Soil Microbial Communities Changes Along Depth and Contrasting Facing Slopes at the Parque Nacional La Campana, Chile
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling Collection
2.2. Soil Sampling
2.3. Vegetation Coverage and Environmental Conditions in PNLC
2.4. Soil Physicochemical Analysis
2.5. DNA Extraction and Sequencing of Phylogenetic Markers of Prokaryotes and Fungi
2.6. Analysis of Microbial Composition and Statistical Analyses
3. Results
3.1. Environmental and Soil Physicochemical Conditions
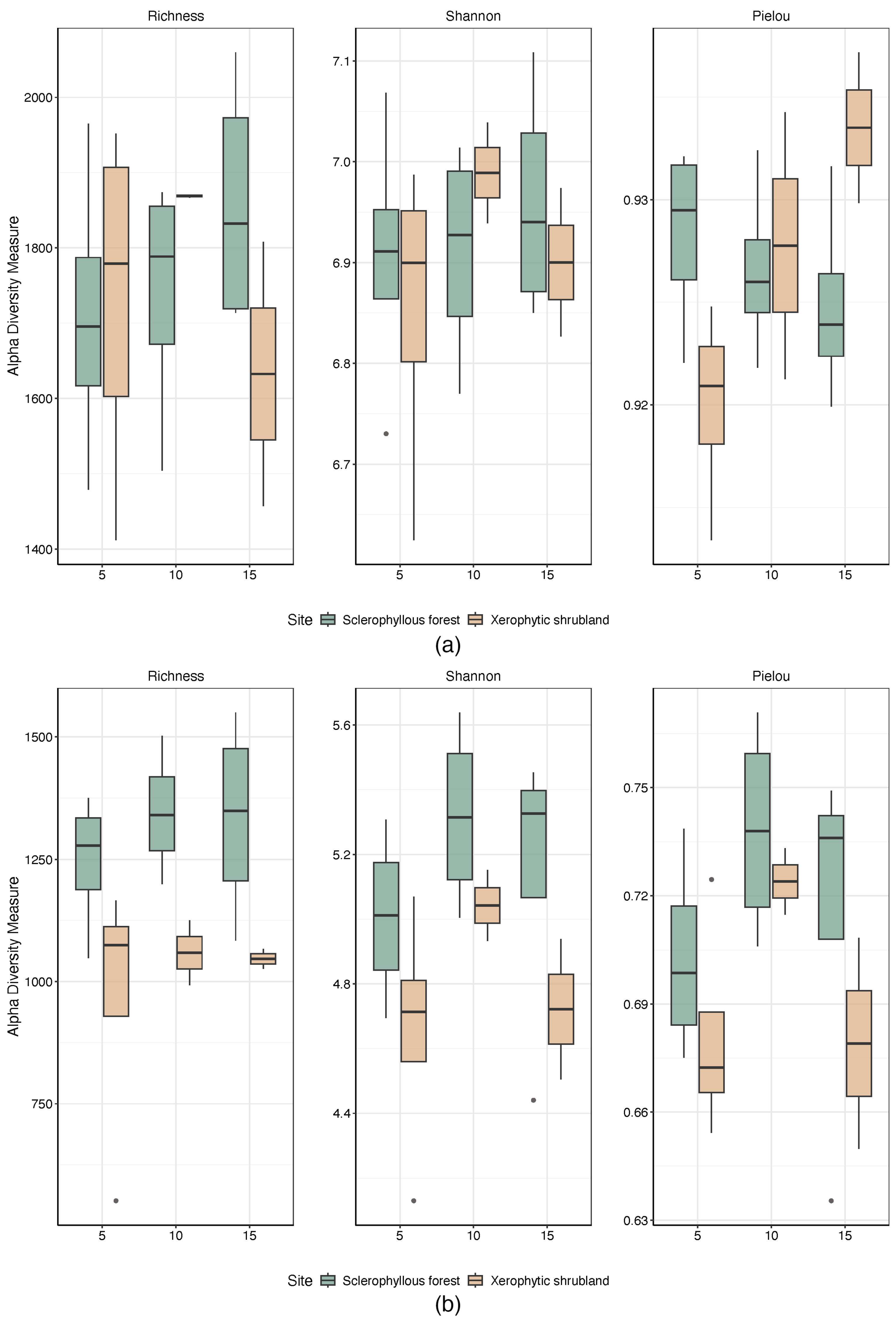
3.2. Microbial Community Alpha and Beta Diversity Spatial Changes (Site and Depth)
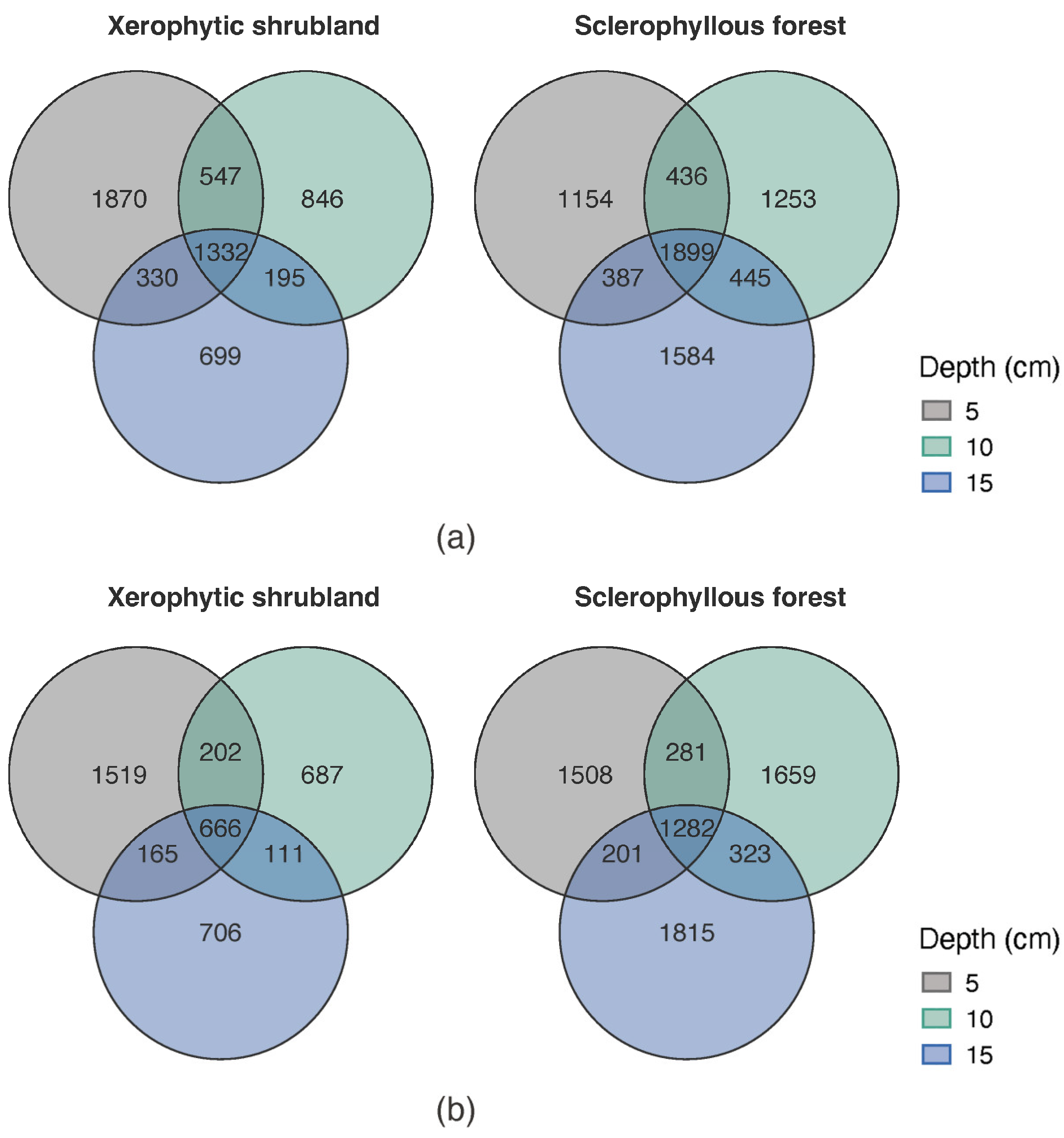
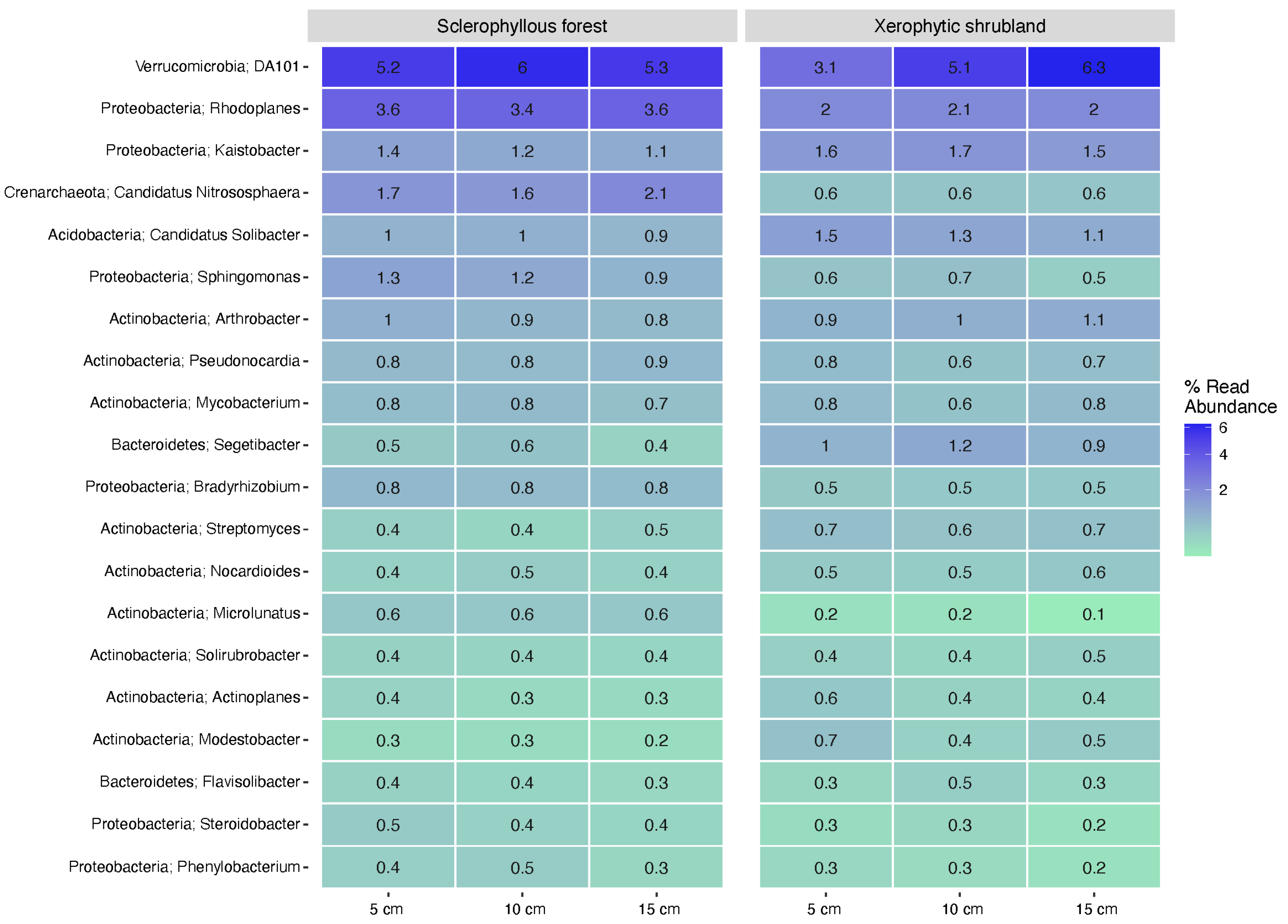
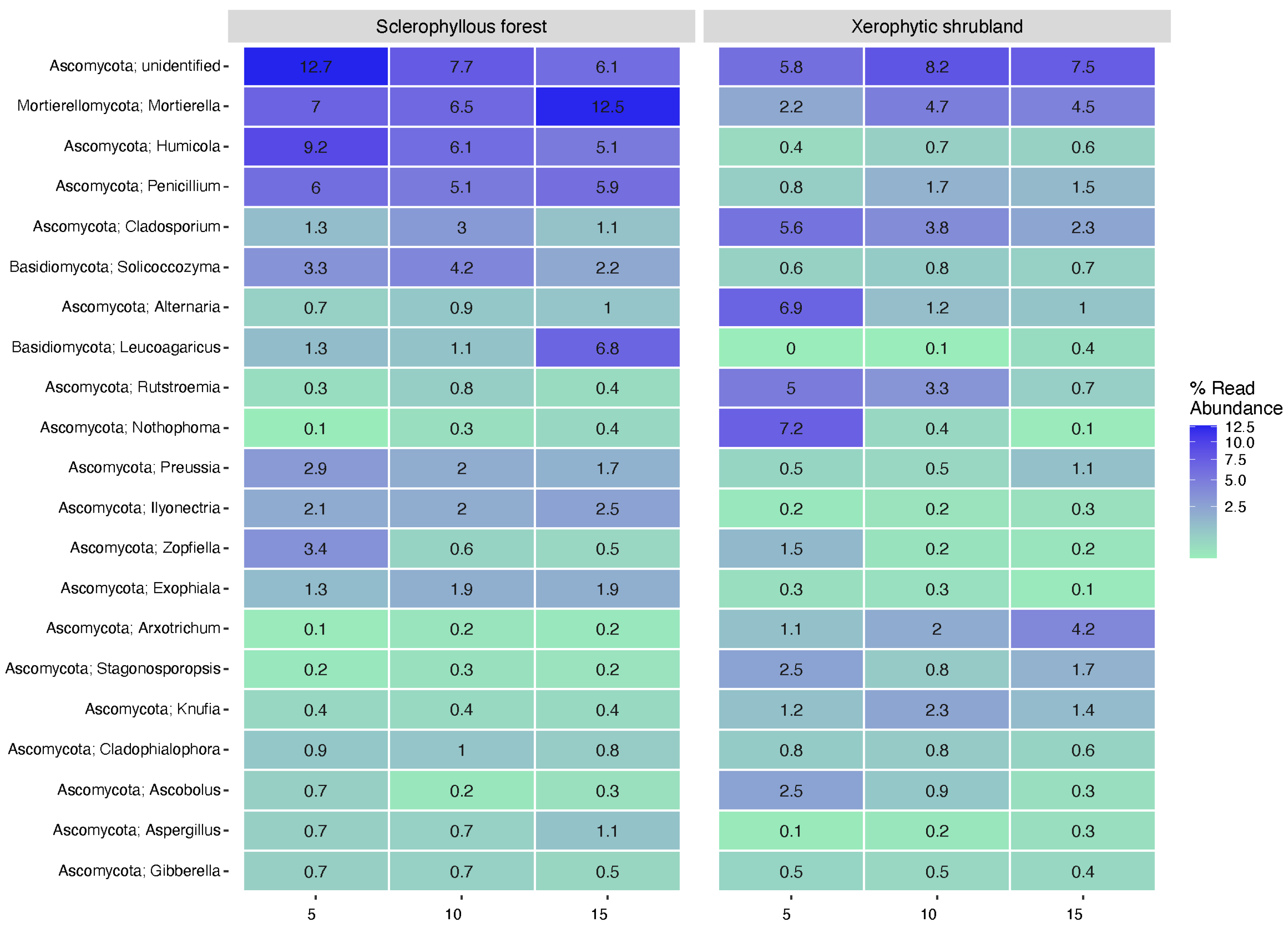
3.3. Microbial Community Composition Spatial Changes (Depth and Sites)
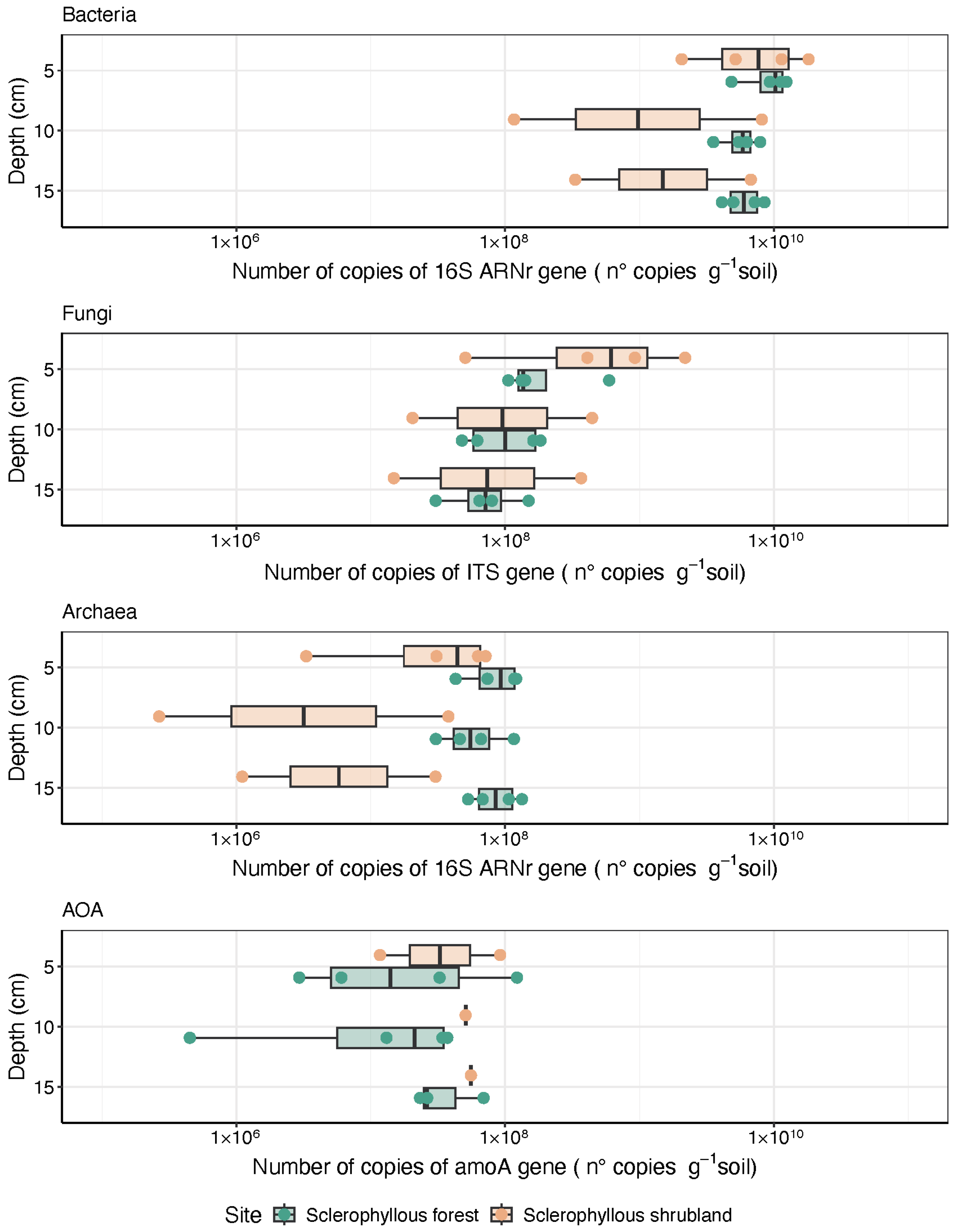
3.4. Microbial Community Abundance and Functional Potential Traits with Depth
4. Discussion
4.1. Spatial Changes in the Microbial Community Composition
4.2. Soil Conditions and Vegetation as Drivers of Microbial Community Spatial Changes Traits in the Soils of PNLC
4.3. Functional and Ecological Microbial Community Potential in PNLC
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopez-Sangil, L.; Rousk, J.; Wallander, H.; Casals, P. Microbial Growth Rate Measurements Reveal That Land-Use Abandonment Promotes a Fungal Dominance of SOM Decomposition in Grazed Mediterranean Ecosystems. Biol. Fertil. Soils 2011, 47, 129–138. [Google Scholar] [CrossRef]
- Maharning, A.R.; Mills, A.A.S.; Adl, S.M. Soil Community Changes during Secondary Succession to Naturalized Grasslands. Appl. Soil Ecol. 2009, 41, 137–147. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going Back to the Roots: The Microbial Ecology of the Rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E. Growth of Saprotrophic Fungi and Bacteria in Soil. FEMS Microbiol. Ecol. 2011, 78, 17–30. [Google Scholar] [CrossRef]
