Effects of Nutritional Mode on the Physiological and Biochemical Characteristics of the Mixotrophic Flagellate Poterioochromonas malhamensis and the Potential Ecological Implications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Cultivation of P. malhamensis
2.2. Purification of P. malhamensis Culture
2.3. Cultivation of P. malhamensis with Different Nutritional Modes
2.4. Growth and Cell Morphology of P. malhamensis with Different Nutritional Modes
2.5. Analysis of Biochemical Composition of P. malhamensis with Different Nutritional Modes
2.6. Grazing Abilities of Paramecium caudatum on P. malhamensis Cells with Different Trophic Modes
2.7. Statistical Analyses
3. Results
3.1. Purification of P. malhamensis Culture
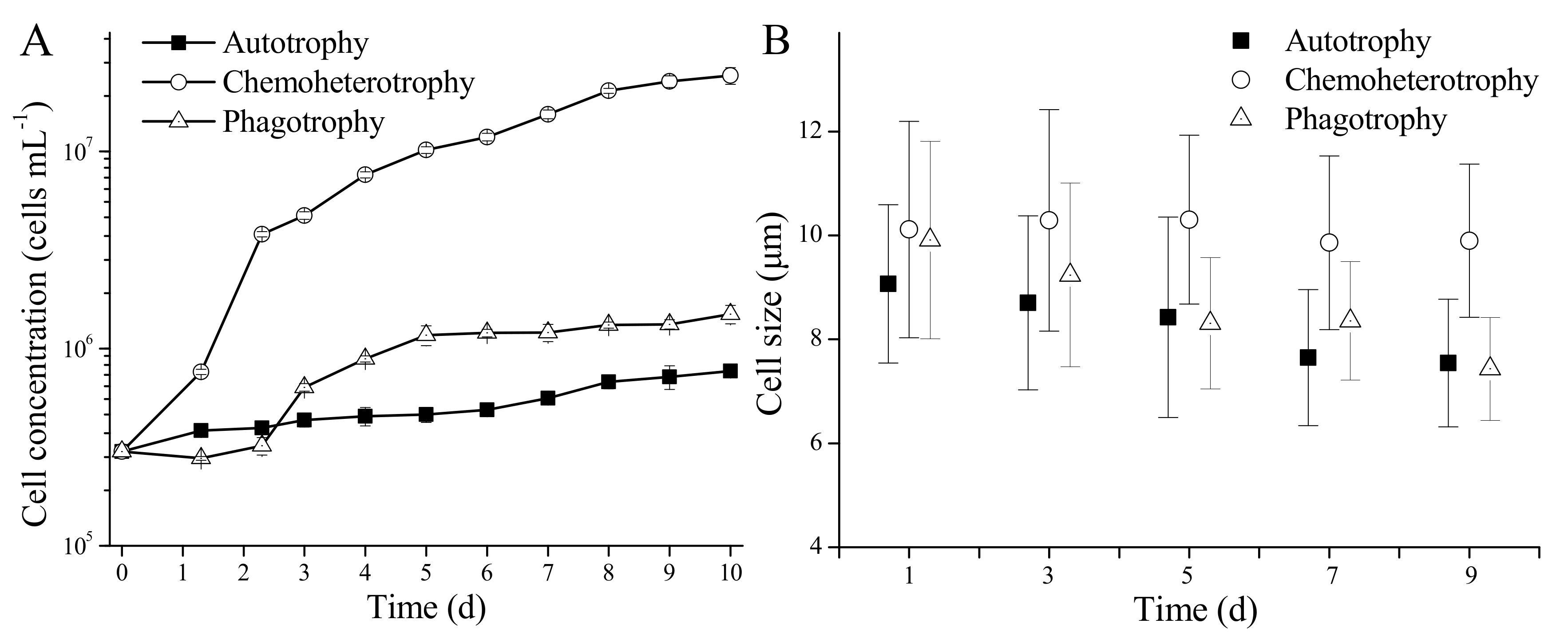
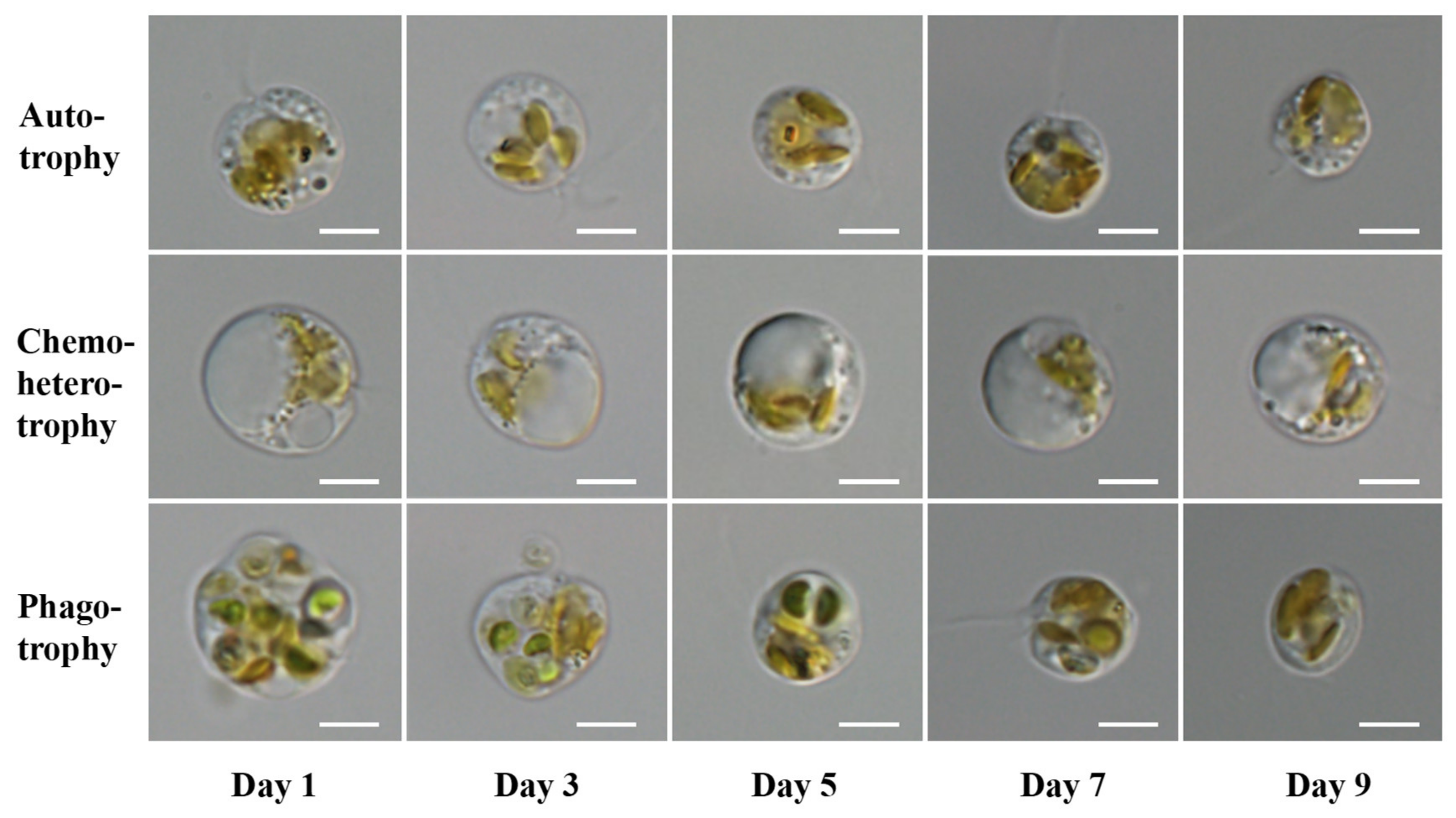
3.2. Growth and Cell Morphology of P. malhamensis with Different Nutritional Modes
3.3. Biochemical Composition of P. malhamensis with Different Nutritional Modes
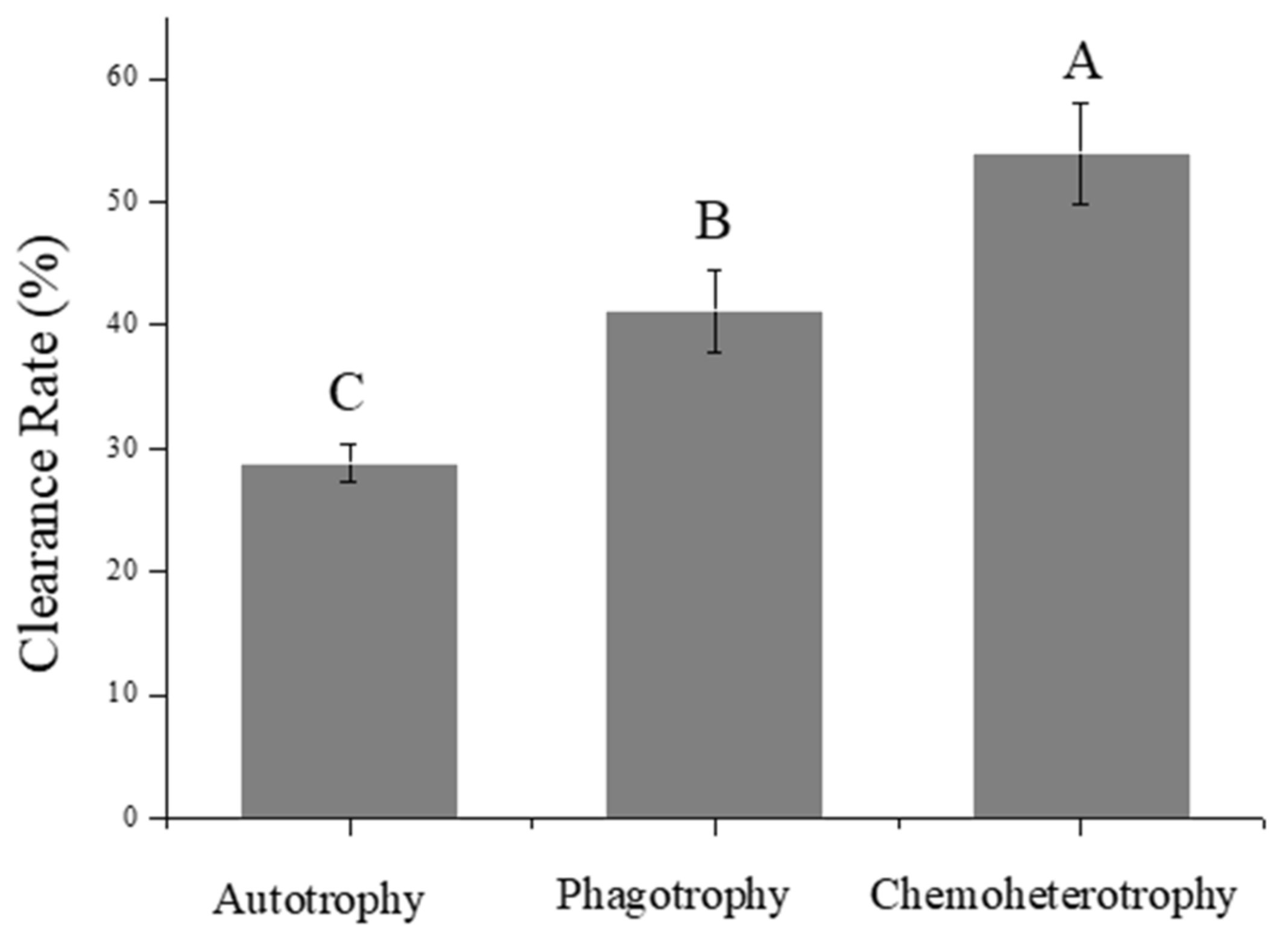
3.4. Grazing Abilities of Paramecium caudatum on P. malhamensis with Different Trophic Modes
4. Discussion
4.1. Purification and Cultivation of P. malhamensis
4.2. Effects of Nutritional Mode on the Morphological and Biochemical Characteristics of P. malhamensis
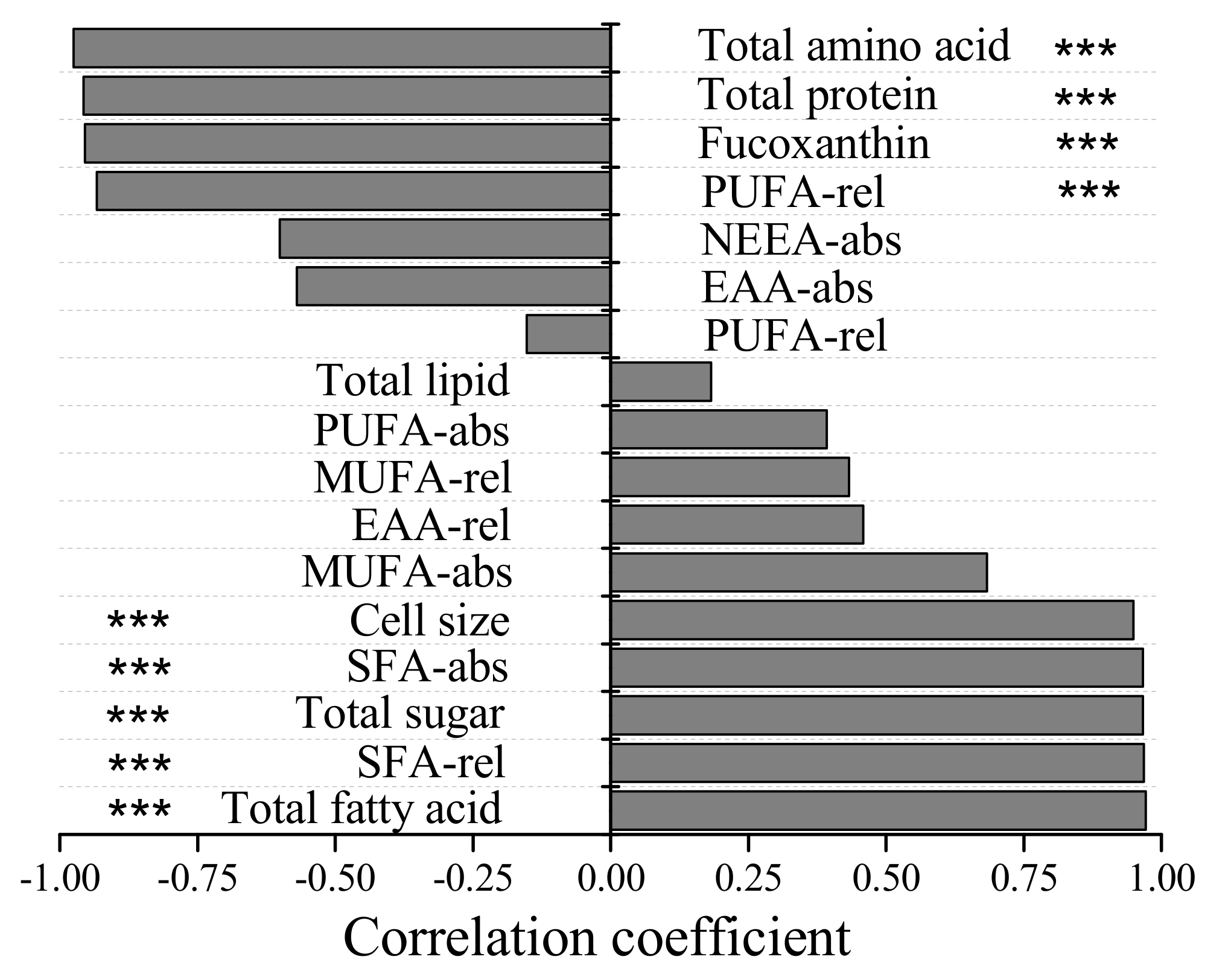
4.3. Effects of Variation in the Biochemical Composition of P. malhamensis, Resulting from Different Nutritional Modes, on Its Predators’ Feeding Behavior and the Implications for Aquatic Food Webs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burkholder, J.M.; Glibert, P.M.; Skelton, H.M. Mixotrophy, a major mode of nutrition for harmful algal species in eutrophic waters. Harmful Algae 2008, 8, 77–93. [Google Scholar] [CrossRef]
- Jones, R.I. Mixotrophy in planktonic protists: An overview. Freshw. Biol. 2000, 45, 219–226. [Google Scholar] [CrossRef]
- Stoecker, D.K.; Hansen, P.J.; Caron, D.A.; Mitra, A. Mixotrophy in the marine plankton. Annu. Rev. Mar. Sci. 2017, 9, 311–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holen, D.A.; Boraas, M.E. Mixotrophy in chrysophytes. In Chrysophyte Algae: Ecology, Phylogeny and Development; Cambridge University Press: Cambridge, UK, 1995; pp. 119–140. [Google Scholar]
