Conservation and Dynamics of Maize Seed Endophytic Bacteria Across Progeny Transmission
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Seed Surface Sterilization
2.3. Identification of Seed Endophytic Bacteria by 16S rDNA Sequencing
2.4. Data Analysis of 16S rDNA Amplicons
3. Results
3.1. Analysis of Pyrosequencing Data
3.2. Community Diversity of Seed Endophytic Bacteria
3.3. Core Endophytic Bacteria Were Common Among Three Seed Generations
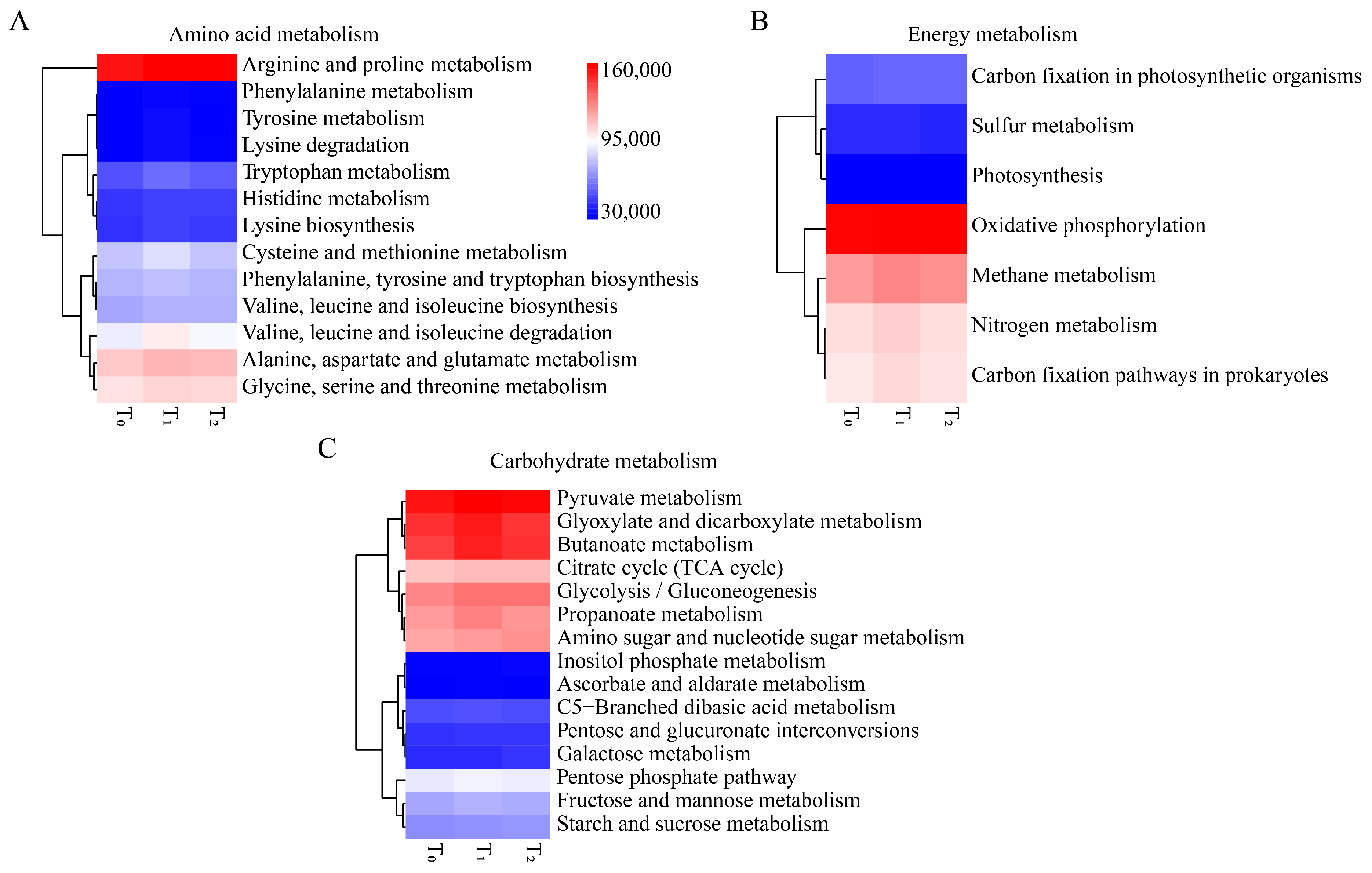
3.4. Three Seed Generations Shared Similar Functional Traits
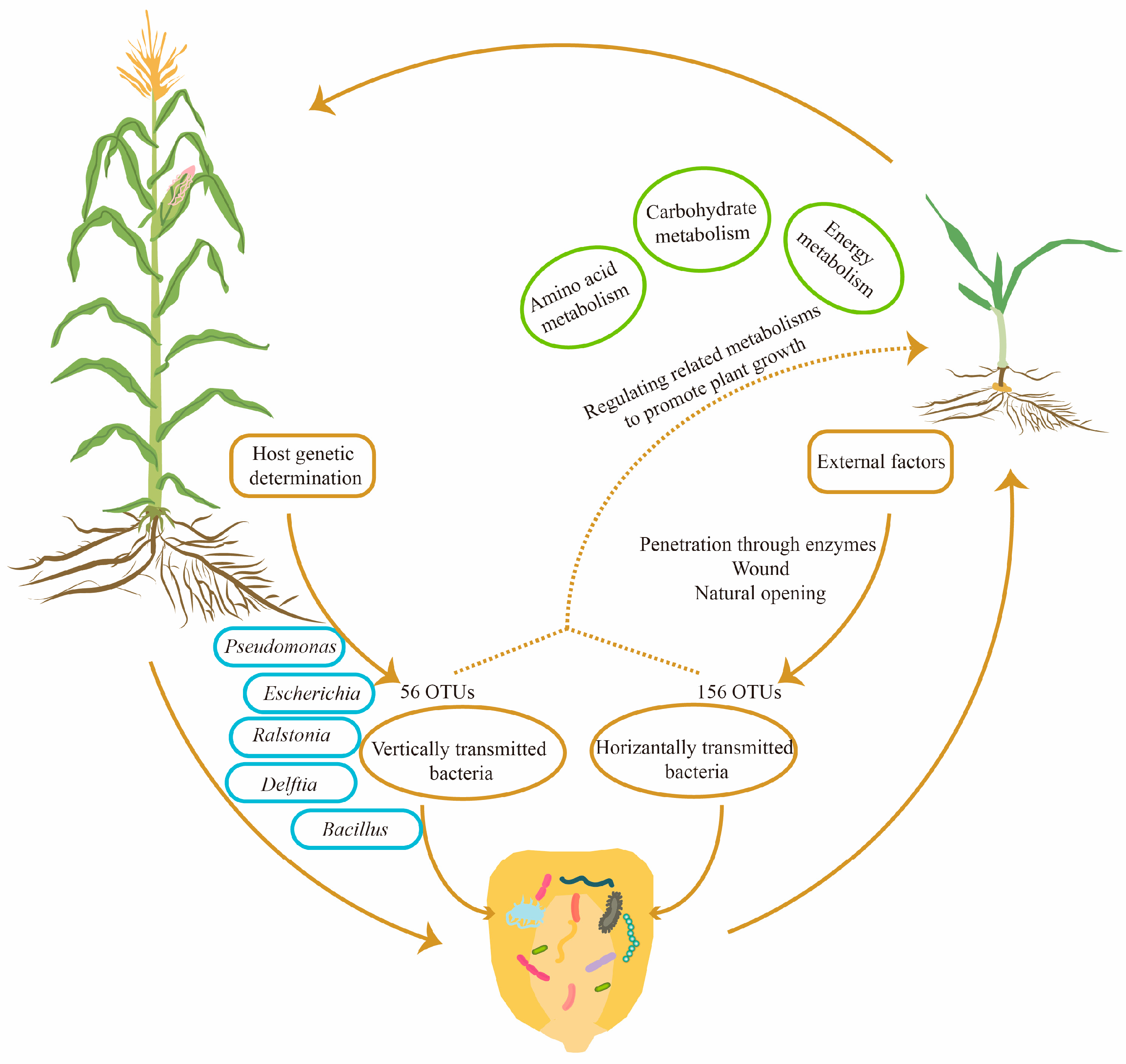
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lodewyckx, C.; Vangronsveld, J.; Porteous, F.; Moore, E.R.B.; Taghavi, S.; Mezgeay, M.; van der Lelie, D. Endophytic bacteria and their potential applications. Crit. Rev. Plant Sci. 2002, 21, 583–606. [Google Scholar] [CrossRef]
- Guha, T.; Biswas, S.M. Recent progress in the role of seed endophytic bacteria as plant growth-promoting microorganisms and biocontrol agents. World J. Microbiol. Biotechnol. 2024, 40, 218. [Google Scholar] [CrossRef] [PubMed]
- Kandel, S.L.; Joubert, P.M.; Doty, S.L. Bacterial endophyte colonization and distribution within plants. Microorganisms 2017, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; van Themaat, E.V.L.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Onyino, J.; Gao, X. Current advances in the functional diversity and mechanisms underlying endophyte-plant interactions. Microorganisms 2024, 12, 779. [Google Scholar] [CrossRef]
- Taghavi, S.; van der Lelie, D.; Hoffman, A.; Zhang, Y.-B.; Walla, M.D.; Vangronsveld, J.; Newman, L.; Monchy, S. Genome sequence of the plant growth promoting endophytic bacterium Enterobacter sp. 638. PLoS Genet. 2010, 6, e1000943. [Google Scholar] [CrossRef]
- Taghavi, S.; Garafola, C.; Monchy, S.; Newman, L.; Hoffman, A.; Weyens, N.; Barac, T.; Vangronsveld, J.; van der Lelie, D. Genome survey and characterization of endophytic bacteria exhibiting a beneficial effect on growth and development of poplar trees. Appl. Environ. Microbiol. 2009, 75, 748–757. [Google Scholar] [CrossRef]