- Zornoza, R.; Acosta, J.A.; Faz, A.; Bååth, E. Microbial Growth and Community Structure in Acid Mine Soils after Addition of Different Amendments for Soil Reclamation. Geoderma 2016, 272, 64–72. [Google Scholar] [CrossRef]
- Custódio, V.; Gonin, M.; Stabl, G.; Bakhoum, N.; Oliveira, M.M.; Gutjahr, C.; Castrillo, G. Sculpting the soil microbiota. Plant J. 2021, 109, 508–522. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the Unknown: Disentangling the Complexities of the Soil Microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Macdonald, C.A.; Thomas, N.; Robinson, L.; Tate, K.R.; Ross, D.J.; Dando, J.; Singh, B.K. Physiological, Biochemical and Molecular Responses of the Soil Microbial Community after Afforestation of Pastures with Pinus radiata. Soil Biol. Biochem. 2009, 41, 1642–1651. [Google Scholar] [CrossRef]
- Zornoza, R.; Guerrero, C.; Mataix-Solera, J.; Scow, K.M.; Arcenegui, V.; Mataix-Beneyto, J. Changes in Soil Microbial Community Structure Following the Abandonment of Agricultural Terraces in Mountainous Areas of Eastern Spain. Appl. Soil Ecol. 2009, 42, 315–323. [Google Scholar] [CrossRef]
- Aponte, H.; Galindo-Castañeda, T.; Yáñez, C.; Hartmann, M.; Rojas, C. Microbial Community-Level Physiological Profiles and Genetic Prokaryotic Structure of Burned Soils Under Mediterranean Sclerophyll Forests in Central Chile. Front. Microbiol. 2022, 13, 824813. [Google Scholar] [CrossRef] [PubMed]
- Escobedo, V.M.; Acuña-Rodríguez, I.S.; García, L.Y.; Torres-Díaz, C.; Atala, C.; Suazo, M.J.; Gómez-González, S.; Newsham, K.K.; Molina-Montenegro, M.A. Native Woody Species Depend on the Soil Microbiome to Establish on Burned Soils, While Non-native Do Not. J. Appl. Ecol. 2024. [Google Scholar] [CrossRef]
- Egli, M.; Sartori, G.; Mirabella, A.; Favilli, F.; Giaccai, D.; Delbos, E. Effect of North and South Exposure on Organic Matter in High Alpine Soils. Geoderma 2009, 149, 124–136. [Google Scholar] [CrossRef]
