Abstract
The contamination of peanuts, with Aspergillus flavus and subsequent aflatoxins (AFs) is considered to be one of the most serious, safety problems in the world. Water activity (aw) and temperature are limiting, factors for fungal growth and aflatoxin production during storage. The objectives of this study were to integrate data on the effects of temperature (34, 37, and 42 °C) and water activity (aw; 0.85, 0.90, and 0.95) on growth rate aflatoxin B1 (AFB1) production and up- or-downregulation of the molecular expression of biosynthetic AFB1 genes divided into three types based on their A. flavus isolate composition and AFB1 capacity in vitro: A. flavus KSU114 (high producer), A. flavus KSU114 (low producer), and A. flavus KSU121 (non-producer). The A. flavus isolates were shown to be resilient in terms of growth on yeast extract sucrose agar media when exposed to temperature and water activity as pivotal environmental factors. The optimal conditions for the fungal growth of three isolates were a temperature of 34 °C and water activity of 0.95 aw; there was very slow fungal growth at the highest temperature of 42 °C, with different aw values causing inhibited fungal growth. The AFB1 production for the three isolates followed the same pattern with one exception: A. flavus KSU114 failed to produce any AFB1 at 42 °C with different aw values. All tested genes of A. flavus were significantly up- or downregulated under three levels of interaction between temperature and aw. The late structural genes of the pathway were significantly upregulated at 34 °C under aw 0.95, although aflR, aflS and most of the early structural genes were upregulated. Compared to 34 °C with an aw value of 0.95, most of the expressed genes were significantly downregulated at 37 and 42 °C with aw values of 0.85 and 0.90. Additionally, two regulatory genes were downregulated under the same conditions. The expression level of laeA was also completely associated with AFB1 production, while the expression level of brlA was linked to A. flavus colonization. This information is required to forecast the actual effects of climate change on A. flavus. The findings can be applied to improve specific food technology processes and create prevention strategies to limit the concentrations of potential carcinogenic substances in peanuts and their derivatives.
1. Introduction
Peanut (Arachis hypogaea L.) is an important economic crop for oil production and a nutritious addition to, human consumption. One of the major problems associated with peanut production worldwide is aflatoxigenic Aspergillus isolates, whose contamination and the aflatoxin (AF) production are massive human health risks that can cause huge economic losses for the peanut industry. AFs are polyketide-derived furanocoumarins with powerful carcinogenicity that are linked with both acute and chronic toxicity for humans and animals [1,2]. Aflatoxin B1 (AFB1), the most risky and toxic of the Afs, is typically produced at high levels by select isolates of aflatoxigenic Aspergillus. Therefore, controlling AFB1 contamination requires a special focus on investigating the growth and metabolism of A. flavus, particularly AFB1 biosynthesis [3]. The 75-Kb gene cluster of A. flavus contains genes encoding the AFs biosynthesis pathway. To date, 34 genes have been discovered and identified, and their functions have been recognized as basic genes of the AFs pathway gene cluster [4]. There has been interest in the implications of climate-change-related abiotic conditions such as water activity (aw) and temperature, which are expected to affect the post-harvest phase of peanut crop, particularly by increasing the risk of fungal growth, aflatoxin production and the expression of aflatoxin pathway genes [5]. Strong effects of environmental conditions, such as temperature and aw modifications, on the functioning of storage ecosystems and deleterious A. flavus and suppression of the AF genes that prevent aflatoxin production lead to AF gene suppression, which represents a key step in risk management [6]. Two factors impact the expression of the AFB1 regulatory system and therefore AFB1 production: first, all biosynthetic genes, enzymes and regulators of AFB1 (an extremely complex molecular system), and second, storage ecosystems such as the aw, temperature and CO2 concentration [7]. It was established that the ratio of the two main regulatory genes (aflS/aflR) is related to temperature and aw interactions. High production of AFB1 is associated with a significant ratio of aflS to aflR [8]. An analysis of the AFB1 gene expression profiles revealed that 9 genes had their maximum levels of expression at 37 °C under an aw of 0.92, while 16 of the 25 genes had their highest levels at 28 °C under an aw of 0.92. At 42 °C, all genes involved in the biosynthesis pathway for AFB1 were downregulated compared to at 37 °C [6]. The transcription levels of two regulatory genes and 19 structural genes in the AF gene cluster in A. flavus at different temperatures showed significantly different expression levels for environmental factors, such as the interaction between the level of aw and temperature [9]. The transcription levels of two regulatory genes and 19 structural genes in the AFB1 gene cluster in A. flavus at different temperatures showed significantly different expression levels for environmental factors, such as the interaction of aw and temperature [9]. Previous research has suggested that interactions between these critical abiotic factors may have a significant impact on fungal diseases of fundamental food crops and possibly on the AFB1 contamination of food and feed. In order to control fungal development and subsequent AFB1 formation on peanut crops, greater effort should be made to obtain optimal storage conditions (especially regarding the aw and temperature) [9,10].
