The Role of ClpV in the Physiology and Pathogenicity of Xanthomonas citri subsp. citri Strain zlm1908
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, and Experimental Plants
2.2. Cloning and Sequencing of clpV Gene from Xcc zlm1908
2.3. Construction of the clpV Deletion Mutant
2.4. Characteristic Analysis of ΔclpV
2.4.1. Genetic Stability Analysis of ΔclpV
2.4.2. Observation of Morphological Characteristics
2.4.3. Growth Curve in Medium and on Leaves
2.4.4. Biofilm Formation and Adhesion Assay
2.4.5. Swarming Motility Analysis
2.4.6. Sensitivity Analysis to Stress and Antibiotics
2.4.7. Extracellular Polysaccharide (EPS) Formation Ability
2.4.8. Extracellular Enzyme Activity
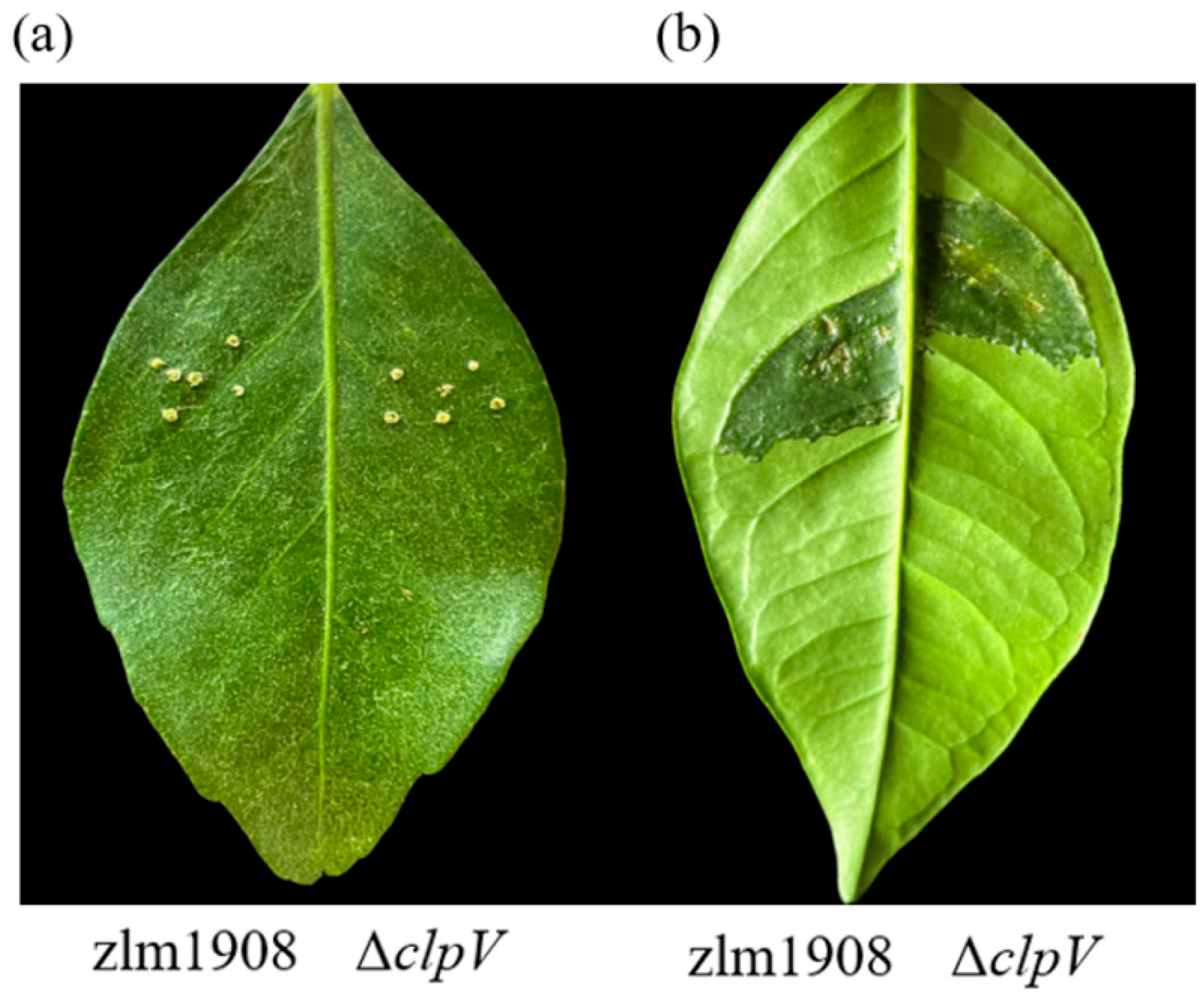
2.4.9. Pathogenicity Assay
2.5. Transcriptome Sequencing
2.5.1. Transcriptome
2.5.2. qRT-PCR Validation
2.6. Statistical Analysis
3. Results
3.1. Cloning of clpV Gene and Construction of ΔclpV
3.2. Characterization of ΔclpV
3.2.1. Genetic Stability of ΔclpV
3.2.2. Morphological Characteristics
3.2.3. Growth Curve
3.2.4. Biofilm Formation and Adhesion Ability
3.2.5. Swarming Motility
3.2.6. Sensitivity to Stress and Antibiotics
3.2.7. Extracellular Polysaccharides Formation
3.2.8. Extracellular Enzyme
3.2.9. Pathogenicity
3.3. Transcriptome Analysis
3.3.1. Identification and Analysis of Differentially Expresses Genes (DEGS)
3.3.2. Gene Ontology Functional Classification of DEGS
3.3.3. The Validation of Transcriptome Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kobayashi, A.K.; Vieira, L.G.E.; Bespalhok Filho, J.C.; Leite, R.P.; Pereira, L.F.P.; Molinari, H.B.C.; Marques, V.V. Enhanced resistance to citrus canker in transgenic sweet orange expressing the sarcotoxin IA gene. Eur. J. Plant Pathol. 2017, 149, 865–873. [Google Scholar] [CrossRef]
- Li, R.F.; Cui, P.; Wei, P.Z.; Liu, X.Y.; Tang, J.L.; Lu, G.T. HprKXcc is a serine kinase that regulates virulence in the Gram-negative phytopathogen Xanthomonas campestris. Environ. Microbiol. 2019, 21, 4504–4520. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.O.; da Silva, J.C.; Soprano, A.S.; Shimo, H.M.; Leme, A.F.P.; Benedetti, C.E. Suppression of citrus canker disease mediated by flagellin perception. Mol. Plant Pathol. 2023, 24, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Asolkar, T.; Ramesh, R. The involvement of the Type Six Secretion System (T6SS) in the virulence of Ralstonia solanacearum on brinjal. 3 Biotech 2020, 10, 324. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, N.; Mannaa, M.; Kim, H.; Park, J.; Jung, H.; Han, G.; Lee, H.H.; Seo, Y.S. Characterization of Type VI Secretion System in Xanthomonas oryzae pv. oryzae and Its Role in Virulence to Rice. Plant Pathol. J. 2020, 36, 289–296. [Google Scholar]
