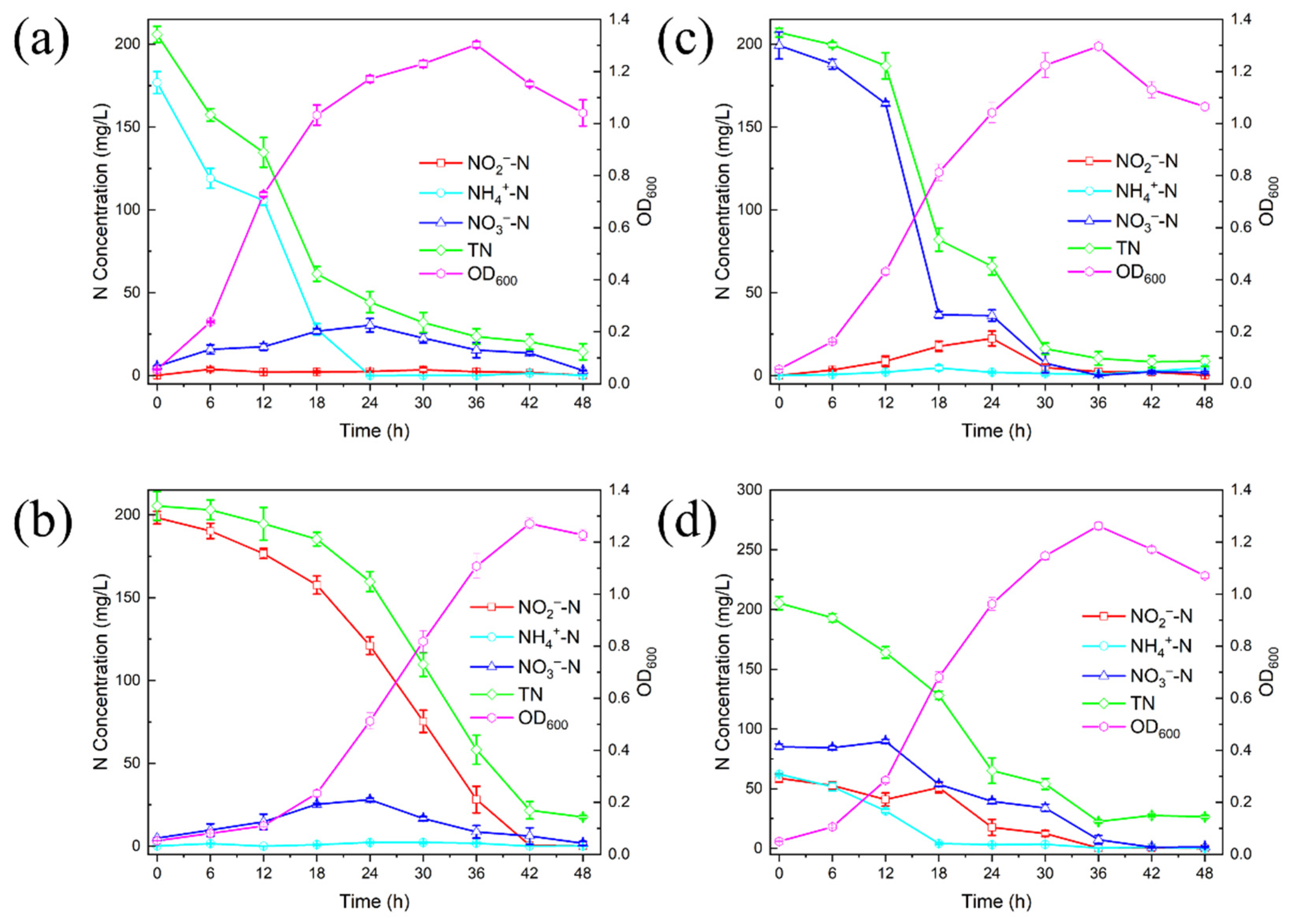
3.2. Nitrogen Removal Characteristics of DGFC5
3.2.1. Removal of NH4+-N by DGFC5
3.2.2. Removal of NO2−-N by DGFC5
3.2.3. Removal of NO3−-N by DGFC5
3.2.4. Removal of Mixed Nitrogen Sources by DGFC5
3.3. Analysis of Nitrogen Balance for DGFC5
3.4. Analysis of Nitrogen Metabolic Pathway for DGFC5
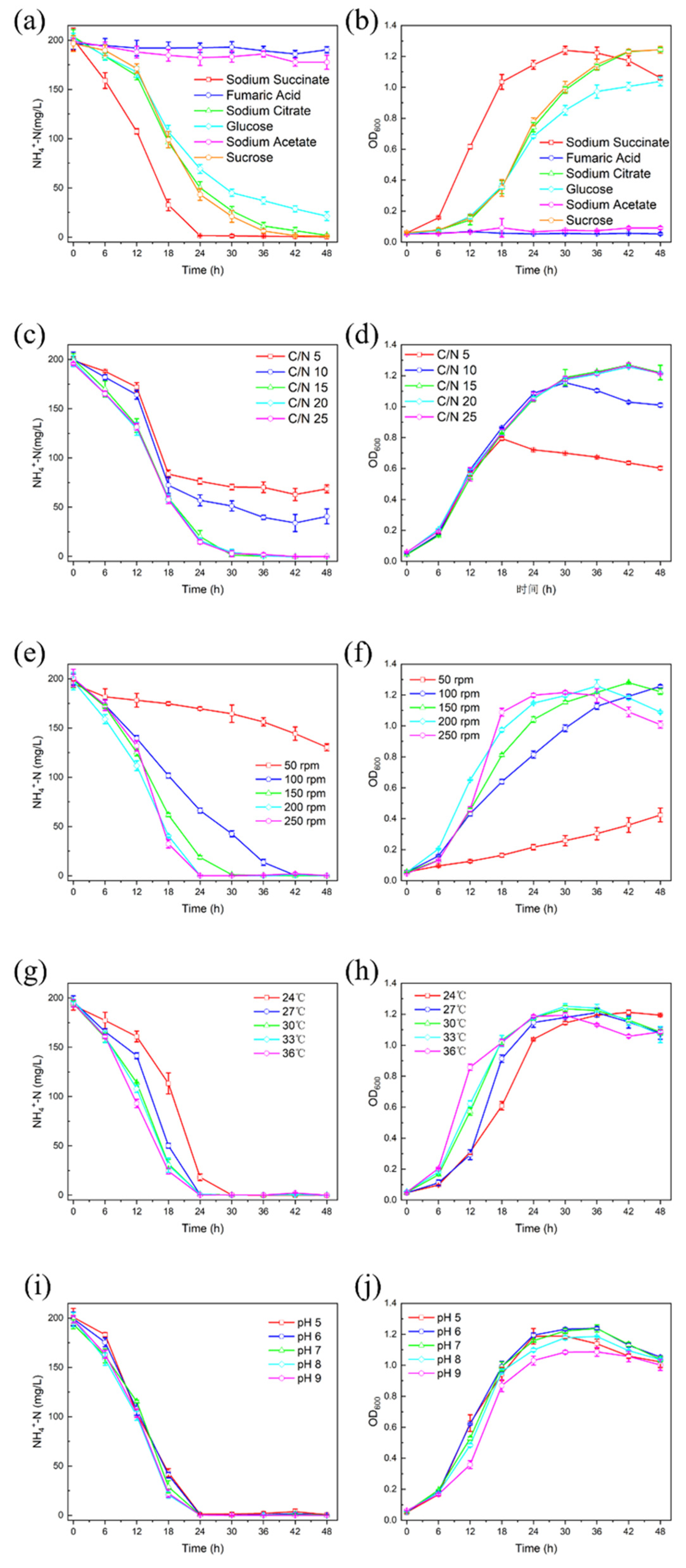
3.5. Influences of Environmental Factors on Heterotrophic Nitrification by DGFC5
3.5.1. Carbon Sources
3.5.2. C/N Ratio
3.5.3. Dissolved Oxygen
3.5.4. Temperature
3.5.5. Initial pH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, S.N.; Li, S.L.; Huang, T.L.; Yang, S.Y.; Liu, K.W.; Ma, B.; Shi, Y.J.; Miao, Y.T. Nitrate reduction by Paracoccus thiophilus strain LSL 251 under aerobic condition: Performance and intracellular central carbon flux pathways. Bioresour. Technol. 2020, 308, 123301. [Google Scholar] [CrossRef]
- Dippong, T.; Hoaghia, M.A.; Senila, M. Appraisal of heavy metal pollution in alluvial aquifers. Study case on the protected area of Ronișoara Forest, Romania. Ecol. Indic. 2022, 143, 109347. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Chen, C.; Tang, Y.; Liu, B. Multi-omics analysis to reveal key pathways involved in low C/N ratio stress response in Pseudomonas sp. LW60 with superior nitrogen removal efficiency. Bioresour. Technol. 2023, 389, 129812. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Yao, T.; Liu, X.; Zhang, A.; Zhang, J.; Pang, L. Efficient heterotrophic nitrification–aerobic denitrification by a novel bacterium Ralstonia pickettii J4: Isolation, identification, and application. Biochem. Eng. J. 2024, 210, 109417. [Google Scholar] [CrossRef]
- Lee, Y.J.; Park, M.K.; Park, G.S.; Lee, S.J.; Lee, S.J.; Kim, B.S.; Shin, J.H.; Lee, D.W. Complete genome sequence of the thermophilic bacterium Geobacillus subterraneus KCTC 3922T as a potential denitrifier. J. Biotechnol. 2017, 251, 141–144. [Google Scholar] [CrossRef]
- Liao, M.; Luo, Y.; Xu, N.; Xie, X.; Gan, X.; Cao, D. Nitrogen removal and metabolic pathway of Enterobacter cloacae DK-6. Environ. Technol. Innov. 2022, 28, 102630. [Google Scholar] [CrossRef]
- Ren, J.; Tang, J.; Min, H.; Tang, D.; Jiang, R.; Liu, Y.; Huang, X. Nitrogen removal characteristics of novel bacterium Klebsiella sp. TSH15 by assimilatory/dissimilatory nitrate reduction and ammonia assimilation. Bioresour. Technol. 2024, 394, 130184. [Google Scholar] [CrossRef]
