Effects of the Symbiotic Chlorella variabilis on the Host Ciliate Paramecium bursaria Phenotypes
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Search Strategy
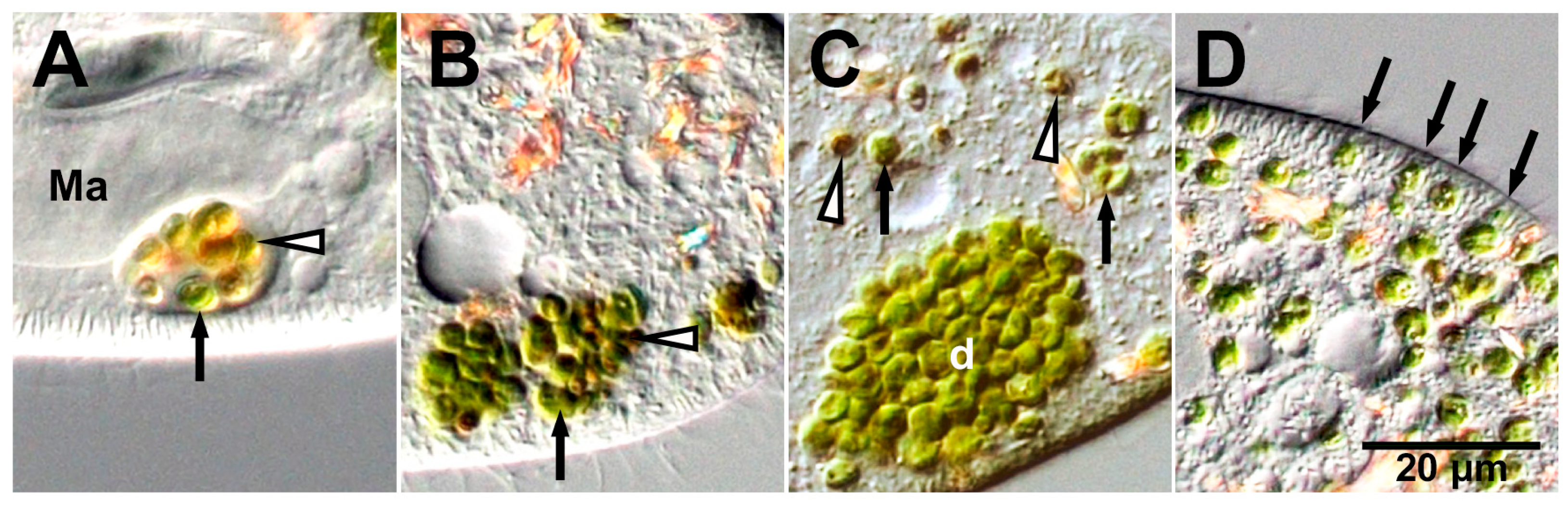
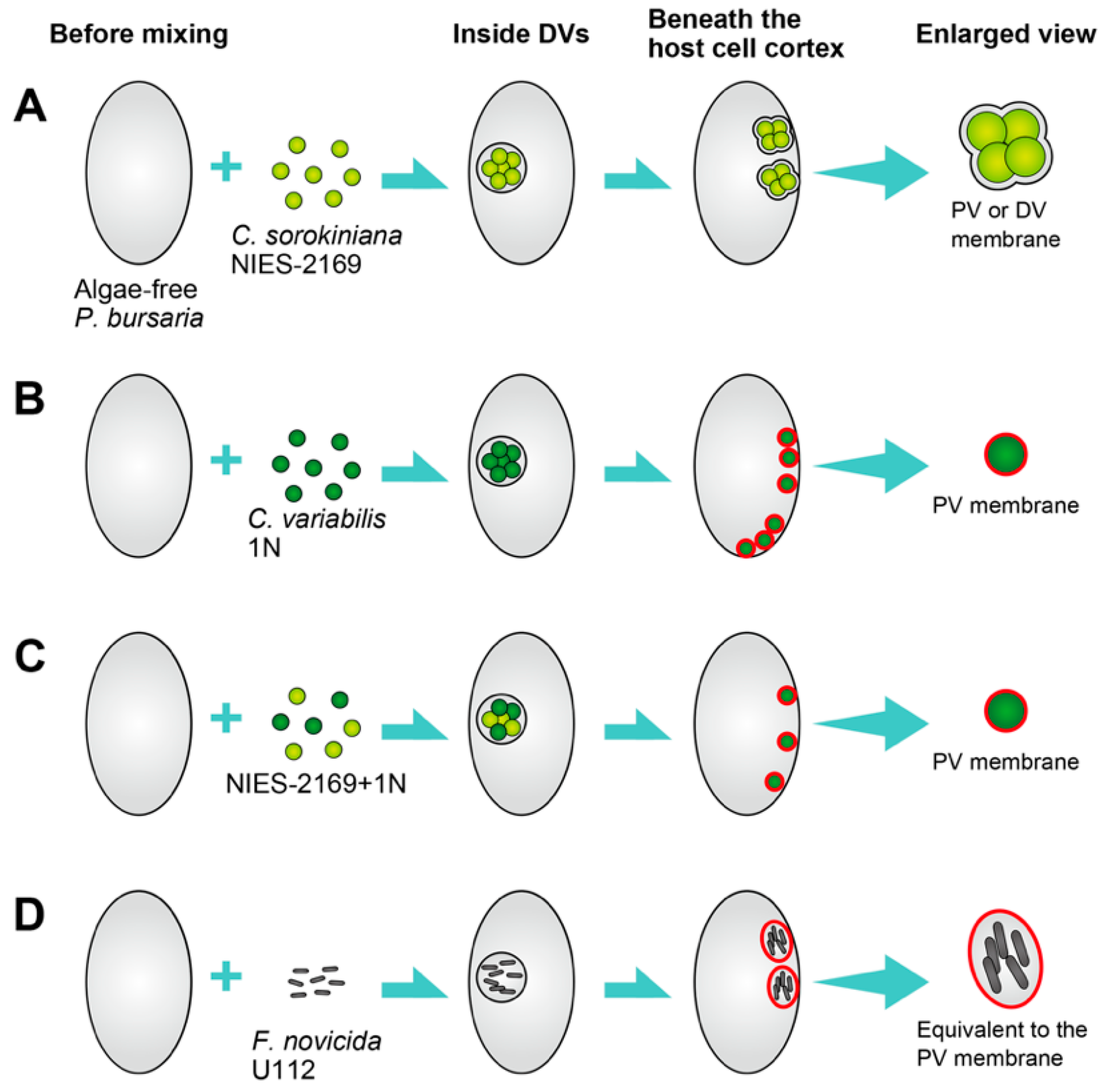
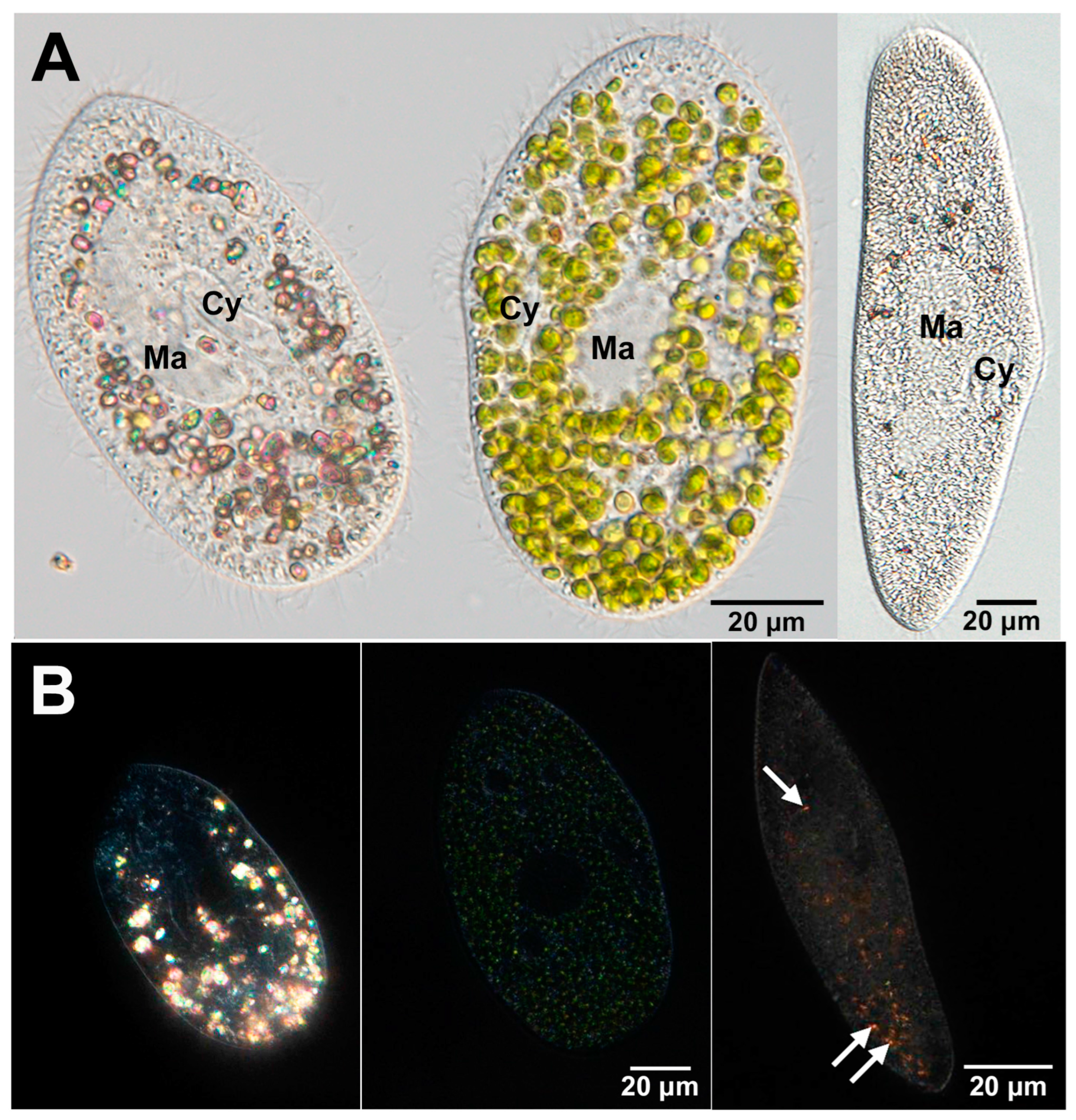
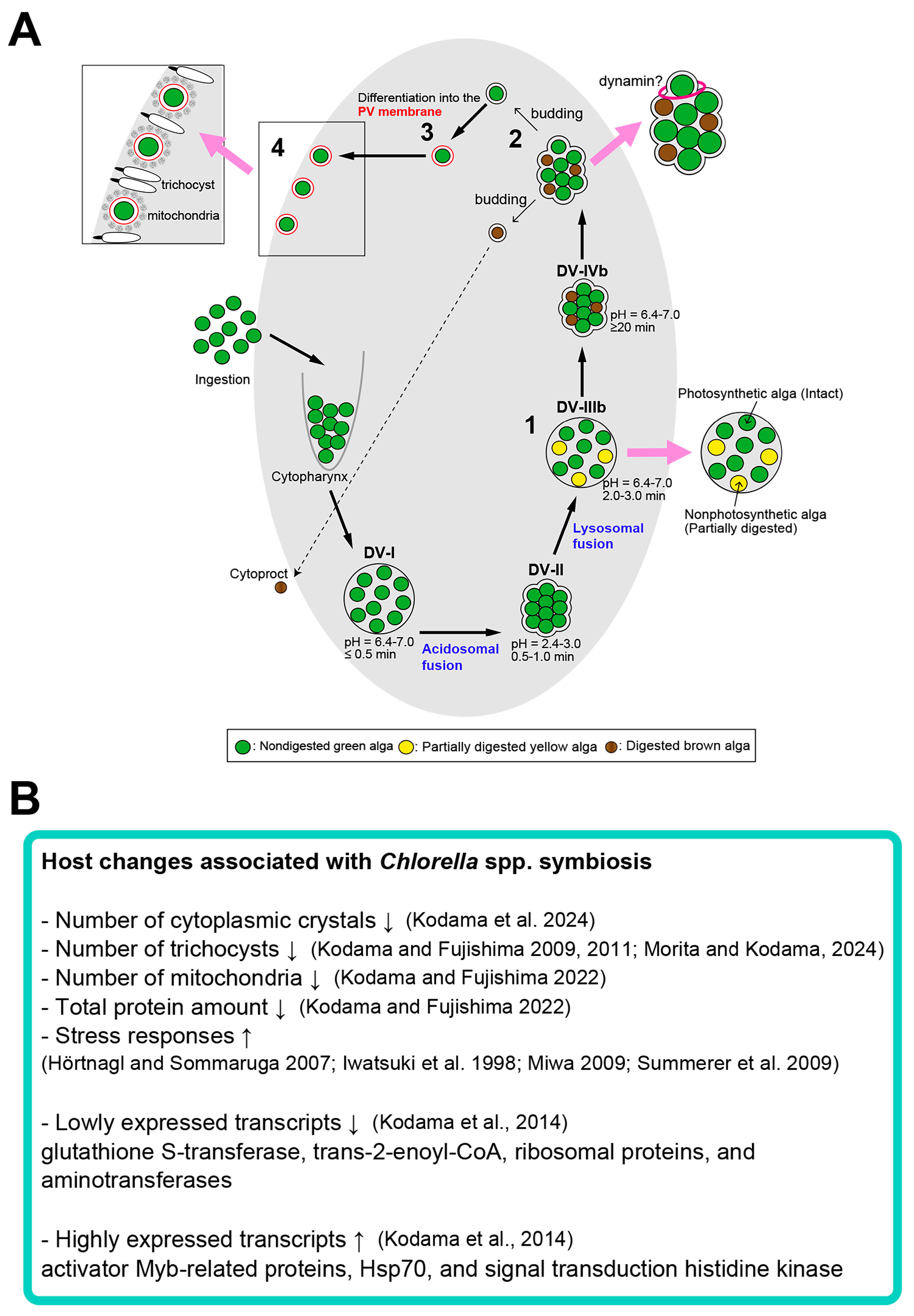
3. Re-Endosymbiosis Process of Symbiotic Chlorella variabilis to the Algae-Free P. bursaria
4. Microorganisms Capable of Symbiosis with Algae-Free P. bursaria Cells
5. Effects of the Symbiotic Chlorella variabilis on Cytoplasmic Crystals and Organelles of the Host Paramecium bursaria
5.1. Algal Effects on Host Cytoplasmic Crystals
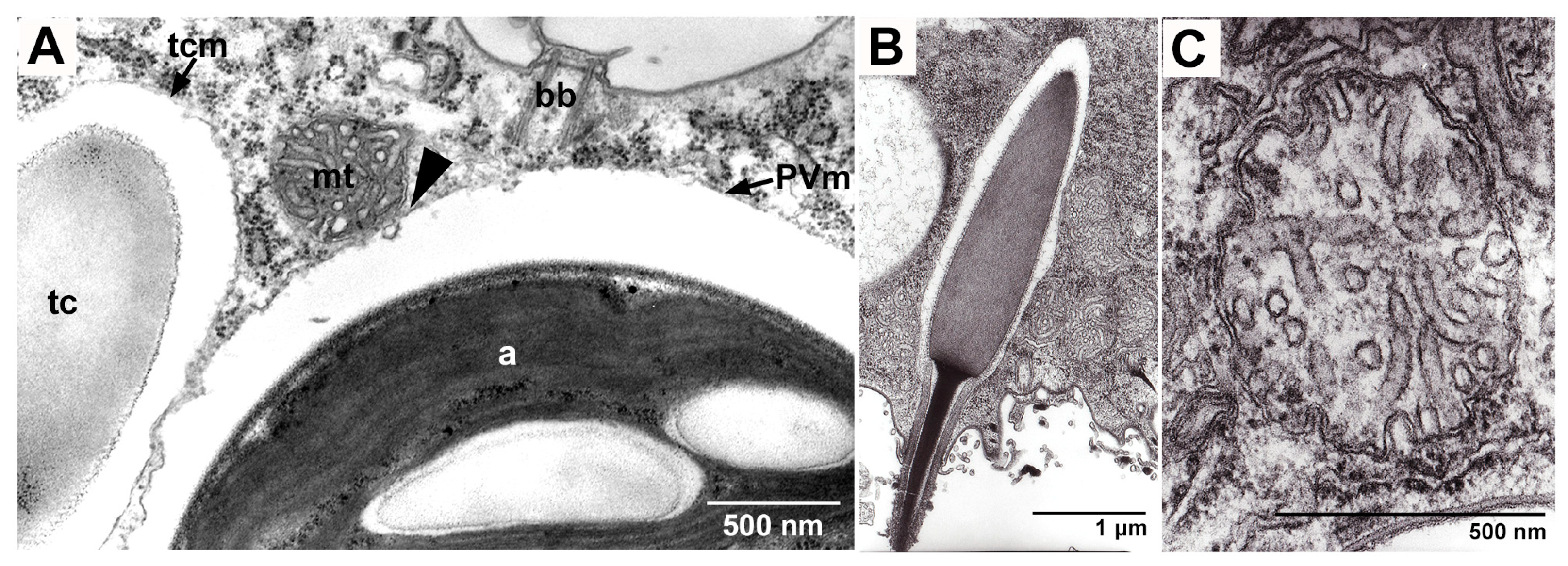
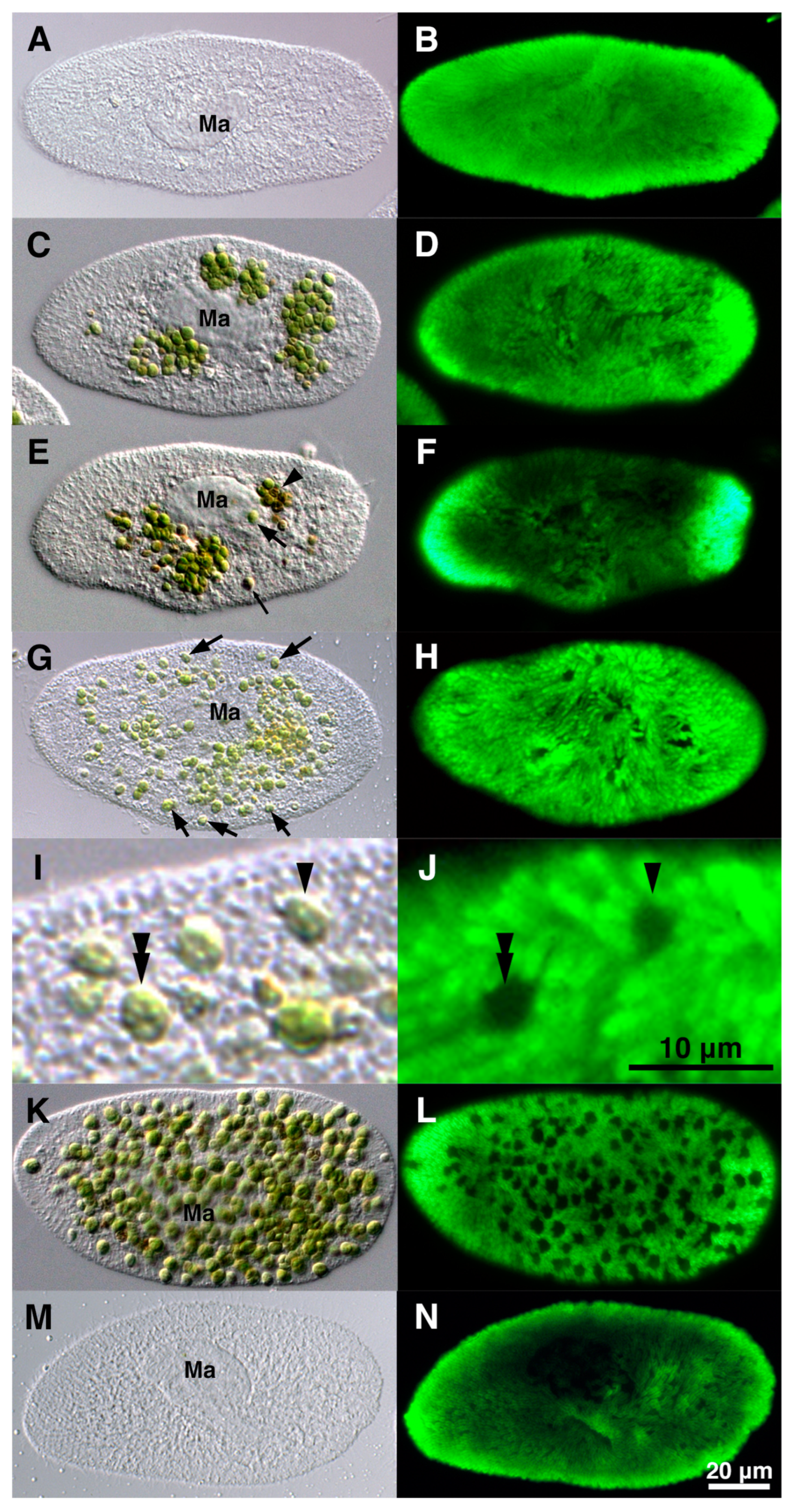
5.2. Algal Effects on Host Trichocysts and Mitochondria
5.2.1. Effects on Host Trichocysts
5.2.2. Effects on Host Mitochondria
5.3. Algal Effects on Host Trichosysts, Mitochondria, Cytoplasmic Crystals, Total Protein Amount, Heat Tolerance, Photoaccumulation, and Circadian Rhythms
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bodył, A.; Mackiewicz, P.; Ciesála, J. Endosymbiotic Theory: Models and Challenges. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Leung, T.L.F.; Poulin, R. Parasitism, commensalism, and mutualism: Exploring the many shades of Symbioses. Vie Milieu/Life Environ. 2008, 58, 107–115. [Google Scholar]
- Davy, S.K.; Allemand, D.; Weis, V.M. Cell Biology of Cnidarian-Dinoflagellate Symbiosis. Microbiol. Mol. Biol. Rev. 2012, 76, 229–261. [Google Scholar] [CrossRef] [PubMed]
- Goetsch, W. Die symbiose der süsswasser-hydroiden und ihre künstliche beeinflussung. Z. Morph Okol. Tiere 1924, 1, 660–751. [Google Scholar] [CrossRef]
- Lee, J.J.; Soldo, A.T.; Reisser, W.; Lee, M.J.; Jeon, K.W.; Görtz, H.-D. The Extent of Algal and Bacterial Endosymbioses in Protozoa. J. Protozool. 1985, 32, 391–403. [Google Scholar] [CrossRef]
- Kreutz, M.; Stoeck, T.; Foissner, W. Morphological and molecular characterization of Paramecium (Viridoparamecium nov. subgen.) chlorelligerum Kahl (Ciliophora). J. Euk. Microbiol. 2012, 59, 548–563. [Google Scholar] [CrossRef]
- Pitsch, G.; Adamec, L.; Dirren, S.; Nitsche, F.; Šimek, K.; Sirová, D.; Posch, T. The Green Tetrahymena utriculariae n. sp. (Ciliophora, Oligohymenophorea) with Its Endosymbiotic Algae (Micractinium sp.), Living in Traps of a Carnivorous Aquatic Plant. J. Euk. Microbiol. 2017, 64, 322–335. [Google Scholar] [CrossRef]
- Pröschold, T.; Pitsch, G.; Darienko, T. Micractinium tetrahymenae (Trebouxiophyceae, Chlorophyta), a New Endosymbiont Isolated from Ciliates. Diversity 2020, 12, 200. [Google Scholar] [CrossRef]
- He, M.; Wang, J.; Fan, X.; Liu, X.; Shi, W.; Huang, N.; Zhao, F.; Miao, M. Genetic basis for the establishment of endosymbiosis in Paramecium. ISME J. 2019, 13, 1360–1369. [Google Scholar] [CrossRef]
- Hoshina, R.; Iwataki, M.; Imamura, N. Chlorella variabilis and Micractinium reisseri sp. nov. (Chlorellaceae, Trebouxiophyceae): Redescription of the endosymbiotic green algae of Paramecium bursaria (Peniculia, Oligohymenophorea) in the 120th year. Phycol. Res. 2010, 58, 188–201. [Google Scholar] [CrossRef]
- Kodama, Y.; Fujishima, M. Cycloheximide Induces Synchronous Swelling of Perialgal Vacuoles Enclosing Symbiotic Chlorella vulgaris and Digestion of the Algae in the Ciliate Paramecium bursaria. Protist 2008, 159, 483–494. [Google Scholar] [CrossRef]
