Realities, Challenges and Benefits of Antimicrobial Stewardship in Dairy Practice in the United States
Abstract
:1. Introduction
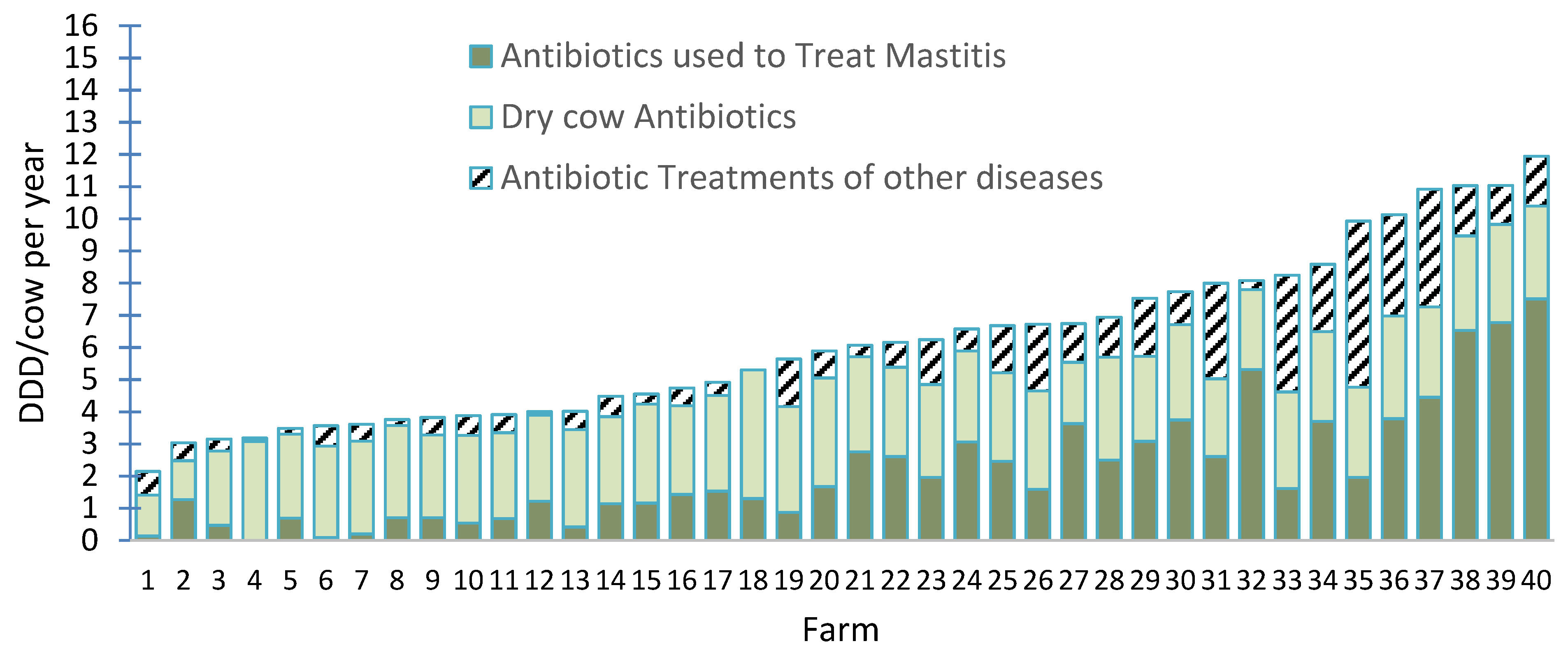
2. Occurrence of Disease and Use of Antimicrobials on Dairy Farms
3. Challenges of Implementing Antimicrobial Stewardship in Dairy Practice
4. Defining and Implementing Antimicrobial Stewardship on Dairy Farms
5. Initiating Antimicrobial Stewardship on Dairy Farms
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hoelzer, K.; Wong, N.; Thomas, J.; Talkington, K.; Jungman, E.; Coukell, A. Antimicrobial drug use in food-producing animals and associated human health risks: What, and how strong, is the evidence? BMC Vet. Res. 2017, 13, 211. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.L.; Caffrey, N.P.; Nóbrega, D.B.; Cork, S.C.; Ronksley, P.E.; Barkema, H.W.; Polachek, A.J.; Ganshorn, H.; Sharma, N.; Kellner, J.D.; et al. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: A systematic review and meta-analysis. Lancet Planet. Health 2017, 1, e316–e327. [Google Scholar] [CrossRef]
- Nobrega, D.B.; Tang, K.L.; Caffrey, N.P.; De Buck, J.; Cork, S.C.; Ronksley, P.E.; Polachek, A.J.; Ganshorn, H.; Sharma, N.; Kastelic, J.P.; et al. Prevalence of antimicrobial resistance genes and its association with restricted antimicrobial use in food-producing animals: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2021, 76, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Ruegg, P.L. Minimizing development of antimicrobial resistance on dairy farms through appropriate use of antibiotics for treatment of mastitis. In Achieving Sustainable Production of Cow’s Milk; Van Belzen, N., Ed.; Burleigh Dodds: Belgium, 2017; Volume 2. [Google Scholar]
- Wemette, M.; Greiner Safi, A.; Wolverton, A.K.; Beauvais, W.; Shapiro, M.; Moroni, P.; Welcome, F.; Ivanek, R. Public perceptions of antibiotic use on dairy farms in the United States. J. Dairy Sci. 2021, 104, 2807–2821. [Google Scholar] [CrossRef] [PubMed]
