Melipona scutellaris Geopropolis: Chemical Composition and Bioactivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
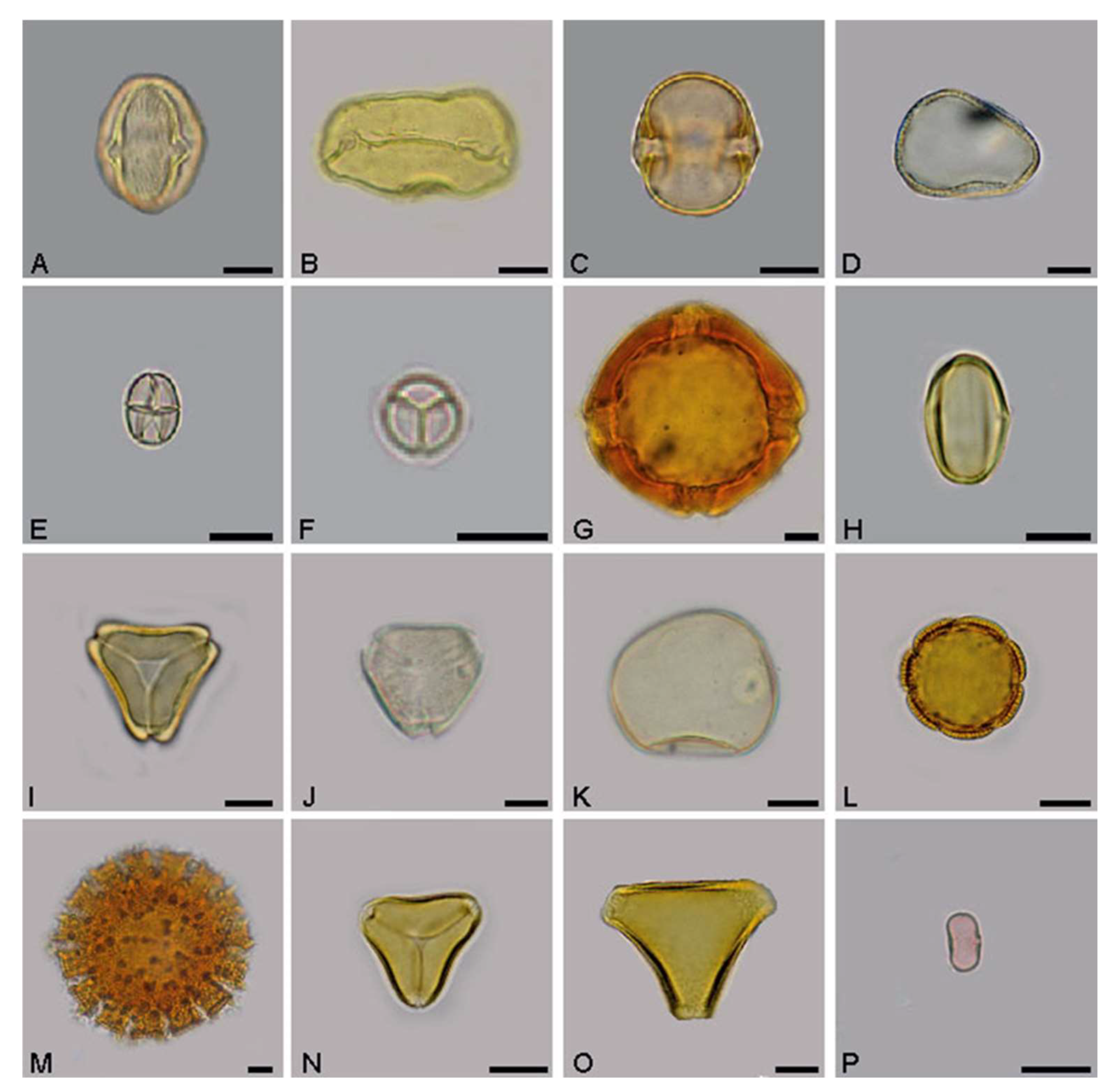
2.2. Palynological Analysis
2.3. Physico-Chemical Analysis
2.4. Preparation of Ethanolic Extracts
2.5. Quantification of Total Phenols and Flavonoid Content
- (a)
- Determination of total phenols
- (b)
- Determination of total flavonoid content
2.6. Antioxidant Activity
- (a)
- Scavenging of DPPH radicals
- (b)
- Reducing power
- (c)
- Discoloration of β-carotene/linoleic acid
2.7. Anti-Inflammatory Activity
2.8. Antimicrobial Activity
2.9. Antimutagenic Activity
2.10. Inhibition of Acetylcholinesterase
2.11. Identification and Quantification of Compounds
3. Results
3.1. Palynological Analysis
3.2. Physicochemical Analysis
3.3. Determination of Phenols
3.4. Biological Activities
3.4.1. Antioxidant Capacity
3.4.2. Anti-Inflammatory Activity
3.4.3. Inhibition of Acetylcholinesterase
3.4.4. Antimicrobial Activity
3.4.5. Antimutagenic Activity
4. Discussion
4.1. Palynological Analysis
4.2. Physicochemical Analysis
4.3. Quantification of Phenolic Compounds
4.4. Antioxidant Activity
4.5. Anti-Inflammatory Activity
4.6. Inhibition of Acetylcholinesterase
4.7. Antimicrobial Activity
4.8. Antimutagenic Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lavinas, F.C.; Macedo, E.H.B.; Sá, G.B.; Amaral, A.C.F.; Silva, J.R.; Azevedo, M.; Vieira, B.A.; Domingos, T.F.S.; Vermelho, A.B.; Carneiro, C.S.; et al. Brazilian stingless bee propolis and geopropolis: Promising sources of biologically active compounds. Rev. Bras. Farmacogn. 2019, 29, 389–399. [Google Scholar] [CrossRef]
- da Silva Cruz, L.F.; de Souza Santos, T.; de Souza, C.O.; dos Santos, L.S.M.; Druzian, J.I.; Tavares, P.P.L.G.; Almeida, L.M.R.; Nascimento, R.Q.; de Andrade Bullos, R.B. Determination of physicochemical characteristics and bioactive compounds in samples of pollen, geopropolis and honey from Melipona Scutellaris bee species. Braz. J. Dev. 2020, 6, 21484–21496. [Google Scholar] [CrossRef]
- Pedro, S.R. The stingless bee fauna in Brazil (Hymenoptera: Apidae). Sociobiology 2014, 61, 348–354. [Google Scholar] [CrossRef]
- Turco, J.F.; Mokochinski, J.B.; Torres, Y.R. Lipidomic analysis of geopropolis of Brazilian stingless bees by LC-HRMS. Food Res. Int. 2023, 167, 112640. [Google Scholar] [CrossRef]
- da Cunha, M.G.; Franchin, M.; de Paula-Eduardo, L.F.; Freires, I.A.; Beutler, J.A.; de Alencar, S.M.; Ikegaki, M.; Tabchoury, C.P.M.; Cunha, T.M.; Rosalen, P.L. Anti-inflammatory and anti-biofilm properties of ent-nemorosone from Brazilian geopropolis. J. Funct. Foods 2016, 26, 27–35. [Google Scholar] [CrossRef]