- Kim, N.; Kim, J.J.; Kim, I.; Mannaa, M.; Park, J.; Kim, J.; Lee, H.; Lee, S.; Park, D.; Sul, J.; et al. Type VI secretion systems of plant-pathogenic Burkholderia glumae BGR1 play a functionally distinct role in interspecies interactions and virulence. Mol. Plant Pathol. 2020, 21, 1055–1069. [Google Scholar] [CrossRef]
- Bleumink-Pluym, N.M.; van Alphen, L.B.; Bouwman, L.I.; Wösten, M.M.; van Putten, J.P. Identification of a functional type VI secretion system in Campylobacter jejuni conferring capsule polysaccharide sensitive cytotoxicity. PLoS Pathog. 2013, 9, e1003393. [Google Scholar] [CrossRef]
- Zheng, J.; Leung, K. Dissection of a type VI secretion system in Edwardsiella tarda. Mol. Microbiol. 2007, 66, 1192–1206. [Google Scholar] [CrossRef]
- Li, Y.; Chen, L.; Zhang, P.; Bhagirath, A.Y.; Duan, K. ClpV3 of the H3-Type VI Secretion System (H3-T6SS) Affects Multiple Virulence Factors in Pseudomonas aeruginosa. Front. Microbiol. 2020, 11, 1096. [Google Scholar] [CrossRef]
- Montenegro, B.N.A.; Alvarez, B.A.; Arrieta-Ortiz, M.L.; Rodriguez, R.L.M.; Botero, D.; Tabima, J.F.; Luisa, C.; Cesar, T.; Silvia, R.; Adriana, B. The type VI secretion system of Xanthomonas phaseoli pv. manihotis is involved in virulence and in vitro motility. BMC Microbiol. 2021, 21, 14. [Google Scholar]
- Wu, C.F.; Lin, J.S.; Shaw, G.C.; Lai, E.M. Acid-induced type VI secretion system is regulated by ExoR-ChvG/ChvI signaling cascade in Agrobacterium tumefaciens. PLoS Pathog. 2012, 8, e1002938. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhuang, Z.; Wang, X.; Huang, H.; Fu, Q.; Yan, Q. Dual RNA-seq reveals the effect of the flgM gene of Pseudomonas plecoglossicida on the immune response of Epinephelus coioides. Fish Shellfish Immunol. 2019, 287, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhuang, Z.; Wang, X.; Huang, H.; Fu, Q.; Yan, Q. Dual RNA-Seq reveals the role of a transcriptional regulator gene in pathogen-host interactions between Pseudomonas plecoglossicida and Epinephelus coioides. Fish Shellfish Immunol. 2019, 87, 778–787. [Google Scholar] [CrossRef]
- Chincinska, I.A. Leaf infiltration in plant science: Old method, new possibilities. Plant Methods 2021, 17, 83. [Google Scholar] [CrossRef]