- Kodama, Y.; Fujishima, M. Synchronous Induction of Detachment and Reattachment of Symbiotic Chlorella spp. from the Cell Cortex of the Host Paramecium bursaria. Protist 2013, 164, 660–672. [Google Scholar] [CrossRef]
- Gu, F.; Chen, L.; Ni, B.; Zhang, X. A comparative study on the electron microscopic enzymo-cytochemistry of Paramecium bursaria from light and dark cultures. Europ J. Protistol. 2002, 38, 267–278. [Google Scholar] [CrossRef]
- Kawakami, H.; Kawakami, N. Behavior of a Virus in a Symbiotic System, Paramecium bursaria—Zoochlorella. J. Protozool. 1978, 25, 217–225. [Google Scholar] [CrossRef]
- Karakashian, S.J. Growth of Paramecium bursaria as Influenced by the Presence of Algal Symbionts. Physiol. Zool. 1963, 36, 52–68. [Google Scholar] [CrossRef]
- Iwai, S.; Fujiwara, K.; Tamura, T. Maintenance of algal endosymbionts in Paramecium bursaria: A simple model based on population dynamics. Environ. Microbiol. 2016, 18, 2435–2445. [Google Scholar] [CrossRef] [PubMed]
- Kodama, Y.; Fujishima, M. Chapter 2—Secondary Symbiosis Between Paramecium and Chlorella Cells. In International Review of Cell and Molecular Biology; Academic Press: Cambridge, MA, USA, 2010; Volume 279, pp. 33–77. [Google Scholar]
- Karakashian, M.W. Symbiosis in Paramecium bursaria. Symp. Soc. Exp. Biol. 1975, 29, 145–173. [Google Scholar]
- Lowe, C.D.; Minter, E.J.; Cameron, D.D.; Brockhurst, M.A. Shining a Light on Exploitative Host Control in a Photosynthetic Endosymbiosis. Curr. Biol. 2016, 26, 207–211. [Google Scholar] [CrossRef]
- Sørensen, M.E.S.; Lowe, C.D.; Minter, E.J.A.; Wood, A.J.; Cameron, D.D.; Brockhurst, M.A. The role of exploitation in the establishment of mutualistic microbial symbioses. FEMS Microbiol. Lett. 2019, 366, fnz148. [Google Scholar] [CrossRef]
- Iwai, S.; Fujita, K.; Takanishi, Y.; Fukushi, K. Photosynthetic Endosymbionts Benefit from Host’s Phagotrophy, Including Predation on Potential Competitors. Curr. Biol. 2019, 29, 3114–3119.e3113. [Google Scholar] [CrossRef]
- Reisser, W. The metabolic interactions between Paramecium bursaria Ehrbg. and Chlorella spec. in the Paramecium bursaria-symbiosis. III. The influence of different CO2-concentrations and of glucose on the photosynthetic and respiratory capacity of the symbiotic unit. Arch. Microbiol. 1980, 125, 291–293. [Google Scholar] [CrossRef]
- Hörtnagl, P.H.; Sommaruga, R. Photo-oxidative stress in symbiotic and aposymbiotic strains of the ciliate Paramecium bursaria. Photochem. Photobiol. Sci. 2007, 6, 842–847. [Google Scholar] [CrossRef]
- Miwa, I. Regulation of Circadian Rhythms of Paramecium bursaria by Symbiotic Chlorella Species. In Endosymbionts in Paramecium; Fujishima, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 83–110. [Google Scholar]
- Summerer, M.; Sonntag, B.; Hörtnagl, P.; Sommaruga, R. Symbiotic Ciliates Receive Protection Against UV Damage from their Algae: A Test with Paramecium bursaria and Chlorella. Protist 2009, 160, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Jennings, H.S. Sex Reaction Types and Their Interrelations in Paramecium bursaria: II. Clones Collected from Natural Habitats. Proc. Natl. Acad. Sci. USA 1938, 24, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Pado, R. Mutual relation of protozoans and symbiotic algae in Paramaecium bursaria. I. The influence of light on the growth of symbionts. Folia Biol. 1965, 13, 173–182. [Google Scholar]