- Moorthi, S.; Caron, D.A.; Gast, R.J.; Sanders, R.W. Mixotrophy: A widespread and important ecological strategy for planktonic and sea-ice nanoflagellates in the Ross Sea, Antarctica. Aquat. Microb. Ecol. 2009, 54, 269–277. [Google Scholar] [CrossRef]
- Sanders, R.W. Alternative nutritional strategies in protists: Symposium introduction and a review of freshwater protists that combine photosynthesis and heterotrophy. J. Eukaryot. Microbiol. 2011, 58, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Sanders, R.W.; Gast, R.J. Bacterivory by phototrophic picoplankton and nanoplankton in Arctic waters. FEMS Microbiol. Ecol. 2012, 82, 242–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, R.W.; Berninger, U.G.; Lim, E.L.; Kemp, P.F.; Caron, D.A. Heterotrophic and mixotrophic nanoplankton predation on picoplankton in the Sargasso Sea and on Georges Bank. Mar. Ecol. Prog. Ser. 2000, 192, 103–118. [Google Scholar] [CrossRef]
- Azam, F.; Fenchel, T.; Field, J.; Grey, J.; Meyer, R.L.; Thingstad, F. The ecological role of water-column microbes. Mar. Ecol. Prog. Ser. 1983, 10, 257–263. [Google Scholar] [CrossRef]
- Unrein, F.; Gasol, J.M.; Not, F.; Forn, I.; Massana, R. Mixotrophic haptophytes are key bacterial grazers in oligotrophic coastal waters. ISME J. 2014, 8, 164. [Google Scholar] [CrossRef] [Green Version]
- Weithoff, G.; Wacker, A. The mode of nutrition of mixotrophic flagellates determines the food quality for their consumers. Funct. Ecol. 2007, 21, 1092–1098. [Google Scholar] [CrossRef]
- Ma, M.; Gong, Y.; Hu, Q. Identification and feeding characteristics of the mixotrophic flagellate Poterioochromonas malhamensis, a microalgal predator isolated from outdoor massive Chlorella culture. Algal Res. 2018, 29, 142–153. [Google Scholar] [CrossRef]
- Zhang, X.; Watanabe, M.M. Light and electron microscopy of grazing by Poterioochromonas malhamensis (Chrysophyceae) on a range of phytoplankton taxa. J. Phycol. 1996, 32, 37–46. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, H.; Men, Y.; Yang, J.; Christoffersen, K. Feeding characteristics of a golden alga (Poterioochromonas sp.) grazing on toxic cyanobacterium Microcystis aeruginosa. Water Res. 2009, 43, 2953–2960. [Google Scholar] [CrossRef]
- Zhang, X.; Watanabe, M.M. Grazing and growth of the mixotrophic chrysomonad Poterioochromonas malhamensis feeding on algae. J. Phycol. 2001, 37, 738–743. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, H.; Warming, T.P.; Christoffersen, K.S. Life history response of Daphnia magna to a mixotrophic golden alga, Poterioochromonas sp., at different food levels. Bull. Environ. Contam. Toxicol. 2011, 87, 117–123. [Google Scholar] [CrossRef]
