Atypical Cutaneous Viral Infections Reveal an Inborn Error of Immunity in 8 Patients
Abstract
1. Introduction
2. Material and Methods
- A-
- Clinical: patients with atypical isolated or syndromic cutaneous viral infections. To be classified as atypical, these infections have to be either chronic or recurrent, profuse (more than 20 lesions in more than one region of the body for warts) or recalcitrant and resistant to treatment (6 months for warts) [7].
- B-
- Immunological: patients with abnormal blood cell, lymphocytes subpopulations or immunoglobulins count, in the absence of any recent immunosuppressive treatment or HIV infection.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bousfiha, A.; Jeddane, L.; Picard, C.; Al-Herz, W.; Ailal, F.; Chatila, T.; Cunningham-Rundles, C.; Etzioni, A.; Franco, J.L.; Holland, S.M.; et al. Human Inborn Errors of Immunity: 2019 Update of the IUIS Phenotypical Classification. J. Clin. Immunol. 2020, 40, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Saeidian, A.H.; Youssefian, L.; Huang, C.Y.; Palizban, F.; Naji, M.; Saffarian, Z.; Mahmoudi, H.; Goodarzi, A.; Sotoudeh, S.; Vahidnezhad, F.; et al. Whole-transcriptome sequencing–based concomitant detection of viral and human genetic determinants of cutaneous lesions. J. Clin. Investig. 2022, 7, e156021. [Google Scholar] [CrossRef]
- Orth, G. Les papillomavirus, “du col à la peau”; Institut Pasteur: Paris, France; 44p, Available online: https://www.college-de-france.fr/sites/default/files/documents/philippe-sansonetti/UPL1600613715525900088_Gerard_Orth.pdf (accessed on 24 December 2022).
- Beauman, J.G. Genital herpes: A review. Am. Fam. Physician 2005, 72, 1527–1534. [Google Scholar] [PubMed]
- Brown, J.; Janniger, C.K.; Schwartz, R.A.; Silverberg, N.B. Childhood molluscum contagiosum. Int. J. Dermatol. 2006, 45, 93–99. [Google Scholar] [CrossRef] [PubMed]
- El Kettani, A.; Ailal, F.; El Bakkouri, J.; Zerouali, K.; Béziat, V.; Jouanguy, E.; Casanova, J.L.; Bousfiha, A.A. HPV-Related Skin Phenotypes in Patients with Inborn Errors of Immunity. Pathogens 2022, 11, 857. [Google Scholar] [CrossRef] [PubMed]
- de Jong, S.J.; Créquer, A.; Matos, I.; Hum, D.; Gunasekharan, V.; Lorenzo, L.; Jabot-Hanin, F.; Imahorn, E.; Arias, A.A.; Vahidnezhad, H.; et al. The human CIB1-EVER1-EVER2 complex governs keratinocyte-intrinsic immunity to β-papillomaviruses. J. Exp. Med. 2018, 215, 2289–2310. [Google Scholar] [CrossRef] [PubMed]
- Béziat, V.; Jouanguy, E. Human inborn errors of immunity to oncogenic viruses. Curr. Opin. Immunol. 2021, 72, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Béziat, V. Human genetic dissection of papillomavirus-driven diseases: New insight into their pathogenesis. Hum. Genet. 2020, 139, 919–939. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.E.; Kilic, S.S.; Aytekin, C.; Kumar, A.; Porras, O.; Kainulainen, L.; Kostyuchenko, L.; Genel, F.; Kütükcüler, N.; Karaca, N.; et al. DOCK8 Deficiency: Clinical and Immunological Phenotype and Treatment Options—A Review of 136 Patients. J. Clin. Immunol. 2015, 35, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Genetic Test Catalog: Genetic Test Panels from Invitae. Available online: https://www.invitae.com/en/providers/test-catalog (accessed on 27 December 2022).
