Examining the Prevalence and Antibiotic Susceptibility of S. aureus Strains in Hospitals: An Analysis of the pvl Gene and Its Co-Occurrence with Other Virulence Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. S. aureusIsolates and Antibiotic Susceptibilities Characterization
2.3. Determination of S. aureus Isolates Genotypes
2.3.1. DNA Isolation for PCR Tests
2.3.2. PCR Reaction for the Detection of mecAGene
2.3.3. Characterization of pvl
2.3.4. Primer Sequences and Identification of sem and seg Genes
2.3.5. Primer Sequences and Identification of agr Alleles
2.4. Statistical Analysis
3. Results
3.1. The Detection of Different Genes among Staphylococcus aureus Isolates
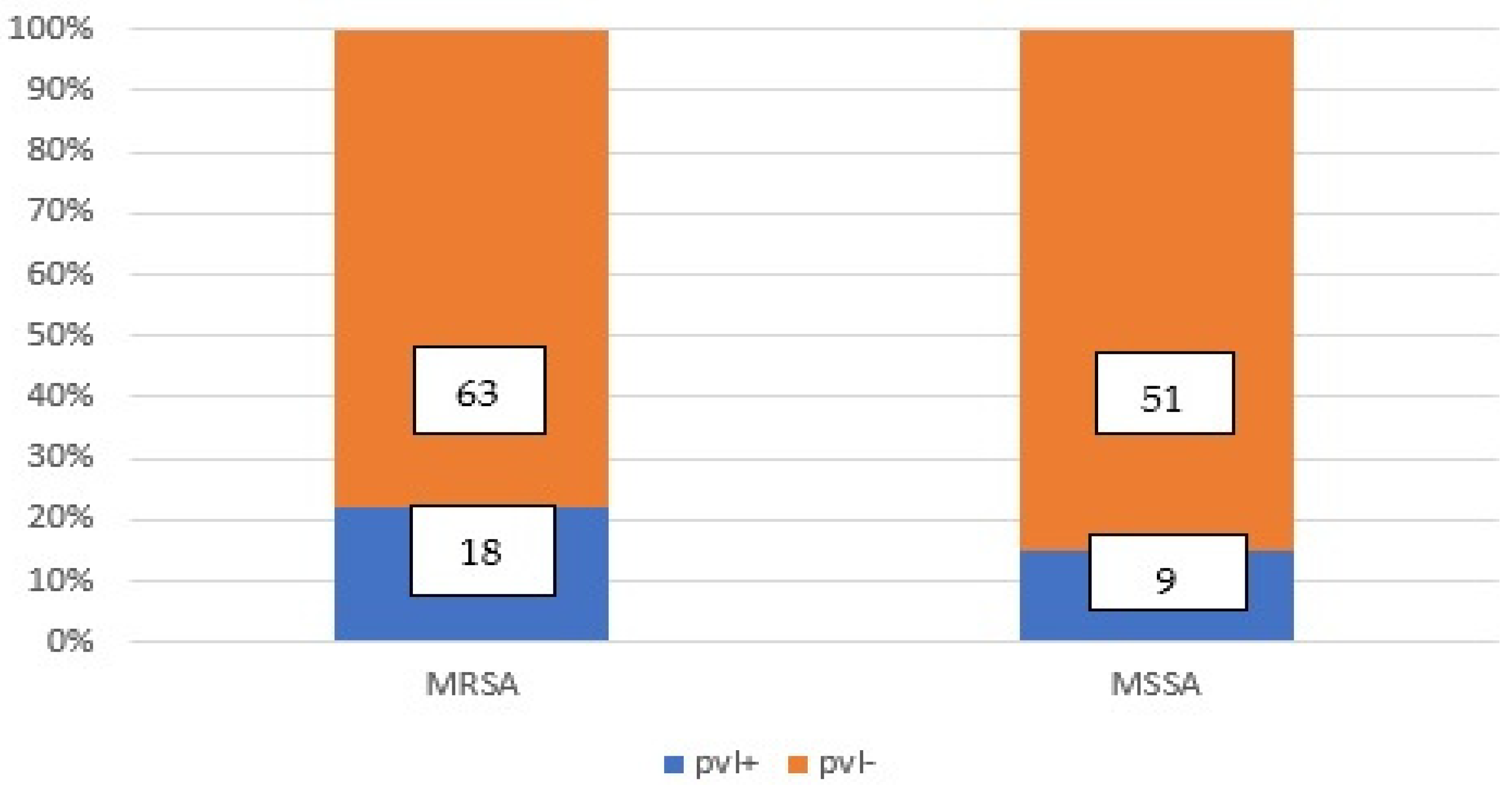
3.1.1. The Panton–Valentine Leukocidine (pvl) among Staphylococcus aureus isolates
3.1.2. The Detection of MRSA and MSSA with or without pvl gene Co-Occurrence Using PCR and PBP2a Latex Tests
3.2. The Genes Content and the Genes Co-Occurrence in pvlProducing S. aureus
3.3. The Resistance Phenotypes of the pvl-Producing MRSA Strains
3.4. The Genes Content, the Genes Co-Occurrence, and the Resistance Phenotypes in pvl-Producing MSSA Strains
3.5. The Genes Content and the Genes Co-Occurrence in the MSSA Strains (mecA-Negative) pvl-Negative
4. Discussions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Archer, G.L. Staphylococcus aureus: A well-armed pathogen. Clin. Infect. Dis. 1998, 26, 1179–1181. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.L.; Novick, R.P. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J. Bacteriol. 1988, 170, 4365–4372. [Google Scholar] [CrossRef] [PubMed]
- Sdougkos, G.; Chini, V. Methicillin-resistant Staphylococcus aureus producing Panton-Valentine leukocidin as a cause of acute osteomyelitis in children. Clin. Microbiol. Infect. 2007, 13, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Chini, V.; Petinaki, E. Spread of Staphylococcus aureus clinical isolates carrying Panton–Valentine leukocidin genes during a 3-year period in Greece. Clin. Microbiol. Infect. 2006, 12, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Gerard, L.; Yves, P. Involvement of Panton-Valentine Leukocidin–Producing Staphylococcus aureus in Primary Skin Infections and Pneumonia. Clin. Infect. Dis. 1999, 29, 1128–1132. [Google Scholar]
- Kaneko, J.; Kimura, T. Complete nucleotide sequence and molecular characterization of the temperate staphylococcal bacteriophage phiPVL carrying Panton-Valentine leukocidin genes. Gene 1998, 215, 57–67. [Google Scholar] [CrossRef]
- Aires de Sousa, M.; Bartzavali, C. Two international methicillin-resistant Staphylococcus aureus clones endemic in a university hospital in Patras, Greece. J. Clin. Microbiol. 2003, 41, 2027–2032. [Google Scholar] [CrossRef]
- CLSI standard M07; Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; pp. 206–246.
- Jarraud, S. Exfoliatin-producing strains define a fourth agr specificity group in Staphylococcus aureus. J. Bacteriol. 2000, 182, 22. [Google Scholar] [CrossRef]
