Abstract
Surplus calves, which consist predominately of male calves born on dairy farms, are an underrecognized source of antimicrobial-resistant (AMR) pathogens. Current production systems for surplus calves have important risk factors for the dissemination of pathogens, including the high degree of commingling during auction and transportation and sometimes inadequate care early in life. These circumstances contribute to an increased risk of respiratory and other infectious diseases, resulting in higher antimicrobial use (AMU) and the development of AMR. Several studies have shown that surplus calves harbor AMR genes and pathogens that are resistant to critically important antimicrobials. This is a potential concern as the resistant pathogens and genes can be shared between animal, human and environmental microbiomes. Although knowledge of AMU and AMR has grown substantially in dairy and beef cattle systems, comparable studies in surplus calves have been mostly neglected in North America. Therefore, the overall goal of this narrative review is to summarize the existing literature regarding AMU and AMR in surplus dairy calf production, highlight the management practices contributing to the increased AMU and the resulting AMR, and discuss potential strategies and barriers for improved antimicrobial stewardship in surplus calf production systems.
1. Introduction
The rise in antimicrobial resistance (AMR) is a major public health crisis [1], and food animals are important reservoirs of AMR bacteria [2]. However, most studies on AMR in food animals focused on either poultry, swine, dairy, or feedlots production systems, with scarce attention paid to surplus calf production. Interestingly, Salaheen et al. [3] found that about 70% of veal calves had resistomes containing AMR genes conferring resistance to multiple medically important antimicrobials. It is therefore important to study the contribution of surplus dairy calves to overall AMR spread.
Surplus dairy calves are calves born on dairy farms that are either unsuitable or not required to replace the milking herd [4]. Most of these calves are male and are either sold for “bob” veal (marketed at <3 weeks of age), veal (milk-fed or formula-fed veal raised mainly only on a milk-based diet with some amount of grain and marketed at 20 weeks of age; grain-fed veal raised mainly on a milk-based diet until 6–8 weeks of age before transitioning to a grain-based diet and marketed at 8 months of age), or dairy beef (marketed at 12–14 months of age). Recently, however, there has been an exponential increase in the use of beef semen in Holstein cows leading to an increase in the number of surplus cross-bred calves [5]. The production stages in surplus calves involve sometimes long-distance transportation to the auction or livestock markets from where the calves are either sent for slaughter or are purchased by the calf raisers to be reared for a certain amount of time depending on the production system type. Throughout these stages, surplus calves experience many health challenges [6,7,8,9,10,11,12,13], leading to an increased risk of disease and subsequent antimicrobial use (AMU) as represented in Figure 1 [4].
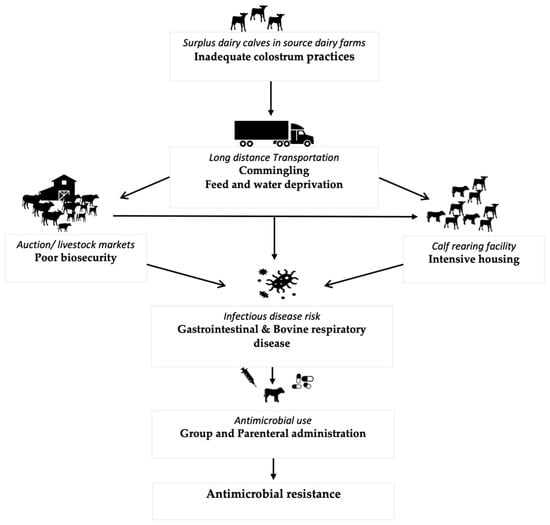
Figure 1.
Events and causal pathways in surplus calf production leading to increased infection risk and subsequent AMU.
AMU in the surplus calf production operations represents an unquantified risk to human health through the foodborne transmission or environmental dissemination of AMR bacteria and the genetic elements mediating resistance. The academic literature has devoted relatively little effort to characterizing the types, indications, and quantity of antimicrobials used and the emerging AMR within surplus calf production systems. In North America, research has characterized AMU among dairy cattle [14,15,16], but few studies focused on AMU in dairy calves [17]. Only a single manuscript in North America has detailed AMU in surplus calf production systems [18]. By comparison, a plethora of research in Europe [19,20,21,22,23] has characterized or identified AMU in surplus calf industries. An overview of the current state of AMU and AMR among commensal and pathogenic bacteria in surplus calves is important to develop scientifically supported and applicable measures to curb AMU and reduce the risk of AMR. Therefore, the overall goal of this review is to (a) summarize the current situation of AMU and AMR in surplus dairy calf production systems, (b) highlight the management practices contributing to the increased AMU and the resulting AMR, and (c) discuss potential strategies and barriers for improved antimicrobial stewardship in surplus calf production systems.
2. Individual and Group Antimicrobial Use in Surplus Calf Production
Individual therapies, primarily consisting of parenteral administration, are mostly used for the treatment of respiratory and gastrointestinal diseases [19], and the majority of AMU happens in the first two months of life [18]. Within the peer-reviewed literature, the reported frequencies of individual AMU in surplus calf systems are typically higher than in other types of animal production. For instance, 61% to 87% of calves received at least one injection of an antimicrobial in their production period [19,24,25]. The level of individual AMU is likely a reflection of the disease incidence. The higher burden of disease has been confirmed through objective health scoring—roughly 85% of calves at a veal facility had at least one day with an abnormal fecal consistency score in the first 28 days after arrival [26]. In addition, others have found a similar disease burden with calves having an abnormal respiratory score for on average 7% of the days the calves were observed at a veal facility [27]. Although prior studies confirm a uniquely high disease burden, AMU could likely be reduced through more targeted AMU. For instance, roughly 40% of replacement heifer producers reported typically using antimicrobials in cases without systemic signs of disease [28]. In another study, Uyama et al. [29] reported that about 96% and 74% of the Canadian dairy producers used antimicrobials to treat respiratory and diarrhea diseases, respectively, in pre-weaned dairy calves. However, less than half of the producers had any written treatment protocol for calf diseases [29].
In addition to individual administration, producers often use group administration of antimicrobials as a prophylactic measure for calf health on arrival due to high disease risks in the first weeks of life. In most contexts, the primary indications for group antimicrobial therapy are (1) metaphylaxis of disease for arriving groups of calves, (2) treatment of gastrointestinal disease and (3) treatment of respiratory disease. In the U.S., group administration of antimicrobials in the feed or water requires a Veterinary Feed Directive or prescription, respectively. Calf producers often use group administration of antimicrobials for the treatment, control, or prevention of disease. In the U.S., 77% of surveyed veal producers reported that they use blanket therapy for the treatment of either diarrhea or pneumonia [30], and group antimicrobial administration constituted 24.1% of the doses of antimicrobials on Ohio veal farms [18]. In Europe, the frequency and/or quantity of group administration of antimicrobials varies widely; investigators reported that 13.4% of farms in Switzerland used group treatments [31], while in France and Belgium, 98% of antimicrobial treatments and antimicrobial doses were group treatments rather than individual treatments [19,32]. By contrast, 85% of antimicrobial doses were administered parenterally rather than in a blanket group therapy on farms in Denmark, potentially reflecting official Danish guidelines that caution against oral administration [23].
2.1. Common Antimicrobials Used in Surplus Calf Production
Beyond the quantity of antimicrobials, the antimicrobial selection is an important consideration for optimal antimicrobial stewardship. The World Health Organization has categorized antimicrobials as either critically important antimicrobials (CIA), highly important (HPA), or important (IA) according to the importance for human medicine. In addition, 3rd generation cephalosporins, fluoroquinolones, and macrolides are categorized as highest-priority (HP-CIAs) [33]. In the U.S., fluoroquinolones (e.g., enrofloxacin) are available to treat respiratory disease in all classes of beef cattle and dairy heifers under 20 months of age but are prohibited for use in veal calves. Third-generation cephalosporins are labeled for bovine respiratory disease and are used on dairy-beef and veal operations for treatment. Veal producers in the U.S. used a mean of 1.0 defined doses of ceftiofur per 100 calves per day, which comprised 3.3% of the individual treatments, and fluoroquinolones were reportedly not used [18]. In European countries, fluoroquinolones and cephalosporins represented 1.9% and 0.1% of the total doses of AMU, respectively [20]. Among the three classes of HP-CIAs (fluoroquinolones, 3rd-generation cephalosporins, and macrolides), macrolides appear to be the most frequently used. Specifically, in a study conducted in Belgium, oral and long-acting parenteral macrolides comprised 11% and 28% of total use, respectively, in part owing to the respiratory disease treatment label and reported effectiveness against Mycoplasma bovis, a common cause of respiratory disease in veal calves [20]. In addition, macrolides were commonly used as individual treatments (4.7% of individual treatments) [18], and macrolides were among the three most used antimicrobials in veal calves in France [22]. Although macrolide use may become a target for improved stewardship, increased use was a protective factor against mortality, and the use of macrolides are likely important for reducing the negative impact of respiratory disease [34].