- Yan, Q.; Wang, N. The ColR/ColS two-component system plays multiple roles in the pathogenicity of the citrus canker pathogen Xanthomonas citri subsp. citri. J. Bacteriol. 2011, 193, 1590–1599. [Google Scholar] [CrossRef] [PubMed]
- Gottig, N.; Garavaglia, B.S.; Garofalo, C.G.; Orellano, E.G.; Ottado, J. A filamentous hemagglutinin-like protein of Xanthomonas axonopodis pv. citri, the phytopathogen responsible for citrus canker, is involved in bacterial virulence. PLoS ONE 2009, 4, e4358. [Google Scholar] [CrossRef]
- Guo, Y.; Sagaram, U.S.; Kim, J.S.; Wang, N. Requirement of the galU gene for polysaccharide production by and pathogenicity and growth In Planta of Xanthomonas citri subsp. citri. Appl. Environ. Microbiol. 2010, 76, 2234–2242. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR and 2−ΔΔCt method. Method 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Li, J.; Wang, N. The wxacO gene of Xanthomonas citri ssp. citri encodes a protein with a role in lipopolysaccharide biosynthesis, biofilm formation, stress tolerance and virulence. Mol. Plant Pathol. 2011, 12, 381–396. [Google Scholar] [CrossRef]
- Rigano, L.A.; Siciliano, F.; Enrique, R.; Sendín, L.; Filippone, P.; Torres, P.S.; Qüest, J.; Dow, J.M.; Castagnaro, A.P.; Vojnow, A.A.; et al. Biofilm formation, epiphytic fitness, and canker development in Xanthomonas axonopodis pv. citri. Mol. Plant Microbe Interact. 2007, 20, 1222–1230. [Google Scholar] [CrossRef]
- Büttner, D.; Bonas, U. Regulation and secretion of Xanthomonas virulence factors. FEMS Microbiol. Rev. 2010, 34, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hu, X.; Li, J.; Wang, N. A Novel Periplasmic Protein, VrpA, Contributes to Efficient Protein Secretion by the Type III Secretion System in Xanthomonas spp. Mol. Plant Microbe Interact. 2015, 28, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Si, M.R.; Wang, Y.; Zhang, B.; Zhao, C.; Kang, Y.W.; Bai, H.N.; Wei, D.W.; Zhu, L.F.; Zhang, L.; Dong, T.G. The Type VI Secretion System Engages a Redox-Regulated Dual-Functional Heme Transporter for Zinc Acquisition. Cell Rep. 2017, 20, 949–959. [Google Scholar] [CrossRef]
- Liu, L.; Hao, S.; Lan, R.; Wang, G.; Xiao, D.; Sun, H.; Xu, J. The Type VI Secretion System Modulates Flagellar Gene Expression and Secretion in Citrobacter freundii and Contributes to Adhesion and Cytotoxicity to Host Cells. Infect. Immun. 2015, 83, 2596–2604. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Kostiuk, B.; Rogers, A.; Teschler, J.; Pukatzki, S.; Yildiz, F.H. Rules of Engagement: The Type VI Secretion System in Vibrio cholerae. Trends Microbiol. 2017, 25, 267–279. [Google Scholar] [CrossRef]