This study contributes to gaining a better understanding of the regulatory mechanisms of multiple combinations of aw and temperature on A. flavus development, biosynthetic, pathway gene expression, and AFB1 production, and this information is useful for reducing AFB1 contamination during peanut storage.
2. Materials and Methods
2.1. Fungal Isolates
Three A. flavus type isolates (KSU107, KSU114 and KSU121) provided by Dr. M. Mahmoud (Central Lab. of Biotechnology, Plant pathology Res. Ins. Agricultural Research Center, Giza, Egypt) were used in this study. The isolates had previously been used for the molecular detection of A. flavus in stored peanut studies [11] with consistent results. They were stored at 4 °C or subcultured on yeast extract sucrose agar (YES; 2% yeast extract, 15% sucrose, 0.05% MgSO4 7H2O).
2.2. Fungal Isolates and Growth Conditions
Three isolates were grown for five days at 25 °C on YES agar (20 g/L yeast extract, 150 g/L sucrose and 15 g/L agar). After covering the agar plates with sterile 8.5 cellophane sheets (Bristol, UK), a single-point inoculation was performed in the center of each plate using 107 of a spore suspension made up of 1 × 107 spores in 0.5% tween 80 and 0.85 g/L NaCl. By utilizing glycerol/water mixes to change the water availability to 0.85, 0.90 and 0.95 aw, as previously stated, the aw of the media was altered [11]. The water activity of the medium was verified by using an Aqualab 3TE instrument (Decagon, Pullman, WA, USA). The plates were inoculated at the temperatures indicated (34, 37 and 42 °C) with replicates (three per treatment). Samples were divided into three parts to carry out (i) a growth assessment, (ii) quantification of AFB1 by HPLC and (iii) determination of the expression of aflatoxin biosynthesis-related genes by real-time PCR (RT-PCR).
2.3. Growth Assessment
The plates of A. flavus were measured by taking two diametric measurements at right angles to each other at 3, 5, 7 and 9 days.
2.4. Quantification of AFB1 by HPLC
The media material was sliced into 4–5 plugs, each weighing about 0.5 g and placed over 9 cm Petri plates. These were weighed after being put into 2 mL Eppendorf tubes. One milliliter of chloroform was added, and AFB1 was extracted by shaking for one hour. After centrifugation, the chloroform was evaporated to dryness, and the biomass was discarded. Aflatoxin-containing dried residues were dissolved in one milliliter of the same liquid mobile phase solution (methanol: acetic acid: water) and kept in opaque vials. AFB1 production was detected and determined using the method presented in [12,13]. The extract was passed through a 0.45 μm micro-filter. A compound analysis was done using HPLC (PerkinElmer® series 200, Walhtam, MA, USA), C18, 100 × 4.6 mm, 3-micron HPLC type. An UV detector was installed in the HPLC, and its wavelength was 365 nm. The mobile phase was composed of acetic acid, methanol and water (20.0/20.0/60.0 v/v/v). At a flow rate of 1 mL/min, the separation took roughly 25 min to complete.
2.5. Extraction of RNA, cDNA Synthesis and the RT-PCR Reaction
Following the manufacturer’s instructions, total RNA was extracted from fungal mycelia using the Plant Mini Kit (QIAGEN, Hilden, Germany). DNA-free DNase was used to process the RNA samples. With the help of the Gen Qunta system, the purity and quantities of RNA were determined by measuring the samples’ absorbance at 260 and 280 nm (Amersham Pharmacia Biotech, Amersham, UK). Takara RNA PCR Kit (AMV) ver. 3.0 was used to reverse-transcribe 5 g of total RNA to produce the cDNA (Takara, Shiga, Japan). The primes were synthesized by GeneLink (Orlando, FL, USA). Table 1 contains a list of the primers used in this study. Reactions using real-time PCR were carried out (Applied Biosystems, Foster City, CA, USA). The amplification detector was the SYBR Green Real-time, PCR Master Mix (Applied Biosystems Foster City, California, USA) with a fluorescent tag. As an internal control, the 18S rRNA sequence was utilized. The following components were used to prepare each reaction (20 μL): 100 ng cDNA, (1 μL), SYBR Premix Ex Taq, (10 μL), Nuclease-Free water (8 μL), and 10 mmol/L primers (0.5 L), both forward and reverse. The PCR protocol had 40 cycles of denaturation at 94 °C for 30 s each, 40 annealing cycles at 57 °C for 30 s, and an initial denaturation phase at 94 °C for 10 min. After the annealing and extension stage, continuous fluorescence signals were produced and monitored once per cycle. At the conclusion of each run, a melting curve was created to guarantee product homogeneity. The 2−ΔΔCt method was used to calculate the relative quantification of changes in gene expression [14]. Each gene’s untreated expression was regarded as the control and given a treatment level of 1.0. In each experiment, each sample was tested in triplicate, and all genes were examined. Each experiment was run three times.