- Hoe, F.G.; Ruegg, P.L. Opinions and practices of wisconsin dairy producers about biosecurity and animal well-being. J. Dairy Sci. 2006, 89, 2297–2308. [Google Scholar] [CrossRef]
- Cobo-Angel, C.; LeBlanc, S.J.; Roche, S.M.; Ritter, C. A Focus Group Study of Canadian Dairy Farmers’ Attitudes and Social Referents on Antimicrobial Use and Antimicrobial Resistance. Front. Vet. Sci. 2021, 8, 645221. [Google Scholar] [CrossRef]
- Wemette, M.; Safi, A.G.; Beauvais, W.; Ceres, K.; Shapiro, M.; Moroni, P.; Welcome, F.L.; Ivanek, R. New York State dairy farmers’ perceptions of antibiotic use and resistance: A qualitative interview study. PLoS ONE 2020, 15, e0232937. [Google Scholar] [CrossRef]
- FDA. GFI#209. The Judicious Use of Medically Important Antmicrobial Drugs in Food-Producing Animals; FDA: Silver Spring, MD, USA, 2012.
- AVMA. Antmicrobial Stewardship Definition and Core Principles. Available online: https://www.avma.org/KB/Policies/Pages/Antimicrobial-Stewardship-Definition-and-Core-Principles.aspx (accessed on 17 June 2022).
- USDA. Health and Management Practices on U.S. Dairy Operations, 2014; USDA: Fort Collins, CO, USA, 2018.
- Goncalves, J.L.; de Campos, J.L.; Steinberger, A.; Safdar, N.; Sethi, A.; Shutske, J.; Rodriguez, Z.; Suen, G.; Goldberg, T.; Ruegg, P.L. Outcomes of first cases of selected bovine disease on 37 large WI dairy farms. In National Mastitis Council; NMC: San Diego, CA, USA, 2022. [Google Scholar]
- USDA. Dairy 2014, Milk Quality, Milking Procedures and Mastitis in the United States, 2014; USDA: Fort Collins, CO, USA, 2016.
- de Campos, J.L.; Kates, A.; Steinberger, A.; Sethi, A.; Suen, G.; Shutske, J.; Safdar, N.; Goldberg, T.; Ruegg, P.L. Quantification of antimicrobial usage in adult cows and preweaned calves on 40 large Wisconsin dairy farms using dose-based and mass-based metrics. J. Dairy Sci. 2021, 104, 4727–4745. [Google Scholar] [CrossRef]
- Anonymous. Critically Important Antimcrobials for Human Health, 6th Revision; WHO: Geneva, Switzerland, 2019.
- FDA. 2020 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals; FDA: Silver Spring, MD, USA, 2021.