The largest number of doses of antimicrobials used on veal farms were those that were more commonly administered in a group fashion. Antimicrobials most used for group therapy and administered in the water included chlortetracycline, neomycin, amoxicillin, and sulfmethoxazole/trimethoprim [18]. Of course, the types of antimicrobials used for group treatments in different countries in part reflect differing regulatory availability of antimicrobials. For instance, doxycycline was the second most used antimicrobial in Belgium [20], and colistin was the second most used antimicrobial in veal herds in the Netherlands [35].
2.2. Quantification of Antimicrobial Use in Surplus Dairy Calves
Quantification of AMU on farms is necessary to measure and monitor the effect of AMU reduction strategies. Quantified estimates of AMU are frequently expressed as a treatment incidence rate, i.e., the number of doses per units of animal-time (e.g., 1000 calf-days). Calculation of the rate requires a definition of a “dose” of antimicrobials (e.g., defined daily doses) and assumed standard animal weight. Methods and assumptions for the calculation have not been standardized in the U.S., but dose definitions have been published in Canada [36] and Europe [37]. Comparisons of AMU estimates between published manuscripts are inherently difficult and particularly difficult for growing calves. For instance, Cheng et al. [18] used an estimate of weight based on carcass weights at slaughter and assumed growth rates, whereas other reports use, for instance, a standard weight of 160 kg [34] or 60 kg [38], likely underestimating the number of doses for lighter calves (i.e., neonates) when AMU is most common. Nonetheless, available research suggests that dosing rates are substantially higher among surplus calf production systems. Among Ohio veal calf herds, veal calves received a mean of 35 doses per 100 calves per day, whereas dosing rates ranged between 1.44 and 2.08 per 100 days on beef or dairy production systems [15,39,40]. Similarly, the animal defined daily dose (ADD) in Belgium veal herds was almost double that of the ADD of dairy and beef herds for parental antimicrobials [41]. The quantity of AMU can substantially vary between farms. For instance, Bokma et al. [34] showed that the dosing rate was approximately 60% higher on farms managed by one veal company relative to a different company. Across 78 farms, the doses used per calf-year ranged from 10 to over 50 [20], and the number of treatments per calf ranged from 2.8 to 15.9 on farms in France [22]. Similarly, the treatment incidence among Ohio, USA veal farms ranged from 20.4 to 54.4 doses per 100 calf-days. The significant between-farm variation suggests there is an opportunity for a substantial reduction in the dosing rate of antimicrobials
2.3. Major Risk Factors Associated with Antimicrobial Use in Surplus Calf Production
Research evaluating the risk factors for AMU in surplus calves has mostly focused on the European and Canadian veal industries. As shown in Table 1, there are multiple risk factors associated with AMU in surplus calves. Clearly, the quality of the arriving calves and the management practices in the calf rearing facility plays a critical role in the occurrence of disease and the use of antimicrobials. Therefore, interventions to reduce the incidence of disease and AMU will require collaborations across multiple segments of the production chain, including dairy farms, transporters, and growers.

Table 1.
Risk factors contributing to increased antimicrobial use in surplus calves.
3. Surplus Calf Production System as Reservoirs of Major Antimicrobial Resistant Pathogens
The selective pressure exerted by antimicrobials is one of the main drivers of AMR in commensals and zoonotic enteropathogens [49,50,51,52]. AMR carriage in surplus calves is a public health concern due to the potential for transmission to humans via direct contact, environmental contamination, or contamination of food. In 2016, the Wisconsin Department of Health and the US Centers for Disease Control and Prevention identified dairy calves sold at livestock markets as the source of the multidrug-resistant Salmonella Heidelberg outbreak in humans [53]. AMR is also a concern for animal health as resistance in animal pathogens reduces the effectiveness of drugs used in veterinary medicine. Direct contact with surplus calves or their environments could transmit AMR to other animals (e.g., via livestock trailers or auctions). Several studies report that among all cattle sectors, the highest levels of AMR in commensals and pathogens are found in veal production systems [54,55,56]. Specifically, bovine respiratory pathogens, enteric pathogens, and foodborne infectious agents are the major group of organisms that have significant health and economic consequences in animals and humans and are widely studied for the presence of AMR in surplus calf production systems. The following paragraphs address each of these categories directly.
3.1. Bovine Respiratory Pathogens Play a Key Role in Overall Antimicrobial Use and Antimicrobial Resistance
Bovine respiratory disease (BRD) has been recorded as the leading cause of morbidity and mortality in pre-weaned calves in the United States [57], wherein in an analysis that involved 43,739 calves, 10.5% were diagnosed with BRD [57]. Bovine respiratory disease (BRD) is a multifactorial disease complex involving numerous bacterial and viral agents which act in synergy with stressors such as nutrition, weaning, transportation, and rearing environment. Members of the Pasteurellaceae family (Mannheimia haemolytica, Pasteurella multocida, Histophilus somni, and Mycoplasma bovis) are often described as secondary infectious agents but are also considered as commensal organisms in the upper respiratory tract [58]. Owing to the high infection pressure after transportation, pro- and metaphylactic treatments are frequently used to control BRD outbreaks in surplus calves [20]. Selection pressure exerted by antimicrobial therapy was shown to influence the prevalence of resistance among commensal and pathogenic respiratory bacteria. Table 2 represents major respiratory pathogens and associated resistance phenotypes isolated from surplus calves.

Table 2.
Summary of research that studied resistance among bovine respiratory pathogens in surplus calves: The table below shows the source of the different isolates included in the study, country in which the study was conducted, panel of antimicrobials that were tested against the bacterial agent and, major findings from the study.
Pasteurella multocida was most frequently isolated (37%) from veal calves in cases associated with BRD followed by Mannhemia spp. [55]. Interestingly, a majority (71.9%) of the resistant strains of the Pasteurellaceae family, and isolates with multi-resistance profiles were restricted to organisms originating from veal calves when compared to the dairy and beef production systems [55]. Similar results were obtained in another study where a substantially higher proportion of AMR P. multocida and M. haemolytica were isolated from intensively reared veal calves in contrast to the more extensively raised beef herds [41]. Antimicrobial susceptibility profiles demonstrated that more than 80% of the resistant Pasteurellaceae strains from veal calves were multidrug-resistant (resistant to four or more antimicrobials). A higher percentage of resistant Pasteurellaceae strains were recovered from veal calves in Switzerland. The study showed that 85% and 95% of M. haemolytica and P. multocida were resistant to at least one of the tested antimicrobials [59]. However, Schönecker et al. [58] reported an overall decrease in resistance in M. haemolytica isolates from veal calves in Switzerland when compared to previous studies [59,62]. Even though there was a decrease in resistance in M. haemolytica, no information was available on the health and treatment status of the calves in the study. Among P. multocida, reports from Swiss veal calves [41] demonstrated a common occurrence of resistance to tetracyclines and macrolides. More recent studies showed a higher prevalence of resistance among Pasteurella spp. To fluoroquinolones and macrolides [58] when compared to earlier studies that had distinctly less resistance towards these antimicrobials [62,63]. Further, group treatment was associated with increased odds of isolating resistant M. haemolytica and P. multocida isolates [59]. Adding to this, another study in veal calves demonstrated large deviations from recommended dosing regimens, with 88% of oral administrations being underdosed [41].
As for Mycoplasma bovis infection, there is no effective vaccine at present, and control is mostly dependent on a variety of husbandry and infection control practices. However, due to the lack of cell wall and its inability to synthesize folic acid, Mycoplasma is intrinsically resistant to beta-lactams and sulfonamide classes of antimicrobials. Hence, antimicrobials that target protein or DNA synthesis (such as fluoroquinolones and macrolides) are commonly used for the treatment of Mycoplasma. However, resistance against these classes of drugs is frequently reported in veal calves [64]. A significant increasing trend in MIC values of macrolides, that are the first choice for the treatment of BRD caused by Mycoplasma spp. Was reported in Netherlands from M. bovis collected from clinical samples between the period 2008–2014 [60]. Similarly, a recent study comparing the antimicrobial susceptibility of Mycoplasma bovis isolated from dairy, beef, and veal cattle sectors in Belgium showed high percentages (50–100%) of acquired resistance for macrolides. However, no significant differences were observed in AMR between production systems except for gamithromycin which was higher in beef cattle [61].
Overall, a higher percentage of AMR respiratory pathogens were isolated from veal calves when compared to dairy and beef cattle [41,55,56]. Furthermore, there have been drastic changes in the prevalence of resistance among respiratory pathogens among surplus calves. Specifically, a trend of elevated resistance towards the macrolide class of antimicrobials in Pasteurellaceae family and Mycoplasma spp. Were observed in recent years. Increased resistance towards macrolides in respiratory pathogens does not come as a surprise, since macrolides (such as tilmicosin and tulathromycin) are frequently administered either prophylactically, metaphylactically, or therapeutically in animals recognized to be at high risk for BRD. The high levels of resistance in animal respiratory pathogens are likely to have a substantial but unmeasured impact on calf health and welfare due to treatment failures. Given the fact that surplus calves are an important reservoir of AMR respiratory bacteria, preventive measures against BRD should be revisited, with good management practices implemented at the farm of origin of the surplus calves, long before the arrival at the fattening unit, rather than actions aimed at limiting the spread of the diseases.