- Boxhorn, J.E.; Holen, D.A.; Boraas, M.E. Toxicity of the chrysophyte flagellate Poterioochromonas malhamensis to the rotifer Brachionus angularis. Hydrobiologia 1998, 387, 283–287. [Google Scholar] [CrossRef]
- Sterner, R.W.; Hagemeier, D.D.; Smith, W.L.; Smith, R.F. Phytoplankton nutrient limitation and food quality for Daphnia. Limnol. Oceanogr. 1993, 38, 857–871. [Google Scholar] [CrossRef] [Green Version]
- Stoecker, D.K.; Capuzzo, J.M. Predation on protozoa: Its importance to zooplankton. J. Plankton Res. 1990, 12, 891–908. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Cui, Y.; Zhou, Y.; Wan, M.; Wang, W.; Xie, J.; Li, Y. The effect of nutrition pattern alteration on Chlorella pyrenoidosa growth, lipid biosynthesis-related gene transcription. Bioresour. Technol. 2014, 164, 214–220. [Google Scholar] [CrossRef]
- Lalibertè, G.; de la Noüie, J. Auto-, hetero-, and mixotrophic growth of Chlamydomonas humicola (chlorophyceae) on acetate. J. Phycol. 1993, 29, 612–620. [Google Scholar] [CrossRef]
- Kryuchkova, M.; Danilushkina, A.; Lvov, Y.; Fakhrullin, R. Evaluation of toxicity of nanoclays and graphene oxide in vivo: A Paramecium caudatum study. Environ. Sci-Nano. 2016, 3, 442–452. [Google Scholar] [CrossRef] [Green Version]
- Gupta, G.S.; Kumar, A.; Senapati, V.A.; Pandey, A.K.; Shanker, R.; Dhawan, A. Laboratory scale microbial food chain to study bioaccumulation, biomagnification, and ecotoxicity of cadmium telluride quantum dots. Environ. Sci. Technol. 2017, 51, 1695–1706. [Google Scholar] [CrossRef]
- Kato, S. Laboratory culture and morphology of Colacium vesiculosum Ehrb.(Euglenophyceae). Jpn. J. Phycol. 1982, 30, 63–67. [Google Scholar]
- Corno, G.; Jurgens, K. Direct and indirect effects of protist predation on population size structure of a bacterial strain with high phenotypic plasticity. Appl. Environ. Microb. 2006, 72, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Destain, J.; Haubruge, É.; Thonart, P.; Portetelle, D.; Francis, F.; Bauwens, J.; Tarayre, C.; Brasseur, C.; Mattéotti, C.; Vandenbol, M. Isolation of an amylolytic chrysophyte, Poterioochromonas sp., from the digestive tract of the termite Reticulitermes santonensis. Biotechnol. Agron. Soc. Environ. 2014, 18, 19–31. [Google Scholar]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Sanders, R.W.; Porter, K.G.; Caron, D.A. Relationship between phototrophy and phagotrophy in the mixotrophic chrysophyte Poterioochromonas malhamensis. Microb. Ecol. 1990, 19, 97–109. [Google Scholar] [CrossRef]
