Updates on Group B Streptococcus Infection in the Field of Obstetrics and Gynecology
Abstract
1. What Is Group B Streptococcus (GBS)?
1.1. Microbiology
1.2. Virulence Factors
1.3. Epidemiology
2. GBS-Related Clinical Diseases
2.1. GBS and Non-Pregnant Women
2.2. GBS in Pregnancy
2.3. GBS and Maternal Microbiome
3. GBS in Gynecology
4. Detection, Prevention, and Treatment of GBS
4.1. Detection: Various Detection Methods for GBS
4.2. Prevention: GBS Vaccination
4.3. Another Possible Treatment Approach, Microbial Therapy
5. Closing and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marió, M.J.S.; Valenzuela, I.; Vásquez, A.E.; Illanes, S.E. Prevention of Early-Onset Neonatal Group B Streptococcal Disease. Rev. Obstet. Gynecol. 2013, 6, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Ben-Taleb, H.; Nayme, K.; Lebrazi, H.; Chamekh, M.; SAILE, R.; Zerouali, K.; Timinouni, M.; Nayeme, K.; Lebrazi, H.; Chamekh, M.; et al. Review A Review of Group B Streptococcus Maternal-Fetal Infection. Moroc. J. Public Heath 2021, 3, 32–39. [Google Scholar]
- Lancefield, B.R.C. A Serological Differentiation of Human and Other Groups of Hemolytic Streptococci. J. Exp. Med. 1933, 1919, 571–595. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.S.; Baker, C.J. Group B Streptococcal Infections in Elderly Adults. Clin. Infect. Dis. 2005, 41, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Slotved, H.C.; Kong, F.; Lambertsen, L.; Sauer, S.; Gilbert, G.L. Serotype IX, a Proposed New Streptococcus Agalactiae Serotype. J. Clin. Microbiol. 2007, 45, 2929–2936. [Google Scholar] [CrossRef] [PubMed]
- Armistead, B.; Oler, E.; Adams Waldorf, K.; Rajagopal, L. The Double Life of Group B Streptococcus: Asymptomatic Colonizer and Potent Pathogen. J. Mol. Biol. 2019, 431, 2914–2931. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, L.C.; Kasper, D.L. Surface Structures of Group B Streptococcus Important in Human Immunity. Gram Positive Pathog. 2019, 204–227. [Google Scholar] [CrossRef] [PubMed]
- Hanna, M.; Noor, A. Streptococcus, Group B. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Randis, T.M.; Baker, J.A.; Ratner, A.J. Group B Streptococcal Infections. Pediatr. Rev. 2017, 38, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Raabe, V.N.; Shane, A.L. Group b Streptococcus (Streptococcus Agalactiae). Gram Positive Pathog. 2019, 228–238. [Google Scholar] [CrossRef]
- Wilkinson, H.W. Group B Streptococcal Infection in Humans. Annu. Rev. Microbiol. 1978, 32, 41–57. [Google Scholar] [CrossRef]
- Anthony, B.F.; Okada, D.M. The Emergence of GBS in Infections of the Newborn Infant. Annu. Rev. Med. 1977, 28, 355–369. [Google Scholar] [CrossRef]
- Group B Strep: Fast Facts and Statistics|CDC. Available online: https://www.cdc.gov/groupbstrep/about/fast-facts.html (accessed on 11 November 2022).
- Gonçalves, B.P.; Procter, S.R.; Paul, P.; Chandna, J.; Lewin, A.; Seedat, F.; Koukounari, A.; Dangor, Z.; Leahy, S.; Santhanam, S.; et al. Group B Streptococcus Infection during Pregnancy and Infancy: Estimates of Regional and Global Burden. Lancet Glob. Health 2022, 10, e807–e819. [Google Scholar] [CrossRef] [PubMed]
- Russell, N.J.; Seale, A.C.; O’Driscoll, M.; O’Sullivan, C.; Bianchi-Jassir, F.; Gonzalez-Guarin, J.; Lawn, J.E.; Baker, C.J.; Bartlett, L.; Cutland, C.; et al. Maternal Colonization with Group B Streptococcus and Serotype Distribution Worldwide: Systematic Review and Meta-Analyses. Clin. Infect. Dis. 2017, 65, S100–S111. [Google Scholar] [CrossRef] [PubMed]
- Farley, M.M. Group B Streptococcal Disease in Nonpregnant Adults. Clin. Infect. Dis. 2001, 33, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Group B Streptococcal Disease in Nonpregnant Adults-UpToDate. Available online: https://www.uptodate.com/contents/group-b-streptococcal-infections-in-nonpregnant-adults (accessed on 15 November 2022).
- Group B Streptococcal Infection in Neonates and Young Infants-UpToDate. Available online: https://www.uptodate.com/contents/group-b-streptococcal-infection-in-neonates-and-young-infants (accessed on 15 November 2022).
- Collin, S.M.; Shetty, N.; Guy, R.; Nyaga, V.N.; Bull, A.; Richards, M.J.; van der Kooi, T.I.I.; Koek, M.B.G.; De Almeida, M.; Roberts, S.A.; et al. Group B Streptococcus in Surgical Site and Non-Invasive Bacterial Infections Worldwide: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2019, 83, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Krohn, M.A.; Hillier, S.L.; Baker, C.J. Maternal Peripartum Complications Associated with Vaginal Group B Streptococci Colonization. J. Infect. Dis. 1999, 179, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Menichini, D.; Chiossi, G.; Monari, F.; De Seta, F.; Facchinetti, F. Supplementation of Probiotics in Pregnant Women Targeting Group B Streptococcus Colonization: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4520. [Google Scholar] [CrossRef]
- Hall, J.; Adams, N.H.; Bartlett, L.; Seale, A.C.; Lamagni, T.; Bianchi-Jassir, F.; Lawn, J.E.; Baker, C.J.; Cutland, C.; Heath, P.T.; et al. Maternal Disease with Group B Streptococcus and Serotype Distribution Worldwide: Systematic Review and Meta-Analyses. Clin. Infect. Dis. 2017, 65, S112–S124. [Google Scholar] [CrossRef]
- Lee, C.C.; Hsu, J.F.; Prasad Janapatla, R.; Chen, C.L.; Zhou, Y.L.; Lien, R.; Chiu, C.H. Clinical and Microbiological Characteristics of Group B Streptococcus from Pregnant Women and Diseased Infants in Intrapartum Antibiotic Prophylaxis Era in Taiwan. Sci. Rep. 2019, 9, 13525. [Google Scholar] [CrossRef]
- Kwatra, G.; Cunnington, M.C.; Merrall, E.; Adrian, P.V.; Ip, M.; Klugman, K.P.; Tam, W.H.; Madhi, S.A. Prevalence of Maternal Colonisation with Group B Streptococcus: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2016, 16, 1076–1084. [Google Scholar] [CrossRef]
- Group B Streptococcal Infection in Pregnant Individuals-UpToDate. Available online: https://www.uptodate.com/contents/group-b-streptococcal-infection-in-pregnant-individuals (accessed on 15 November 2022).