3.2. Enteric Bacteria in Surplus Calves Contribute to Antimicrobial Resistance Burden
Enteric bacteria, such as E. coli, are known to be reservoirs of AMR genes as shown in Table 3. Studies show that surplus calves can carry higher levels of resistant E. coli strains compared to older animals [65,66,67,68,69]. For instance, the proportion of extended-spectrum beta-lactamase (ESBL) carrying E. coli isolates was higher in veal calves relative to adult cattle in France [32]. Further, Schönecker et al. [59] demonstrated that ~70% of the E. coli isolated from veal calves were resistant to at least one of the tested antimicrobials. However, only a few studies studied the prevalence and distribution of AMR in E. coli within surplus dairy calves in the U.S. In this context, Salaheen et al. [70] showed that multidrug-resistant E. coli (resistant to more than three antimicrobial classes) was isolated from 75–100% of veal calf samples in Pennsylvania. The authors showed that E. coli isolated from those calves were resistant to antimicrobials belonging to multiple antimicrobial classes [70]. Hutchinson et al. [71] also documented a higher percentage of multidrug resistance (97%) among E. coli recovered from farm isolates in a vertically integrated veal production system in the U.S. Consistent with the study in veal calves in Europe [32,72], resistance to quinolones and macrolides remained low at all times among enteric E. coli in the U.S. Similarly, Berge et al. [73] reported lower resistance percentages to quinolones among veal calves in California. Furthermore, a follow-up study from the same authors [3], characterizing the gut microbiota of veal calves by shotgun metagenomic sequencing showed that 70% of veal calves had resistomes containing AMR genes conferring resistance to aminoglycosides, tetracyclines, and MLS.

Table 3.
Summary of research that studied resistance among enteric bacteria in surplus calves: The table below shows the source of the different isolates included in the study, the country in which the study was conducted, the panel of antimicrobials that were tested against the bacterial agent, antimicrobials to which highest proportion of resistance was observed and major antibiotic resistance genes that were identified in the study.
Studies also show age-related changes in the fecal carriage of AMR in E. coli among surplus calf production systems [69]. For instance, resistance to critically important antimicrobials (quinolone resistance) decreased significantly by the end of the fattening process in veal calves in Europe [32]. However, resistance towards non critically important antimicrobials and MDR in E. coli increased in parallel to a decrease in resistance to critically important antimicrobials by the end of the fattening process. These results imply an increase in the use of non-critically important antimicrobials during the fattening process. Concurring with these observations, higher tetracycline use was reported in fattening farms when compared to the farms of birth [32]. In contrast, higher quantities of antimicrobial administration were observed in the first three weeks of rearing in veal calves in Ohio [18]. Similar to the studies in Europe, studies in U.S. have found an age-related decline in the carriage of ampicillin-resistant E. coli in calves sampled within the first month after birth until up to the eighth month of sampling [74]. In contrast, Salaheen et al. [70] observed higher percentages of resistance in samples collected from animals before slaughter (16–18 weeks) when compared to samples collected from bob calves (<1-month-old) at the auction houses. Further, fluoroquinolone resistance was found in 75% of farm fecal isolates, despite veal calves never receiving fluoroquinolones from veal growers [71]. It is not clear why fluoroquinolone resistance was found in E. coli isolates recovered from veal calves. One possibility is that the resistant bacteria were acquired at birth from the dam or dairy-farm environment, as fluoroquinolones are not used in U.S. veal operations. Alternatively, contact with other animals or contaminated environments during auction or transport could have resulted in colonization. Similarly, another study showed the presence of E. coli strains with a similar resistance pattern (resistance to ampicillin, streptomycin, sulfisoxazole, tetracycline, and trimethoprim-sulfamethoxazole) isolated from both auction houses and farms suggesting a common source of origin of the isolates [70].
Resistance towards extended-spectrum cephalosporins is a common occurrence in young dairy calves. Despite their public health significance, fecal carriage of extended-spectrum cephalosporins/AmpC is not well described in surplus calves. The presence of blaCTX-M, a group of class A ESBL, conferring resistance to second and third-generation cephalosporins was observed in 13.3–17.5% of veal calf samples in the U.S. [70]. In a similar study from Pennsylvania, Donaldson et al. [75] reported that 100% of E. coli isolated from dairy calves that were resistant to ceftiofur were also MDR. Additionally, Hordijk et al. [66] evaluated the fecal samples taken from veal calves and found that 83% of the E. coli isolates that were resistant to cefotaxime carried the blaCTX-M gene family. Researchers in France also identified CTX-M group 1 enzyme (71.5%) in veal calf isolates with ESBL phenotype. [32]. This is consistent with the data from other European countries where blaCTX-M-1 was identified as the main gene responsible for ESBL spread in food-producing animals including veal, poultry, cattle, and swine [66,76,77]. Interestingly, 26% of the E. coli that harbored ESBL/AmpC genes carried multiple plasmid types (both IncI1 and IncF plasmids) within the same isolate, suggesting horizontal gene transfer events occurring within those isolates. Further, the authors also reported a rare combination of blaCTX-M-1 with IncF-type plasmid in ESBL E. coli. [76].
Overall, a higher prevalence of resistance observed among enteric bacteria in young surplus calves relative to older calves is a consistent feature across Europe and the U.S. This can be attributed to several factors such as increased fecal–oral transmission, in vivo fitness advantage of resistant E. coli in neonatal calves, or due to the higher levels of AMU in younger animals due to the increased infection risk in those animals [78,79]. Age related changes in the occurrence of AMR is a common feature among calves which is partially a result of changes in management practices and reduced incidence of disease in older calves. Furthermore, the emergence of enteric E. coli carrying ESBL such as blaCTX-M-55 in surplus calves is of significant concern as those isolates are known to carry horizontally transferable genes which mediate resistance to aminoglycoside (rmtB) and colistin (mcr-3) [80]. High trafficking of calves from different locations across dairy farms along with the presence of resistant determinants capable of horizontal transfer between bacterial populations might have resulted in a diverse set of plasmid/gene combinations among surplus calves. In any case, resistance to CIA antimicrobials among enteric commensal organisms has significant public health implications due to their ability for the widespread dissemination of AMR genes among animal and human bacterial populations.
3.3. Major AMR Foodborne Pathogens Recovered from Meat from Surplus Calves
Integrated surveillance efforts have been used to monitor AMR transmission from food animals to humans. This includes sampling on-farm, harvest facilities, and from retail cuts of meat [81]. Much information on AMR bacteria in ground beef and other food products are available. However, information on AMR bacteria in surplus dairy and veal meat is very limited. The first estimates of bacterial contamination and AMR prevalence in retail veal meat in the United States were generated as part of the 2018 U.S. National Antimicrobial Resistance Monitoring System (NARMS) [82]. The study showed that 14% of the E. coli and 39% of Enterococcus veal-derived isolates were resistant to antimicrobials, and resistance was more likely to be found in isolates derived from veal samples compared to dairy cattle samples [82]. Although a wide variety of AMR bacteria of public health significance are detected in veal meats, major AMR foodborne pathogens of concern include Salmonella, Escherichia coli, and Campylobacter [83].
MDR (resistant to five or more antimicrobials) Salmonella was detected in 24% of the total Salmonella isolated from grain-fed veal meat [84]. Studies also show that bob veal samples harbored higher concentrations of Salmonella compared to special-fed veal [85]. While Salmonella isolated from healthy adult cattle are typically pan susceptible and are not of high public health significance [86], the opposite is true for Salmonella isolated from surplus calves. For instance, special-fed veal was found to harbor Salmonella serovars of greater clinical importance (such as monophasic Typhimurium 4,[5],12:i:-, Heidelberg, and Agona) than those recovered from bob veal samples. Additionally, Salmonella recovered from special-fed veal was found to be more resistant than those from bob veal [85]. Further, samples from mesenteric lymph nodes collected from a vertically integrated veal production company revealed the presence of Salmonella in 21.9% (35/160) of the samples [87]. In addition, serotypes of high public health importance were identified in the study and include Salmonella Typhimurium, and Newport [87]. MDR Salmonella Dublin was also recovered from ground veal [82]. Salmonella Dublin is a cattle-adapted serovar with the potential for causing severe disease in humans [88]. The incidence of human Dublin infections has been increasing over the last few decades [89] and multidrug resistance is a hallmark of American isolates [90]. Work within a veal production company revealed that over 70.8% of lymph node isolates (34/48) were MDR and Salmonella Dublin was the most common serovar recovered from the samples. [91]. Further genomic analysis of Dublin isolates recovered from those veal lymph nodes suggested lymph node infection that could be traced to the farm of origin [91].
