Endophytic Plant Growth-Promoting Bacterium Bacillus subtilis Reduces the Toxic Effect of Cadmium on Wheat Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Bacterial Strain
2.2. Inoculum Preparation and Seed Treatment
2.3. Cd Stress Tolerance: Bacterial Strain Growth and Seed Germination In Vitro
2.4. Design of the Experiments and Growth Conditions
2.5. Growth Parameters
2.6. Leaves Photosynthetic Pigments
2.7. Lignin Content and Deposition in the Cell Wall of the Basal Part of the Roots
2.8. Lipid Peroxidation (MDA)
2.9. Hydrogen Peroxide (H2O2)
2.10. Electrolytes Leakage (EL)
2.11. Cd Content in Plants Tissues
2.12. Statistical Analysis
3. Results
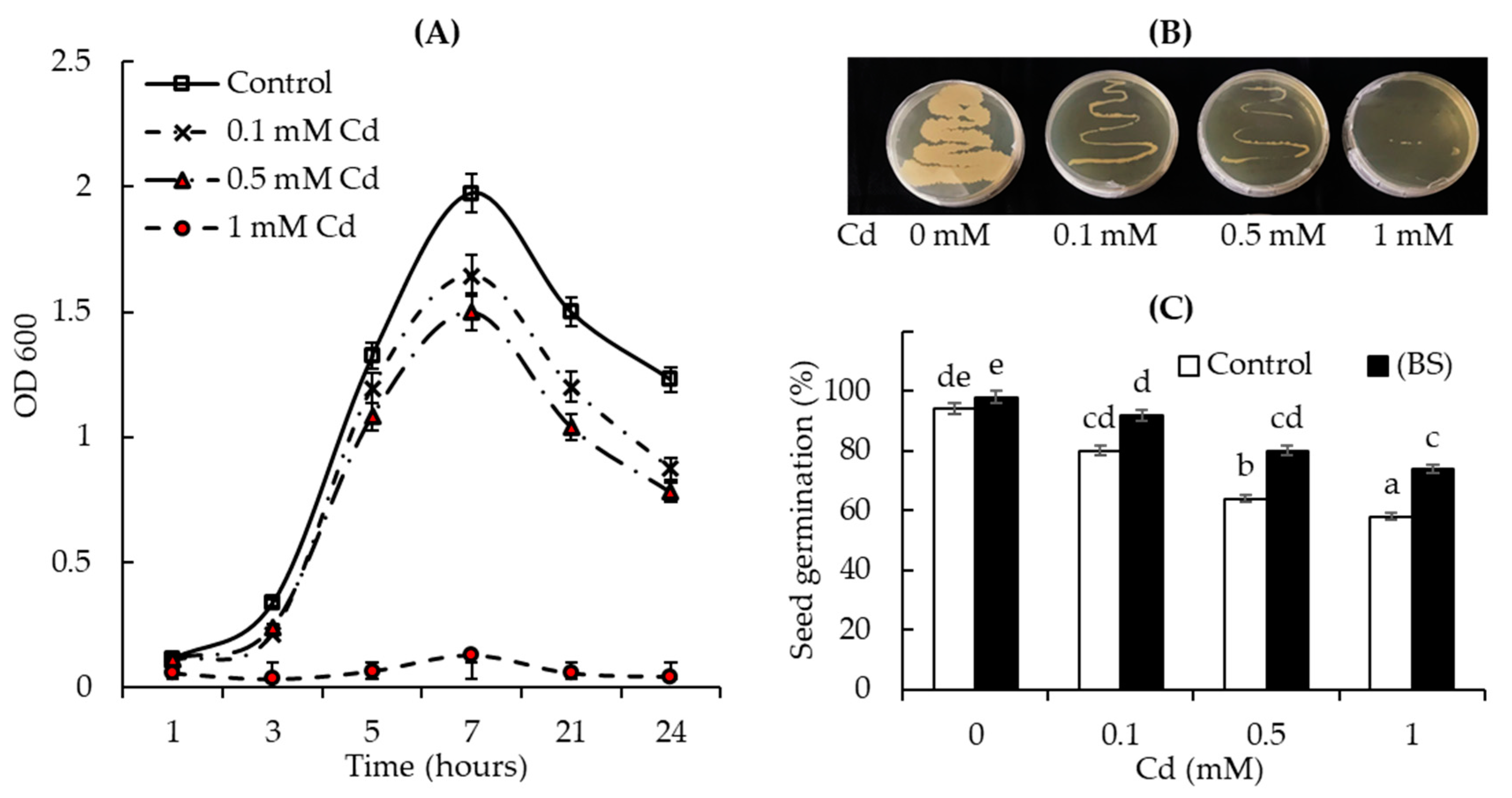
3.1. Cd Tolerance Test: Ability of Bacterial Cells and Wheat Seeds to Grow in the Presence of Different Concentrations of Cd Acetate
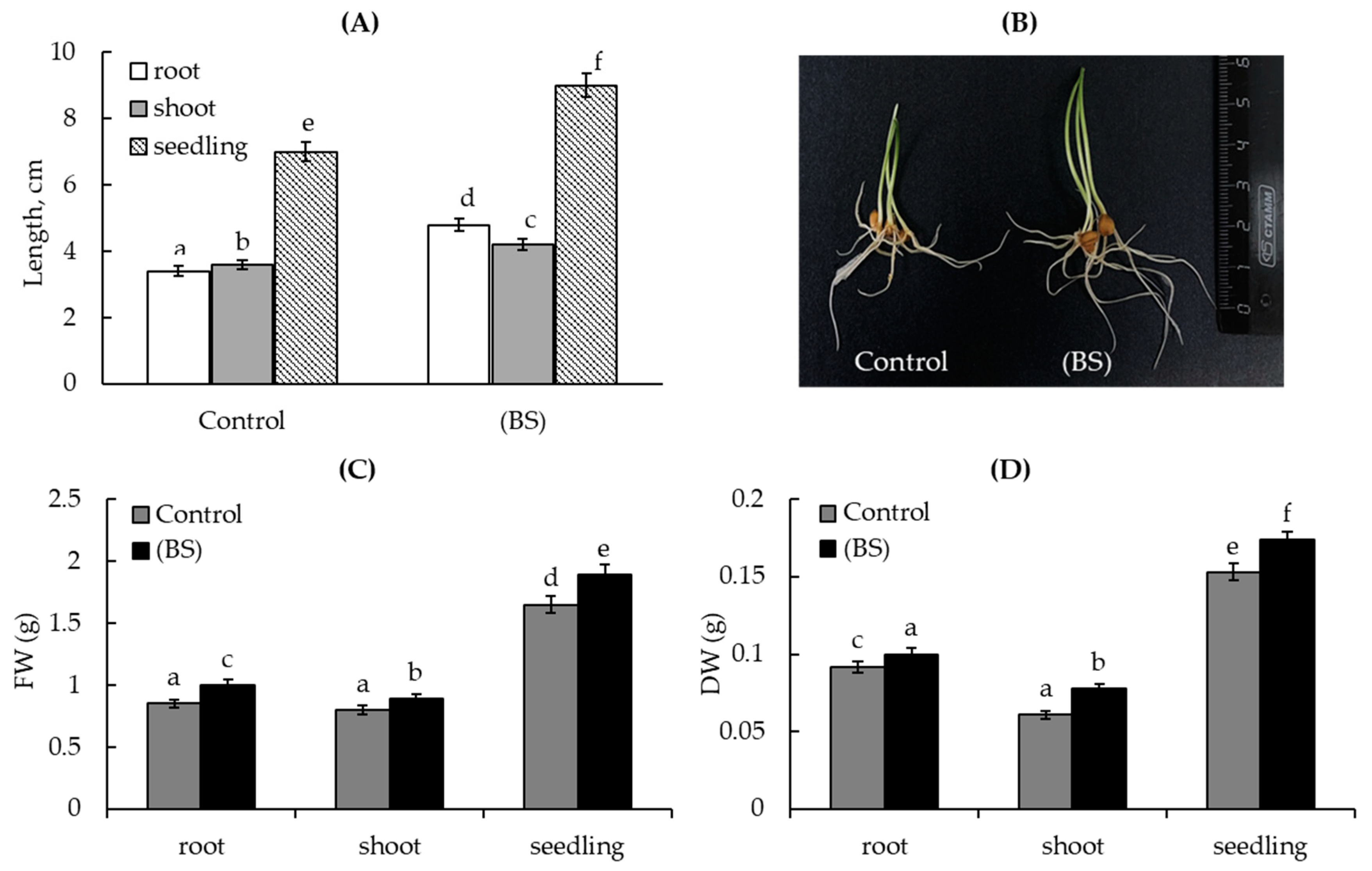
3.2. The Endophyte B. subtilis Improved Wheat Growth under Normal and Cd Acetate Stress Conditions
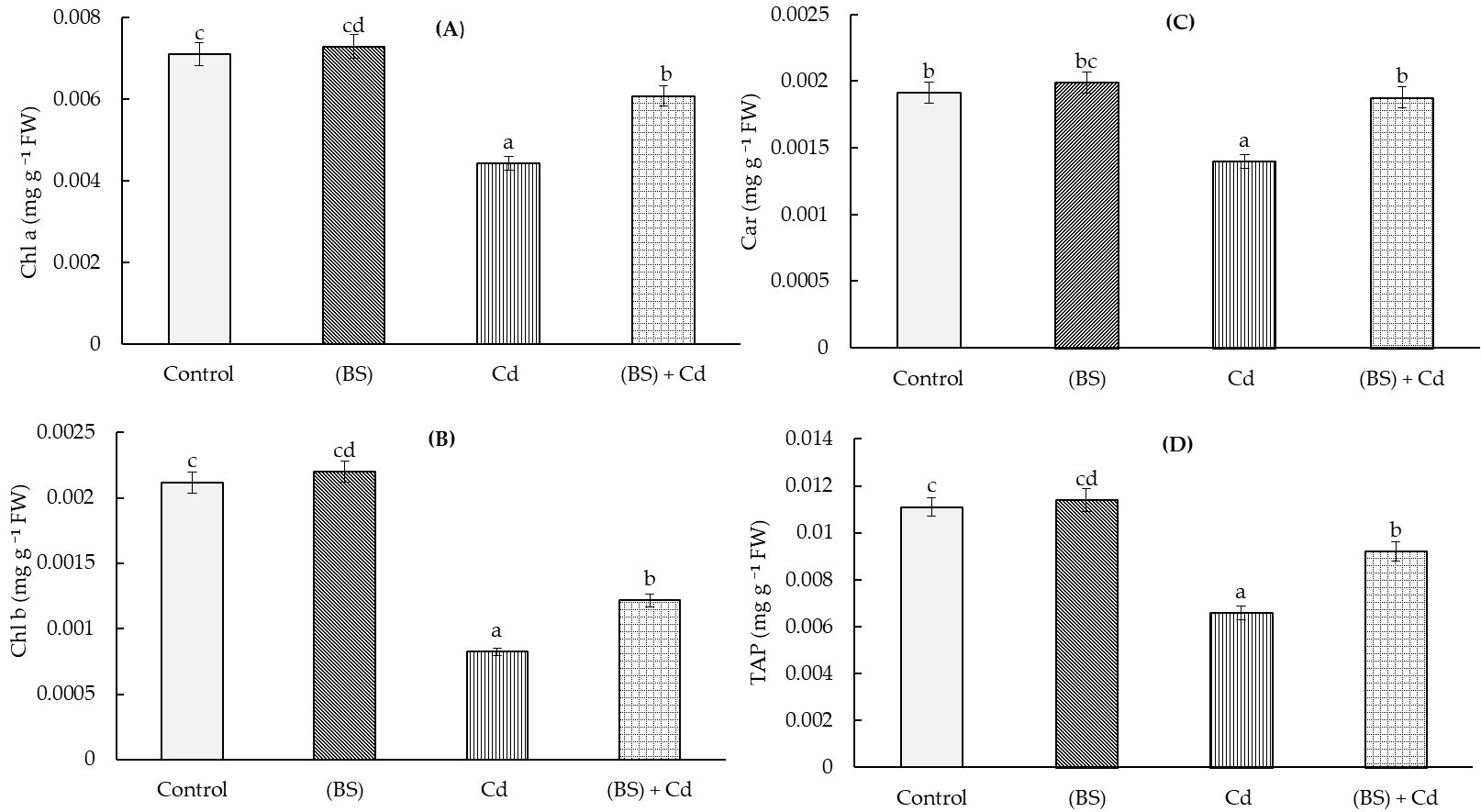
3.3. Effect of Cd Stress on Leaf Photosynthetic Pigments in B. subtilis-Treated Wheat
3.4. Effect of the Endophyte B. subtilis on Lignin Deposition and Content in Wheat Roots under Cd Stress
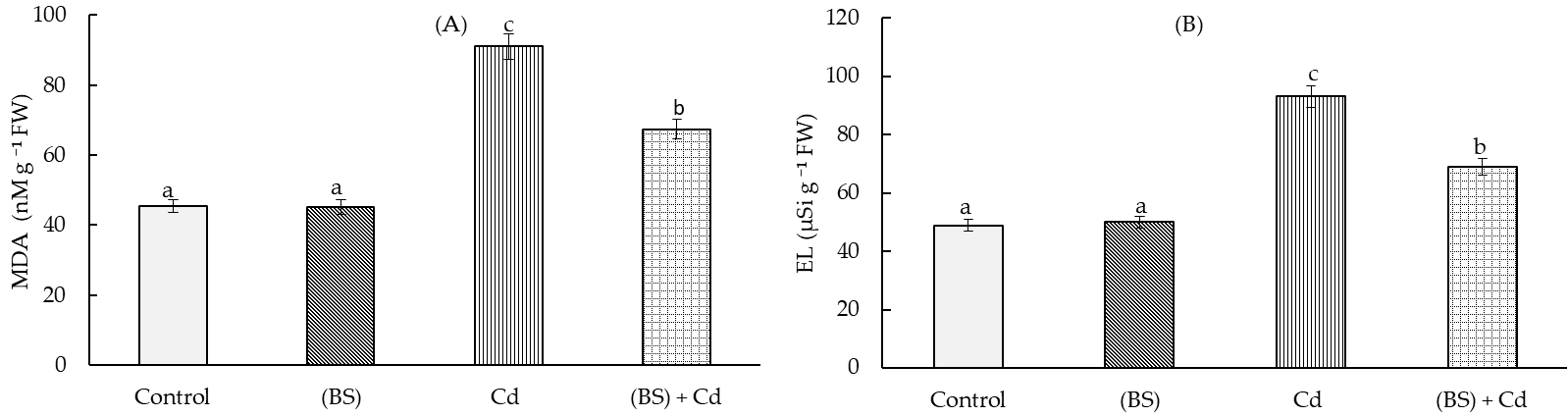
3.5. Levels of Oxidative Stress Markers in the Presence and Absence of the Endophyte B. subtilis under Cd Stress
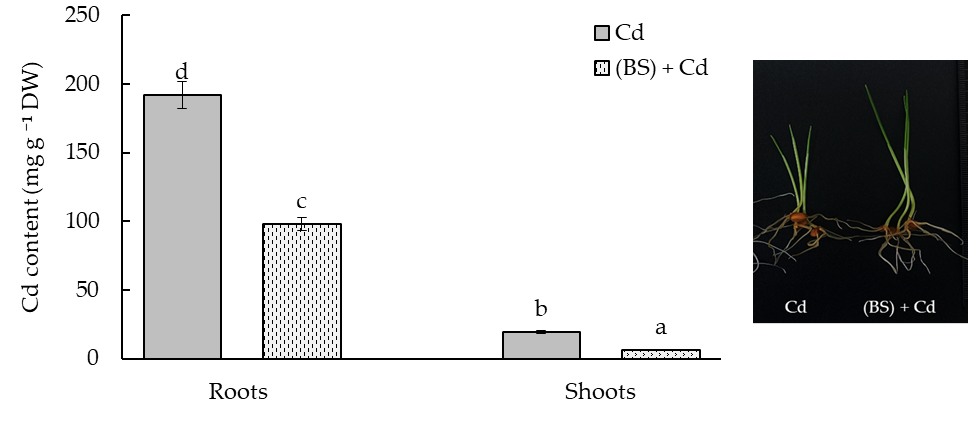
3.6. Effect of the Endophyte B. subtilis on Cd Uptake by the Wheat Seedlings
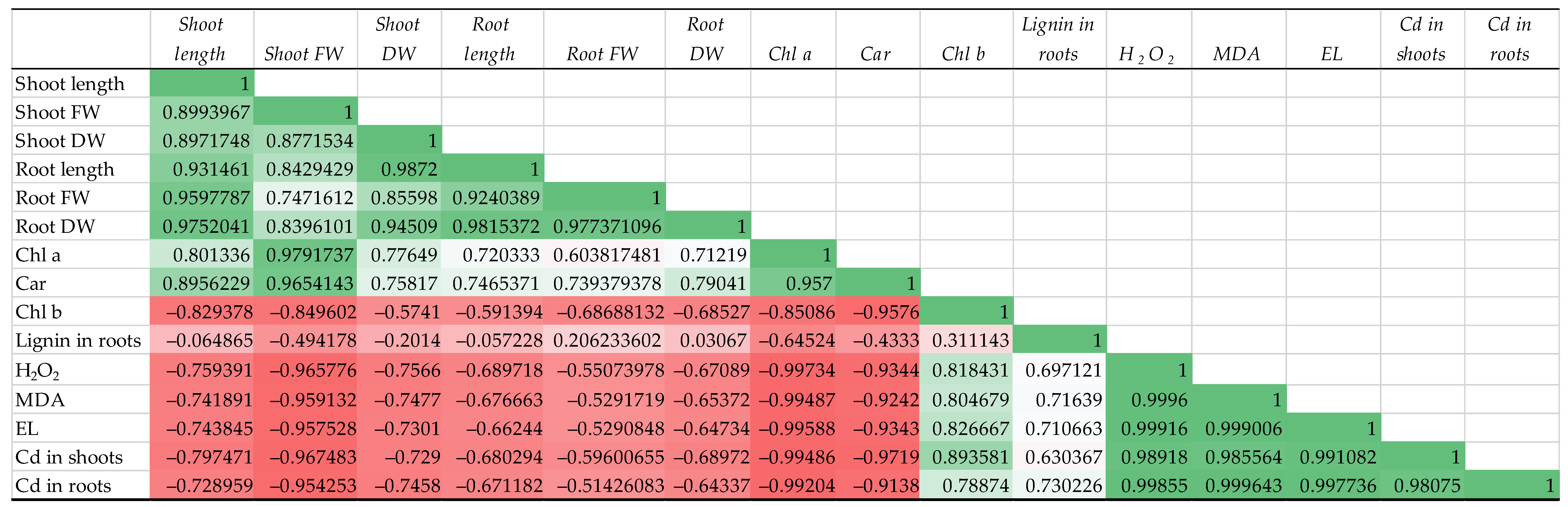
3.7. Correlation Matrices
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.A.; Bashir, O.; Haq, S.A.U.; Amin, T.; Rafiq, A.; Ali, M.; Américo-Pinheiro, J.H.P.; Sher, F. Phytoremediation of heavy metals in soil and water: An eco-friendly, sustainable and multidisciplinary approach. Chemosphere 2022, 303, 134788. [Google Scholar] [CrossRef] [PubMed]
- Henao, S.G.; Ghneim-Herrera, T. Heavy Metals in Soils and the Remediation Potential of Bacteria Associated with the Plant Microbiome. Front. Environ. Sci. 2021, 9, 604216. [Google Scholar] [CrossRef]
- Guo, G.; Lei, M.; Wang, Y.; Song, B.; Yang, J. Accumulation of As, Cd, and Pb in Sixteen Wheat Cultivars Grown in Contaminated Soils and Associated Health Risk Assessment. Int. J. Environ. Res. Public Health 2018, 15, 2601. [Google Scholar] [CrossRef]
- Sobolewska, M.; Wenda-Piesik, A.; Jaroszewska, A.; Stankowski, S. Effect of Habitat and Foliar Fertilization with K, Zn and Mn on Winter Wheat Grain and Baking Qualities. Agronomy 2020, 10, 276. [Google Scholar] [CrossRef]