- Sarioglu, O.F.; Suluyayla, R.; Tekinay, T. Heterotrophic ammonium removal by a novel hatchery isolate Acinetobacter calcoaceticus STB1. Int. Biodeterior. Biodegrad. 2012, 71, 67–71. [Google Scholar] [CrossRef]
- Yang, Y.; Gui, X.; Chen, L.; Li, H.; Li, Z.; Liu, T. Acid-tolerant Pseudomonas citronellolis YN-21 exhibits a high heterotrophic nitrification capacity independent of the amo and hao genes. Ecotox. Environ. Safe 2024, 279, 116385. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Cheng, D.Y.; Tan, P.; An, Q.; Guo, J.S. Characterization of an aerobic denitrifier Pseudomonas stutzeri strain XL-2 to achieve efficient nitrate removal. Bioresour. Technol. 2018, 250, 564–573. [Google Scholar] [CrossRef]
- Fang, J.; Liao, S.; Gu, T.; Lu, W.; Lu, X.; Yu, M.; Li, B.; Ye, J. Efficient nitrogen removal by heterotrophic nitrification-aerobic denitrification yeast Candida boidinii L21: Performance, pathway and application. Bioresour. Technol. 2024, 414, 131621. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Li, X.; Fan, W.; Wang, J. Heterotrophic nitrification and aerobic denitrification by a novel Acinetobacter sp. ND7 isolated from municipal activated sludge. Bioresour. Technol. 2020, 301, 122749. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.X.; Wang, W.C.; Feng, Y.; Zhu, X.H.; Zhou, H.Z.; Tan, Z.L.; Li, X.D. Impact resistance of different factors on ammonia removal by heterotrophic nitrification–aerobic denitrification bacterium Aeromonas sp. HN-02. Bioresour. Technol. 2014, 167, 456–461. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Q.; Wang, Y.; Zhan, W.; Wu, Z.; Zhou, H.; Cheng, H.; Chen, Z. Effects of Cd(II) on nitrogen removal by a heterotrophic nitrification aerobic denitrification bacterium Pseudomonas sp. XF-4. Ecotox Environ. Safe 2024, 280, 116588. [Google Scholar] [CrossRef]
- Yang, J.R.; Wang, Y.; Chen, H.; Lyu, Y.K. Ammonium removal characteristics of an acid-resistant bacterium Acinetobacter sp. JR1 from pharmaceutical wastewater capable of heterotrophic nitrification-aerobic denitrification. Bioresour. Technol. 2019, 274, 56–64. [Google Scholar] [CrossRef]
- Vijay, A.; Arora, S.; Gupta, S.; Chhabra, M. Halophilic starch degrading bacteria isolated from Sambhar Lake, India, as potential anode catalyst in microbial fuel cell: A promising process for saline water treatment. Bioresour. Technol. 2018, 256, 391–398. [Google Scholar] [CrossRef]
- Chen, P.P.; Zhang, F.P.; Zhang, L.J.; Liu, H.; Zhang, Q.; Xing, Z.L.; Zhao, T.T. Characterization of a novel salt-tolerant strain Sphingopyxis sp. CY-10 capable of heterotrophic nitrification and aerobic denitrification. Bioresour. Technol. 2022, 358, 127353. [Google Scholar] [CrossRef]