Similar to the occurrence of resistance in Salmonella, AMR was also reported in E. coli and Campylobacter in veal meat. Resistance to five or more antimicrobials was detected in 33% and 10% of the total E. coli isolates isolated from milk-fed and grain-fed veal samples, respectively [84,92]. Work within a veal production system in the U.S. that harvested bob and special-fed veal recovered MDR (resistance to three or more antimicrobial classes) E. coli from 61% (51/84) pre-evisceration isolates, and 22% (5/21) final carcass isolates [71]. Additionally, an epidemiologic investigation to identify the source of sporadic Campylobacteriosis outbreaks in humans linked the veal liver as a potential source of infection [93]. In a study in Switzerland, 27% of the Campylobacter spp. isolated from veal calves at slaughter were resistant to at least one of the tested antimicrobials [94]. A low prevalence of Campylobacter was observed in retail grain-fed veal meat in Canada. However, 50% of the samples were resistant to one or more tested antimicrobials [94].
To summarize, antimicrobial resistance is typically found in isolates recovered from surplus calves. The consistent recovery of MDR Salmonella and E. coli from veal meat implicates this population as a reservoir for MDR pathogens. Furthermore, the Salmonella serotypes that are recovered from surplus calf meat have higher public health importance relative to isolates from other cattle production classes. Surplus veal calves (both bob and formula-fed) are unique populations with differing challenges from beef cattle, but the lack of knowledge on the source and prevalence of AMR foodborne pathogens in surplus calf meat hinders the creation of appropriate interventions. Therefore, to assess potential food safety risks and efficiently target mitigation efforts, further research into surplus calf meat production is prudent.
4. Antimicrobial Resistance Control Strategies Focused on Reduced Antimicrobial Use in Surplus Calf Production
Given the high levels of AMR within bacteria from surplus calves and the likely, albeit unmeasured public health impact, improvements in antimicrobial stewardship among veal and dairy beef farms is necessary for long-term sustainability and maintenance of a social license to operate [4,95]. The most often stated goal for antimicrobial stewardship programs is to reduce the total quantity of antimicrobials used throughout the production process [96]. Based on limited evidence in North America, veal and dairy-beef production systems have higher levels of AMU dosing rates relative to other cattle production systems, and a higher prevalence of AMR commensals and pathogens relative to other production classes of cattle. Undoubtedly, reducing AMU and/or AMR will be particularly challenging within surplus calf production systems. Reductions in the total quantity of AMU can be achieved through some combination of reduced disease incidence and judicious (i.e., selective) antimicrobial use [23,31,97,98]. However, the disaggregated nature of the production chain makes changes difficult. For instance, dairy farmers responsible for early-life care have little financial motivation for investments in the care of calves sold at auctions [4,9]. Collaboration across sectors of the production system will be necessary to remove some of the causes of disease. Conflicting messages and a lack of harmony among different advisors were major barriers to applying novel management practices to reduce unnecessary use [99,100]. Additionally, high production costs combined with unstable calf prices, calf quality, and scarce labor keep surplus calf producers under constant financial pressure [101], which makes motivating changes difficult.
Improving Judicious Application of Antimicrobials
In addition to reduced disease incidence, reductions in AMR can be achieved by adopting strategies for judicious AMU (Figure 2).
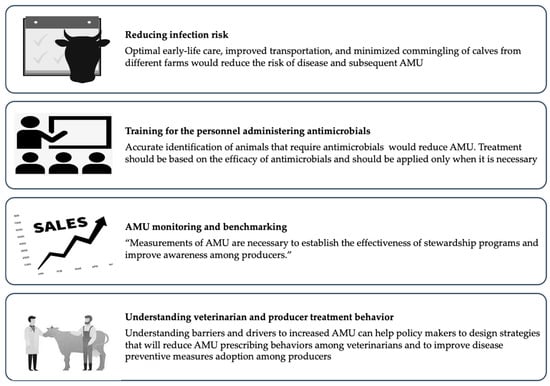
Figure 2.
Strategies for reducing antimicrobial use in surplus calf production.
Selective application of antimicrobials or reserving AMU where it is necessary for animal health is likely to be effective in reducing AMU. However, some routine applications of antimicrobials lack documented evidence of efficacy. Metaphylactic treatment based on oral administration of antimicrobials for healthy and diseased animals within the same group can be a frequent practice [42], and for instance, U.S. producers sometimes use extra-label administration of antimicrobials in the water to treat or prevent gastrointestinal disease. The efficacy of this practice has not been documented, and optimal antimicrobial stewardship will require evidence to support routine use. Similarly, appropriate training is necessary for farm personnel to accurately identify instances requiring antimicrobial therapy and to follow veterinary written treatment protocols. Two-thirds of veal producers in the Midwestern United States reported routinely using antimicrobials when presented with uncomplicated cases of diarrhea that could be managed with non-antimicrobial interventions [102]. Indeed, training on the accurate identification of calves with diarrhea and pneumonia led to better-targeted therapy and a 50% reduction in the quantity of antimicrobials on Ohio veal herds [30]. Routine implementation of more selective application of antimicrobials will require an additional understanding of behavioral drivers and barriers among farm personnel and veterinarians responsible for AMU. Research has begun to identify the drivers and barriers that influence veterinarians’ and farm owners’ intentions to make treatment decisions [103]. The risk aversion to the high mortality rate among veal calves has been found to facilitate the start of antimicrobial therapy among veterinarians in Flanders, Belgium [20]. Additionally, veterinarians acknowledged that they were afraid of legal action from their employers in the event of negative clinical outcomes if they refuse to treat with antimicrobials [103]. Veterinarians wanted to make responsible usage of antimicrobials, but sometimes they responded to the uncertainty of clinical cases and the need for favorable results by prescribing antimicrobials [104].
Furthermore, routine measurement and monitoring of antimicrobial consumption are necessary to document patterns and temporal changes in the consumption of antimicrobials. Standardized and consistent methods for AMU monitoring have not yet been adopted in the U.S, but the methods will need to be scalable, efficient, and able to document farm-level variability. Importantly, systematic monitoring should protect the confidentiality of participant farms. Candidate methods for AMU monitoring on cattle operations in the U.S. are either based on farm treatment records [105] or sales data [106]. Using treatment records to quantify AMU would be particularly challenging on surplus calf operations as meat residue violations are not a concern for early life treatments, and treatments are recorded less frequently relative to other cattle production classes. Therefore, veterinary sales data are likely the only viable option, which would be enabled by the forthcoming FDA rules eliminating over-the-counter sales of medically important antimicrobials [107]. Given the availability of consistent farm-level AMU measurements, benchmarking tools could be used to improve the awareness of producers on the quantity of AMU and motivate the behavioral changes necessary for meaningful reductions [108,109].
A combination of contextual and psychosocial factors serves as important barriers to a reduction in AMU either through reducing disease incidence or improving selectivity for application of antimicrobials. Future research incorporating social science approaches is necessary to understand the barriers to the implementation of antimicrobial stewardship. Coordination across components of the surplus calf production chain and collaboration between actors in the industry, academics, and regulators are additionally necessary to facilitate or implement stewardship activities, including antimicrobial use monitoring.
5. Conclusions
Surplus dairy calves are likely an underrecognized source of AMR bacteria, and reducing the global AMU in the surplus calf sector will be important to mitigate public health risks and assuage consumer concerns. Several challenges faced by surplus calves during their early life (such as long-distance transportation, commingling, and inadequate nutrition) predispose them to respiratory and enteric diseases, necessitating relatively high dosing rates of antimicrobials. Treatment strategies in veal calves often depend on group administration of antimicrobials rather than treating calves individually. Very few studies have characterized AMU in North American surplus calf production systems, and the lack of standardized dosing metrics for AMU quantification hinders AMU reduction strategies. A variety of factors, including young age, high exposures, and antimicrobial use result in substantial levels of resistance to medically important antimicrobials. Coordination across the disaggregated sectors of surplus calf production will be required to reduce disease incidence and AMU. A better understanding of psychosocial and contextual barriers faced by veterinarians and farm owners will facilitate the implementation of economically feasible antimicrobial stewardship practices that maintain animal health and welfare. However, it is still unclear to what magnitude surplus calves affect AMR in livestock production; therefore, additional work will be necessary to characterize and mitigate the impact on public health.