- Ascher, J.; Sartori, G.; Graefe, U.; Thornton, B.; Ceccherini, M.T.; Pietramellara, G.; Egli, M. Are Humus Forms, Mesofauna and Microflora in Subalpine Forest Soils Sensitive to Thermal Conditions? Biol. Fertil. Soils 2012, 48, 709–725. [Google Scholar] [CrossRef]
- Baldrian, P. Forest Microbiome: Diversity, Complexity and Dynamics. FEMS Microbiol. Rev. 2016, 41, fuw040. [Google Scholar] [CrossRef] [PubMed]
- López-Mondéjar, R.; Voříšková, J.; Větrovský, T.; Baldrian, P. The Bacterial Community Inhabiting Temperate Deciduous Forests Is Vertically Stratified and Undergoes Seasonal Dynamics. Soil Biol. Biochem. 2015, 87, 43–50. [Google Scholar] [CrossRef]
- Ekblad, A.; Wallander, H.; Godbold, D.L.; Cruz, C.; Johnson, D.; Baldrian, P.; Björk, R.G.; Epron, D.; Kieliszewska-Rokicka, B.; Kjøller, R.; et al. The Production and Turnover of Extramatrical Mycelium of Ectomycorrhizal Fungi in Forest Soils: Role in Carbon Cycling. Plant Soil 2013, 366, 1–27. [Google Scholar] [CrossRef]
- Schulze-Makuch, D.; Wagner, D.; Kounaves, S.P.; Mangelsdorf, K.; Devine, K.G.; de Vera, J.-P.; Schmitt-Kopplin, P.; Grossart, H.-P.; Parro, V.; Kaupenjohann, M.; et al. Transitory Microbial Habitat in the Hyperarid Atacama Desert. Proc. Natl. Acad. Sci. USA 2018, 115, 2670–2675. [Google Scholar] [CrossRef]
- Eilers, K.G.; Debenport, S.; Anderson, S.; Fierer, N. Digging Deeper to Find Unique Microbial Communities: The Strong Effect of Depth on the Structure of Bacterial and Archaeal Communities in Soil. Soil Biol. Biochem. 2012, 50, 58–65. [Google Scholar] [CrossRef]
- Bernhard, N.; Moskwa, L.M.; Schmidt, K.; Oeser, R.A.; Aburto, F.; Bader, M.Y.; Baumann, K.; von Blanckenburg, F.; Boy, J.; van den Brink, L.; et al. Pedogenic and Microbial Interrelations to Regional Climate and Local Topography: New Insights from a Climate Gradient (Arid to Humid) along the Coastal Cordillera of Chile. Catena 2018, 170, 335–355. [Google Scholar] [CrossRef]
- Oeser, R.A.; Stroncik, N.; Moskwa, L.M.; Bernhard, N.; Schaller, M.; Canessa, R.; van den Brink, L.; Köster, M.; Brucker, E.; Stock, S.; et al. Chemistry and Microbiology of the Critical Zone along a Steep Climate and Vegetation Gradient in the Chilean Coastal Cordillera. Catena 2018, 170, 183–203. [Google Scholar] [CrossRef]
