Infectious Uveitis in Horses and New Insights in Its Leptospiral Biofilm-Related Pathogenesis
Abstract
1. Introduction
2. Equine Leptospirosis
3. Historical and Preliminary Remarks on the Etiology of ERU
3.1. Leptospirosis and Autoimmune Disease
4. Types of Equine Uveitis and Differential Diagnosis
4.1. Equine Recurrent Uveitis (ERU)
- (a)
- Acute uveitis
- Epiphora, blepharospasm, and photophobia;
- Concomitant conjunctivitis;
- Gossamer to smoky corneal opacity (Supplementary Material S1, Figures S1 and S2);
- After a short time, circular vascular ingrowth into the cornea may begin (Supplementary Material S1, Figures S1–S4);
- Inflammatory products in the anterior chamber of the eye (positive Tyndall effect, fibrin, leukocytes and/or erythrocytes) (Supplementary Material S1, Figures S3–S5);
- More rarely, also a hypopyon (Supplementary Material S1, Figure S3);
- Pain-related and often complete miosis; mydriasis can often only be achieved with a striking delay, and possibly incompletely with medication (Supplementary Material S1, Figures S1–S4);
- Diffuse vitreous haze (Supplementary Material S1, Figures S6 and S7);
- In rare cases, rubeosis iridis (Supplementary Material S1, Figure S8).
- (b)
- Chronic recurrent uveitis, inflammation-free interval
- Atrophy of the globe (often resulting in the development of a so-called “3rd corner of the eye” occurring on the upper eyelid, Supplementary Material S1, Figure S9);
- Chronic keratitis;
- Posterior (more rarely, also anterior) synechiae, sometimes only iris residues on the anterior lens capsule (Supplementary Material S1, Figures S10 and S11);
- Vesicular cataract, typically in the periphery of the posterior surface of the lens (Supplementary Material S1, Figure S12);
- Cataract formation (cataract typically originating from the lens capsule: either as a result of posterior synechiae or as a result of inflammatory products adhering to the posterior surface of the lens) (Supplementary Material S1, Figures S8–S11);
- Vitreous liquefaction;
- Sometimes chronic diffuse vitreous haziness, which does not completely resolve in the inflammation-free interval (Supplementary Material S1, Figures S10 and S13);
- Over time, increasing accumulation of dense cloudy to membranous inflammatory deposits in the vitreous body (“floaters”), which are not resorbed in the inflammation-free interval, typically starting high dorsal in the liquefied vitreous, floating with eye-movements (Supplementary Material S1, Figure S13);
- Centrifugal retinal folds surrounding the optic nerve disc, bullous, planar, or complete retinal detachments (Supplementary Material S1, Figures S14 and S15);
- More rarely also lens (sub-)luxations,
- In later stages, significant bulbar atrophy, corneal opacities due to collapsed anterior chamber and extensive contact between iris and corneal endothelium, phthisis bulbi (Supplementary Material S1, Figures S9 and S11);
- Very rarely: glaucoma (e.g., in later ERU stages after cataract formation and subsequent subluxations or luxations of the lens).
4.2. “Leopard Coat Pattern”—Uveitis
4.3. Traumatic Uveitis
4.4. Phacogenic Uveitis
4.5. Chronic Iritis, Similar to “Fuchs’ Heterochromic Iritis” in Humans
4.6. Uveitis during Septicemia
4.7. Uveitis Accompanying Intraocular Tumors
4.8. Uveitis during Parasitic Infections
4.9. Uveitis Accompanying Severe Keratitis
4.10. Endophthalmitis
4.11. Autoimmune Uveitis and Uveitis of Unknown Etiology
5. Therapy
5.1. Acute Uveitis
- Topical mydriatics, as often as needed for dilation of the pupil to prevent posterior synechiae and thereby cataract formation (in equids, atropine is by far the most effective substance for this purpose, and to the best of the authors’ knowledge, atropine can be used as frequently as needed without any concern (Supplementary Material S2));
- Topical treatment with corticosteroids to reduce inflammation (most effective are ophthalmic ointments containing dexamethasone)—if corneal defects are present, these are a contraindication for ophthalmic ointments containing corticosteroids, because otherwise the development of serious corneal ulcers may be favored;
- Systemic administration of non-steroidal anti-inflammatory drugs;
- Palliative measures: the avoidance of bright light, limited exercise, or even stall rest;
- Systemic administration of antibiotics can be considered in severe cases when a bacterial infection is involved (e.g., hypopyon–keratitis or ERU with severe haziness of intraocular fluids); thus, this is rarely indicated.