- Davy, C.; Doorbar, J. Human Papillomaviruses: Methods and Protocols; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2005; 495p. [Google Scholar]
- Picard, C.; Bobby Gaspar, H.; Al-Herz, W.; Bousfiha, A.; Casanova, J.L.; Chatila, T.; Crow, Y.J.; Cunningham-Rundles, C.; Etzioni, A.; Franco, J.L.; et al. International Union of Immunological Societies: 2017 Primary Immunodeficiency Diseases Committee Report on Inborn Errors of Immunity. J. Clin. Immunol. 2018, 38, 96–128. [Google Scholar] [CrossRef] [PubMed]
- Orth, G. Genetics of epidermodysplasia verruciformis: Insights into host defense against papillomaviruses. Semin. Immunol. 2006, 18, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Abdollahpour, H.; Appaswamy, G.; Kotlarz, D.; Diestelhorst, J.; Beier, R.; Schäffer, A.A.; Gertz, E.M.; Schambach, A.; Kreipe, H.H.; Pfeifer, D.; et al. The phenotype of human STK4 deficiency. Blood 2012, 119, 3450–3457. [Google Scholar] [CrossRef] [PubMed]
- Vece, T.J.; Watkin, L.B.; Nicholas, S.K.; Canter, D.; Braun, M.C.; Guillerman, R.P.; Eldin, K.W.; Bertolet, G.; McKinley, S.D.; de Guzman, M.; et al. Copa Syndrome: A Novel Autosomal Dominant Immune Dysregulatory Disease. J. Clin. Immunol. 2016, 36, 377–387. [Google Scholar] [CrossRef] [PubMed]
