A 2-Year Retrospective Case Series on Isolates of the Emerging Pathogen Actinotignum schaalii from a Canadian Tertiary Care Hospital
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Demographics
3.2. Patient Comorbidity Profile
3.3. Management Prior to Microbial Culture
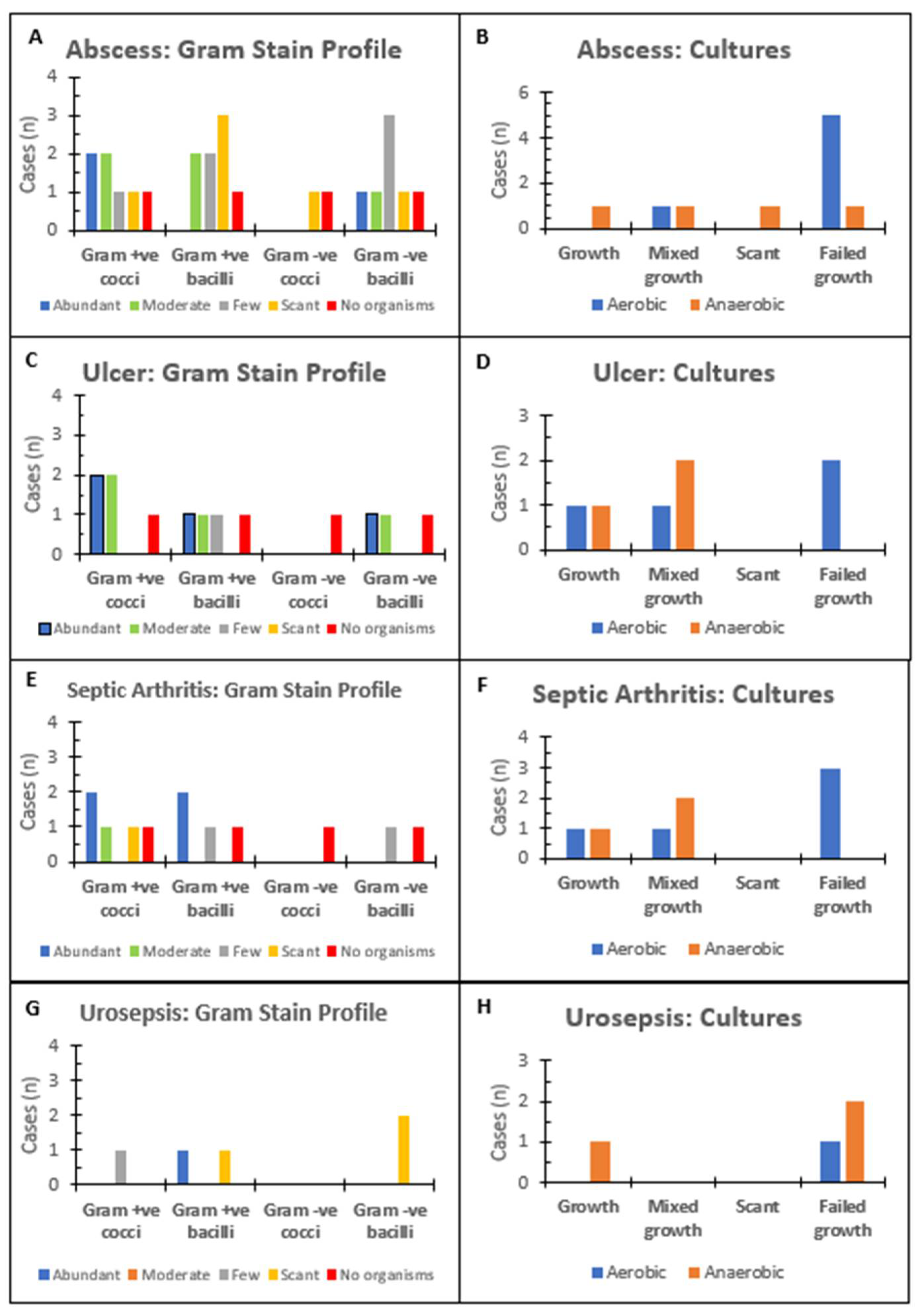
3.4. Microbiology Laboratory Investigations
3.5. Antimicrobial Tailoring after Microbial Culture
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lawson, P.A.; Falsen, E.; Åkervall, E.; Vandamme, P.; Collins, M.D. Characterization of Some Actinomyces-Like Isolates from Human Clinical Specimens: Reclassification of Actinomyces suis (Soltys and Spratling) as Actinobaculum suis comb. nov. and Description of Actinobaculum schaalii sp. nov. Int. J. Syst. Bacteriol. 1997, 47, 899–903. [Google Scholar] [CrossRef]
- Molitor, E. Obituary. Rev. Med Microbiol. 2016, 27, 172. [Google Scholar] [CrossRef]
- Lotte, R.; Lotte, L.; Ruimy, R. Actinotignum schaalii (formerly Actinobaculum schaalii): A newly recognized pathogen-review of the literature. Clin. Microbiol. Infect. 2016, 22, 28–36. [Google Scholar] [CrossRef]
- Yassin, A.F.; Spröer, C.; Pukall, R.; Sylvester, M.; Siering, C.; Schumann, P. Dissection of the genus Actinobaculum: Reclassification of Actinobaculum schaalii Lawson et al. 1997 and Actinobaculum urinale Hall et al. 2003 as Actinotignum schaalii gen. nov., comb. nov. and Actinotignum urinale comb. nov., description of Actinotignum sanguinis sp. nov. and emended descriptions of the genus Actinobaculum and Actinobaculum suis; and re-examination of the culture deposited as Actinobaculum massiliense CCUG 47753T (= DSM 19118T), revealing that it does not represent a strain of this species. Int. J. Syst. Evol. Microbiol. 2015, 65 Pt 2, 615–624. [Google Scholar]
- Reinhard, M.; Prag, J.; Kemp, M.; Andresen, K.; Klemmensen, B.; Højlyng, N.; Sørensen, S.H.; Christensen, J.J. Ten cases of Actinobaculum schaalii infection: Clinical relevance, bacterial identification, and antibiotic susceptibility. J. Clin. Microbiol. 2005, 43, 5305–5308. [Google Scholar] [CrossRef]
- Hilt, E.E.; McKinley, K.; Pearce, M.M.; Rosenfeld, A.B.; Zilliox, M.J.; Mueller, E.R.; Brubaker, L.; Gai, X.; Wolfe, A.J.; Schreckenberger, P.C. Urine Is Not Sterile: Use of Enhanced Urine Culture Techniques To Detect Resident Bacterial Flora in the Adult Female Bladder. J. Clin. Microbiol. 2014, 52, 871–876. [Google Scholar] [CrossRef]
- Lewis, D.A.; Brown, R.; Williams, J.; White, P.; Jacobson, S.K.; Marchesi, J.R.; Drake, M.J. The human urinary microbiome; bacterial DNA in voided urine of asymptomatic adults. Front. Cell. Infect. Microbiol. 2013, 3, 41. [Google Scholar] [CrossRef]