- Abedi, T.; Mojiri, A. Cadmium Uptake by Wheat (Triticum aestivum L.): An Overview. Plants 2020, 9, 500. [Google Scholar] [CrossRef]
- Gao, X.; Grant, C.A. Cadmium and Zinc Concentration in Grain of Durum Wheat in Relation to Phosphorus Fertilization, Crop Sequence and Tillage Management. Appl. Environ. Soil Sci. 2012, 2012, 817107. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Abbas, T.; Zia-ur-Rehman, M.; Hannan, F.; Keller, C.; Al-Wabel, M.I.; Ok, Y.S. Cadmium minimization in wheat: A critical review. Ecotoxicol. Environ. Saf. 2016, 130, 43–53. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Z. Recent Advances in Minimizing Cadmium Accumulation in Wheat. Toxics 2022, 10, 187. [Google Scholar] [CrossRef]
- DalCorso, G.; Farinati, S.; Maistri, S.; Furini, A. How Plants Cope with Cadmium: Staking All on Metabolism and Gene Expression. J. Integr. Plant Biol. 2008, 50, 1268–1280. [Google Scholar] [CrossRef]
- Saleh, S.R.; Kandeel, M.M.; Ghareeb, D.; Ghoneim, T.M.; Talha, N.I.; Alaoui-Sossé, B.; Aleya, L.; Abdel-Daim, M.M. Wheat biological responses to stress caused by cadmium, nickel and lead. Sci. Total Environ. 2020, 706, 136013. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rahman, S.U.; Qiu, Z.; Shahzad, S.M.; Nawaz, M.F.; Huang, J.; Naveed, S.; Li, L.; Wang, X.; Cheng, H. Toxic effects of cadmium on the physiological and biochemical attributes of plants, and phytoremediation strategies: A review. Environ. Pollut. 2023, 325, 121433. [Google Scholar] [CrossRef] [PubMed]
- Ahemad, M. Remediation of metalliferous soils through the heavy metal resistant plant growth promoting bacteria: Paradigms and prospects. Arab. J. Chem. 2019, 12, 1365–1377. [Google Scholar] [CrossRef]
- Devi, R.; Kaur, T.; Kour, D.; Hricovec, M.; Mohan, R.; Yadav, N.; Rai, P.K.; Rai, A.K.; Yadav, A.; Kumar, M.; et al. Microbes-mediated alleviation of heavy metal stress in crops: Current research and future challenges. J. Appl. Biol. Biotechnol. 2022, 10, 25–37. [Google Scholar] [CrossRef]
- Wang, X.H.; Nie, Z.; He, L.; Wang, Q.; Sheng, X. Isolation of As-tolerant bacteria and their potentials of reducing As and Cd accumulation of edible tissues of vegetables in metal(loid)-contaminated soils. Sci. Total Environ. 2017, 579, 179–189. [Google Scholar] [CrossRef]
- Wang, X.-H.; Luo, W.-W.; Wang, Q.; He, L.-Y.; Sheng, X.-F. Metal(loid)-resistant bacteria reduce wheat Cd and As uptake in metal(loid)-contaminated soil. Environ. Pollut. 2018, 241, 529–539. [Google Scholar] [CrossRef]