- Maharaja, P.; Sivashankaran, S.; Nagabalaji, V.; Srinivasan, S.V.; Swarnalatha, S.; Karthikeyan, S.; Sekaran, G. Simultaneous brine recovery and water depollution in highly saline leather industry wastewater using a united halophiles supported nano porous carbon catalyst. J. Clean. Prod. 2021, 326, 129419. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Ren, J.; Bai, X.; Liu, Y.; Huang, X. Simultaneous nitrification and aerobic denitrification by a novel isolated Ochrobactrum anthropi HND19. Bioresour. Technol. 2021, 340, 125582. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Luo, L.; Huang, X.; Jiang, D.; Wu, X.; Li, Z. Characterization and metabolic pathway of Pseudomonas fluorescens 2P24 for highly efficient ammonium and nitrate removal. Bioresour. Technol. 2023, 382, 129189. [Google Scholar] [CrossRef]
- Ke, X.; Liu, C.; Tang, S.Q.; Guo, T.T.; Pan, L.; Xue, Y.P.; Zheng, Y.G. Characterization of Acinetobacter indicus ZJB20129 for heterotrophic nitrification and aerobic denitrification isolated from an urban sewage treatment plant. Bioresour. Technol. 2022, 347, 126423. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Liao, M.; Liang, Y.; Guo, J.; Zhang, Y.; Xie, X.; Fan, Q.; Zhu, Y. Biological nitrogen removal capability and pathways analysis of a novel low C/N ratio heterotrophic nitrifying and aerobic denitrifying bacterium (Bacillus thuringiensis strain WXN-23). Environ. Res. 2021, 195, 110797. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, Y.Q.; Liu, H.J.; Xi, C.W.; Song, L.Y. A novel heterotrophic nitrifying and aerobic denitrifying bacterium, Zobellella taiwanensis DN-7, can remove high-strength ammonium. Appl. Microbiol. Biotechnol. 2016, 100, 4219–4229. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cui, Y.; Li, D.; Yin, M.; Pei, Y.; Wang, X.; Li, J.; Zhu, Y. Fulvic acid mediated highly efficient heterotrophic nitrification-aerobic denitrification by Paracoccus denitrificans XW11 with reduced C/N ratio. Water Res. 2024, 267, 122557. [Google Scholar] [CrossRef] [PubMed]
- Barathi, S.; Meng, Y.; Yu, Z.; Ni, S.Q.; Meng, F. Roles of nitrite in mediating the composition and metacommunity of multispecies biofilms. J. Water Process. Eng. 2021, 40, 101764. [Google Scholar] [CrossRef]