- Santos, H.F.d.; Campos, J.F.; Santos, C.M.D.; Balestieri, J.B.P.; Silva, D.B.; Carollo, C.A.; De Picoli Souza, K.; Estevinho, L.M.; Dos Santos, E.L. Chemical profile and antioxidant, anti-inflammatory, antimutagenic and antimicrobial activities of geopropolis from the stingless bee Melipona orbignyi. Int. J. Mol. Sci. 2017, 18, 953. [Google Scholar] [CrossRef]
- Dutra, R.P.; Abreu, B.V.D.B.; Cunha, M.S.; Batista, M.C.A.; Torres, L.M.B.; Nascimento, F.R.F.; Ribeiro, M.N.S.; Guerra, R.N.M. Phenolic acids, hydrolyzable tannins, and antioxidant activity of geopropolis from the stingless bee Melipona fasciculata Smith. J. Agric. Food Chem. 2014, 62, 2549–2557. [Google Scholar] [CrossRef]
- Alves de Souza, S.; Camara, C.A.; Monica Sarmento da Silva, E.; Silva, T.M.S. Composition and antioxidant activity of geopropolis collected by Melipona subnitida (Jandaíra) bees. Evid.-Based Complement. Altern. Med. 2013, 2013, 801383. [Google Scholar] [CrossRef]
- Guzmán-Gutiérrez, S.L.; Nieto-Camacho, A.; Castillo-Arellano, J.I.; Huerta-Salazar, E.; Hernández-Pasteur, G.; Silva-Miranda, M.; Argüello-Nájera, O.; Sepúlveda-Robles, O.; Espitia, C.I.; Reyes-Chilpa, R. Mexican propolis: A source of antioxidants and anti-inflammatory compounds, and isolation of a novel chalcone and ε-caprolactone derivative. Molecules 2018, 23, 334. [Google Scholar] [CrossRef]
- Bartolomeu, A.R.; Frión-Herrera, Y.; Da Silva, L.M.; Romagnoli, G.G.; De Oliveira, D.E.; Sforcin, J.M. Combinatorial effects of geopropolis produced by Melipona fasciculata Smith with anticancer drugs against human laryngeal epidermoid carcinoma (HEp-2) cells. Biomed. Pharmacother. 2016, 81, 48–55. [Google Scholar] [CrossRef]
- Rocha, V.M.; Portela, R.D.; dos Anjos, J.P.; de Souza, C.O.; Umsza-Guez, M.A. Stingless bee propolis: Composition, biological activities and its applications in the food industry. Food Prod. Process. Nutr. 2023, 5, 29. [Google Scholar] [CrossRef]
- IBGE. Brazilian Institute of Geography and Statistics; Cities Series; IBGE: Rio de Janeiro, Brazil, 2023.
- Matos, V.R.; Alencar, S.M.; Santos, F.A. Pollen types and levels of total phenolic compounds in propolis produced by Apis mellifera L. (Apidae) in an area of the Semiarid Region of Bahia, Brazil. An. Acad. Bras. Ciênc. 2014, 86, 407–418. [Google Scholar] [CrossRef]
- Santos, F.A.R. Botanical identification of bee pollen. Magistra 2011, 23, 5–9. [Google Scholar]
