Effects of Short-Term Nitrogen Addition on Soil Fungal Community Increase with Nitrogen Addition Rate in an Alpine Steppe at the Source of Brahmaputra
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Design
2.2. Vegetation Community Examination, Soil Sampling, and Analysis
2.3. Statistical Analysis
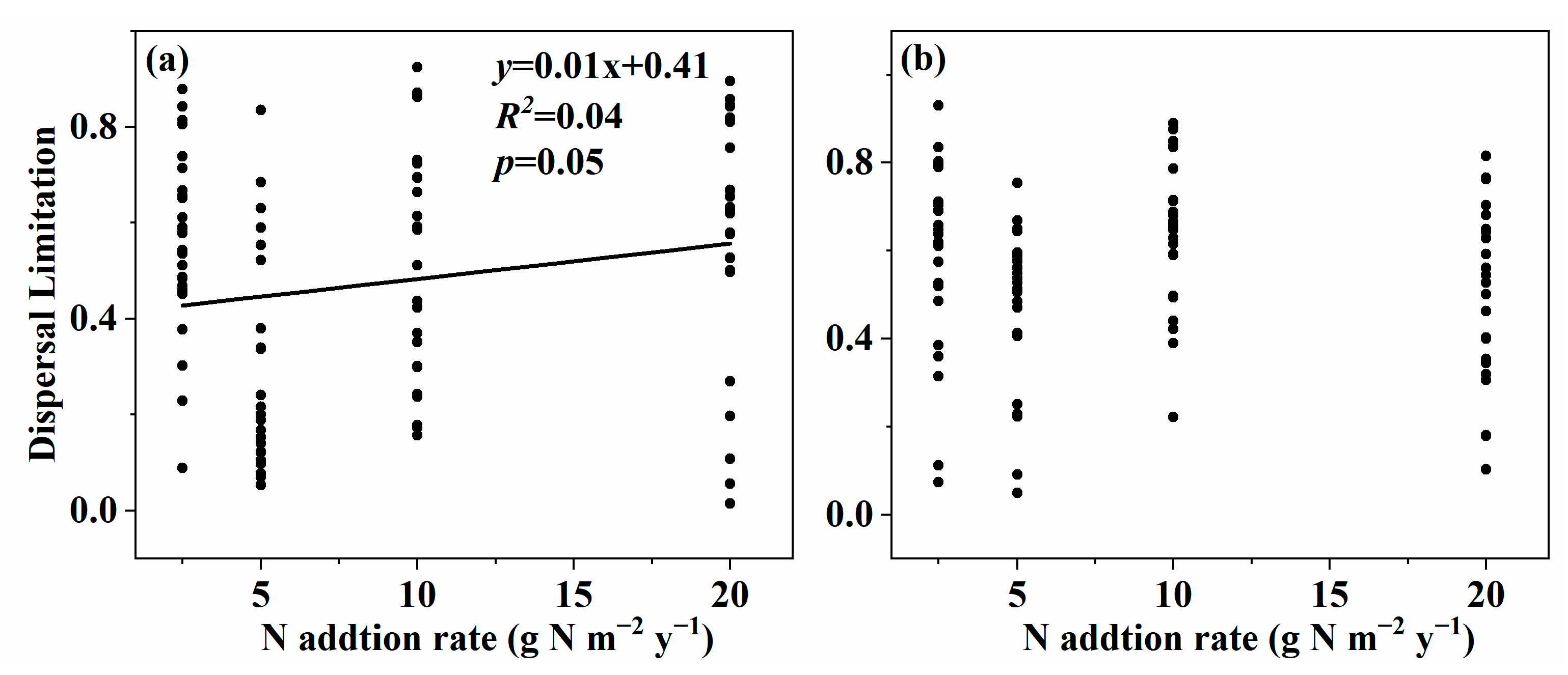
3. Results
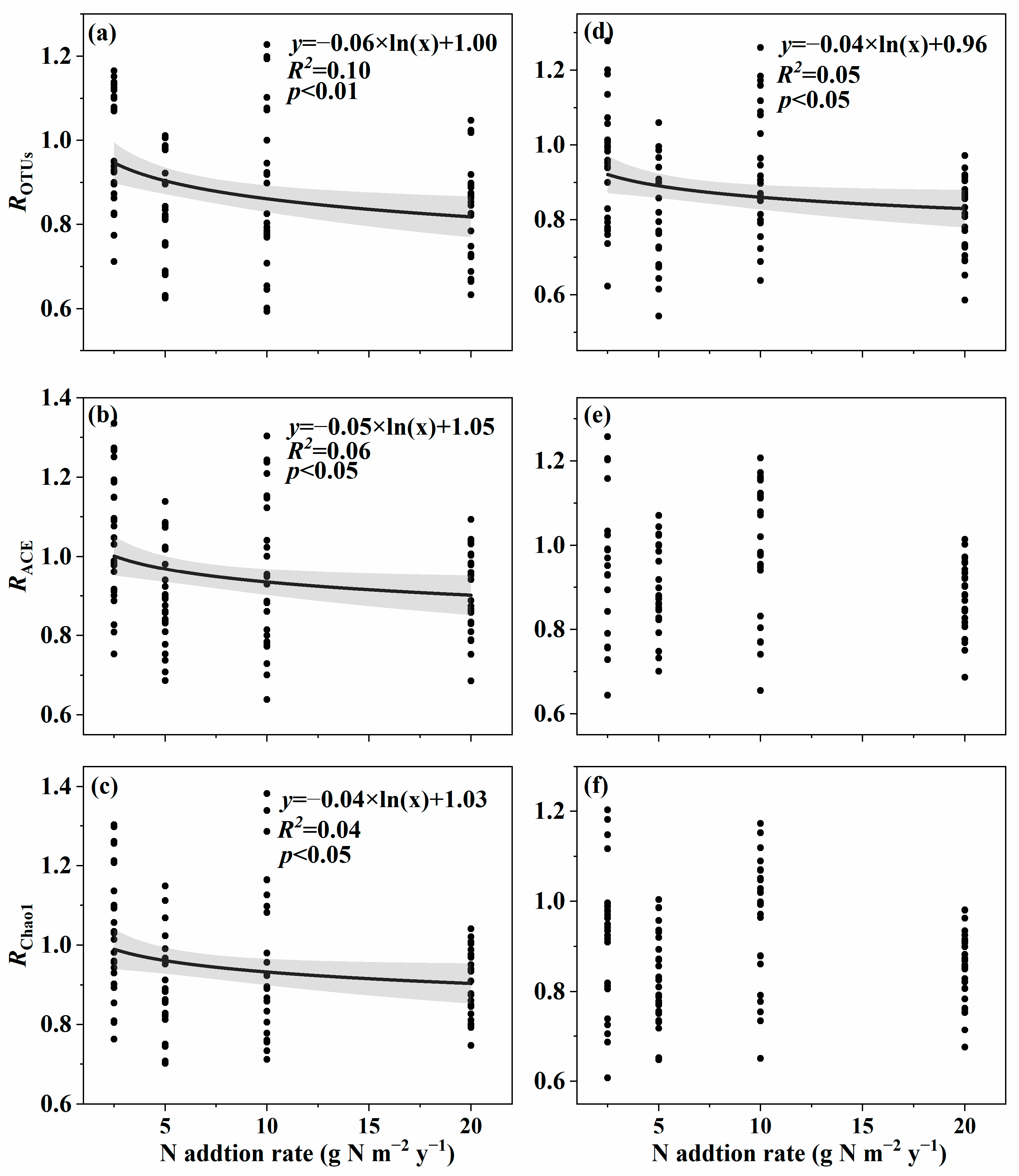
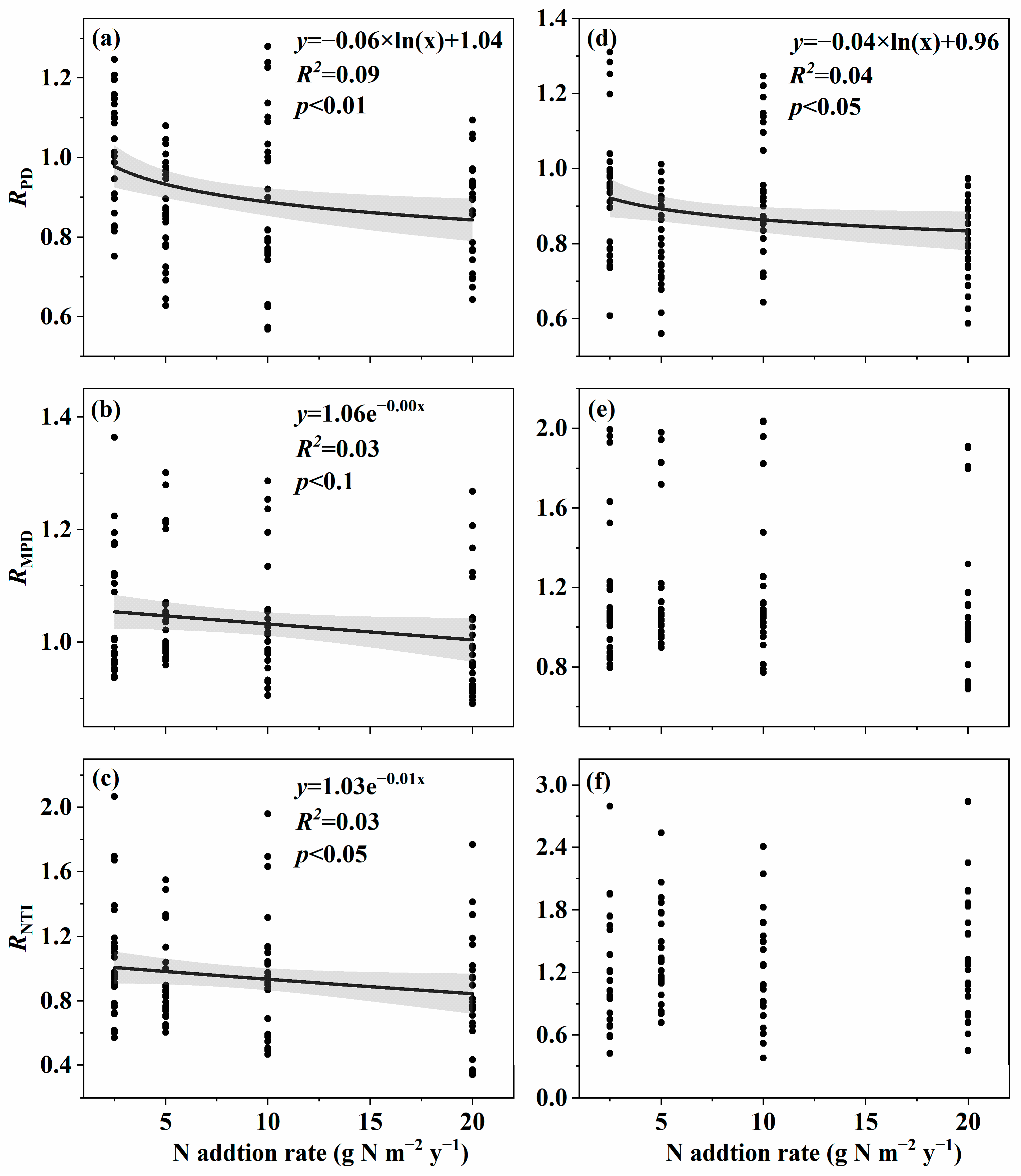
3.1. Changes in Soil Fungal Species and Phylogenetic α-Diversity with the Nitrogen Addition Gradient and the Relation with Environmental Variables
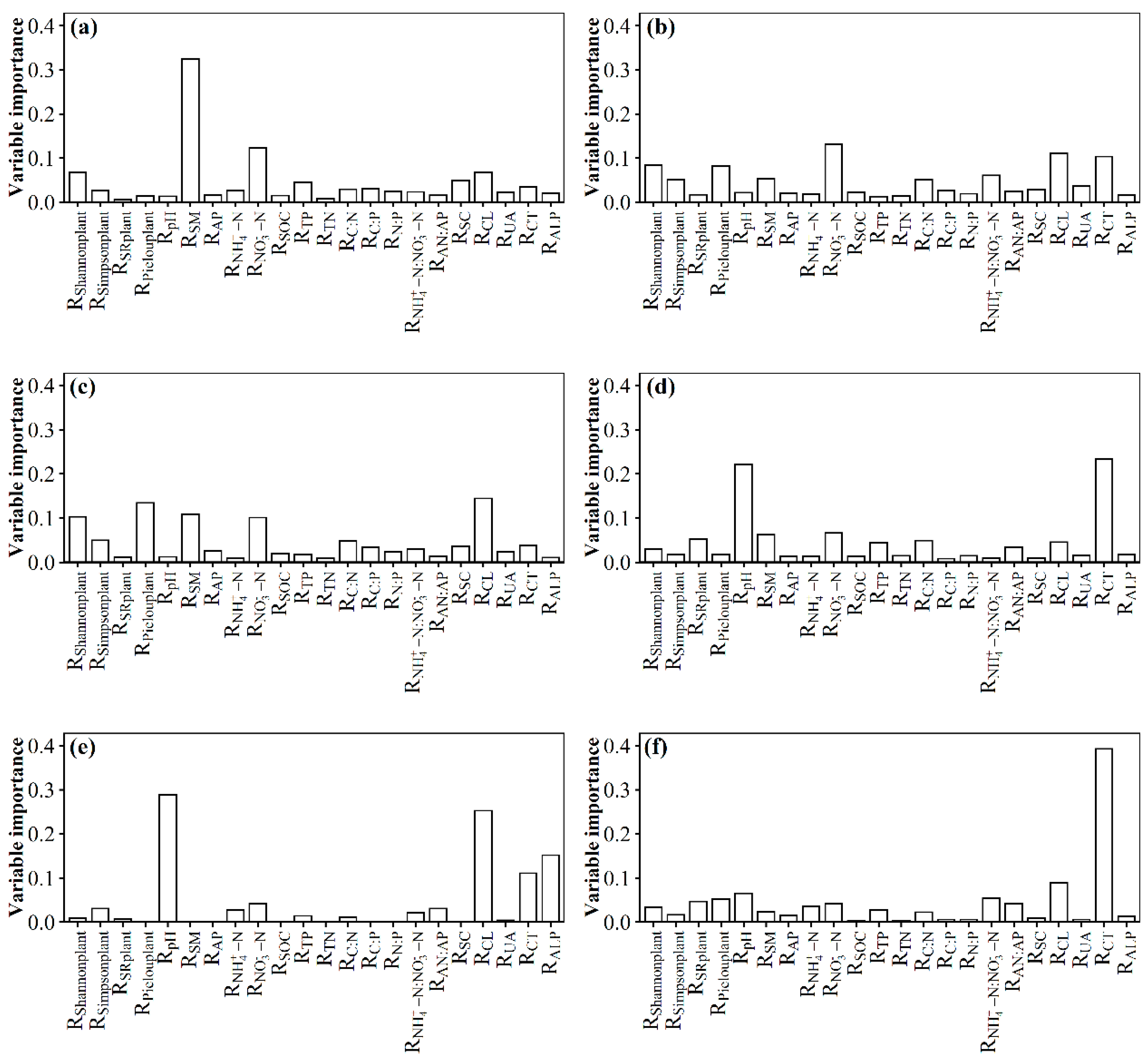
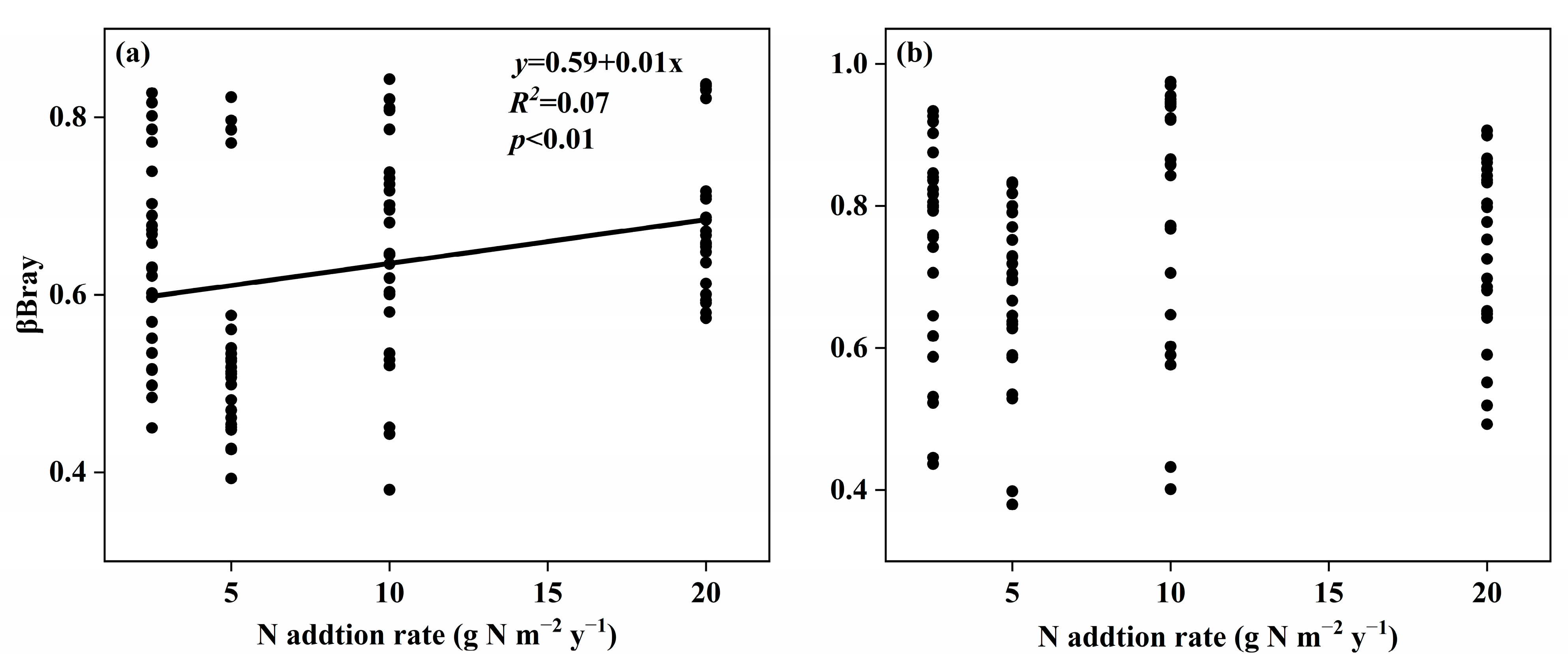
3.2. Changes in Soil Fungal Species and Phylogenetic Community Composition with the Nitrogen Addition Gradient and the Relation with Environmental Variables
3.3. Effect of Nitrogen Addition on Community Assembly of Soil Fungal Community
4. Discussion
4.1. Changes in Species and Phylogenetic α-Diversity with the Nitrogen Addition Gradient
4.2. Changes in Species and Phylogenetic Community Composition with the Nitrogen Addition Gradient
4.3. Response Strength Is Stronger in Surface Soil than Subsurface Soil
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A.; et al. Nitrogen cycles: Past, present, and future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Bobbink, R.; Hicks, K.; Galloway, J.; Spranger, T.; Alkemade, R.; Ashmore, M.; Bustamante, M.; Cinderby, S.; Davidson, E.; Dentener, F.; et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol. Appl. 2010, 20, 30–59. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Du, Y.H.; Guo, P.; Guo, L.D.; Qu, K.Y.; He, J.P. Effects of different types of N deposition on the fungal decomposition activities of temperate forest soils. Sci. Total Environ. 2014, 497, 91–96. [Google Scholar] [CrossRef]
- Gotosa, J.; Kodzwa, J.; Nyamangara, J.; Gwenzi, W. Effect of Nitrogen Fertiliser Application on Maize Yield Across Agro-Ecological Regions and Soil Types in Zimbabwe: A Meta-analysis Approach. Int. J. Plant Prod. 2019, 13, 251–266. [Google Scholar] [CrossRef]
- Bradley, K.; Drijber, R.A.; Knops, J. Increased N availability in grassland soils modifies their microbial communities and decreases the abundance of arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2006, 38, 1583–1595. [Google Scholar] [CrossRef]