- Oteino, N.; Lally, R.D.; Kiwanuka, S.; Lloyd, A.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015, 6, 745. [Google Scholar] [CrossRef]
- Castillo, U.F.; Strobel, G.A.; Ford, E.J.; Hess, W.M.; Porter, H.; Jensen, J.B.; Albert, H.; Robison, R.; Condron, M.A.M.; Teplow, D.B.; et al. Munumbicins, wide-spectrum antibiotics produced by Streptomyces NRRL 30562, endophytic on Kennedia nigriscans. Microbiology 2002, 148, 2675–2685. [Google Scholar] [CrossRef]
- Semenzato, G.; Bernacchi, A.; Amata, S.; Bechini, A.; Berti, F.; Calonico, C.; Catania, V.; Esposito, A.; Puglia, A.M.; Piccionello, A.P.; et al. Antibacterial properties of bacterial endophytes isolated from the medicinal plant Origanum heracleoticum L. Front. Biosci. 2024, 29, 111. [Google Scholar] [CrossRef]
- He, Y.; Miao, X.; Xia, Y.; Chen, X.; Liu, J.; Zhou, G. The research of antagonistic endophytic bacterium Bacillus velezensis CSUFT-BV4 for growth promotion and induction of resistance to anthracnose in Camellia oleifera. Microorganisms 2024, 12, 763. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.B. Microbial dynamics and interactions in the spermosphere. Annu. Rev. Phytopathol. 2004, 42, 271–309. [Google Scholar] [CrossRef] [PubMed]
- Truyens, S.; Weyens, N.; Cuypers, A.; Vangronsveld, J. Bacterial seed endophytes: Genera, vertical transmission and interaction with plants. Environ. Microbiol. Rep. 2015, 7, 40–50. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, H.; Zhang, X.X.; Zhang, R.Y.; Li, M.; Xu, T.J.; Yang, F.Z.; Zheng, H.J.; Zhao, J.R. Investigating the endophytic bacterial diversity and community structures in seeds of genetically related maize (Zea mays L.) genotypes. 3 Biotech 2020, 10, 27. [Google Scholar] [CrossRef]
- Diaz Herrera, S.; Grossi, C.; Zawoznik, M.; Daniela Groppa, M. Wheat seeds harbour bacterial endophytes with potential as plant growth promoters and biocontrol agents of Fusarium graminearum. Microbiol. Res. 2016, 186, 37–43. [Google Scholar] [CrossRef]