- Weis, D.S. Regulation of host and symbiont population size in Paramecium bursaria. Experientia 1969, 25, 664–666. [Google Scholar] [CrossRef]
- Wichterman, R. The Biological Effects of X-Rays on Mating Types and Conjugation of Paramecium bursaria. Biol. Bull. 1948, 94, 113–127. [Google Scholar] [CrossRef]
- Hosoya, H.; Kimura, K.; Matsuda, S.; Kitaura, M.; Takahashi, T.; Kosaka, T. Symbiotic Algae-free Strains of The Green Paramecium Paramecium bursaria Produced by Herbicide Paraquat. Zool. Sci. 1995, 12, 807–810. [Google Scholar] [CrossRef]
- Reisser, W. The metabolic interactions between Paramecium bursaria Ehrbg. and Chlorella spec. in the Paramecium bursaria-symbiosis. I. The nitrogen and the carbon metabolism (author’s transl). Arch. Microbiol. 1976, 107, 357–360. [Google Scholar] [CrossRef]
- Weis, D.S. The effect of accumulation time of separate cultivation on the frequency of infection of aposymbiotic ciliates by symbiotic algae in Paramecium bursaria. J. Protozool. 1984, 31, 13A–14A. [Google Scholar]
- Pringsheim, E.G. Physiologische Untersuchungen an Paramecium bursaria: Ein Beitrag zur Symbioseforschung. Arch. Protistenkd. 1928, 64, 289–418. [Google Scholar]
- Siegel, R.W.; Karakashian, S.J. Dissociation and restoration of endocellular symbiosis in Paramecium bursaria. Anat. Rec. 1959, 134, 639. [Google Scholar]
- Blanc, G.; Duncan, G.; Agarkova, I.; Borodovsky, M.; Gurnon, J.; Kuo, A.; Lindquist, E.; Lucas, S.; Pangilinan, J.; Polle, J.; et al. The Chlorella variabilis NC64A genome reveals adaptation to photosymbiosis, coevolution with viruses, and cryptic sex. Plant Cell 2010, 22, 2943–2955. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-H.; Liu, C.-F.J.; Yu, Y.-H.; Jhou, Y.-T.; Fujishima, M.; Tsai, I.J.; Leu, J.-Y. Genome plasticity in Paramecium bursaria revealed by population genomics. BMC Biol. 2020, 18, 180. [Google Scholar] [CrossRef] [PubMed]
- Dohra, H.; Fujishima, M.; Suzuki, H. Analysis of amino acid and codon usage in Paramecium bursaria. FEBS Lett. 2015, 589, 3113–3118. [Google Scholar] [CrossRef] [PubMed]
- Galvani, A.; Sperling, L. RNA interference by feeding in Paramecium. Trends Genet. 2002, 18, 11–12. [Google Scholar] [CrossRef]
- Arnaiz, O.; Cain, S.; Cohen, J.; Sperling, L. ParameciumDB: A community resource that integrates the Paramecium tetraurelia genome sequence with genetic data. Nucleic Acids Res. 2006, 35, D439–D444. [Google Scholar] [CrossRef] [PubMed]
- Hori, M.; Tominaga, T.; Ishida, M.; Kawano, M. RNA interference reveals the escape response mechanism of Paramecium to mechanical stimulation. Biophys. Physicobiol. 2023, 20, e200025. [Google Scholar] [CrossRef]
- Jenkins, B.H.; Maguire, F.; Leonard, G.; Eaton, J.D.; West, S.; Housden, B.E.; Milner, D.S.; Richards, T.A. Characterization of the RNA-interference pathway as a tool for reverse genetic analysis in the nascent phototrophic endosymbiosis, Paramecium bursaria. R. Soc. Open Sci. 2021, 8, 210140. [Google Scholar] [CrossRef]
- Kodama, Y.; Suzuki, H.; Dohra, H.; Sugii, M.; Kitazume, T.; Yamaguchi, K.; Shigenobu, S.; Fujishima, M. Comparison of gene expression of Paramecium bursaria with and without Chlorella variabilis symbionts. BMC Genom. 2014, 15, 183. [Google Scholar] [CrossRef]
- Gentil, J.; Hempel, F.; Moog, D.; Zauner, S.; Maier, U.G. Review: Origin of complex algae by secondary endosymbiosis: A journey through time. Protoplasma 2017, 254, 1835–1843. [Google Scholar] [CrossRef]