- Prevention of Group B Streptococcal Early-Onset Disease in Newborns: ACOG Committee Opinion, Number 797. Obstet. Gynecol. 2020, 135, e51–e72. [CrossRef] [PubMed]
- Puopolo, K.M.; Lynfield, R.; Cummings, J.J. Management of Infants at Risk for Group B Streptococcal Disease. Pediatrics 2019, 144, e20191881. [Google Scholar] [CrossRef] [PubMed]
- Nanduri, S.A.; Petit, S.; Smelser, C.; Apostol, M.; Alden, N.B.; Harrison, L.H.; Lynfield, R.; Vagnone, P.S.; Burzlaff, K.; Spina, N.L.; et al. Epidemiology of Invasive Early-Onset and Late-Onset Group B Streptococcal Disease in the United States, 2006 to 2015: Multistate Laboratory and Population-Based Surveillance. JAMA Pediatr. 2019, 173, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Berardi, A.; Tzialla, C.; Riva, M.; Cerbo, R.M.; Creti, R. Group B Streptococcus: Early- and Late-Onset Infections. J. Chemother. 2007, 19 (Suppl. S2), 24–27. [Google Scholar] [CrossRef] [PubMed]
- Berardi, A.; Trevisani, V.; Di Caprio, A.; Bua, J.; China, M.; Perrone, B.; Pagano, R.; Lucaccioni, L.; Fanaro, S.; Iughetti, L.; et al. Understanding Factors in Group b Streptococcus Late-Onset Disease. Infect. Drug Resist. 2021, 14, 3207–3218. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.Y.C.; Weisman, L.E.; Troendle, J.; Adams, K. Prematurity Is the Major Risk Factor for Late-Onset Group B Streptococcus Disease. J. Infect. Dis. 2003, 188, 267–271. [Google Scholar] [CrossRef]
- Le Doare, K.; Heath, P.T.; Plumb, J.; Owen, N.A.; Brocklehurst, P.; Chappell, L.C. Uncertainties in Screening and Prevention of Group B Streptococcus Disease. Clin. Infect. Dis. 2019, 69, 720–725. [Google Scholar] [CrossRef]
- Bartlett, A.W.; Smith, B.; George, C.R.R.; McMullan, B.; Kesson, A.; Lahra, M.M.; Palasanthiran, P. Epidemiology of Late and Very Late Onset Group B Streptococcal Disease: Fifteen-Year Experience from Two Australian Tertiary Pediatric Facilities. Pediatr. Infect. Dis. J. 2017, 36, 20–24. [Google Scholar] [CrossRef]
- Yeo, K.T.; Lahra, M.; Bajuk, B.; Hilder, L.; Abdel-Latif, M.E.; Wright, I.M.; Oei, J.L. Long-Term Outcomes after Group B Streptococcus Infection: A Cohort Study. Arch. Dis. Child. 2019, 104, 172–178. [Google Scholar] [CrossRef]
- Libster, R.; Edwards, K.M.; Levent, F.; Edwards, M.S.; Rench, M.A.; Castagnini, L.A.; Cooper, T.; Sparks, R.C.; Baker, C.J.; Shah, P.E. Long-Term Outcomes of Group B Streptococcal Meningitis. Pediatrics 2012, 130, e8–e15. [Google Scholar] [CrossRef]
- Verani, J.R.; McGee, L.; Schrag, S.J. Prevention of Perinatal Group B Streptococcal Disease—Revised Guidelines from CDC, 2010. MMWR Recomm. Rep. 2010, 59, 1–36. [Google Scholar] [PubMed]
- Schrag, S.; Gorwitz, R.; Fultz-Butts, K.; Schuchat, A. Prevention of Perinatal Group B Streptococcal Disease. Revised Guidelines from CDC. MMWR Recomm. Rep. 2002, 51, 1–22. [Google Scholar] [PubMed]
- Schrag, S.J.; Zywicki, S.; Farley, M.M.; Reingold, A.L.; Harrison, L.H.; Lefkowitz, L.B.; Hadler, J.L.; Danila, R.; Cieslak, P.R.; Schuchat, A. Group B Streptococcal Disease in the Era of Intrapartum Antibiotic Prophylaxis. N. Engl. J. Med. 2000, 342, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Dermer, P.; Lee, C.; Eggert, J.; Few, B. A History of Neonatal Group B Streptococcus with Its Related Morbidity and Mortality Rates in the United States. J. Pediatr. Nurs. 2004, 19, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Edmond, K.M.; Kortsalioudaki, C.; Scott, S.; Schrag, S.J.; Zaidi, A.K.; Cousens, S.; Heath, P.T. Group B Streptococcal Disease in Infants Aged Younger than 3 Months: Systematic Review and Meta-Analysis. Lancet 2012, 379, 547–556. [Google Scholar] [CrossRef]
- Kristeva, M.; Tillman, C.; Goordeen, A. Immunization Against Group B Streptococci vs. Intrapartum Antibiotic Prophylaxis in Peripartum Pregnant Women and Their Neonates: A Review. Cureus 2017, 9, e1775. [Google Scholar] [CrossRef]
- University Hospitals of Leicester NHS. Group B Streptococcus in Pregnancy and the Newborn UHL Obstetric Guideline. 2008. Available online: https://secure.library.leicestershospitals.nhs.uk/PAGL/Shared%20Documents/Group%20B%20Streptococcus%20in%20Pregnancy%20and%20the%20Newborn%20UHL%20Obstetric%20Guideline.pdf (accessed on 31 August 2022).
- Group B Strep Suppprt. Group B Streptococcus (GBS) in Pregnancy and Newborn Babies. 2017, Volume 2017. Available online: https://gbss.org.uk/wp-content/uploads/2018/01/2017-Joint-RCOG-GBSS-PIL_final.pdf (accessed on 13 September 2022).
- Group B Streptococcus (GBS) Infections Guidelines: GBS Prophylaxis in Preterm Labor. Available online: https://emedicine.medscape.com/article/229091-guidelines (accessed on 15 November 2022).
- Pangerl, S.; Sundin, D.; Geraghty, S. Group B Streptococcus Screening Guidelines in Pregnancy: A Critical Review of Compliance. Matern. Child Health J. 2021, 25, 257–267. [Google Scholar] [CrossRef]
- NHS Joint Clinical Guideline for: Group B Sreptococcus in Pregnancy. The Management of Women known to be carriers of Group B Streptococcus. Ver 6.4. 2021; 1–7.