- Zong, N.; Shi, P.L.; Song, M.H.; Zhang, X.Z.; Jiang, J.; Chai, X. Nitrogen Critical Loads for an Alpine Meadow Ecosystem on the Tibetan Plateau. Environ. Manag. 2016, 57, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Shen, Z.X. Response of Alpine Plants to Nitrogen Addition on the Tibetan Plateau: A Meta-analysis. J. Plant Growth Regul. 2016, 35, 974–979. [Google Scholar] [CrossRef]
- Humbert, J.Y.; Dwyer, J.M.; Andrey, A.; Arlettaz, R. Impacts of nitrogen addition on plant biodiversity in mountain grasslands depend on dose, application duration and climate: A systematic review. Glob. Chang. Biol. 2016, 22, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Nave, L.E.; Vance, E.D.; Swanston, C.W.; Curtis, P.S. Impacts of elevated N inputs on north temperate forest soil C storage, C/N, and net N-mineralization. Geoderma 2009, 153, 231–240. [Google Scholar] [CrossRef]
- Geisseler, D.; Lazicki, P.A.; Scow, K.M. Mineral nitrogen input decreases microbial biomass in soils under grasslands but not annual crops. Appl. Soil Ecol. 2016, 106, 1–10. [Google Scholar] [CrossRef]
- Lucas, R.W.; Klaminder, J.; Futter, M.N.; Bishop, K.H.; Egnell, G.; Laudon, H.; Hogberg, P. A meta-analysis of the effects of nitrogen additions on base cations: Implications for plants, soils, and streams. For. Ecol. Manag. 2011, 262, 95–104. [Google Scholar] [CrossRef]
- Li, W.B.; Jin, C.J.; Guan, D.X.; Wang, Q.K.; Wang, A.Z.; Yuan, F.H.; Wu, J.B. The effects of simulated nitrogen deposition on plant root traits: A meta-analysis. Soil Biol. Biochem. 2015, 82, 112–118. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Belnap, J.; Rudgers, J.; Kuske, C.R.; Martinez, N.; Sandquist, D. Soil microbial responses to nitrogen addition in arid ecosystems. Front. Microbiol. 2015, 6, 819. [Google Scholar] [CrossRef]
- Philippot, L.; Spor, A.; Henault, C.; Bru, D.; Bizouard, F.; Jones, C.M.; Sarr, A.; Maron, P.A. Loss in microbial diversity affects nitrogen cycling in soil. ISME J. 2013, 7, 1609–1619. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2003, 54, 655–670. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Polme, S.; Koljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 458–463. [Google Scholar] [CrossRef]
