Targeted Therapy of Severe Infections Caused by Staphylococcus aureus in Critically Ill Adult Patients: A Multidisciplinary Proposal of Therapeutic Algorithms Based on Real-World Evidence
Abstract
1. Introduction
2. Materials and Methods
3. Targeted Treatment of Infections Caused by Staphylococcus aureus in Critically Ill Patients
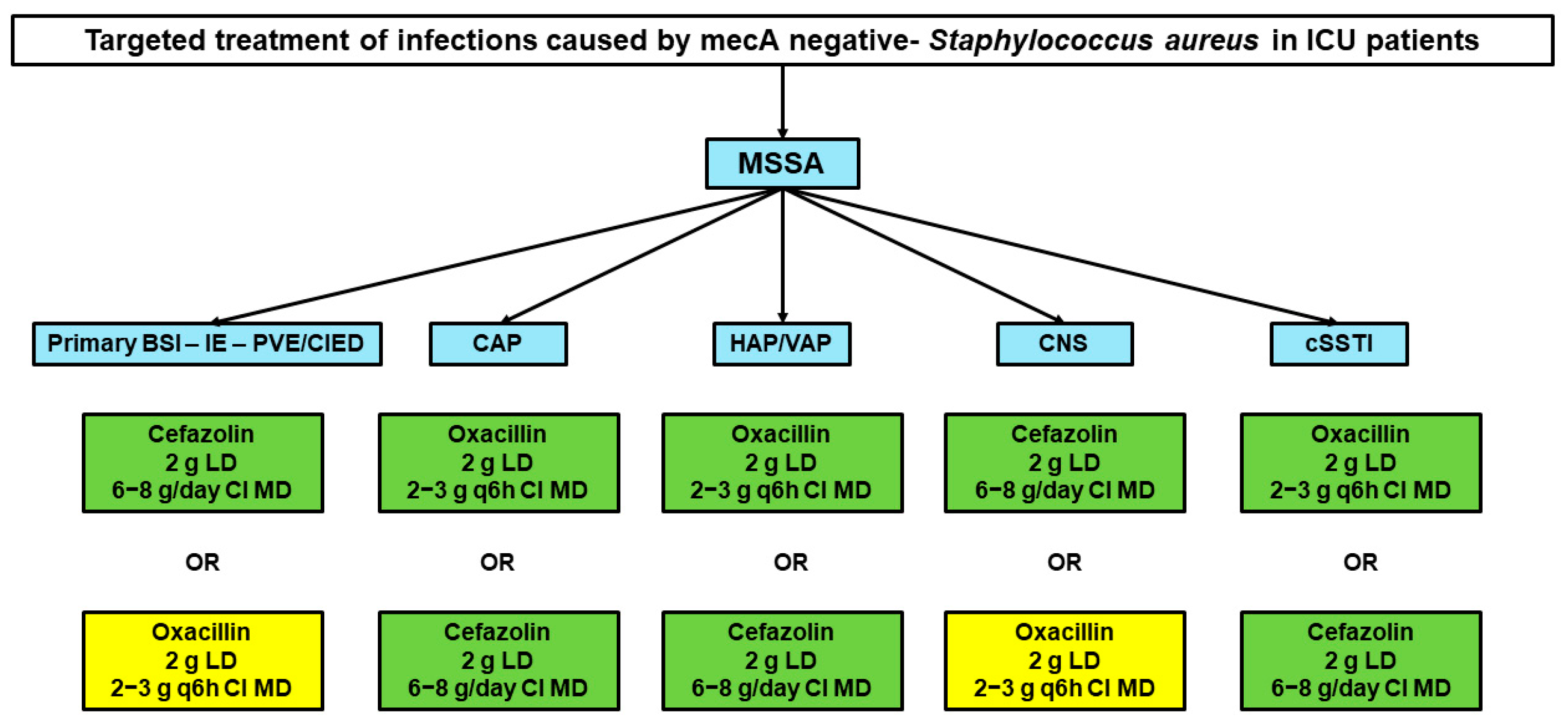
3.1. Targeted Treatment of MSSA Infections
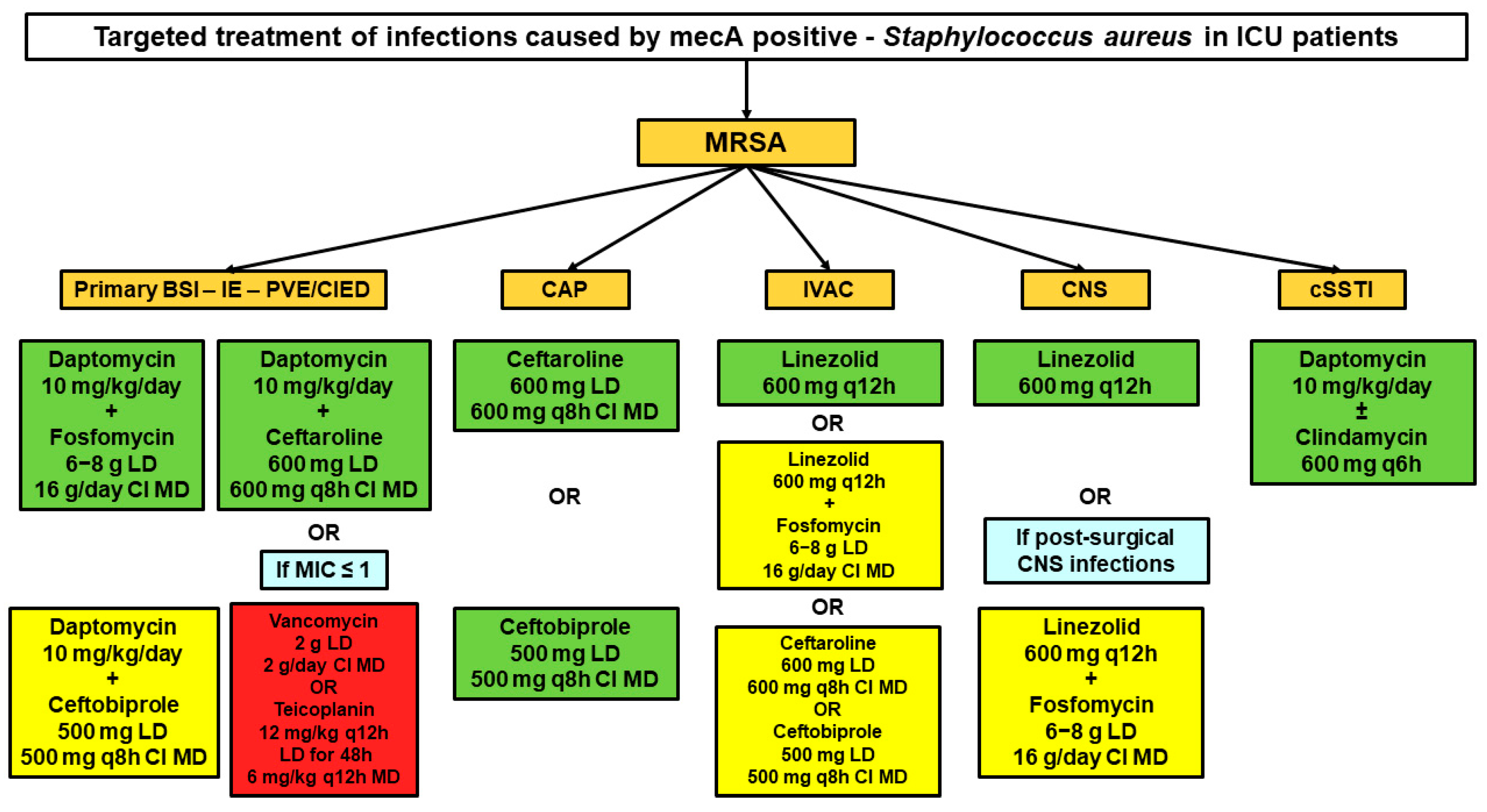
3.2. Targeted Treatment of MRSA Infections
3.2.1. Primary BSIs, Infective Endocarditis, and Intracardiac/Intravascular Devices Infections
3.2.2. Community-Acquired Pneumonia
3.2.3. Infection-Related Ventilator-Associated Complications
3.2.4. Central Nervous System Infections
3.2.5. Necrotizing Skin and Soft Tissue Infections
4. Expert Opinion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khader, K.; Thomas, A.; Huskins, W.C.; Stevens, V.; Keegan, L.T.; Visnovsky, L.; Samore, M.H. Effectiveness of Contact Precautions to Prevent Transmission of Methicillin-Resistant Staphylococcus Aureus and Vancomycin-Resistant Enterococci in Intensive Care Units. Clin. Infect. Dis. 2021, 72, S42–S49. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus Aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Sakr, Y.; Singer, M.; Martin-Loeches, I.; Machado, F.R.; Marshall, J.C.; Finfer, S.; Pelosi, P.; Brazzi, L.; Aditianingsih, D.; et al. Prevalence and Outcomes of Infection Among Patients in Intensive Care Units in 2017. JAMA 2020, 323, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report for 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-eueea-ears-net-annual-epidemiological-report-2020 (accessed on 31 October 2022).