- de Jong, S.J.; Imahorn, E.; Itin, P.; Uitto, J.; Orth, G.; Jouanguy, E.; Casanova, J.L.; Burger, B. Epidermodysplasia Verruciformis: Inborn Errors of Immunity to Human Beta-Papillomaviruses. Front. Microbiol. 2018, 9, 1222. [Google Scholar] [CrossRef] [PubMed]
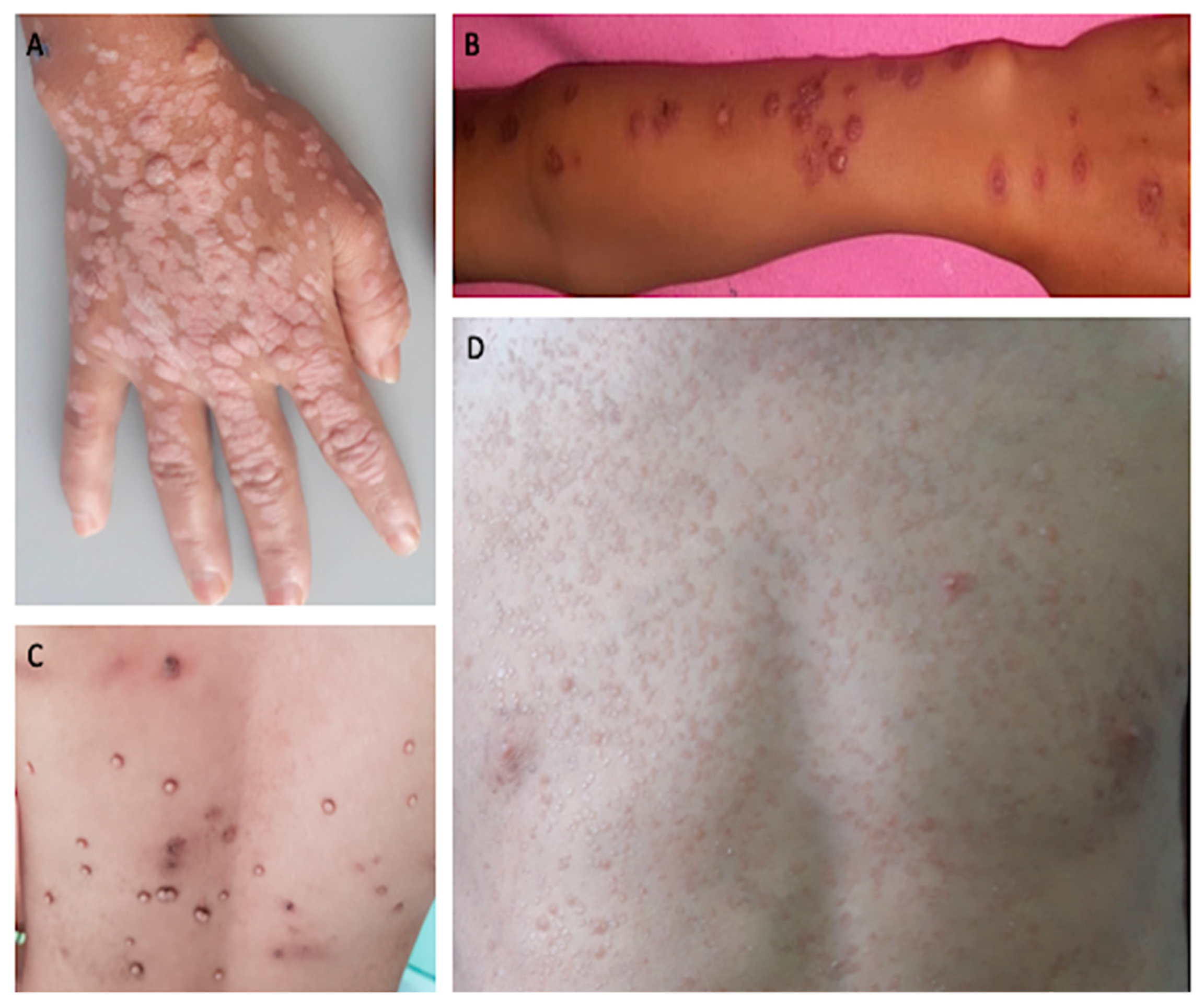
| Pt | Age of Onset (yrs) | Sex | Reason for Consultation | Site of the Lesions | Family and Personal History | Blood Cell Count Unit: cells/mm3 (Pathological) | Lymphocyte/Immunoglobulin Subpopulations (Pathological) | Histology | Genetic Diagnosis (Mutation) | Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|
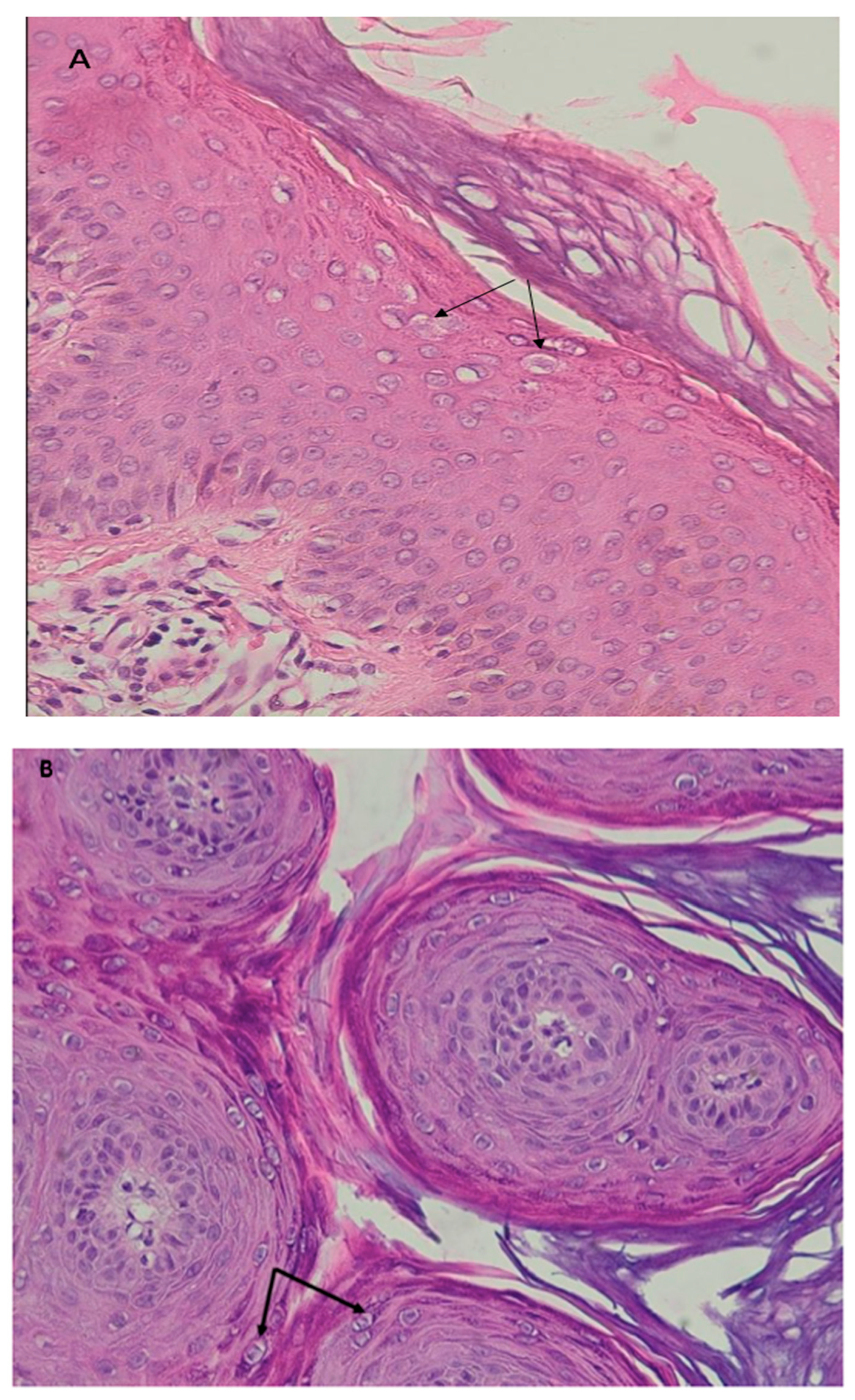
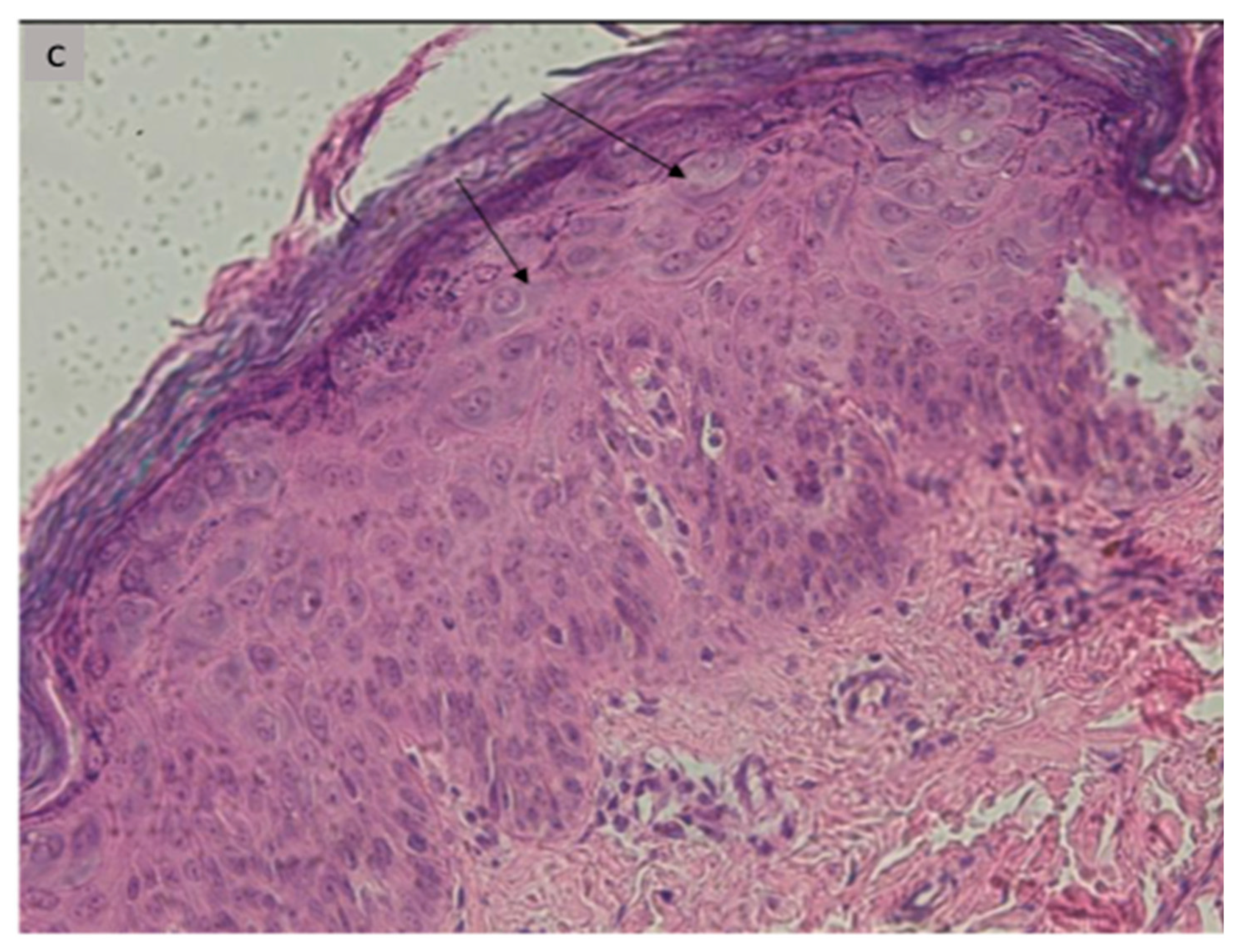
| 1 | 12 | M | Recalcitrant profuse verrucous lesions (flat and pityriasiform warts) since the age of 6 years | Face, neck, trunk, arms | Sister followed for CVID Recurrent diarrhea since the age of 10 | Lymphopenia 900/mm3 | CD3+: 771/mm3 CD4+: 253/mm3 CD19+: 128/mm3 CD16+, CD56+: 743/mm3 | Koilocytes, keratinocytes with pale blue cytoplasm in the upper epidermis associated with high levels of intranuclear viral replication | STK4 (homozygous, c.1305 + 1G > A) | LOCID+ Syndromic epidermo- dysplasia verruciformis |