- Gomori, G. Microscopic Histochemistry; Principles and Practice; University of Chicago Press: Chicago, IL, USA, 1952. [Google Scholar]
- Ramoino, P.; Beltrame, F.; Fato, M. Image analysis of lysosomal activity during the early clonal life of Paramecium primaurelia. FEMS Microbiol. Lett. 1995, 125, 57–61. [Google Scholar] [CrossRef]
- Steinhoff, U.; Golecki, J.R.; Kazda, J.; Kaufmann, S.H. Evidence for phagosome-lysosome fusion in Mycobacterium leprae-infected murine Schwann cells. Infect. Immun. 1989, 57, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Michetti, K.M.; Leonardi, P.I.; Cáceres, E.J. Cytochemical localization of acid phosphatase in Stigeoclonium tenue (Chaetophorales, Chlorophyceae). Biocell 2006, 30, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, M.; Kodama, Y. Insights into the Paramecium-Holospora and Paramecium-Chlorella Symbioses. In Cilia/Flagella and Ciliates/Flagellates; Hausmann, K., Radek, R., Eds.; Schweizerbart Science Publisher: Stuttgart, Germany, 2014; pp. 203–227. [Google Scholar]
- Kodama, Y.; Fujishima, M. Infection of Paramecium bursaria by Symbiotic Chlorella Species. In Endosymbionts in Paramecium; Microbiology Monographs; Springer: Berlin/Heidelberg, Germany, 2009; pp. 31–55. [Google Scholar]
- Kodama, Y.; Fujishima, M. Paramecium as a Model Organism for Studies on Primary and Secondary Endosymbioses. In Biocommunication of Ciliates; Witzany, G., Nowacki, M., Eds.; Springer: Cham, Switzerland, 2016; pp. 277–304. [Google Scholar]
- Kodama, Y.; Fujishima, M. Symbiotic Chlorella sp. of the ciliate Paramecium bursaria do not prevent acidification and lysosomal fusion of host digestive vacuoles during infection. Protoplasma 2005, 225, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Fok, A.K.; Allen, R.D. The lysosome system. In Paramecium; Görtz, H.-D., Ed.; Springer: Berlin/Heidelberg, Germany, 1988; pp. 301–324. [Google Scholar]
- Kodama, Y.; Fujishima, M. Symbiotic Chlorella variabilis incubated under constant dark conditions for 24 hours loses the ability to avoid digestion by host lysosomal enzymes in digestive vacuoles of host ciliate Paramecium bursaria. FEMS Microbiol. Ecol. 2014, 90, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Kodama, Y.; Fujishima, M. Characteristics of the Digestive Vacuole Membrane of the Alga-Bearing Ciliate Paramecium bursaria. Protist 2012, 163, 658–670. [Google Scholar] [CrossRef]
- Obayashi, K.; Kodama, Y. Dynamics of digestive vacuole differentiation clarified by the observation of living Paramecium bursaria. Protoplasma 2024. [Google Scholar] [CrossRef]
- Kodama, Y.; Sumita, H. The ciliate Paramecium bursaria allows budding of symbiotic Chlorella variabilis cells singly from the digestive vacuole membrane into the cytoplasm during algal reinfection. Protoplasma 2022, 259, 117–125. [Google Scholar] [CrossRef]
- Kodama, Y.; Fujishima, M. Localization of Perialgal Vacuoles beneath the Host Cell Surface is not a Prerequisite Phenomenon for Protection from the Host’s Lysosomal Fusion in the Ciliate Paramecium bursaria. Protist 2009, 160, 319–329. [Google Scholar] [CrossRef]
- Kodama, Y.; Fujishima, M. Timing of Perialgal Vacuole Membrane Differentiation from Digestive Vacuole Membrane in Infection of Symbiotic Algae Chlorella vulgaris of the Ciliate Paramecium bursaria. Protist 2009, 160, 65–74. [Google Scholar] [CrossRef]