- Beguelin, C.; Genne, D.; Varca, A.; Tritten, M.L.; Siegrist, H.H.; Jaton, K.; Lienhard, R. Actinobaculum schaalii: Clinical observation of 20 cases. Clin Microbiol Infect. 2011, 17, 1027–1031. [Google Scholar] [CrossRef]
- Lotte, L.; Lotte, R.; Durand, M.; Degand, N.; Ambrosetti, D.; Michiels, J.F.; Amiel, J.; Cattoir, V.; Ruimy, R. Infections related to Actinotignum schaalii (formerly Actinobaculum schaalii): A 3-year prospective observational study on 50 cases. Clin. Microbiol. Infect. 2016, 22, 388–390. [Google Scholar] [CrossRef]
- Maraki, S.; Evangelou, G.; Stafylaki, D.; Scoulica, E. Actinotignum schaalii subcutaneous abscesses in a patient with hidradenitis suppurativa: Case report and literature review. Anaerobe 2017, 43, 43–46. [Google Scholar] [CrossRef]
- Siller Ruiz, M.; Hernández Egido, S.; Calvo Sánchez, N.; Muñoz Bellido, J.L. Unusual clinical presentations of Actinotignum (Actinobaculum) schaalii infection. Enferm. Infecc. Microbiol. Clin. 2017, 35, 197–198. [Google Scholar] [CrossRef]
- Vanden Bempt, I.; Van Trappen, S.; Cleenwerck, I.; De Vos, P.; Camps, K.; Celens, A.; Van De Vyvere, M. Actinobaculum schaalii causing Fournier’s gangrene. J. Clin. Microbiol. 2011, 49, 2369–2371. [Google Scholar] [CrossRef]
- Gomez, E.; Gustafson, D.R.; Rosenblatt, J.E.; Patel, R. Actinobaculum Bacteremia: A Report of 12 Cases. J. Clin. Microbiol. 2011, 49, 4311–4313. [Google Scholar] [CrossRef]
- Tena, D.; Fernández, C.; Lago, M.R.; Arias, M.; Medina, M.J.; Sáez-Nieto, J.A. Skin and soft-tissue infections caused by Actinobaculum schaalii: Report of two cases and literature review. Anaerobe 2014, 28, 95–97. [Google Scholar] [CrossRef]
- Sturm, P.D.; Van Eijk, J.; Veltman, S.; Meuleman, E.; Schulin, T. Urosepsis with Actinobaculum schaalii and Aerococcus urinae. J. Clin. Microbiol. 2006, 44, 652–654. [Google Scholar] [CrossRef]
- Sandlund, J.; Glimåker, M.; Svahn, A.; Brauner, A. Bacteraemia caused by Actinobaculum schaalii: An overlooked pathogen? Scand. J. Infect. Dis. 2014, 46, 605–608. [Google Scholar] [CrossRef]
- Non, L.R.; Nazinitsky, A.; Gonzalez, M.D.; Burnham, C.-A.D.; Patel, R. Actinobaculum schaalii bacteremia: A report of two cases. Anaerobe 2015, 34, 84–85. [Google Scholar] [CrossRef]
- Hashimoto, T.; Ando, M.; Komiya, K.; Usagawa, Y.; Yamasue, M.; Umeki, K.; Nureki, S.-I.; Hiramatsu, K.; Kadota, J.-I. Presumed Septic Shock Caused by Actinotignum schaalii Bacteremia. Intern. Med. 2021, 60, 1915–1919. [Google Scholar] [CrossRef]
- Fendukly, F.; Osterman, B. Isolation of Actinobaculum schaalii and Actinobaculum urinale from a Patient with Chronic Renal Failure. J. Clin. Microbiol. 2005, 43, 3567–3569. [Google Scholar] [CrossRef]