- Grasselli, G.; Scaravilli, V.; Mangioni, D.; Scudeller, L.; Alagna, L.; Bartoletti, M.; Bellani, G.; Biagioni, E.; Bonfanti, P.; Bottino, N.; et al. Hospital-Acquired Infections in Critically Ill Patients with COVID-19. Chest 2021, 160, 454–465. [Google Scholar] [CrossRef]
- De Pascale, G.; De Maio, F.; Carelli, S.; De Angelis, G.; Cacaci, M.; Montini, L.; Bello, G.; Cutuli, S.L.; Pintaudi, G.; Tanzarella, E.S.; et al. Staphylococcus Aureus Ventilator-Associated Pneumonia in Patients with COVID-19: Clinical Features and Potential Inference with Lung Dysbiosis. Crit. Care 2021, 25, 197. [Google Scholar] [CrossRef] [PubMed]
- Sampedro, G.R.; Bubeck Wardenburg, J. Staphylococcus Aureus in the Intensive Care Unit: Are These Golden Grapes Ripe for a New Approach? J. Infect. Dis. 2017, 215, S64–S70. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical Practice Guidelines by the Infectious Diseases Society of America for the Treatment of Methicillin-Resistant Staphylococcus Aureus Infections in Adults and Children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [CrossRef] [PubMed]
- López-Cortés, L.E.; Del Toro, M.D.; Gálvez-Acebal, J.; Bereciartua-Bastarrica, E.; Fariñas, M.C.; Sanz-Franco, M.; Natera, C.; Corzo, J.E.; Lomas, J.M.; Pasquau, J.; et al. Impact of an Evidence-Based Bundle Intervention in the Quality-of-Care Management and Outcome of Staphylococcus Aureus Bacteremia. Clin. Infect. Dis. 2013, 57, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Pea, F.; Viale, P. Bench-to-Bedside Review: Appropriate Antibiotic Therapy in Severe Sepsis and Septic Shock--Does the Dose Matter? Crit. Care 2009, 13, 214. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Alffenaar, J.-W.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.-A.; Pea, F.; Sjovall, F.; et al. Antimicrobial Therapeutic Drug Monitoring in Critically Ill Adult Patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef] [PubMed]
- Poole, S.; Clark, T.W. Rapid Syndromic Molecular Testing in Pneumonia: The Current Landscape and Future Potential. J. Infect. 2020, 80, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bartoletti, M.; Tedeschi, S.; Scudeller, L.; Pascale, R.; Rosselli Del Turco, E.; Trapani, F.; Tumietto, F.; Virgili, G.; Marconi, L.; Ianniruberto, S.; et al. Impact on Mortality of a Bundle for the Management of Enterococcal Bloodstream Infection. Open Forum Infect. Dis. 2019, 6, ofz473. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Barnini, S.; Guarracino, F.; Parisio, E.M.; Spinicci, M.; Viaggi, B.; D’Arienzo, S.; Forni, S.; Galano, A.; Gemmi, F. Orthopaedic Implant-Associated Staphylococcal Infections: A Critical Reappraisal of Unmet Clinical Needs Associated with the Implementation of the Best Antibiotic Choice. Antibiotics 2022, 11, 406. [Google Scholar] [CrossRef] [PubMed]
- Murad, M.H.; Asi, N.; Alsawas, M.; Alahdab, F. New Evidence Pyramid. Evid. Based Med. 2016, 21, 125–127. [Google Scholar] [CrossRef]
- Rindone, J.P.; Mellen, C.K. Meta-Analysis of Trials Comparing Cefazolin to Antistaphylococcal Penicillins in the Treatment of Methicillin-Sensitive Staphylococcus Aureus Bacteraemia. Br. J. Clin. Pharmacol. 2018, 84, 1258–1266. [Google Scholar] [CrossRef]
- McDanel, J.S.; Roghmann, M.-C.; Perencevich, E.N.; Ohl, M.E.; Goto, M.; Livorsi, D.J.; Jones, M.; Albertson, J.P.; Nair, R.; O’Shea, A.M.J.; et al. Comparative Effectiveness of Cefazolin Versus Nafcillin or Oxacillin for Treatment of Methicillin-Susceptible Staphylococcus Aureus Infections Complicated by Bacteremia: A Nationwide Cohort Study. Clin. Infect. Dis. 2017, 65, 100–106. [Google Scholar] [CrossRef]
- Davis, J.S.; Turnidge, J.; Tong, S. A Large Retrospective Cohort Study of Cefazolin Compared with Flucloxacillin for Methicillin-Susceptible Staphylococcus Aureus Bacteraemia. Int. J. Antimicrob. Agents 2018, 52, 297–300. [Google Scholar] [CrossRef]
- Beganovic, M.; Cusumano, J.A.; Lopes, V.; LaPlante, K.L.; Caffrey, A.R. Comparative Effectiveness of Exclusive Exposure to Nafcillin or Oxacillin, Cefazolin, Piperacillin/Tazobactam, and Fluoroquinolones Among a National Cohort of Veterans with Methicillin-Susceptible Staphylococcus Aureus Bloodstream Infection. Open Forum Infect. Dis. 2019, 6, ofz270. [Google Scholar] [CrossRef]
- Rao, S.N.; Rhodes, N.J.; Lee, B.J.; Scheetz, M.H.; Hanson, A.P.; Segreti, J.; Crank, C.W.; Wang, S.K. Treatment Outcomes with Cefazolin versus Oxacillin for Deep-Seated Methicillin-Susceptible Staphylococcus Aureus Bloodstream Infections. Antimicrob. Agents Chemother. 2015, 59, 5232–5238. [Google Scholar] [CrossRef]
- Bai, A.D.; Findlater, A.; Irfan, N.; Singhal, N.; Loeb, M. Cefazolin versus Cloxacillin as Definitive Antibiotic Therapy for Methicillin-Susceptible Staphylococcus Aureus Spinal Epidural Abscess: A Retrospective Cohort Study. Int. J. Antimicrob. Agents 2021, 58, 106429. [Google Scholar] [CrossRef]
- Corsini Campioli, C.; Go, J.R.; Abu Saleh, O.; Challener, D.; Yetmar, Z.; Osmon, D.R. Antistaphylococcal Penicillin vs Cefazolin for the Treatment of Methicillin-Susceptible Staphylococcus Aureus Spinal Epidural Abscesses. Open Forum Infect. Dis. 2021, 8, ofab071. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, B.; Hoen, B.; Goehringer, F.; Sime, W.N.; Aissa, N.; Alauzet, C.; Jeanmaire, E.; Hénard, S.; Filippetti, L.; Selton-Suty, C.; et al. Antistaphylococcal Penicillins vs. Cefazolin in the Treatment of Methicillin-Susceptible Staphylococcus Aureus Infective Endocarditis: A Quasi-Experimental Monocentre Study. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 2605–2616. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Echevarria, K.L.; Hughes, D.W.; Cadena, J.A.; Bowling, J.E.; Lewis, J.S. Comparison of Cefazolin versus Oxacillin for Treatment of Complicated Bacteremia Caused by Methicillin-Susceptible Staphylococcus Aureus. Antimicrob. Agents Chemother. 2014, 58, 5117–5124. [Google Scholar] [CrossRef]
- Le Turnier, P.; Gregoire, M.; Deslandes, G.; Lakhal, K.; Deschanvres, C.; Lecomte, R.; Talarmin, J.-P.; Dubée, V.; Bellouard, R.; Boutoille, D.; et al. Should We Reconsider Cefazolin for Treating Staphylococcal Meningitis? A Retrospective Analysis of Cefazolin and Cloxacillin Cerebrospinal Fluid Levels in Patients Treated for Staphylococcal Meningitis. Clin. Microbiol. Infect. 2020, 26, 1415.e1–1415.e4. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.W.; Frei, C.R.; Maxwell, P.R.; Green, K.; Patterson, J.E.; Crawford, G.E.; Lewis, J.S. Continuous versus Intermittent Infusion of Oxacillin for Treatment of Infective Endocarditis Caused by Methicillin-Susceptible Staphylococcus Aureus. Antimicrob. Agents Chemother. 2009, 53, 2014–2019. [Google Scholar] [CrossRef]
- Grillo, S.; Puig-Asensio, M.; Schweizer, M.L.; Cuervo, G.; Oriol, I.; Pujol, M.; Carratalà, J. The Effectiveness of Combination Therapy for Treating Methicillin-Susceptible Staphylococcus Aureus Bacteremia: A Systematic Literature Review and a Meta-Analysis. Microorganisms 2022, 10, 848. [Google Scholar] [CrossRef]
- Grillo, S.; Cuervo, G.; Carratalà, J.; Grau, I.; Pallarès, N.; Tebé, C.; Guillem Tió, L.; Murillo, O.; Ardanuy, C.; Domínguez, M.A.; et al. Impact of β-Lactam and Daptomycin Combination Therapy on Clinical Outcomes in Methicillin-Susceptible Staphylococcus Aureus Bacteremia: A Propensity Score-Matched Analysis. Clin. Infect. Dis. 2019, 69, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- Pujol, M.; Miró, J.-M.; Shaw, E.; Aguado, J.-M.; San-Juan, R.; Puig-Asensio, M.; Pigrau, C.; Calbo, E.; Montejo, M.; Rodriguez-Álvarez, R.; et al. Daptomycin Plus Fosfomycin Versus Daptomycin Alone for Methicillin-Resistant Staphylococcus Aureus Bacteremia and Endocarditis: A Randomized Clinical Trial. Clin. Infect. Dis. 2021, 72, 1517–1525. [Google Scholar] [CrossRef]
- Miró, J.M.; Entenza, J.M.; Del Río, A.; Velasco, M.; Castañeda, X.; Garcia de la Mària, C.; Giddey, M.; Armero, Y.; Pericàs, J.M.; Cervera, C.; et al. High-Dose Daptomycin plus Fosfomycin Is Safe and Effective in Treating Methicillin-Susceptible and Methicillin-Resistant Staphylococcus Aureus Endocarditis. Antimicrob. Agents Chemother. 2012, 56, 4511–4515. [Google Scholar] [CrossRef]
- García-de-la-Mària, C.; Gasch, O.; García-Gonzalez, J.; Soy, D.; Shaw, E.; Ambrosioni, J.; Almela, M.; Pericàs, J.M.; Tellez, A.; Falces, C.; et al. The Combination of Daptomycin and Fosfomycin Has Synergistic, Potent, and Rapid Bactericidal Activity against Methicillin-Resistant Staphylococcus Aureus in a Rabbit Model of Experimental Endocarditis. Antimicrob. Agents Chemother. 2018, 62, e02633-17. [Google Scholar] [CrossRef]
- Geriak, M.; Haddad, F.; Rizvi, K.; Rose, W.; Kullar, R.; LaPlante, K.; Yu, M.; Vasina, L.; Ouellette, K.; Zervos, M.; et al. Clinical Data on Daptomycin plus Ceftaroline versus Standard of Care Monotherapy in the Treatment of Methicillin-Resistant Staphylococcus Aureus Bacteremia. Antimicrob. Agents Chemother. 2019, 63, e02483-18. [Google Scholar] [CrossRef] [PubMed]
- McCreary, E.K.; Kullar, R.; Geriak, M.; Zasowski, E.J.; Rizvi, K.; Schulz, L.T.; Ouellette, K.; Vasina, L.; Haddad, F.; Rybak, M.J.; et al. Multicenter Cohort of Patients With Methicillin-Resistant Staphylococcus Aureus Bacteremia Receiving Daptomycin Plus Ceftaroline Compared With Other MRSA Treatments. Open Forum Infect. Dis. 2020, 7, ofz538. [Google Scholar] [CrossRef] [PubMed]