- Terrado, R.; Pasulka, A.L.; Lie, A.A.; Orphan, V.J.; Heidelberg, K.B.; Caron, D.A. Autotrophic and heterotrophic acquisition of carbon and nitrogen by a mixotrophic chrysophyte established through stable isotope analysis. ISME J. 2017, 11, 2022–2034. [Google Scholar] [CrossRef] [Green Version]
- Leakey, R.; Burkill, P.; Sleigh, M. A comparison of fixatives for the estimation of abundance and biovolume of marine planktonic ciliate populations. J. Plankton Res. 1994, 16, 375–389. [Google Scholar] [CrossRef]
- Ma, M.; Wei, C.; Wang, H.; Sha, C.; Chen, M.; Gong, Y.; Hu, Q. Isolation and evaluation of a novel strain of Chlorella sorokiniana that resists grazing by the predator Poterioochromonas malhamensis. Algal Res. 2019, 38, 101429. [Google Scholar] [CrossRef]
- Wahlen, B.D.; Willis, R.M.; Seefeldt, L.C. Biodiesel production by simultaneous extraction and conversion of total lipids from microalgae, cyanobacteria, and wild mixed-cultures. Bioresour. Technol. 2011, 102, 2724–2730. [Google Scholar] [CrossRef] [PubMed]
- Billakanti, J.M.; Catchpole, O.J.; Fenton, T.A.; Mitchell, K.A.; MacKenzie, A.D. Enzyme-assisted extraction of fucoxanthin and lipids containing polyunsaturated fatty acids from Undaria pinnatifida using dimethyl ether and ethanol. Process Biochem. 2013, 48, 1999–2008. [Google Scholar] [CrossRef]
- Ye, J.; Liang, J.; Wang, L.; Markou, G. The mechanism of enhanced wastewater nitrogen removal by photo-sequencing batch reactors based on comprehensive analysis of system dynamics within a cycle. Bioresour. Technol. 2018, 260, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Laurens, L.M.; Quinn, M.; Van Wychen, S.; Templeton, D.W.; Wolfrum, E.J. Accurate and reliable quantification of total microalgal fuel potential as fatty acid methyl esters by in situ transesterification. Anal. Bioanal. Chem. 2012, 403, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Boëchat, I.G.; Weithoff, G.; Krüger, A.; Gücker, B.; Adrian, R. A biochemical explanation for the success of mixotrophy in the flagellate Ochromonas sp. Limnol. Oceanogr. 2007, 52, 1624–1632. [Google Scholar] [CrossRef]