| 2 | 26 | F | Recalcitrant profuse verrucous lesions (flat and vulgar warts) since the age of 15 years | Face, forearms, hands, legs, feet | No special features | Monocytopenia 100/mm3 | CD19+: 9/mm3 CD16+, CD56+: 64/mm3 Absence of CD14+ monocytic cells | Papillomatous epidermis related to common warts | GATA2 (c.1070C > T, p.T357I) | DCML deficiency |
| 3 | 10 | M | Recurrent post-herpetic erythema multiforme (cocade lesions) since the age of 9 | Face, arms, forearms, hands | Two identical episodes of post-herpetic erythema multiforme in the current year | Lymphopenia 1050/mm3 | CD3+: 945/mm3 CD4+: 453/mm3 CD8+: 338/mm3 | - | - | CID |
| 4 | 9 | F | Profuse chronic Molluscum contagiosum since the age of 2 | Face, trunk, arms, forearms, hands | Twin sister of patient 5 Since the age of one: Recurrent respiratory infections At 2 years old: sarcoidosis | Microcytic hypochromic anemia | Normal | - | COPA (c.3653T > C, p.Ile1218Thr) | CID |
| 5 | 9 | F | Profuse chronic Molluscum contagiosum since the age of 2 | Face, trunk, arms, forearms, hands | Twin sister of patient 4 Since the age of one: Recurrent respiratory infections At 2 years old: sarcoidosis | Microcytic hypochromic anemia | Normal | - | COPA (c.3653T > C p.Ile1218Thr) | CID |