- Group B Strep Suppprt. Updated Group B Strep Guidelines. Available online: https://gbss.org.uk/wp-content/uploads/2018/06/2018_06_RCOG_Summary_Leaflet.pdf (accessed on 22 October 2022). [CrossRef]
- Committee on Infectious Diseases; Committee on Fetus and Newborn; Baker, C.J.; Byington, C.L.; Polin, R.A. Policy Statement-Recommendations for the Prevention of Perinatal Group B Streptococcal (GBS) Disease. Pediatrics 2011, 128, 611–616. [Google Scholar] [CrossRef]
- Ahmadzia, H.K.; Heine, R.P. Diagnosis and Management of Group B Streptococcus in Pregnancy. Obstet. Gynecol. Clin. North Am. 2014, 41, 629–647. [Google Scholar] [CrossRef]
- Dhudasia, M.B.; Flannery, D.D.; Pfeifer, M.R.; Puopolo, K.M. Updated Guidance: Prevention and Management of Perinatal Group b Streptococcus Infection. Neoreviews 2021, 22, e177–e188. [Google Scholar] [CrossRef]
- Liou, J.M.; Malfertheiner, P.; Lee, Y.C.; Sheu, B.S.; Sugano, K.; Cheng, H.C.; Yeoh, K.G.; Hsu, P.I.; Goh, K.L.; Mahachai, V.; et al. Screening and Eradication of Helicobacter Pylori for Gastric Cancer Prevention: The Taipei Global Consensus. Gut 2020, 69, 2093–2112. [Google Scholar] [CrossRef] [PubMed]
- Fallone, C.A.; Chiba, N.; van Zanten, S.V.; Fischbach, L.; Gisbert, J.P.; Hunt, R.H.; Jones, N.L.; Render, C.; Leontiadis, G.I.; Moayyedi, P.; et al. The Toronto Consensus for the Treatment of Helicobacter Pylori Infection in Adults. Gastroenterology 2016, 151, 51–69.e14. [Google Scholar] [CrossRef] [PubMed]
- Goderska, K.; Agudo Pena, S.; Alarcon, T. Helicobacter Pylori Treatment: Antibiotics or Probiotics. Appl. Microbiol. Biotechnol. 2018, 102, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Piscione, M.; Mazzone, M.; Di Marcantonio, M.C.; Muraro, R.; Mincione, G. Eradication of Helicobacter Pylori and Gastric Cancer: A Controversial Relationship. Front. Microbiol. 2021, 12, 630852. [Google Scholar] [CrossRef] [PubMed]
- Freedberg, D.E.; Abrams, J.A.; Wang, T.C. Prevention of Gastric Cancer with Antibiotics: Can It Be Done without Eradicating Helicobacter Pylori? J. Natl. Cancer Inst. 2014, 106, 1995–1996. [Google Scholar] [CrossRef][Green Version]
- Gao, Y.; Shang, Q.; Li, W.; Guo, W.; Stojadinovic, A.; Mannion, C.; Man, Y.G.; Chen, T. Antibiotics for Cancer Treatment: A Double-Edged Sword. J. Cancer 2020, 11, 5135–5149. [Google Scholar] [CrossRef]
- Schistosomes, liver flukes and Helicobacter pylori. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 1994; Volume 61, pp. 1–241.
- Hayes, K.; O’Halloran, F.; Cotter, L. A Review of Antibiotic Resistance in Group B Streptococcus: The Story so Far. Crit. Rev. Microbiol. 2020, 46, 253–269. [Google Scholar] [CrossRef]
- Davies, H.G.; Carreras-Abad, C.; Le Doare, K.; Heath, P.T. Group B Streptococcus: Trials and Tribulations. Pediatr. Infect. Dis. J. 2019, 38, S72–S76. [Google Scholar] [CrossRef]
- Madhi, S. Group B Streptococcus (GBS) Vaccine. Int. J. Infect. Dis. 2018, 73, 31. [Google Scholar] [CrossRef]
- Dominguez, K.; Randis, T.M. Toward the Development of a Protein-Based Group B Streptococcus Vaccine. Cell Rep. Med. 2022, 3, 100536. [Google Scholar] [CrossRef]
- Garcia, V.R. Impact of Intrapartum Antibiotic Prophylaxis for Group B Streptococcus on the Term Infant Gut Microbiome: A State of the Science Review. J. Midwifery Women’s Health 2021, 66, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Ainonen, S.; Tejesvi, M.V.; Mahmud, M.R.; Paalanne, N.; Pokka, T.; Li, W.; Nelson, K.E.; Salo, J.; Renko, M.; Vänni, P.; et al. Antibiotics at Birth and Later Antibiotic Courses: Effects on Gut Microbiota. Pediatr. Res. 2022, 91, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Rosen, G.H.; Randis, T.M.; Desai, P.V.; Sapra, K.J.; Ma, B.; Gajer, P.; Humphrys, M.S.; Ravel, J.; Gelber, S.E.; Ratner, A.J. Group B Streptococcus and the Vaginal Microbiota. J. Infect. Dis. 2017, 216, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Brzychczy-Wloch, M.; Pabian, W.; Majewska, E.; Zuk, M.; Kielbik, J.; Gosiewski, T.; Bulanda, M. Dynamics of Colonization with Group B Streptococci in Relation to Normal Flora in Women during Subsequent Trimesters of Pregnancy. New Microbiol. 2014, 37, 307–319. [Google Scholar] [PubMed]
- Svare, J.A.; Schmidt, H.; Hansen, B.B.; Lose, G. Bacterial Vaginosis in a Cohort of Danish Pregnant Women: Prevalence and Relationship with Preterm Delivery, Low Birthweight and Perinatal Infections. BJOG An Int. J. Obstet. Gynaecol. 2006, 113, 1419–1425. [Google Scholar] [CrossRef]
- Fettweis, J.M.; Serrano, M.G.; Brooks, J.P.; Edwards, D.J.; Girerd, P.H.; Parikh, H.I.; Huang, B.; Arodz, T.J.; Edupuganti, L.; Glascock, A.L.; et al. The Vaginal Microbiome and Preterm Birth. Nat. Med. 2019, 25, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Leitich, H.; Kiss, H. Asymptomatic Bacterial Vaginosis and Intermediate Flora as Risk Factors for Adverse Pregnancy Outcome. Best Pract. Res. Clin. Obstet. Gynaecol. 2007, 21, 375–390. [Google Scholar] [CrossRef]
- Hillier, S.L.; Nugent, R.P.; Eschenbach, D.A.; Krohn, M.A.; Gibbs, R.; Martin, D.H.; Cotch, M.F.; Edelman, R. Association between Bacterial Vaginosis and Preterm Delivery of a Low- Birth-Weight Infant. Stud. Fam. Plann. 1996, 27, 57. [Google Scholar] [CrossRef]
- Guerra, B.; Ghi, T.; Quarta, S.; Morselli-Labate, A.M.; Lazzarotto, T.; Pilu, G.; Rizzo, N. Pregnancy Outcome after Early Detection of Bacterial Vaginosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 128, 40–45. [Google Scholar] [CrossRef]
- Guaschino, S.; De Seta, F.; Piccoli, M.; Maso, G.; Alberico, S. Aetiology of Preterm Labour: Bacterial Vaginosis. BJOG An Int. J. Obstet. Gynaecol. 2006, 113, 46–51. [Google Scholar] [CrossRef]