- Pal, G.; Kumar, K.; Verma, A.; Verma, S.K. Seed inhabiting bacterial endophytes of maize promote seedling establishment and provide protection against fungal disease. Microbiol. Res. 2022, 255, 126926. [Google Scholar] [CrossRef]
- Sanchez-Lopez, A.S.; Pintelon, I.; Stevens, V.; Imperato, V.; Timmermans, J.-P.; Gonzalez-Chavez, C.; Carrillo-Gonzalez, R.; Van Hamme, J.; Vangronsveld, J.; Thijs, S. Seed endophyte microbiome of Crotalaria pumila unpeeled: Identification of plant-beneficial Methylobacteria. Int. J. Mol. Sci. 2018, 19, 291. [Google Scholar] [CrossRef]
- Hernandez, I.; Taule, C.; Perez-Perez, R.; Battistoni, F.; Fabiano, E.; Villanueva-Guerrero, A.; Napoles, M.C.; Herrera, H. Endophytic seed-associated bacteria as plant growth promoters of cuban rice (Oryza sativa L.). Microorganisms 2023, 11, 2317. [Google Scholar] [CrossRef]
- Verma, S.K.; Kingsley, K.; Irizarry, I.; Bergen, M.; Kharwar, R.N.; White, J.F., Jr. Seed-vectored endophytic bacteria modulate development of rice seedlings. J. Appl. Microbiol. 2017, 122, 1680–1691. [Google Scholar] [CrossRef]
- Johnston-Monje, D.; Raizada, M.N. Conservation and diversity of seed associated endophytes in Zea across boundaries of evolution, ethnography and ecology. PLoS ONE 2011, 6, e20396. [Google Scholar] [CrossRef]
- Kaga, H.; Mano, H.; Tanaka, F.; Watanabe, A.; Kaneko, S.; Morisaki, H. Rice seeds as sources of endophytic bacteria. Microbes Environ. 2009, 24, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Ewald, P.W. Transmission modes and evolution of the parasitism-mutualism continuum. Ann. N. Y. Acad. Sci. 1987, 503, 295–306. [Google Scholar] [CrossRef]
- Hardoim, P.R.; Hardoim, C.C.P.; van Overbeek, L.S.; van Elsas, J.D. Dynamics of seed-borne rice endophytes on early plant growth stages. PLoS ONE 2012, 7, e30438. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Fan, X.; Wang, Y.; Kusstatscher, P.; Duan, J.; Wu, S.; Chen, S.; Qiao, K.; Wang, Y.; Ma, B.; et al. Bacterial seed endophyte shapes disease resistance in rice. Nat. Plants 2021, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Martiny, J.B.H.; Jones, S.E.; Lennon, J.T.; Martiny, A.C. Microbiomes in light of traits: A phylogenetic perspective. Science 2015, 350, aac9323. [Google Scholar] [CrossRef] [PubMed]