- Bank, S.; Søby, K.M.; Kristensen, L.H.; Voldstedlund, M.; Prag, J. A validation of the Danish microbiology database (MiBa) and incidence rate of Actinotignum schaalii (Actinobaculum schaalii) bacteraemia in Denmark. Clin. Microbiol. Infect. 2015, 21, 1097.e1–1097.e4. [Google Scholar] [CrossRef]
- Jacquier, H.; Benmansour, H.; Zadegan, F.; Hannouche, D.; Micaelo, M.; Mongiat-Artus, P.; Salomon, E.; Cambau, E.; Bercot, B. Actinobaculum schaalii, a new cause of knee prosthetic joint infection in elderly. Infection 2016, 44, 547–549. [Google Scholar] [CrossRef]
- Haller, P.; Bruderer, T.; Schaeren, S.; Laifer, G.; Frei, R.; Battegay, M.; Fluckiger, U.; Bassetti, S. Vertebral osteomyelitis caused by Actinobaculum schaalii: A difficult-to-diagnose and potentially invasive uropathogen. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 667–670. [Google Scholar] [CrossRef]
- Loïez, C.; Pilato, R.; Mambie, A.; Hendricx, S.; Faure, K.; Wallet, F. Native aortic endocarditis due to an unusual pathogen: Actinotignum schaalii. APMIS 2018, 126, 171–173. [Google Scholar] [CrossRef]
- Diallo, K.; Ferrand, J.; Goehringer, F.; Selton-Suty, C.; Folliguet, T.; Alauzet, C.; Lozniewski, A. The Brief Case: An Unusual Cause of Infective Endocarditis after a Urological Procedure. J. Clin. Microbiol. 2018, 56, e01400-17. [Google Scholar]
- Hoenigl, M.; Leitner, E.; Valentin, T.; Zarfel, G.; Salzer, H.J.; Krause, R.; Grisold, A.J. Endocarditis Caused by Actinobaculum schaalii, Austria. Emerg. Infect. Dis. 2010, 16, 1171–1173. [Google Scholar] [CrossRef]
- Olsen, A.B.; Andersen, P.K.; Bank, S.; Søby, K.M.; Lund, L.; Prag, J. Actinobaculum schaalii, a commensal of the urogenital area. Br. J. Urol. 2013, 112, 394–397. [Google Scholar] [CrossRef]
- Lotte, R.; Durand, M.; Mbeutcha, A.; Ambrosetti, D.; Pulcini, C.; Degand, N.; Loeffler, J.; Ruimy, R.; Amiel, J. A rare case of histopathological bladder necrosis associated with Actinobaculum schaalii: The incremental value of an accurate microbiological diagnosis using 16S rDNA sequencing. Anaerobe 2014, 26, 46–48. [Google Scholar] [CrossRef]
- Barberis, C.; Cittadini, R.; Del Castillo, M.; Acevedo, P.; Roig, C.G.; Ramirez, M.S.; Perez, S.; Almuzara, M.; Vay, C. Actinobaculum schaalii causing urinary tract infections: Report of four cases from Argentina. J. Infect. Dev. Ctries. 2014, 8, 240–244. [Google Scholar] [CrossRef][Green Version]
- Gupta, A.; Gupta, P.; Khaira, A. Actinobaculum schaalii pyelonephritis in a kidney allograft recipient. Iran. J. Kidney Dis. 2012, 6, 386–388. [Google Scholar]
- Pajkrt, D.; Simoons-Smit, A.M.; Savelkoul, P.H.M.; Hoek, J.V.D.; Hack, W.W.M.; Van Furth, A.M. Pyelonephritis Caused by Actinobaculum schaalii in a Child with Pyeloureteral Junction Obstruction. Eur. J. Clin. Microbiol. 2003, 22, 438–440. [Google Scholar] [CrossRef]