- Khatri, S.; Sharma, R.K.; Shridhar, V. Influence of Cadmium-Tolerant and Plant Growth-Promoting Rhizobacteria on Cadmium Accumulation and Growth Response of Wheat Seedlings Under Mountain Ecosystem. Agric. Res. 2020, 9, 56–65. [Google Scholar] [CrossRef]
- Awan, S.A.; Ilyas, N.; Khan, I.; Raza, M.A.; Rehman, A.U.; Rizwan, M.; Rastogi, A.; Tariq, R.; Brestic, M. Bacillus siamensis Reduces Cadmium Accumulation and Improves Growth and Antioxidant Defense System in Two Wheat (Triticum aestivum L.) Varieties. Plants 2020, 9, 878. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, S. Bioremediation of heavy metals from industrial effluents by endophytes and their metabolic activity: Recent advances. Bioresour. Technol. 2021, 339, 125589. [Google Scholar] [CrossRef]
- Maslennikova, D.; Nasyrova, K.; Chubukova, O.; Akimova, E.; Baymiev, A.; Blagova, D.; Ibragimov, A.; Lastochkina, O. Effects of Rhizobium leguminosarum Thy2 on the Growth and Tolerance to Cadmium Stress of Wheat Plants. Life 2022, 12, 1675. [Google Scholar] [CrossRef]
- Shahabivand, S.; Maivan, H.Z.; Goltapeh, E.M.; Sharifi, M.; Aliloo, A.A. The effects of root endophyte and arbuscular mycorrhizal fungi on growth and cadmium accumulation in wheat under cadmium toxicity. Plant Physiol. Biochem. 2012, 60, 53–58. [Google Scholar] [CrossRef]
- Wang, X.-H.; Wang, Q.; Nie, Z.-W.; He, L.-Y.; Sheng, X.-F. Ralstonia eutropha Q2-8 reduces wheat plant above-ground tissue cadmium and arsenic uptake and increases the expression of the plant root cell wall organization and biosynthesis-related proteins. Environ. Pollut. 2018, 242, 1488–1499. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Ali, N.; Jan, G.; Jan, F.G.; Rahman, I.U.; Iqbal, A.; Hamayun, M. IAA producing fungal endophyte Penicillium roqueforti Thom., enhances stress tolerance and nutrients uptake in wheat plants grown on heavy metal contaminated soils. PLoS ONE 2018, 13, e0208150. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.A.; Asaf, S.; Lubna, N.; Lee, I.-J.; Kim, K.M. Metal Resistant Endophytic Bacteria Reduces Cadmium, Nickel Toxicity, and Enhances Expression of Metal Stress Related Genes with Improved Growth of Oryza Sativa, via Regulating Its Antioxidant Machinery and Endogenous Hormones. Plants 2019, 8, 363. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Chatterjee, S.; Kataki, S.; Rastogi, R.P.; Gupta, D.K. Bacterial tolerance strategies against lead toxicity and their relevance in bioremediation application. Environ. Sci. Pollut. Res. 2021, 28, 14271–14284. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Bhattacharjee, S.; Saha, J.; Sarkar, M.; Mandal, P. Bacterial survival strategies and responses under heavy metal stress: A comprehensive overview. Crit. Rev. Microbiol. 2022, 48, 327–355. [Google Scholar] [CrossRef] [PubMed]
