A Lectin AtTL-2 Obtained from Acropora aff. tenuis Induced Stimualation of Phagocytosis of Symbiodiniaceae
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
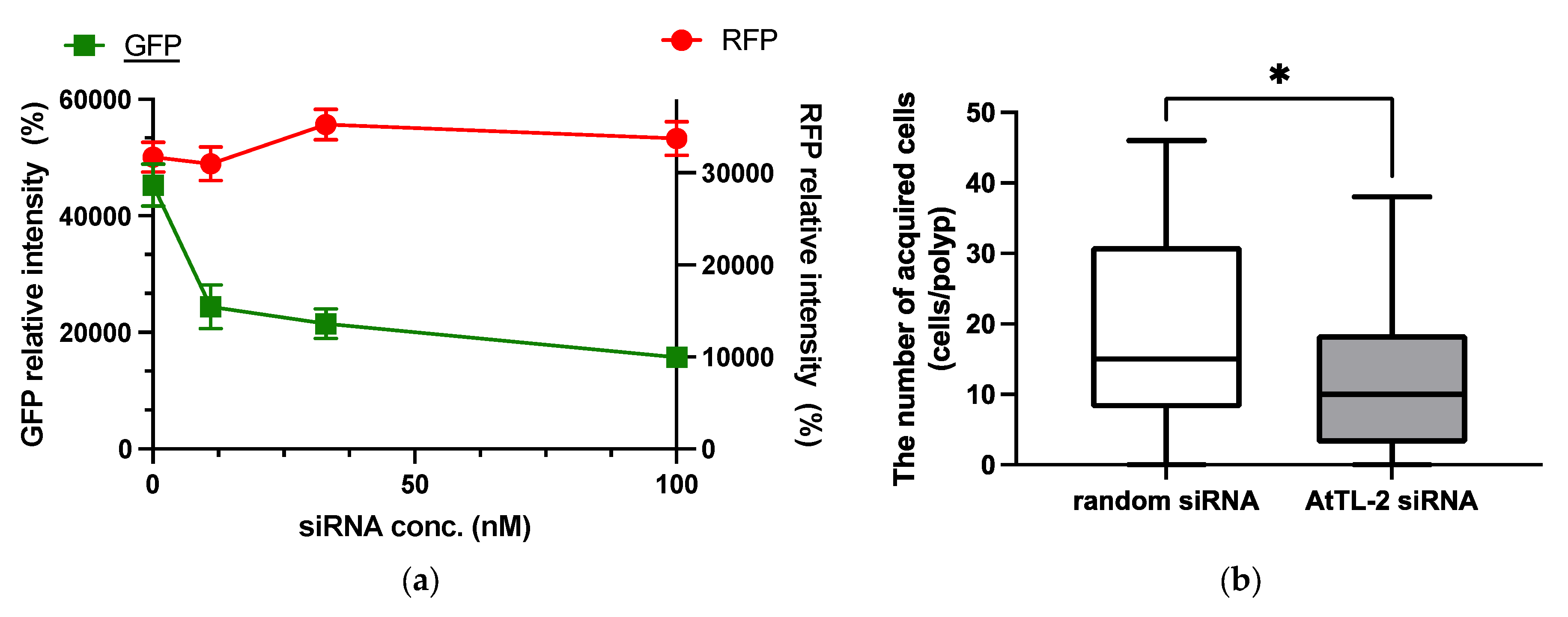
2.2. RNA Interference
2.3. Immunohistochemistry
2.4. Preparation of AtTL-2–Fixed Beads
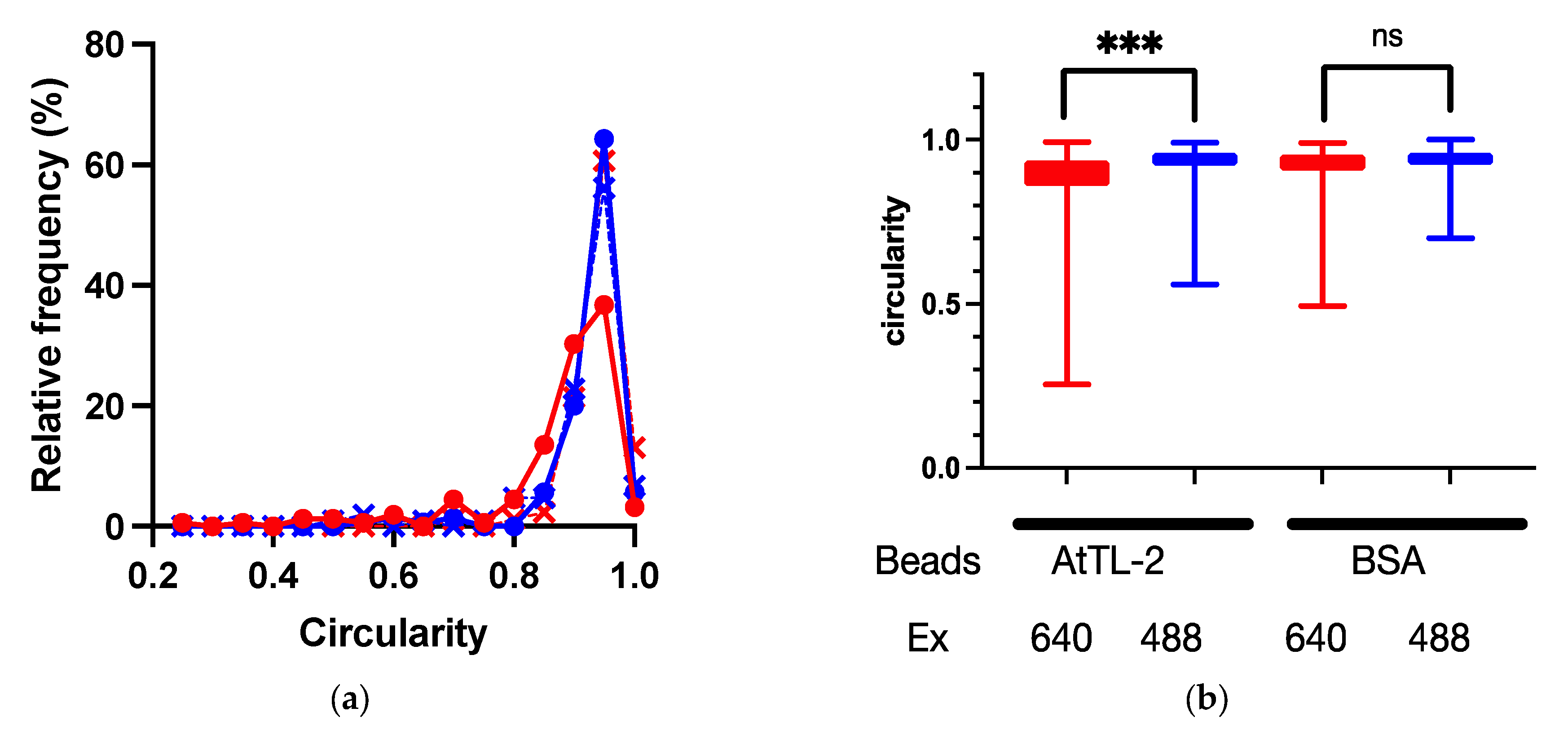
2.5. Evaluation of Phagocytosis of AtTL-2–Fixed Beads
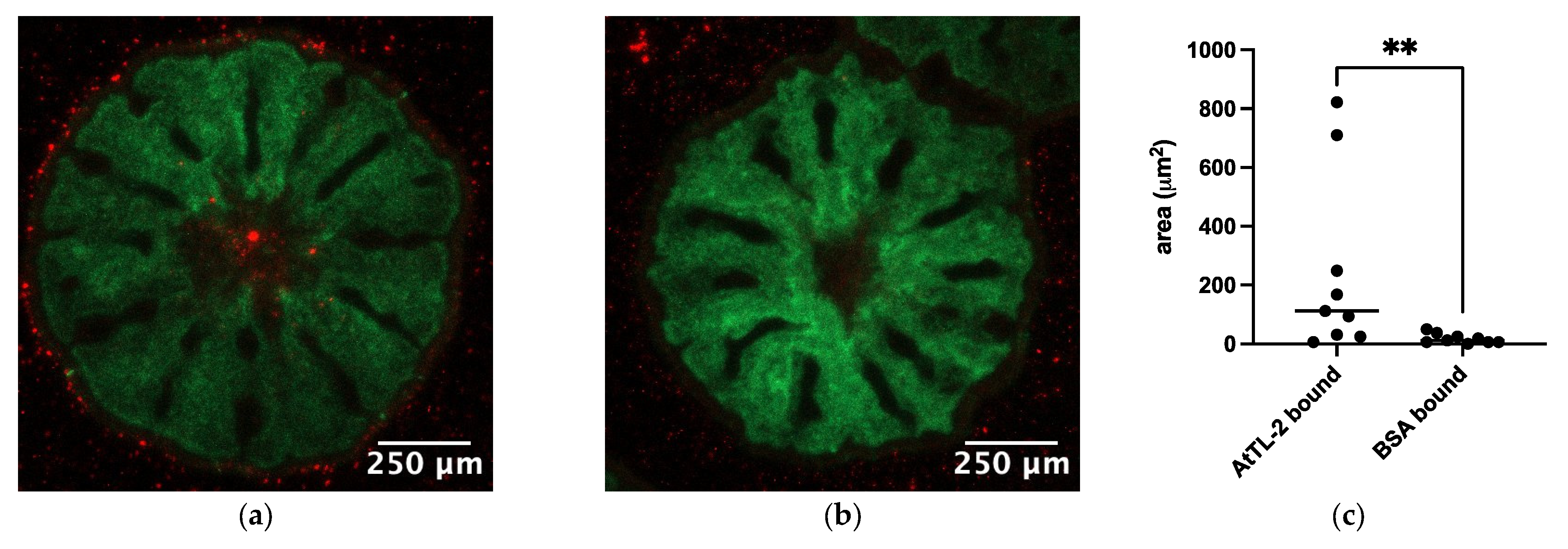
2.6. Binding of AtTL-2–Fixed Beads to Polyp Cells
2.7. Statistical Analysis
3. Results
3.1. Distribution of AtTL-2
3.2. RNAi Analysis of AtTL-2
3.3. Acquisition of AtTL-2–Fixed Beads by Juvenile Polyps
3.4. Binding of AtTL-2–Fixed Beads to Symbiodiniaceae-Containing Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fisher, R.; O’Leary, R.A.; Low-Choy, S.; Mengersen, K.; Knowlton, N.; Brainard, R.E.; Caley, M.J. Species richness on coral reefs and the pursuit of convergent global estimates. Curr. Biol. 2015, 25, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Burriesci, M.S.; Raab, T.K.; Pringle, J.R. Evidence that glucose is the major transferred metabolite in dinoflagellate-cnidarian symbiosis. J. Exp. Biol. 2012, 215, 3467–3477. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Suzuki, G.; Hayashibara, T.; Koike, K. Acropora recruits harbor “rare” Symbiodinium in the environmental pool. Coral Reefs 2013, 32, 355–366. [Google Scholar] [CrossRef]
- Bridge, T.C.L.; Cowman, P.F.; Quattrini, A.M.; Bonito, V.E.; Sinniger, F.; Harii, S.; Head, C.E.I.; Hung, J.Y.; Halafihi, T.; Rongo, T.; et al. A tenuis relationship: Traditional taxonomy obscures systematics and biogeography of the ‘Acropora tenuis’ (scleractinia: Acroporidae) species complex. Zool. J. Linn. Soc. 2023, 202, zlad062. [Google Scholar] [CrossRef]
- Aihara, Y.; Maruyama, S.; Baird, A.H.; Iguchi, A.; Takahashi, S.; Minagawa, J. Green fluorescence from cnidarian hosts attracts symbiotic algae. Proc. Natl. Acad. Sci. USA 2019, 116, 2118–2123. [Google Scholar] [CrossRef]