- Jones, G.D.; Bryant, V.M., Jr. Melissopalynology. In Palynology: Principles and Applications; Jansonius, J., McGregor, P.C., Eds.; AASP—The Palynological Society: Salt Lake City, UT, USA, 1996. [Google Scholar]
- Dias, L.G.; Pereira, A.P.; Estevinho, L.M. Comparative study of different Portuguese samples of propolis: Pollinic, sensorial, physicochemical, microbiological characterization and antibacterial activity. Food Chem. Toxicol. 2012, 50, 4246–4253. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.; Rodrigues, S.; Feás, X.; Estevinho, L.M. Antimicrobial activity, phenolic profile and role in the inflammation of propolis. Food Chem. Toxicol. 2012, 50, 1790–1795. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.L.; Cury, J.A.; Rosalen, P.L.; Alencar, S.M.; Ikegaki, M.; Duarte, S.; Koo, H. Propolis from southeastern and northeastern of Brazil: The influence of seasonality in antibacterial activity and phenolic composition. Quim. Nova 2007, 30, 1512–1516. [Google Scholar] [CrossRef]
- Morais, M.; Moreira, L.; Feás, X.; Estevinho, L.M. Honeybee-collected pollen from five Portuguese Natural Parks: Palynological origin, phenolic content, antioxidant properties and antimicrobial activity. Food Chem. Toxicol. 2011, 49, 1096–1101. [Google Scholar] [CrossRef]
- Moreira, L.; Dias, L.G.; Pereira, J.A.; Estevinho, L.M. Antioxidant properties, total phenols and pollen analysis of propolis samples from Portugal. Food Chem. Toxicol. 2008, 46, 3482–3485. [Google Scholar] [CrossRef]
- Ahn, M.R.; Kumazawa, S.; Hamasaka, T.; Bang, K.S.; Nakayama, T. Antioxidant activity and constituents of propolis collected in various areas of Korea. J. Agric. Food Chem. 2004, 52, 7286–7292. [Google Scholar] [CrossRef]
- Sahasrabudhe, A.; Deodhar, M. Anti-hyaluronidase, anti-elastase activity of Garcinia indica. Int. J. Bot. 2010, 6, 299–303. [Google Scholar] [CrossRef]
- Pascoal, A.; Rodrigues, S.; Teixeira, A.; Feás, X.; Estevinho, L.M. Biological activities of commercial bee pollens: Antimicrobial, antimutagenic, antioxidant and anti-inflammatory. Food Chem. Toxicol. 2014, 63, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Mata, A.T.; Proença, C.; Ferreira, A.R.; Serralheiro, M.L.M.; Nogueira, J.M.F.; Araújo, M.E.M. Antioxidant and antiacetylcholinesterase activities of five plants used as Portuguese food spices. Food Chem. 2007, 103, 778–786. [Google Scholar] [CrossRef]