Author Contributions
Writing—original draft preparation, P.G.V., S.R.L., R.P.-G., D.L.R. and G.G.H.; writing—review and editing, P.G.V., S.R.L., R.P.-G., D.L.R. and G.G.H.; supervision, G.G.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog. Glob. Health 2015, 109, 309. [Google Scholar] [CrossRef] [PubMed]
- Acar, J.F.; Moulin, G. Antimicrobial Resistance at Farm Level. Rev. Sci. Tech. 2006, 25, 775–792. [Google Scholar] [CrossRef] [PubMed]
- Salaheen, S.; Kim, S.W.; Hovingh, E.; Kessel, J.A.S.; Haley, B.J. Metagenomic Analysis of the Microbial Communities and Resistomes of Veal Calf Feces. Front. Microbiol. 2021, 11, 3342. [Google Scholar] [CrossRef]
- Creutzinger, K.; Pempek, J.; Habing, G.; Proudfoot, K.; Locke, S.; Wilson, D.; Renaud, D. Perspectives on the Management of Surplus Dairy Calves in the United States and Canada. Front. Vet. Sci. 2021, 8, 661453. [Google Scholar] [CrossRef]
- Ettema, J.F.; Thomasen, J.R.; Hjortø, L.; Kargo, M.; Østergaard, S.; Sørensen, A.C. Economic Opportunities for Using Sexed Semen and Semen of Beef Bulls in Dairy Herds. J. Dairy Sci. 2017, 100, 4161–4171. [Google Scholar] [CrossRef] [PubMed]
- Fecteau, G.; Baillargeon, P.; Higgins, R.; Paré, J.; Fortin, M. Bacterial Contamination of Colostrum Fed to Newborn Calves in Québec Dairy Herds. Can. Vet. J. 2002, 43, 523. [Google Scholar]
- Hall, J.B.; Roberts Lew, M.C.; Smith, W.K.; Shivley, C.B.; Urie, N.; Lombard, J.E. 1210 Management of Dairy Bull Calves on U.S. Dairy Operations. J. Anim. Sci. 2016, 94, 581. [Google Scholar] [CrossRef]
- Renaud, D.; Steele, M.; Genore, R.; Roche, S.; Winder, C. Passive Immunity and Colostrum Management Practices on Ontario Dairy Farms and Auction Facilities: A Cross-Sectional Study. J. Dairy Sci. 2020, 103, 8369–8377. [Google Scholar] [CrossRef]
- Wilson, D.J.; Stojkov, J.; Renaud, D.L.; Fraser, D. Risk Factors for Poor Health Outcomes for Male Dairy Calves Undergoing Transportation in Western Canada. Can. Vet. J. 2020, 61, 1265. [Google Scholar]
- Ballou, M.A.; Cobb, C.J.; Earleywine, T.J.; Obeidat, B.S. Breed and Plane of Milk-Replacer Nutrition Influence the Performance of Pre-and Postweaned Dairy Calves. Prof. Anim. Sci. 2013, 29, 116–123. [Google Scholar] [CrossRef]
- Rosenberger, K.; Costa, J.H.C.; Neave, H.W.; Keyserlingk, M.A.G.; Weary, D.M. The Effect of Milk Allowance on Behavior and Weight Gains in Dairy Calves. J. Dairy Sci. 2017, 100, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Renaud, D.; Pardon, B. Preparing Male Dairy Calves for the Veal and Dairy Beef Industry. Vet. Clin. N. Am.-Food Anim. Pract. 2022, 38, 77–92. [Google Scholar] [CrossRef]
- USDA. Dairy Heifer Raiser, 2011: An Overview of Operations that Specialize in Raising Dairy Heifers. Available online: https://www.aphis.usda.gov/animal_health/nahms/dairy/downloads/dairyheifer11/HeiferRaiser_1.pdf (accessed on 22 April 2021).
- Lardé, H.; Dufour, S.; Archambault, M.; Massé, J.; Roy, J.P.; Francoz, D. An Observational Cohort Study on Antimicrobial Usage on Dairy Farms in Quebec, Canada. J. Dairy Sci. 2021, 104, 1864–1880. [Google Scholar] [CrossRef]
- Saini, V.; McClure, J.; Léger, D.; Dufour, S.; Sheldon, A.; Scholl, D.; Barkema, H. Antimicrobial Use on Canadian Dairy Farms. J. Dairy Sci. 2012, 95, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.L.; Kates, A.; Steinberger, A.; Sethi, A.; Suen, G.; Shutske, J.; Safdar, N.; Goldberg, T.; Ruegg, P.L. Quantification of Antimicrobial Usage in Adult Cows and Preweaned Calves on 40 Large Wisconsin Dairy Farms Using Dose-Based and Mass-Based Metrics. J. Dairy Sci. 2021, 104, 4727–4745. [Google Scholar] [CrossRef] [PubMed]
- Waldner, C.L.; Parker, S.; Gow, S.; Wilson, D.J.; Campbell, J.R. Antimicrobial Usage in Western Canadian Cow-Calf Herds. Can. Vet. J. 2019, 60, 255. [Google Scholar]
- Cheng, Y.; Almeida, B.G.; Pempek, J.A.; Masterson, M.A.; Habing, G.G. The Use of Common Antimicrobial Agents in US Veal Calves. Zoonoses Pub. Health 2022, 64, 359–369. [Google Scholar] [CrossRef]
- Pardon, B.; Catry, B.; Dewulf, J.; Persoons, D.; Hostens, M.; Bleecker, K.; Deprez, P. Prospective Study on Quantitative and Qualitative Antimicrobial and Anti-Inflammatory Drug Use in White Veal Calves. J. Antimicrob. Chemother. 2012, 67, 1027–1038. [Google Scholar] [CrossRef]
- Bokma, J.; Boone, R.; Deprez, P.; Pardon, B. Risk Factors for Antimicrobial Use in Veal Calves and the Association with Mortality. J. Dairy Sci. 2019, 102, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Mallioris, P.; Dohmen, W.; Luiken, R.E.C.; Wagenaar, J.A.; Stegeman, A.; Mughini-Gras, L. Factors Associated with Antimicrobial Use in Pig and Veal Calf Farms in the Netherlands: A Multi-Method Longitudinal Data Analysis. Prev. Vet. Med. 2022, 199, 105563. [Google Scholar] [CrossRef]
- Jarrige, N.; Cazeau, G.; Morignat, E.; Chanteperdrix, M.; Gay, E. Quantitative and Qualitative Analysis of Antimicrobial Usage in White Veal Calves in France. Prev. Vet. Med. 2017, 144, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Fertner, M.; Toft, N.; Martin, H.L.; Boklund, A. A Register-Based Study of the Antimicrobial Usage in Danish Veal Calves and Young Bulls. Prev. Vet. Med. 2016, 131, 41–47. [Google Scholar] [CrossRef]
- Sandelin, A.; Hälli, O.; Härtel, H.; Herva, T.; Kaartinen, L.; Tuunainen, E.; Rautala, H.; Soveri, T.; Simojoki, H. Effect of Farm Management Practices on Morbidity and Antibiotic Usage on Calf Rearing Farms. Antibiotics 2022, 11, 270. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.; Kelton, D.F.; Duffield, T.F.; Renaud, D.L. Risk Factors Identified on Arrival Associated with Morbidity and Mortality at a Grain-Fed Veal Facility: A Prospective, Single-Cohort Study. J. Dairy Sci. 2019, 102, 9224–9235. [Google Scholar] [CrossRef] [PubMed]
- Schinwald, M.; Creutzinger, K.; Keunen, A.; Winder, C.B.; Haley, D.; Renaud, D.L. Predictors of Diarrhea, Mortality, and Weight Gain in Male Dairy Calves. J. Dairy Sci. 2022, 105, 5296–5309. [Google Scholar] [CrossRef] [PubMed]
- Buss, L.N.; Yohe, T.T.; Cangiano, L.R.; Renaud, D.L.; Keunen, A.J.; Guan, L.L.; Steele, M.A. The Effect of Neomycin Inclusion in Milk Replacer on the Health, Growth, and Performance of Male Holstein Calves during Preweaning. J. Dairy Sci. 2021, 104, 8188–8201. [Google Scholar] [CrossRef]
- Habing, G.; Djordjevic, C.; Schuenemann, G.M.; Lakritz, J. Understanding Antimicrobial Stewardship: Disease Severity Treatment Thresholds and Antimicrobial Alternatives among Organic and Conventional Calf Producers. Prev. Vet. Med. 2016, 130, 77–85. [Google Scholar] [CrossRef]
- Uyama, T.; Kelton, D.F.; Morrison, E.I.; Jong, E.; McCubbin, K.D.; Barkema, H.W.; Dufour, S.; Sanchez, J.; Heider, L.C.; LeBlanc, S.J.; et al. Cross-Sectional Study of Antimicrobial Use and Treatment Decision for Preweaning Canadian Dairy Calves. JDS Commun. 2022, 3, 72–77. [Google Scholar] [CrossRef]
- Pempek, J.; Masterson, M.; Portillo-Gonzalez, R.; Creutzinger, K.; Cheng, T.-Y.; Habing, G. The Impact of Antimicrobial Stewardship Training on Calf Producers Knowledge, Treatment Behaviors and Quantified Antimicrobial Use. Microorganisms 2022, 10, 1525. [Google Scholar] [CrossRef]
- Lava, M.; Pardon, B.; Schüpbach-Regula, G.; Keckeis, K.; Deprez, P.; Steiner, A.; Meylan, M. Effect of Calf Purchase and Other Herd-Level Risk Factors on Mortality, Unwanted Early Slaughter, and Use of Antimicrobial Group Treatments in Swiss Veal Calf Operations. Prev. Vet. Med. 2016, 126, 81–88. [Google Scholar] [CrossRef]
- Gay, E.; Bour, M.; Cazeau, G.; Jarrige, N.; Martineau, C.; Madec, J.Y.; Haenni, M. Antimicrobial Usages and Antimicrobial Resistance in Commensal Escherichia Coli from Veal Calves in France: Evolution during the Fattening Process. Front. Microbiol. 2019, 10, 792. [Google Scholar] [CrossRef]
- Critically Important Antimicrobials for Human Medicine 3rd Revision 2011. Available online: https://apps.who.int/iris/bitstream/handle/10665/77376/9789241504485_eng.pdf;jsessionid=E257391C432A4141F5496157F269EC75?sequence=1 (accessed on 4 August 2022).