- Aoyagi, S.; Kodama, Y.; Passarelli, M.K.; Vorng, J.-L.; Kawashima, T.; Yoshikiyo, K.; Yamamoto, T.; Gilmore, I.S. OrbiSIMS Imaging Identifies Molecular Constituents of the Perialgal Vacuole Membrane of Paramecium bursaria with Symbiotic Chlorella variabilis. Anal. Chem. 2019, 91, 14545–14551. [Google Scholar] [CrossRef]
- Kodama, Y. Localization of attachment area of the symbiotic Chlorella variabilis of the ciliate Paramecium bursaria during the algal removal and reinfection. Symbiosis 2013, 60, 25–36. [Google Scholar] [CrossRef]
- Kodama, Y.; Fujishima, M. Endosymbiosis of Chlorella species to the ciliate Paramecium bursaria alters the distribution of the host’s trichocysts beneath the host cell cortex. Protoplasma 2011, 248, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Kodama, Y.; Fujishima, M. Infectivity of Chlorella species for the ciliate Paramecium bursaria is not based on sugar residues of their cell wall components, but on their ability to localize beneath the host cell membrane after escaping from the host digestive vacuole in the early infection process. Protoplasma 2007, 231, 55–63. [Google Scholar] [CrossRef]
- Karakashian, S.J.; Karakashian, M.W. Evolution and Symbiosis in the Genus Chlorella and Related Algae. Evolution 1965, 19, 368–377. [Google Scholar] [CrossRef]
- Kodama, Y.; Fujishima, M. Differences in infectivity between endosymbiotic Chlorella variabilis cultivated outside host Paramecium bursaria for 50 years and those immediately isolated from host cells after one year of reendosymbiosis. Biol. Open 2015, 5, 55–61. [Google Scholar] [CrossRef]
- Bomford, R. Infection of Alga-Free Paramecium bursaria with Strains of Chlorella, Scenedesmus, and a Yeast. J. Protozool. 1965, 12, 221–224. [Google Scholar] [CrossRef]
- Görtz, H.-D. Infections of Paramecium bursaria with bacteria and yeasts. J. Cell Sci. 1982, 58, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Suzaki, T.; Omura, G.; Görtz, H.-D. Infection of symbiont-free Paramecium bursaria with yeasts (in Japanese). Jpn. J. Protozool. 2003, 36, 17–18. [Google Scholar] [CrossRef]
- Ohkawa, H.; Hashimoto, N.; Furukawa, S.; Kadono, T.; Kawano, T. Forced symbiosis between synechocystis spp. PCC 6803 and apo-symbiotic Paramecium bursaria as an experimental model for evolutionary emergence of primitive photosynthetic eukaryotes. Plant Signal Behav 2011, 6, 773–776. [Google Scholar] [CrossRef]
- Kodama, Y.; Endoh, Y. Comparative Analyses of the Symbiotic Associations of the Host Paramecium bursaria with Free-Living and Native Symbiotic Species of Chlorella. Curr. Microbiol. 2024, 81, 66. [Google Scholar] [CrossRef]
- Watanabe, K.; Motonaga, A.; Tachibana, M.; Shimizu, T.; Watarai, M. Francisella novicida can utilize Paramecium bursaria as its potential host. Environ. Microbiol. Rep. 2022, 14, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Kinkead, L.C.; Fayram, D.C.; Allen, L.H. Francisella novicida inhibits spontaneous apoptosis and extends human neutrophil lifespan. J. Leukoc. Biol. 2017, 102, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Higuchi, Y.; Shimmura, M.; Tachibana, M.; Fujishima, M.; Shimizu, T.; Watarai, M. Peculiar Paramecium Hosts Fail to Establish a Stable Intracellular Relationship With Legionella pneumophila. Front. Microbiol. 2020, 11, 596731. [Google Scholar] [CrossRef]