- Nastić, N.; Švarc-Gajić, J.; Delerue-Matos, C.; Barroso, M.F.; Soares, C.; Moreira, M.M.; Morais, S.; Mašković, P.; Srček, V.G.; Slivac, I.; et al. Subcritical water extraction as an environmentally-friendly technique to recover bioactive compounds from traditional Serbian medicinal plants. Ind. Crop. Prod. 2018, 111, 579–589. [Google Scholar] [CrossRef]
- Rubilar, M.; Pinelo, M.; Shene, C.; Sineiro, J.; Nuñez, M.J. Separation and HPLC-MS identification of phenolic antioxidants from agricultural residues: Almond hulls and grape pomace. J. Agric. Food Chem. 2007, 55, 10101–10109. [Google Scholar] [CrossRef]
- Oliveira, R.C.; Contrera, F.A.L.; Arruda, H.; Jaffe, R.; Costa, L.; Pessin, G.; Venturieri, G.C.; De Souza, P.; Imperatriz-Fonseca, V.L. Foraging and drifting patterns of the highly eusocial neotropical stingless bee Melipona fasciculata assessed by radio-frequency identification tags. Front. Ecol. Evol. 2021, 9, 708178. [Google Scholar] [CrossRef]
- Shanahan, M.; Spivak, M. Resin use by stingless bees: A review. Insects 2021, 12, 719. [Google Scholar] [CrossRef]
- Campos, J.F.; Santos, U.P.D.; Rocha, P.D.S.D.; Damião, M.J.; Balestieri, J.B.P.; Cardoso, C.A.L.; Paredes-Gamero, E.J.; Estevinho, L.M.; de Picoli Souza, K.; Santos, E.L.D. Antimicrobial, antioxidant, anti-inflammatory, and cytotoxic activities of propolis from the stingless bee Tetragonisca fiebrigi (Jataí). Evid.-Based Complement. Alternat. Med. 2015, 2015, 296186. [Google Scholar] [CrossRef]
- Barros, M.H.M.R.; Luz, C.F.P.D.; Albuquerque, P.M.C.D. Pollen analysis of geopropolis of Melipona (Melikerria) fasciculata Smith, 1854 (Meliponini, Apidae, Hymenoptera) in areas of Restinga, Cerrado and flooded fields in the state of Maranhão, Brazil. Grana 2013, 52, 81–92. [Google Scholar] [CrossRef]
- Barth, O.M.; Luz, C.F.P.D. Palynological analysis of Brazilian geopropolis sediments. Grana 2003, 42, 121–127. [Google Scholar] [CrossRef]
- Araújo, C.V.; Alves, L.D.J.; Santos, O.M.; Alves, J.M. Arbuscular mycorrhiza in Eucalyptus cloeziana F. Muell plantations on the north coast of Bahia, Brasil. Acta Bot. Bras. 2004, 18, 513–520. [Google Scholar] [CrossRef]
- Sawaya, A.C.H.F.; Calado, J.C.P.; Santos, L.D.; Marcucci, M.C.; Akatsu, I.P.; Soares, A.E.E.; Eberlin, M.N. Composition and antioxidant activity of propolis from three species of Scaptotrigona stingless bees. J. ApiProd. ApiMed. Sci. 2009, 1, 37–42. [Google Scholar] [CrossRef]
- Oliveira, M.S. Evaluation of Three Physical-Chemical Quality Parameters in Propolis and Geopropoly Samples from Native Amazon Stingless Bees. Agroecol. Noteb. 2016, 10. [Google Scholar]