- Yamashita, H.; Koike, K.; Shinzato, C.; Jimbo, M.; Suzuki, G. Can Acropora tenuis larvae attract native Symbiodiniaceae cells by green fluorescence at the initial establishment of symbiosis? PLoS ONE 2021, 16, e0252514. [Google Scholar] [CrossRef]
- Davy, S.K.; Allemand, D.; Weis, V.M. Cell Biology of Cnidarian-Dinoflagellate Symbiosis. Microbiol. Mol. Biol. Rev. 2012, 76, 229–261. [Google Scholar] [CrossRef]
- Hagedorn, M.; Carter, V.; Zuchowicz, N.; Phillips, M.; Penfield, C.; Shamenek, B.; Vallen, E.A.; Kleinhans, F.W.; Peterson, K.; White, M.; et al. Trehalose is a chemical attractant in the establishment of coral symbiosis. PLoS ONE 2015, 10, e0117087. [Google Scholar] [CrossRef]
- Lin, K.; Wang, J.; Fang, L. Participation of glycoproteins on zooxanthellal cell walls in the establishment of a symbiotic relationship with the sea anemone, Aiptasia Pulchella. Zool. Stud. 2000, 39, 172–178. [Google Scholar]
- Wood-Charlson, E.M.; Hollingsworth, L.L.; Krupp, D.A.; Weis, V.M. Lectin/glycan interactions play a role in recognition in a coral/dinoflagellate symbiosis. Cell. Microbiol. 2006, 8, 1985–1993. [Google Scholar] [CrossRef]
- Mansfield, K.M.; Gilmore, T.D. Innate immunity and cnidarian-Symbiodiniaceae mutualism. Dev. Comp. Immunol. 2019, 90, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Iwao, K.; Fujisawa, T.; Hatta, M. A cnidarian neuropeptide of the GLWamide family induces metamorphosis of reef-building corals in the genus Acropora. Coral Reefs 2002, 21, 127–129. [Google Scholar] [CrossRef]
- Yuyama, I.; Hayakawa, H.; Endo, H.; Iwao, K.; Takeyama, H.; Maruyama, T.; Watanabe, T. Identification of symbiotically expressed coral mRNAs using a model infection system. Biochem. Biophys. Res. Commun. 2005, 336, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Kuniya, N.; Jimbo, M.; Tanimoto, F.; Yamashita, H.; Koike, K.; Harii, S.; Nakano, Y.; Iwao, K.; Yasumoto, K.; Watabe, S. Possible involvement of tachylectin-2-like lectin from Acropora tenuis in the process of symbiodinium acquisition. Fish. Sci. 2015, 81, 473–483. [Google Scholar] [CrossRef]