- Silva, T.F.C.V.; Fonseca, A.; Saraiva, I.; Vilar, V.J.P.; Boaventura, R.A.R. Biodegradability enhancement of a leachate after biological lagooning using a solar driven photo-Fenton reaction, and further combination with an activated sludge biological process, at pre-industrial scale. Water Res. 2013, 47, 3543–3557. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhang, Y.; Zhou, J.; Chen, M.; Wang, X. Biological removal of nitrate and ammonium under aerobic atmosphere by Paracoccus versutus LYM. Bioresour. Technol. 2013, 148, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; He, Y.L.; Hughes, J.; Zhang, X.F. Heterotrophic nitrogen removal by a newly isolated Acinetobacter calcoaceticus HNR. Bioresour. Technol. 2010, 101, 5194–5200. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Fu, L.; Gao, Y.; Zhao, H.; Zhou, W. Aerobic-heterotrophic nitrogen removal through nitrate reduction and ammonium assimilation by marine bacterium Vibrio sp. Y1-5. Bioresour. Technol. 2017, 230, 103–111. [Google Scholar] [CrossRef]
- Wu, T.; Li, J.; Cao, R.; Chen, X.; Wang, B.; Huang, T.; Wen, G. Nitrate removal by a novel aerobic denitrifying Pelomonas puraquae WJ1 in oligotrophic condition: Performance and carbon source metabolism. Sci. Total Environ. 2024, 954, 176614. [Google Scholar] [CrossRef]
- Sun, Y.; Feng, L.; Li, A.; Zhang, X.; Yang, J.; Ma, F. Ammonium assimilation: An important accessory during aerobic denitrification of Pseudomonas stutzeri T13. Bioresour. Technol. 2017, 234, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Hu, Y.Y.; Qiu, G.L.; Liang, D.H.; Li, Y.Y.; Cheng, J.H.; Chen, Y.C.; Wang, G.B.; Xie, J.Y.; Zhu, X.Q. Genomics and metabolic characteristics of simultaneous heterotrophic nitrification aerobic denitrification and aerobic phosphorus removal by Acinetobacter indicus CZH-5. Bioresour. Technol. 2024, 395, 130322. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ding, X.; Lin, Y.; Lu, X.; Lv, H.; Zhao, M.; Yu, R. Nitrogen removal by a novel heterotrophic nitrification and aerobic denitrification bacterium Acinetobacter calcoaceticus TY1 under low temperatures. Bioresour. Technol. 2022, 353, 127148. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zhang, D.; Cao, H.; Zhang, Y.; Zhao, D.; Zeng, W.; Lei, H.; Bai, Y. Identification of aerobic-denitrifying Psychrobacter cryohalolentis strain F5-6 and its nitrate removal at low temperature. Int. Biodeterior. Biodegrad. 2022, 172, 105426. [Google Scholar] [CrossRef]