- Pereira, L.R.L.; Salatino, M.L.F.; Salatino, A. Production of propolis and geopropolis by stingless bees. MOJ Food Process Technol. 2020, 8, 1–3. [Google Scholar] [CrossRef]
- Ferreira, B.L.; Gonzaga, L.V.; Vitali, L.; Micke, G.A.; Baggio, D.; de Oliveira Costa, A.C.; Fett, R. Dataset about Southern-Brazilian geopropolis: Physical and chemical perspectives. Data Brief 2020, 29, 105109. [Google Scholar] [CrossRef] [PubMed]
- Araújo, K.S.D.S.; Santos Júnior, J.F.D.; Sato, M.O.; Finco, F.D.B.A.; Soares, I.M.; Barbosa, R.D.S.; Alvim, T.d.C.; Ascêncio, S.D.; Mariano, S.M.B. Physicochemical properties and antioxidant capacity of propolis of stingless bees (Meliponinae) and Apisfrom two regions of Tocantins, Brazil. Acta Amaz. 2016, 46, 61–68. [Google Scholar] [CrossRef]
- Cardozo, D.V.; Mokochinski, J.B.; Machado, C.S.; Sawaya, A.C.H.F.; Caetano, I.K.; Felsner, M.L.; Torres, Y.R. Chemical variability of geopropolis produced by Jataí, Mandaçaia and Mandurí stingless bees. Rev. Virtual Quim. 2015, 7, 2456–2474. [Google Scholar] [CrossRef]
- Araújo, M.J.A.M.; Búfalo, M.C.; Conti, B.J.; Fernandes, A.R.Y., Jr.; Trusheva, B.; Bankova, V.; Sforcin, J.M. The chemical composition and pharmacological activities of geopropolis produced by Melipona fasciculata Smith in Northeast Brazil. J. Mol. Pathophysiol. 2015, 4, 12–20. [Google Scholar] [CrossRef]
- Oliveira, L.P.G.; Conte, F.L.; de Oliveira Cardoso, E.; Conti, B.J.; Santiago, K.B.; de Assis Golim, M.; Feltran, G.d.S.; Zambuzzi, W.F.; Sforcin, J.M. A new chemotherapeutic approach using doxorubicin simultaneously with geopropolis favoring monocyte functions. Life Sci. 2019, 217, 81–90. [Google Scholar] [CrossRef]
- Júnior, U.P.S.; Cabrera, S.P.; da Silva, T.M.G.; da Silva, E.M.S.; Camara, C.A.; Silva, T.M.S. Geopropolis gel for the adjuvant treatment of candidiasis-formulation and in vitro release assay. Rev. Bras. Farmacogn. 2019, 29, 278–286. [Google Scholar] [CrossRef]
- Dutra, R.P. Physical-Chemical Characteristics of Geopropolis from Melipona fasciculata Smith (tiúba) Produced in the State of Maranhão. Master’s Thesis, Federal University of Maranhão, São Luis, Brazil, 2006. [Google Scholar]
- Righi, A.A.; Negri, G.; Salatino, A. Comparative chemistry of propolis from eight Brazilian localities. Evid.-Based Complement. Alternat. Med. 2013, 2013, 267878. [Google Scholar] [CrossRef]
- Kasote, D.M.; Pawar, M.V.; Gundu, S.S.; Bhatia, R.; Nandre, V.S.; Jagtap, S.D.; Mahajan, S.G.; Kulkarni, M.V. Chemical profiling, antioxidant, and antimicrobial activities of Indian stingless bees propolis samples. J. Apic. Res. 2019, 58, 617–625. [Google Scholar] [CrossRef]