- Schrag, N.F.D.; Godden, S.M.; Apley, M.D.; Singer, R.S.; Lubbers, B.V. Antimicrobial use quantification in adult dairy cows—Part 3—Use measured by standardized regimens and grams on 29 dairies in the United States. Zoonoses Public Health 2020, 67 (Suppl. 1), 82–93. [Google Scholar] [CrossRef]
- Scott, H.M.; Acuff, G.; Bergeron, G.; Bourassa, M.W.; Gill, J.; Graham, D.W.; Kahn, L.H.; Morley, P.S.; Salois, M.J.; Simjee, S.; et al. Critically important antibiotics: Criteria and approaches for measuring and reducing their use in food animal agriculture. Ann. N. Y. Acad. Sci. 2019, 1441, 8–16. [Google Scholar] [CrossRef]
- Sato, T.; Okubo, T.; Usui, M.; Yokota, S.; Izumiyama, S.; Tamura, Y. Association of veterinary third-generation cephalosporin use with the risk of emergence of extended-spectrum-cephalosporin resistance in Escherichia coli from dairy cattle in Japan. PLoS ONE 2014, 9, e96101. [Google Scholar] [CrossRef] [PubMed]
- Awosile, B.B.; McClure, J.T.; Sanchez, J.; VanLeeuwen, J.; Rodriguez-Lecompte, J.C.; Keefe, G.; Heider, L.C. Short communication: Extended-spectrum cephalosporin-resistant Escherichia coli in colostrum from New Brunswick, Canada, dairy cows harbor blaCMY-2 and blaTEM resistance genes. J. Dairy Sci. 2017, 100, 7901–7905. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.A.; Ossa-Trujillo, C.; Vinasco, J.; Jordan, E.R.; Garcia Buitrago, J.A.; Hagevoort, R.; Norman, K.N.; Lawhon, S.D.; Pineiro, J.M.; Levent, G.; et al. Use of critically important antimicrobial classes early in life may adversely impact bacterial resistance profiles during adult years: Potential co-selection for plasmid-borne fluoroquinolone and macrolide resistance via extended-spectrum beta-lactam use in dairy cattle. Lett. Appl. Microbiol. 2021, 72, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Koops, W.J.; Ekstrom, J.; Armstrong, D. 5.8 Report on Practical Strategies to Reduce Antimicrobial Use in Dairy Farming; EuroDairy, 2018. [Google Scholar]
- Lam, T.; Heuvelink, A.E.; Gonggrijp, M.A.; Santman-Berends, I. Antimicrobial use in dairy cattle in the Netherlands. J. Anim. Sci. 2020, 98, S9–S14. [Google Scholar] [CrossRef]
- Ruegg, P.L. Practical approaches to mastitis therapy on large dairy herds. In Large Dairy Herd Management, 3rd ed.; Hogan, J., Ed.; American Dairy Science Association: Champaign, IL, USA, 2017. [Google Scholar]
- Ruegg, P.L. Making Antibiotic Treatment Decisions for Clinical Mastitis. Vet. Clin. N. Am. Food Anim. Pract. 2018, 34, 413–425. [Google Scholar] [CrossRef]
- Ruegg, P.L.; Erskine, R.D. Mammary Gland Health and Disorders. In Large Animal Veterinary Internal Medicine, 6th ed.; Smith, B.P., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1018–1150. [Google Scholar]
- Oliveira, L.; Hulland, C.; Ruegg, P.L. Characterization of clinical mastitis occurring in cows on 50 large dairy herds in Wisconsin. J. Dairy Sci. 2013, 96, 7538–7549. [Google Scholar] [CrossRef]
- Fuenzalida, M.J.; Ruegg, P.L. Negatively controlled, randomized clinical trial to evaluate intramammary treatment of nonsevere, gram-negative clinical mastitis. J. Dairy Sci. 2019, 102, 5438–5457. [Google Scholar] [CrossRef]
- Fuenzalida, M.J.; Ruegg, P.L. Negatively controlled, randomized clinical trial to evaluate use of intramammary ceftiofur for treatment of nonsevere culture-negative clinical mastitis. J. Dairy Sci. 2019, 102, 3321–3338. [Google Scholar] [CrossRef]
- Ruegg, P.L. What Is Success? A Narrative Review of Research Evaluating Outcomes of Antibiotics Used for Treatment of Clinical Mastitis. Front. Vet. Sci. 2021, 8, 639641. [Google Scholar] [CrossRef]
- Nobrega, D.B.; Naqvi, S.A.; Dufour, S.; Deardon, R.; Kastelic, J.P.; De Buck, J.; Barkema, H.W. Critically important antimicrobials are generally not needed to treat nonsevere clinical mastitis in lactating dairy cows: Results from a network meta-analysis. J. Dairy Sci. 2020, 103, 10585–10603. [Google Scholar] [CrossRef]