- Martynenko, E.; Arkhipova, T.; Safronova, V.; Seldimirova, O.; Galin, I.; Akhtyamova, Z.; Veselov, D.; Ivanov, R.; Kudoyarova, G. Effects of Phytohormone-Producing Rhizobacteria on Casparian Band Formation, Ion Homeostasis and Salt Tolerance of Durum Wheat. Biomolecules 2022, 12, 230. [Google Scholar] [CrossRef] [PubMed]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Praveen, S.; Divte, P.R.; Mitra, R.; Kumar, M.; Gupta, C.K.; Kalidindi, U.; Bansal, R.; Roy, S.; Anand, A.; et al. Metal tolerance in plants: Molecular and physicochemical interface determines the “not so heavy effect” of heavy metals. Chemosphere 2022, 287, 131957. [Google Scholar] [CrossRef]
- Dalvi, A.A.; Bhalerao, S.A. Response of plants towards heavy metal toxicity: An overview of avoidance, tolerance and uptake mechanism. Ann. Plant Sci. 2013, 2, 362–368. [Google Scholar]
- Baker, A.J.M. Metal tolerance. New Phytol. 1987, 106, 93–111. [Google Scholar] [CrossRef]
- Pál, M.; Horváth, E.; Janda, T.; Páldi, E.; Szalai, G. Physiological changes and defense mechanisms induced by cadmium stress in maize. J. Plant Nutr. Soil Sci. 2006, 169, 239–246. [Google Scholar] [CrossRef]
- Shrivastava, M.; Khandelwal, A.; Srivastava, S. Heavy metal hyperaccumulator plants: The resource to under-stand the extreme adaptations of plants towards heavy metals. In Plant-Metal Interactions; Springer: Cham, Switzerland, 2019; pp. 79–97. [Google Scholar] [CrossRef]
- Lastochkina, O. Bacillus subtilis-Mediated Abiotic Stress Tolerance in Plants. In Bacilli and Agrobiotechnology: Phytostimulation and Biocontrol; Islam, M., Rahman, M., Pandey, P., Boehme, M., Haesaert, G., Eds.; Springer: Cham, Switzerland, 2019; pp. 97–133. [Google Scholar] [CrossRef]
- Mallick, I.; Bhattacharyya, C.; Mukherji, S.; Dey, D.; Sarkar, S.C.; Mukhopadhyay, U.K.; Ghosh, A. Effective rhizoinoculation and biofilm formation by arsenic immobilizing halophilic plant growth promoting bacteria (PGPB) isolated from mangrove rhizosphere: A step towards arsenic rhizoremediation. Sci. Total Environ. 2018, 610–611, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Zhang, J.; Tschaplinski, T.J.; Tuskan, G.A.; Chen, J.-G.; Muchero, W. Regulation of Lignin Biosynthesis and Its Role in Growth-Defense Tradeoffs. Front. Plant Sci. 2018, 9, 1427. [Google Scholar] [CrossRef]