- Takeuchi, R.; Jimbo, M.; Tanimoto, F.; Iijima, M.; Yamashita, H.; Suzuki, G.; Harii, S.; Nakano, Y.; Yasumoto, K.; Watabe, S. N-acetyl-D-glucosamine-binding lectin in Acropora tenuis attracts specific Symbiodiniaceae cell culture strains. Mar. Drugs 2021, 19, 146. [Google Scholar] [CrossRef]
- Takeuchi, R.; Jimbo, M.; Tanimoto, F.; Tanaka, C.; Harii, S.; Nakano, Y.; Yasumoto, K.; Watabe, S. Establishment of a model for chemoattraction of Symbiodinium and characterization of chemotactic compounds in Acropora tenuis. Fish. Sci. 2017, 83, 479–487. [Google Scholar] [CrossRef]
- Fitt, W.K.; Trench, R.K. Endocytosis of the symbiotic dinoflagellate Symbiodinium microadriaticum freudenthal by endodermal cells of the Scyphistomae of Cassiopeia xamachana and resistance of the algae to host digestion. J. Cell Sci. 1983, 64, 195–212. [Google Scholar] [CrossRef]
- Suzuki, G. Optimization of a Spawning-induction Protocol for the Prediction of Natural Coral Spawning. Fish. Sci. 2020, 86, 665–671. [Google Scholar] [CrossRef]
- Hayashibara, T.; Iwao, K.; Omori, M. Induction and Control of Spawning in Okinawan Staghorn Corals. Coral Reefs 2004, 23, 406–409. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Yuyama, I.; Higuchi, T.; Hidaka, M. application of RNA interference technology to Acroporid juvenile corals. Front. Mar. Sci. 2021, 8, 688876. [Google Scholar] [CrossRef]
- Mariscal, R.N.; Bigger, C.H.; McLean, R.B. The form and function of cnidarian spirocysts. Cell Tissue Res. 1976, 168, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Gundlach, K.A.; Watson, G.M. The effects of symbiotic state and nutrient availability on the cnidom in the model sea anemone, Exaiptasia diaphana. Mar. Biol. 2019, 166, 31. [Google Scholar] [CrossRef]
- Kamiya, H.; Jimbo, M.; Koike, K.; Sakai, R. Roles of coral lectins in morphological change of zooxanthellae. In Animal Lectins; Vasta, G.R., Moustafa, A., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 231–238. ISBN 978-0-8493-7269-8. [Google Scholar]