- Chen, J.L.; Proteau, P.J.; Roberts, M.A.; Gerwick, W.H.; Slate, D.L.; Lee, R.H. Structure of malhamensilipin A, an inhibitor of protein tyrosine kinase, from the cultured chrysophyte Poterioochromonas malhamensis. J. Nat. Prod. 1994, 57, 524–527. [Google Scholar] [CrossRef]
- Ma, M.; Li, Y.; Chen, J.; Wang, F.; Yuan, L.; Li, Y.; Zhang, B.; Ye, D.; Han, D.; Jin, H.; et al. High-cell-density cultivation of the flagellate alga Poterioochromonas malhamensis for biomanufacturing the water-soluble β-1, 3-glucan with multiple biological activities. Bioresour. Technol. 2021, 337, 125447. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, H.; Hong, Y.; Yang, J. Isolation of a Poterioochromonas capable of feeding on Microcystis aeruginosa and degrading microcystin-LR. FEMS Microbiol. Lett. 2008, 288, 241–246. [Google Scholar] [CrossRef] [Green Version]
- Ma, M.; Wang, F.; Wei, C.; Chen, J.; Jin, H.; Wang, H.; Song, L.; Hu, Q.; Gong, Y. Establishment of high-cell-density heterotrophic cultivation of Poterioochromonas malhamensis contributes to achieving biological control of Microcystis. J. Appl. Phycol. 2022, 34, 423–434. [Google Scholar] [CrossRef]
- Giordano, M.; Beardall, J.; Raven, J.A. CO2 concentrating mechanisms in algae: Mechanisms, environmental modulation, and evolution. Annu. Rev. Plant Biol. 2005, 56, 99–131. [Google Scholar] [CrossRef] [Green Version]
- Lewitus, A.J.; Caron, D.A. Physiological responses of phytoflagellates to dissolved organic substrate additions. 1. Dominant role of heterotrophic nutrition in Poterioochromonas malhamensis (Chrysophyceae). Plant Cell Physiol. 1991, 32, 671–680. [Google Scholar] [CrossRef]
- Aaronson, S.; Behrens, U. Ultrastructure of an unusual contractile vacuole in several chrysomonad phytoflagellates. J. Cell Sci. 1974, 14, 1–9. [Google Scholar] [CrossRef]
- Marchello, A.E.; dos Santos, A.C.; Lombardi, A.T.; de Souza, C.W.O.; Montanhim, G.C. Physiological and ecological aspects of Chlorella sorokiniana (Trebouxiophyceae) under photoautotrophic and mixotrophic conditions. Microb. Ecol. 2018, 76, 791–800. [Google Scholar] [CrossRef]
- Zhang, L.; Li, B.; Wu, Z.; Gu, L.; Yang, Z. Changes in growth and photosynthesis of mixotrophic Ochromonas sp. in response to different concentrations of glucose. J. Appl. Phycol. 2016, 28, 2671–2678. [Google Scholar] [CrossRef]