- Morfeldt, E.; Janzon, L. Cloning of a chromosomal locus (exp) which regulates the expression of several exoprotein genes in Staphylococcus aureus. Mol. Gen. Genet. 1988, 211, 435–440. [Google Scholar] [CrossRef]
- Vandenesch, F.; Naimi, T. Community acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: Worldwide emergence. Emerg. Infect. Dis. 2003, 9, 978–984. [Google Scholar] [CrossRef]
- Recsei, P.; Kreiswirth, B. Regulation of exoprotein gene expression in Staphylococcus aureus by agar. Mol. Gen. Genet. 1986, 202, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Bronner, S.P.; Stoessel, A. Variable expressions of Staphylococcus aureus bicomponent leucotoxins semiquantified by competitive reverse transcription-PCR. Appl. Environ. Microbiol. 2000, 66, 3931–3938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Stewart, G.C. Characterization of the promoter elements for the staphylococcal enterotoxin D gene. J. Bacteriol. 2000, 182, 2321. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, Y.; Okii, K. Changes in the agr Locus Affect Enteritis Caused by Methicillin-Resistant Staphylococcus aureus. J. Clin. Microbiol. 2009, 47, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.Y.; Koh, Y.L. Establishment of ST30 as the predominant clonal type among community-associated methicillin-resistant Staphylococcus aureus isolates in Singapore. J. Clin. Microbiol. 2006, 44, 1090–1093. [Google Scholar] [CrossRef]
- Heyer, G.S.; Saba, R.A. Staphylococcus aureus agr and sarA functions are required for invasive Infection but not inflammatory responses in the lung. Infect. Immun. 2002, 70, 127–133. [Google Scholar] [CrossRef]
- Dziewanowska, K.; Carson, A.M. Staphylococcal fibronectin binding protein interacts with heat shock protein 60 and integrins: Role in internalization by epithelial cells. Infect. Immun. 2000, 68, 6321–6328. [Google Scholar] [CrossRef]
- Ligozzi, M.; Bernini, C.; Bonora, M.G. Evaluation of the VITEK 2 system for identification and antimicrobial susceptibility testing of medically relevant gram-positive cocci. J. Clin. Microbiol. 2002, 40, 1681–1686. [Google Scholar] [CrossRef]
- Lowy, F.D. Staphylococcus aureusInfections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef]
- Goerke, C.; Campana, S. Direct quantitative transcript analysis of the agr regulon of Staphylococcus aureus during human infection in comparison to the expression profile in vitro. Infect. Immun. 2000, 68, 1304–1311. [Google Scholar] [CrossRef]
- Gajic, I.; Kabic, J. Antimicrobial Susceptibility Testing: A Comprehensive Review of Currently Used Methods. Antibiotics 2022, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Stoakes, L. Correlation of oxacillin MIC with mecA gene carriage in coagulase-negative staphylococci. J. Clin. Microbiol. 2000, 38, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Vassiliki, C.; Petinaki, E. Emergence of a new clone carrying Panton-Valentine leukocidin genes and staphylococcal cassette chromosome mec type V among methicillin-resistant Staphylococcus aureus in Greece. Scand. J. Infect. Dis. 2008, 40, 368–372. [Google Scholar]