- Huang, M.Q.; Cui, Y.W.; Yang, H.J.; Xu, M.J.; Cui, Y.; Chen, Z. A halophilic aerobic-heterotrophic strain Halomonas venusta SND-01: Nitrogen removal by ammonium assimilation and heterotrophic nitrification-aerobic denitrification. Bioresour. Technol. 2023, 374, 128758. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Tan, X.; Sheng, Y.; Li, Y.; Zhang, Q.; Xu, J.; Shi, Z. Genomics and nitrogen metabolic characteristics of a novel heterotrophic nitrifying-aerobic denitrifying bacterium Acinetobacter oleivorans AHP123. Bioresour. Technol. 2023, 375, 128822. [Google Scholar] [CrossRef]
- Forchhammer, K.; Lüddecke, J. Sensory properties of the P signalling protein family. FEBS J. 2016, 283, 425–437. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.X.; Lin, W.; Wang, S.S.; Zhang, W.X.; Jiang, Y.M.; Zhang, Y.; Zhang, H.; Han, Y.H. Full evaluation of assimilatory and dissimilatory nitrate reduction in a new denitrifying bacterium Leclercia adecarboxylata strain AS3-1: Characterization and functional gene analysis. Environ. Technol. Innov. 2021, 23, 101731. [Google Scholar] [CrossRef]
- Sparacino-Watkins, C.; Stolz, J.F.; Basu, P. Nitrate and periplasmic nitrate reductases. Chem. Soc. Rev. 2014, 43, 676–706. [Google Scholar] [CrossRef]
- Huang, X.; Weisener, C.G.; Ni, J.; He, B.; Xie, D.; Li, Z. Nitrate assimilation, dissimilatory nitrate reduction to ammonium, and denitrification coexist in Pseudomonas putida Y-9 under aerobic conditions. Bioresour. Technol. 2020, 312, 123597. [Google Scholar] [CrossRef]
- Pandey, C.B.; Kumar, U.; Kaviraj, M.; Minick, K.J.; Mishra, A.K.; Singh, J.S. DNRA: A short-circuit in biological N-cycling to conserve nitrogen in terrestrial ecosystems. Sci. Total Environ. 2020, 738, 139710. [Google Scholar] [CrossRef]
- Sanford, R.A.; Wagner, D.D.; Wu, Q.; Chee-Sanford, J.C.; Thomas, S.H.; Cruz-Garcia, C.; Rodriguez, G.; Massol-Deya, A.; Krishnani, K.K.; Ritalahti, K.M.; et al. Unexpected nondenitrifier nitrous oxide reductase gene diversity and abundance in soils. Proc. Natl. Acad. Sci. USA 2012, 109, 19709–19714. [Google Scholar] [CrossRef]
- Wang, H.; Chen, N.; Feng, C.; Deng, Y. Insights into heterotrophic denitrification diversity in wastewater treatment systems: Progress and future prospects based on different carbon sources. Sci. Total Environ. 2021, 780, 146521. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Wang, K.; Liu, X.; Wong, M.H.; Hu, Z.; Chen, H.; Yang, X.; Li, S. A study on the nitrogen removal efficacy of bacterium Acinetobacter tandoii MZ-5 from a contaminated river of Shenzhen, Guangdong Province, China. Bioresour. Technol. 2020, 315, 123888. [Google Scholar] [CrossRef]