- Galili, G.; Amir, R.; Fernie, A.R. The regulation of essential amino acid synthesis and accumulation in plants. Annu. Rev. Plant Biol. 2016, 67, 153–178. [Google Scholar] [CrossRef]
- Mitra, A.; Flynn, K.J.; Burkholder, J.M.; Berge, T.; Calbet, A.; Raven, J.A.; Granéli, E.; Glibert, P.M.; Hansen, P.J.; Stoecker, D.K. The role of mixotrophic protists in the biological carbon pump. Biogeosciences 2014, 11, 995–1005. [Google Scholar] [CrossRef] [Green Version]
- Caron, D.A. Mixotrophy stirs up our understanding of marine food webs. Proc. Natl. Acad. Sci. USA 2016, 113, 2806–2808. [Google Scholar] [CrossRef] [Green Version]
- Wichterman, R. The nutritional requirements of Paramecium: Axenic Media. In The Biology of Paramecium; Springer: Boston, MA, USA, 1986; pp. 181–196. [Google Scholar]
- Johnson, W.; Brennan, M.; Berard, D.; Morrow, J.; Hudson, K. Proteins and polysaccharides in the nutrition of Paramecium multimicronucleatun. J. Protozool. 1980, 27, 332–336. [Google Scholar] [CrossRef]
- Boenigk, J.; Stadler, P. Potential toxicity of chrysophytes affiliated with Poterioochromonas and related ‘Spumella-like’ flagellates. J. Plankton Res. 2004, 26, 1507–1514. [Google Scholar] [CrossRef] [Green Version]

| Total Lipid (%) | Total Protein (%) | Total Sugar (%) | Fucoxanthin (%) | |
|---|---|---|---|---|
| Autotrophy | 28.2 ± 0.8 | 42.1 ± 1.0 | 11.3 ± 0.5 | 0.34 ± 0.03 |
| Chemoheterotrophy | 28.4 ± 0.6 | 23.0 ± 1.6 | 42.8 ± 3.4 | 0.12 ± 0.00 |
| Phagotrophy | 27.4 ± 0.3 | 30.1 ± 0.8 | 24.7 ± 1.1 | 0.20 ± 0.01 |
| Amino Acid | % of Dry Weight (Absolute) | % of Total Amino Acid (Relative) | |||||
|---|---|---|---|---|---|---|---|
| Auto | Chemo | Phago | Auto | Chemo | Phago | ||
| Essential amino acid (EAA) | Thr | 1.80 ± 0.00 | 1.11 ± 0.05 | 1.43 ± 0.07 | 5.31 ± 0.01 | 5.15 ± 0.22 | 4.95 ± 0.24 |
| Val | 2.01 ± 0.06 | 1.22 ± 0.03 | 1.57 ± 0.05 | 5.93 ± 0.19 | 5.67 ± 0.14 | 5.42 ± 0.19 | |
| Met | 0.39 ± 0.02 | 0.12 ± 0.02 | 0.35 ± 0.05 | 1.15 ± 0.66 | 0.57 ± 0.07 | 1.21 ± 0.16 | |
| Ile | 1.72 ± 0.06 | 1.14 ± 0.08 | 1.11 ± 0.11 | 5.06 ± 0.16 | 5.31 ± 0.37 | 3.84 ± 0.37 | |
| Leu | 3.02 ± 0.04 | 1.97 ± 0.08 | 2.23 ± 0.15 | 8.88 ± 0.11 | 9.18 ± 036 | 7.69 ± 0.51 | |
| Phe | 2.19 ± 0.04 | 1.41 ± 0.03 | 1.59 ± 0.08 | 6.45 ± 0.13 | 6.55 ± 0.14 | 5.48 ± 0.28 | |
| His | 0.96 ± 0.04 | 0.71 ± 0.02 | 0.66 ± 0.03 | 2.82 ± 0.11 | 3.29 ± 0.10 | 2.28 ± 0.10 | |