- Song, B.; Li, Y.; Yang, L.Y.; Shi, H.Q.; Li, L.H.; Bai, W.M.; Zhao, Y. Soil Acidification Under Long-Term N Addition Decreases the Diversity of Soil Bacteria and Fungi and Changes Their Community Composition in a Semiarid Grassland. Microb. Ecol. 2021, 85, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xu, R.; Chen, J.; Yuan, X.; Zhou, L.; Tan, T.; Fan, J.; Zhang, Y.; Hu, T. Consistent responses of surface- and subsurface soil fungal diversity to N enrichment are mediated differently by acidification and plant community in a semi-arid grassland. Soil Biol. Biochem. 2018, 127, 110–119. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Wang, C.K.; Jin, Y.; Gu, J.C. Effects of long-term nitrogen addition on soil fungal communities in two temperate plantations with different mycorrhizal associations. Appl. Soil Ecol. 2021, 168, 104111. [Google Scholar] [CrossRef]
- Wang, C.; Liu, D.W.; Bai, E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol. Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Chen, X.; Hao, B.H.; Jing, X.; He, J.S.; Ma, W.H.; Zhu, B. Minor responses of soil microbial biomass, community structure and enzyme activities to nitrogen and phosphorus addition in three grassland ecosystems. Plant Soil 2019, 444, 21–37. [Google Scholar] [CrossRef]
- He, D.; Xiang, X.; He, J.S.; Wang, C.; Cao, G.; Adams, J.; Chu, H. Composition of the soil fungal community is more sensitive to phosphorus than nitrogen addition in the alpine meadow on the Qinghai-Tibetan Plateau. Biol. Fertil. Soils 2016, 52, 1059–1072. [Google Scholar] [CrossRef]
- He, W.Y.; Zhang, M.M.; Jin, G.Z.; Sui, X.; Zhang, T.; Song, F.Q. Effects of Nitrogen Deposition on Nitrogen-Mineralizing Enzyme Activity and Soil Microbial Community Structure in a Korean Pine Plantation. Microb. Ecol. 2021, 81, 410–424. [Google Scholar] [CrossRef] [PubMed]
- McHugh, T.A.; Morrissey, E.M.; Mueller, R.C.; Gallegos-Graves, L.V.; Kuske, C.R.; Reed, S.C. Bacterial, fungal, and plant communities exhibit no biomass or compositional response to two years of simulated nitrogen deposition in a semiarid grassland. Environ. Microbiol. 2017, 19, 1600–1611. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Wang, C.K.; Zheng, M.H.; Jiang, L.F.; Luo, Y.Q. Patterns and mechanisms of responses by soil microbial communities to nitrogen addition. Soil Biol. Biochem. 2017, 115, 433–441. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, H.; Gao, H.; An, S.S. Response and driving factors of soil microbial diversity related to global nitrogen addition. Land Degrad. Dev. 2020, 31, 190–204. [Google Scholar] [CrossRef]