- Velikova, M.; Bankova, V.; Tsvetkova, I.; Kujumgiev, A.; Marcucci, M.C. Antibacterial ent-kaurene from Brazilian propolis of native stingless bees. Fitoterapia 2000, 71, 693–696. [Google Scholar] [CrossRef]
- Giannuzzo, A.N.; Nazareno, M.A.; Mishima, H.T.; López, D.E.; Mishima, B.A. Naringin extraction of Citrus paradisi L. comparative study and optimization of extractives techniques. Food Sci. Technol. 2000, 20, 257–261. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.M.; Fernandes-Silva, C.C.; Salatino, A.; Negri, G. Antioxidant activity of a geopropolis from northeast Brazil: Chemical characterization and likely botanical origin. Evid.-Based Complement. Alternat. Med. 2017, 2017, 4024721. [Google Scholar] [CrossRef]
- Alves, C.Q.; Brandão, H.N.; David, J.M.; David, J.P.; Lima, L.D.S. Evaluation of the antioxidant activity of flavonoids. Dialogues Sci. 2007, 12, 1–8. [Google Scholar]
- Duarte-Almeida, J.M.; Santos, R.J.D.; Genovese, M.I.; Lajolo, F.M. Assessment of antioxidant activity using the beta-carotene/linoleic acid system and DPPH radical scavenging method. Food Sci. Technol. 2006, 26, 446–452. [Google Scholar] [CrossRef]
- Batista, M.C.A.; Abreu, B.V.D.B.; Dutra, R.P.; Cunha, M.S.; Amaral, F.M.M.D.; Torres, L.M.B.; Ribeiro, M.N.D.S. Chemical composition and antioxidant activity of geopropolis produced by Melipona fasciculata (Meliponinae) in flooded fields and cerrado areas of Maranhão State, northeastern Brazil. Acta Amaz. 2016, 46, 315–322. [Google Scholar] [CrossRef]
- Liberio, S.A.; Pereira, A.L.A.; Dutra, R.P.; Reis, A.S.; Araújo, M.J.A.; Mattar, N.S.; Silva, L.A.; Ribeiro, M.N.; Nascimento, F.R.; Guerra, R.N.; et al. Antimicrobial activity against oral pathogens and immunomodulatory effects and toxicity of geopropolis produced by the stingless bee Melipona fasciculata Smith. BMC Complement. Altern. Med. 2011, 11, 108. [Google Scholar] [CrossRef]
- da Cunha, M.G.; Franchin, M.; Galvão, L.; de Ruiz, A.; de Carvalho, J.E.; Ikegaki, M.; de Alencar, S.M.; Koo, H.; Rosalen, P.L. Antimicrobial and antiproliferative activities of stingless bee Melipona scutellaris geopropolis. BMC Complement. Altern. Med. 2013, 13, 23. [Google Scholar] [CrossRef]