| Lys | 2.70 ± 0.04 | 1.78 ± 0.06 | 1.73 ± 0.09 | 7.90 ± 0.11 | 8.30 ± 0.27 | 5.98 ± 0.32 | |
| Arg | 2.15 ± 0.08 | 1.31 ± 0.06 | 3.35 ± 0.13 | 6.30 ± 0.22 | 6.10 ± 0.29 | 11.57 ± 0.46 | |
| Total | 16.94 ± 0.12 | 10.78 ± 0.31 | 14.04 ± 0.74 | 49.80 ± 0.34 | 50.11 ± 1.42 | 48.41 ± 2.56 | |
| Non-essential amino acid (NEAA) | Asp | 4.21 ± 0.10 | 2.53 ± 0.05 | 3.27 ± 0.19 | 12.38 ± 0.29 | 11.76 ± 0.24 | 11.27 ± 0.65 |
| Ser | 1.77 ± 0.05 | 1.12 ± 0.05 | 1.31 ± 0.07 | 5.21 ± 0.15 | 5.23 ± 0.23 | 4.52 ± 0.24 | |
| Glu | 4.23 ± 0.09 | 2.87 ± 0.12 | 4.26 ± 0.18 | 12.42 ± 0.27 | 13.33 ± 0.56 | 14.70 ± 0.61 | |
| Gly | 1.77 ± 0.06 | 1.14 ± 0.05 | 1.59 ± 0.07 | 5.20 ± 0.19 | 5.32 ± 0.22 | 5.49 ± 0.23 | |
| Ala | 2.13 ± 0.04 | 1.26 ± 0.04 | 2.06 ± 0.12 | 6.24 ± 0.12 | 5.88 ± 0.20 | 7.11 ± 0.40 | |
| Cys | 0.14 ± 0.01 | 0.06 ± 0.03 | 0.18 ± 0.05 | 0.27 ± 0.24 | 0.30 ± 0.12 | 0.62 ± 0.17 | |
| Tyr | 1.27 ± 0.04 | 0.76 ± 0.04 | 1.04 ± 0.03 | 3.72 ± 0.12 | 3.55 ± 0.18 | 3.60 ± 0.11 | |
| Pro | 1.62 ± 0.12 | 0.97 ± 0.05 | 1.24 ± 0.05 | 4.76 ± 0.34 | 4.51 ± 0.22 | 4.28 ± 0.19 | |
| Total | 17.14 ± 0.31 | 10.73 ± 0.34 | 14.96 ± 0.72 | 50.20 ± 0.91 | 49.89 ± 1.58 | 51.59 ± 2.48 | |
| Total | 34.08 ± 0.31 | 21.51 ± 0.63 | 29.00 ± 1.44 | 100 | 100 | 100 | |
| Fatty Acid | %, of Dry Weight (Absolute) | %, of Total Fatty Acid (Relative) | ||||
|---|---|---|---|---|---|---|
| Auto | Chemo | Phago | Auto | Chemo | Phago | |
| C14:0 | 1.66 ± 0.04 | 6.29 ± 0.64 | 1.18 ± 0.10 | 12.20 ± 0.16 | 24.38 ± 0.06 | 5.74 ± 0.34 |
| C15:0 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.03 ± 0.00 | 0.14 ± 0.01 | 0.02 ± 0.01 | 0.14 ± 0.00 |
| C16:0 | 1.36 ± 0.03 | 4.51 ± 0.45 | 5.17 ± 0.13 | 10.06 ± 0.13 | 17.49 ± 0.01 | 25.07 ± 0.69 |
| C17:0 | 0.03 ± 0.00 | 0.04 ± 0.00 | 0.08 ± 0.00 | 0.21 ± 0.01 | 0.16 ± 0.01 | 0.39 ± 0.01 |
| C18:0 | 0.14 ± 0.00 | 2.01 ± 0.20 | 0.86 ± 0.02 | 1.03 ± 0.02 | 7.80 ± 0.01 | 4.16 ± 0.09 |
| C22:0 | 0.08 ± 0.00 | 0.10 ± 0.01 | 0.05 ± 0.01 | 0.56 ± 0.03 | 0.39 ± 0.01 | 0.24 ± 0.02 |
| ∑SFA | 3.29 ± 0.08 | 12.96 ± 1.31 | 7.37 ± 0.25 | 24.22 ± 0.35 | 50.24 ± 0.11 | 35.74 ± 1.15 |
| C16:1 | 0.05 ± 0.00 | 0.49 ± 0.04 | 0.16 ± 0.00 | 0.40 ± 0.02 | 1.89 ± 0.02 | 0.78 ± 0.04 |
| C17:1 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.08 ± 0.00 | 0.078 ± 0.09 | 0.04 ± 0.02 | 0.38 ± 0.01 |