- Gomes Bomfim, C.S.; da Silva, V.B.; Santos Cursino, L.H.; Mattos, W.D.S.; Souza Santos, J.C.; Barbosa de Souza, L.S.; Dantas, B.F.; Santiago de Freitas, A.D.; Fernandes-Junior, P.I. Endophytic bacteria naturally inhabiting commercial maize seeds occupy different niches and are efficient plant growth-promoting agents. Symbiosis 2020, 81, 255–269. [Google Scholar] [CrossRef]
- Wallace, J.G. Maize seed endophytes. Mol. Plant Pathol. 2022, 24, 801–810. [Google Scholar] [CrossRef]
- Sanchez-Lopez, A.S.; Thijs, S.; Beckers, B.; Carmen Gonzalez-Chavez, M.; Weyens, N.; Carrillo-Gonzalez, R.; Vangronsveld, J. Community structure and diversity of endophytic bacteria in seeds of three consecutive generations of Crotalaria pumila growing on metal mine residues. Plant Soil 2018, 422, 51–66. [Google Scholar] [CrossRef]
- Johnston-Monje, D.; Gutierrez, J.P.; Lopez-Lavalle, L.A.B. Seed-transmitted bacteria and fungi dominate juvenile plant microbiomes. Front. Microbiol. 2021, 12, 737616. [Google Scholar] [CrossRef]
- Chen, Y.L.; Liang, J.P.; Zia, A.; Gao, X.; Wang, Y.; Zhang, L.Z.; Xiang, Q.J.; Zhao, K.; Yu, X.M.; Chen, Q.; et al. Culture dependent and independent characterization of endophytic bacteria in the seeds of highland barley. Front. Microbiol. 2022, 13, 981158. [Google Scholar] [CrossRef]
- Bergna, A.; Cernava, T.; Raendler, M.; Grosch, R.; Zachow, C.; Berg, G. Tomato seeds preferably transmit plant beneficial endophytes. Phytobiomes J. 2018, 2, 183–193. [Google Scholar] [CrossRef]
- Ai, J.M.; Yu, T.F.; Liu, X.D.; Jiang, Y.Y.; Wang, E.T.; Deng, Z.S. Seed associated microbiota and vertical transmission of bacterial communities from seed to nodule in Sophora davidii. Plant Soil 2023, 491, 285–302. [Google Scholar] [CrossRef]