- Bokma, J.; Boone, R.; Deprez, P.; Pardon, B. Short Communication: Herd-Level Analysis of Antimicrobial Use and Mortality in Veal Calves: Do Herds with Low Usage Face Higher Mortality? J. Dairy Sci. 2020, 103, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Bosman, A.B.; Wagenaar, J.; Stegeman, A.; Vernooij, H.; Mevius, D. Quantifying Antimicrobial Resistance at Veal Calf Farms. PLoS ONE 2012, 7, e44831. [Google Scholar] [CrossRef] [PubMed]
- Lardé, H.; Dufour, S.; Archambault, M.; Léger, D.; Loest, D.; Roy, J.P.; Francoz, D. Assignment of Canadian Defined Daily Doses and Canadian Defined Course Doses for Quantification of Antimicrobial Usage in Cattle. Front. Vet. Sci. 2020, 7, 10. [Google Scholar] [CrossRef]
- Catry, B.; Chauvin, C.; Greko, C.; Heederik, D.; Jacobsen, E.; Geijlswijk, I.; Mcewen, S.; Müntener, C.; Litleskare, I. Revised ESVAC Reflection Paper on Collecting Data on Consumption of Antimicrobial Agents per Animal Species, on Technical Units of Measurement and Indicators for Reporting Consumption of Antimicrobial Agents in Animals. 2013. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/revised-european-surveillance-veterinary-antimicrobial-consumption-esvac-reflection-paper-collecting_en.pdf (accessed on 4 August 2022).
- González Pereyra, V.; Pol, M.; Pastorino, F.; Herrero, A. Quantification of Antimicrobial Usage in Dairy Cows and Preweaned Calves in Argentina. Prev. Vet. Med. 2015, 122, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Hyde, R.M.; Remnant, J.G.; Bradley, A.J.; Breen, J.E.; Hudson, C.D.; Davies, P.L.; Clarke, T.; Critchell, Y.; Hylands, M.; Linton, E.; et al. Quantitative Analysis of Antimicrobial Use on British Dairy Farms. Vet. Rec. 2017, 181, 683. [Google Scholar] [CrossRef]
- Stevens, M.; Piepers, S.; Supré, K.; Vliegher, S. Antimicrobial Consumption on Dairy Herds and Its Association with Antimicrobial Inhibition Zone Diameters of Non-Aureus Staphylococci and Staphylococcus Aureus Isolated from Subclinical Mastitis. J. Dairy Sci. 2018, 101, 3311–3322. [Google Scholar] [CrossRef]
- Catry, B.; Dewulf, J.; Maes, D.; Pardon, B.; Callens, B.; Vanrobaeys, M.; Opsomer, G.; Kruif, A.; Haesebrouck, F. Effect of Antimicrobial Consumption and Production Type on Antibacterial Resistance in the Bovine Respiratory and Digestive Tract. PLoS ONE 2016, 11, e0146488. [Google Scholar] [CrossRef]
- Schnyder, P.; Schönecker, L.; Schüpbach-Regula, G.; Meylan, M. Effects of Management Practices, Animal Transport and Barn Climate on Animal Health and Antimicrobial Use in Swiss Veal Calf Operations. Prev. Vet. Med. 2019, 167, 146–157. [Google Scholar] [CrossRef]
- Brscic, M.; Leruste, H.; Heutinck, L.F.M.; Bokkers, E.A.M.; Wolthuis-Fillerup, M.; Stockhofe, N.; Gottardo, F.; Lensink, B.J.; Cozzi, G.; Reenen, C.G. Prevalence of Respiratory Disorders in Veal Calves and Potential Risk Factors. J. Dairy Sci. 2012, 95, 2753–2764. [Google Scholar] [CrossRef]
- Rot, C.; Creutzinger, K.; Goetz, H.; Winder, C.; Morrison, J.; Conboy, M.; Bajus, A.; Renaud, D.L. Factors Associated with Body Weight of Young Surplus Dairy Calves on Arrival to a Calf Rearing Facility. Prev. Vet. Med. 2022, 203, 105630. [Google Scholar] [CrossRef]
- Marcato, F.; Brand, H.; Kemp, B.; Engel, B.; Wolthuis-Fillerup, M.; Reenen, K. Effects of Pretransport Diet, Transport Duration, and Type of Vehicle on Physiological Status of Young Veal Calves. J. Dairy Sci. 2020, 103, 3505–3520. [Google Scholar] [CrossRef] [PubMed]
- Renaud, D.L.; Duffield, T.F.; LeBlanc, S.J.; Ferguson, S.; Haley, D.B.; Kelton, D.F. Risk Factors Associated with Mortality at a Milk-Fed Veal Calf Facility: A Prospective Cohort Study. J. Dairy Sci. 2018, 101, 2659–2668. [Google Scholar] [CrossRef]
- Goetz, H.M.; Kelton, D.F.; Costa, J.H.C.; Winder, C.B.; Renaud, D.L. Identification of Biomarkers Measured upon Arrival Associated with Morbidity, Mortality, and Average Daily Gain in Grain-Fed Veal Calves. J. Dairy Sci. 2021, 104, 874–885. [Google Scholar] [CrossRef]
- Bähler, C.; Steiner, A.; Luginbühl, A.; Ewy, A.; Posthaus, H.; Strabel, D.; Kaufmann, T.; Regula, G. Risk Factors for Death and Unwanted Early Slaughter in Swiss Veal Calves Kept at a Specific Animal Welfare Standard. Res. Vet. Sci. 2012, 92, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Linton, A.H.; Howe, K.; Osborne, A.D. The Effects of Feeding Tetracycline, Nitrovin and Quindoxin on the Drug-Resistance of Coli-Aerogenes Bacteria from Calves and Pigs. J. Appl. Bacteriol. 1975, 38, 255–275. [Google Scholar] [CrossRef]
- Graveland, H.; Wagenaar, J.A.; Heesterbeek, H.; Mevius, D.; Duijkeren, E.; Heederik, D. Methicillin Resistant Staphylococcus Aureus ST398 in Veal Calf Farming: Human MRSA Carriage Related with Animal Antimicrobial Usage and Farm Hygiene. PLoS ONE 2010, 5, e10990. [Google Scholar] [CrossRef]
- Dorado-García, A.; Bos, M.E.H.; Graveland, H.; Cleef, B.A.G.L.; Verstappen, K.M.; Kluytmans, J.A.J.W.; Wagenaar, J.A.; Heederik, D.J.J. Risk Factors for Persistence of Livestock-Associated MRSA and Environmental Exposure in Veal Calf Farmers and Their Family Members: An Observational Longitudinal Study. BMJ Open 2013, 3, e003272. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.H.; Moore, L.S.P.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J.V. Understanding the Mechanisms and Drivers of Antimicrobial Resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- Nichols, M.; Gollarza, L.; Sockett, D.; Aulik, N.; Patton, E.; Francois Watkins, L.K.; Gambino-Shirley, K.J.; Folster, J.P.; Chen, J.C.; Tagg, K.A.; et al. Outbreak of Multidrug-Resistant Salmonella Heidelberg Infections Linked to Dairy Calf Exposure, United States, 2015–2018. Foodborne Pathog. Dis. 2022, 19, 199–208. [Google Scholar] [CrossRef]
- Pardon, B.; Bleecker, K.; Dewulf, J.; Callens, J.; Boyen, F.; Catry, B.; Deprez, P. Prevalence of Respiratory Pathogens in Diseased, Non-Vaccinated, Routinely Medicated Veal Calves. Vet. Rec. 2011, 169, 278. [Google Scholar] [CrossRef] [PubMed]
- Catry, B.; Haesebrouck, F.; Vliegher, S.; Feyen, B.; Vanrobaeys, M.; Opsomer, G.; Schwarz, S.; Kruif, A. Variability in Acquired Resistance of Pasteurella and Mannheimia Isolates from the Nasopharynx of Calves, with Particular Reference to Different Herd Types. Microb. Drug Resist. 2005, 11, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Catry, B.; Laevens, H.; Devriese, L.A.; Opsomer, G.; Kruif, A. Antimicrobial Resistance in Livestock. J. Vet. Pharmacol. Ther. 2003, 26, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Chakravarty, A.K. Disease Resistance for Different Livestock Species. Genet. Breed. Dis. Resist. Livest. 2020, 271–296. [Google Scholar] [CrossRef]
- Schönecker, L.; Schnyder, P.; Schüpbach-Regula, G.; Meylan, M.; Overesch, G. Prevalence and Antimicrobial Resistance of Opportunistic Pathogens Associated with Bovine Respiratory Disease Isolated from Nasopharyngeal Swabs of Veal Calves in Switzerland. Prev. Vet. Med. 2020, 185, 105182. [Google Scholar] [CrossRef]