- Nichols, C.N.; Wardlow, L.C.; Coe, K.E.; Sobhanie, M.M.E. Clinical Outcomes With Definitive Treatment of Methicillin-Resistant Staphylococcus Aureus Bacteremia With Retained Daptomycin and Ceftaroline Combination Therapy vs De-Escalation to Monotherapy With Vancomycin, Daptomycin, or Ceftaroline. Open Forum Infect. Dis. 2021, 8, ofab327. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.M.; Molina, K.C.; Miller, M.A.; Kiser, T.H.; Huang, M.; Mueller, S.W. Combination Ceftaroline and Daptomycin Salvage Therapy for Complicated Methicillin-Resistant Staphylococcus Aureus Bacteraemia Compared with Standard of Care. Int. J. Antimicrob. Agents 2021, 57, 106310. [Google Scholar] [CrossRef]
- Ahmad, O.; Crawford, T.N.; Myint, T. Comparing the Outcomes of Ceftaroline Plus Vancomycin or Daptomycin Combination Therapy Versus Monotherapy in Adults with Complicated and Prolonged Methicillin-Resistant Staphylococcus Aureus Bacteremia Initially Treated with Supplemental Ceftaroline. Infect. Dis. Ther. 2020, 9, 77–87. [Google Scholar] [CrossRef]
- Sakoulas, G.; Moise, P.A.; Casapao, A.M.; Nonejuie, P.; Olson, J.; Okumura, C.Y.M.; Rybak, M.J.; Kullar, R.; Dhand, A.; Rose, W.E.; et al. Antimicrobial Salvage Therapy for Persistent Staphylococcal Bacteremia Using Daptomycin plus Ceftaroline. Clin. Ther. 2014, 36, 1317–1333. [Google Scholar] [CrossRef]
- Cortes-Penfield, N.; Oliver, N.T.; Hunter, A.; Rodriguez-Barradas, M. Daptomycin and Combination Daptomycin-Ceftaroline as Salvage Therapy for Persistent Methicillin-Resistant Staphylococcus Aureus Bacteremia. Infect. Dis. 2018, 50, 643–647. [Google Scholar] [CrossRef]
- Hornak, J.P.; Anjum, S.; Reynoso, D. Adjunctive Ceftaroline in Combination with Daptomycin or Vancomycin for Complicated Methicillin-Resistant Staphylococcus Aureus Bacteremia after Monotherapy Failure. Ther. Adv. Infect. Dis. 2019, 6, 2049936119886504. [Google Scholar] [CrossRef]
- Duss, F.-R.; Garcia de la Mària, C.; Croxatto, A.; Giulieri, S.; Lamoth, F.; Manuel, O.; Miró, J.M. Successful Treatment with Daptomycin and Ceftaroline of MDR Staphylococcus Aureus Native Valve Endocarditis: A Case Report. J. Antimicrob. Chemother. 2019, 74, 2626–2630. [Google Scholar] [CrossRef]
- Cunha, B.A.; Gran, A. Successful Treatment of Meticillin-Resistant Staphylococcus Aureus (MRSA) Aortic Prosthetic Valve Endocarditis with Prolonged High-Dose Daptomycin plus Ceftaroline Therapy. Int. J. Antimicrob. Agents 2015, 46, 225–226. [Google Scholar] [CrossRef]
- Tascini, C.; Attanasio, V.; Ripa, M.; Carozza, A.; Pallotto, C.; Bernardo, M.; Francisci, D.; Oltolini, C.; Palmiero, G.; Scarpellini, P. Ceftobiprole for the Treatment of Infective Endocarditis: A Case Series. J. Glob. Antimicrob. Resist. 2020, 20, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Oltolini, C.; Castiglioni, B.; Tassan Din, C.; Castiglioni, A.; Ossi, C.; La Canna, G.; Pajoro, U.; Scarpellini, P. Meticillin-Resistant Staphylococcus Aureus Endocarditis: First Report of Daptomycin plus Ceftobiprole Combination as Salvage Therapy. Int. J. Antimicrob. Agents 2016, 47, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Barber, K.E.; Werth, B.J.; Ireland, C.E.; Stone, N.E.; Nonejuie, P.; Sakoulas, G.; Pogliano, J.; Rybak, M.J. Potent Synergy of Ceftobiprole plus Daptomycin against Multiple Strains of Staphylococcus Aureus with Various Resistance Phenotypes. J. Antimicrob. Chemother. 2014, 69, 3006–3010. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, M.L.; Richardson, K.; Vaughan Sarrazin, M.S.; Goto, M.; Livorsi, D.J.; Nair, R.; Alexander, B.; Beck, B.F.; Jones, M.P.; Puig-Asensio, M.; et al. Comparative Effectiveness of Switching to Daptomycin Versus Remaining on Vancomycin Among Patients with Methicillin-Resistant Staphylococcus Aureus (MRSA) Bloodstream Infections. Clin. Infect. Dis. 2021, 72, S68–S73. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Lye, D.C.; Yahav, D.; Sud, A.; Robinson, J.O.; Nelson, J.; Archuleta, S.; Roberts, M.A.; Cass, A.; Paterson, D.L.; et al. Effect of Vancomycin or Daptomycin With vs Without an Antistaphylococcal β-Lactam on Mortality, Bacteremia, Relapse, or Treatment Failure in Patients With MRSA Bacteremia: A Randomized Clinical Trial. JAMA 2020, 323, 527–537. [Google Scholar] [CrossRef]
- Ryder, J.H.; Tong, S.Y.C.; Gallagher, J.C.; McDonald, E.G.; Thevarajan, I.; Lee, T.C.; Cortés-Penfield, N.W. Deconstructing the Dogma: Systematic Literature Review and Meta-Analysis of Adjunctive Gentamicin and Rifampin in Staphylococcal Prosthetic Valve Endocarditis. Open Forum Infect. Dis. 2022, 9, ofac583. [Google Scholar] [CrossRef]
- Sotgiu, G.; Aliberti, S.; Gramegna, A.; Mantero, M.; Di Pasquale, M.; Trogu, F.; Saderi, L.; Blasi, F. Efficacy and Effectiveness of Ceftaroline Fosamil in Patients with Pneumonia: A Systematic Review and Meta-Analysis. Respir. Res. 2018, 19, 205. [Google Scholar] [CrossRef]
- Bassetti, M.; Russo, A.; Cilloniz, C.; Giacobbe, D.R.; Vena, A.; Amaro, R.; Graziano, E.; Soriano, A.; Torres, A. Ceftaroline for Severe Community-Acquired Pneumonia: A Real-World Two-Centre Experience in Italy and Spain. Int. J. Antimicrob. Agents 2020, 55, 105921. [Google Scholar] [CrossRef]
- Nicholson, S.C.; Welte, T.; File, T.M.; Strauss, R.S.; Michiels, B.; Kaul, P.; Balis, D.; Arbit, D.; Amsler, K.; Noel, G.J. A Randomised, Double-Blind Trial Comparing Ceftobiprole Medocaril with Ceftriaxone with or without Linezolid for the Treatment of Patients with Community-Acquired Pneumonia Requiring Hospitalisation. Int. J. Antimicrob. Agents 2012, 39, 240–246. [Google Scholar] [CrossRef]
- Durante-Mangoni, E.; Andini, R.; Mazza, M.C.; Sangiovanni, F.; Bertolino, L.; Ursi, M.P.; Paradiso, L.; Karruli, A.; Esposito, C.; Murino, P.; et al. Real-Life Experience with Ceftobiprole in a Tertiary-Care Hospital. J. Glob. Antimicrob. Resist. 2020, 22, 386–390. [Google Scholar] [CrossRef]
- Riccobene, T.A.; Pushkin, R.; Jandourek, A.; Knebel, W.; Khariton, T. Penetration of Ceftaroline into the Epithelial Lining Fluid of Healthy Adult Subjects. Antimicrob. Agents Chemother. 2016, 60, 5849–5857. [Google Scholar] [CrossRef] [PubMed]
- Rodvold, K.A.; Nicolau, D.P.; Lodise, T.P.; Khashab, M.; Noel, G.J.; Kahn, J.B.; Gotfried, M.; Murray, S.A.; Nicholson, S.; Laohavaleeson, S.; et al. Identifying Exposure Targets for Treatment of Staphylococcal Pneumonia with Ceftobiprole. Antimicrob. Agents Chemother. 2009, 53, 3294–3301. [Google Scholar] [CrossRef]
- Edlinger-Stanger, M.; Al Jalali, V.; Andreas, M.; Jäger, W.; Böhmdorfer, M.; Zeitlinger, M.; Hutschala, D. Plasma and Lung Tissue Pharmacokinetics of Ceftaroline Fosamil in Patients Undergoing Cardiac Surgery with Cardiopulmonary Bypass: An In Vivo Microdialysis Study. Antimicrob. Agents Chemother. 2021, 65, e0067921. [Google Scholar] [CrossRef] [PubMed]
- Chauzy, A.; Gregoire, N.; Ferrandière, M.; Lasocki, S.; Ashenoune, K.; Seguin, P.; Boisson, M.; Couet, W.; Marchand, S.; Mimoz, O.; et al. Population Pharmacokinetic/Pharmacodynamic Study Suggests Continuous Infusion of Ceftaroline Daily Dose in Ventilated Critical Care Patients with Early-Onset Pneumonia and Augmented Renal Clearance. J. Antimicrob. Chemother. 2022, 77, 3173–3179. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, J.A.; Maggiore, C.R.; Cole, P.; Smith, A.; Jandourek, A.; Friedland, H.D. Ceftaroline Fosamil for the Treatment of Staphylococcus Aureus Bacteremia Secondary to Acute Bacterial Skin and Skin Structure Infections or Community-Acquired Bacterial Pneumonia. Infect. Dis. Clin. Pr. 2015, 23, 39–43. [Google Scholar] [CrossRef]
- Kato, H.; Hagihara, M.; Asai, N.; Shibata, Y.; Koizumi, Y.; Yamagishi, Y.; Mikamo, H. Meta-Analysis of Vancomycin versus Linezolid in Pneumonia with Proven Methicillin-Resistant Staphylococcus Aureus. J. Glob. Antimicrob. Resist. 2021, 24, 98–105. [Google Scholar] [CrossRef]
- Jiang, H.; Tang, R.-N.; Wang, J. Linezolid versus Vancomycin or Teicoplanin for Nosocomial Pneumonia: Meta-Analysis of Randomised Controlled Trials. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Antonello, R.M.; Principe, L.; Maraolo, A.E.; Viaggi, V.; Pol, R.; Fabbiani, M.; Montagnani, F.; Lovecchio, A.; Luzzati, R.; Di Bella, S. Fosfomycin as Partner Drug for Systemic Infection Management. A Systematic Review of Its Synergistic Properties from In Vitro and In Vivo Studies. Antibiotics 2020, 9, E500. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, H.; Liu, Y.; Xu, S.; Wu, M.; Liu, Z.; Qi, C.; Zhang, G.; Li, J.; Huang, X. Synergistic Effect of Linezolid with Fosfomycin against Staphylococcus Aureus in Vitro and in an Experimental Galleria Mellonella Model. J. Microbiol. Immunol. Infect. 2020, 53, 731–738. [Google Scholar] [CrossRef]
- Chen, H.; Li, L.; Liu, Y.; Wu, M.; Xu, S.; Zhang, G.; Qi, C.; Du, Y.; Wang, M.; Li, J.; et al. In Vitro Activity and Post-Antibiotic Effects of Linezolid in Combination with Fosfomycin against Clinical Isolates of Staphylococcus Aureus. Infect. Drug Resist. 2018, 11, 2107–2115. [Google Scholar] [CrossRef]