- Oliveira, L.; Ruegg, P.L. Treatments of clinical mastitis occurring in cows on 51 large dairy herds in Wisconsin. J. Dairy Sci. 2014, 97, 5426–5436. [Google Scholar] [CrossRef]
- Pinzon-Sanchez, C.; Cabrera, V.E.; Ruegg, P.L. Decision tree analysis of treatment strategies for mild and moderate cases of clinical mastitis occurring in early lactation. J. Dairy Sci. 2011, 94, 1873–1892. [Google Scholar] [CrossRef] [PubMed]
- Kolar, Q.K.; Godden, S.M.; Ruegg, P.L. Results from a Negatively-Controlled Randomized Clinical Trial Evaluating Antimicrobial Treatments for Mastitis Caused by Gram-Positive Pathogens; American Dairy Science Association: Champaign, IL, USA, 2021; p. 398. [Google Scholar]
- Rowe, S.M.; Godden, S.M.; Nydam, D.V.; Gorden, P.J.; Lago, A.; Vasquez, A.K.; Royster, E.; Timmerman, J.; Thomas, M.J. Randomized controlled non-inferiority trial investigating the effect of 2 selective dry-cow therapy protocols on antibiotic use at dry-off and dry period intramammary infection dynamics. J. Dairy Sci. 2020, 103, 6473–6492. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.M.; Godden, S.M.; Nydam, D.V.; Gorden, P.J.; Lago, A.; Vasquez, A.K.; Royster, E.; Timmerman, J.; Thomas, M.J. Randomized controlled trial investigating the effect of 2 selective dry-cow therapy protocols on udder health and performance in the subsequent lactation. J. Dairy Sci. 2020, 103, 6493–6503. [Google Scholar] [CrossRef] [PubMed]
- Alzayn, M.; Findlay, J.; Schubert, H.; Mounsey, O.; Gould, V.C.; Heesom, K.J.; Turner, K.M.; Barrett, D.C.; Reyher, K.K.; Avison, M.B. Characterization of AmpC-hyperproducing Escherichia coli from humans and dairy farms collected in parallel in the same geographical region. J. Antimicrob. Chemother. 2020, 75, 2471–2479. [Google Scholar] [CrossRef]
- Haimerl, P.; Arlt, S.; Borchardt, S.; Heuwieser, W. Antibiotic treatment of metritis in dairy cows—A meta-analysis. J. Dairy Sci. 2017, 100, 3783–3795. [Google Scholar] [CrossRef]
- Gonzalez, S.M.; Steiner, A.; Gassner, B.; Regula, G. Antimicrobial use in Swiss dairy farms: Quantification and evaluation of data quality. Prev. Vet. Med. 2010, 95, 50–63. [Google Scholar] [CrossRef]
- Hyde, R.M.; Remnant, J.G.; Bradley, A.J.; Breen, J.E.; Hudson, C.D.; Davies, P.L.; Clarke, T.; Critchell, Y.; Hylands, M.; Linton, E.; et al. Quantitative analysis of antimicrobial use on British dairy farms. Vet. Rec. 2017, 181, 683. [Google Scholar] [CrossRef] [PubMed]
- Laroche, J.; Ferrouillet, C.; DesCoteaux, L. Analysis of antimicrobial sales data of the main distributor in Quebec from 2016 to 2019: An estimate of usage in dairy cattle, horses, and small animals. Can. Vet. J. 2022, 63, 379–385. [Google Scholar]
- Mills, H.L.; Turner, A.; Morgans, L.; Massey, J.; Schubert, H.; Rees, G.; Barrett, D.; Dowsey, A.; Reyher, K.K. Evaluation of metrics for benchmarking antimicrobial use in the UK dairy industry. Vet. Rec. 2018, 182, 379. [Google Scholar] [CrossRef] [PubMed]
- More, S.J.; Clegg, T.A.; McCoy, F. The use of national-level data to describe trends in intramammary antimicrobial usage on Irish dairy farms from 2003 to 2015. J. Dairy Sci. 2017, 100, 6400–6413. [Google Scholar] [CrossRef] [PubMed]
- Schrag, N.F.D.; Apley, M.D.; Godden, S.M.; Lubbers, B.V.; Singer, R.S. Antimicrobial use quantification in adult dairy cows—Part 1—Standardized regimens as a method for describing antimicrobial use. Zoonoses Public Health 2020, 67 (Suppl. 1), 51–68. [Google Scholar] [CrossRef] [PubMed]
- Redding, L.E.; Bender, J.; Baker, L. Quantification of antibiotic use on dairy farms in Pennsylvania. J. Dairy Sci. 2019, 102, 1494–1507. [Google Scholar] [CrossRef]