- Kumar, S.; Johnson, L.J.J.; Teasdale, S.; Morozova, Y.; de Bonth, A.C.M.; Jauregui, R.; Hannaford, R.; Card, S.D.D. Survey of the endophytic bacteria inhabiting wild daucus seed using 16S rRNA gene amplicon sequencing. Microbiol. Resour. Announc. 2023, 12, 23. [Google Scholar] [CrossRef]
- Guo, J.X.; Bowatte, S.; Hou, F.J. Diversity of endophytic bacteria and fungi in seeds of Elymus nutans growing in four locations of Qinghai Tibet Plateau, China. Plant Soil 2021, 459, 49–63. [Google Scholar] [CrossRef]
- Petrosyan, K.; Thijs, S.; Piwowarczyk, R.; Ruraz, K.; Kaca, W.; Vangronsveld, J. Diversity and potential plant growth promoting capacity of seed endophytic bacteria of the holoparasite Cistanche phelypaea (Orobanchaceae). Sci. Rep. 2023, 13, 11835. [Google Scholar] [CrossRef]
- Zeng, K.; Li, Y.; Wang, Z.; Du, Y.; Fan, M.; Xie, L. Community structure and diversity of endophytic bacteria in Melon (Cucumis melo L.) seeds. Horticulturae 2023, 9, 1195. [Google Scholar] [CrossRef]
- Bodhankar, S.; Grover, M.; Hemanth, S.; Reddy, G.; Rasul, S.; Yadav, S.K.; Desai, S.; Mallappa, M.; Mandapaka, M.; Srinivasarao, C. Maize seed endophytic bacteria: Dominance of antagonistic, lytic enzyme-producing Bacillus spp. 3 Biotech 2017, 7, 232. [Google Scholar] [CrossRef]
- Yan, K.; He, L.M.; Zhou, W.H.; Feng, R.Z.; Meng, L.N.; Wei, Q.; Liu, Y. Determination of the community structure and diversity of endophytic bacteria from Alpinia zerumbet seeds. Int. J. Agric. Biol. 2020, 24, 420–428. [Google Scholar]
- Pal, G.; Saxena, S.; Kumar, K.; Verma, A.; Kumar, D.; Shukla, P.; Pandey, A.; Verma, S.K. Seed endophytic bacterium Bacillus velezensis and its lipopeptides acts as elicitors of defense responses against Fusarium verticillioides in maize seedlings. Plant Soil 2023, 492, 109–124. [Google Scholar] [CrossRef]
- Etesami, H.; Alikhani, H.A. Suppression of the fungal pathogen Magnaporthe grisea by Stenotrophomonas maltophilia, a seed-borne rice (Oryza sativa L.) endophytic bacterium. Arch. Agron. Soil Sci. 2016, 62, 1271–1284. [Google Scholar] [CrossRef]