- Campisciano, G.; Zanotta, N.; Petix, V.; Giangreco, M.; Ricci, G.; Maso, G.; Comar, M.; De Seta, F. Vaginal Dysbiosis and Partial Bacterial Vaginosis: The Interpretation of the “Grey Zones” of Clinical Practice. Diagnostics 2021, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Kaambo, E.; Africa, C.; Chambuso, R.; Passmore, J.A.S. Vaginal Microbiomes Associated with Aerobic Vaginitis and Bacterial Vaginosis. Front. Public Health 2018, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, M.A.; Hillier, S.L.; Nugent, R.P.; MacPherson, C.A.; Hauth, J.C.; Carey, J.C.; Harper, M.; Wapner, R.J.; Trout, W.; Moawad, A.; et al. Is Bacterial Vaginosis a Stronger Risk Factor for Preterm Birth When It Is Diagnosed Earlier in Gestation? Am. J. Obstet. Gynecol. 2005, 192, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Donders, G.G.; Van Calsteren, K.; Bellen, G.; Reybrouck, R.; Van Den Bosch, T.; Riphagen, I.; Van Lierde, S. Predictive Value for Preterm Birth of Abnormal Vaginal Flora, Bacterial Vaginosis and Aerobic Vaginitis during the First Trimester of Pregnancy. BJOG An Int. J. Obstet. Gynaecol. 2009, 116, 1315–1324. [Google Scholar] [CrossRef]
- Donders, G.G.G.; Bellen, G.; Rezeberga, D. Aerobic Vaginitis in Pregnancy. BJOG An Int. J. Obstet. Gynaecol. 2011, 118, 1163–1170. [Google Scholar] [CrossRef]
- Donders, G.G.; Van Calsteren, C.; Bellen, G.; Reybrouck, R.; Van den Bosch, T.; Riphagen, I.; Van Lierde, S. Association between Abnormal Vaginal Flora and Cervical Length as Risk Factors for Preterm Birth. Ultrasound Obstet. Gynecol. 2010. [Google Scholar] [CrossRef]
- Mohamed, I.; Zakeer, S.; Azab, M.; Hanora, A. Changes in Vaginal Microbiome in Pregnant and Nonpregnant Women with Bacterial Vaginosis: Toward Microbiome Diagnostics? Omi. A J. Integr. Biol. 2020, 24, 602–614. [Google Scholar] [CrossRef]
- Daskalakis, G.; Papapanagiotou, A.; Mesogitis, S.; Papantoniou, N.; Mavromatis, K.; Antsaklis, A. Bacterial Vaginosis and Group B Streptococcal Colonization and Preterm Delivery in a Low-Risk Population. Fetal Diagn. Ther. 2006, 21, 172–176. [Google Scholar] [CrossRef]
- Rick, A.M.; Aguilar, A.; Cortes, R.; Gordillo, R.; Melgar, M.; Samayoa-Reyes, G.; Frank, D.N.; Asturias, E.J. Group B Streptococci Colonization in Pregnant Guatemalan Women: Prevalence, Risk Factors, and Vaginal Microbiome. Open Forum Infect. Dis. 2017, 4, ofx020. [Google Scholar] [CrossRef]
- Kim, D.H.; Min, B.J.; Jung, E.J.; Byun, J.M.; Jeong, D.H.; Lee, K.B.; Sung, M.S.; Kim, K.T.; Kim, Y.N. Prevalence of Group B Streptococcus Colonization in Pregnant Women in a Tertiary Care Center in Korea. Obstet. Gynecol. Sci. 2018, 61, 575–583. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, Y.J.; Jung, S.Y.; Kim, S.Y.; Son, D.W.; Seo, I.H. Pregnancy and Neonatal Outcomes of Group B Streptococcus Infection in Preterm Births. Perinatology 2018, 29, 147. [Google Scholar] [CrossRef]
- Ling, Z.; Kong, J.; Liu, F.; Zhu, H.; Chen, X.; Wang, Y.; Li, L.; Nelson, K.E.; Xia, Y.; Xiang, C. Molecular Analysis of the Diversity of Vaginal Microbiota Associated with Bacterial Vaginosis. BMC Genom. 2010, 11, 488. [Google Scholar] [CrossRef]
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal Microbiota and the Potential of Lactobacillus Derivatives in Maintaining Vaginal Health. Microb. Cell Fact. 2020, 19, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, Y.; Chen, T.; Li, R. The Female Vaginal Microbiome in Health and Bacterial Vaginosis. Front. Cell. Infect. Microbiol. 2021, 11, 631972. [Google Scholar] [CrossRef] [PubMed]
- Shipitsyna, E.; Roos, A.; Datcu, R.; Hallén, A.; Fredlund, H.; Jensen, J.S.; Engstrand, L.; Unemo, M. Composition of the Vaginal Microbiota in Women of Reproductive Age-Sensitive and Specific Molecular Diagnosis of Bacterial Vaginosis Is Possible? PLoS ONE 2013, 8, e60670. [Google Scholar] [CrossRef] [PubMed]
- Patras, K.A.; Rösler, B.; Thoman, M.L.; Doran, K.S. Characterization of Host Immunity during Persistent Vaginal Colonization by Group B Streptococcus. Mucosal Immunol. 2015, 8, 1339–1348. [Google Scholar] [CrossRef]
- Xu, J.; Peng, J.-J.; Yang, W.; Fu, K.; Zhang, Y. Vaginal Microbiomes and Ovarian Cancer: A Review. Am. J. Cancer Res. 2020, 10, 743–756. [Google Scholar]
- Kyrgiou, M.; Mitra, A.; Moscicki, A.B. Does the Vaginal Microbiota Play a Role in the Development of Cervical Cancer? Transl. Res. 2017, 179, 168–182. [Google Scholar] [CrossRef]
- Xie, Y.; Feng, Y.; Li, W.; Zhan, F.; Huang, G.; Hu, H.; Xiong, Y.; Tan, B.; Chen, T. Revealing the Disturbed Vaginal Micobiota Caused by Cervical Cancer Using High-Throughput Sequencing Technology. Front. Cell. Infect. Microbiol. 2020, 10, 538336. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Y.; Yu, L.; Shi, X.; Min, M.; Xiong, L.; Pan, J.; Liu, P.; Wu, G.; Gao, G. A Cross-Sectional Analysis about Bacterial Vaginosis, High-Risk Human Papillomavirus Infection, and Cervical Intraepithelial Neoplasia in Chinese Women. Sci. Rep. 2022, 12, 6609. [Google Scholar] [CrossRef]
- Di Paola, M.; Sani, C.; Clemente, A.M.; Iossa, A.; Perissi, E.; Castronovo, G.; Tanturli, M.; Rivero, D.; Cozzolino, F.; Cavalieri, D.; et al. Characterization of Cervico-Vaginal Microbiota in Women Developing Persistent High-Risk Human Papillomavirus Infection. Sci. Rep. 2017, 7, 10200. [Google Scholar] [CrossRef]
- Oh, H.Y.; Kim, B.S.; Seo, S.S.; Kong, J.S.; Lee, J.K.; Park, S.Y.; Hong, K.M.; Kim, H.K.; Kim, M.K. The Association of Uterine Cervical Microbiota with an Increased Risk for Cervical Intraepithelial Neoplasia in Korea. Clin. Microbiol. Infect. 2015, 21, 674.e1–674.e9. [Google Scholar] [CrossRef] [PubMed]