- Förster, A.; Planamente, S.; Manoli, E.; Lossi, N.S.; Freemont, P.S.; Filloux, A. Coevolution of the ATPase ClpV, the sheath proteins TssB and TssC, and the accessory protein TagJ/HsiE1 distinguishes type VI secretion classes. J. Biol. Chem. 2014, 289, 33032–33043. [Google Scholar] [CrossRef]
- Douzi, B.; Brunet, Y.R.; Spinelli, S.; Lensi, V.; Legrand, P.; Blangy, S.; Kumar, A.; Journet, L.; Cascales, E.; Cambillau, C. Structure and specificity of the Type VI secretion system ClpV-TssC interaction in enteroaggregative Escherichia coli. Sci. Rep. 2016, 6, 34405. [Google Scholar] [CrossRef] [PubMed]
- Masum, M.M.I.; Yang, Y.; Li, B.; Olaitan, O.S.; Chen, J.; Zhang, Y.; Fang, Y.; Qiu, W.; Wang, Y.; Sun, G. Role of the Genes of Type VI Secretion System in Virulence of Rice Bacterial Brown Stripe Pathogen Acidovorax avenae subsp. avenae Strain RS-2. Int. J. Mol. Sci. 2017, 18, 2024. [Google Scholar] [CrossRef]
- O’Toole, G.; Kaplan, H.B.; Kolter, R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000, 54, 49–79. [Google Scholar] [CrossRef]
- Schell, M.A.; Ulrich, R.L.; Ribot, W.J.; Brueggemann, E.E.; Hines, H.B. Type VI secretion is a major virulence determinant in Burkholderia mallei. Mol. Microbiol. 2007, 64, 1466–1485. [Google Scholar] [CrossRef]
- Soria-Bustos, J.; Ares, M.A.; Gómez-Aldapa, C.A.; González-Y-Merchand, J.A.; Girón, J.A.; De la Cruz, M.A. Two Type VI Secretion Systems of Enterobacter cloacae Are Required for Bacterial Competition, Cell Adherence, and Intestinal Colonization. Front. Microbiol. 2020, 11, 560488. [Google Scholar] [CrossRef] [PubMed]
- Altegoer, F.; Schuhmacher, J.; Pausch, P.; Bange, G. From molecular evolution to biobricks and synthetic modules: A lesson by the bacterial flagellum. Biotechnol. Genet. Eng. Rev. 2014, 30, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial Extracellular Polysaccharides in Biofilm Formation and Function. Microbiol. Spectr. 2015, 3, 223–247. [Google Scholar] [CrossRef] [PubMed]
- Caggianiello, G.; Kleerebezem, M.; Spano, G. Exopolysaccharides produced by lactic acid bacteria: From health-promoting benefits to stress tolerance mechanisms. Appl. Microbiol. Biotechnol. 2016, 100, 3877–3886. [Google Scholar] [CrossRef]
- Yin, R.; Cheng, J.; Lin, J. The role of the type VI secretion system in the stress resistance of plant-associated bacteria. Stress Biol. 2024, 4, 16. [Google Scholar] [CrossRef]