- Rodriguez, V.; Moskwa, L.M.; Oses, R.; Kühn, P.; Riveras-Muñoz, N.; Seguel, O.; Scholten, T.; Wagner, D. Impact of Climate and Slope Aspects on the Composition of Soil Bacterial Communities Involved in Pedogenetic Processes along the Chilean Coastal Cordillera. Microorganisms 2022, 10, 847. [Google Scholar] [CrossRef] [PubMed]
- Quinteros-Urquieta, C.; Francois, J.-P.; Aguilar-Muñoz, P.; Orellana, R.; Villaseñor, R.; Moreira-Muñoz, A.; Molina, V. Microbial Diversity of Soil in a Mediterranean Biodiversity Hotspot: Parque Nacional La Campana, Chile. Microorganisms 2024, 12, 1569. [Google Scholar] [CrossRef] [PubMed]
- Hauck, L.; Moreira-Muñoz, A.; Nezadal, W. La Flora Exótica Ruderal Del Parque Nacional La Campana, Región de Valparaíso, Chile Central. Gayana Bot. 2016, 73, 206–219. [Google Scholar] [CrossRef]
- Norman, M. Biodiversity Hotspots Revisited. Bioscience 2003, 53, 916–917. [Google Scholar] [CrossRef]
- Mittermeier, R.; Myers, N.; Gill, P.; Mittermeier, C. Hotspots: Earth’s Biologically Richest and Most Endangered Terrestrial Ecoregions. J. Mammal. 2002, 83, 630–633. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla, V. Situación Postincendio En Ecosistemas de Un Macizo Montañoso de Gran Valor Geobotánico, En La Cordillera Meditarránea de Chile. Cad. Geografia. Coimbra FLUC 2009, 28/29, 149–150. [Google Scholar]
- Elórtegui, S.; Moreira-Muñoz, A. Parque Nacional La Campana: Origen de Una Reserva de La Biósfera En Chile Central, 2nd ed.; Elórtegui, S., Moreira-Muñoz, A., Eds.; Taller la Era: Santiago, Chile, 2009. [Google Scholar]
- Quintanilla, V.G.; Cadiñanos, J.A.; Latasa, I.; Lozano, P.J. Aproximación Biogeográfica a Los Bosques de La Zona Mediterránea de Chile: Caracterización e Inventario. Boletín Asoc. Geógrafos Españoles 2012, 60, 91–114. [Google Scholar] [CrossRef]