- Valcanaia, C.P.; Masote, J.B.B.; Sommer, H.F.; Schiquet, S.; Padilha, B.; Krepsky, L.; Paganelli, C.J.; Borges, P.P.; Danielli, L.J.; Apel, M.A.; et al. Antimicrobial Activity of Volatile Oils from Brazilian Stingless Bees Melipona quadrifasciata quadrifasciata and Tetragonisca angustula Propolis. Chem. Biodivers. 2022, 19, e202200369. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Luo, W.; Sun, D.; Bi, X.; Zeng, X.; Xiao, G.; Xu, A.; Chen, W.; Jiang, J.; Li, X.; et al. Selection and evaluation of quality control markers in propolis based on its hyperlipidemia therapy via regulating PXR/CYP3A4 expression. Phytomedicine Plus 2021, 1, 100006. [Google Scholar] [CrossRef]
- Costa, G.; Francisco, V.; Lopes, M.C.; Cruz, M.T.; Batista, M.T. Intracellular signaling pathways modulated by phenolic compounds: Application for new anti-inflammatory drugs discovery. Curr. Med. Chem. 2012, 19, 2876–2900. [Google Scholar] [CrossRef] [PubMed]
| ASamples | pH | Conductivity (mS/cm) | Moisture (%) | Ash (%) | Waxes (%) | Phenolic Compounds (%) | Flavonoid Compounds (%) |
|---|---|---|---|---|---|---|---|
| Rainy geopropolis (summer) * | 4.110 ± 0.060 a | 36.000 ± 0.000 a | 2.630 ± 0.070 a | 76.130 ± 0.860 a | 3.370 ± 0.290 a | 25.130 ± 0.140 a | 3.920 ± 0.090 a |
| Dry geopropolis (winter) * | 4.200 ± 0.040 a | 34.000 ± 0.820 b | 2.660 ± 0.020 a | 76.340 ± 0.070 a | 3.170 ± 0.720 a | 19.30 ± 0.150 b | 2.090 ± 0.370 b |
| Compound | Retention Time (min) | Rainy Geopropolis | Dry Geopropolis |
|---|---|---|---|
| gallic acid | 7.14 | 5.820 | 5.840 |
| protocatechuic acid | 12.74 | 2.530 | 2.460 |
| Catechin | 18.30 | 8.390 | 2.640 |
| vanillic acid | 22.79 | 2.430 | ND |
| caffeic acid | 24.40 | ND | ND |
| Epicatechin | 25.38 | 1.720 | 1.320 |
| p-coumaric acid | 31.48 | <LOQ | <LOD |
| ferulic acid | 34.38 | ND | ND |
| Naringin | 42.17 | 45.390 | 29.870 |
| Rutin | 45.56 | ND | ND |
| cinnamic acid | 49.71 | 2.540 | <LOD |
| Naringenin | 55.25 | 2.110 | <LOQ |
| Quercetin | 54.07 | ND | ND |
| Scavenging of DPPH Radicals—IC50 (mg/mL) | Reducing Power—IC50 (mg/mL) | β-Carotene/Linoleic Acid (%) | Hyaluronidase (30 mg/mL) (%) | Acetylcholinesterase—IC50 (µg/mL) | |
|---|---|---|---|---|---|
| Rainy Geopropolis * | 0.058 ± 0.005 a | 0.310 ± 0.008 a | 89.52 ± 0.57 a | 30.95 ± 1.28 a | 0.28 ± 0.01 b |
| Dry Geopropolis * | 0.064 ± 0.004 a | 0.220 ± 0.001 b | 75.54 ± 1.10 b | 26.62 ± 0.90 b | 0.354 ± 0.006 a |
| Ascorbic acid | 0.015 ± 0.001 | 0.074 ± 0.013 | - | - | - |
| BHA | - | - | 92.450 ± 0.001 | - | |
| Eserine | 0.005 ± 0.001 |
| Microorganisms | Rainy Geopropolis (mg/mL) | Dry Geopropolis (mg/mL) | Gentamicin (mg/mL) | Amphoterecin (mg/mL) | ||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |||
| Gram-positive bacteria | ||||||