- Schönecker, L.; Schnyder, P.; Overesch, G.; Schüpbach-Regula, G.; Meylan, M. Associations between Antimicrobial Treatment Modalities and Antimicrobial Susceptibility in Pasteurellaceae and E. Coli Isolated from Veal Calves under Field Conditions. Vet. Microbiol. 2019, 236, 108363. [Google Scholar] [CrossRef]
- Heuvelink, A.; Reugebrink, C.; Mars, J. Antimicrobial Susceptibility of Mycoplasma Bovis Isolates from Veal Calves and Dairy Cattle in the Netherlands. Vet. Microbiol. 2016, 189, 1–7. [Google Scholar] [CrossRef]
- Bokma, J.; Gille, L.; Bleecker, K.; Callens, J.; Haesebrouck, F.; Pardon, B.; Boyen, F. Antimicrobial Susceptibility of Mycoplasma Bovis Isolates from Veal, Dairy and Beef Herds. Antibiotics 2020, 9, 882. [Google Scholar] [CrossRef]
- Rérat, M.; Albini, S.; Jaquier, V.; Hüssy, D. Bovine Respiratory Disease: Efficacy of Different Prophylactic Treatments in Veal Calves and Antimicrobial Resistance of Isolated Pasteurellaceae. Prev. Vet. Med. 2012, 103, 265–273. [Google Scholar] [CrossRef]
- Vogel, G.; Nicolet, J.; Martig, J.; Tschudi, P.; Meylan, M. Pneumonia in Calves: Characterization of the Bacterial Spectrum and the Resistance Patterns to Antimicrobial Drugs. Schweiz. Arch. Tierheilkd. 2001, 143, 341–350. [Google Scholar]
- Lysnyansky, I.; Ayling, R.D. Mycoplasma Bovis: Mechanisms of Resistance and Trends in Antimicrobial Susceptibility. Front. Microbiol. 2016, 7, 595. [Google Scholar] [CrossRef] [PubMed]
- Madec, J.Y.; Lazizzera, C.; Châtre, P.; Meunier, D.; Martin, S.; Lepage, G.; Ménard, M.F.; Lebreton, P.; Rambaud, T. Prevalence of Fecal Carriage of Acquired Expanded-Spectrum Cephalosporin Resistance in Enterobacteriaceae Strains from Cattle in France. J. Clin. Microbiol. 2008, 46, 1566–1567. [Google Scholar] [CrossRef] [PubMed]
- Hordijk, J.; Wagenaar, J.A.; Giessen, A.; Dierikx, C.; Essen-Zandbergen, A.; Veldman, K.; Kant, A.; Mevius, D. Increasing Prevalence and Diversity of ESBL/AmpC-Type β-Lactamase Genes in Escherichia Coli Isolated from Veal Calves from 1997 to 2010. J. Antimicrob. Chemother. 2013, 68, 1970–1973. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Hörmansdorfer, S.; Messelhäusser, U.; Käsbohrer, A.; Sauter-Louis, C.; Mansfeld, R. Prevalence of Extended-Spectrum β-Lactamase-Producing Escherichia Coli on Bavarian Dairy and Beef Cattle Farms. Appl. Environ. Microbiol. 2013, 79, 3027–3032. [Google Scholar] [CrossRef] [PubMed]
- DeFrancesco, K.A.; Cobbold, R.N.; Rice, D.H.; Besser, T.E.; Hancock, D.D. Antimicrobial Resistance of Commensal Escherichia Coli from Dairy Cattle Associated with Recent Multi-Resistant Salmonellosis Outbreaks. Vet. Microbiol. 2004, 98, 55–61. [Google Scholar] [CrossRef]
- Springer, H.R.; Denagamage, T.N.; Fenton, G.D.; Haley, B.J.; Kessel, J.A.S.; Hovingh, E.P. Antimicrobial Resistance in Fecal Escherichia Coli and Salmonella Enterica from Dairy Calves: A Systematic Review. Foodborne Pathog. Dis. 2019, 16, 23–34. [Google Scholar] [CrossRef]
- Salaheen, S.; Kim, S.W.; Cao, H.; Wolfgang, D.R.; Hovingh, E.; Karns, J.S.; Haley, B.J.; Kessel, J.A.S. Antimicrobial Resistance among Escherichia Coli Isolated from Veal Calf Operations in Pennsylvania. Foodborne Pathog. Dis. 2019, 16, 74–80. [Google Scholar] [CrossRef]
- Hutchinson, H.; Finney, S.; Muñoz-Vargas, L.; Feicht, S.; Masterson, M.; Habing, G. Prevalence and Transmission of Antimicrobial Resistance in a Vertically Integrated Veal Calf Production System. Foodborne Pathog Dis 2017, 14, 711–718. [Google Scholar] [CrossRef]
- Ceccarelli, D.; Hesp, A.; Goot, J.; Joosten, P.; Sarrazin, S.; Wagenaar, J.A.; Dewulf, J.; Mevius, D.J. Antimicrobial Resistance Prevalence in Commensal Escherichia Coli from Broilers, Fattening Turkeys, Fattening Pigs and Veal Calves in European Countries and Association with Antimicrobial Usage at Country Level. J. Med. Microbiol. 2020, 69, 537–547. [Google Scholar] [CrossRef]
- Berge, A.C.B.; Atwill, E.R.; Sischo, W.M. Animal and Farm Influences on the Dynamics of Antibiotic Resistance in Faecal Escherichia Coli in Young Dairy Calves. Prev. Vet. Med. 2005, 69, 25–38. [Google Scholar] [CrossRef]
- Hoyle, D.V.; Shaw, D.J.; Knight, H.I.; Davison, H.C.; Pearce, M.C.; Low, C.; Gunn, G.J.; Woolhouse, M.E.J. Age-Related Decline in Carriage of Ampicillin-Resistant Escherichia Coli in Young Calves. Appl. Environ. Microbiol. 2004, 70, 6927–6930. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, S.C.; Straley, B.A.; Hegde, N.V.; Sawant, A.A.; DebRoy, C.; Jayarao, B.M. Molecular Epidemiology of Ceftiofur-Resistant Escherichia Coli Isolates from Dairy Calves. Appl. Environ. Microbiol. 2006, 72, 3940–3948. [Google Scholar] [CrossRef]
- Hordijk, J.; Wagenaar, J.A.; Kant, A.; Essen-Zandbergen, A.; Dierikx, C.; Veldman, K.; Wit, B.; Mevius, D. Cross-Sectional Study on Prevalence and Molecular Characteristics of Plasmid Mediated ESBL/AmpC-Producing Escherichia Coli Isolated from Veal Calves at Slaughter. PLoS ONE 2013, 8, e65681. [Google Scholar] [CrossRef] [PubMed]
- Hordijk, J.; Mevius, D.J.; Kant, A.; Bos, M.E.H.; Graveland, H.; Bosman, A.B.; Hartskeerl, C.M.; Heederik, D.J.J.; Wagenaar, J.A. Within-Farm Dynamics of ESBL/AmpC-Producing Escherichia Coli in Veal Calves: A Longitudinal Approach. J. Antimicrob. Chemother. 2013, 68, 2468–2476. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Linton, A.H.; Osborne, A.D. A Longitudinal Study of Escherichia Coli in Cows and Calves with Special Reference to the Distribution of O-Antigen Types and Antibiotic Resistance. J. Appl. Bacteriol. 1976, 40, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Hinton, M.; Linton, A.M.; Hedges, A.J. The Ecology of Escherichia Coli in Calves Reared as Dairy-Cow Replacements. J. Appl. Bacteriol. 1985, 58, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Haenni, M.; Beyrouthy, R.; Lupo, A.; Châtre, P.; Madec, J.Y.; Bonnet, R. Epidemic Spread of Escherichia Coli ST744 Isolates Carrying Mcr-3 and BlaCTX-M-55 in Cattle in France. J. Antimicrob. Chemother. 2018, 73, 533. [Google Scholar] [CrossRef]
- Karp, B.E.; Tate, H.; Plumblee, J.R.; Dessai, U.; Whichard, J.M.; Thacker, E.L.; Hale, K.R.; Wilson, W.; Friedman, C.R.; Griffin, P.M.; et al. National Antimicrobial Resistance Monitoring System: Two Decades of Advancing Public Health through Integrated Surveillance of Antimicrobial Resistance. Foodborne Pathog. Dis. 2017, 14, 545–557. [Google Scholar] [CrossRef]
- Tate, H.; Li, C.; Nyirabahizi, E.; Tyson, G.H.; Zhao, S.; Rice-Trujillo, C.; Jones, S.B.; Ayers, S.; M’Ikanatha, N.M.; Hanna, S.; et al. A National Antimicrobial Resistance Monitoring System Survey of Antimicrobial-Resistant Foodborne Bacteria Isolated from Retail Veal in the United States. J. Food Prot. 2021, 84, 1749–1759. [Google Scholar] [CrossRef]
- Lianou, A.; Panagou, E.Z.; Nychas, G.J.E. Meat Safety—I Foodborne Pathogens and Other Biological Issues. In Lawrie’s Meat Science; Woodhead Publishing: Sawston, UK, 2017; pp. 521–552. [Google Scholar] [CrossRef]