- Ramette, A.; Tiedje, J.M. Multiscale responses of microbial life to spatial distance and environmental heterogeneity in a patchy ecosystem. Proc. Natl. Acad. Sci. USA 2007, 104, 2761–2766. [Google Scholar] [CrossRef]
- Stegen, J.C.; Lin, X.; Konopka, A.E.; Fredrickson, J.K. Stochastic and deterministic assembly processes in subsurface microbial communities. Isme J. 2012, 6, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ning, D. Stochastic Community Assembly: Does It Matter in Microbial Ecology? Microbiol. Mol. Biol. Rev. 2017, 81, e00002-17. [Google Scholar] [CrossRef]
- Zeng, C.; Zhang, F.; Wang, Q.J.; Chen, Y.Y.; Joswiak, D.R. Impact of alpine meadow degradation on soil hydraulic properties over the Qinghai-Tibetan Plateau. J. Hydrol. 2013, 478, 148–156. [Google Scholar] [CrossRef]
- Lu, C.Q.; Tian, H.Q. Spatial and temporal patterns of nitrogen deposition in China: Synthesis of observational data. J. Geophys. Res.-Atmos. 2007, 112, D22. [Google Scholar] [CrossRef]
- Sun, J.; Hou, G.; Liu, M.; Fu, G.; Zhan, T.; Zhou, H.; Tsunekawa, A.; Haregeweyn, N. Effects of climatic and grazing changes on desertification of alpine grasslands, Northern Tibet. Ecol. Indic. 2019, 107, 105647. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, H.; Yao, B.; Wang, W.; Dong, S.; Shang, Z.; She, Y.; Ma, L.; Huang, X.; Zhang, Z.; et al. Effects of nutrient addition on degraded alpine grasslands of the Qinghai-Tibetan Plateau: A meta-analysis. Agric. Ecosyst. Environ. 2020, 301, 106970. [Google Scholar] [CrossRef]
- Li, J.J.; Yang, C.; Zhou, H.K.; Shao, X.Q. Responses of plant diversity and soil microorganism diversity to water and nitrogen additions in the Qinghai-Tibetan Plateau. Glob. Ecol. Conserv. 2020, 22, e01003. [Google Scholar] [CrossRef]
- Chen, W.J.; Zhou, H.K.; Wu, Y.; Wang, J.; Zhao, Z.W.; Li, Y.Z.; Qiao, L.L.; Chen, K.L.; Liu, G.B.; Xue, S. Direct and indirect influences of long-term fertilization on microbial carbon and nitrogen cycles in an alpine grassland. Soil Biol. Biochem. 2020, 149, 107922. [Google Scholar] [CrossRef]
- Barua, A.; Vij, S.; Rahman, M.Z. Powering or sharing water in the Brahmaputra River basin. Int. J. Water Resour. Dev. 2018, 34, 829–843. [Google Scholar] [CrossRef]
- Huang, S.; Yu, C.; Fu, G.; Sun, W.; Li, S.; Xiao, J. Different responses of soil bacterial species diversity and phylogenetic diversity to short-term nitrogen input in an alpine steppe at the source of Brahmaputra. Front. Ecol. Evol. 2022, 10, 1073177. [Google Scholar] [CrossRef]