- Chai, D.; Liu, X.; Wang, R.; Bai, Y.; Cai, Y. Efficacy of Linezolid and Fosfomycin in Catheter-Related Biofilm Infection Caused by Methicillin-Resistant Staphylococcus Aureus. Biomed Res. Int. 2016, 2016, 6413982. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Jiang, L.; Chen, M.; Zhang, G.; Liu, Y.; Li, J.; Huang, X. In Vitro and in Vivo Antibacterial Activity of Linezolid Plus Fosfomycin Against Staphylococcus Aureus with Resistance to One Drug. Infect. Drug Resist. 2021, 14, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Kaye, K.S.; Udeani, G.; Cole, P.; Friedland, H.D. Ceftaroline Fosamil for the Treatment of Hospital-Acquired Pneumonia and Ventilator-Associated Pneumonia. Hosp. Pr. 2015, 43, 144–149. [Google Scholar] [CrossRef]
- Scheeren, T.W.L.; Welte, T.; Saulay, M.; Engelhardt, M.; Santerre-Henriksen, A.; Hamed, K. Early Improvement in Severely Ill Patients with Pneumonia Treated with Ceftobiprole: A Retrospective Analysis of Two Major Trials. BMC Infect. Dis. 2019, 19, 195. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Giani, T.; Coppi, M.; Di Pilato, V.; Arena, F.; Colavecchio, O.L.; Conte, V.; Santerre Henriksen, A.; Rossolini, G.M.; MRSA-HAP Study Group; et al. Staphylococcus Aureus from Hospital-Acquired Pneumonia from an Italian Nationwide Survey: Activity of Ceftobiprole and Other Anti-Staphylococcal Agents, and Molecular Epidemiology of Methicillin-Resistant Isolates. J. Antimicrob. Chemother. 2019, 74, 3453–3461. [Google Scholar] [CrossRef] [PubMed]
- Conte, J.E.; Golden, J.A.; Kipps, J.; Zurlinden, E. Intrapulmonary Pharmacokinetics of Linezolid. Antimicrob. Agents Chemother. 2002, 46, 1475–1480. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, X.; Xie, J.; Li, Q.; He, J.; Hu, L.; Wang, H.; Liu, A.; Xu, J.; Yang, C.; et al. Pharmacokinetic/Pharmacodynamic Parameters of Linezolid in the Epithelial Lining Fluid of Patients With Sepsis. J. Clin. Pharmacol. 2022, 62, 891–897. [Google Scholar] [CrossRef]
- Boselli, E.; Breilh, D.; Caillault-Sergent, A.; Djabarouti, S.; Guillaume, C.; Xuereb, F.; Bouvet, L.; Rimmelé, T.; Saux, M.-C.; Allaouchiche, B. Alveolar Diffusion and Pharmacokinetics of Linezolid Administered in Continuous Infusion to Critically Ill Patients with Ventilator-Associated Pneumonia. J. Antimicrob. Chemother. 2012, 67, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Matzi, V.; Lindenmann, J.; Porubsky, C.; Kugler, S.A.; Maier, A.; Dittrich, P.; Smolle-Jüttner, F.M.; Joukhadar, C. Extracellular Concentrations of Fosfomycin in Lung Tissue of Septic Patients. J. Antimicrob. Chemother. 2010, 65, 995–998. [Google Scholar] [CrossRef]
- Chen, H.-A.; Yang, C.-J.; Tsai, M.-S.; Liao, C.-H.; Lee, C.-H. Linezolid as Salvage Therapy for Central Nervous System Infections Due to Methicillin-Resistant Staphylococcus Aureus at Two Medical Centers in Taiwan. J. Microbiol. Immunol. Infect. 2020, 53, 909–915. [Google Scholar] [CrossRef]
- Pintado, V.; Pazos, R.; Jiménez-Mejías, M.E.; Rodríguez-Guardado, A.; Díaz-Pollán, B.; Cabellos, C.; García-Lechuz, J.M.; Lora-Tamayo, J.; Domingo, P.; Muñez, E.; et al. Linezolid for Therapy of Staphylococcus Aureus Meningitis: A Cohort Study of 26 Patients. Infect. Dis. 2020, 52, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Sipahi, O.R.; Bardak-Ozcem, S.; Turhan, T.; Arda, B.; Ruksen, M.; Pullukcu, H.; Aydemir, S.; Dalbasti, T.; Yurtseven, T.; Sipahi, H.; et al. Vancomycin versus Linezolid in the Treatment of Methicillin-Resistant Staphylococcus Aureus Meningitis. Surg. Infect. 2013, 14, 357–362. [Google Scholar] [CrossRef]
- Rebai, L.; Fitouhi, N.; Daghmouri, M.A.; Bahri, K. Linezolid for the Treatment of Postneurosurgical Infection Caused by Methicillin-Resistant Staphylococcus. Surg. Neurol. Int. 2019, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Aoki, K.; Sakurai, T.; Ito, K.; Hayashi, M.; Hirata, Y.; Sato, K.; Harashina, J.; Akahata, M.; Iwabuchi, S. Linezolid Treatment for Intracranial Abscesses Caused by Methicillin-Resistant Staphylococcus Aureus--Two Case Reports. Neurol. Med. Chir. 2010, 50, 515–517. [Google Scholar] [CrossRef] [PubMed]
- Kallweit, U.; Harzheim, M.; Marklein, G.; Welt, T.; Pöhlau, D. Successful Treatment of Methicillin-Resistant Staphylococcus Aureus Meningitis Using Linezolid without Removal of Intrathecal Infusion Pump. Case Report. J. Neurosurg. 2007, 107, 651–653. [Google Scholar] [CrossRef]
- Kessler, A.T.; Kourtis, A.P. Treatment of Meningitis Caused by Methicillin-Resistant Staphylococcus Aureus with Linezolid. Infection 2007, 35, 271–274. [Google Scholar] [CrossRef]
- Viale, P.; Pagani, L.; Cristini, F.; Stefini, R.; Bergomi, R.; Colombini, P.; Carosi, G. Linezolid for the Treatment of Central Nervous System Infections in Neurosurgical Patients. Scand. J. Infect. Dis. 2002, 34, 456–459. [Google Scholar] [CrossRef]
- Viaggi, B.; Paolo, A.D.; Danesi, R.; Polillo, M.; Ciofi, L.; Del Tacca, M.; Malacarne, P. Linezolid in the Central Nervous System: Comparison between Cerebrospinal Fluid and Plasma Pharmacokinetics. Scand. J. Infect. Dis. 2011, 43, 721–727. [Google Scholar] [CrossRef]
- Pfausler, B.; Spiss, H.; Dittrich, P.; Zeitlinger, M.; Schmutzhard, E.; Joukhadar, C. Concentrations of Fosfomycin in the Cerebrospinal Fluid of Neurointensive Care Patients with Ventriculostomy-Associated Ventriculitis. J. Antimicrob. Chemother. 2004, 53, 848–852. [Google Scholar] [CrossRef]
- Samura, M.; Kitahiro, Y.; Tashiro, S.; Moriyama, H.; Hamamura, Y.; Takahata, I.; Kawabe, R.; Enoki, Y.; Taguchi, K.; Takesue, Y.; et al. Efficacy and Safety of Daptomycin versus Vancomycin for Bacteremia Caused by Methicillin-Resistant Staphylococcus aureus with Vancomycin Minimum Inhibitory Concentration > 1 µg/mL: A Systematic Review and Meta-Analysis. Pharmaceutics 2022, 14, 714. [Google Scholar] [CrossRef]
- Cogo, A.; Gonzalez-Ruiz, A.; Pathan, R.; Hamed, K. Real-World Treatment of Complicated Skin and Soft Tissue Infections with Daptomycin: Results from a Large European Registry (EU-CORE). Infect. Dis. Ther. 2015, 4, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Heath, D.M.; Boyer, B.J.; Ghali, A.N.; Momtaz, D.A.; Nagel, S.C.; Brady, C.I. Use of Clindamycin for Necrotizing Soft Tissue Infection Decreases Amputation Rate. J. Orthop. Trauma 2022, 36, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Gasparini, L.E.; Laratta, M.; Sigurtà, A.; Rossi, A.; Brioschi, P.; Chiara, O.; Vismara, C.; Scaglione, F.; Arlati, S. Intensive Multidisciplinary Management in Critical Care Patients Affected by Severe Necrotizing Soft Tissue Infections: A Cooperative Method to Improve the Efficacy of Treatment. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Suecof, L.A.; Sutherland, C.A.; Gao, L.; Kuti, J.L.; Nicolau, D.P. In Vivo Microdialysis Study of the Penetration of Daptomycin into Soft Tissues in Diabetic versus Healthy Volunteers. Antimicrob. Agents Chemother. 2008, 52, 3941–3946. [Google Scholar] [CrossRef] [PubMed]
- Traunmüller, F.; Schintler, M.V.; Metzler, J.; Spendel, S.; Mauric, O.; Popovic, M.; Konz, K.H.; Scharnagl, E.; Joukhadar, C. Soft Tissue and Bone Penetration Abilities of Daptomycin in Diabetic Patients with Bacterial Foot Infections. J. Antimicrob. Chemother. 2010, 65, 1252–1257. [Google Scholar] [CrossRef]
- Ramanan, P.; Bryson, A.L.; Binnicker, M.J.; Pritt, B.S.; Patel, R. Syndromic Panel-Based Testing in Clinical Microbiology. Clin. Microbiol. Rev. 2018, 31, e00024-17. [Google Scholar] [CrossRef]
- Gatti, M.; Pea, F. Pharmacokinetic/Pharmacodynamic Target Attainment in Critically Ill Renal Patients on Antimicrobial Usage: Focus on Novel Beta-Lactams and Beta Lactams/Beta-Lactamase Inhibitors. Expert Rev. Clin. Pharmacol. 2021, 14, 583–599. [Google Scholar] [CrossRef]
- Galar, A.; Weil, A.A.; Dudzinski, D.M.; Muñoz, P.; Siedner, M.J. Methicillin-Resistant Staphylococcus Aureus Prosthetic Valve Endocarditis: Pathophysiology, Epidemiology, Clinical Presentation, Diagnosis, and Management. Clin. Microbiol. Rev. 2019, 32, e00041-18. [Google Scholar] [CrossRef]
- Mishra, N.N.; Lew, C.; Abdelhady, W.; Lapitan, C.K.; Proctor, R.A.; Rose, W.E.; Bayer, A.S. Synergy Mechanisms of Daptomycin-Fosfomycin Combinations in Daptomycin-Susceptible and -Resistant Methicillin-Resistant Staphylococcus Aureus: In Vitro, Ex Vivo, and In Vivo Metrics. Antimicrob. Agents Chemother. 2022, 66, e0164921. [Google Scholar] [CrossRef]
- Eliazar, J.; Johnson, T.; Chbib, C. Pre-Clinical Impact of the Synergistic Mechanism of Daptomycin and Ceftaroline on Patients with Methicillin-Resistant Staphylococcus Aureus Bacteremia Infections. Curr. Rev. Clin. Exp. Pharmacol. 2021, 16, 296–299. [Google Scholar] [CrossRef]
- Fu, Z.; Ma, Y.; Chen, C.; Guo, Y.; Hu, F.; Liu, Y.; Xu, X.; Wang, M. Prevalence of Fosfomycin Resistance and Mutations in MurA, GlpT, and UhpT in Methicillin-Resistant Staphylococcus Aureus Strains Isolated from Blood and Cerebrospinal Fluid Samples. Front. Microbiol. 2015, 6, 1544. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Chen, P.-Y.; Wang, J.-T.; Chang, S.-C. Prevalence of Fosfomycin Resistance and Gene Mutations in Clinical Isolates of Methicillin-Resistant Staphylococcus Aureus. Antimicrob. Resist. Infect. Control. 2020, 9, 135. [Google Scholar] [CrossRef]
- Harrison, E.M.; Ba, X.; Blane, B.; Ellington, M.J.; Loeffler, A.; Hill, R.L.R.; Holmes, M.A.; Peacock, S.J. PBP2a Substitutions Linked to Ceftaroline Resistance in MRSA Isolates from the UK. J. Antimicrob. Chemother. 2016, 71, 268–269. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yoon, E.-J.; Kim, D.; Kim, J.W.; Lee, K.-J.; Kim, H.S.; Kim, Y.R.; Shin, J.H.; Shin, J.H.; Shin, K.S.; et al. Ceftaroline Resistance by Clone-Specific Polymorphism in Penicillin-Binding Protein 2a of Methicillin-Resistant Staphylococcus Aureus. Antimicrob. Agents Chemother. 2018, 62, e00485-18. [Google Scholar] [CrossRef]