- Wang, N.; Han, N.; Tian, R.; Chen, J.; Gao, X.; Wu, Z.; Liu, Y.; Huang, L. Role of the Type VI Secretion System in the Pathogenicity of Pseudomonas syringae pv. actinidiae, the Causative Agent of Kiwifruit Bacterial Canker. Front. Microbiol. 2021, 12, 627785. [Google Scholar]
- Tian, Y.; Zhao, Y.; Wu, X.; Liu, F.; Hu, B.; Walcott, R.R. The type VI protein secretion system contributes to biofilm formation and seed-to-seedling transmission of Acidovorax citrulli on melon. Mol. Plant Pathol. 2015, 16, 38–47. [Google Scholar] [CrossRef]
- Luo, G.; Xu, X.; Zhao, L.; Qin, Y.; Huang, L.; Su, Y.; Yan, Q. clpV is a key virulence gene during in vivo Pseudomonas plecoglossicida infection. J. Fish Dis. 2019, 42, 991–1000. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, P.; Chen, Y.; Wang, H.; Li, J.; Li, J.; Zhu, G.; Cui, L.; Meng, X. ClpV1 in avian pathogenic Escherichia coli is a crucial virulence factor contributing to meningitis in a mouse model in vivo. Vet. Microbiol. 2021, 263, 109273. [Google Scholar] [CrossRef]
- Chen, S.; Du, F.; Shang, K.; Chen, H.; Guo, R.; Liao, C.; Jia, Y.; Yu, Z.; Li, J.; Zhang, C.; et al. Colonization Mediated by T6SS-ClpV Disrupts Host Gut Microbiota and Enhances Virulence of Salmonella enterica serovar Typhimurium. J. Agric. Food Chem. 2024, 72, 19155–19166. [Google Scholar] [CrossRef]
- Lundgren, B.R.; Shoytush, J.M.; Scheel, R.A.; Sain, S.; Sarwar, Z.; Nomura, C.T. Utilization of L-glutamate as a preferred or sole nutrient in Pseudomonas aeruginosa PAO1 depends on genes encoding for the enhancer-binding protein AauR, the sigma factor RpoN and the transporter complex AatJQMP. BMC Microbiol. 2021, 21, 83. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Guo, X.; Li, J.; Li, Y.; Sun, H.; Li, A.; Cao, B. RpoN is required for the motility and contributes to the killing ability of Plesiomonas shigelloides. BMC Microbiol. 2022, 22, 299. [Google Scholar] [CrossRef] [PubMed]





| Strains and Plasmids | Relevant Characteristic | Source or Reference |
|---|---|---|
| Xcc strains | ||
| zlm1908 | Wild type, isolated from the leaf of diseased Citrus sinensis ‘hongjiang’ | Stored in our lab |
| ΔclpV | zlm1908, in-frame deletion of clpV51-1432 | This study |
| C-clpV | ΔclpV with clpV-pBAD33CM-rp4 Recombinant vector | This study |
| E. coli strains | ||
| DH5α | Competent cell | Takara |
| β2163 | Competent cell | Takara |
| Plasmids | ||
| pLP12 | Suicide vector, Cm+ | This study |
| pBAD33 | Suicide vector, Cm+ | This study |
| pMD18-T | Cloning vector, Amp+ | Takara |
| Primer Name | Primer Sequence (5′-3′) |
|---|---|