- Luebert, F.; Pliscoff, P. Sinopsis Bioclimática y Vegetacional de Chile; Editorial Universitaria: Santiago, Chile, 2006. [Google Scholar]
- Dierschke, H. Pflanzensoziologie: Grundlagen und Methoden; Ulmer: Stuttgart, Germany, 1994. [Google Scholar]
- Knapp, R. Sampling Methods and Taxon Analysis in Vegetation Science; Springer: Dordrecht, The Netherlands, 1984; Volume 4. [Google Scholar]
- San Martín, C.; Ramírez, C.; San Martín, J.; Villaseñor, R. Flora y vegetacion del estero Reñaca (V Region, Chile). Gayana Bot. 2001, 58. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture (USDA), Soil Survey Laboratory Methods Manual. Soil Survey Investigations Report No 42; Washington DC. 2004. Available online: https://www.nrcs.usda.gov/sites/default/files/2023-01/SSIR42.pdf (accessed on 1 September 2024).
- Al-Shammary, A.A.G.; Kouzani, A.Z.; Kaynak, A.; Khoo, S.Y.; Norton, M.; Gates, W. Soil Bulk Density Estimation Methods: A Review. Pedosphere 2018, 28, 581–596. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Costello, E.K.; Berg-Lyons, D.; Gonzalez, A.; Stombaugh, J.; Knights, D.; Gajer, P.; Ravel, J.; Fierer, N.; et al. Moving Pictures of the Human Microbiome. Genome Biol. 2011, 12, R50. [Google Scholar] [CrossRef] [PubMed]
- Degnan, P.H.; Ochman, H. Illumina-Based Analysis of Microbial Community Diversity. ISME J. 2012, 6, 183–194. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a Chimera-Checked 16S RRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef]
- Abarenkov, K.; Zirk, A.; Piirmann, T.; Pöhönen, R.; Ivanov, F.; Nilsson, R.H.; Kõljalg, U. UNITE QIIME release for Fungi 2. Version 10.05.2021. UNITE Community 2021, 7, 1264763. [Google Scholar]
- Team, R.C. A Language and Environment for Statistical Computing. 2023. Available online: https://www.r-project.org/ (accessed on 1 September 2024).
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Lahti, L.; Shetty, S. Introduction to the Microbiome R Package. 2018. Available online: https://microbiome.github.io/tutorials (accessed on 1 September 2024).