Table 1.
Primers used for real-time PCR.
2.6. Statistical Analysis
In all experimental trials, three replicates of each treatment were used. The standard error of the means (SE), along with the average of each of the three measures, was used to calculate the means. In order to analyses the variance of means with a 95% confidence interval, the Analysis of Variance (ANOVA) was used. Using the same statistical software, the Least Significant Difference (LSD) was used to determine differences between the means, with p 0.05 indicating a significant difference.
3. Results
3.1. Effect of aw × Temperature Interactions on Three Isolates of A. flavus Grown on YES Agar Media
On YES medium, growth cultures of A. flavus KSU107, A. flavus KSU114 and A. flavus KSU121 was investigated in relation to three different levels of aw and temperature. Figure 1a–c show the combined effect of three different levels of aw and temperature on the growth rate. The growth of the three Aspergillus isolates generally increased gradually, especially at the medium temperatures of 34 and 37 °C compared with the high temperature of 42 °C. This shows that the optimal colonization conditions for three isolates were 34 °C and 0.95 aw after nine days; there was very slow fungal growth at the highest temperature of 42 °C, and all aw treatments were inhibited.
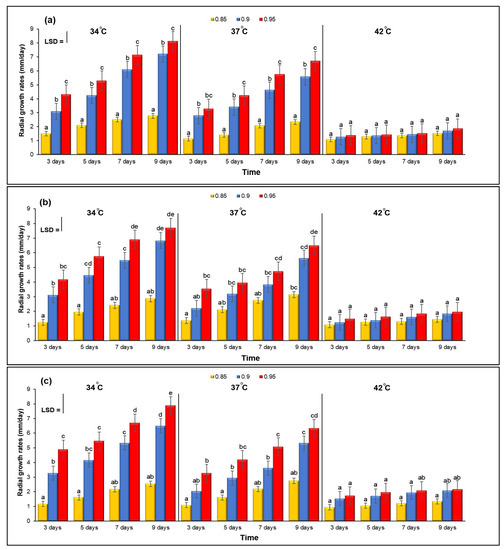
Figure 1.
(a–c) The combined effect of three different levels of aw and temperature on the growth rates. Bars indicate the Least Significant Difference (LSD, p ≤ 0.05). Error bars represent the ±Standard Error of the mean of three replicates (n = 3). Different letters indicate significant differences.
3.2. Effect of aw × Temperature Interactions on AB1 Production by Three A. flavus Isolates on YES Agar Media
Figure 2a,b show the combined effect of three different levels of aw and temperature on the concentration of AFB1 in two isolates of A. flavus (A. flavus KSU107 and A. flavus KSU114) examined on YES agar media. Generally, AFB1 production was higher at 34 °C with 0.95 aw for both isolates. When comparing samples grown at 34 °C to those grown at other temperatures, AFB1 production was significantly increased at 34 °C and 0.95 aw. At 42 °C with 0.85 and 0.90 aw, both isolates failed to produce any amount of AFB1. A. flavus KSU121 failed to produce any AFB1 at different temperatures and aw levels.
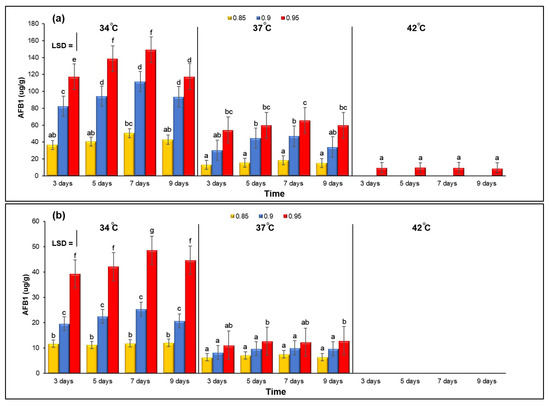
Figure 2.
The combined effect of three different levels of aw and temperature on AFB1 production by A. flavus KSU107 (a), A. flavus KSU114 (b) grown on YES agar media and incubated at 34, 37 and 42 °C under 0.85, 0.90 and –0.95 aw. Error bars represent the ±Standard Error of the mean of three replicates (n = 3). Different letters indicate significant differences.