- Jacob, J.T.; DiazGranados, C.A. High Vancomycin Minimum Inhibitory Concentration and Clinical Outcomes in Adults with Methicillin-Resistant Staphylococcus Aureus Infections: A Meta-Analysis. Int. J. Infect. Dis. 2013, 17, e93–e100. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Takata, T.; Yoshimura, H.; Matsunaga, A.; Ohta, D.; Ishikura, H.; Futo, M.; Hara, S.; Kamimura, H.; Tamura, K.; et al. Vancomycin Bactericidal Activity as a Predictor of 30-Day Mortality in Patients with Methicillin-Resistant Staphylococcus Aureus Bacteremia. Antimicrob. Agents Chemother. 2011, 55, 1819–1820. [Google Scholar] [CrossRef]
- Kan, W.-C.; Chen, Y.-C.; Wu, V.-C.; Shiao, C.-C. Vancomycin-Associated Acute Kidney Injury: A Narrative Review from Pathophysiology to Clinical Application. Int. J. Mol. Sci. 2022, 23, 2052. [Google Scholar] [CrossRef]
- Luque, S.; Grau, S.; Alvarez-Lerma, F.; Ferrández, O.; Campillo, N.; Horcajada, J.P.; Basas, M.; Lipman, J.; Roberts, J.A. Plasma and Cerebrospinal Fluid Concentrations of Linezolid in Neurosurgical Critically Ill Patients with Proven or Suspected Central Nervous System Infections. Int. J. Antimicrob. Agents 2014, 44, 409–415. [Google Scholar] [CrossRef]
- Sartelli, M.; Guirao, X.; Hardcastle, T.C.; Kluger, Y.; Boermeester, M.A.; Raşa, K.; Ansaloni, L.; Coccolini, F.; Montravers, P.; Abu-Zidan, F.M.; et al. 2018 WSES/SIS-E Consensus Conference: Recommendations for the Management of Skin and Soft-Tissue Infections. World J. Emerg. Surg. 2018, 13, 58. [Google Scholar] [CrossRef]
- Gatti, M.; Pea, F. Should the Clinical Pharmacologist Play a Role in the Multidisciplinary Team Managing Severe Necrotizing Soft-Tissue Infections? Clin. Pharmacokinet. 2021, 60, 403–407. [Google Scholar] [CrossRef]
| Author, Year and Reference | Study Design | No. of Patients | Antibiotic and Dosing | Source of Infection | Isolates | Severity | Clinical Outcomes | Relapse Rate—Resistance Development | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Oxacillin or Cefazolin | |||||||||
| Rindone et al., 2018 | Systematic review with meta-analysis | 10 observational studies (one prospective and nine retrospective) | CEF 3–8 g/day vs. OXA 10–12 g/day | BSIs caused by MSSA Secondary BSI:BJI 9–59% CR-BSI 3–38% SSTI 3–34% LTRI 1–18% IE 0–27% | Significantly lower mortality rate was found with cefazolin vs. oxacillin (RR 0.78; 95%CI 0.69–0.88). Additionally, cefazolin showed significant higher clinical cure rate (RR 1.09; 95%CI 1.02–1.17) and lower risk of withdrawal for AEs (RR 0.27; 95%CI 0.16–0.47). No difference between cefazolin and oxacillin in terms of relapse BSIs was found (RR 1.29; 95%CI 0.96–1.74). | ||||
| Davis et al., 2018 | Multicentric retrospective cohort | 7312 (6520 flucloxacillin vs. 792 CEF) | flucloxacillin vs.CEF | CR-BSI33.2–37.1% SSTI 18.2–18.7% BJI 16.2–18.3% primary 12.4–12.5% IE 5.9–8.0% deep abscess 2.5–2.8% CNS 1.5–2.5% | 100% MSSA | ICU admission 13.5–13.9% IHD 8.9–20.8% | 30-day mortality rate:11.2% (flucloxacillin) vs. 10.7% (cefazolin)(OR 0.93; 95%CI 0.72–1.17) 30-day mortality rate in propensity adjusted analysis: aOR 0.86 (95%CI 0.65–1.14) | NA | Cefazolin is likely to have equivalent or superior outcomes to ASPs for MSSA bacteraemia. |
| McDanel et al., 2017 | Multicentric retrospective cohort | 3167 (1163 CEF vs. 2004 NAF/OXA) | CEF vs. NAF/OXA | SSTI 23–25% BJI 12–13% IE 4–7% | 100% MSSA | ICU admission 17.6% APACHE III score > 34 52–56% | 30-day mortality rate:aHR 0.63 (95%CI 0.51–0.78) 90-day mortality rate:aHR 0.77 (95%CI 0.66–0.90) | Recurrence: aOR 1.13 (95%CI 0.94–1.36). 90-day MSSA relapse: 2% (CEF) vs. 1% (NAF/OXA)p = 0.47 1-year MSSA relapse: 3% (CEF) vs. 2% (NAF/OXA)p = 0.07 | Patients who received cefazolin had a lower risk of mortality and similar odds of recurrent infections compared with nafcillin or oxacillin for MSSA infections complicated by bacteremia. |
| Beganovic et al., 2019 | Retrospective cohort | 212 (105 NAF/OXA vs. 107 CEF) | NAF/OXA vs. CEF | SSTI 21.7% BJI 12.3% surgical site 11.3% IE 7.1% UTI 6.1% LRTI 4.6% | 100% MSSA | ICU admission 5.2% Median APACHE score 22–25.5 | 30-day mortality rate:4.6% vs. 6.8% HR 0.67 (95%CI 0.11–4.00) Discharge: 97.7% vs. 93.2% HR 0.80 (95%CI 0.44–1.44) | 30-day readmission: 20.9% vs. 19.5%HR 0.75 (95%CI 0.26–2.16) 30-day reinfection: 9.3% vs. 0.0% p = NS | In hospitalized patients with BSIs caused by MSSA, no difference in mortality was observed between NAF/OXA and CEF. |
| Rao et al., 2015 | Multicentric retrospective cohort | 161 (103 CEF vs. 58 OXA) | CEF vs. OXA | CR-BSI24.1–45.6% SSTI 14.6–22.4%BJI 13.8–20.4%IE 18.0% LRTI 1.7–1.9% UTI 1.7–1.9% CNS 0.0–1.7% | 100% MSSA | ICU admission 32.8–41.8% | Treatment failure rate for deep-seated MSSA infections: 15.6% vs. 20%p = 0.72 In-hospital mortality:1% vs. 5.2% p = 0.13 | BSI recurrence: 4.9% vs. 5.2% p = 0.99 | Treatment with cefazolin or oxacillin was not independently associated with treatment failure (aOR 3.76; 95%CI, 0.98 to 14.4). Cefazolin was not associated with higher rates of treatment failure and appears to be an effective alternative to oxacillin for treatment of deep-seated MSSA BSI. |
| Bai et al., 2021 | Retrospective cohort | 98 (50 CEF vs. 48 cloxacillin) | CEF vs. cloxacillin | Spinal epidural abscesses 100% | 100% MSSA | Septic shock 11.2% | 90-day mortality rate:8% vs. 13% p = 0.52 Failure rate: 12% vs. 19% p = 0.21 | Recurrence rate: 2% vs. 9% p = 0.20 Serious AEs: 0% vs. 4% p = 0.24 | Cefazolin is likely as effective as an ASP and may be considered as a first-line treatment for MSSA spinal epidural abscesses. |
| Li et al., 2014 | Multicentric retrospective cohort | 93 (59 CEF vs. 34 OXA) | CEF 2–8 g/day II or CIvs. OXA 10–12 g/day II or CI | 100% complicated BSIBJI 41% IE 20% SSTI 10% CR-BSI 8% UTI 6% LRTI 4% Unknown 11% | 100% MSSA | ICU admission 11% Immunosuppression 6% | Clinical cure at EOT:95% vs. 88%p = 0.25 30-day mortality rate:0% vs. 3% p = 0.37 Overall failure at 90-day: 24% (CEF) vs. 47% (OXA) p = 0.04 | BSI recurrence:2% vs. 6% p = 0.55 AEs rate: 3% vs. 30% p < 0.001 | Cefazolin appears similar to oxacillin for the treatment of complicated MSSA bacteremia but with significantly improved safety.The higher rates of failure with oxacillin may have been confounded by other patient factors and warrant further investigation. |
| Corsini Campioli et al., 2021 | Retrospective cohort | 79 (45 CEF vs. 34 ASPs) | CEF 2 g q8 h vs. OXA 2 g q4 h or NAF 2 g q4 h | Spinal epidural abscesses 100% | 100% MSSA | ICU admission 19% | 30-day mortality rate:2% vs. 5.9% p = 0.57 6-week clinical failure: 75.6% vs. 82.4% p = 0.58 12-week clinical failure: 33.3% vs. 44.1% p = 0.35 | 90-day recurrence: 11.4% vs. 9.4%p = 0.99 | Cefazolin was equally as effective as ASPs, suggesting that it can be an alternative to ASPs in the treatment of MSSA spinal epidural abscesses. |
| Lefevre et al., 2021 | Retrospective cohort | 73 (35 ASPs vs. 38 CEF) | ASPs 12 g/day vs. CEF 6 g/day | IE 100% | 100% MSSA | Septic shock 30.1% | 90-day mortality rate:28.6% (ASPs) vs. 21.1% (CEF) p = 0.57 | Relapse 0.0% vs. 5.3%p = 0.49 | Efficacy and safety did not statistically differ between ASPs and cefazolin for MSSA-IE treatment. |
| Le Turnier et al., 2020 | Retrospective cohort | 17 (8 CEF vs. 9 cloxacillin) | CEF 2 g q6 h CI vs. cloxacillin 2 g q4 h CI | CNS 100% | 58.8% MSSA 35.3% MSSE 5.9% S. lugdunensis | NA | Ratio concentration CSF/plasma: 4.3% (CEF) vs. 1.8% (cloxacillin) | Clinical failure:0% (CEF) vs. 22.2% (cloxacillin) | Patients with staphylococcal meningitis treated with high-dose continuous intravenous infusion of CEF achieved therapeutic concentrations in CSF. CEF appears to be a therapeutic candidate which should be properly evaluated in this indication. |
| Hughes et al., 2009 | Retrospective cohort | 107 | CI OXA (78 patients) vs.II OXA (29 patients) | IE 100% | 100% MSSA | IHD 7% | 30-day mortality rate:8% (CI) vs. 10% (II) p = 0.7 30-day microbiological cure: 94% (CI) vs. 79% (II)p = 0.03 | NA | CI emerged as the only independent variable associated with 30-day microbiological cure at multivariate analysis (p = 0.01). CI oxacillin is an effective alternative to II oxacillin for the treatment of IE caused by MSSA and may improve microbiological cure. This convenient and pharmacodynamically optimized dosing regimen for oxacillin deserves consideration for patients with IE caused by MSSA. |
| Author, Year and Reference | Study Design | No. of Patients | Antibiotic and Dosing | Source of Infection | Isolates | Severity | Clinical Outcomes | Relapse Rate— Resistance Development | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Primary or CR-BSI; IE; Infections Associated with Intracardiac Device—Daptomycin + Fosfomycin | |||||||||
| Pujol et al., 2021 | RCT | 155 (74 DAP + FOS vs. 81 DAP) | DAP 10 mg/kg/day + FOS 2 g q6 h vs. DAP 10 mg/kg/day | 100% BSIs CR-BSI 41.9–48.1% SSTI 13.5–23.5% IE 11.1–12.2% surgical site 4.9–9.5% UTI 3.7–8.1% other 7.4–9.9% unknown 9.9–18.9% | 100% MRSA | Median CCI: 3–4 Mean Pitt score: 1.15–1.22 | Treatment success at 6-week: 54.1% vs. 42.0% (RR 1.29; 95%CI 0.93–1.80; p = 0.14) Complicated BSI: 16.2% vs. 32.1% p = 0.022 | 6-week microbiological failure rate: 0% vs. 11.1% p = 0.003 AEs rate: 17.6% vs. 4.9%p = 0.018 | Daptomycin plus fosfomycin provided a 12% higher rate of treatment success than daptomycin alone. This antibiotic combination prevented microbiological failure and complicated BSI, but it was more often associated with AEs. |