- Rajkumar, M.; Ma, Y.; Freitas, H. Characterization of metal-resistant plant-growth promoting Bacillus weihenstephanensis isolated from serpentine soil in Portugal. J. Basic Microbiol. 2008, 48, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.-F.; Xia, J.-J. Improvement of rape (Brassica napus) plant growth and cadmium uptake by cadmium-resistant bacteria. Chemosphere 2006, 64, 1036–1042. [Google Scholar] [CrossRef]
- Zaidi, S.; Usmani, S.; Singh, B.R.; Musarrat, J. Significance of Bacillus subtilis strain SJ-101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea. Chemosphere 2006, 64, 991–997. [Google Scholar] [CrossRef]
- Dourado, M.N.; Martins, P.F.; Quecine, M.C.; Piotto, F.A.; Souza, L.A.; Franco, M.R.; Tezotto, T.; Azevedo, R.A. Burkholderia sp. SCMS54 reduces cadmium toxicity and promotes growth in tomato. Ann. Appl. Biol. 2013, 163, 494–507. [Google Scholar] [CrossRef]
- Lin, X.; Mou, R.; Cao, Z.; Xu, P.; Wu, X.; Zhu, Z.; Chen, M. Characterization of cadmium-resistant bacteria and their potential for reducing accumulation of cadmium in rice grains. Sci. Total Environ. 2016, 569–570, 97–104. [Google Scholar] [CrossRef]
- Wiewióra, B.; Żurek, G. The Response of the Associations of Grass and Epichloë Endophytes to the Increased Content of Heavy Metals in the Soil. Plants 2021, 10, 429. [Google Scholar] [CrossRef]
- Żurek, G.; Wiewióra, B.; Rybka, K.; Prokopiuk, K. Different response of perennial ryegrass—Epichloë endophyte symbiota to the elevated concentration of heavy metals in soil. J. Appl. Genet. 2021, 63, 47–59. [Google Scholar] [CrossRef]
- Lastochkina, O.; Aliniaeifard, S.; Garshina, D.; Garipova, S.; Pusenkova, L.; Allagulova, C.; Fedorova, K.; Baymiev, A.; Koryakov, I.; Sobhani, M. Seed priming with endophytic Bacillus subtilis strain-specifically improves growth of Phaseolus vulgaris plants under normal and salinity conditions and exerts anti-stress effect through induced lignin deposition in roots and decreased oxidative and osmotic damages. J. Plant Physiol. 2021, 263, 153462. [Google Scholar] [CrossRef] [PubMed]
- Lastochkina, O.; Pusenkova, L.; Yuldashev, R.; Babaev, M.; Garipova, S.; Blagova, D.; Khairullin, R.; Aliniaeifard, S. Effects of Bacillus subtilis on some physiological and biochemical parameters of Triticum aestivum L. (wheat) under salinity. Plant Physiol. Biochem. 2017, 121, 80–88. [Google Scholar] [CrossRef]
- Lastochkina, O.; Ivanov, S.; Petrova, S.; Garshina, D.; Lubyanova, A.; Yuldashev, R.; Kuluev, B.; Zaikina, E.; Maslennikova, D.; Allagulova, C.; et al. Role of Endogenous Salicylic Acid as a Hormonal Intermediate in the Bacterial Endophyte Bacillus subtilis-Induced Protection of Wheat Genotypes Contrasting in Drought Susceptibility under Dehydration. Plants 2022, 11, 3365. [Google Scholar] [CrossRef] [PubMed]