- Vallet, A.; Noël, N.; Bahi, R.; Teicher, E.; Quertainmont, Y.; Delfraissy, J.-F.; Ferlicot, S.; Potron, A.; Goujard, C.; Lambotte, O. Recurrent obstructive acute pyelonephritis: A rare form of Actinotignum (Actinobaculum) schaalii infection in a HIV-1 infected patient. Anaerobe 2017, 43, 75–77. [Google Scholar] [CrossRef]
- Kitano, H.; Hieda, K.; Kitagawa, H.; Nakaoka, Y.; Koba, Y.; Ota, K.; Shigemoto, N.; Hayashi, T.; Kashiyama, S.; Teishima, J.; et al. Case Report: Emphysematous Pyelonephritis With a Congenital Giant Ureterocele. Front. Pediatr. 2021, 9, 775468. [Google Scholar] [CrossRef]
- Larios, O.E.; Bernard, K.A.; Manickam, K.; Ng, B.; Alfa, M.; Ronald, A. First report of Actinobaculum schaalii urinary tract infection in North America. Diagn. Microbiol. Infect. Dis. 2010, 67, 282–285. [Google Scholar] [CrossRef]
- Vasquez, M.A.; Marne, C.; López-Calleja, A.I.; Martín-Saco, G.; Revillo, M.J. Actinobaculum schaalii recurrent urinary infection in a centenarian patient. Geriatr. Gerontol. Int. 2013, 13, 807–808. [Google Scholar] [CrossRef]
- Van Aarle, S.; Arents, N.L.A.; De Laet, K. Actinobaculum schaalii causing epididymitis in an elderly patient. J. Med Microbiol. 2013, 62, 1092–1093. [Google Scholar] [CrossRef][Green Version]
- Andersen, L.B.; Bank, S.; Hertz, B.; Søby, K.M.; Prag, J. Actinobaculum schaalii, a cause of urinary tract infections in children? Acta Paediatr 2012, 101, e232–e234. [Google Scholar] [CrossRef]
- Zimmermann, P.; Berlinger, L.; Liniger, B.; Grunt, S.; Agyeman, P.; Ritz, N. Actinobaculum schaalii an emerging pediatric pathogen? BMC Infect Dis. 2012, 12, 201. [Google Scholar] [CrossRef]
| Clinical Diagnosis (n) | Site/Etiology (n) | Comorbidities (n) |
|---|---|---|
| Abscess (9) | Breast (4) | Endocrine: T2DM (2), obesity (2), SLE (1) Gastrointestinal: hemorrhoids (2), appendicitis (2), GERD (1) Cardio: HTN (3), HF (1) Neuro/Psych: anxiety (2), depression (2), spina bifida (1), idiopathic intracranial hypertension (1) Breast: Recurrent breast infection (2), breast cancer on chemotherapy (1) Gynecology/GU: Endometriosis (3) Others: CKD (1), OA/RA (1), COPD (1), DVT (1) |
| Neck (1) | ||
| Pfannenstiel Line (1) | ||
| Periclitoral (1) | ||
| Umbilicus (1) | ||
| Thigh (1) | ||
| Ulcer (5) | Toe base (post-amputation) (1) | Endocrine: T2DM (3), hypothyroidism (1) Gastrointestinal: GERD (2), IBD (1), hernia (1) Cardio: HTN (3), arrhythmia (2) Neuro/Psych: Dementia (2), TIA (1) Others: RA (1), peripheral vascular disease (1), recurrent foot infection (1) |
| Diabetic foot ulcer (3) | ||
| Coccygeal pressure ulcer (1) | ||