- Jensen, V.F.; Jacobsen, E.; Bager, F. Veterinary antimicrobial-usage statistics based on standardized measures of dosage. Prev. Vet. Med. 2004, 64, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Pol, M.; Ruegg, P.L. Treatment practices and quantification of antimicrobial drug usage in conventional and organic dairy farms in Wisconsin. J. Dairy Sci. 2007, 90, 249–261. [Google Scholar] [CrossRef]
- Saini, V.; McClure, J.T.; Leger, D.; Dufour, S.; Sheldon, A.G.; Scholl, D.T.; Barkema, H.W. Antimicrobial use on Canadian dairy farms. J. Dairy Sci. 2012, 95, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Pereyra, V.; Pol, M.; Pastorino, F.; Herrero, A. Quantification of antimicrobial usage in dairy cows and preweaned calves in Argentina. Prev. Vet. Med. 2015, 122, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, A.; Koops, W.J.; Wemmenhove, H. Antibiotic use in dairy herds in the Netherlands from 2005 to 2012. J. Dairy Sci. 2016, 99, 1632–1648. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.; Piepers, S.; Supre, K.; Dewulf, J.; De Vliegher, S. Quantification of antimicrobial consumption in adult cattle on dairy herds in Flanders, Belgium, and associations with udder health, milk quality, and production performance. J. Dairy Sci. 2016, 99, 2118–2130. [Google Scholar] [CrossRef]
- USDA. Dairy 2014. Dairy Cattle Managment Practices in the U.S., 2014; USDA: Fort Collins, CO, USA, 2017.
- Moore, D.A.; McConnel, C.S.; Busch, R.; Sischo, W.M. Dairy veterinarians’ perceptions and experts’ opinions regarding implementation of antimicrobial stewardship on dairy farms in the western United States. J. Am. Vet. Med. Assoc. 2021, 258, 515–526. [Google Scholar] [CrossRef]
- Richert, R.M.; Cicconi, K.M.; Gamroth, M.J.; Schukken, Y.H.; Stiglbauer, K.E.; Ruegg, P.L. Management factors associated with veterinary usage by organic and conventional dairy farms. JAVMA-J. Am. Vet. Med. A 2013, 242, 1732–1743. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, E.M.; Ekong, P.S.; Okello, E.; Williams, D.R.; Karle, B.M.; Rowe, J.D.; Marshall, E.S.; Lehenbauer, T.W.; Aly, S.S. 2019 Survey of Antimicrobial Drug Use and Stewardship Practices in Adult Cows on California Dairies: Post Senate Bill 27. Microorganisms 2021, 9, 1507. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.J.; Marier, E.A.; Tranter, R.B.; Wu, G.; Watson, E.; Teale, C.J. Factors affecting dairy farmers’ attitudes towards antimicrobial medicine usage in cattle in England and Wales. Prev. Vet. Med. 2015, 121, 30–40. [Google Scholar] [CrossRef]
- Gozdzielewska, L.; King, C.; Flowers, P.; Mellor, D.; Dunlop, P.; Price, L. Scoping review of approaches for improving antimicrobial stewardship in livestock farmers and veterinarians. Prev. Vet. Med. 2020, 180, 105025. [Google Scholar] [CrossRef]
- Golding, S.E.; Ogden, J.; Higgins, H.M. Shared Goals, Different Barriers: A Qualitative Study of UK Veterinarians’ and Farmers’ Beliefs about Antimicrobial Resistance and Stewardship. Front. Vet. Sci 2019, 6, 132. [Google Scholar] [CrossRef]
- Vaarst, M.; Paarup-Laursen, B.; Houe, H.; Fossing, C.; Andersen, H.J. Farmers’ choice of medical treatment of mastitis in Danish dairy herds based on qualitative research interviews. J. Dairy Sci. 2002, 85, 992–1001. [Google Scholar] [CrossRef]
- Swinkels, J.M.; Hilkens, A.; Zoche-Golob, V.; Kromker, V.; Buddiger, M.; Jansen, J.; Lam, T.J. Social influences on the duration of antibiotic treatment of clinical mastitis in dairy cows. J. Dairy Sci. 2015, 98, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Members of the AVMA Committee on Antimicrobials; Fajt, V.R.; Lehenbauer, T.W.; Plummer, P.J.; Robbins, R.C.; Scheftel, J.M.; Singer, R.S.; Canon, A.J.; Frey, E.; Gaunt, P.S.; et al. A call to action for veterinarians and partners in animal health to collect antimicrobial use data for the purposes of supporting medical decision-making and antimicrobial stewardship. J. Am. Vet. Med. Assoc. 2022, 260, 853–859. [Google Scholar] [CrossRef] [PubMed]