- Uddin, M.A.; Akter, S.; Ferdous, M.; Haidar, B.; Amin, A.; Molla, A.H.M.S.I.; Khan, H.; Islam, M.R. A plant endophyte Staphylococcus hominis strain MBL_AB63 produces a novel lantibiotic, homicorcin and a position one variant. Sci. Rep. 2021, 11, 11211. [Google Scholar]
- Thomas, P.; Shaik, S.P. Molecular profiling on surface-disinfected tomato seeds reveals high diversity of cultivation-recalcitrant endophytic bacteria with low shares of spore-forming Firmicutes. Microb. Ecol. 2020, 79, 910–924. [Google Scholar] [CrossRef]





| OTU | Reference Species | Abundance |
|---|---|---|
| OTU217 | Acidovorax avenae | 0.75% |
| OTU169 | Bacillus velezensis | 1.31% |
| OTU198 | Delftia tsuruhatensis | 4.32% |
| OTU120 | Pseudomonas mosselii | 0.56% |
| OTU158 | Ralstonia solanacearum | 54.31% |
| OTU82 | Sanguibacte rinulinus | 0.21% |
| OTU132 | Shigella boydii | 1.12% |
| OTU32 | Sphingomonas paucimobilis | 0.23% |
| OTU1 | Staphylococcus hominis | 0.31% |
| OTU219 | Stenotrophomonas maltophiliag | 0.49% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, K.; Zhang, Y.; Zhao, C.; Wang, Q.; Gao, X. Conservation and Dynamics of Maize Seed Endophytic Bacteria Across Progeny Transmission. Microorganisms 2024, 12, 2399. https://doi.org/10.3390/microorganisms12122399
Zhai K, Zhang Y, Zhao C, Wang Q, Gao X. Conservation and Dynamics of Maize Seed Endophytic Bacteria Across Progeny Transmission. Microorganisms. 2024; 12(12):2399. https://doi.org/10.3390/microorganisms12122399
Chicago/Turabian StyleZhai, Kaihui, Yingying Zhang, Caihong Zhao, Qing Wang, and Xiquan Gao. 2024. "Conservation and Dynamics of Maize Seed Endophytic Bacteria Across Progeny Transmission" Microorganisms 12, no. 12: 2399. https://doi.org/10.3390/microorganisms12122399
APA StyleZhai, K., Zhang, Y., Zhao, C., Wang, Q., & Gao, X. (2024). Conservation and Dynamics of Maize Seed Endophytic Bacteria Across Progeny Transmission. Microorganisms, 12(12), 2399. https://doi.org/10.3390/microorganisms12122399