- Piyathilake, C.J.; Ollberding, N.J.; Kumar, R.; Macaluso, M.; Alvarez, R.D.; Morrow, C.D. Cervical Microbiota Associated with Higher Grade Cervical Intraepithelial Neoplasia in Women Infected with High-Risk Human Papillomaviruses. Cancer Prev. Res. 2016, 9, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; MacIntyre, D.A.; Lee, Y.S.; Smith, A.; Marchesi, J.R.; Lehne, B.; Bhatia, R.; Lyons, D.; Paraskevaidis, E.; Li, J.V.; et al. Cervical Intraepithelial Neoplasia Disease Progression Is Associated with Increased Vaginal Microbiome Diversity. Sci. Rep. 2015, 5, 16865. [Google Scholar] [CrossRef] [PubMed]
- Audirac-Chalifour, A.; Torres-Poveda, K.; Bahena-Román, M.; Téllez-Sosa, J.; Martínez-Barnetche, J.; Cortina-Ceballos, B.; López-Estrada, G.; Delgado-Romero, K.; Burguete-García, A.I.; Cantú, D.; et al. Cervical Microbiome and Cytokine Profile at Various Stages of Cervical Cancer: A Pilot Study. PLoS ONE 2016, 11, e0153274. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Gao, W.; Pan, Y.; Gao, Y.; Shen, J.; Xiong, H. The Direct and Indirect Association of Cervical Microbiota with the Risk of Cervical Intraepithelial Neoplasia. Cancer Med. 2018, 7, 2172–2179. [Google Scholar] [CrossRef]
- Kang, G.U.; Jung, D.R.; Lee, Y.H.; Jeon, S.Y.; Han, H.S.; Chong, G.O.; Shin, J.H. Potential Association between Vaginal Microbiota and Cervical Carcinogenesis in Korean Women: A Cohort Study. Microorganisms 2021, 9, 294. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kang, G.U.; Jeon, S.Y.; Tagele, S.B.; Pham, H.Q.; Kim, M.S.; Ahmad, S.; Jung, D.R.; Park, Y.J.; Han, H.S.; et al. Vaginal Microbiome-Based Bacterial Signatures for Predicting the Severity of Cervical Intraepithelial Neoplasia. Diagnostics 2020, 10, 1013. [Google Scholar] [CrossRef]
- Verteramo, R.; Pierangeli, A.; Mancini, E.; Calzolari, E.; Bucci, M.; Osborn, J.; Nicosia, R.; Chiarini, F.; Antonelli, G.; Degener, A.M. Human Papillomaviruses and Genital Co-Infections in Gynaecological Outpatients. BMC Infect. Dis. 2009, 9, 16. [Google Scholar] [CrossRef]
- Hakimjavadi, H.; George, S.H.; Taub, M.; Dodds, L.V.; Sanchez-Covarrubias, A.P.; Huang, M.; Pearson, J.M.; Slomovitz, B.M.; Kobetz, E.N.; Gharaibeh, R.; et al. The Vaginal Microbiome Is Associated with Endometrial Cancer Grade and Histology. Cancer Res. Commun. 2022, 2, 447–455. [Google Scholar] [CrossRef]
- Boutriq, S.; González-González, A.; Plaza-Andrades, I.; Laborda-Illanes, A.; Sánchez-Alcoholado, L.; Peralta-Linero, J.; Domínguez-Recio, M.E.; Bermejo-Pérez, M.J.; Lavado-Valenzuela, R.; Alba, E.; et al. Gut and Endometrial Microbiome Dysbiosis: A New Emergent Risk Factor for Endometrial Cancer. J. Pers. Med. 2021, 11, 659. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.L.; Chen, L.X.; Shu, W.S.; Yao, S.Z.; Wang, S.W.; Chen, Y.Q. Barcoded Sequencing Reveals Diverse Intrauterine Microbiomes in Patients Suffering with Endometrial Polyps. Am. J. Transl. Res. 2016, 8, 1581–1592. [Google Scholar] [PubMed]
- Nené, N.R.; Reisel, D.; Leimbach, A.; Franchi, D.; Jones, A.; Evans, I.; Knapp, S.; Ryan, A.; Ghazali, S.; Timms, J.F.; et al. Association between the Cervicovaginal Microbiome, BRCA1 Mutation Status, and Risk of Ovarian Cancer: A Case-Control Study. Lancet Oncol. 2019, 20, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Sipos, A.; Ujlaki, G.; Mikó, E.; Maka, E.; Szabó, J.; Uray, K.; Krasznai, Z.; Bai, P. The Role of the Microbiome in Ovarian Cancer: Mechanistic Insights into Oncobiosis and to Bacterial Metabolite Signaling. Mol. Med. 2021, 27, 33. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Tian, T.; Wei, Z.; Shih, N.; Feldman, M.D.; Alwine, J.C.; Coukos, G.; Robertson, E.S. The Ovarian Cancer Oncobiome. Oncotarget 2017, 8, 36225–36245. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.L.; Perez, A.V.; Machado, M.M.; Kayser, M.L.; Vettori, D.V.; Alegretti, A.P.; Ferreira, C.F.; Vettorazzi, J.; Valério, E.G. Group B Streptococcus Detection in Pregnant Women: Comparison of QPCR Assay, Culture, and the Xpert GBS Rapid Test. BMC Pregnancy Childbirth 2019, 19, 532. [Google Scholar] [CrossRef]
- Song, K.E.; Hwang, N.; Ham, J.Y.; Cha, H.H.; Chong, G.O.; Lee, N.Y. Prevalence of Group B Streptococcus Colonization in Pregnant Women at a University Hospital in Korea. Clin. Lab. 2022, 68, 1711–1716. [Google Scholar] [CrossRef]
- Larsen, J.W.; Sever, J.L. Group B Streptococcus and Pregnancy: A Review. Am. J. Obstet. Gynecol. 2008, 198, 440–450. [Google Scholar] [CrossRef]
- Walker, K.F.; Morris, E.; Plumb, J.; Gray, J.; Thornton, J.G.; Daniels, J. Universal Testing for Group B Streptococcus during Pregnancy: Need for a Randomised Trial. BJOG An Int. J. Obstet. Gynaecol. 2020, 127, 693. [Google Scholar] [CrossRef]
- Filkins, L.; Hauser, J.R.; Robinson-Dunn, B.; Tibbetts, R.; Boyanton, B.L.; Revell, P. American Society for Microbiology Provides 2020 Guidelines for Detection and Identification of Group B Streptococcus. J. Clin. Microbiol. 2021, 59, 2020–2021. [Google Scholar] [CrossRef]
- Rosa-fraile, M.; Spellerberg, B. Reliable Detection of Group B Streptococcus in the Clinical Laboratory. J. Clin. Microbiol. 2017, 55, 2590–2598. [Google Scholar] [CrossRef]
- Ke, D.; Bergeron, M.G. Molecular Methods for Rapid Detection of Group B Streptococci. Expert Rev. Mol. Diagn. 2001, 1, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.W. CAMP Disk Test for Presumptive Identification of Group B Streptococci. J. Clin. Microbiol. 1977, 6, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, C.; Martínez, J.; Menasalvas, A.; Blázquez, R.; Rodríguez, T.; Segovia, M. Use of Direct Latex Agglutination Testing of Selective Broth in the Detection of Group B Strepptococcal Carriage in Pregnant Women. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 61–62. [Google Scholar] [CrossRef] [PubMed]
- Hensler, M.E.; Quach, D.; Hsieh, C.-J.; Doran, K.S.; Nizet, V. CAMP Factor Is Not Essential for Systemic Virulence of Group B Streptococcus. Microb. Pathog. 2008, 44, 84–88. [Google Scholar] [CrossRef]