- More, S.J. European perspectives on efforts to reduce antimicrobial usage in food animal production. Ir. Vet. J. 2020, 73, 2. [Google Scholar] [CrossRef]
- Speksnijder, D.C.; Jaarsma, D.A.; Verheij, T.J.; Wagenaar, J.A. Attitudes and perceptions of Dutch veterinarians on their role in the reduction of antimicrobial use in farm animals. Prev. Vet. Med. 2015, 121, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Speksnijder, D.C.; Wagenaar, J.A. Reducing antimicrobial use in farm animals: How to support behavioral change of veterinarians and farmers. Anim. Front. 2018, 8, 4–9. [Google Scholar] [CrossRef] [PubMed]
- AABP. AABP Guidelines: Key Elements for Implementing Antimicrobial Stewardship Plans in Bovine Veterinary Practices Working with Beef and Dairy Operations; American Association of Bovine Practitioners: Ashland, OH, USA, 2022. [Google Scholar]
- AABP. Drug Use Guidelines for Bovine Practice. 2015. Available online: http://aabp.org/resources/aabp_guidelines/druguseguidelines_2015-4-8-1.pdf (accessed on 23 June 2022).
- Page, S.; Prescott, J.; Weese, S. The 5Rs approach to antimicrobial stewardship. Vet. Rec. 2014, 175, 207–208. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S.; Page, S.W.; Prescott, J.F. Antimicrobial stewardship in animals. In Antimicrobial Therapy; Giguere, S., Prescott, J.F., Dowling, P.M., Eds.; Wiley Blackwell: Ames, IA, USA, 2013; pp. 117–133. [Google Scholar]
- McDougall, S.; Niethammer, J.; Graham, E.M. Antimicrobial usage and risk of retreatment for mild to moderate clinical mastitis cases on dairy farms following on-farm bacterial culture and selective therapy. N. Zeal. Vet. J. 2018, 66, 98–107. [Google Scholar] [CrossRef] [PubMed]

| Usage | Active Ingredient of Products Approved for Specific Indications Highest Priority, Critically Important Antimicrobials [15] | |
|---|---|---|
| Intramammary treatment and control of mastitis | Lactating cows—7 products Ceftiofur; Cephapirin; Amoxicillin; Hetacillin; Pirlimycin; Cloxacillin; Penicillin | Dry cows—7 products Ceftiofur; Cephapirin; Penicillin/Streptomycin; Penicillin; Albacillin; Penicillin/Novobiocin; Cloxacillin |
| Systemic a,b treatments | Cattle ≥ 20 months of age—7 approved formulations of 5 antimicrobials Ampicillin; Ceftiofur (3 formulations); Oxytetracycline. Penicillin and Sulfadimethoxine (restricted usage) | |
| Calves a,b and replacement heifers | Calves and cattle < 20 months of age—10 approved antimicrobials Ampicillin; Ceftiofur, Enrofloxacin; Florfenicol; Gamithromycin; Oxytetracycline; Penicillin; Sulfadimethoxine (restricted use); Tildipirosin; Tulathromycin | |
| Pol & Ruegg [47] | Saini et al. [48] | Gonzalez Pereyra et al. [49] | Kuipers et al. [50] | Stevens et al. [51] | de Campos et al. [14] | |
|---|---|---|---|---|---|---|
| Year | 2007 | 2012 | 2015 | 2016 | 2016 | 2021 |
| Country | WI, USA | Canada | Argentina | Netherlands | Belgium | WI USA |
| Data Collection method | On-farm survey | Packaging audit | On-farm survey | Retrieval of sales data | Packaging audit | Farm visit and record analysis |
| Herd (n) | 40 1 | 89 | 18 | 94 | 57 | 40 |
| Lactating Cows/herd | 197 | 69 | 219 | 110 | 69 | 1163 |
| DDD/cow/year | 5.4 | 5.2 | 5.2 | 5.5 | 7.6 | 6.1 |
| % IMM route 2 | 66% | 35% | 85% | 72% | 63% | 78% |
| Element of AMS | |||
|---|---|---|---|
| AABP, 2022 [66] | Weese et al., 2013 [68] | ||
| Core Principle of Judicious Use 1 | Examples of Common Practices Related to Antimicrobial Stewardship | Leadership; Drug Expertise; Tracking AMU; Reporting and Action | Responsibility; Reduction; Replacement; Refinement; Review |
| Prevention |