- Koike, K.; Jimbo, M.; Sakai, R.; Kaeriyama, M.; Muramoto, K.; Ogata, T.; Maruyama, T.; Kamiya, H. Octocoral chemical signaling selects and controls dinoflagellate symbionts. Biol. Bull. 2004, 207, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Mariscal, R. Nematocysts. In Coelenterate Biology: Reviews and New Perspectives; Muscatin, L., Lenhoff, H., Eds.; Academic Press: New York, NY, USA, 1974; pp. 29–178. [Google Scholar]
- Avian, M.; Mancini, L.; Voltolini, M.; Bonnet, D.; Dreossi, D.; Macaluso, V.; Pillepich, N.; Prieto, L.; Ramšak, A.; Terlizzi, A.; et al. A novel endocast technique providing a 3D quantitative analysis of the gastrovascular system in Rhizostoma pulmo: An unexpected through-gut in cnidaria. PLoS ONE 2022, 17, e0272023. [Google Scholar] [CrossRef]
- Fujita, T.; Matsushita, M.; Endo, Y. The lectin-complement pathway—Its role in innate immunity and evolution. Immunol. Rev. 2004, 198, 185–202. [Google Scholar] [CrossRef]
- Inamori, K.; Saito, T.; Iwaki, D.; Nagira, T.; Iwanaga, S.; Arisaka, F.; Kawabata, S. A newly identified horseshoe crab lectin with specificity for blood group a antigen recognizes specific O-antigens of bacterial lipopolysaccharides. J. Biol. Chem. 1999, 274, 3272–3278. [Google Scholar] [CrossRef]
- Okino, N.; Kawabata, S.; Saito, T.; Hirata, M.; Takagi, T.; Iwanaga, S. Purification, characterization, and cDNA cloning of a 27-kda lectin (l10) from horseshoe crab hemocytes. J. Biol. Chem. 1995, 270, 31008–31015. [Google Scholar] [CrossRef]
- Tagawa, K.; Yoshihara, T.; Shibata, T.; Kitazaki, K.; Endo, Y.; Fujita, T.; Koshiba, T.; Kawabata, S. Microbe-specific C3b deposition in the horseshoe crab complement system in a C2/factor B-dependent or -independent manner. PLoS ONE 2012, 7, e36783. [Google Scholar] [CrossRef]
- Kvennefors, E.C.E.; Leggat, W.; Kerr, C.C.; Ainsworth, T.D.; Hoegh-Guldberg, O.; Barnes, A.C. Analysis of evolutionarily conserved innate immune components in coral links immunity and symbiosis. Dev. Comp. Immunol. 2010, 34, 1219–1229. [Google Scholar] [CrossRef]