- Lastochkina, O.; Yakupova, A.; Avtushenko, I.; Lastochkin, A.; Yuldashev, R. Effect of Seed Priming with Endophytic Bacillus subtilis on Some Physio-Biochemical Parameters of Two Wheat Varieties Exposed to Drought after Selective Herbicide Application. Plants 2023, 12, 1724. [Google Scholar] [CrossRef]
- Maslennikova, D.; Lastochkina, O. Contribution of Ascorbate and Glutathione in Endobacteria Bacillus subtilis-Mediated Drought Tolerance in Two Triticum aestivum L. Genotypes Contrasting in Drought Sensitivity. Plants 2021, 10, 2557. [Google Scholar] [CrossRef]
- Berlanga-Clavero, M.V.; Molina-Santiago, C.; Caraballo-Rodríguez, A.M.; Petras, D.; Díaz-Martínez, L.; Pérez-García, A.; de Vicente, A.; Carrión, V.J.; Dorrestein, P.C.; Romero, D. Bacillus subtilis biofilm matrix components target seed oil bodies to promote growth and anti-fungal resistance in melon. Nat. Microbiol. 2022, 7, 1001–1015. [Google Scholar] [CrossRef]
- Netrusov, A.I.; Egorova, M.A.; Zakharchuk, L.M. Praktikum po Mikrobiologii (A Practical Course in Microbiology); Izdat. Tsentr Akademiya: Moscow, Russia, 2005; 608p. [Google Scholar]
- Mokronosova, A.T. Small Workshop on Plant Physiology; Moscow State University: Moscow, Russia, 1994; p. 184. [Google Scholar]
- Jeffrey, S.W.; Humphrey, G.F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Shakirova, F.M.; Allagulova, C.R.; Maslennikova, D.R.; Klyuchnikova, E.O.; Avalbaev, A.M.; Bezrukova, M.V. Salicylic acid-induced protection against cadmium toxicity in wheat plants. Environ. Exp. Bot. 2016, 122, 19–28. [Google Scholar] [CrossRef]
- Sedlarova, M.; Lebeda, A. Histochemical Detection and Role of Phenolic Compounds in the Defense Response of Lactuca spp. to Lettuce Downy Mildew (Bremia lactucae). J. Phytopathol. 2001, 149, 693–697. [Google Scholar] [CrossRef]
- Sancho, M.A.; De Forchetti, S.M.; Pliego, F.; Valpuesta, V.; Quesada, M.A. Peroxidase activity and isoenzymes in the culture medium of NaCl adapted tomato suspension cells. Plant Cell Tissue Organ Cult. 1996, 44, 161–167. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Bindschedler, L.V.; Minibayeva, F.; Gardner, S.L.; Gerrish, C.; Davies, D.R.; Bolwell, G.P. Early signalling events in the apoplastic oxidative burst in suspension cultured French bean cells involve cAMP and Ca2+. New Phytol. 2001, 151, 185–194. [Google Scholar] [CrossRef]