- Gerhardt, P.; Murray, R.G.E. Methods for General and Molecular Bacteriology; American Society for Microbiology: Washington, DC, USA, 1994. [Google Scholar]
- Grisold, A.J.; Leitner, E. Detection of methicillin-resistant Staphylococcus aureus and simultaneous confirmation by automated nucleic acid extraction and real-time PCR. J. Clin. Microbiol. 2002, 40, 2392–2397. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Chen, L. Current methodologies on genotyping for nosocomial pathogen methicillin-resistant Staphylococcus aureus (MRSA). Microb. Pathog. 2017, 107, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Prévost, G.; Cribier, B. Panton-Valentine leucocidin, and gamma-hemolysin from Staphylococcus aureus ATCC 49775 are encoded by distinct genetic loci and have different biological activities. Infect. Immun. 1995, 63, 4121–4129. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, D.R.; Cavaco, L.M. Association of Panton Valentine Leukocidin (PVL) genes with methicillin resistant Staphylococcus aureus (MRSA) in Western Nepal: A matter of concern for community infections (a hospital based prospective study). BMC Infect. Dis. 2016, 16, 199. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Yoshida, H.; Bogaki-Shonai, M.; Niga, T.; Hattori, H.; Nakamura, S. Quinolone resistance mutations in the DNA gyrase gyrA and gyrB genes of Staphylococcus aureus. Antimicrob. Agents Chemother. 1994, 38, 2014–2023. [Google Scholar] [CrossRef] [PubMed]
- Wannet, W.J.B.; Spalburg, E. Emergence of virulent methicillin-resistant Staphylococcus aureus strains carrying Panton-Valentine leucocidin genes in the Netherlands. J. Clin. Microbiol. 2005, 43, 3341–3345. [Google Scholar] [CrossRef]
- Chui, L.; Drebot, M. Comparison of 9 different PCR primers for the rapid detection of severe acute respiratory syndrome coronavirus using 2 RNA extraction methods. Diagn. Microbiol. Infect. Dis. 2005, 53, 47–55. [Google Scholar] [CrossRef]
- Shopsin, B.; Drlica-Wagner, A. Prevalence of agr dysfunction among colonizing Staphylococcus aureus strains. J. Infect. Dis. 2008, 198, 8. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.A.; Brown, P.D. Association between the agr locus and the presence of virulence genes and pathogenesis in Staphylococcus aureus using a Caenorhabditis elegans model. Int. J. Infect. Dis. 2017, 54, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Gillet, Y.; Issartel, B. Association between Staphylococcus aureus strains carrying genes for Panton-Valentine leukocidin and highly lethal necrotizing pneumonia in young immunocompetent patients. Lancet 2002, 359, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Robert, J.; Etienne, J. Methicillin- resistant Staphylococcus aureus producing Panton-Valentine leukocidin in a retrospective case series from 12 French hospital laboratories. Clin. Microbiol. Infect. 2005, 11, 585–587. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.; Ganner, M. Staphylococcus aureus isolates carrying Panton-Valentine leucocidin genes in England and Wales: Frequency, characterization, and association with clinical disease. J. Clin. Microbiol. 2005, 43, 2384–2390. [Google Scholar] [CrossRef] [PubMed]