- Tian, Y.; Fu, G. Quantifying Plant Species α-Diversity Using Normalized Difference Vegetation Index and Climate Data in Alpine Grasslands. Remote Sens. 2022, 14, 5007. [Google Scholar] [CrossRef]
- Fu, G.; Shen, Z.X. Response of alpine soils to nitrogen addition on the Tibetan Plateau: A meta-analysis. Appl. Soil Ecol. 2017, 114, 99–104. [Google Scholar] [CrossRef]
- Zong, N.; Fu, G. Variations in species and function diversity of soil fungal community along a desertification gradient in an alpine steppe. Ecol. Indic. 2021, 131, 108197. [Google Scholar] [CrossRef]
- Chao, A. Nonparametric Estimation of the Number of Classes in a Population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Fu, G.; Wang, J.H.; Li, S.W. Response of forage nutritional quality to climate change and human activities in alpine grasslands. Sci. Total Environ. 2022, 845, 157552. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Fu, G. Response of soil fungal species, phylogenetic and functional diversity to diurnal asymmetric warming in an alpine agricultural ecosystem. Agric. Ecosyst. Environ. 2022, 335, 107993. [Google Scholar] [CrossRef]
- Han, F.; Yu, C.; Fu, G. Warming alters elevation distributions of soil bacterial and fungal communities in alpine grasslands. Glob. Ecol. Conserv. 2022, 39, e02306. [Google Scholar] [CrossRef]
- Gao, X.H.; Fan, P.S.; Wang, J.L.; Ruan, Y.Z.; Wang, Q. Nitrogen inputs reduce fungal diversity and alter community composition in a Chinese fir plantation. Appl. Ecol. Environ. Res. 2021, 19, 2605–2615. [Google Scholar] [CrossRef]
- He, J.; Jiao, S.; Tan, X.; Wei, H.; Ma, X.; Nie, Y.; Liu, J.; Lu, X.; Mo, J.; Shen, W. Adaptation of Soil Fungal Community Structure and Assembly to Long- Versus Short-Term Nitrogen Addition in a Tropical Forest. Front. Microbiol. 2021, 12, 689674. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Wang, C.K.; Luo, Y.Q. Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality. Nat. Commun. 2020, 11, 3072. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Eldridge, D.J.; Ochoa, V.; Gozalo, B.; Singh, B.K.; Maestre, F.T. Soil microbial communities drive the resistance of ecosystem multifunctionality to global change in drylands across the globe. Ecol. Lett. 2017, 20, 1295–1305. [Google Scholar] [CrossRef]