- Bajji, M.; Kinet, J.-M.; Lutts, S. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul. 2001, 36, 61–70. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Zhang, C.; Freitas, H. Beneficial role of bacterial endophytes in heavy metal phytoremediation. J. Environ. Manag. 2016, 174, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Wang, Q.; Lv, M.; Chen, L. Microorganism remediation strategies towards heavy metals. J. Chem. Eng. 2019, 360, 1553–1563. [Google Scholar] [CrossRef]
- Singh, Y.; Ramteke, P.; Shukla, P.K. Isolation and characterization of heavy metal resistant Pseudomonas spp. and their plant growth promoting activities. Adv. Appl. Sci. Res. 2013, 4, 269–272. [Google Scholar]
- Lastochkina, O.; Garshina, D.; Ivanov, S.; Yuldashev, R.; Khafizova, R.; Allagulova, C.; Fedorova, K.; Avalbaev, A.; Maslennikova, D.; Bosacchi, M. Seed Priming with Endophytic Bacillus subtilis Modulates Physiological Responses of Two Different Triticum aestivum L. Cultivars under Drought Stress. Plants 2020, 9, 1810. [Google Scholar] [CrossRef]
- Paul, M.J. Improving Photosynthetic Metabolism for Crop Yields: What Is Going to Work? Front. Plant Sci. 2021, 12, 743862. [Google Scholar] [CrossRef]
- El-Shahir, A.A.; El-Tayeh, N.A.; Ali, O.M.; Latef, A.A.H.A.; Loutfy, N. The Effect of Endophytic Talaromyces pinophilus on Growth, Absorption and Accumulation of Heavy Metals of Triticum aestivum Grown on Sandy Soil Amended by Sewage Sludge. Plants 2021, 10, 2659. [Google Scholar] [CrossRef]
- Tamás, L.; Mistrík, I.; Huttová, J.; Halušková, L.; Valentovičová, K.; Zelinová, V. Role of reactive oxygen species-generating enzymes and hydrogen peroxide during cadmium, mercury and osmotic stresses in barley root tip. Planta 2010, 231, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, J.V.; Kuramshina, Z.M.; Horohorina, E.E.; Khairullin, R.M. Influence of Bacteria Bacillus subtilis Antioxidant Enzymes Triticum aestivum under the Joint Action of Cadmium and Lead. Mod. Probl. Sci. Educ. J. 2016, 6, 541. [Google Scholar]
- Kuramshina, Z.M.; Smirnova, Y.V.; Khairullin, R.M. Cadmium and Nickel Toxicity for Sinapis alba Plants Inoculated with Endophytic Strains of Bacillus subtilis. Russ. J. Plant Physiol. 2018, 65, 269–277. [Google Scholar] [CrossRef]
- Treesubsuntorn, C.; Dhurakit, P.; Khaksar, G.; Thiravetyan, P. Effect of microorganisms on reducing cadmium uptake and toxicity in rice (Oryza sativa L.). Environ. Sci. Pollut. Res. 2017, 25, 25690–25701. [Google Scholar] [CrossRef] [PubMed]
- Jha, Y. Cell water content and lignification in maize regulated by rhizobacteria under salinity. Braz. J. Biol. Sci. 2017, 4, 9–18. [Google Scholar] [CrossRef]