- Picard, F.J.; Bergeron, M.G. Laboratory Detection of Group B Streptococcus for Prevention of Perinatal Disease. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 665–671. [Google Scholar] [CrossRef]
- Berry, G.J.; Zhang, F.; Manji, R.; Juretschko, S. Comparison of the Panther Fusion and BD MAX Group B Streptococcus (GBS) Assays for Detection of GBS in Prenatal Screening Specimens. J. Clin. Microbiol. 2019, 57, e01034-19. [Google Scholar] [CrossRef]
- Ellem, J.A.; Kovacevic, D.; Olma, T.; Chen, S.C.A. Rapid Detection of Group B Streptococcus Directly from Vaginal–Rectal Specimens Using Liquid Swabs and the BD Max GBS Assay. Clin. Microbiol. Infect. 2017, 23, 948–951. [Google Scholar] [CrossRef]
- Andreasen, T.; Møller, J.K.; Khalil, M.R. Comparison of BD MAX GBS and GenomEra GBS Assays for Rapid Intrapartum PCR Detection of Vaginal Carriage of Group B Streptococci. PLoS ONE 2019, 14, e0215314. [Google Scholar] [CrossRef]
- Gao, K.; Deng, Q.; Huang, L.; Chang, C.Y.; Zhong, H.; Xie, Y.; Guan, X.; Liu, H. Diagnostic Performance of Various Methodologies for Group B Streptococcus Screening in Pregnant Woman in China. Front. Cell. Infect. Microbiol. 2021, 11, 651968. [Google Scholar] [CrossRef]
- Sung, J.H.; Cha, H.H.; Lee, N.Y.; Lee, W.K.; Choi, Y.; Han, H.S.; Lee, Y.Y.; Chong, G.O.; Seong, W.J. Diagnostic Accuracy of Loop-Mediated Isothermal Amplification Assay for Group B Streptococcus Detection in Recto-Vaginal Swab: Comparison with Polymerase Chain Reaction Test and Conventional Culture. Diagnostics 2022, 12, 1569. [Google Scholar] [CrossRef] [PubMed]
- Silbert, S.; Rocchetti, T.T.; Gostnell, A.; Kubasek, C.; Widen, R. Detection of Group B Streptococcus Directly from Collected ESwab Samples by Use of the BD Max GBS Assay. J. Clin. Microbiol. 2016, 54, 1660–1663. [Google Scholar] [CrossRef] [PubMed]
- Clifford, V.; Garland, S.M.; Grimwood, K. Prevention of Neonatal Group B Streptococcus Disease in the 21st Century. J. Paediatr. Child Health 2012, 48, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Group B Streptococcus Vaccine: Full Value of Vaccine Assessment. In Executive Summary; World Health Organization: Geneva, Switzerland, 2021.
- Vornhagen, J.; Adams Waldorf, K.M.; Rajagopal, L. Perinatal Group B Streptococcal Infections: Virulence Factors, Immunity, and Prevention Strategies. Trends Microbiol. 2017, 25, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.L.; Avci, F.Y.; Kasper, D.L. A Maternal Vaccine against Group B Streptococcus: Past, Present, and Future. Vaccine 2013, 31, D13–D19. [Google Scholar] [CrossRef]
- Dangor, Z.; Kwatra, G.; Izu, A.; Lala, S.G.; Madhi, S.A. Review on the Association of Group B Streptococcus Capsular Antibody and Protection against Invasive Disease in Infants. Expert Rev. Vaccines 2014, 14, 135–149. [Google Scholar] [CrossRef]
- Melin, P. Neonatal Group B Streptococcal Disease: From Pathogenesis to Preventive Strategies. Clin. Microbiol. Infect. 2011, 17, 1294–1303. [Google Scholar] [CrossRef]
- Carreras-Abad, C.; Ramkhelawon, L.; Heath, P.T.; Doare, K. Le A Vaccine against Group b Streptococcus: Recent Advances. Infect. Drug Resist. 2020, 13, 1263–1272. [Google Scholar] [CrossRef]
- Urgent Need for Vaccine to Prevent Deadly Group B Streptococcus. Available online: https://www.who.int/news/item/02-11-2021-urgent-need-for-vaccine-to-prevent-deadly-group-b-streptococcus (accessed on 14 November 2022).
- Kobayashi, M.; Schrag, S.J.; Alderson, M.R.; Madhi, S.A.; Baker, C.J.; Sobanjo-Ter Meulen, A.; Kaslow, D.C.; Smith, P.G.; Moorthy, V.S.; Vekemans, J. WHO Consultation on Group B Streptococcus Vaccine Development: Report from a Meeting Held on 27–28 April 2016. Vaccine 2016, 37, 7307–7314. [Google Scholar] [CrossRef]
- Heath, P.T. An Update on Vaccination against Group B Streptococcus. Expert Rev. Vaccines 2011, 10, 685–694. [Google Scholar] [CrossRef]
- Puopolo, K.M. Current Status of Vaccine Development for Group b Streptococcus. Neoreviews 2014, 15, e430–e438. [Google Scholar] [CrossRef]
- Group B Streptococcus Vaccine Development Technology Roadmap. In Priority Activities for Development, Testing, Licensure and Global Availability of Group B Streptococcus Vaccines; World Health Organization: Geneva, Switzerland, 2017.
- Nuccitelli, A.; Rinaudo, C.D.; Maione, D. Group B Streptococcus Vaccine: State of the Art. Ther. Adv. Vaccines 2015, 3, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Bianchi-Jassir, F.; Paul, P.; To, K.N.; Carreras-Abad, C.; Seale, A.C.; Jauneikaite, E.; Madhi, S.A.; Russell, N.J.; Hall, J.; Madrid, L.; et al. Systematic Review of Group B Streptococcal Capsular Types, Sequence Types and Surface Proteins as Potential Vaccine Candidates. Vaccine 2020, 38, 6682–6694. [Google Scholar] [CrossRef] [PubMed]
- FDA Grants Breakthrough Therapy Designation to Pfizer’s Group B Streptococcus Vaccine Candidate to Help Prevent Infection in Infants Via Immunization of Pregnant Women|Pfizer. Available online: https://www.pfizer.com/news/press-release/press-release-detail/fda-grants-breakthrough-therapy-designation-pfizers-group-b (accessed on 14 November 2022).
- Product Pipeline-MinervaX. Available online: https://www.minervax.com/product-pipeline/ (accessed on 14 November 2022).
- MinervaX Raises $57M for Group B Streptococcus Vaccine Race with Pfizer|Fierce Biotech. Available online: https://www.fiercebiotech.com/biotech/minervax-raises-57m-for-group-b-streptococcus-vaccine-race-pfizer (accessed on 14 November 2022).
- Group B Streptococcus Vaccine in Healthy Females-Full Text View-ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03807245 (accessed on 11 November 2022).
- Safety and Immunogenicity of a Trivalent Group B Streptococcus Vaccine in Healthy Pregnant Women-Full Text View-ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02046148 (accessed on 11 November 2022).