| ACTION “Review the disease prevention programs such as vaccination, nutrition, and environmental management programs for specific disease conditions to assure optimal husbandry.” | REDUCTION “…requires consideration of the entire spectrum of possible reduction approaches, which also include genetic selection for disease resistance, use of vaccines…, identifying modifiable risk factors and of course, measuring current practice.” |
| Diagnosis |
| ACTION “Review diagnosis/treatment protocols developed for different disease syndromes.” | REFINEMENT “… improved culture-based diagnostic tests are allowing selective treatment of dairy cattle with purulent vaginal discharge or clinical mastitis, and improved use of SCC data is guiding selective dry cow treatment of dairy cattle, each decreasing amu.” |
| Drug Selection and Management |
| DRUG EXPERTISE “Bovine practitioners should provide AMU protocols and treatment guidelines specific for each operation as described in the AABP Guideline” “Establishing and maintaining the veterinarian-client-patient relationship in bovine practice” and “Drug use guidelines for bovine practice.” | REFINE and REPLACEMENT “Replacement of the use of antimicrobials with alternative, nonantimicrobial measures, wherever possible and appropriate, is another critical AMS tenet” |
| Treatment Protocols |
| LEADERSHIP “Am I committed to complete the cycle of disease management by following the judicious use of antimicrobial drugs with reevaluation of their need?” “Have I followed the legal requirements for using antimicrobial drugs by selecting approved products when available or choosing legally acceptable extra-label use?” | REFINEMENT “Use all antimicrobial agents, … only after careful review and reasonable justification.” “Use narrow-spectrum in preference to broad-spectrum antimicrobials whenever appropriate.” “Minimize therapeutic exposure to antimicrobials by treating for only as long as needed to meet the therapeutic objective.” |
| Monitoring |
| TRACKING and REPORTING “Bovine practitioners should periodically review treatment records, drugs present on the farm in relation to treatment protocols, and on-farm antimicrobial drug dispensing and usage.” “Bovine practitioners should support efforts to report AMU across farms in order to benchmark and compare usage...” | REVIEW “Review includes the measurement of progress toward each objective. Information on the use of antimicrobials can be obtained from both quantitative and qualitative assessments.” |
| Leadership |
| LEADERSHIP “It includes accepting responsibility and accountability for antimicrobial prescribing, dispensing, and administration. This commitment also includes identifying leaders within the practice and client operations to share in antimicrobial stewardship.” | RESPONSIBILITY “There are many enabling mechanisms to ensure that a collaborative and participatory team approach is taken with effective communication with all stakeholders.” |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruegg, P.L. Realities, Challenges and Benefits of Antimicrobial Stewardship in Dairy Practice in the United States. Microorganisms 2022, 10, 1626. https://doi.org/10.3390/microorganisms10081626
Ruegg PL. Realities, Challenges and Benefits of Antimicrobial Stewardship in Dairy Practice in the United States. Microorganisms. 2022; 10(8):1626. https://doi.org/10.3390/microorganisms10081626
Chicago/Turabian StyleRuegg, Pamela L. 2022. "Realities, Challenges and Benefits of Antimicrobial Stewardship in Dairy Practice in the United States" Microorganisms 10, no. 8: 1626. https://doi.org/10.3390/microorganisms10081626
APA StyleRuegg, P. L. (2022). Realities, Challenges and Benefits of Antimicrobial Stewardship in Dairy Practice in the United States. Microorganisms, 10(8), 1626. https://doi.org/10.3390/microorganisms10081626