- Bernheimer, A.W. A Comparative Study of the Crystalline Inclusions of Protozoa. Trans. Am. Microsc. Soc. 1938, 57, 336–343. [Google Scholar] [CrossRef]
- Pautard, F.G.E. Calcification in Unicellular Organisms. In Biological Calcification: Cellular and Molecular Aspects; Schraer, H., Ed.; Springer: Boston, MA, USA, 1970; pp. 105–201. [Google Scholar]
- Creutz, C.E.; Mohanty, S.; Defalco, T.; Kretsinger, R. Purine Composition of the Crystalline Cytoplasmic Inclusions of Paramecium tetraurelia. Protist 2002, 153, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, K. Kristalle in Wimpertieren. Mikrokosmos 1982, 71, 33–39. (In German) [Google Scholar]
- Pilátová, J.; Pánek, T.; Oborník, M.; Čepička, I.; Mojzeš, P. Revisiting biocrystallization: Purine crystalline inclusions are widespread in eukaryotes. ISME J. 2022, 16, 2290–2294. [Google Scholar] [CrossRef]
- Kodama, Y.; Kitatani, A.; Morita, Y. Characterization of Crystals in Ciliate Paramecium bursaria Harboring Endosymbiotic Chlorella variabilis. Curr. Microbiol. 2024, 81, 265. [Google Scholar] [CrossRef]
- Fujishima, M.; Kodama, Y. Endosymbionts in Paramecium. Europ J. Protistol. 2012, 48, 124–137. [Google Scholar] [CrossRef]
- Song, C.; Murata, K.; Suzaki, T. Intracellular symbiosis of algae with possible involvement of mitochondrial dynamics. Sci. Rep. 2017, 7, 1221. [Google Scholar] [CrossRef]
- Kodama, Y.; Fujishima, M. Endosymbiotic Chlorella variabilis reduces mitochondrial number in the ciliate Paramecium bursaria. Sci. Rep. 2022, 12, 8216. [Google Scholar] [CrossRef] [PubMed]
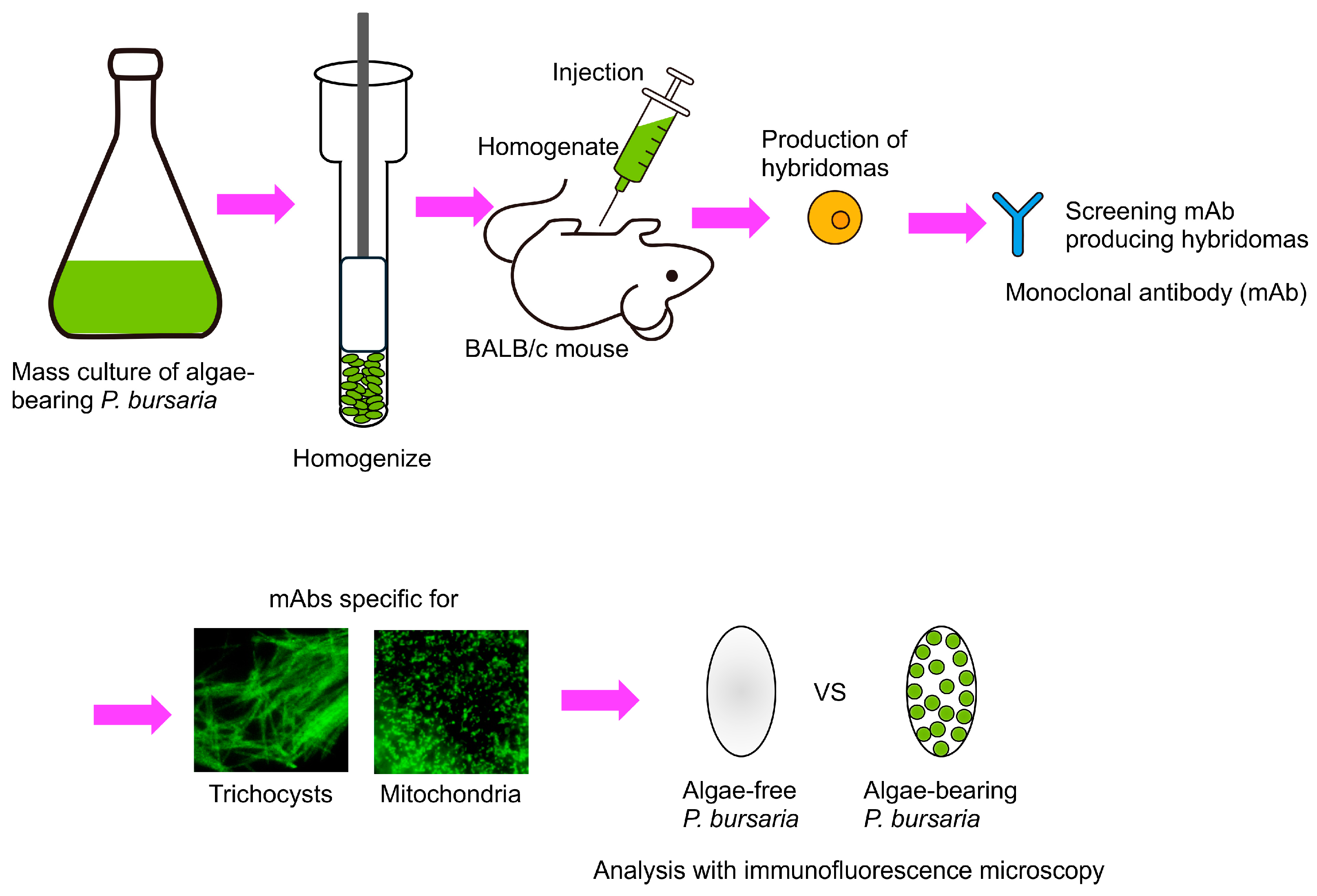
- Galfrè, G.; Milstein, C. Preparation of monoclonal antibodies: Strategies and procedures. Methods Enzymol. 1981, 73, 3–46. [Google Scholar] [CrossRef] [PubMed]
- Harumoto, T.; Miyake, A. Defensive function of trichocysts in Paramecium. J. Exp. Zool. 1991, 260, 84–92. [Google Scholar] [CrossRef]
- Kodama, Y.; Miyazaki, S. Autolysis of Chlorella variabilis in Starving Paramecium bursaria Help the Host Cell Survive Against Starvation Stress. Curr. Microbiol. 2021, 78, 558–565. [Google Scholar] [CrossRef]
- Morita, H.; Kodama, Y. Quantitative analysis of trichocysts in Paramecium bursaria following artificial removal and infection with the symbiotic Chlorella variabilis. Europ J. Protistol. 2024, 95, 126115. [Google Scholar] [CrossRef]
- Ehret, C.D.; McArdle, E.W. The structure of Paramecium as viewed from its constituent levels of organization. In Paramecium: A Current Survey; Wagtendonk, W.J.V., Ed.; Elsevier: Amsterdam, The Netherlands, 1974; pp. 263–338. [Google Scholar]
- Berger, J. Feeding Behaviour of Didinium nasutum on Paramecium bursaria with Normal or Apochlorotic Zoochlorellae. J. Gen. Microbiol. 1980, 118, 397–404. [Google Scholar] [CrossRef]
- Vakifahmetoglu-Norberg, H.; Ouchida, A.T.; Norberg, E. The role of mitochondria in metabolism and cell death. Biochem. Biophys. Res. Commun. 2017, 482, 426–431. [Google Scholar] [CrossRef]
- Medeiros, T.C.; Mehra, C.; Pernas, L. Contact and competition between mitochondria and microbes. Curr. Opin. Microbio 2021, 63, 189–194. [Google Scholar] [CrossRef]
- Cann, J.P. An ultrastructural study of Mayorella viridis (Leidy) (Amoebida: Paramoebidae), a Rhizopod containing zoochlorellae. Arch. Protistenkd. 1981, 124, 353–360. [Google Scholar] [CrossRef]
- Kawai, S.; Araki, S.; Kodama, Y. No mutual symbiosis following infection of algae-free Paramecium bursaria with symbiotic algae from Mayorella viridis. Symbiosis 2018, 75, 51–59. [Google Scholar] [CrossRef]