- Wang, J.T.; Zheng, Y.M.; Hu, H.W.; Zhang, L.M.; Li, J.; He, J.Z. Soil pH determines the alpha diversity but not beta diversity of soil fungal community along altitude in a typical Tibetan forest ecosystem. J. Soils Sediments 2015, 15, 1224–1232. [Google Scholar] [CrossRef]
- Xiao, Y.M.; Li, C.B.; Yang, Y.; Peng, Y.F.; Yang, Y.H.; Zhou, G.Y. Soil Fungal Community Composition, Not Assembly Process, Was Altered by Nitrogen Addition and Precipitation Changes at an Alpine Steppe. Front. Microbiol. 2020, 11, 579072. [Google Scholar] [CrossRef]
- Peay, K.G.; Kennedy, P.G.; Talbot, J.M. Dimensions of biodiversity in the Earth mycobiome. Nat. Rev. Microbiol. 2016, 14, 434–447. [Google Scholar] [CrossRef]
- Prober, S.M.; Leff, J.W.; Bates, S.T.; Borer, E.T.; Firn, J.; Harpole, W.S.; Lind, E.M.; Seabloom, E.W.; Adler, P.B.; Bakker, J.D.; et al. Plant diversity predicts beta but not alpha diversity of soil microbes across grasslands worldwide. Ecol. Lett. 2015, 18, 85–95. [Google Scholar] [CrossRef]
- Chen, W.; Xu, R.; Wu, Y.; Chen, J.; Zhang, Y.; Hu, T.; Yuan, X.; Zhou, L.; Tan, T.; Fan, J. Plant diversity is coupled with beta not alpha diversity of soil fungal communities following N enrichment in a semi-arid grassland. Soil Biol. Biochem. 2018, 116, 388–398. [Google Scholar] [CrossRef]
- Yang, P.; Luo, Y.; Gao, Y.; Gao, X.L.; Gao, J.F.; Wang, P.K.; Feng, B.L. Soil properties, bacterial and fungal community compositions and the key factors after 5-year continuous monocropping of three minor crops. PLoS ONE 2020, 15, e0237164. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Nie, C.; Liu, Y.H.; Du, W.; He, P. Soil microbial community composition closely associates with specific enzyme activities and soil carbon chemistry in a long-term nitrogen fertilized grassland. Sci. Total Environ. 2019, 654, 264–274. [Google Scholar] [CrossRef]
- Cassman, N.A.; Leite, M.F.; Pan, Y.; de Hollander, M.; van Veen, J.A.; Kuramae, E.E. Plant and soil fungal but not soil bacterial communities are linked in long-term fertilized grassland. Sci. Rep. 2016, 6, 23680. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Sui, Y.Y.; Yu, Z.H.; Shi, Y.; Chu, H.Y.; Jin, J.; Liu, X.B.; Wang, G.H. Soil carbon content drives the biogeographical distribution of fungal communities in the black soil zone of northeast China. Soil Biol. Biochem. 2015, 83, 29–39. [Google Scholar] [CrossRef]