| Mirò et al., 2012 | Case series + In vitro study | 3 (+14 in vitro tested isolates) | DAP 10 mg/kg/day + FOS 2 g q6 h | 100% IEs | 67% MRSA 33% MSSA | NA | Clinical cure: 100% | AEs rate: 0.0% | This combination was tested in vitro against 7 MSSA, 5 MRSA, and 2 intermediately glycopeptide-resistant S. aureus isolates and proved to be synergistic against 11 (79%) strains and bactericidal against 8 (57%) strains. |
| Garcia-de-la-Maria et al., 2018 | In vivo study (rabbit model) | 5 MRSA strains | DAP 6–10 mg/kg + FOS 2 g q6 h or cloxacillin 2 g q4 h | Daptomycin plus fosfomycin significantly improved the efficacy of daptomycin monotherapy at 6 mg/kg/day in terms of both the proportion of sterile vegetations (100% versus 72%, p = 0.046) and the decrease in the density of bacteria within the vegetations (p = 0.025). Daptomycin plus fosfomycin was as effective as daptomycin monotherapy at 10 mg/kg/day (100% versus 93%, p = 1.00) and had activity similar to that of daptomycin plus cloxacillin when daptomycin was administered at 6 mg/kg/day (100% versus 88%, p = 0.48). Daptomycin nonsusceptibility was not detected in any of the isolates recovered from vegetations. In conclusion, for the treatment of MRSA experimental endocarditis, the combination of daptomycin plus fosfomycin showed synergistic and bactericidal activity. | |||||
| Primary or CR-BSI; IE; Infections Associated with Intracardiac Device—Daptomycin + Ceftaroline or Ceftobiprole | |||||||||
| Geriak et al., 2019 | RCT | 40 (17 DAP + ceftaroline vs. 23 DAP/VAN monotherapy) | DAP 6–8 mg/kg/day + ceftaroline 600 mg q8 h | 100% BSIs SSTIs 35–53% BJI 17–29% LRTI 6–26% UTI 17–18% IE 4–19% CR-BSI 6–13% IAI 9% | 100% MRSA | ICU admission 13–18%Median CCI 5–6 Immunosuppressed 4% | In-hospital mortality rate: 0% vs. 26% p = 0.029 | NA | This exploratory study showed with a very small number of patients that initial therapy with DAP + ceftaroline may be associated with reduced in-hospital mortality compared with the treatment standards of VAN or DAP monotherapy in patients with MRSA bacteremia. The survival benefit, if any, may be limited to patients with high-risk endovascular sources and those with IL-10 of >5 pg/mL on the day of first positive blood culture. |
| McCreary et al., 2019 | Retrospective matched cohort study | 171 (58 DAP + ceftaroline vs. 113 standard of care) | DAP 8 mg/kg/day + ceftaroline 600 mg q8 h | 100% BSIs Endovascular 53% Secondary 42% CR-BSI 5% | 100% MRSA | ICU admission 16% CCI >3 49–57% Immunosuppressed 9% | 30-day mortality rate: 8.3% vs. 14.2% p = NS Lower 30-day mortality rate in subgroup of patients receiving combination therapy with a CCI ≥3, endovascular source, and receipt of DAP-ceftaroline within 72 h of index culture | Relapse: 8.6% vs. 9.7% p= NS | DAP-ceftaroline treatment is often delayed in MRSA BSI. Combination therapy may be more beneficial if initiated earlier, particularly in patients at higher risk for mortality. |
| Nichols et al., 2021 | Retrospective case-control study | 140 (66 DAP + ceftaroline vs. 74 DAP/VAN or ceftaroline monotherapy) | DAP + ceftaroline | 100% BSIs | 100% MRSA | ICU admission: 57–64% MV 11–18% Vasopressors: 15–17%Median CCI: 2–3 Immunosuppressed 13% | Primary outcome (infection-related mortality, 60-day readmission, 60-day BSI recurrence): 21% vs. 24% p = 0.66 | BSI recurrence: 3% vs. 7% p = 0.45 | No difference was found in the composite outcome of 60-day bacteremia recurrence, readmission, or inpatient infection-related mortality for patients with MRSA bacteremia retained on combination therapy versus those de-escalated to monotherapy. |
| Johnson et al., 2021 | Retrospective cohort study | 60 (30 DAP + ceftaroline vs. 30 DAP/VAN ± GEN/RIF) | DAP 10 mg/kg/day + ceftaroline 600 mg q8 h | 100% BSIs Endovascular 37–40% IE 23–50% CR-BSI 6.7–37% SSTI 13–23% LRTI 13% IAI 3.3% UTI 3.3% | 100% MRSA | ICU admission 53–57% Immunocompromised 10–13% Median CCI 5 | Clinical failure rate: 20% vs. 43% p = 0.052 At multivariate analysis, DAP + ceftaroline was associated with 77% lower odds of clinical failure (OR 0.23; 95%CI 0.06–0.89) | 60-day recurrence: 0% vs. 30% p < 0.01 | In patients with complicated MRSA-BSI with delayed clearance, DAP + ceftaroline trended towards lower rates of clinical failure than SoC and was significantly associated with decreased clinical failure after adjustment for baseline differences. |
| Ahmad et al., 2020 | Retrospective case-control study | 30 (15 DAP/VAN + ceftaroline vs. 15 switched to DAP/VAN monotherapy following BSI resolution) | VAN 15–20 mg/kg q8–12 h or DAP 8–10 mg/kg/day + ceftaroline 600 mg q8–12 h | 100% BSIs IE 33–87% BJI 7–47% CNS infection 7% | 100% MRSA | Median CCI 0 | Mortality rate: 20% vs. 7% p = 0.24 | Recurrence: 0% vs. 27% p = 0.27 | In subjects with complicated and prolonged MRSA bacteremia requiring supplemental ceftaroline, clinical outcomes did not differ among patients prescribed DAP/VAN alone following bacteremia resolution vs. patients who continued combination therapy. |
| Sakoulas et al., 2014 | Retrospective multicenter study + in vitro analysis | 26 | DAP 4–10 mg/kg/day + ceftaroline 200 mg q12 h– 600 mg q8 h | 100% BSIs 54% IEs 42% BJI 4% SSTI | 76.9% MRSA 7.7% MSSA 7.7% VISA 7.7% MRSE | NA | Mortality rate: 4% Time to bacteremia clearance: 10 (previous therapeutic regimens) vs. 2 days (DAP + ceftaroline) | Ceftaroline plus daptomycin may be an option to hasten clearance of refractory staphylococcal bacteremia. Ceftaroline offers dual benefit via synergy with both daptomycin and sensitization to innate host defense peptide cathelicidin LL37, which could attenuate virulence of the pathogen. | |
| Cortes-Penfield et al., 2019 | Retrospective cohort study | 17 (5 DAP monotherapy vs. 12 DAP + ceftaroline 2–3° line) | DAP 8 mg/kg/day + ceftaroline | 100% persistent BSIs BJI 47.1% IE 29.4% SSTI 23.5% | 100% MRSA | ICU admission 64.7% Mean CCI: 3.2–5 | Mortality rate: 53% Duration of BSI: 11.1 (early combination therapy) vs. 17.3 days p = 0.11 | NA | Early combination therapy with daptomycin and ceftaroline shortens prolonged MRSA bacteremia and may be helpful in securing favorable clinical outcomes. |
| Hornak et al., 2019 | Case series | 11 (6 DAP + ceftaroline; 5 VAN + ceftaroline) | DAP or VAN + ceftaroline 200 mg q12 h– 600 mg q8 h | 100% BSIs | 100% MRSA | Median CCI 4.5 Immunosuppressed 20% | Microbiological cure rate:100% 30-day mortality rate: 11.1% | 30-/60-day relapse: 0.0% | Combination therapy demonstrated success in diverse cases of refractory MRSA BSIs, including instances of persistent bacteremia paired with incomplete source control. |
| Duss et al., 2019 | Case report + in vitro analysis | 1 | DAP 10 mg/kg/day + ceftaroline600 mg q8 h | IE | MRSA | NA | Clinical cure In in vitro analysis, at high inoculum only combination between DAP and ceftaroline provides synergistic and bactericidal activity | No relapse | A synergistic effect between daptomycin plus ceftaroline and increased bactericidal activity against MRSA was reported, suggesting that this combination may be effective for the treatment of invasive MRSA infection. |
| Cunha et al., 2015 | Case report | 1 | DAP 12 mg/kg/day + Ceftaroline 600 mg q12 h | PVE | 100% MRSA | NA | Clinical cure | No relapse | Ceftaroline plus high-dose daptomycin could be a treatment option for PVE sustained by difficult-to- treat MRSA strains. |
| Tascini et al., 2020 | Case series | 12 | Ceftobiprole + DAP (in 11 patients) | 100% IEs 67% PVEs | 33.3% MRSA 33.3% MSSA 33.3% CoNS | Immunosuppressed 16.7% | Clinical cure rate 83% | Relapse 0.0% | Ceftobiprole, especially in combination, could be a promising alternative treatment for infective endocarditis. |
| Oltolini et al., 2016 | Case report | 1 | DAP 10 mg/kg/day + ceftobiprole 500 mg q8 h | IE | MRSA | NA | Clinical cure | No relapse | Ceftobiprole plus daptomycin could be a treatment option for IE sustained by difficult-to- treat MRSA strains. |
| Barber et al., 2014 | In vitro study | 20 MRSA isolates | DAP + ceftobiprole | Ceftobiprole plus daptomycin represented the most potent combination with a 4-fold decrease in MIC and synergy against all strains evaluated in time–kill evaluations. Additionally, binding studies demonstrated enhanced daptomycin binding in the presence of subinhibitory concentrations of ceftobiprole. The use of combination therapy with ceftobiprole may provide a needed addition for the treatment of Gram-positive infections resistant to daptomycin or vancomycin. | |||||
| Primary or CR-BSI; IE; Infections Associated with Intracardiac Device—Vancomycin or Teicoplanin | |||||||||
| Schweizer et al., 2021 | Multicenter retrospective cohort | 7411 of which 606 switched to DAP during the first hospitalization and 108 within the first 3 days | VAN vs. switch to DAP | 100% BSIs SSTI 46.4–48.5%BJI 20.4–29.2% Endovascular 18.1–29.9% LRTI 2.3–5.0% | 100% MRSA MIC > 1 mg/L for VAN: 8.2–16.0% | ICU admission 5.5–7.1% Immunosuppressed 76.2% | 30-day mortality rate: 8.3% (early switch to DAP) vs. 17.4% (VAN) aHR 0.48 (95%CI 0.25–0.92) 30-day mortality rate: 12.9% (any switch) vs. 17.4% (VAN) aHR 0.87 (95%CI 0.69–1.09) | NA | Switching to daptomycin within 3 days of initial receipt of vancomycin is associated with lower 30-day mortality among patients with MRSA BSI. |