- Coombs, G.W.; Nimmo, G.R.; Bell, J.M.; Huygens, F.; O’brien, F.G.; Malkowski, M.J.; Pearson, J.C.; Stephens, A.J.; Giffard, P.M. Genetic diversity among community methicillin-resistant Staphylococcus aureus strains causing outpatient infections in Australia. J. Clin. Microbiol. 2004, 42, 4735–4743. [Google Scholar] [CrossRef]
- Boyle-Vavra, S.; Ereshefsky, B. Successful multiresistant community associated methicillin-resistant Staphylococcus aureus lineage from Taipei, Taiwan, that carries either the novel staphylococcal chromosome cassette mec (SCCmec) type VT or SCCmec type IV. J. Clin. Microbiol. 2005, 43, 4719–4730. [Google Scholar] [CrossRef]
- Tristan, A.; Bes, M. Global distribution of Panton-Valentine leukocidin-positive methicillin-resistant Staphylococcus aureus. Emerg. Infect. Dis. 2007, 13, 594–600. [Google Scholar] [CrossRef]
- Faria, N.A.; Oliveira, D.C. Epidemiology of emerging methicillin-resistant Staphylococcus aureus (MRSA) in Denmark: A nationwide 16 study in a country with low prevalence of MRSA infection. J. Clin. Microbiol. 2005, 43, 1836–1842. [Google Scholar] [CrossRef]
- Diep, B.A.; Sensabaugh, G.F. Widespread skin and soft-tissue infections due to 2 methicillin- resistant Staphylococcus aureus strains harbouring the genes for Panton-Valentine leucocidin. J. Clin. Microbiol. 2004, 42, 2080–2084. [Google Scholar] [CrossRef]
- Witte, W.; Braulke, C. Emergence of methicillin-resistant Staphylococcus aureus with Panton-Valentine leukocidin genes in central Europe. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, H.; Aires-de-Sousa, M.; Boyce, J. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet 2006, 368, 874–885. [Google Scholar] [CrossRef] [PubMed]

| S. aureus Strains | agr Gene | agr, sem and seg Genes | agr and sem Genes | sem and seg Genes | sem Gene | seg Gene | agr, sem, seg Genes- | Total |
|---|---|---|---|---|---|---|---|---|
| MRSA (mec A +) 81 strains | 6 pvl+ | 6pvl+ | 6pvl+ | − | − | 18pvl+ | ||
| MSSA: (mec A −) 60 strains | − | − | − | 6pvl+ | − | − | 3pvl | 9pvl + |
| Total | 6 | 6 | 6 | 6 | − | − | 27 |
| agr, sem and seg Genes | pvl + |
|---|---|
| agr | 6 MRSA |
| agr+ sem | 6 MRSA |
| agr+ sem+ seg+ | 6 MRSA |
| sem+ seg+ | 6 MSSA |
| agr–sem– seg− | 3 MSSA |
| Total | 27 |
| Genes | agrI | agrIII | agrIV | Total |
|---|---|---|---|---|
| Agr | 9 | 9 | − | 18 |
| Agr and sem+seg+ | 9 | 9 | 9 | 27 |
| Agr and sem+ | 9 | 9 | 9 | |
| Total | 18 (4.3%) | 27 (6.38%) | 9 (2.12%) |
| Strain | pvl | agr | Resistance Pattern |
|---|---|---|---|
| MRSA | Positive | agrI | Β-lactamins, Ka, To, Ge, Er.,Clin., Ci., sensitive to glycopeptides, GISA/GRSA |
| MRSA | Positive | agrI, sem+ | Β-lactamins, Ka., Ge., Er., Clin., Ci., sensitive to glycopep-tides, GISA |
| MRSA | Positive | agrIII, sem+, seg+ | Β-lactamins, Ka, To, Ge, Er.,Clin., Ci., sensitive to glycopep-tides |