| Urosepsis (4) | Traumatic catheterization (1) | Endocrine: hypothyroidism (2), OSA (1) Gastrointestinal: GERD (2), hernia (1), cholecystitis (1), Cardio: HTN (2), arrhythmia (1), CAD (1) Neuro/Psych: Dementia (1), ET (1), CVA/TIA (1), CP (1) Gynecology/GU: Urolithiasis (2), BPH (2), UTIs (1), trabeculated hypotonic bladder (1) Others: COPD (2), CKD (1), OA/RA (1), DVT (1), anemia (1), knee replacement (1) |
| Ureteric stone (2) | ||
| Catheterization of atonic bladder (1) | ||
| UTIs (17) | Dementia (6) | Endocrine: Dyslipidemia (3), T2DM (1) Cardio: Arrhythmia (1), CAD (1) Neuro/Psych: Dementia (6), CVA/TIA (3), PD (1), NPH (1), vertigo (1), spina bifida (1), arachnoid cyst (1) Gyne/GU: UTIs (2), cystocele (2), BPH (1), endometriosis (1) Others: OA/RA (1), GERD (1) |
| Acute urinary retention (4) | ||
| Cystocele (2) | ||
| CVA (3) | ||
| Pyelonephritis (2) | Castration-resistant prostate cancer (1) | Gynecology/GU: Prostate cancer (2) |
| Obstructive uropathy with ARF (1) | ||
| Septic arthritis (5) | Hip (4) | Endocrine: T2DM (3), OSA (1), hyperthyroidism (1), hypothyroidism (1) Cardio: HTN (3), HF (3), aortic disease (2), CABG (1), arrhythmia (1) Renal: CKD (3), renal transplant (1) Gastrointestinal: GERD (2), diverticulitis (1), colitis (1), cirrhosis (1), chronic pancreatitis (1) Orthopedic: elective hip replacement (1), hardware-associated osteomyelitis (1), hip fracture (1) Others: Gout (2), COPD (1), Anemia (1), peripheral neuropathy (1), pulmonary embolism (1), |
| Knee (1) | ||
| Perforated Viscus (1) | Toxic Megacolon (1) | Gastrointestinal: Appendicitis (1), IBD (1), GERD (1) cholecystitis (1) Hematology: DVT (1), PE (1), anemia (1) Others: HTN (1) |
| Clinical Diagnosis (n) and Specimen | Site/Etiology (n) | Treatment Strategy (n) | Antimicrobials before Cultures (Dosage) (n) |
|---|---|---|---|
| Abscess (9) Specimen Swabs (6), Aspirate (3) | Breast (4) | Abx (3), I/D (2) | Daptomycin (IV 6 mg/kg OD for 2/52) (1), ceftriaxone (2 g for 3/7) (2) |
| Neck (1) | Abx for HAP | Piperacillin–tazobactam (IV 2.25 mg q6h, 3/7) deescalated to azithromycin (PO 250 mg OD 3/7) | |
| Pfannenstiel Line (1) | I/D | NA | |
| Periclitoral (1) | I/D | NA | |
| Umbilicus (1) | I/D | NA | |
| Thigh (1) | Abx, I/D | Doxycycline (PO 100 mg BID 7/7) | |
| Ulcer (5) Specimen Swabs (3), Tissue (2) | Toe base (post-amputation) (1) | Abx (1) | Piperacillin–tazobactam (IV 2.25 mg q6h) |
| Diabetic foot ulcer (3) | Debridement (3) | NA | |
| Coccygeal pressure ulcer (1) | Repositioning (1) | NA | |
| Urosepsis (4) Specimen Aspirate (3), Venipuncture (2) | Traumatic catheterization (1) | Abx (1) | Piperacillin–tazobactam (IV 4.5 g q6h) |