| Staphylococcus aureus ATCC 4330 | 0.05 ± 0.12 | 2.01 ± 0.07 | 0.10 ± 0.02 | 1.25 ± 0.06 | 0.03 ± 0.01 | * |
| Staphylococcus aureus ESA 321 | 0.05 ± 0.02 | 2.25 ± 0.01 | 0.12 ± 0.01 | 1.75 ± 0.01 | 0.13 ± 0.02 | * |
| Gram-negative bacteria | ||||||
| Proteus mirabilis ATCC 25933 | 1.00 ± 0.03 | 1.50 ± 0.03 | 0.50 ± 0.15 | 1.34 ± 0.04 | 0.06 ± 0.01 | * |
| Proteus mirabilis ESA 229 | 0.07 ± 0.01 | 1.76 ± 0.07 | 0.10 ± 0.05 | 1.12 ± 0.01 | 0.005 ± 0.001 | * |
| Fungi | ||||||
| Candida albicans ATCC 10231 | 1.35 ± 0.01 | 5.01 ± 0.17 | 2.5 ± 0.04 | 5.00 ± 0.06 | * | 0.002 ± 0.001 |
| Candida albicans ESA 115 | 2.50 ± 0.11 | 5.22 ± 0.02 | 5.00 ± 0.01 | 5.00 ± 0.04 | * | 0.021 ± 0.008 |
| Sample | Treatment | Survivals (%) | Gene Conversion Colonies/105 | Mutant Colonies/106 | |
|---|---|---|---|---|---|
| [Extract] (mg/mL) | EMS (mg/mL) | ||||
| Dry geopropolis | 0 | 5.0 | 88.18 ± 1.60 | 51.55 ± 0.60 a | 380.56 ± 7.67 |
| 2.5 | 5.0 | 36.24 ± 2.72 | 37.33 ± 1.27 b | 323.07 ± 1.36 | |
| 5.0 | 5.0 | 28.84 ± 2.19 | 31.07 ± 0.98 c | 302.58 ± 3.43 | |
| Rainy geopropolis | 0 | 5.0 | 88.59 ± 1.71 | 51.34 ± 0.63 d | 404.04 ± 9.46 |
| 2.5 | 5.0 | 30.64 ± 2.38 | 43.82 ± 3.95 e | 354.95 ± 17.78 | |
| 5.0 | 5.0 | 23.01 ± 2.05 | 37.21 ± 1.87 f | 311.88 ± 1.52 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coutinho, S.; Matos, V.; Seixas, N.; Rodrigues, H.; Paula, V.B.; Freitas, L.; Dias, T.; Santos, F.d.A.R.; Dias, L.G.; Estevinho, L.M. Melipona scutellaris Geopropolis: Chemical Composition and Bioactivity. Microorganisms 2023, 11, 2779. https://doi.org/10.3390/microorganisms11112779
Coutinho S, Matos V, Seixas N, Rodrigues H, Paula VB, Freitas L, Dias T, Santos FdAR, Dias LG, Estevinho LM. Melipona scutellaris Geopropolis: Chemical Composition and Bioactivity. Microorganisms. 2023; 11(11):2779. https://doi.org/10.3390/microorganisms11112779
Chicago/Turabian StyleCoutinho, Sónia, Vanessa Matos, Natália Seixas, Hellen Rodrigues, Vanessa B. Paula, Lais Freitas, Teresa Dias, Francisco de Assis Ribeiro Santos, Luís G. Dias, and Letícia M. Estevinho. 2023. "Melipona scutellaris Geopropolis: Chemical Composition and Bioactivity" Microorganisms 11, no. 11: 2779. https://doi.org/10.3390/microorganisms11112779
APA StyleCoutinho, S., Matos, V., Seixas, N., Rodrigues, H., Paula, V. B., Freitas, L., Dias, T., Santos, F. d. A. R., Dias, L. G., & Estevinho, L. M. (2023). Melipona scutellaris Geopropolis: Chemical Composition and Bioactivity. Microorganisms, 11(11), 2779. https://doi.org/10.3390/microorganisms11112779