- Guo, L.; Chen, Q.; Fang, F.; Hu, Z.; Wu, J.; Miao, A.; Xiao, L.; Chen, X.; Yang, L. Application potential of a newly isolated indigenous aerobic denitrifier for nitrate and ammonium removal of eutrophic lake water. Bioresour. Technol. 2013, 142, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Lv, Y.; Liu, Y.; Ren, R. Removal of nitrogen by heterotrophic nitrification-aerobic denitrification of a novel metal resistant bacterium Cupriavidus sp. S1. Bioresour. Technol. 2016, 220, 142–150. [Google Scholar] [CrossRef]
- Zhao, K.; Tian, X.; Li, H.; Dong, S.; Jiang, W. Characterization of a novel marine origin aerobic nitrifying–denitrifying bacterium isolated from shrimp culture ponds. Aquac. Res. 2019, 50, 1770–1781. [Google Scholar] [CrossRef]
- Zheng, H.Y.; Liu, Y.; Gao, X.Y.; Ai, G.M.; Miao, L.L.; Liu, Z.P. Characterization of a marine origin aerobic nitrifying–denitrifying bacterium. J. Biosci. Bioeng. 2012, 114, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Lee, D.I.; Keller, J. Effect of temperature and free ammonia on nitrification and nitrite accumulation in landfill leachate and analysis of its nitrifying bacterial community by FISH. Bioresour. Technol. 2006, 97, 459–468. [Google Scholar] [CrossRef]
- Deng, M.; Zhao, X.; Senbati, Y.; Song, K.; He, X. Nitrogen removal by heterotrophic nitrifying and aerobic denitrifying bacterium Pseudomonas sp. DM02: Removal performance, mechanism and immobilized application for real aquaculture wastewater treatment. Bioresour. Technol. 2021, 322, 124555. [Google Scholar] [CrossRef]
- Ren, J.; Wei, C.; Ma, H.; Dai, M.; Fan, J.; Liu, Y.; Wu, Y.; Han, R. The nitrogen-removal efficiency of a novel high-efficiency salt-tolerant aerobic denitrifier, Halomonas alkaliphile HRL-9, isolated from a seawater biofilter. Int. J. Environ. Res. Public Health 2019, 16, 4451. [Google Scholar] [CrossRef]
- Liu, L.; Dai, Y. Strong adsorption of metolachlor by biochar prepared from walnut shells in water. Environ. Sci. Pollut. Res. 2021, 28, 48379–48391. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.L.; Liu, Y.; Ai, G.M.; Miao, L.L.; Zheng, H.Y.; Liu, Z.P. The characteristics of a novel heterotrophic nitrification–aerobic denitrification bacterium, Bacillus methylotrophicus strain L7. Bioresour. Technol. 2012, 108, 35–44. [Google Scholar] [CrossRef] [PubMed]

| Substrate | Initial TN (mg/L) | Nitrogen Concentrations After 48 h (mg/L) | Intracellular Nitrogen (mg/L) | Gaseous Nitrogen (mg/L) | |||
|---|---|---|---|---|---|---|---|
| NH4+-N | NO2−-N | NO3−-N | Organonitrogen | ||||
| Ammonia nitrogen | 205.84 ± 4.94 | 0.16 ± 0.03 | 0.16 ± 0.02 | 3.05 ± 0.17 | 10.96 ± 4.85 | 121.07 ± 3.86 | 70.44 ± 6.43 |