| Ureteric stone (2) | Cystoscopy (2), stent (2), Abx (2) | Nitrofurantoin (100 mg BID 7/7) (1) | |
| Catheterization of atonic bladder (1) | Abx (1) | Ceftriaxone (IV 2 g for 4/7), ciprofloxacin (IV 500 mg bid 3/7) | |
| UTIs (17) Specimen Sterile catheter (6), voided midstream (5), cystoscopy collected (3) | Dementia (6) | Abx (2), NA (4) | NA (2) |
| Acute urinary retention (4) | Abx/self-catheterization (3), NA (1) | Ciprofloxacin (IV 500 mg bid 7/7), NA | |
| Cystocele (2) | Cystoscopy (2), Abx (1) | Nitrofurantoin (PO 400 mg bid 3/7) | |
| CVA (3) | Abx (1), NA (2) | Ceftriaxone (IV 2 g q12h) and metronidazole (IV 500 mg TID 2/52) | |
| Pyelonephritis (2) Specimen Sterile catheter (1), Venipuncture (1) | Castration-resistant prostate cancer (1) | Nephrostomy (1) | NA |
| Obstructive uropathy with ARF (1) | Bil. nephrostomy + ante stents (1) | NA | |
| Septic arthritis (5) Specimen Aspirate (1), Swab (4) | Hip (4) | Abx (3), Debridement (1), THR (1), NA (1) | Cefazolin (1), daptomycin (IV 6 mg/kg OD for 6/52) (2), amoxiclav (PO 875–125 mg BID, 2/52) (1), TMP-SMX (IV 4 mg/kg OD for 8/52) (1) |
| Knee (1) | Debridement (1) | NA (1) | |
| Perforated Viscus (1) Specimen Aspirate (1), | Toxic Megacolon (1) | subtotal colectomy and end ileostomy | NA (1) |
| Clinical Diagnosis (n) | Culture Type (n) | No. of Coisolates Mode [Range] | Organism Identity (n) | Antimicrobials After Cultures (Dosage) (n) |
|---|---|---|---|---|
| Abscess (9) | Mono (2) | NA | Actinotignum schaalii (2) | Metronidazole (250 mg, qid, 10/7) (1), cephalexin (500 mg, qid, 10/7) (1) |
| Poly (7) | 3 [2 to 4] | A. schaalii (7), Propionibacterium avidum (2), Finegoldia magna (1), Escherichia coli (1), Peptoniphilus asaccharolyticus (1), Klebsiella pneumoniae (1), Morganella morganii (1), Staphylococcus hominis (1), Streptococcus intermedius (1), Actinomyces neuii (1), Bacillus circulans (1), Staphylococcus aureus (1), Staphylococcus epidermidis (1), Pseudomonas aeruginosa (1) | Clindamycin (300 mg, qid, 7/7) (2), cefixime (400 mg, od, 14/7) (1), TMP (200 mg, bid, 7/7) (1), doxyclicine (100 mg, bid, 7/7) (1), amoxicillin–clavulanate (250 mg, tid, 10/7) (1) | |
| Ulcer (5) | Mono (1) | NA | A. schaalii (1) | Cephalexin (500 mg, qid, 8/7) (1) |
| Poly (4) | 2 [2 to 5] | A. schaalii (4), Enterococcus faecalis (2), Staphylococcus lugdunensis (1), S. aureus (1), P. aeruginosa (1), Bacteroides fragilis (1), Proteus mirabilis (1), S. epidermis (1), F. magna (1), Staphylococcus pettenkoferi (1) | Clindamycin (300 mg, qid, 10/7) (1), amoxicillin–clavulanate (500–125 mg, bid, 14/7) (1) | |
| Pyelonephritis (2) | Mono (1) | NA | A. schaalii * (1) | Ampicillin (2 g q6h, 10/7) |
| Poly (1) | 2 | A. schaalii * (1), Candida albicans (1) | NA | |