- Xu, S.; Zhan, L.; Tang, W.; Wang, Q.; Dai, Z.; Zhou, L.; Feng, T.; Chen, M.; Wu, T.; Hu, E.; et al. MicrobiotaProcess: A comprehensive R package for deep mining microbiome. Innovation 2023, 4, 100388. [Google Scholar] [CrossRef]
- Andersen, K.S.; Kirkegaard, R.H.; Karst, S.M.; Albertsen, M. Ampvis2: An R Package to Analyse and Visualise 16S RRNA Amplicon Data. bioRxiv 2018, 299537. [Google Scholar] [CrossRef]
- Oksanen, J. Design Decisions and Implementation Details in Vegan. Vignette Package Vegan R Package Version 2016, 2016, 2–4. [Google Scholar]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Liu, C.; Cui, Y.; Li, X.; Yao, M. Microeco: An R Package for Data Mining in Microbial Community Ecology. FEMS Microbiol. Ecol. 2021, 97, fiaa255. [Google Scholar] [CrossRef] [PubMed]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling Function and Taxonomy in the Global Ocean Microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An Open Annotation Tool for Parsing Fungal Community Datasets by Ecological Guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Casanova, M.; Salazar, O.; Seguel, O.; Luzio, W. The Soils of Chile; Springer: Dordrecht, The Netherlands, 2013; ISBN 978-94-007-5948-0. [Google Scholar]
- Luebert, F.; Muñoz Schick, M.; Moreira-Muñoz, A. Vegetación y Flora. In Parque Nacional La Campana: Origen de una Reserva de la biósfera en Chile Central; Elórtegui, S., Moreira-Muñoz, A., Eds.; Taller La Era: Viña del Mar, Chile, 2009. [Google Scholar]
- Graham, R.C.; Buol, S.W. Soil-Geomorphic Relations on the Blue Ridge Front: II. Soil Characteristics and Pedogenesis. Soil Sci. Soc. Am. J. 1990, 54, 1367–1377. [Google Scholar] [CrossRef]
- Barbosa, W.R.; Romero, R.E.; de Souza Júnior, V.S.; Cooper, M.; Sartor, L.R.; de Moya Partiti, C.S.; Jorge, F.d.O.; Cohen, R.; de Jesus, S.L.; Ferreira, T.O. Effects of Slope Orientation on Pedogenesis of Altimontane Soils from the Brazilian Semi-Arid Region (Baturité Massif, Ceará). Environ. Earth Sci. 2015, 73, 3731–3743. [Google Scholar] [CrossRef]
- Bao, Y.; Dolfing, J.; Guo, Z.; Chen, R.; Wu, M.; Li, Z.; Lin, X.; Feng, Y. Important Ecophysiological Roles of Non-Dominant Actinobacteria in Plant Residue Decomposition, Especially in Less Fertile Soils. Microbiome 2021, 9, 84. [Google Scholar] [CrossRef]
- Bardelli, T.; Gómez-Brandón, M.; Ascher-Jenull, J.; Fornasier, F.; Arfaioli, P.; Francioli, D.; Egli, M.; Sartori, G.; Insam, H.; Pietramellara, G. Effects of Slope Exposure on Soil Physico-Chemical and Microbiological Properties along an Altitudinal Climosequence in the Italian Alps. Sci. Total Environ. 2017, 575, 1041–1055. [Google Scholar] [CrossRef]
- Ward, L.M.; Hemp, J.; Shih, P.M.; McGlynn, S.E.; Fischer, W.W. Evolution of Phototrophy in the Chloroflexi Phylum Driven by Horizontal Gene Transfer. Front. Microbiol. 2018, 9, 260. [Google Scholar] [CrossRef]
- Frenk, S.; Hadar, Y.; Minz, D. Resilience of Soil Bacterial Community to Irrigation with Water of Different Qualities under M Editerranean Climate. Environ. Microbiol. 2014, 16, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Pii, Y.; Borruso, L.; Brusetti, L.; Crecchio, C.; Cesco, S.; Mimmo, T. The Interaction between Iron Nutrition, Plant Species and Soil Type Shapes the Rhizosphere Microbiome. Plant Physiol. Biochem. 2016, 99, 39–48. [Google Scholar] [CrossRef]
- Shen, C.; Ge, Y.; Yang, T.; Chu, H. Verrucomicrobial Elevational Distribution Was Strongly Influenced by Soil PH and Carbon/Nitrogen Ratio. J. Soils Sediments 2017, 17, 2449–2456. [Google Scholar] [CrossRef]
- Siles, J.A.; Rachid, C.T.C.C.; Sampedro, I.; García-Romera, I.; Tiedje, J.M. Microbial Diversity of a Mediterranean Soil and Its Changes after Biotransformed Dry Olive Residue Amendment. PLoS ONE 2014, 9, e103035. [Google Scholar] [CrossRef]
- Egidi, E.; Wood, J.L.; Celestina, C.; May, T.W.; Mele, P.; Edwards, J.; Powell, J.; Bissett, A.; Franks, A.E. Delving into the Dark Ecology: A Continent-Wide Assessment of Patterns of Composition in Soil Fungal Communities from Australian Tussock Grasslands. Fungal Ecol. 2019, 39, 356–370. [Google Scholar] [CrossRef]
- Egidi, E.; Delgado-Baquerizo, M.; Plett, J.M.; Wang, J.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K. A Few Ascomycota Taxa Dominate Soil Fungal Communities Worldwide. Nat. Commun. 2019, 10, 2369. [Google Scholar] [CrossRef]
