The Cell Wall Deacetylases Spy1094 and Spy1370 Contribute to Streptococcus pyogenes Virulence
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Generation of S. pyogenes and L. lactis Mutants
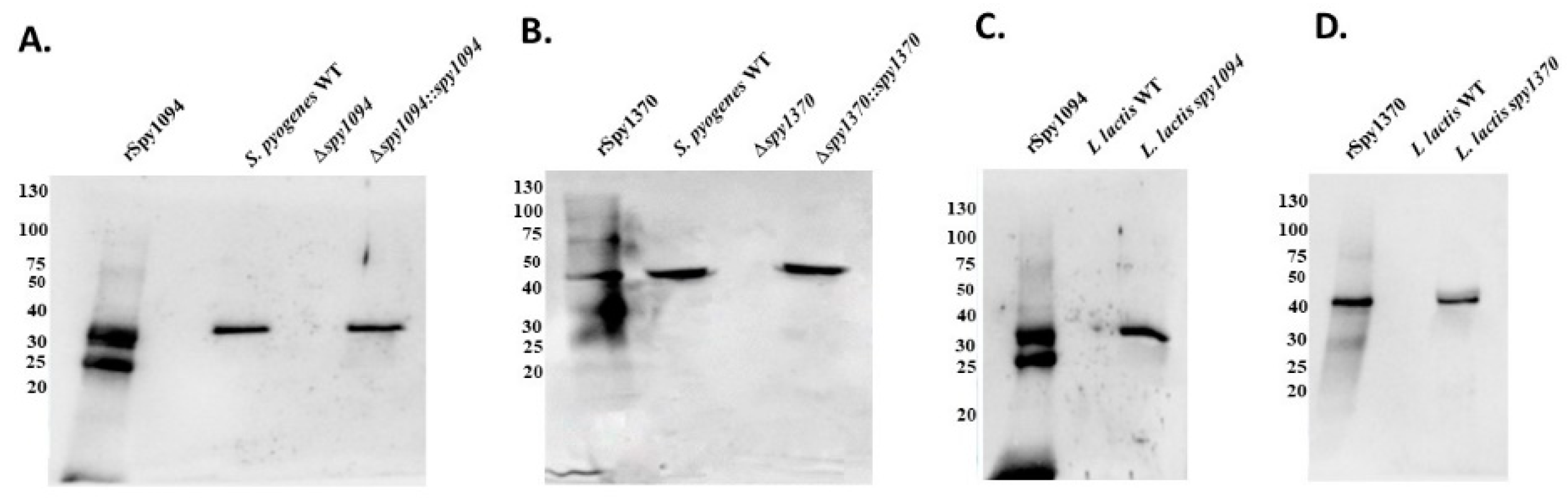
2.3. Western Blots
2.4. Effects of Lysozyme on the Survival of S. pyogenes and L. lactis Strains
2.5. Effects of Cecropin B on the Survival of GAS Mutants
2.6. Effects of H2O2 on the Survival of GAS Mutants
2.7. Biofilm Formation in GAS Mutants
2.8. Whole Blood Killing Assay
2.9. Galleria Mellonella Larvae Infection Model
2.10. Statistical Analysis
3. Results
3.1. Generation of S. pyogenes Gene Deletion Strain and L. lactis Gain-of-Function Strains
3.2. Deletion of spy1094 and spy1370 Decreases Lysozyme Resistance
3.3. Deletion of spy1370 Confers Resistance to Cationic AMP, but Not to Oxidative Killing
3.4. Deletion of spy1094 and spy1370 Decreases Biofilm Formation
3.5. Spy1094 and Spy1370 Promote Survival in Human Blood
3.6. Deletion of spy1094 and spy1370 Reduces Virulence of S. pyogenes in a Galleria Mellonella Infection Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anjos, L.M.; Marcondes, M.B.; Lima, M.F.; Mondelli, A.L.; Okoshi, M.P. Streptococcal acute pharyngitis. Rev. Soc. Bras. Med. Trop. 2014, 47, 409–413. [Google Scholar] [CrossRef]
- Stevens, D.L.; Bryant, A.E. Impetigo, Erysipelas and Cellulitis. In Streptococcus pyogenes: Basic Biology to Clinical Manifestations; Ferretti, J.J., Stevens, D.L., Fischetti, V.A., Eds.; University of Oklahoma Health Sciences Center: Oklahoma City, OK, USA, 2016. [Google Scholar]
- Botteaux, A.; Budnik, I.; Smeesters, P.R. Group A Streptococcus infections in children: From virulence to clinical management. Curr. Opin. Infect. Dis. 2018, 31, 224–230. [Google Scholar] [CrossRef]
- Cunningham, M.W. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 2000, 13, 470–511. [Google Scholar] [CrossRef]
- Stevens, D.L. Streptococcal toxic-shock syndrome: Spectrum of disease, pathogenesis, and new concepts in treatment. Emerg. Infect. Dis. 1995, 1, 69–78. [Google Scholar] [CrossRef]
- Stevens, D.L.; Bryant, A.E. Severe Group A Streptococcal Infections. In Streptococcus pyogenes: Basic Biology to Clinical Manifestations; Ferretti, J.J., Stevens, D.L., Fischetti, V.A., Eds.; The University of Oklahoma Health Sciences Center: Oklahoma City, OK, USA, 2016. [Google Scholar]
- Cunningham, M.W. Streptococcus and rheumatic fever. Curr. Opin. Rheumatol. 2012, 24, 408–416. [Google Scholar] [CrossRef]
- Martin, W.J.; Steer, A.C.; Smeesters, P.R.; Keeble, J.; Inouye, M.; Carapetis, J.; Wicks, I.P. Post-infectious group A streptococcal autoimmune syndromes and the heart. Autoimmun. Rev. 2015, 14, 710–725. [Google Scholar] [CrossRef]