| Nitrite nitrogen | 205.37 ± 8.62 | 0.13 ± 0.10 | 0.08 ± 0.01 | 1.70 ± 1.16 | 15.71 ± 1.54 | 52.82 ± 5.50 | 134.93 ± 7.66 |
| Nitrate nitrogen | 207.01 ± 5.65 | 4.67 ± 0.81 | - | 1.83 ± 0.67 | 8.17 ± 2.77 | 61.65 ± 3.13 | 130.69 ± 4.61 |
| HNAD Strain Name | Optimum Carbon Source | Optimum Shaking Speed (rpm) | Optimum C/N | Optimum Temperature (°C) | Optimum pH | Salinity (%) | Initial NH4+-N (mg/L) | Reaction Time (h) | NH4+-N Removal Efficiency (%) | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Cupriavidus sp. S1 | Sodium pyruvate | - | 14.0 | - | - | 0 | 100.00 | 36 | 99.68 | [47] |
| Pseudomonas citronellolis YN-21 | Succinate | - | 10.0 | 30 | 5.0 | 0 | 100.00 | 18 | 100.00 | [9] |
| Halomonas venusta SND-01 | Sodium citrate | - | 10.0 | - | 8.5 | 3 | 100.00 | 24 | 98.00 | [36] |
| Acinetobacter sp. JR1 | Fumaric acid | 120 rpm | 16.0 | 30 | 4.5 | 0 | 100.00 | 24 | 98.50 | [15] |
| Acinetobacter calcoaceticus TY1 | Sodium citrate | 90 rpm | 10.0 | - | 7.0 | 0 | 106.00 | 60 | 97.70 | [34] |
| Acinetobacter sp. ND7 | Sodium citrate | 150 rpm | 8.0 | 35 | - | 0 | 50.00 | 24 | 93.23 | [12] |
| Aeromonas sp. HN-02 | - | - | - | 30 | 10.0 | 0 | 35.00 | 10 | 100.00 | [13] |
| Pseudomonas sp. DM02 | - | - | 8.0 | - | 7.0 | 0 | 10.00 | 24 | 100.00 | [51] |
| Enterobacter quasihormaechei DGFC5 | Sodium succinate | 200 rpm | 15.0 | 30 | 7.0 | 5 | 198.33 | 48 | 100.00 | In this study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Peng, H.; Liu, W. The Nitrogen Removal Characteristics of a Novel Salt-Tolerant Bacterium, Enterobacter quasihormaechei DGFC5, Isolated from Municipal Sludge. Microorganisms 2024, 12, 2652. https://doi.org/10.3390/microorganisms12122652
Wang B, Peng H, Liu W. The Nitrogen Removal Characteristics of a Novel Salt-Tolerant Bacterium, Enterobacter quasihormaechei DGFC5, Isolated from Municipal Sludge. Microorganisms. 2024; 12(12):2652. https://doi.org/10.3390/microorganisms12122652
Chicago/Turabian StyleWang, Bingguo, Huanlong Peng, and Wei Liu. 2024. "The Nitrogen Removal Characteristics of a Novel Salt-Tolerant Bacterium, Enterobacter quasihormaechei DGFC5, Isolated from Municipal Sludge" Microorganisms 12, no. 12: 2652. https://doi.org/10.3390/microorganisms12122652
APA StyleWang, B., Peng, H., & Liu, W. (2024). The Nitrogen Removal Characteristics of a Novel Salt-Tolerant Bacterium, Enterobacter quasihormaechei DGFC5, Isolated from Municipal Sludge. Microorganisms, 12(12), 2652. https://doi.org/10.3390/microorganisms12122652