3.3. Effect of aw × Temperature Interactions on the Expression of 15 Genes Involved in the Biosynthesis of AFB1 Production
The RT-PCR investigation of A. flavus KSU107 (high AFB1 producer) and A. flavus KSU114 (low AFB1 producer) revealed the transcription levels of 15 genes implicated in the production of AFB1 with combinations of three levels of aw and temperatures after 7 days of cultivation. Figure 3 and Figure 4 show the combined effect of three different levels of aw and temperature on the transcription levels of 11 structural genes in the AFB1 gene cluster, two regulatory genes (aflR and aflS) and two fungal development regulators (abaA and brlA) in both A. flavus isolates at different aw × temperature combinations to indicate the molecular mechanism underlying the up-and-downregulation of the molecular expression profile of the tested AFB1 biosynthetic genes. Figure 3 indicates the transcription levels of previously mentioned regulatory genes in A. flavus KSU107 (high AFB1 producer) at various aw levels and temperatures. In general, the majority of the examined genes had significantly different expression levels under various aw x temperature combinations. All of the AFB1 biosynthesis-related genes were evaluated and showed the highest levels of expression at 34 °C with 0.95 aw compared to at 37 °C and 42 °C with various aw values. The five most upregulated genes were aflO, aflQ, aflX, abaA, and brlA; these genes were upregulated by an average of 2.1-fold at the same temperature and 0.80 aw. At 37 °C with 0.95 aw, all of the tested genes were linked to AFB1 production. When converting Versicolorin A (VERA) to AFB1, the middle and late genes involved in the -AFB1 biosynthetic pathway were significantly upregulated, with a 1.25- to 5.5-fold increase when compared to conditions of 37 °C and 0.85 aw. At 40 °C with 0.85 and 0.90 aw, none of the genes in the biosynthetic pathway, which involved regulatory and structural genes, were expressed.
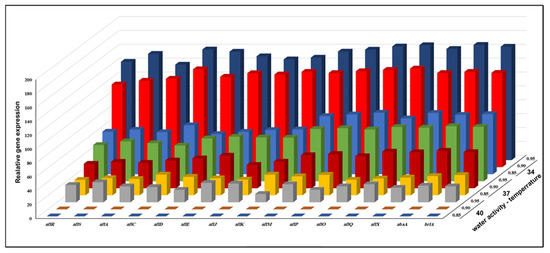
Figure 3.
The combined effect of three different levels of aw and temperature on the relative expression of fifteen AFB1 genes of A. flavus KSU107 (high AFB1 producer) grown on a YES agar medium.
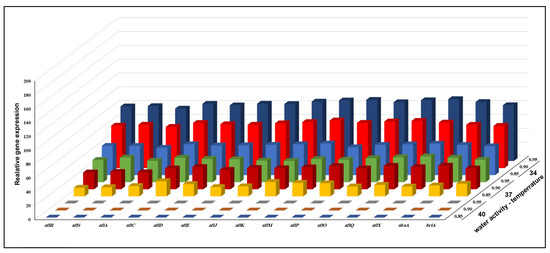
Figure 4.
The combined effect of three different levels of aw and temperature on the relative expression of fifteen AFB1 genes of A. flavus KSU114 (low AFB1 producer) grown on a YES agar medium.
Changes in the gene expression of all genes by KSU114 (a low AFB1 producer) after a 7-day incubation period based on interactions between different temperatures and aw values are shown in Figure 4. Overall, combinations of three levels of aw and temperature had a substantial impact on the majority of the detected genes. The highest levels of gene expression were observed at 34 °C and 0.95 aw, while at 40 °C, with all aw levels, nonexpression was observed for all genes. At 37 °C with 0.95 aw, tall AFB1 biosynthetic pathway genes were highly upregulated with 3.25- and 2.50-fold increases compared with the levels at 37 °C with an aw of 0.95 and 78.8- and 80.5-fold increases compared with the levels at 42 °C with an aw of 0.95, respectively. The four AFB1 genes with the highest levels of expression were aflM, aflP, aflQ and aflX at 34 °C with an aw of 0.95. Additionally, at 37 °C with an aw of 0.95, the same effect was observed for the aflK, aflM, aflO and aflQ genes.