- Mosquera, J.; Pedreanez, A. Acute post-streptococcal glomerulonephritis: Analysis of the pathogenesis. Int. Rev. Immunol. 2020, 1–20. [Google Scholar] [CrossRef]
- Carapetis, J.R.; Steer, A.C.; Mulholland, E.K.; Weber, M. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 2005, 5, 685–694. [Google Scholar] [CrossRef]
- Brouwer, S.; Barnett, T.C.; Rivera-Hernandez, T.; Rohde, M.; Walker, M.J. Streptococcus pyogenes adhesion and colonization. FEBS Lett. 2016, 590, 3739–3757. [Google Scholar] [CrossRef]
- Raynes, J.M.; Young, P.G.; Proft, T.; Williamson, D.A.; Baker, E.N.; Moreland, N.J. Protein adhesins as vaccine antigens for Group A Streptococcus. Pathog. Dis. 2018, 76, fty016. [Google Scholar] [CrossRef]
- Nakata, M.; Kreikemeyer, B. Genetics, Structure, and Function of Group A Streptococcal Pili. Front. Microbiol. 2021, 12, 616508. [Google Scholar] [CrossRef]
- Proft, T.; Fraser, J.D. Streptococcal superantigens. Chem. Immunol. Allergy 2007, 93, 1–23. [Google Scholar] [CrossRef]
- Tabata, A.; Nagamune, H. Diversity of beta-hemolysins produced by the human opportunistic streptococci. Microbiol. Immunol. 2021, 65, 512–529. [Google Scholar] [CrossRef]
- Verhamme, I.M.; Panizzi, P.R.; Bock, P.E. Pathogen activators of plasminogen. J. Thromb. Haemost. 2015, 13 (Suppl. 1), S106–S114. [Google Scholar] [CrossRef]
- Laabei, M.; Ermert, D. Catch Me if You Can: Streptococcus pyogenes Complement Evasion Strategies. J. Innate Immun. 2019, 11, 3–12. [Google Scholar] [CrossRef]
- Moynihan, P.J.; Sychantha, D.; Clarke, A.J. Chemical biology of peptidoglycan acetylation and deacetylation. Bioorg. Chem. 2014, 54, 44–50. [Google Scholar] [CrossRef]
- Glick, A.D.; Ranhand, J.M.; Cole, R.M. Degradation of group A streptococcal cell walls by egg-white lysozyme and human lysosomal enzymes. Infect. Immun. 1972, 6, 403–413. [Google Scholar] [CrossRef]
- di Luzio, N.R. Lysozyme activity: An index of macrophage functional status. Front. Biol. 1979, 48, 447–462. [Google Scholar]
- Vollmer, W.; Tomasz, A. Peptidoglycan N-acetylglucosamine deacetylase, a putative virulence factor in Streptococcus pneumoniae. Infect. Immun. 2002, 70, 7176–7178. [Google Scholar] [CrossRef]
- Planas, A. Peptidoglycan Deacetylases in Bacterial Cell Wall Remodeling and Pathogenesis. Curr. Med. Chem. 2022, 29, 1293–1312. [Google Scholar] [CrossRef]
- Milani, C.J.E.; Aziz, R.K.; Locke, J.B.; Dahesh, S.; Nizet, V.; Buchanan, J.T. The novel polysaccharide deacetylase homologue Pdi contributes to virulence of the aquatic pathogen Streptococcus iniae. Microbiology (Reading) 2010, 156, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Branda, S.S.; Vik, S.; Friedman, L.; Kolter, R. Biofilms: The matrix revisited. Trends Microbiol. 