| Tong et al., 2020 | RCT | 352 (174 DAP/VAN + beta-lactam vs. 178 DAP/VAN) | DAP 6–10 mg/kg/day or VAN 1 g q12 h + Oxacillin 2 g q6 h or Cloxacillin 2 g q6 h or CEF 2 g q8 h vs. DAP 6–10 mg/kg/day or VAN 1 g q12 h | 100% BSI SSTI 23–28% primary BSI 20% BJI 15–18% CR-BSI 12–14% LRTI 6–7% IE 3–5% other 7–10% | 100% MRSA | Median CCI: 5 Median SOFA score: 1–2 | Primary outcome (90-day mortality, relapse, persistent BSI, microbiological failure): 35% vs. 39% (−4.2%; 95%CI −14.3% to 6%) 90-day mortality: 21% vs. 16% (4.5%; 95%CI −3.7% to 12.7%) | Persistent BSI: 11% vs. 20% (−8.9%; 95%CI −16.6% to −1.2%) AKI: 23% vs. 6% (17.2%; 95%CI 9.3% vs. 25.2%) | Among patients with MRSA bacteremia, addition of an antistaphylococcal β-lactam to standard antibiotic therapy with vancomycin or daptomycin did not result in significant improvement in the primary composite end point of mortality, persistent bacteremia, relapse, or treatment failure. Early trial termination for safety concerns and the possibility that the study was underpowered to detect clinically important differences in favor of the intervention should be considered when interpreting the findings. |
| Community-Acquired Pneumonia—Ceftaroline or Ceftobiprole | |||||||||
| Sotgiu et al., 2018 | Systematic review with meta-analysis | 6 retrospective observational studies providing data on patients with documented MRSA pneumonia (345 patients) | Ceftaroline 600 mg q12 h | CAP/HAP/VAP caused by MRSA | Pooled success rate in CAP subgroup: 81.3% (95%CI 80.0–82.7) Pooled success rate in MRSA subgroup: 71.7% (95%CI 59.7–82.3) | ||||
| Bassetti et al., 2020 | Retrospective cohort study | 89 | Ceftaroline 600 mg q8 h (60% combination therapy) | 100% severe CAP 12% bacteraemic | Isolated pathogens in 34.8% of included cases 10.1% MRSA | ICU admission 37% Septic shock 12% Immunosuppressed 40% Mean CCI 4 ± 3 | 30-day mortality rate: 20% Clinical failure rate: 36% The only independent predictor of clinical failure was the time elapsing from severe CAP diagnosis to ceftaroline therapy (OR for each passing day 1.5, 95%CI 1.1–1.9, p = 0.003). | NA | Ceftaroline could represent an important therapeutic option for severe CAP. |
| Nicholson et al., 2012 | RCT | 638 (314 ceftobiprole vs. 324 ceftriaxone plus LIN) | Ceftobiprole 500 mg q8 h vs. Ceftriaxone 2 g/day ± Linezolid 600 mg q12 h | 100% CAP 4% Bacteraemic | Isolated pathogens in 28.8% of included cases | PSI ≥ 4: 22% SIRS 52–55% | Clinical cure: 86.6% vs. 87.4% (95%CI −6.9% to 5.3%) Microbiological eradication: 88.2% vs. 90.8% (95%CI −12.6% to 7.5%) | NA | Ceftobiprole was non-inferior to the comparator (ceftriaxone ± linezolid) in all clinical and microbiological analyses conducted, suggesting that ceftobiprole has a potential role in treating hospitalized patients with CAP. |
| Durante-Mangoni et al., 2020 | Retrospective cohort study | 29 | Ceftobiprole 250 mg/die– 500 mg q8 h | 19.3% CAP | 24.1% MRSA | Septic shock 13.8% | Clinical cure rate: 68.9% (66.7% in CAP subgroup) | NA | Ceftobiprole, even outside current indications, may be a safe and effective treatment for resistant Gram-positive cocci infections where other drugs are inactive or poorly tolerated and for salvage therapy. |
| Infection-Related Ventilator-Associated Complications—Linezolid or Linezolid + Fosfomycin | |||||||||
| Kato et al., 2021 | Systematic review with meta-analysis | 7 RCTs (1239 patients) and 8 retrospective observational studies (6125 patients) | LIN 600 mg q12 h vs. VAN 1 g q12 h or 15 mg/kg q12 h | HAP/VAP caused by MRSA | Clinical cure and microbiological eradication rates were significantly increased in patients treated with LIN in RCTs (clinical cure: RR 0.81; 95%CI 0.71–0.92; microbiological eradication: RR 0.71; 95%CI 0.62–0.81) and retrospective studies (clinical cure: OR 0.35; 95%CI 0.18–0.69). However, mortality was comparable between patients treated with VAN and LIN in RCTs (RR 1.08; 95%CI 0.88–1.32) and retrospective studies (OR 1.20; 95%CI 0.94–1.53). Likewise, there was no significant difference in AEs between VAN and LIN in retrospective studies (thrombocytopenia: OR 0.95; 95%CI 0.50–1.82; nephrotoxicity: OR 1.72; 95%CI 0.85–3.45). According to our meta-analysis of RCTs and retrospective studies conducted worldwide, we found robust evidence to corroborate the IDSA guidelines for the treatment of proven MRSA pneumonia. | ||||
| Jiang et al., 2013 | Systematic review with meta-analysis | 12 RCTs (4725 patients) | LIN vs. VAN or TEI | HAP | There was no statistically significant difference between the two groups in the treatment of nosocomial pneumonia regarding the clinical cure rate (RR 1.08; 95%CI 1.00–1.17; p = 0.06). Linezolid was associated with better microbiological eradication rate in nosocomial pneumonia patients compared with glycopeptide antibiotics (RR 1.16; 95%CI 1.03–1.31; p = 0.01). There were no differences in the all-cause mortality (RR 0.95; 95%CI 0.83–1.09; p = 0.46) between the two groups. However, the risks of rash (RR 0.41; 95%CI 0.24–0.71; p = 0.001) and renal dysfunction (RR 0.41; 95%CI = 0.27–0.64; p < 0.0001) were higher with glycopeptide antibiotics. | ||||
| Antonello et al., 2020 | Systematic review of in vitro studies | 9 in vitro/in vivo preclinical studies | LIN + FOS | S. aureus isolates (166 strains) | Combination therapy including FOS and LIN had a synergistic effect in vitro approximately in 95% of cases (synergistic effect of the combination against 100% of the tested isolates was reported in 6 in vitro studies) and even against staphylococcal biofilm cultures. Furthermore, the only 2 in vivo studies performed proved FOS + LZD combination to have higher efficacy than FOS or LIN alone. | ||||
| Chen et al., 2018 | In vitro study | 11 S. aureus strains (5 MSSA and 6 MRSA) | LIN + FOS | Synergistic effects were observed for eight strains, and no antagonism was found with any combination. Moreover, LIN combined with FOS at 4× MIC showed the best synergistic antibacterial effect, and this effect was retained after 24 h. In addition, both the antibiotics alone and in combination showed increased post-antibiotic effect and post-antibiotic subminimum inhibitory concentration effect values in a concentration- and time-dependent manner. | |||||
| Li et al., 2020 | In vitro/in vivo preclinical study | 4 S. aureus strains (2 MSSA and 2 MRSA) | LIN 10 mg/kg + FOS 200 mg/kg | The combination of linezolid and fosfomycin was synergistic and bacteriostatic against four tested strains. Treatment of Galleria mellonella larvae infected with lethal doses of S. aureus resulted in significantly enhanced survival rates when low-dose of combination has no significant differences with high-dose combination. Combination therapy including linezolid and fosfomycin has synergistic effect against S. aureus in vitro and in an experimental G. mellonella model, and it suggests that a high dose of linezolid and fosfomycin may not be necessary. | |||||
| Chai et al., 2016 | In vitro/in vivo preclinical study | 3 MRSA strains | LIN 40 mg/kg q12 h + FOS 300 mg/kg q12 h | A FICI ≤ 0.5 was found for LIN + FOS combination, showing the best synergistic effect in all strains. The combination of LIN and FOS in a catheter-related biofilm rat model found that viable bacteria counts in biofilm were significantly reduced after treatment (p < 0.05). | |||||
| Xie et al., 2021 | In vitro/in vivo preclinical study | One MRSA strain | LIN 2.5–10 mg/kg + FOS 50–200 mg/kg | Antibiotic combination showed excellent synergistic or additive effects on the original and the linezolid-resistant strain but showed indifferent effect for fosfomycin-resistant strain. In the Galleria mellonella infection model, the survival rate of the antibiotic combined was improved compared with that of the single drug. There was a good correlation between in vivo efficacy and in vitro susceptibility. | |||||
| Infection-Related Ventilator-Associated Complications—Ceftaroline or Ceftobiprole | |||||||||
| Sotgiu et al., 2018 | Systematic review with meta-analysis | 6 retrospective observational studies providing data on patients with documented MRSA pneumonia (345 patients) | Ceftaroline 600 mg q12 h | CAP/HAP/VAP caused by MRSA | Pooled success rate in HAP/VAP subgroup: 83.0% (95%CI 65.0–95.0) Pooled success rate in MRSA subgroup: 71.7% (95%CI 59.7–82.3) | ||||
| Kaye et al., 2015 | Retrospective cohort study | 40 | Ceftaroline | 67.5% HAP 32.5% VAP | 47.5% MRSA | ICU admission 42.5% | Overall clinical cure rate: 75.0% (61.5% in VAP subgroup) Clinical success rate in MRSA subgroup: 57.9% | NA | Ceftaroline is an effective treatment option for HAP and VAP when a susceptible etiologic pathogen is identified, including MRSA. |
| Scheeren et al., 2019 | Retrospective analysis of an RCT | 307 (169 ceftobiprole vs.138 comparator) | Ceftobiprole 500 mg q8 h vs. Ceftazidime + Linezolid | 100% HAP | 23.7% S. aureus | Mechanical ventilation 22.5% | Early clinical response (at day 4): difference 12.5% (95%CI 3.5–21.4) | NA | Ceftobiprole treatment may have advantages over other antibiotics in terms of achieving early improvement in high-risk patients with HAP. |