| Septic Arthritis (5) | Mono (3) | NA | A. schaalii (3) | TMP-SMX (800–160 mg, qid, 42/7) (1), cephalexin (500 mg, qid, 4/7) (2), NA (1) |
| Poly (2) | 2 | A. schaalii (2), F. magna (1), E. faecalis (1) | Amoxicillin–clavulanate (875–125 mg, bid, 14/7) (1), NA (1) | |
| Perforated viscus (1) | Poly (1) | 2 | A. schaalii (2), B. fragilis (1) | Ciprofloxacin (500 mg, bid, 30/7) (1), metronidazole (500 mg, bid, 30/7) (1) |
| Clinical Diagnosis | Site/Etiology | Culture Results (n); Coisolates | Treatment Strategy (n) | Source |
|---|---|---|---|---|
| Abscess (42) Mean age = 38 ± 2.9, M:F (21:21) Aspirate (40), Blood (1), Tissue (1) | Abdominal (3) | Mono (2), Poly (1); CoNS | I/D (3), linezolid (2), pristinamycin (1) | [9,10] |
| Breast (9) | Mono (3), Poly (6); Streptococcus constellatus, Gemella haemolysans, CoNS, Escherichia coli, Klebsiella pneumoniae, Morganella morganii, Staphylococcus hominis, Propionibacterium avidum, Finegoldia magna, Peptoniphilus asaccharolyticus | I/D (6), daptomycin (1), ceftriaxone (2), amoxiclav (2) | [10,11], * | |
| Fournier gangrene (3) | Mono (3) | I/D (1), vancomycin (1), ciprofloxacin (1), metronidazole (2), amoxiclav (2) | [11,12,13] | |
| Perineal hidradenitis suppurativa (3) | Poly (3); Prevotella melaninogenica (2), Fusobacterium spp., Aerococcus spp. | I/D (3), amoxiclav (1), clindamycin (1), minocycline (1) | [10] | |
| Pilonidal (3) | Mono (3) | I/D (1), cloxacillin (2), vancomycin (1) | [10,14] | |
| Vagina (3) | Mono (1), Poly (2); Actinomyces turicensis, Bacteroides fragilis | I/D (3), iodine (1) | [10], * | |
| Surgical site (2) | Poly (2); Acinetobacter spp., Helcococcus spp., Anaerococcus spp., Actinomyces neuii | I/D (1), amoxiclav (1) | [10], * | |
| Groin (12) | Mono (6), Poly (6); Arcanobacterium pyogenes (2), Aerococcus sp., Enterococcus Faecalis (2), Propionibacterium avidum, Bacillus circulans | I/D (10), amoxiclav (3), imipenem (2), pristinamycin (3), linezolid (2), doxycycline | [9,10], * | |
| Inguinal (1) | Mono (1) | I/D, cloxacillin | [14] | |
| Malleolus (1) | Poly (1); Peptostreptococcus spp. | I/D | [10] | |
| Neck (1) | Poly (1); Streptococcus intermedius | I/D, amoxiclav | * | |
| Cauda equina, Intradural (1) | Poly (1); Non-hemolytic streptococci | I/D, PCN, metronidazole | [5] | |
| Bacteremia (38) Mean age = 71.7 ± 2.1, M:F (27:11), Aspirate (6), Blood (16), Urine (10), Urine and Blood (10) | Unknown source (9) | Mono (5), Poly (4); Peptostreptococcus asaccharolyticus, Mixed Anaerobe, Urethral flora, streptococcus, Bacteroides fragilis | Surgical management (3), CTX (3), cefixime, PIP-TZN (2), amoxiclav, ofloxacin, ciprofloxacin, metronidazole, daptomycin, cefepime, amoxicillin | [9,13,15,16,17], * |