| MRSA | Positive | agrIV, sem+, seg+ | Β-lactamins, Ka, To, Ge, Er.,Clin., Ci., sensitive to glycopeptides |
| MRSA | Positive | agrIII+, sem+ | Β-lactamins, Ka, To, Ge, Er.,Clin., VISA |
| MRSA | Positive | agrIII+ | Β-lactamins (β-lactamase+) Κa., Fusidic acid, VISA/GISA |
| Nr | S. aureus | pvl | agr | sem | seg | Resistance Phenotype |
|---|---|---|---|---|---|---|
| 1 | MSSA | + | − | + | + | (9 strains) VISA |
| 2 | MSSA | + | − | + | + | (9 strains) GISA |
| 3 | MSSA | + | − | − | − | GS-MRSA |
| pvl (-) mec (-) | sem and seg Genes | Number | % (from the Total of S. aureus Strains) |
|---|---|---|---|
| MSSA | sem+ seg− | 3 | 2.13% |
| MSSA | sem+ seg+ | 21 | 14.89% |
| MSSA | sem− seg+ | 3 | 2.13% |
| MSSA | sem− seg− | 24 | 17.02% |
| Total | 51 | 36.17% |
| S. aureus | agr | sem | seg | Resistance Phenotype | Nr of strains |
|---|---|---|---|---|---|
| MSSA | − | + | + | S, GISA, β lactamase ND, | 3 |
| MSSA | − | + | + | S, GS, β lactamase ND | 6 |
| MSSA | − | + | − | GS-MRSA, β lactamase ND | 3 |
| MSSA | − | + | + | S, β lactamase +, GRSA | 3 |
| MSSA | − | − | + | E, Clind, GS, β lactamase + | 3 |
| MSSA | − | + | + | E, GS-MRSA, β lactamase ND | 6 |
| MSSA | − | + | + | E, CIP, β lactamase - | 3 |
| 27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vittorakis, E.; Vică, M.L.; Zervaki, C.O.; Vittorakis, E.; Maraki, S.; Mavromanolaki, V.E.; Schürger, M.E.; Neculicioiu, V.S.; Papadomanolaki, E.; Sinanis, T.; et al. Examining the Prevalence and Antibiotic Susceptibility of S. aureus Strains in Hospitals: An Analysis of the pvl Gene and Its Co-Occurrence with Other Virulence Factors. Microorganisms 2023, 11, 841. https://doi.org/10.3390/microorganisms11040841
Vittorakis E, Vică ML, Zervaki CO, Vittorakis E, Maraki S, Mavromanolaki VE, Schürger ME, Neculicioiu VS, Papadomanolaki E, Sinanis T, et al. Examining the Prevalence and Antibiotic Susceptibility of S. aureus Strains in Hospitals: An Analysis of the pvl Gene and Its Co-Occurrence with Other Virulence Factors. Microorganisms. 2023; 11(4):841. https://doi.org/10.3390/microorganisms11040841
Chicago/Turabian StyleVittorakis, Eftychios, Mihaela Laura Vică, Calina Oana Zervaki, Evangelos Vittorakis, Sofia Maraki, Viktoria Eirini Mavromanolaki, Michael Ewald Schürger, Vlad Sever Neculicioiu, Evangelia Papadomanolaki, Theodoros Sinanis, and et al. 2023. "Examining the Prevalence and Antibiotic Susceptibility of S. aureus Strains in Hospitals: An Analysis of the pvl Gene and Its Co-Occurrence with Other Virulence Factors" Microorganisms 11, no. 4: 841. https://doi.org/10.3390/microorganisms11040841
APA StyleVittorakis, E., Vică, M. L., Zervaki, C. O., Vittorakis, E., Maraki, S., Mavromanolaki, V. E., Schürger, M. E., Neculicioiu, V. S., Papadomanolaki, E., Sinanis, T., Giannoulaki, G., Xydaki, E., Kastanakis, S. G., & Junie, L. M. (2023). Examining the Prevalence and Antibiotic Susceptibility of S. aureus Strains in Hospitals: An Analysis of the pvl Gene and Its Co-Occurrence with Other Virulence Factors. Microorganisms, 11(4), 841. https://doi.org/10.3390/microorganisms11040841