| 6 | 8 | F | Profuse recalcitrant warty lesions (flat and pityriasiform warts) Since the age of 3 years | Face, neck trunk, arms, forearms, hands | Recurrent respiratory infections since the age of 3 years, with stature weight repercussions, Diarrhea | Neutropenia 580/mm3 Lymphopenia: 1750/mm3 | CD4+: 254/mm3 CD19+:130/mm3 CD16+, CD56+: 64/mm3 | Koilocytes, keratinocytes with pale blue cytoplasm in the upper epidermis associated with high levels of intranuclear viral replication | STK4 (homozygous, c.750G > A, p.W250) | CID+ Syndromic epidermo- dysplasia verruciformis |
| 7 | 18 | F | Recalcitrant profuse warty lesions (flat and vulgar warts) since the age of 10 years | Face, neck, trunk, arms, forearms, hands, thighs, legs, feet Recurrent diarrhea | Recurrent respiratory infections | Leukocytes: 3160/mm3 lymphopenia: 430/mm3 | CD4+: 171/mm3 CD8+: 182/mm3 CD19+: 22/mm3 CD16+, CD56+: 90/mm3 | Papillomatous epidermis related to common warts with no particularity | - | LOCID |
| 8 | 7 | F | Profuse chronic Molluscum contagiosum lesions | Trunk, arms, forearms | For the past year and a half: -Recurrent respiratory infections -leukorrhea, vulvitis -Eczema lesions | High PNE: 1070/mm3 | IgE: 162 kUI/l | - | - | Hyper IgE syndrome |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Kettani, A.; Ailal, F.; Marnissi, F.; Hali, F.; El Bakkouri, J.; Benhsaien, I.; Le