2005, 13, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Vuong, C.; Voyich, J.M.; Fischer, E.R.; Braughton, K.R.; Whitney, A.R.; DeLeo, F.R.; Otto, M. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell. Microbiol. 2004, 6, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Aspell, T.; Khemlani, A.H.J.; Loh, J.M.S.; Tsai, C.J. Functional characterisation of two novel deacetylases from Streptococcus pyogenes. Microbiol. Res. 2022, 13, 323–331. [Google Scholar] [CrossRef]
- Wang, J.; Ma, K.; Ruan, M.; Wang, Y.; Li, Y.; Fu, Y.V.; Song, Y.; Sun, H.; Wang, J. A novel cecropin B-derived peptide with antibacterial and potential anti-inflammatory properties. PeerJ 2018, 6, e5369. [Google Scholar] [CrossRef]
- Pericone, C.D.; Park, S.; Imlay, J.A.; Weiser, J.N. Factors contributing to hydrogen peroxide resistance in Streptococcus pneumoniae include pyruvate oxidase (SpxB) and avoidance of the toxic effects of the fenton reaction. J. Bacteriol. 2003, 185, 6815–6825. [Google Scholar] [CrossRef]
- Kimura, K.R.; Nakata, M.; Sumitomo, T.; Kreikemeyer, B.; Podbielski, A.; Terao, Y.; Kawabata, S. Involvement of T6 pili in biofilm formation by serotype M6 Streptococcus pyogenes. J. Bacteriol. 2012, 194, 804–812. [Google Scholar] [CrossRef]
- Aghababa, H.; Ting, Y.T.; Pilapitiya, D.; Loh, J.M.S.; Young, P.G.; Proft, T. Complement evasion factor (CEF), a novel immune evasion factor of Streptococcus pyogenes. Virulence 2022, 13, 225–240. [Google Scholar] [CrossRef]
- Loh, J.M.; Adenwalla, N.; Wiles, S.; Proft, T. Galleria mellonella larvae as an infection model for group A streptococcus. Virulence 2013, 4, 419–428. [Google Scholar] [CrossRef]
- Tsai, C.J.; Loh, J.M.S.; Proft, T. The Use of Galleria mellonella (Wax Moth) as an Infection Model for Group A Streptococcus. Methods Mol. Biol 2020, 2136, 279–286. [Google Scholar] [CrossRef]
- Que, Y.A.; Haefliger, J.A.; Francioli, P.; Moreillon, P. Expression of Staphylococcus aureus clumping factor A in Lactococcus lactis subsp. cremoris using a new shuttle vector. Infect. Immun. 2000, 68, 3516–3522. [Google Scholar] [CrossRef]
- Clarke, A.J.; Dupont, C. O-acetylated peptidoglycan: Its occurrence, pathobiological significance, and biosynthesis. Can. J. Microbiol. 1992, 38, 85–91. [Google Scholar] [CrossRef]
- Pokrovskaya, V.; Poloczek, J.; Little, D.J.; Griffiths, H.; Howell, P.L.; Nitz, M. Functional characterization of Staphylococcus epidermidis IcaB, a de-N-acetylase important for biofilm formation. Biochemistry 2013, 52, 5463–5471. [Google Scholar] [CrossRef]
- Xanthopoulos, K.G.; Lee, J.Y.; Gan, R.; Kockum, K.; Faye, I.; Boman, H.G. The structure of the gene for cecropin B, an antibacterial immune protein from Hyalophora cecropia. Eur. J. Biochem. 1988, 172, 371–376. [Google Scholar] [CrossRef]