- Swamy, G.K.; Metz, T.D.; Edwards, K.M.; Soper, D.E.; Beigi, R.H.; Campbell, J.D.; Grassano, L.; Buffi, G.; Dreisbach, A.; Margarit, I.; et al. Safety and Immunogenicity of an Investigational Maternal Trivalent Group B Streptococcus Vaccine in Pregnant Women and Their Infants: Results from a Randomized Placebo-Controlled Phase II Trial. Vaccine 2020, 38, 6930–6940. [Google Scholar] [CrossRef] [PubMed]
- Safety and Immunogenicity of a Group B Streptococcus Vaccine in Non Pregnant and Pregnant Women 18–40 Years of Age-Full Text View-ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01193920 (accessed on 11 November 2022).
- A Phase 1/2, Randomized, Placebo-Controlled, Observer-Blinded Trial to Evaluate the Safety, Tolerability, and Immunogenicity of a Multivalent Group B Streptococcus Vaccine in Healthy Adults 18 to 49 Years of Age-Full Text View-ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03170609 (accessed on 11 November 2022).
- Absalon, J.; Segall, N.; Block, S.L.; Center, K.J.; Scully, I.L.; Giardina, P.C.; Peterson, J.; Watson, W.J.; Gruber, W.C.; Jansen, K.U.; et al. Safety and Immunogenicity of a Novel Hexavalent Group B Streptococcus Conjugate Vaccine in Healthy, Non-Pregnant Adults: A Phase 1/2, Randomised, Placebo-Controlled, Observer-Blinded, Dose-Escalation Trial. Lancet Infect. Dis. 2021, 21, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Study of a Group B Streptococcus Vaccine in Pregnant Women Living with HIV and in Pregnant Women Who Do Not Have HIV-Full Text View-ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04596878 (accessed on 11 November 2022).
- Sorbara, M.T.; Pamer, E.G. Microbiome-Based Therapeutics. Nat. Rev. Microbiol. 2022, 20, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shah, K. The Potential of the Gut Microbiome to Reshape the Cancer Therapy Paradigm: A Review. JAMA Oncol. 2022, 8, 1059–1067. [Google Scholar] [CrossRef]
- Peterson, S.N.; Bradley, L.M.; Ronai, Z.A. The Gut Microbiome: An Unexpected Player in Cancer Immunity. Curr. Opin. Neurobiol. 2020, 62, 48–52. [Google Scholar] [CrossRef]
- Yu, Z.K.; Xie, R.L.; You, R.; Liu, Y.P.; Chen, X.Y.; Chen, M.Y.; Huang, P.Y. The Role of the Bacterial Microbiome in the Treatment of Cancer. BMC Cancer 2021, 21, 934. [Google Scholar] [CrossRef]
- Gulliver, E.L.; Young, R.B.; Chonwerawong, M.; D’Adamo, G.L.; Thomason, T.; Widdop, J.T.; Rutten, E.L.; Rossetto Marcelino, V.; Bryant, R.V.; Costello, S.P.; et al. Review Article: The Future of Microbiome-Based Therapeutics. Aliment. Pharmacol. Ther. 2022, 56, 192–208. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Ng, S.C.; Schnabl, B. Promises of Microbiome-Based Therapies. J. Hepatol. 