- Yang, Y.; Dou, Y.X.; Huang, Y.M.; An, S.S. Links between Soil Fungal Diversity and Plant and Soil Properties on the Loess Plateau. Front. Microbiol. 2017, 8, 2198. [Google Scholar] [CrossRef]
- Yang, T.; Adams, J.M.; Shi, Y.; Sun, H.; Cheng, L.; Zhang, Y.; Chu, H. Fungal community assemblages in a high elevation desert environment: Absence of dispersal limitation and edaphic effects in surface soil. Soil Biol. Biochem. 2017, 115, 393–402. [Google Scholar] [CrossRef]
- Jobbagy, E.G.; Jackson, R.B. The distribution of soil nutrients with depth: Global patterns and the imprint of plants. Biogeochemistry 2001, 53, 51–77. [Google Scholar] [CrossRef]
- Powell, J.R.; Karunaratne, S.; Campbell, C.D.; Yao, H.Y.; Robinson, L.; Singh, B.K. Deterministic processes vary during community assembly for ecologically dissimilar taxa. Nat. Commun. 2015, 6, 8444. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Liu, X.; Song, L.; Lin, X.; Zhang, H.; Shen, C.; Chu, H. Nitrogen fertilization directly affects soil bacterial diversity and indirectly affects bacterial community composition. Soil Biol. Biochem. 2016, 92, 41–49. [Google Scholar] [CrossRef]
- Zhang, H.R.; Fu, G. Responses of plant, soil bacterial and fungal communities to grazing vary with pasture seasons and grassland types, northern Tibet. Land Degrad. Dev. 2021, 32, 1821–1832. [Google Scholar] [CrossRef]

| Species | Surface | N0 | N2.5 | N5 | N10 | |
| N2.5 | 0.81 (0.86) | |||||
| Adonis2 | N5 | 0.97 (0.51) | 1.22 (0.11) | |||
| F (p value) | N10 | 0.96 (0.53) | 0.88 (0.71) | 1.27 (0.15) | ||
| N20 | 1.82 (0.02) | 1.66 (0.04) | 2.46 (0.01) | 1.06 (0.44) | ||
| Subsurface | N0 | N2.5 | N5 | N10 | ||
| N2.5 | 0.77 (0.94) | |||||
| Adonis2 | N5 | 0.95 (0.62) | 0.78 (0.91) | |||
| F (p value) | N10 | 1.17 (0.33) | 0.74 (0.82) | 1.55 (0.11) | ||
| N20 | 1.43 (0.06) | 0.95 (0.51) | 1.36 (0.09) | 1.54 (0.07) | ||
| Phylogenetic | Surface | N0 | N2.5 | N5 | N10 | |
| N2.5 | 0.01 (0.79) | |||||
| Adonis2 | N5 | 0.20 (0.78) | 0.11 (0.91) | |||
| F (p value) | N10 | 1.60 (0.34) | 0.98 (0.52) | 0.21 (0.78) | ||
| N20 | 2.71 (0.04) | 1.89 (0.16) | 1.09 (0.53) | 0.35 (0.83) | ||
| Subsurface | N0 | N2.5 | N5 | N10 | ||
| N2.5 | 1.00 (0.55) | |||||
| Adonis2 | N5 | −1.16 (0.90) | 0.53 (0.77) | |||
| F (p value) | N10 | 3.00 (0.17) | 1.06 (0.22) | 3.47 (0.07) | ||
| N20 | −0.50 (0.74) | 0.20 (0.91) | 1.66 (0.18) | 2.53 (0.20) | ||