| Urosepsis (29) | Mono (10), Poly (19); Enterococcus faecalis (4), Actinotignum urinale (2), CoNS, Aerococcus urinae (3), Urethral flora (2), Staphylococcus epidermidis, Prevotella, alpha-hemolytic streptococci, Actinomyces sp. and Peptostreptococcus anaerobius (2), Citrobacter braakii, Pseudomonas aeruginosa, Escherichia coli (2), Non-hemolytic Streptococcus, Aeromicrobium massiliense, peptostreptococcus asaccharolyticus (2), Proteus mirabilis | PIP-TZM (6), metronidazole (3), cefuroxime (3), gentamicin (3), meropenem, ciprofloxacin (9), metronidazole, CTX (3), amoxicillin, amoxiclav (3), ampicillin, nitrofurantoin, TMP-SMX (2), cefotaxime (5), vancomycin, cefepime, levofloxacin, nephrectomy, nephrostomy catheter (2) | [5,9,13,15,16,18,19,20], * | |
| Septic Joint (6) Mean age = 68.3 ± 4.5, M:F (3:3) Tissue (3), Aspirate (2), Disc Biopsy (1) | Incidental findings during elective hip replacement surgery (2) | Mono (1), Poly (1); Finegoldia magna | Debridement, cefazolin | * |
| Hip hardware-associated osteomyelitis (3) | Mono (2), Poly (1); Enterococcus faecalis | Daptomycin (2), amoxiclav, TMP-SMX, amoxicillin, gentamicin, rifampin | [21], * | |
| Vertebral Osteomyelitis (1) | Poly (1); Corynebacterium striatum | Amoxiclav | [22] | |
| Infective Endocarditis (3) Mean age = 68 ± 10.9, M:F (3:0) Blood (2), Urine and blood (1) | Native valve (2) | Mono (1), Poly (1); Escherichia coli | Amoxicillin (2), amoxiclav, gentamicin (2), aortic valve replacement surgery (2), tricuspid valve replacement | [23,24] |
| Prosthetic valve (1) | Mono (1) | PIP-TZM, amoxiclav | [25] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakodkar, P.; Hamula, C. A 2-Year Retrospective Case Series on Isolates of the Emerging Pathogen Actinotignum schaalii from a Canadian Tertiary Care Hospital. Microorganisms 2022, 10, 1608. https://doi.org/10.3390/microorganisms10081608
Kakodkar P, Hamula C. A 2-Year Retrospective Case Series on Isolates of the Emerging Pathogen Actinotignum schaalii from a Canadian Tertiary Care Hospital. Microorganisms. 2022; 10(8):1608. https://doi.org/10.3390/microorganisms10081608
Chicago/Turabian StyleKakodkar, Pramath, and Camille Hamula. 2022. "A 2-Year Retrospective Case Series on Isolates of the Emerging Pathogen Actinotignum schaalii from a Canadian Tertiary Care Hospital" Microorganisms 10, no. 8: 1608. https://doi.org/10.3390/microorganisms10081608
APA StyleKakodkar, P., & Hamula, C. (2022). A 2-Year Retrospective Case Series on Isolates of the Emerging Pathogen Actinotignum schaalii from a Canadian Tertiary Care Hospital. Microorganisms, 10(8), 1608. https://doi.org/10.3390/microorganisms10081608