| Durante-Mangoni et al., 2020 | Retrospective cohort study | 29 | Ceftobiprole 250 mg/die– 500 mg q8 h | 47.8% HAP/VAP | 24.1% MRSA | Septic shock 13.8% | Clinical cure rate: 68.9% (85.7% in HAP/VAP subgroup) No clinical failure in MRSA subgroup | NA | Ceftobiprole, even outside current indications, may be a safe and effective treatment for resistant Gram-positive cocci infections where other drugs are inactive or poorly tolerated and for salvage therapy. |
| Antonelli et al., 2019 | In vitro study | 66 MRSA isolates from HAP | Ceftobiprole | Overall susceptibility to ceftobiprole: 95.5%; MIC50: 1 mg/L; MIC90: 2 mg/L | |||||
| Central Nervous System Infections—Linezolid or Linezolid + Fosfomycin | |||||||||
| Chen et al., 2020 | Retrospective cohort study | 66 | LIN | Brain abscess 28.8% Spinal epidural abscess 27.3% Meningitis 18.2% Meningitis + brain/epidural abscess 13.6% Spine device-related infection 7.6% | 100% MRSA Bacteraemic 78.8% | Liver cirrhosis 21.2% | In-hospital mortality rate: 13.6% | Relapse rate: 16.7% | LIN demonstrated promising effect as a salvage therapy for central nervous system infection caused by MRSA, whether due to drug allergy or glycopeptide treatment failure. |
| Pintado et al., 2020 | Retrospective multicentric cohort study | 26 | LIN 600 mg q12 h (62% monotherapy) | 100% meningitis 81% post-operative | 15 MRSA 11 MSSA Bacteraemic 8% | Immunosuppressed 8% | Clinical cure rate: 69% Microbiological cure rate: 93% 30-day mortality rate: 23% | NA | Linezolid appears to be effective and safe for therapy of S. aureus meningitis. |
| Sipahi et al., 2013 | Retrospective case-control study | 17 | LIN 600 mg q12 h vs. VAN 500 mg q6 h | Meningitis 100% | 17 MRSA | NA | Microbiological cure at 5-day: 77.8% vs. 25.0% p = 0.044 | NA | Findings suggested that LIN is superior to VAN for treating MRSA meningitis, especially in cases in which there is a high MIC (2 mg/L) for VAN. |
| Rebai et al., 2019 | Case series | 10 | LIN 600 mg q12 h | Meningitis 60%Ventriculitis 20% Subdural empyema 20% | 7 MRSA 3 MRSE | NA | Microbiological cure rate: 100% | NA | LIN could be an alternative to VAN for the treatment of post-neurosurgical infections caused by MRSA with a high rate of efficacy. |
| Viaggi et al., 2011 | PK study | 7 | LIN 600 mg q12 h | 100% external ventricular drainage | Prophylaxis of CNS infection | ICU admission 100% | AUCCSF/AUCpkasma 0.57 | NA | The wide variability in the CSF concentration profile and drug PK among patients suggests the adoption of TDM-guided strategy. |
| Saito et al., 2010 | Case series | 2 | LIN 600 mg q12 h | 100% intracranial abscess | 2 MRSA | ICU admission 100% | Clinical cure 100% | None | LIN showed high CSF penetration allowing for the effective treatment of post-neurosurgical infections caused by MRSA. |
| Kallweit et al., 2007 | Case report | 1 | LIN 600 mg q12 h | Meningitis | MRSA | NA | Clinical cure | None | LIN showed high CSF penetration allowing for the effective treatment of post-neurosurgical infections caused by MRSA. |
| Kessler et al., 2007 | Case report | 1 | LIN 600 mg q12 h | Meningitis | MRSA | NA | Clinical cure | None | LIN showed high CSF penetration allowing for the effective treatment of post-neurosurgical infections caused by MRSA. |
| Pfausler et al., 2004 | PK study | 6 | FOS 8 g q8 h | 100% ventriculitis | 2 MSSA 2 MSSE 2 NA | ICU admission 100% | AUCCSF/AUCpkasma 0.27 ± 0.08 | NA | High-dose FOS could provide sufficient antimicrobial concentrations in the CSF for the overall treatment period. |
| Antonello et al., 2020 | Systematic review of in vitro studies | 9 in vitro/in vivo preclinical studies | LIN + FOS | S. aureus isolates (166 strains) | Combination therapy including FOS and LIN had a synergistic effect in vitro approximately in 95% of cases (synergistic effect of the combination against 100% of the tested isolates was reported in 6 in vitro studies) and even against staphylococcal biofilm cultures. Furthermore, the only 2 in vivo studies performed proved FOS + LZD combination to have higher efficacy than FOS or LIN alone. | ||||
| Chen et al., 2018 | In vitro study | 11 S. aureus strains (5 MSSA and 6 MRSA) | LIN + FOS | Synergistic effects were observed for eight strains, and no antagonism was found with any combination. Moreover, LIN combined with FOS at 4× MIC showed the best synergistic antibacterial effect, and this effect was retained after 24 h. In addition, both the antibiotics alone and in combination showed increased post-antibiotic effect and post-antibiotic subminimum inhibitory concentration effect values in a concentration- and time-dependent manner. | |||||
| Li et al., 2020 | In vitro/in vivo preclinical study | 4 S. aureus strains (2 MSSA and 2 MRSA) | LIN 10 mg/kg + FOS 200 mg/kg | The combination of linezolid and fosfomycin was synergistic and bacteriostatic against four tested strains. Treatment of Galleria mellonella larvae infected with lethal doses of S. aureus resulted in significantly enhanced survival rates when low-dose of combination has no significant differences with high-dose combination. Combination therapy including linezolid and fosfomycin has synergistic effect against S. aureus in vitro and in an experimental G. mellonella model, and it suggests that a high dose of linezolid and fosfomycin may not be necessary. | |||||
| Chai et al., 2016 | In vitro/in vivo preclinical study | 3 MRSA strains | LIN 40 mg/kg q12 h + FOS 300 mg/kg q12 h | A FICI ≤ 0.5 was found for LIN + FOS combination, showing the best synergistic effect in all strains. The combination of LIN and FOS in a catheter-related biofilm rat model found that viable bacteria counts in biofilm were significantly reduced after treatment (p < 0.05). | |||||
| Xie et al., 2021 | In vitro/in vivo preclinical study | One MRSA strain | LIN 2.5–10 mg/kg + FOS 50–200 mg/kg | Antibiotic combination showed excellent synergistic or additive effects on the original and the linezolid-resistant strain but showed indifferent effect for fosfomycin-resistant strain. In the Galleria mellonella infection model, the survival rate of the antibiotic combined was improved compared with that of the single drug. There was a good correlation between in vivo efficacy and in vitro susceptibility. | |||||
| Necrotizing Fasciitis—Daptomycin ± Clindamycin | |||||||||
| Samura et al., 2022 | Systematic review with meta-analysis | 7 studies (2 RCTs and 5 retrospective observational; 907 patients) | DAP vs. VAN | 100% BSI due to MRSA (28–32.8% complicated SSTI) | DAP was associated with significantly lower mortality (OR 0.53, 95%CI 0.29–0.98) and higher treatment success (OR 2.20, 95%CI 1.63–2.96) compared to VAN. For intermediate-risk sources (including complicated SSTI), DAP was a factor increasing treatment success compared with VAN (OR 4.40, 95%CI 2.06–9.40). | ||||
| Cogo et al., 2015 | Multicenter retrospective registry | 1927 | DAP ≥ 4–6 mg/kg/day | 100% complicated SSTI | S. aureus 51.9% (MRSA 31.5%) | NA | Overall clinical success rate: 84.7% Clinical success rate in MRSA subgroup: 87.0% | NA | DAP treatment resulted in high clinical success rates in patients with different complicated SSTI subtypes, the majority of whom having failed previous antibiotic therapy, with no safety issues. |
| Gatti et al., 2019 | Retrospective case-control study | 62 (32 receiving IMM vs. 30 SM) | DAP 8–10 mg/kg/day vs. VAN 20 mg/kg/day | 100% NSTI | S. aureus 22.6% (MRSA 3.2%) | ICU admission 100%MV 90.3% Vasopressors 83.9% Immunosuppression 24.2% | ICU mortality rate: 15.6% vs. 40% (p = 0.032) 7-day mortality rate: 3.1% vs. 20% (p = 0.049) | NA | IMM was more effective than SM as it allowed the earlier control of infection and the faster reduction of multiple organ-dysfunction (ΔSOFA −5.2 ± 3.5 pts. versus −2.1 ± 3.0 pts.; p = 0.003). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatti, M.; Viaggi, B.; Rossolini, G.M.; Pea, F.; Viale, P. Targeted Therapy of Severe Infections Caused by Staphylococcus aureus in Critically Ill Adult Patients: A Multidisciplinary Proposal of Therapeutic Algorithms Based on Real-World Evidence. Microorganisms 2023, 11, 394. https://doi.org/10.3390/microorganisms11020394
Gatti M, Viaggi B, Rossolini GM, Pea F, Viale P. Targeted Therapy of Severe Infections Caused by Staphylococcus aureus in Critically Ill Adult Patients: A Multidisciplinary Proposal of Therapeutic Algorithms Based on Real-World Evidence. Microorganisms. 2023; 11(2):394. https://doi.org/10.3390/microorganisms11020394
Chicago/Turabian StyleGatti, Milo, Bruno Viaggi, Gian Maria Rossolini, Federico Pea, and Pierluigi Viale. 2023. "Targeted Therapy of Severe Infections Caused by Staphylococcus aureus in Critically Ill Adult Patients: A Multidisciplinary Proposal of Therapeutic Algorithms Based on Real-World Evidence" Microorganisms 11, no. 2: 394. https://doi.org/10.3390/microorganisms11020394
APA StyleGatti, M., Viaggi, B., Rossolini, G. M., Pea, F., & Viale, P. (2023). Targeted Therapy of Severe Infections Caused by Staphylococcus aureus in Critically Ill Adult Patients: A Multidisciplinary Proposal of Therapeutic Algorithms Based on Real-World Evidence. Microorganisms, 11(2), 394. https://doi.org/10.3390/microorganisms11020394