4. Discussion
According to the literature, A. flavus can develop and produce AFB1 across a wide range of aw values and temperatures [6]. Numerous studies have described the ideal aw temperature for A. flavus growth and AFB1 production on various food substrates and formula media [15,16,17]. A. flavus growth and AFB1 production on polished rice can occur over a broad range of aw × temperature levels. For fungal growth, the optimal conditions were found to be an aw of 0.92–0.96 and a temperature of 28–37 °C. The maximum quantity of AFB1 was observed at 33 °C and an aw of 0.96 [18,19]. The A. flavus isolates were elastic for the duration of their growth on pistachio-based media and the colonization of pistachio nuts with no significant difference when exposed to temperature (35 vs. 37 °C) and water activity (0.98–0.93) as abiotic factors related to climate change (CC). AFB1 production was significantly promoted, particularly when exposed to a temperature of 35 °C and an aw of 0.98–0.95 and occasionally at 37 °C and 0.98 aw [20]. The analysis of variance for the effects of temperature and aw on the radial growth of two isolates of A. flavus revealed that these variables as well as their interactions had substantial impacts on fungal growth and the formation of AFB1. The response surface analysis revealed that both isolates could develop at temperatures between 22 and 37 °C and aw values between 0.88 and 0.98, although the production of AFB1 did not occur at temperatures below 37 °C at 0.90 aw. The authors suggest that the aw in harvested chili should be immediately decreased to less than 0.90 by an effective drying method to prevent fungal growth and AFB1 production [21]. Two A. flavus isolates isolated from imported raw peanuts were studied for growth and AFB1 production using a full factorial design with seven aw levels (0.85–0.98 aw) and five temperature levels (20–40 °C). The findings showed that both isolates were unable to grow at 20 °C under aw values of 0.94 and 0.85. At 30 °C with an aw of 0.98, both isolates showed the maximum growth rate. According to the analysis of variance (p < 0.05), temperature and aw had significant impacts on A. flavus growth and AFB1 production. AFB1 production showed a similar pattern, but at a lower temperature range (25–35 °C) and higher aw (0.92–0.98) [22]. A. flavus grew on shelled peanuts at a lower rate when the water activity was ≤0.85 or the temperature was ≤20 °C. A temperature of 37 °C and aw of 0.98 were the best conditions for fungal growth in shelled peanuts. The maximum amount of AFB1 in peanuts was obtained at aw of 0.96 and 28 °C [6]. Temperature and aw represent two key environmental factors that influence both the rate of fungal colonization of food and feed and the production of AFs. Previous studies have shown that aw and temperature significantly affect the expression of genes implicated in the production of AFB1, particularly the regulatory genes aflR and aflS [12]. Gene expression continuously precedes the appearance of phenotypic traits. Genome sequencing and many annotations of A. flavus have paved the way for various molecular approaches, such as reverse transcription real-time PCR (RT-qPCR) and RT-PCR, which may be useful to obtain crucial information on the up-and downregulation of specific genes involved in AFB1 biosynthesis under various environmental conditions [23]. RNA-Seq technology was used to determine the effect of temperature on aflatoxin biosynthesis, and it was discovered that most genes involved in the aflatoxin cluster were highly expressed at 30 °C but not at 37 °C, because the very low concentration of salts in the medium which supported A. flavus did not produce a high concentration of AFs [24]. In comparison to 37 °C, [25] found that a medium temperature (30 °C) upregulated the expression of the genes aflR and aflS, activating the AFB1 biosynthesis gene. The transcriptional activation and inactivation of the AFB1 gene cluster appear to be modulated by slight variations in the expression levels of aflR and aflS. Additionally, [6] found a similar result for AFB1 production in peanut kernels. At 28 °C, a higher ratio of aflS/aflR led to a higher level of AFB1 in peanut kernels compared with 42 °C and 37 °C. According to qRT-PCR results, the two regulating genes (aflR and aflS) were significantly expressed at varied conditions such as (20 and 28 °C and 0.96–0.99 aw) during AFB1 production. Additionally, under the conditions required to achieve the highest capacity for AFB1 production, two structural genes (aflD and aflO) were significantly expressed [26]. At various aw and temperatures, the majority of the examined AFB1 production genes displayed noticeably differential expression levels. All of the AFB1 biosynthetic development-related genes evaluated showed the highest transcription levels at 25 °C under an aw of 0.96, compared to at 33 °C and 37 °C. The two most highly expressed genes were aflR and aflS (regulatory genes), followed by aflE, aflJ, and aflH (structural genes) [19]. In the study, the relative transcription levels of abaA and brlA were significantly upregulated at 37 °C compared to at 40 °C, and they were highly significantly upregulated at 34 °C. In comparison with at aw 0.95, the transcription levels of both genes were also significantly reduced at aw values of 0.85 and 0.90 under different temperatures. Similar findings were observed by [6]. When A. flavus grew on peanut kernels at 33 °C under different aw conditions, a sufficiently favorable correlation between these conditions and the expression levels of the abaA and brlA genes was observed [6], whereas a similar correlation was not found for polished rice [19]. To trigger the transcription of each of the structural genes in the AFB1 pathway in the AFB1 gene cluster, aflS and aflR join forces to form a complex known as the AflR binding molecule, which then binds to the promoter region of each structural gene and activates it. Upregulation of aflS resulted in high AflS production, which could subsequently provide a good opportunity for the formation of the AflR-AflS complex, causing increases in AFB1 gene transcription and AFB1 production [27]. The aflR and aflP genes experienced substantial peaks in expression for both A. flavus and A. parasiticus species in maize