5.2. Chronic Course of Uveitis
6. Diagnostic Value of Testing Serum Samples for Anti-Leptospira Antibodies in Horses Suffering from ERU and in Horses with Healthy Eyes
7. Examination of Intraocular Specimens in Horses Suffering from ERU
7.1. Breakthrough with Vitrectomy
7.2. Antibody Detection in Aqueous Humor and Vitreous Samples
Blood–Aqueous Barrier and Intraocular Protein Content
- Vitrectomy is performed exclusively in the inflammation-free interval, not during an acute attack of uveitis;
- A disturbed blood–ocular barrier especially plays a role during acute inflammation;
- Depending on the time of sampling and stage of ERU, the leakage of proteins from the blood into the intraocular fluids is of minor importance at the time of surgery (quiet interval and mostly an early stage of the disease);
- The immunoglobulin content in the serum is about ten times greater than the immunoglobulin content in the vitreous; nevertheless, the intraocular antibody titer is usually higher (Supplementary Material S6, Tables S3–S5);
- The more significant the diffuse haziness in the vitreous, the higher the protein content in the intraocular fluids, and the more obvious a “leakage” would be from the serum—but, in fact, the antibody titer in intraocular samples is then usually also significantly higher in relation to the antibody titer in the serum (Section 7.3) (Supplementary Material S6, Table S3);
- Low MAT titers in intraocular samples are mainly present when the eye ophthalmoscopically shows hardly any or no changes in the sense of ERU (especially no diffuse opacity of the intraocular fluids); then, the protein content in the intraocular samples is also very low;
- Electrophoresis shows that the eye usually contains less albumin than the immunoglobulin content would suggest; compared with serum samples, the albumin/immunoglobulin ratio in intraocular samples from eyes affected by ERU is shifted in favor of the immunoglobulin content;
- In “non-ERU” uveitis, glaucoma and a few days after damage of the blood–ocular barrier due to trauma with intraocular bleeding, typically, no intraocular anti-Leptospira antibodies are detectable even if MAT titers are detectable in corresponding serum samples.
7.3. Intraocular Antibody Production
7.3.1. Calculation of the Goldmann–Witmer Coefficient (GWC)
7.3.2. Interpretation of the GWC
7.3.3. Misleading Interpretation of the GWC in Recent Publications on Equine Ophthalmology
7.3.4. Application of the GWC
7.4. Cultural Detection of Pathogenic Leptospira spp.
7.5. PCR
7.6. Comparison of Laboratory Results of Vitreous and Aqueous Humor Samples
7.7. Ultrastructural and Histological Examinations of Vitreous Specimens
7.8. Cells and Cell Dynamics in Vitreous Specimens from ERU Eyes
7.9. Neutrophil Extracellular Traps (NETs)
7.10. AA Amyloid
7.11. Samples from Healthy Eyes
8. Bacterial Biofilm
8.1. Steps of Biofilm Formation and Dissemination of Infection
8.2. Characteristics of Biofilm-Associated Diseases
- The direct examination of infected tissue reveals bacteria living in cell aggregates or microcolonies surrounded by an extracellular matrix;
- The infection is generally confined to a specific location or organ;
- The infection is impossible or difficult to eliminate using antibiotics, to which the responsible organisms are sensitive when in their planktonic or free-living state;
- Often, no organism can be cultured despite a strong presumption of infection with the pathogen of interest;
- Immune responses are ineffective, as evidenced by bacterial aggregates surrounded by inflammatory cells within host tissue.
8.3. Biofilm Formation of Leptospira spp.
8.4. Vitreous Structure, ERU, and Biofilm
- Hardly any nutrients are present in the healthy vitreous body (Section 7.2);
- The collagen fiber scaffold in the vitreous body could serve as a surface, analogous to plant fibers [481], where biofilm formation begins; the vitreous fibrils in the horse [422] have a diameter of approximately 10–12 nm, as in other species [547], and several fibrils can attach to each other to form fibers [546];
- Some vitreous areas are very viscous (hyaluronic acid and collagen fibers), which also promotes biofilm formation [533];
- The equine vitreous represents an avascular “immunological niche” of approximately 28 mL [11], resulting in delayed and reduced host defense responses; thus, biofilm formation can occur before the immune defense has any chance to eliminate the infection;
- As in the renal tubules, there are fenestrated capillaries in the area of the Pars plicata of the ciliary body under the respective single-layered unpigmented and pigmented epithelium [5], which can promote passage of the leptospires into the vitreous cavity during hematogenous infection.
- Fibrin: the fibrin scaffold looks very similar to the collagen fiber scaffold of the vitreous body by electron microscopy: see picture p. 10 in [550] (https://publications.rwth-aachen.de/record/466223/files/466223.pdf; accessed on 5 December 2021);
- NETs (Section 7.9, vivid picture on: https://scitechdaily.com/are-overactive-immune-cells-the-cause-of-COVID-19-deaths/; accessed on 5 December 2021);
- AA amyloid: amyloid fibrils are 8–15 nm in diameter [551], similar in thickness to those of the vitreous scaffold, and amyloid fibrils can also assemble into fibers; the curli fibers formed by some bacteria in the biofilm (Section 7.10) are only slightly thinner (6–12 nm in diameter) [552].