| C18:1 | 0.19 ± 0.00 | 1.47 ± 0.13 | 2.30 ± 0.07 | 1.41 ± 0.05 | 5.71 ± 0.08 | 11.15 ± 0.41 |
| C22:1 | 0.23 ± 0.01 | 0.78 ± 0.09 | 0.21 ± 0.03 | 1.68 ± 0.07 | 3.02 ± 0.05 | 1.03 ± 0.12 |
| ∑MUFA | 0.48 ± 0.04 | 2.75 ± 0.27 | 2.75 ± 0.11 | 3.56 ± 0.24 | 10.66 ± 0.18 | 13.35 ± 0.57 |
| C18:2 | 2.44 ± 0.07 | 5.56 ± 0.55 | 5.38 ± 0.14 | 18.01 ± 0.13 | 21.57 ± 0.01 | 26.11 ± 0.14 |
| C18:3 | 3.39 ± 0.08 | 1.02 ± 0.10 | 3.64 ± 0.19 | 24.96 ± 0.08 | 3.94 ± 0.01 | 17.67 ± 0.50 |
| C18:4 | 0.14 ± 0.00 | 0.06 ± 0.01 | 0.03 ± 0.01 | 1.02 ± 0.01 | 0.22 ± 0.01 | 0.16 ± 0.02 |
| C20:5 | 0.45 ± 0.01 | 0.25 ± 0.02 | 0.23 ± 0.02 | 3.33 ± 0.05 | 0.98 ± 0.01 | 1.09 ± 0.06 |
| C22:5 | 2.99 ± 0.07 | 2.97 ± 0.30 | 0.99 ± 0.08 | 22.01 ± 0.29 | 11.50 ± 0.03 | 4.79 ± 0.29 |
| C22:6 | 0.39 ± 0.01 | 0.23 ± 0.01 | 0.22 ± 0.00 | 2.87 ± 0.06 | 0.89 ± 0.03 | 1.07 ± 0.01 |
| ∑PUFA | 9.80 ± 0.25 | 10.09 ± 1.00 | 10.49 ± 0.43 | 72.21 ± 0.61 | 39.10 ± 0.10 | 50.89 ± 1.03 |
| Total | 13.57 ± 0.32 | 25.80 ± 2.57 | 20.61 ± 0.56 | 100 | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, M.; Wei, C.; Chen, M.; Wang, H.; Gong, Y.; Hu, Q. Effects of Nutritional Mode on the Physiological and Biochemical Characteristics of the Mixotrophic Flagellate Poterioochromonas malhamensis and the Potential Ecological Implications. Microorganisms 2022, 10, 852. https://doi.org/10.3390/microorganisms10050852
Ma M, Wei C, Chen M, Wang H, Gong Y, Hu Q. Effects of Nutritional Mode on the Physiological and Biochemical Characteristics of the Mixotrophic Flagellate Poterioochromonas malhamensis and the Potential Ecological Implications. Microorganisms. 2022; 10(5):852. https://doi.org/10.3390/microorganisms10050852
Chicago/Turabian StyleMa, Mingyang, Chaojun Wei, Man Chen, Hongxia Wang, Yingchun Gong, and Qiang Hu. 2022. "Effects of Nutritional Mode on the Physiological and Biochemical Characteristics of the Mixotrophic Flagellate Poterioochromonas malhamensis and the Potential Ecological Implications" Microorganisms 10, no. 5: 852. https://doi.org/10.3390/microorganisms10050852
APA StyleMa, M., Wei, C., Chen, M., Wang, H., Gong, Y., & Hu, Q. (2022). Effects of Nutritional Mode on the Physiological and Biochemical Characteristics of the Mixotrophic Flagellate Poterioochromonas malhamensis and the Potential Ecological Implications. Microorganisms, 10(5), 852. https://doi.org/10.3390/microorganisms10050852