- Kimura, A.; Sakaguchi, E.; Nonaka, M. Multi-component complement system of cnidaria: C3, Bf, and MASP genes expressed in the endodermal tissues of a sea anemone, Nematostella Vectensis. Immunobiol. 2009, 214, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Ganot, P.; Moya, A.; Magnone, V.; Allemand, D.; Furla, P.; Sabourault, C. Adaptations to endosymbiosis in a cnidarian-dinoflagellate association: Differential gene expression and specific gene duplications. PLoS Genet. 2011, 7, e1002187. [Google Scholar] [CrossRef] [PubMed]

| siRNA Name | Target mRNA (Accession No.) | Sequences |
|---|---|---|
| AtTL-2 | Attl-2 (AB972924) | CCGCAUAUGCGAGUGACAAdTdT UUGUCACUCGCAUAUGCGGdTdT |
| GFP | Green fluorescent protein (AB626608) | GAGGUGAUCUGGCUAUGUUdTdT AACAUAGCCAGAUCACCUCdTdT |
| random | - | AAUUGGGUCUGUUGAGGCUdTdT AGCCUCAACAGACCCAAUUdTdT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jimbo, M.; Kuniya, N.; Fujimaki, Y.; Yoshikawa, D.; Kamiya, N.; Amano, H.; Yasumoto, K.; Yuyama, I.; Suzuki, G.; Harii, S. A Lectin AtTL-2 Obtained from Acropora aff. tenuis Induced Stimualation of Phagocytosis of Symbiodiniaceae. Microorganisms 2025, 13, 1095. https://doi.org/10.3390/microorganisms13051095
Jimbo M, Kuniya N, Fujimaki Y, Yoshikawa D, Kamiya N, Amano H, Yasumoto K, Yuyama I, Suzuki G, Harii S. A Lectin AtTL-2 Obtained from Acropora aff. tenuis Induced Stimualation of Phagocytosis of Symbiodiniaceae. Microorganisms. 2025; 13(5):1095. https://doi.org/10.3390/microorganisms13051095
Chicago/Turabian StyleJimbo, Mitsuru, Nami Kuniya, Yuna Fujimaki, Daiki Yoshikawa, Naoki Kamiya, Haruna Amano, Ko Yasumoto, Ikuko Yuyama, Go Suzuki, and Saki Harii. 2025. "A Lectin AtTL-2 Obtained from Acropora aff. tenuis Induced Stimualation of Phagocytosis of Symbiodiniaceae" Microorganisms 13, no. 5: 1095. https://doi.org/10.3390/microorganisms13051095
APA StyleJimbo, M., Kuniya, N., Fujimaki, Y., Yoshikawa, D., Kamiya, N., Amano, H., Yasumoto, K., Yuyama, I., Suzuki, G., & Harii, S. (2025). A Lectin AtTL-2 Obtained from Acropora aff. tenuis Induced Stimualation of Phagocytosis of Symbiodiniaceae. Microorganisms, 13(5), 1095. https://doi.org/10.3390/microorganisms13051095