- Cook, A.; Reid-Smith, R.J.; Irwin, R.J.; Mcewen, S.A.; Young, V.; Butt, K.; Ribble, C. Antimicrobial Resistance in Escherichia Coli Isolated from Retail Milk-Fed Veal Meat from Southern Ontario, Canada. J. Food Prot. 2011, 74, 1328–1333. [Google Scholar] [CrossRef]
- Bosilevac, J.M.; Zhilyaev, S.; Wang, R.; Luedtke, B.E.; Wheeler, T.L.; Koohmaraie, M. Prevalence and Characterization of Salmonella Present during Veal Harvest. J. Food Prot. 2019, 82, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Webb, H.E.; Brichta-Harhay, D.M.; Brashears, M.M.; Nightingale, K.K.; Arthur, T.M.; Bosilevac, J.M.; Kalchayanand, N.; Schmidt, J.W.; Wang, R.; Granier, S.A.; et al. Salmonella in Peripheral Lymph Nodes of Healthy Cattle at Slaughter. Front. Microbiol. 2017, 8, 2214. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Vargas, L.; Finney, S.K.; Hutchinson, H.; Masterson, M.A.; Habing, G. Impact of Clinical Salmonellosis in Veal Calves on the Recovery of Salmonella in Lymph Nodes at Harvest. Foodborne Pathog. Dis. 2017, 14, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Varma, J.K.; Mølbak, K.; Barrett, T.J.; Beebe, J.L.; Jones, T.F.; Rabatsky-Ehr, T.; Smith, K.E.; Vugia, D.J.; Chang, H.G.H.; Angulo, F.J. Antimicrobial-Resistant Nontyphoidal Salmonella Is Associated with Excess Bloodstream Infections and Hospitalizations. J. Infect. Dis. 2005, 191, 554–561. [Google Scholar] [CrossRef]
- Harvey, R.R.; Friedman, C.R.; Crim, S.M.; Judd, M.; Barrett, K.A.; Tolar, B.; Folster, J.P.; Griffin, P.M.; Brown, A.C. Epidemiology of Salmonella Enterica Serotype Dublin Infections among Humans, United States, 1968–2013. Emerg. Infect. Dis. 2017, 23, 1493–1501. [Google Scholar] [CrossRef]
- McDonough, P.L.; Fogelman, D.; Shin, S.J.; Brunner, M.A.; Lein, D.H. Salmonella Enterica Serotype Dublin Infection: An Emerging Infectious Disease for the Northeastern United States. J. Clin. Microbiol. 1999, 37, 2418–2427. [Google Scholar] [CrossRef]
- Locke, S.R.; Pempek, J.A.; Meyer, R.; Portillo-Gonzalez, R.; Sockett, D.; Aulik, N.; Habing, G. Prevalence and Sources of Salmonella Lymph Node Infection in Special-Fed Veal Calves. J. Food Prot. 2022, 85, 906–917. [Google Scholar] [CrossRef]
- Cook, A.; Reid-Smith, R.J.; Irwin, R.J.; Mcewen, S.; Young, V.; Ribble, C. Antimicrobial Resistance in Campylobacter, Salmonella, and Escherichia Coli Isolated from Retail Grain-Fed Veal Meat from Southern Ontario, Canada. J. Food Prot. 2011, 74, 1245–1251. [Google Scholar] [CrossRef]
- Gaulin, C.; Ramsay, D.; Dion, R.; Simard, M.; Gariépy, C.; Levac, É.; Hammond-Collins, K.; Michaud-Dumont, M.; Gignac, M.; Fiset, M. Veal Liver as Food Vehicle for Human Campylobacter Infections. Emerg. Infect. Dis. 2018, 24, 1130–1133. [Google Scholar] [CrossRef]
- Labio, E.; Regula, G.; Steiner, A.; Miserez, R.; Thomann, A.; Ledergerber, U. Antimicrobial Resistance in Bacteria from Swiss Veal Calves at Slaughter. Zoonoses Pub. Health 2007, 54, 344–352. [Google Scholar] [CrossRef]
- Barkema, H.W.; Keyserlingk, M.A.G.; Kastelic, J.P.; Lam, T.J.G.M.; Luby, C.; Roy, J.P.; LeBlanc, S.J.; Keefe, G.P.; Kelton, D.F. Invited Review: Changes in the Dairy Industry Affecting Dairy Cattle Health and Welfare. J. Dairy Sci. 2015, 98, 7426–7445. [Google Scholar] [CrossRef] [PubMed]
- Santinello, M.; Diana, A.; Marchi, M.; Scali, F.; Bertocchi, L.; Lorenzi, V.; Alborali, G.L.; Penasa, M. Promoting Judicious Antimicrobial Use in Beef Production: The Role of Quarantine. Animals 2022, 12, 116. [Google Scholar] [CrossRef] [PubMed]
- Menéndez González, S.; Steiner, A.; Gassner, B.; Regula, G. Antimicrobial Use in Swiss Dairy Farms: Quantification and Evaluation of Data Quality. Prev. Vet. Med. 2010, 95, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Pardon, B.; Catry, B.; Boone, R.; Theys, H.; Bleecker, K.; Dewulf, J.; Deprez, P. Characteristics and Challenges of the Modern Belgian Veal Industry. Vlaams Diergeneeskd. Tijdschr. 2014, 83, 155–163. [Google Scholar] [CrossRef]
- Speksnijder, D.C.; Jaarsma, A.D.C.; Gugten, A.C.; Verheij, T.J.M.; Wagenaar, J.A. Determinants Associated with Veterinary Antimicrobial Prescribing in Farm Animals in the Netherlands: A Qualitative Study. Zoonoses Pub. Health 2015, 62 (Suppl. 1), 39–51. [Google Scholar] [CrossRef]
- Rell, J.; Wunsch, N.; Home, R.; Kaske, M.; Walkenhorst, M.; Vaarst, M. Stakeholders’ Perceptions of the Challenges to Improving Calf Health and Reducing Antimicrobial Use in Swiss Veal Production. Prev. Vet. Med. 2020, 179, 104970. [Google Scholar] [CrossRef]
- Speksnijder, D.C.; Jaarsma, D.A.C.; Verheij, T.J.M.; Wagenaar, J.A. Attitudes and Perceptions of Dutch Veterinarians on Their Role in the Reduction of Antimicrobial Use in Farm Animals. Prev. Vet. Med. 2015, 121, 365–373. [Google Scholar] [CrossRef]
- Pempek, J.A.; Cheng, T.Y.; Masterson, M.A.; Habing, G.G. A Survey of Antimicrobial Use Practices on Veal Farms in the United States; The Ohio State University: Columbus, OH, USA, 2022; manuscript in preparation. [Google Scholar]
- Hockenhull, J.; Turner, A.E.; Reyher, K.K.; Barrett, D.C.; Jones, L.; Hinchliffe, S.; Buller, H.J. Antimicrobial Use in Food-Producing Animals: A Rapid Evidence Assessment of Stakeholder Practices and Beliefs. Vet. Rec. 2017, 181, 510. [Google Scholar] [CrossRef]
- Golding, S.E.; Ogden, J.; Higgins, H.M. Shared Goals, Different Barriers: A Qualitative Study of UK Veterinarians’ and Farmers’ Beliefs about Antimicrobial Resistance and Stewardship. Front. Vet. Sci. 2019, 6, 132. [Google Scholar] [CrossRef]
- Apley, M.D.; Godden, S.; Hope, K.J.; Schrag, N.F.D. Antimicrobial Use Monitoring. Am. Assoc. Bov. Pract. Conf. Proc. 2018, 91–95. [Google Scholar] [CrossRef]
- Sanders, P.; Vanderhaeghen, W.; Fertner, M.; Fuchs, K.; Obritzhauser, W.; Agunos, A.; Carson, C.; Borck Høg, B.; Dalhoff Andersen, V.; Chauvin, C.; et al. Monitoring of Farm-Level Antimicrobial Use to Guide Stewardship: Overview of Existing Systems and Analysis of Key Components and Processes. Front. Vet. Sci. 2020, 7, 540. [Google Scholar] [CrossRef] [PubMed]
- FDA. Finalizes Guidance to Bring Remaining Approved Over-the-Counter Medically Important Antimicrobial Drugs Used for Animals Under Veterinary Oversight|FDA. Available online: https://www.fda.gov/animal-veterinary/cvm-updates/fda-finalizes-guidance-bring-remaining-approved-over-counter-medically-important-antimicrobial-drugs (accessed on 26 June 2022).
- Cobo-angel, C.; Gohar, B.; Leblanc, S.J. Values and Risk Perception Shape Canadian Dairy Farmers’ Attitudes toward Prudent Use of Antimicrobials. Antibiotics 2022, 11, 550. [Google Scholar] [CrossRef] [PubMed]
- Ekakoro, J.E.; Caldwell, M.; Strand, E.B.; Okafor, C.C. Drivers, Alternatives, Knowledge, and Perceptions towards Antimicrobial Use among Tennessee Beef Cattle Producers: A Qualitative Study. BMC Vet. Res. 2019, 15, 16. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).