2022, 76, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.; Brotman, R.M. Translating the Vaginal Microbiome: Gaps and Challenges. Genome Med. 2016, 8, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Zwittink, R.D.; van den Munckhof, E.H.A.; Leverstein-van Hall, M.A.; Boers, K.; Molijn, A.; Knetsch, C.W.; Kuijper, E.J. The Vaginal Microbiota in the Course of Bacterial Vaginosis Treatment. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Vieira-Baptista, P.; De Seta, F.; Verstraelen, H.; Ventolini, G.; Lonnee-Hoffmann, R.; Lev-Sagie, A. The Vaginal Microbiome: V. Therapeutic Modalities of Vaginal Microbiome Engineering and Research Challenges. J. Low. Genit. Tract Dis. 2022, 26, 99–104. [Google Scholar] [CrossRef]
- Wu, S.; Hugerth, L.W.; Schuppe-Koistinen, I.; Du, J. The Right Bug in the Right Place: Opportunities for Bacterial Vaginosis Treatment. NPJ Biofilms Microbiomes 2022, 8, 34. [Google Scholar] [CrossRef]
- Ma, B.; Forney, L.; Ravel, J. Tha Vaginal Microbiome: Rethinking Health and Diseases. Annu. Rev. Microbiol. 2013, 66, 371–389. [Google Scholar] [CrossRef]
- Lev-Sagie, A.; Goldman-Wohl, D.; Cohen, Y.; Dori-Bachash, M.; Leshem, A.; Mor, U.; Strahilevitz, J.; Moses, A.E.; Shapiro, H.; Yagel, S.; et al. Vaginal Microbiome Transplantation in Women with Intractable Bacterial Vaginosis. Nat. Med. 2019, 25, 1500–1504. [Google Scholar] [CrossRef]
- Junca, H.; Pieper, D.H.; Medina, E. The Emerging Potential of Microbiome Transplantation on Human Health Interventions. Comput. Struct. Biotechnol. J. 2022, 20, 615–627. [Google Scholar] [CrossRef]
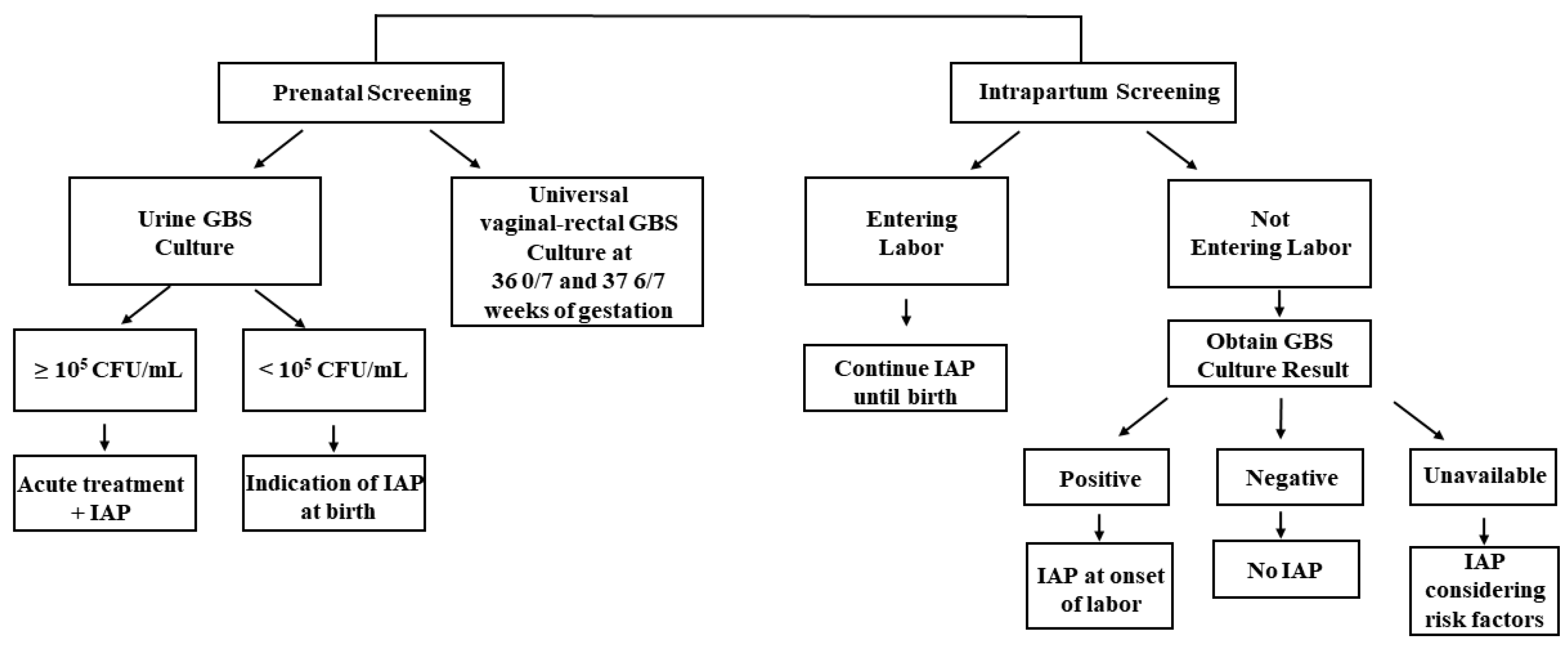
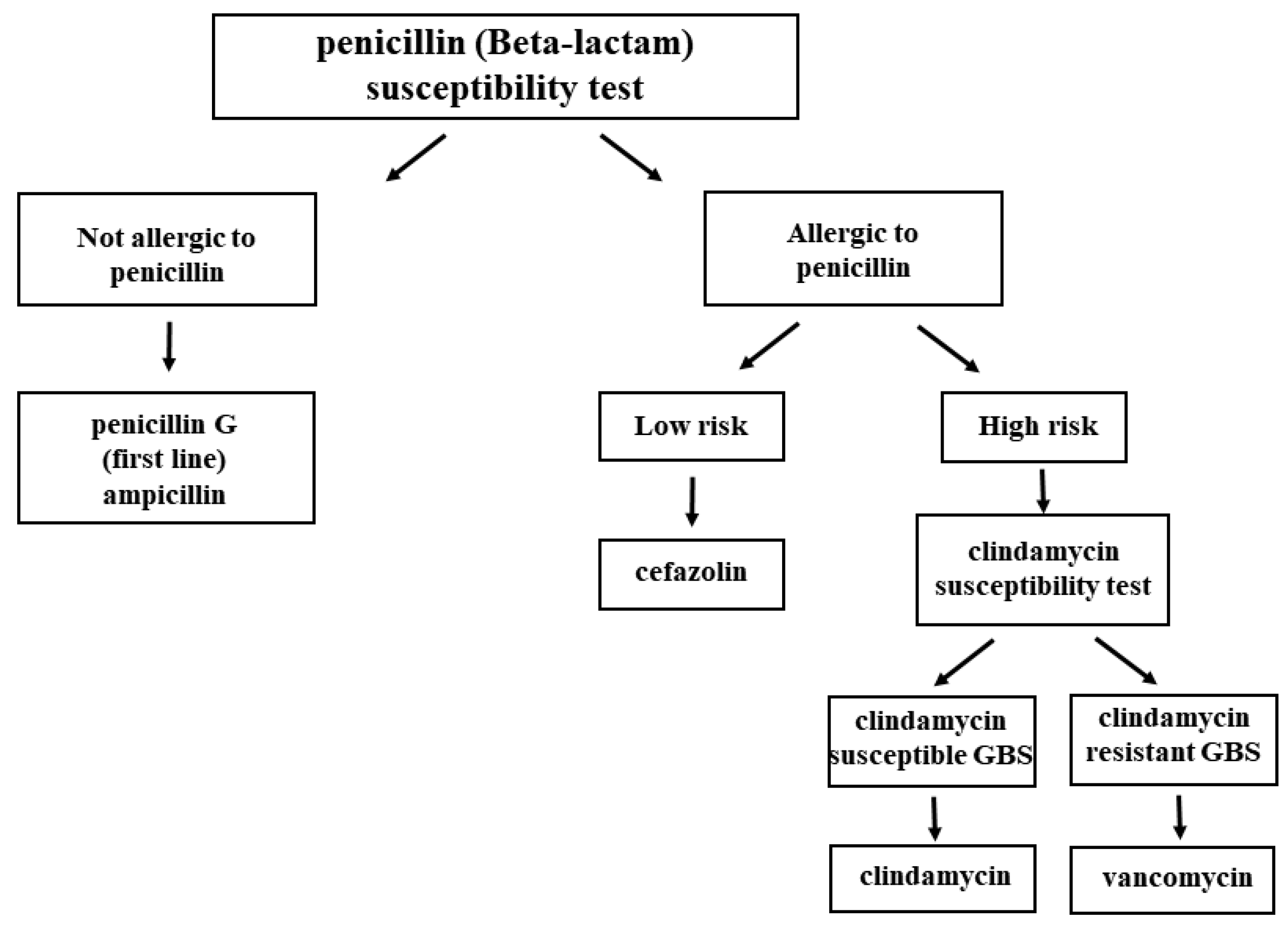
| GBS IAP Indicated | GBS IAP Not Indicated |
|---|---|
Maternal History
|
|
Current Pregnancy
|
|
Intrapartum
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.; Han, H.-S.; Chong, G.O.; Le, T.M.; Nguyen, H.D.T.; Lee, O.E.; Lee, D.; Seong, W.J.; Seo, I.; Cha, H.-H. Updates on Group B Streptococcus Infection in the Field of Obstetrics and Gynecology. Microorganisms 2022, 10, 2398. https://doi.org/10.3390/microorganisms10122398
Choi Y, Han H-S, Chong GO, Le TM, Nguyen HDT, Lee OE, Lee D, Seong WJ, Seo I, Cha H-H. Updates on Group B Streptococcus Infection in the Field of Obstetrics and Gynecology. Microorganisms. 2022; 10(12):2398. https://doi.org/10.3390/microorganisms10122398
Chicago/Turabian StyleChoi, Yeseul, Hyung-Soo Han, Gun Oh Chong, Tan Minh Le, Hong Duc Thi Nguyen, Olive EM Lee, Donghyeon Lee, Won Joon Seong, Incheol Seo, and Hyun-Hwa Cha. 2022. "Updates on Group B Streptococcus Infection in the Field of Obstetrics and Gynecology" Microorganisms 10, no. 12: 2398. https://doi.org/10.3390/microorganisms10122398
APA StyleChoi, Y., Han, H.-S., Chong, G. O., Le, T. M., Nguyen, H. D. T., Lee, O. E., Lee, D., Seong, W. J., Seo, I., & Cha, H.-H. (2022). Updates on Group B Streptococcus Infection in the Field of Obstetrics and Gynecology. Microorganisms, 10(12), 2398. https://doi.org/10.3390/microorganisms10122398