- Young, C.; Holder, R.C.; Dubois, L.; Reid, S.D. Streptococcus pyogenes Biofilm. In Streptococcus pyogenes: Basic Biology to Clinical Manifestations; Ferretti, J.J., Stevens, D.L., Fischetti, V.A., Eds.; University of Oklahoma Health Sciences Center: Oklahoma City, OK, USA, 2016. [Google Scholar]
- Skutlaberg, D.H.; Wiker, H.G.; Mylvaganam, H.; Group, I.S.; Norrby-Teglund, A.; Skrede, S. Consistent Biofilm Formation by Streptococcus pyogenes emm 1 Isolated from Patients with Necrotizing Soft Tissue Infections. Front. Microbiol. 2022, 13, 822243. [Google Scholar] [CrossRef]
- Tsai, C.J.; Loh, J.M.; Proft, T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 2016, 7, 214–229. [Google Scholar] [CrossRef]
- Vollmer, W.; Tomasz, A. The pgdA gene encodes for a peptidoglycan N-acetylglucosamine deacetylase in Streptococcus pneumoniae. J. Biol. Chem. 2000, 275, 20496–20501. [Google Scholar] [CrossRef]
- Ragland, S.A.; Criss, A.K. From bacterial killing to immune modulation: Recent insights into the functions of lysozyme. PLoS Pathog. 2017, 13, e1006512. [Google Scholar] [CrossRef]
- Deng, D.M.; Urch, J.E.; ten Cate, J.M.; Rao, V.A.; van Aalten, D.M.; Crielaard, W. Streptococcus mutans SMU.623c codes for a functional, metal-dependent polysaccharide deacetylase that modulates interactions with salivary agglutinin. J. Bacteriol. 2009, 191, 394–402. [Google Scholar] [CrossRef]
- Fittipaldi, N.; Sekizaki, T.; Takamatsu, D.; de la Cruz Dominguez-Punaro, M.; Harel, J.; Bui, N.K.; Vollmer, W.; Gottschalk, M. Significant contribution of the pgdA gene to the virulence of Streptococcus suis. Mol. Microbiol. 2008, 70, 1120–1135. [Google Scholar] [CrossRef]
- Rush, J.S.; Parajuli, P.; Ruda, A.; Li, J.; Pohane, A.A.; Zamakhaeva, S.; Rahman, M.M.; Chang, J.C.; Gogos, A.; Kenner, C.W.; et al. PplD is a de-N-acetylase of the cell wall linkage unit of streptococcal rhamnopolysaccharides. Nat. Commun. 2022, 13, 590. [Google Scholar] [CrossRef] [PubMed]
- Meyrand, M.; Boughammoura, A.; Courtin, P.; Mezange, C.; Guillot, A.; Chapot-Chartier, M.P. Peptidoglycan N-acetylglucosamine deacetylation decreases autolysis in Lactococcus lactis. Microbiology (Reading) 2007, 153, 3275–3285. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.J.; Underhill, D.M. Peptidoglycan recognition by the innate immune system. Nat. Rev. Immunol. 2018, 18, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.R.; Virtaneva, K.; Porcella, S.F.; Barry, W.T.; Gowen, B.B.; Johnson, C.R.; Wright, F.A.; Musser, J.M. Group A Streptococcus transcriptome dynamics during growth in human blood reveals bacterial adaptive and survival strategies. Am. J. Pathol. 2005, 166, 455–465. [Google Scholar] [CrossRef]