- Sinai, A.P.; Webster, P.; Joiner, K.A. Association of host cell endoplasmic reticulum and mitochondria with the Toxoplasma gondii parasitophorous vacuole membrane: A high affinity interaction. J. Cell Sci. 1997, 110, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Mehra, C.; Pernas, L. Contact sites between host organelles and pathogens: Boon or bane? mSphere 2023, 8, e0044823. [Google Scholar] [CrossRef]
- Kudryashev, M.; Lepper, S.; Stanway, R.; Bohn, S.; Baumeister, W.; Cyrklaff, M.; Frischknecht, F. Positioning of large organelles by a membrane-associated cytoskeleton in Plasmodium sporozoites. Cell Microbiol. 2010, 12, 362–371. [Google Scholar] [CrossRef]
- Scanlon, M.; Leitch, G.J.; Visvesvara, G.S.; Shaw, A.P. Relationship between the host cell mitochondria and the parasitophorous vacuole in cells infected with Encephalitozoon microsporidia. J. Eukaryot. Microbiol. 2004, 51, 81–87. [Google Scholar] [CrossRef]
- Stavru, F.; Riemer, J.; Jex, A.; Sassera, D. When bacteria meet mitochondria: The strange case of the tick symbiont Midichloria mitochondrii(†). Cell Microbiol. 2020, 22, e13189. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, L.; Bigliardi, E.; Corona, S.; Beninati, T.; Lo, N.; Franceschi, A. A symbiont of the tick Ixodes ricinus invades and consumes mitochondria in a mode similar to that of the parasitic bacterium Bdellovibrio bacteriovorus. Tissue Cell 2004, 36, 43–53. [Google Scholar] [CrossRef]
- Fokin, S.I.; Schweikert, M.; Görtz, H.-D.; Fujishima, M. Bacterial endocytobionts of Ciliophora. Diversity and some interactions with the host. Europ J. Protistol. 2003, 39, 475–480. [Google Scholar] [CrossRef]
- Yamataka, S.; Hayashi, R. Electron microscopic studies on the mitochondria and intramitochondrial microorganisms of Halteria geleiana. J. Electron. Microsc. 1970, 19, 50–62. [Google Scholar]
- de Puytorac, P.; Grain, J. Intramitochondrial bacteria and pecularities of cytostomopharyngeal ultrastructure in the ciliate, Urotricha ovata Kahl (Ciliata). Comptes Rendus Seances Soc. Biol. Ses Fil. 1972, 166, 604–607. [Google Scholar]
- Dunn, S.R.; Pernice, M.; Green, K.; Hoegh-Guldberg, O.; Dove, S.G. Thermal Stress Promotes Host Mitochondrial Degradation in Symbiotic Cnidarians: Are the Batteries of the Reef Going to Run Out? PLoS ONE 2012, 7, e39024. [Google Scholar] [CrossRef]
- Wang, Z.; Yong, H.; Zhang, S.; Liu, Z.; Zhao, Y. Colonization Resistance of Symbionts in Their Insect Hosts. Insects 2023, 14, 594. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.; Capodanno, B.; Xu, S.; Hamilton, M.; Strassmann, J.; Queller, D. Reduced and Nonreduced Genomes in Paraburkholderia Symbionts of Social Amoebas. mSystems 2022, 7, e00562-00522. [Google Scholar] [CrossRef] [PubMed]
- Iwatsuki, K.; Nishidoi, M.; Suehiro, K. Symbiotic Chlorella enhances the thermal tolerance in Paramecium bursaria. Comp. Biochem. Physiol. Part A 1998, 121, 405–409. [Google Scholar] [CrossRef]
- Iwatsuki, K.; Naitoh, Y. Behavioural Responses to Light in Paramecium Bursaria in Relation to its Symbiotic Green Alga Chlorella. J. Exp. Biol. 1988, 134, 43–60. [Google Scholar] [CrossRef]
- Matsuoka, K.; Nakaoka, Y. Photoreceptor Potential Causing Phototaxis of Paramecium Bursaria. J. Exp. Biol. 1988, 137, 477–485. [Google Scholar] [CrossRef]