kernels by day 6 of incubation, while the aflS gene reached its peak by day 8. Despite the fact that the relative gene expression levels were relatively similar, the A. parasiticus aflP gene expression values in this food matrix were greater than those for A. flavus. In peanut seeds, greatest expression values for the aflR, aflS and aflP genes were seen at 7, 9 and 8 days of incubation for A. flavus. However, the three AF-related genes’ greatest expression levels were observed in A. parasiticus by day 7 of incubation. A. parasiticus obtained greater relative expression values for the aflP gene than did A. flavus. A. flavus was discovered to have the highest expression levels of the aflS gene [28]. Senna pods are very sensitive to A. flavus and AFB1 contamination, and the regulatory restrictions of importing countries make it difficult for pods to be successfully exported. The diametric growth rate was shown to be most optimal at 28 and 37 °C with an aw of 0.96. However, regulatory gene expression (aflR and aflS) was highly expressed and AFB1 production was at its highest at 28 °C with an aw of 0.96. The maximum growth rate was seen at 37 °C, which was favorable for neither gene expression nor the formation of AFB1. However, at 28 °C, it was positively correlated with gene expression and AFB1 production. The difference in the growth rate and AFB1 production at the highest temperature may have been caused by the suppressed molecules’ effect on the expression of regulatory genes. The rapid drying of senna, pods with a low aw (≤0.87 aw) and storage at a low temperature (20 °C) are ideal conditions for avoiding AFB1 and ensuring the quality of produce for export [29]. The A. flavus isolate NRRL3357 was inoculated in peanuts with diverse aw values (0.90, 0.95 and 0.99). The changes in AFB1 production were the highest with aw 0.90, followed by aw 0.95 and were minimal at aw 0.99. According to the transcriptome data and RT-qPCR analyses, the middle- and later-stage genes involved in AFB1 biosynthesis in particular were considerably upregulated in aw 0.90 compared to at aw 0.95 and 0.99. The AtfB factor may play a key role in regulating downstream genes, especially AFB1 biosynthetic genes, in response to aw changes [5]. The stress of the environmental factors associated with climate change, such as drought, temperature and oxidative stress, can aggravate aflatoxin contamination in agricultural crops [30]. Over the last decade, abundant research efforts have been undertaken to confirm that environmental stress affects aflatoxigenic A. flavus and AF production. Environmental stress-responding compounds, such as reactive oxygen species (ROS), which are generated by host cells in oxidative bursts associated with fungal stress-responsive signaling and secondary metabolites, can stimulate the production of AFs by A. flavus. Both temperature and aw changes and oxidative stress are tightly linked to ROS metabolism in fungal cells. Given that ROS play important roles in the regulation of both fungal metabolites and AF production, a serious analysis of the responses of A. flavus to all stresses and the regulation of AFs production is supported, thereby aiding efforts to prevent AF contamination under climate change scenarios [31,32]. Indeed, fungal cells have a complex “temporal switch” that regulates the activation of AF genes involved in AFs biosynthetic pathways and controls their activity. The transcriptomic responses of A. flavus to temperature, aw and oxidative stresses at the AF production stage revealed that AF biosynthetic genes were strongly affected, which means that AF genes were upregulated under stress conditions [33]. Advanced genomic techniques have greatly improved our knowledge of the molecular aspects of the regulation of AF biosynthesis genes. However, data on AF regulation at the molecular level under climate change scenarios are still very scarce. The availability of up-to-date techniques, including genomics and transcriptomics, has contributed to a prompt expansion of data on the biology of A. flavus as a mycotoxigenic fungus [34]. The availability of up-to-date techniques, including genomics and transcriptomics, has contributed to a prompt expansion of data on the biology of A. flavus as a mycotoxigenic fungus. Indeed, the use of transcriptomic data is a better way to understand AF modelling, climate change and development. Additionally, transcriptomics explains how chromatin remodeling is influenced by interacting stresses of environmental factors and a more comprehensive view of climate change scenarios. Transcriptional regulation of AF production is dependent on diverse factors, including AF cluster genes, regulators, transcription factors, environmental factors, and crop substrates. Transcriptomic and proteomic analyses produce accurate data that can be used to identify the genes or pathways involved in the regulation of AF biosynthesis [35,36].
5. Conclusions
The current study examined the impacts of temperature and aw on the development of three isolates of A. flavus, the transcription of important regulatory and structural genes and the biosynthesis of AFB1 on YES agar media. The findings showed that aw and temperature have significant effects on the expression of the critical genes involved in AFB1 biosynthesis in all isolates of A. flavus as well as having effects on AFB1 production and fungal growth. The expression of structural genes is more strongly influenced by aw and temperature than by AF’s regulatory genes, aflR and aflS. The findings presented here may provide readers with a deeper understanding of the decrease in AFB1 production caused by peanut contamination. The two main environmental parameters that affect the speed of fungal growth and AFB1 formation in the storage and marketing processes are aw and temperature. AFB1 production and fungal spoiling of peanuts and peanut products may be prevented with the help of the information that is provided here regarding crucial control.
Funding
This research was funded by Princess Nourah bint Abdulrahman University Researcher Supporting Project under grant number PNURSP2023R84.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R84), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflicts of Interest
The author declares no conflict of interest.