- He, M.-Q.; Zhao, R.-L.; Liu, D.-M.; Denchev, T.T.; Begerow, D.; Yurkov, A.; Kemler, M.; Millanes, A.M.; Wedin, M.; McTaggart, A.R.; et al. Species Diversity of Basidiomycota. Fungal Divers. 2022, 114, 281–325. [Google Scholar] [CrossRef]
- Schüβler, A.; Schwarzott, D.; Walker, C. A New Fungal Phylum, the Glomeromycota: Phylogeny and Evolution. Mycol. Res. 2001, 105, 1413–1421. [Google Scholar] [CrossRef]
- Gleason, F.H.; Letcher, P.M.; Commandeur, Z.; Jeong, C.E.; McGee, P.A. The Growth Response of Some Chytridiomycota to Temperatures Commonly Observed in the Soil. Mycol. Res. 2005, 109, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Hanrahan-Tan, D.G.; Lilje, O.; Henderson, L. Chytrids in Soil Environments: Unique Adaptations and Distributions. Encyclopedia 2023, 3, 642–664. [Google Scholar] [CrossRef]
- Vega, F.E.; Meyling, N.V.; Luangsa-ard, J.J.; Blackwell, M. Fungal Entomopathogens. In Insect Pathology; Elsevier: Amsterdam, The Netherlands, 2012; pp. 171–220. [Google Scholar]
- Nahidan, S.; Nourbakhsh, F.; Mosaddeghi, M.R. Variation of Soil Microbial Biomass C and Hydrolytic Enzyme Activities in a Rangeland Ecosystem: Are Slope Aspect and Position Effective? Arch. Agron. Soil Sci. 2015, 61, 797–811. [Google Scholar] [CrossRef]
- Aponte, C.; Marañón, T.; García, L.V. Microbial C, N and P in Soils of Mediterranean Oak Forests: Influence of Season, Canopy Cover and Soil Depth. Biogeochemistry 2010, 101, 77–92. [Google Scholar] [CrossRef]
- Lucas-Borja, M.E.; Hedo de Santiago, J.; Yang, Y.; Shen, Y.; Candel-Pérez, D. Nutrient, Metal Contents and Microbiological Properties of Litter and Soil along a Tree Age Gradient in Mediterranean Forest Ecosystems. Sci. Total Environ. 2019, 650, 749–758. [Google Scholar] [CrossRef]
- Šnajdr, J.; Valášková, V.; Merhautová, V.; Herinková, J.; Cajthaml, T.; Baldrian, P. Spatial Variability of Enzyme Activities and Microbial Biomass in the Upper Layers of Quercus Petraea Forest Soil. Soil Biol. Biochem. 2008, 40, 2068–2075. [Google Scholar] [CrossRef]
- Shahin, O.; Paul, N.M.-S.; Rambal, S.; Joffre, R.; Richard, F. Ectomycorrhizal Fungal Diversity in Quercus Ilex Mediterranean Woodlands: Variation among Sites and over Soil Depth Profiles in Hyphal Exploration Types, Species Richness and Community Composition. Symbiosis 2013, 61, 1–12. [Google Scholar] [CrossRef]
- O’Brien, H.E.; Parrent, J.L.; Jackson, J.A.; Moncalvo, J.-M.; Vilgalys, R. Fungal Community Analysis by Large-Scale Sequencing of Environmental Samples. Appl. Environ. Microbiol. 2005, 71, 5544–5550. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.-R.; Doyle, A.; Holden, P.A.; Schimel, J.P. Drying and Rewetting Effects on C and N Mineralization and Microbial Activity in Surface and Subsurface California Grassland Soils. Soil Biol. Biochem. 2008, 40, 2281–2289. [Google Scholar] [CrossRef]
- Kuikman, P.; Van Vuure, M.; Van Veen, J. Effect of soil moisture regime on predation by protozoa of bacterial biomass and the release of bacterial nitrogen. Agric. Ecosyst. Environ. 1989, 27, 271–279. [Google Scholar] [CrossRef]
- Augusto, L.; Ranger, J.; Binkley, D.; Rothe, A. Impact of several common tree species of European temperate forests on soil fertility. Ann. For. Sci. 2002, 59, 233–253. [Google Scholar] [CrossRef]
- Billore, S.K.; Ohsawa, M.; Numata, M.; Okano, S. Microbial biomass nitrogen pool in soils from a warm temperate grassland, and from deciduous and evergreen forests in Chiba, central Japan. Biol. Fertil. Soils 1995, 19, 124–128. [Google Scholar] [CrossRef]
- Schumann, P. Conexibacteraceae. In Bergey’s Manual of Systematics of Archaea and Bacteria; Wiley: Hoboken, NJ, USA, 2015; pp. 1–3. [Google Scholar]
- Eo, J.; Park, K.-C.; Kim, M.-H. Plant-Specific Effects of Sunn Hemp (Crotalaria juncea) and Sudex (Sorghum bicolor × Sorghum bicolor var. Sudanense) on the Abundance and Composition of Soil Microbial Community. Agric. Ecosyst. Environ. 2015, 213, 86–93. [Google Scholar] [CrossRef]
- Zou, J.; Dong, Y.; Wang, H.; Liang, W.; Li, D. Identification and Characterization of Nothophoma Quercina Causing Bud Blight on Photinia × Fraseri in China. Plant Dis. 2021, 105, 1356–1364. [Google Scholar] [CrossRef]
- Lombard, L.; van der Merwe, N.A.; Groenewald, J.Z.; Crous, P.W. Generic Concepts in Nectriaceae. Stud. Mycol. 2015, 80, 189–245. [Google Scholar] [CrossRef] [PubMed]
- Ahmadpour, S.A.; Mehrabi-Koushki, M.; Farokhinejad, R.; Asgari, B.; Javadi Estahbanati, A.; Mirabolfathy, M.; Rahnama, K. New Records of Fungal Species of the Family Didymellaceae from Iran. Mycol. Iran. 2021, 8, 119–133. [Google Scholar]