- Taniguchi, M.; Lindsey, J.S. Absorption and Fluorescence Spectral Database of Chlorophylls and Analogues. Photochem. Photobiol. 2021, 97, 136–165. [Google Scholar] [CrossRef]
- Johnson, C.H.; Miwa, I.; Kondo, T.; Hastings, J.W. Circadian rhythm of photoaccumulation in Paramecium bursaria. J. Biol. Rhythms 1989, 4, 405–415. [Google Scholar] [CrossRef]
- Miwa, I.; Fujimori, N.; Tanaka, M. Effects of symbiotic Chlorella on the period length and the phase shift of circadian rhythms in Paramecium bursaria. Europ J. Protistol. 1996, 32, 102–107. [Google Scholar] [CrossRef]
- Tanaka, M.; Miwa, I. Correlation of Photosynthetic Products of Symbiotic Chlorella with the Mating Reactivity Rhythms in a Mutant Strain of genus-species Paramecium bursaria. Zool. Sci. 2000, 17, 735–742. [Google Scholar] [CrossRef]
- Hussa, E.A.; Goodrich-Blair, H. It takes a village: Ecological and fitness impacts of multipartite mutualism. Annu. Rev. Microbiol. 2013, 67, 161–178. [Google Scholar] [CrossRef]
- Fujishima, M.; Kodama, Y. Mechanisms for establishing primary and secondary endosymbiosis in Paramecium. J. Euk. Microbiol. 2022, 69, e12901. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, M.; Hiwatashi, K. Transplantation of germ nucleus in Paramecium caudatum I: Nuclei in the pre-meiotic S phase can enter into mitotic cycle. Exp. Cell Res. 1978, 111, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, M.; Hiwatashi, K. Transplantation of germ nuclei in Paramecium caudatum: II. Induction of meiosis in transplanted interphase nucleus. Exp. Cell Res. 1981, 131, 63–71. [Google Scholar] [CrossRef]
- Fujishima, M.; Watanabe, T. Transplantation of germ nuclei in Paramecium caudatum: III. Role of germinal micronucleus in vegetative growth. Exp. Cell Res. 1981, 132, 47–56. [Google Scholar] [CrossRef] [PubMed]


| Phenomena | Algae-Free P. bursaria | Algae-Bearing P. bursaria | References |
|---|---|---|---|
| Abundance of trichocysts | Increase | Decrease | [57,61,81] |
| Trichocyst regeneration speed | Increase | Decrease | [85] |
| Abundance of mitochondria | Increase | Decrease | [81] |
| Abundance of cytoplasmic crystals | Increase | Decrease | [78] |
| Abundance of total cell protein | Increase | Decrease | [81] |
| Heat tolerance | Decrease | Increase | [24,104] |
| Photoaccumulation | Almost no change | Almost no change | [105,106] |
| Circadian rhythms on photoaccumulation and mating reactivity | Depend on the host clock | Depend on the Chlorella clock under constant light conditions | [24,109,110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kodama, Y.; Fujishima, M. Effects of the Symbiotic Chlorella variabilis on the Host Ciliate Paramecium bursaria Phenotypes. Microorganisms 2024, 12, 2537. https://doi.org/10.3390/microorganisms12122537
Kodama Y, Fujishima M. Effects of the Symbiotic Chlorella variabilis on the Host Ciliate Paramecium bursaria Phenotypes. Microorganisms. 2024; 12(12):2537. https://doi.org/10.3390/microorganisms12122537
Chicago/Turabian StyleKodama, Yuuki, and Masahiro Fujishima. 2024. "Effects of the Symbiotic Chlorella variabilis on the Host Ciliate Paramecium bursaria Phenotypes" Microorganisms 12, no. 12: 2537. https://doi.org/10.3390/microorganisms12122537
APA StyleKodama, Y., & Fujishima, M. (2024). Effects of the Symbiotic Chlorella variabilis on the Host Ciliate Paramecium bursaria Phenotypes. Microorganisms, 12(12), 2537. https://doi.org/10.3390/microorganisms12122537