| Variables | Species | Phylogenetic | ||
|---|---|---|---|---|
| Surface | Subsurface | Surface | Subsurface | |
| Shannonplant | 0.07 | 0.14 | −0.02 | 0.17 + |
| Simpsonplant SRplant | 0.13 | 0.13 | 0.01 | 0.14 |
| −0.07 | 0.12 | −0.08 | 0.14 | |
| Pielouplant | 0.16 | 0.14 | 0.00 | 0.12 |
| pH | −0.09 | −0.17 | 0.00 | −0.06 |
| SM | 0.09 | 0.14 | 0.28 + | 0.07 |
| AP (mg kg−1) | −0.04 | −0.14 | 0.07 | −0.14 |
| NH4+-N | 0.29 * | −0.11 | 0.20 + | −0.07 |
| NO3−-N | 0.25 ** | −0.07 | 0.14 + | −0.10 |
| SOC | 0.19 | 0.24 + | −0.01 | 0.16 |
| TP | 0.14 | 0.15 | 0.11 | 0.12 |
| TN | 0.20 + | 0.24 * | 0.04 | 0.21 + |
| C:N | −0.14 | −0.07 | 0.06 | −0.12 |
| C:P | 0.18 | 0.04 | 0.04 | −0.01 |
| N:P | 0.16 | 0.10 | 0.03 | 0.11 |
| NH4+-N:NO3−-N | −0.13 | −0.13 | −0.13 | −0.10 |
| AN:AP | 0.24 + | −0.08 | 0.24 + | −0.10 |
| SC (mg g−1 d−1) | 0.26 ** | 0.26 * | 0.26 ** | 0.18 + |
| CL (mg g−1 3 d−1) | −0.10 | 0.02 | 0.04 | −0.07 |
| UA (mg g−1 d−1) | 0.06 | 0.34 ** | 0.01 | 0.27 + |
| CT (ml g−1 20 min−1) | 0.15 + | 0.09 | 0.03 | −0.05 |
| ALP (mg g−1 2 h−1) | −0.09 | −0.08 | −0.11 | −0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Yu, C.; Fu, G.; Sun, W.; Li, S.; Han, F.; Xiao, J. Effects of Short-Term Nitrogen Addition on Soil Fungal Community Increase with Nitrogen Addition Rate in an Alpine Steppe at the Source of Brahmaputra. Microorganisms 2023, 11, 1880. https://doi.org/10.3390/microorganisms11081880
Huang S, Yu C, Fu G, Sun W, Li S, Han F, Xiao J. Effects of Short-Term Nitrogen Addition on Soil Fungal Community Increase with Nitrogen Addition Rate in an Alpine Steppe at the Source of Brahmaputra. Microorganisms. 2023; 11(8):1880. https://doi.org/10.3390/microorganisms11081880
Chicago/Turabian StyleHuang, Shaolin, Chengqun Yu, Gang Fu, Wei Sun, Shaowei Li, Fusong Han, and Jianyu Xiao. 2023. "Effects of Short-Term Nitrogen Addition on Soil Fungal Community Increase with Nitrogen Addition Rate in an Alpine Steppe at the Source of Brahmaputra" Microorganisms 11, no. 8: 1880. https://doi.org/10.3390/microorganisms11081880
APA StyleHuang, S., Yu, C., Fu, G., Sun, W., Li, S., Han, F., & Xiao, J. (2023). Effects of Short-Term Nitrogen Addition on Soil Fungal Community Increase with Nitrogen Addition Rate in an Alpine Steppe at the Source of Brahmaputra. Microorganisms, 11(8), 1880. https://doi.org/10.3390/microorganisms11081880