- Hongsanan, S.; Hyde, K.D.; Phookamsak, R.; Wanasinghe, D.N.; McKenzie, E.H.C.; Sarma, V.V.; Lücking, R.; Boonmee, S.; Bhat, J.D.; Liu, N.-G.; et al. Refined Families of Dothideomycetes: Orders and Families Incertae sedis in Dothideomycetes. Fungal Divers. 2020, 105, 17–318. [Google Scholar] [CrossRef]
- Tahtamouni, M.E.; Khresat, S.; Lucero, M.; Sigala, J.; Unc, A. Diversity of Endophytes across the Soil-Plant Continuum for Atriplex spp. in Arid Environments. J. Arid Land 2016, 8, 241–253. [Google Scholar] [CrossRef]
- Schulz, B.; Boyle, C. The Endophytic Continuum. Mycol. Res. 2005, 109, 661–686. [Google Scholar] [CrossRef]
- Hou, L.W.; Groenewald, J.Z.; Pfenning, L.H.; Yarden, O.; Crous, P.W.; Cai, L. The Phoma-like Dilemma. Stud. Mycol. 2020, 96, 309–396. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Jiang, J.R.; Zhang, G.Z.; Cai, L.; Crous, P.W. Resolving the Phoma Enigma. Stud. Mycol. 2015, 82, 137–217. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.; Zhou, J.; Chen, L.; Li, Y.-X.; Yang, A.-L.; Dong, X.-F.; Zhang, H.-B. Virulence and Community Dynamics of Fungal Species with Vertical and Horizontal Transmission on a Plant with Multiple Infections. PLoS Pathog. 2021, 17, e1009769. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xu, L.; Bai, X.; Zhang, G.; Zhang, M.; Huang, Y. Differential Fungal Assemblages and Functions between the Plastisphere of Biodegradable and Conventional Microplastics in Farmland. Sci. Total Environ. 2024, 906, 167478. [Google Scholar] [CrossRef]
- Hartmann, M.; Lee, S.; Hallam, S.J.; Mohn, W.W. Bacterial, Archaeal and Eukaryal Community Structures throughout Soil Horizons of Harvested and Naturally Disturbed Forest Stands. Environ. Microbiol. 2009, 11, 3045–3062. [Google Scholar] [CrossRef]
- Zeglin, L.H.; Taylor, A.E.; Myrold, D.D.; Bottomley, P.J. Bacterial and Archaeal AmoA Gene Distribution Covaries with Soil Nitrification Properties across a Range of Land Uses. Environ. Microbiol. Rep. 2011, 3, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Szukics, U.; Hackl, E.; Zechmeister-Boltenstern, S.; Sessitsch, A. Rapid and Dissimilar Response of Ammonia Oxidizing Archaea and Bacteria to Nitrogen and Water Amendment in Two Temperate Forest Soils. Microbiol. Res. 2012, 167, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Sher, Y.; Zaady, E.; Nejidat, A. Spatial and Temporal Diversity and Abundance of Ammonia Oxidizers in Semi-Arid and Arid Soils: Indications for a Differential Seasonal Effect on Archaeal and Bacterial Ammonia Oxidizers. FEMS Microbiol. Ecol. 2013, 86, 544–556. [Google Scholar] [CrossRef]

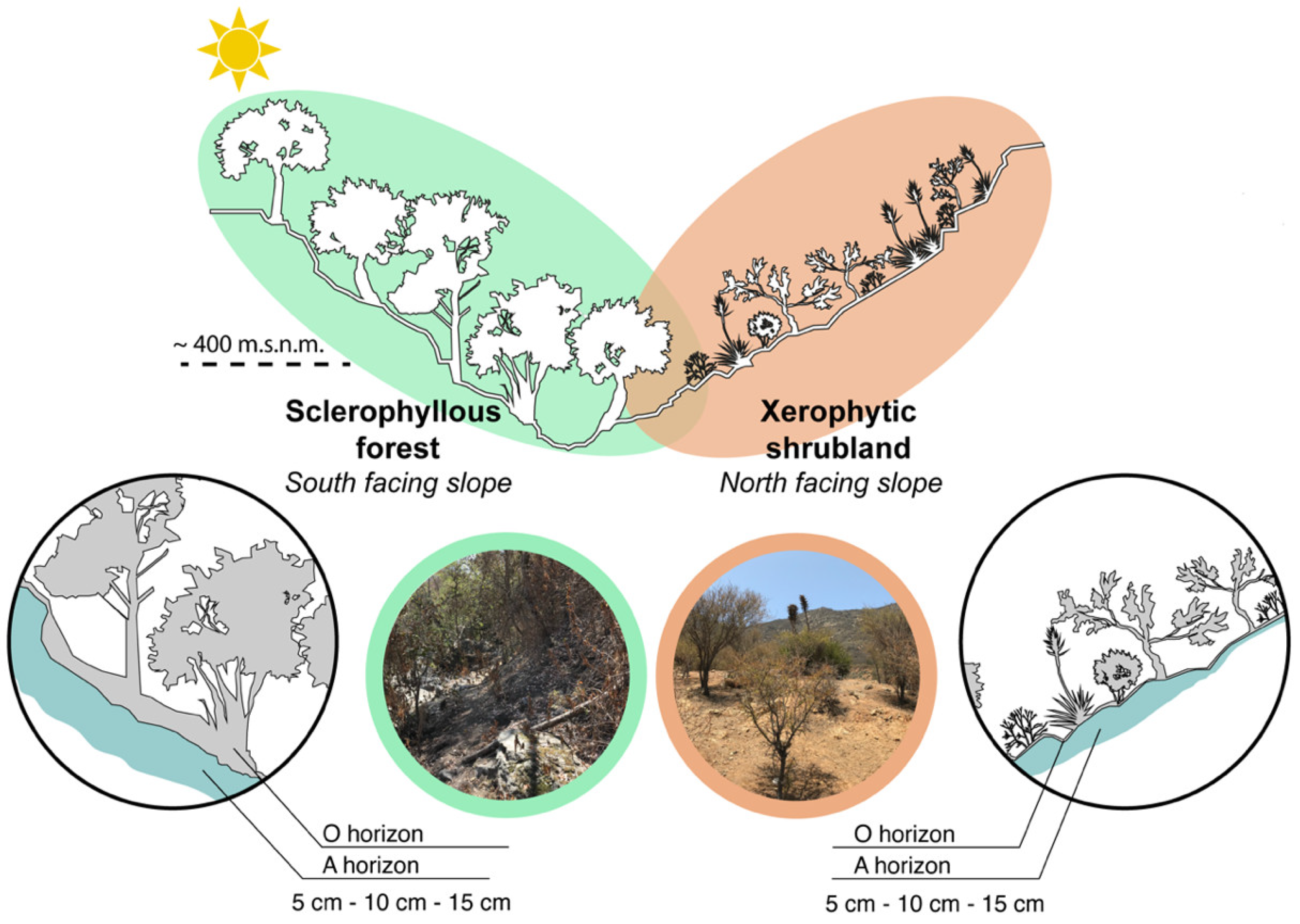

| Site | Species | Coverage | Site | Species | Coverage |
|---|---|---|---|---|---|
| SF | Peumus boldus | 70% | XS | Vachellia caven | 60% |
| SF | Cryptocarya alba | 45% | XS | Conyza bonariensis | 30% |
| SF | Citronella mucronata | 35% | XS | Retanilla trinervia | 30% |
| Lithraea caustica | 15% | ||||
| SF | Quillaja saponaria | 30% | XS | Puya chilensis | 15% |
| SF | Adiantum chilense | 15% | XS | Trichocereus chiloensis | 5% |
| SF | Adenopeltis serrata | 10% | XS | Senna stipulacea | 2% |
| SF | Chusquea cumingii | + | XS | Asteraceae indet | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quinteros-Urquieta, C.; Francois, J.P.; Aguilar-Muñoz, P.; Molina, V. Soil Microbial Communities Changes Along Depth and Contrasting Facing Slopes at the Parque Nacional La Campana, Chile. Microorganisms 2024, 12, 2487. https://doi.org/10.3390/microorganisms12122487
Quinteros-Urquieta C, Francois JP, Aguilar-Muñoz P, Molina V. Soil Microbial Communities Changes Along Depth and Contrasting Facing Slopes at the Parque Nacional La Campana, Chile. Microorganisms. 2024; 12(12):2487. https://doi.org/10.3390/microorganisms12122487
Chicago/Turabian StyleQuinteros-Urquieta, Carolina, Jean Pierre Francois, Polette Aguilar-Muñoz, and Verónica Molina. 2024. "Soil Microbial Communities Changes Along Depth and Contrasting Facing Slopes at the Parque Nacional La Campana, Chile" Microorganisms 12, no. 12: 2487. https://doi.org/10.3390/microorganisms12122487
APA StyleQuinteros-Urquieta, C., Francois, J. P., Aguilar-Muñoz, P., & Molina, V. (2024). Soil Microbial Communities Changes Along Depth and Contrasting Facing Slopes at the Parque Nacional La Campana, Chile. Microorganisms, 12(12), 2487. https://doi.org/10.3390/microorganisms12122487