References
- Tengey, T.K.; Kankam, F.; Ndela, D.N.; Frempong, D.; Appaw, W.O. Growth and Toxigenicity of A. flavus on Resistant and Susceptible Peanut Genotypes. Toxins 2022, 14, 536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhong, H.; Han, X.; Guo, Z.; Yang, W.; Liu, Y.; Yang, K.; Zhuang, Z.; Wang, S. Proteomic profile of Aspergillus flavus in response to water activity. Fungal Biol. 2015, 119, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Wu, F. Perspective: Time to face the fungal threat. Nature 2014, 516, S7. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, T.E.; Yu, J.; Fedorova, N.; Bhatnagar, D.; Payne, G.A.; Nierman, W.C. Potential of Aspergillus flavus genomics for applications in biotechnology. Trends Biotechnol. 2009, 27, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, X.; Ma, X.; Yu, Q.; Yu, X.; Liu, Y.; Nie, C.; Zhang, Y.; Xing, F. The regulatory mechanism of water activities on aflatoxins biosynthesis and conidia development, and transcription factor AtfB is involved in this regulation. Toxins 2021, 13, 431. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Guan, X.; Xing, F.; Lv, C.; Dai, X.; Liu, Y. Effect of water activity and temperature on the growth of Aspergillus flavus, the expression of aflatoxin biosynthetic genes and aflatoxin production in shelled peanuts. Food Control 2017, 82, 325–332. [Google Scholar] [CrossRef]
- Medina, A.; Gilbert, M.K.; Mack, B.M.; Obrian, G.R.; Rodríguez, A.; Bhatnagar, D.; Payne, G.; Magan, N. Interactions between water activity and temperature on the Aspergillus flavus transcriptome and aflatoxin B (1) production. Int. J. Food Microbiol. 2017, 256, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Heydt, M.; Rüfer, C.E.; Abdel-Hadi, A.; Magan, N.; Geisen, R. The production of aflatoxin B1 or G1 by Aspergillus parasiticus at various combinations of temperature and water activity is related to the ratio of aflS to aflR expression. Mycotoxin 2010, 26, 241–246. [Google Scholar] [CrossRef]
- Lv, C.; Jin, J.; Wang, P.; Dai, X.; Liu, Y.; Zheng, M.; Xing, F. Interaction of water activity and temperature on the growth, gene expression and aflatoxin production by Aspergillus flavus on paddy and polished rice. Food Chem. 2019, 293, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.; Rodríguez, A.; Sultan, Y.; Magan, N. Climate change factors and A. flavus: Effects on gene expression, growth and aflatoxin production. World Mycotoxin J. 2015, 8, 171–179. [Google Scholar] [CrossRef]
- Mahmoud, M.A. Detection of Aspergillus flavus in stored peanuts using real-time PCR and the expression of aflatoxin genes in toxigenic and atoxigenic A. flavus isolates. Foodborne Pathog. Dis. 2015, 12, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Heydt, M.; Abdel-Hadi, A.; Magan, N.; Geisen, R. Complex regulation of the aflatoxin biosynthesis gene cluster of Aspergillus flavus in relation to various combinations of water activity and temperature. Int. J. Food Microbiol. 2009, 135, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Christian, G. HPLC Tips and Tricks; Great Britain at the Iden Press: Oxford, UK, 1990; p. 608. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.S.T.; Sarreal, S.B.L.; Chang, P.-K.; Yu, J. Transcriptional regulation of aflatoxin biosynthesis and conidiation in Aspergillus flavus by Wickerhamomyces anomalus WRL-076 for reduction of aflatoxin contamination. Toxins 2019, 11, 81. [Google Scholar] [CrossRef]
- Priesterjahn, E.-M.; Geisen, R.; Schmidt-Heydt, M. Influence of light and water activity on growth and mycotoxin formation of selected isolates of Aspergillus flavus and Aspergillus parasiticus. Microorganisms 2020, 8, 2000. [Google Scholar] [CrossRef]
- Zingales, V.; Taroncher, M.; Martino, P.A.; Ruiz, M.-J.; Caloni, F. Climate Change and Effects on Molds and Mycotoxins. Toxins 2022, 14, 445. [Google Scholar] [CrossRef]
- Donato, C.J.R.; Cendoya, E.; Demonte, L.D.; Repetti, M.R.; Chulze, S.N.; Ramirez, M.L. Influence of abiotic factors (water activity and temperature) on growth and aflatoxin production by Aspergillus flavus in a chickpea-based medium. Int. J. Food Microbiol. 2022, 379, 109841. [Google Scholar] [CrossRef]
- Khan, R.; Ghazali, F.M.; Mahyudin, N.A.; Samsudin, N.I.P. Aflatoxin Biosynthesis, Genetic Regulation, Toxicity, and Control Strategies: A Review. J. Fungi 2021, 7, 606. [Google Scholar] [CrossRef]