- Virtaneva, K.; Porcella, S.F.; Graham, M.R.; Ireland, R.M.; Johnson, C.A.; Ricklefs, S.M.; Babar, I.; Parkins, L.D.; Romero, R.A.; Corn, G.J.; et al. Longitudinal analysis of the group A Streptococcus transcriptome in experimental pharyngitis in cynomolgus macaques. Proc. Natl. Acad. Sci. USA 2005, 102, 9014–9019. [Google Scholar] [CrossRef]
- Freiberg, J.A.; Le Breton, Y.; Tran, B.Q.; Scott, A.J.; Harro, J.M.; Ernst, R.K.; Goo, Y.A.; Mongodin, E.F.; Goodlett, D.R.; McIver, K.S.; et al. Global Analysis and Comparison of the Transcriptomes and Proteomes of Group A Streptococcus Biofilms. mSystems 2016, 1, e00149-16. [Google Scholar] [CrossRef]






| A: Primers Used to Generate and Confirm S. pyogenesΔspy1094 and S. pyogenesΔspy1370 | ||
| Primer | Sequence (5′-3′) | Restriction enzyme |
| spy1094FR1.fw | tgcctcgagtcgagctgacgggttttc | XhoI |
| spy1094FR1.rev | aggtaagcttgaaaatgagggtcaaaccaa | HindIII |
| spy1094FR2.fw | agctgcagccaaatcatactcactgtaaac | PstI |
| spy1094FR2.rev | agcccgggttcagttcaagacctgttgac | XmaI |
| spy1370FR1.fw | cggtcgaccagttggtttagttcttgcc | SalI |
| spy1370FR1.rev | cgggatccaataaacacaatagctaaac | BamHI |
| spy1370FR2.fw | agctgcaggtaatatcgttatgtttc | PstI |
| spy1370FR2.rev | atacccgggcttagcttatgtctttccta | XmaI |
| aad9.fw | ccttattggtacttacatgtttg | none |
| aad9.rev | ccattcaatattctctccaag | none |
| B: Primers used to generate L. lactisspy1094 and L. lactisspy1370 | ||
| Primer | Sequence (5′-3′) | Restriction enzyme |
| spy1094RBS.fw | tcaggatccgattggagcaaataaatatgaacaatagacataaacggc | BamHI |
| spy1094.rev | gaattcttatggttccattgtttg | EcoRI |
| spy1370RBS.fw | tcaggatccgattggagcaaataaatatgaaaaaattaaatgttattcttgttg | BamHI |
| spy1370.rev | ccgctcgagttactgatgcgcatagag | XhoI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aspell, T.; Khemlani, A.H.J.; Tsai, C.J.-Y.; Loh, J.M.S.; Proft, T. The Cell Wall Deacetylases Spy1094 and Spy1370 Contribute to Streptococcus pyogenes Virulence. Microorganisms 2023, 11, 305. https://doi.org/10.3390/microorganisms11020305
Aspell T, Khemlani AHJ, Tsai CJ-Y, Loh JMS, Proft T. The Cell Wall Deacetylases Spy1094 and Spy1370 Contribute to Streptococcus pyogenes Virulence. Microorganisms. 2023; 11(2):305. https://doi.org/10.3390/microorganisms11020305
Chicago/Turabian StyleAspell, Tiger, Adrina Hema J. Khemlani, Catherine Jia-Yun Tsai, Jacelyn Mei San Loh, and Thomas Proft. 2023. "The Cell Wall Deacetylases Spy1094 and Spy1370 Contribute to Streptococcus pyogenes Virulence" Microorganisms 11, no. 2: 305. https://doi.org/10.3390/microorganisms11020305
APA StyleAspell, T., Khemlani, A. H. J., Tsai, C. J.-Y., Loh, J. M. S., & Proft, T. (2023). The Cell Wall Deacetylases Spy1094 and Spy1370 Contribute to Streptococcus pyogenes Virulence. Microorganisms, 11(2), 305. https://doi.org/10.3390/microorganisms11020305