| Primers for construction of the clpV deletion mutant | |
| clpV-CF/CR | TGTGGAATTGGTGCATTGGC/TGCATCGCGAGATCACTAGC |
| clpV-F/R | ACCGCATGCTGAGCCTGTTC/CAAGGGTTCATTAACTCGGG |
| clpV-F/R | ACCGCATGCTGAGCCTGTTC/CAAGGGTTCATTAACTCGGG |
| clpV-UF | GGAATCTAGACCTTGAGTCGCGAGCGACCAGTTCCGTGCG |
| clpV-UR | CATAGGTTGCCATTCTTAGTGAACAGGGAGCTGCGGGAGA |
| clpV-DF | TCTCCCGCAGCTCCCTGTTCACTAAGAATGGCAACCTATG |
| clpV-DR | ACAGCTAGCGACGATATGTCTCAAAGACCAGACCCCAGAC |
| pLP-UF/UR | GACACAGTTGTAACTGGTCCA/CAGGAACACTTAACGGCTGAC |
| pLP-UTF/UTR | CAGGAACACTTAACGGCTGAC/CAGGAATCTAGACCTTGAGTCG |
| Primers for qRT-PCR | |
| flgH-F/R | TGAACCTGGTCGAAAGCACC/TTCTCAAGCACGTTCAAGCC |
| fliM-F/R | TGATCTGCTTTCCCAGGACG/TCCTGGCTGGACAGATCGTA |
| mcp-F/R | AGTCGCTCATCACGTCCATC/CAGGAACACTTAACGGCTGAC |
| mexH-F/R | CAGCACCTACGTCAACGACT/AATGGTCGATGTCCTGTGGG |
| OD600 | Pinprick | Infiltration | ||||
|---|---|---|---|---|---|---|
| ΔclpV | WT | C-clpV | ΔclpV | WT | C-clpV | |
| 0.1 | 5.24 ± 0.01 | 5.26 ± 0.01 | 5.23 ± 0.01 | 75.47 ± 2.15 | 76.19 ± 3.32 | 78.32 ± 3.65 |
| 0.2 | 5.56 ± 0.02 | 5.62 ± 0.03 | 5.57 ± 0.02 | 79.32 ± 6.12 | 83.19 ± 4.65 | 84.33 ± 4.76 |
| 0.5 | 5.44 ± 0.02 | 5.65 ± 0.03 | 5.54 ± 0.03 | 80.32 ± 6.43 | 83.16 ± 7.21 | 82.76 ± 4.68 |
| 0.7 | 5.97 ± 0.03 | 5.92 ± 0.04 | 5.88 ± 0.02 | 83.43 ± 7.53 | 82.65 ± 8.32 | 89.54 ± 8.32 |
| 0.9 | 6.09 ± 0.04 | 6.24 ± 0.04 | 6.13 ± 0.05 | 85.43 ± 9.02 | 82.14 ± 10.32 | 86.45 ± 9.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wu, Z.; Liu, D.; Cong, K.; Dai, J.; Xu, W.; Ke, Y.; He, X. The Role of ClpV in the Physiology and Pathogenicity of Xanthomonas citri subsp. citri Strain zlm1908. Microorganisms 2024, 12, 2536. https://doi.org/10.3390/microorganisms12122536
Li Y, Wu Z, Liu D, Cong K, Dai J, Xu W, Ke Y, He X. The Role of ClpV in the Physiology and Pathogenicity of Xanthomonas citri subsp. citri Strain zlm1908. Microorganisms. 2024; 12(12):2536. https://doi.org/10.3390/microorganisms12122536
Chicago/Turabian StyleLi, Ya, Zilin Wu, Dengyan Liu, Kexin Cong, Jiajun Dai, Wenjie Xu, Yingtong Ke, and Xinyi He. 2024. "The Role of ClpV in the Physiology and Pathogenicity of Xanthomonas citri subsp. citri Strain zlm1908" Microorganisms 12, no. 12: 2536. https://doi.org/10.3390/microorganisms12122536
APA StyleLi, Y., Wu, Z., Liu, D., Cong, K., Dai, J., Xu, W., Ke, Y., & He, X. (2024). The Role of ClpV in the Physiology and Pathogenicity of Xanthomonas citri subsp. citri Strain zlm1908. Microorganisms, 12(12), 2536. https://doi.org/10.3390/microorganisms12122536