- Diba, F.; Alam, F.; Talukder, A.A. Screening of Acetic Acid Producing Microorganisms from Decomposed Fruits for Vinegar Production. Adv. Microbiol. 2015, 05, 291–297. [Google Scholar] [CrossRef]
- Mesa, J.; Mateos-Naranjo, E.; Caviedes, M.A.; Redondo-Gómez, S.; Pajuelo, E.; Rodríguez-Llorente, I.D. Endophytic Cultivable Bacteria of the Metal Bioaccumulator Spartina maritima Improve Plant Growth but Not Metal Uptake in Polluted Marshes Soils. Front. Microbiol. 2015, 6, 1450. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Zia-Ur-Rehman, M.; Qayyum, M.F.; Abbas, F.; Hannan, F.; Rinklebe, J.; Ok, Y.S. Effect of biochar on cadmium bioavailability and uptake in wheat (Triticum aestivum L.) grown in a soil with aged contamination. Ecotoxicol. Environ. Saf. 2017, 140, 37–47. [Google Scholar] [CrossRef]
- Sessitsch, A.; Kuffner, M.; Kidd, P.; Vangronsveld, J.; Wenzel, W.W.; Fallmann, K.; Puschenreiter, M. The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol. Biochem. 2013, 60, 182–194. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Yusof, M.L.M.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Lignin Content (ΔA540 nm/g FW) | Visual Assessment of Lignin Deposition in Roots | |
|---|---|---|---|
| 4-Day-Old | 5-Day-Old | ||
| Control | 0 a | 0 a |  |
| (BS) | 1.12 ± 0.045 b | 1.21 ± 0.049 b |  |
| Cd | N/A | 1.88 ± 0.079 c |  |
| (BS) + Cd | N/A | 2.1 ± 0.094 d |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maslennikova, D.; Koryakov, I.; Yuldashev, R.; Avtushenko, I.; Yakupova, A.; Lastochkina, O. Endophytic Plant Growth-Promoting Bacterium Bacillus subtilis Reduces the Toxic Effect of Cadmium on Wheat Plants. Microorganisms 2023, 11, 1653. https://doi.org/10.3390/microorganisms11071653
Maslennikova D, Koryakov I, Yuldashev R, Avtushenko I, Yakupova A, Lastochkina O. Endophytic Plant Growth-Promoting Bacterium Bacillus subtilis Reduces the Toxic Effect of Cadmium on Wheat Plants. Microorganisms. 2023; 11(7):1653. https://doi.org/10.3390/microorganisms11071653
Chicago/Turabian StyleMaslennikova, Dilara, Igor Koryakov, Ruslan Yuldashev, Irina Avtushenko, Albina Yakupova, and Oksana Lastochkina. 2023. "Endophytic Plant Growth-Promoting Bacterium Bacillus subtilis Reduces the Toxic Effect of Cadmium on Wheat Plants" Microorganisms 11, no. 7: 1653. https://doi.org/10.3390/microorganisms11071653
APA StyleMaslennikova, D., Koryakov, I., Yuldashev, R., Avtushenko, I., Yakupova, A., & Lastochkina, O. (2023). Endophytic Plant Growth-Promoting Bacterium Bacillus subtilis Reduces the Toxic Effect of Cadmium on Wheat Plants. Microorganisms, 11(7), 1653. https://doi.org/10.3390/microorganisms11071653