- Baazeem, A.; Rodriguez, A.; Medina, A.; Magan, N. Impacts of climate change interacting abiotic factors on growth, aflD and aflR gene expression and aflatoxin B1 production by Aspergillus flavus strains in vitro and on pistachio nuts. Toxins 2021, 13, 385. [Google Scholar] [CrossRef]
- Chananya, C.; Warapa, M.; Thanapoom, M. Comparative study on the effect of temperature and water activity on Aspergillus flavus and Aspergillus carbonarius isolates growth and mycotoxin production on a chili powder medium. Cogent Food Agric. 2020, 6, 1782097. [Google Scholar]
- Norlia, M.; Jinap, S.; Nor-Khaizura, M.A.R.; Radu, S.; John, J.M.; Rahman, M.A.H.; Mshelia, L.P.; Zawiyah, S. Modelling the effect of temperature and water activity on the growth rate of Aspergillus flavus and aflatoxin production in peanut meal extract agar. Int. J. Food Microbiol. 2020, 335, 108836. [Google Scholar] [CrossRef] [PubMed]
- Shabeer, S.; Asad, S.; Jamal, A.; Ali, A. Aflatoxin contamination, its impact and management strategies: An updated review. Toxins 2022, 14, 307. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Payne, G.A.; Nierman, W.C.; Machida, M.; Bennett, J.W.; Campbell, B.C. Aspergillus flavus genomics as a tool for studying the mechanism of aflatoxin formation. Food Addit. Contam. Part A 2008, 25, 1152–1157. [Google Scholar]
- Yu, J.; Fedorova, N.D.; Montalbano, B.G.; Bhatnagar, D.; Cleveland, T.E.; Bennett, J.W.; William, C.N. Tight control of mycotoxin biosynthesis gene expression in Aspergillus flavus by temperature as revealed by RNA-Seq. FEMS Microbiol. Lett. 2011, 322, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Solfrizzo, M.; Epifani, F.; Panzarini, G.; Perrone, G. Effect of temperature and water activity on gene expression and aflatoxin biosynthesis in Aspergillus flavus on almond medium. International. Int. J. Food Microbiol. 2016, 217, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Chi, C.; Yu, J.; Shan, S.; Li, Q.; Guan, B.; Nieman, W.C.; Bennett, J.W. The inhibitory effect of Bacillus megaterium on aflatoxin and cyclopiazonic acid biosynthetic pathway gene expression in Aspergillus flavus. Appl. Microbiol. Biotechnol. 2014, 98, 5161–5172. [Google Scholar] [CrossRef] [PubMed]
- Bernáldez, V.; Córdoba, J.J.; Delgado, J.; Bermúdez, E.; Rodríguez, A. Gene expression analysis to predict aflatoxins B1 and G1 contamination in some plant origin foods. LWT 2018, 93, 517–524. [Google Scholar] [CrossRef]
- Natarajan, S.; Balachandar, D.; Senthil, N.; Paranidharan, V. Interaction of water activity and temperature on growth, gene expression, and aflatoxin B1 production in Aspergillus flavus on Indian senna (Cassia angustifolia Vahl.). Int. J. Food Microbiol. 2022, 361, 109457. [Google Scholar] [CrossRef] [PubMed]
- Kebede, H.; Abbas, H.K.; Fisher, D.K.; Bellaloui, N. Relationship between aflatoxin contamination and physiological responses of corn plants under drought and heat stress. Toxins 2012, 4, 1385–1403. [Google Scholar] [CrossRef] [PubMed]
- Segal, L.M.; Wilson, R.A. Reactive oxygen species metabolism and plant-fungal interactions. Fungal Genet Biol. 2018, 110, 1–9. [Google Scholar] [CrossRef]
- Gao, X.; Kolomiets, M.V. Host-derived lipids and oxylipins are crucial signals in modulating mycotoxin production by fungi. Toxin Rev. 2009, 28, 79–88. [Google Scholar] [CrossRef]
- Roze, L.V.; Chanda, A.; Linz, J.E. Compartmentalization and molecular traffic in secondary metabolism: A new understanding of established cellular processes. Fungal Genet. Biol. 2011, 48, 35–48. [Google Scholar] [CrossRef]
- Perrone, G.; Massimo, F.; Angel, M.; Michelangelo, P.; Magan, N. Toxigenic fungi and mycotoxins in a climate change scenario: Ecology, genomics, distribution, prediction and prevention of the risk. Microorganisms 2020, 8, 1496. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.K.; Ji, I.K.; Vivek, K.B.; Kwanwoo, K.; Hoomin, L.; Sonam, S.; Jesus, S.-G.; Jianbo, X.; Sajad, A.; Yun, S.H.; et al. Mycotoxins in food and feed: Toxicity, preventive challenges, and advanced detection techniques for associated diseases. Crit. Rev. Food Sci. Nutr. 2022, 21, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Sang, Y.L.; So, Y.W.; Hwa, Y.C.; Seongeun, H.; Gyoungju, N.; Hyang, S.C. Transcriptomic responses of Aspergillus flavus to temperature and oxidative stresses during aflatoxin production. Sci. Rep. 2021, 11, 2803. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).