Voyer, T.; Guèye, M.S.; Chevalier, R.; Chiheb, S.; et al. Atypical Cutaneous Viral Infections Reveal an Inborn Error of Immunity in 8 Patients. Microorganisms 2023, 11, 1202. https://doi.org/10.3390/microorganisms11051202
El Kettani A, Ailal F, Marnissi F, Hali F, El Bakkouri J, Benhsaien I, Le Voyer T, Guèye MS, Chevalier R, Chiheb S, et al. Atypical Cutaneous Viral Infections Reveal an Inborn Error of Immunity in 8 Patients. Microorganisms. 2023; 11(5):1202. https://doi.org/10.3390/microorganisms11051202
Chicago/Turabian StyleEl Kettani, Assiya, Fatima Ailal, Farida Marnissi, Fouzia Hali, Jalila El Bakkouri, Ibtihal Benhsaien, Tom Le Voyer, Mame Sokhna Guèye, Rémi Chevalier, Soumiya Chiheb, and et al. 2023. "Atypical Cutaneous Viral Infections Reveal an Inborn Error of Immunity in 8 Patients" Microorganisms 11, no. 5: 1202. https://doi.org/10.3390/microorganisms11051202
APA StyleEl Kettani, A., Ailal, F., Marnissi, F., Hali, F., El Bakkouri, J., Benhsaien, I., Le Voyer, T., Guèye, M. S., Chevalier, R., Chiheb, S., Zerouali, K., Jouanguy, E., Casanova, J.-L., & Bousfiha, A. A. (2023). Atypical Cutaneous Viral Infections Reveal an Inborn Error of Immunity in 8 Patients. Microorganisms, 11(5), 1202. https://doi.org/10.3390/microorganisms11051202