9. Prophylaxis
10. Discussion
- Clinically noticeable episodes of uveitis not until many months or even years after systemic infection;
- Cultural leptospiral detection is difficult and has often failed;
- Remarkably, positive cultures, despite high intraocular antibody titers and (although less frequently) antibiotic concentrations in vitreous samples that were well above the MIC for planktonic leptospires;
- Persistence of leptospiral infection in the vitreous cavity for many years;
- Chronic recurrent inflammatory episodes at unpredictable intervals;
11. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dwyer, A.E.; Crockett, R.S.; Kalsow, C.M. Association of leptospiral seroreactivity and breed with uveitis and blindness in horses: 372 cases (1986–1993). J. Am. Vet. Med. Assoc. 1995, 207, 1327–1331. [Google Scholar] [PubMed]
- Gerhards, H.; Wollanke, B.; Brem, S. Vitrectomy as a diagnostic and therapeutic approach for equine recurrent uveitis (ERU). In Proceedings of the 45th Annual Convention of the American Association of Equine Practitioners (AAEP), Albuquerque, NM, USA, 8 December 1999; pp. 89–93. [Google Scholar]
- Gilger, B.C. Equine recurrent uveitis: The viewpoint from the USA. Equine Vet. J. Suppl. 2010, 37, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Gerding, J.C.; Gilger, B.C. Prognosis and impact of equine recurrent uveitis. Equine Vet. J. 2016, 48, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Gilger, B.C.; Hollingsworth, S.R. Diseases of the uvea, uveitis, and recurrent uveitis. In Equine Ophthalmology, 3rd ed.; Gilger, B.C., Ed.; Wiley Blackwell: Ames, IA, USA, 2017; pp. 369–415. [Google Scholar]
- Curling, A. Equine recurrent uveitis: Treatment. Compend. Contin. Educ. Vet. 2011, 33, E1. [Google Scholar]
- Sandmeyer, L.S.; Kingsley, N.B.; Walder, C.; Archer, S.; Leis, M.L.; Bellone, R.R.; Bauer, B.S. Risk factors for equine recurrent uveitis in a population of Appaloosa horses in western Canada. Vet. Ophthalmol. 2020, 23, 515–525. [Google Scholar] [CrossRef]
- Gagnon, N.A.; Hartley, C.; Gilger, B.C. Efficacy and safety of suprachoroidal triamcinolone injection in horses with poorly responsive equine recurrent uveitis. Vet. Ophthalmol. 2021, 24, 308–312. [Google Scholar] [CrossRef]
- Alexander, C.S.; Keller, H. Ätiologie und Vorkommen der periodischen Augenentzündung des Pferdes im Raum Berlin [Etiology and occurrence of periodic eye inflammation of horses in the area of Berlin]. Tierarztl. Prax. 1990, 18, 623–627. [Google Scholar]
- Szemes, P.; Gerhards, H. Untersuchungen zur Prävalenz der equinen rezidivierenden Uveitis im Großraum Köln-Bonn [Study on the prevalence of equine recurrent uveitis in the Cologne-Bonn area]. Praktische Tierarzt 2000, 81, 408–420. [Google Scholar]
- Lavach, J.D. Periodic ophthalmia. In Large Animal Ophthalmology; C.W. Mosby Company: St. Louis, MO, USA, 1990; pp. 162–171. [Google Scholar]
- Sandmeyer, L.S.; Bauer, B.S.; Feng, C.X.; Grahn, B.H. Equine recurrent uveitis in western Canadian prairie provinces: A retrospective study (2002–2015). Can. Vet. J. 2017, 58, 717–722. [Google Scholar]
- Lowe, R.C. Equine uveitis: A UK perspective. Equine Vet. J. Suppl. 2010, 37, 46–49. [Google Scholar] [CrossRef]
- Himebaugh, N.; Gilger, B. Role of Leptospira spp. testing and ocular examination in horses with equine recurrent uveitis: A retrospective study of 63 horses. Equine Vet. Educ. 2021. epub. [Google Scholar] [CrossRef]
- Baumgart, A.; Gerhards, H. Besonderheiten der Tigerschecken-Uveitis und möglicher Cyclosporin A-Einsatz in deren Therapie in Deutschland [Characteristics of uveitis in horses with leopard coat color and potential use of cyclosporine A in its therapy in Germany]. Pferdeheilkunde 2014, 30, 626–632. [Google Scholar]
- Wollanke, B.; Gerhards, H.; Schinagl, C. Results of 654 trans-pars plana vitrectomies of equine eyes with recurrent uveitis—Follow-up until 18 years after surgery. Pferdeheilkunde—Equine Med. 2021, 37, 204–214. [Google Scholar] [CrossRef]
- Wollanke, B.; Rohrbach, B.W.; Gerhards, H. Serum and vitreous humor antibody titers in and isolation of Leptospira interrogans from horses with recurrent uveitis. J. Am. Vet. Med. Assoc. 2001, 219, 795–800. [Google Scholar] [CrossRef]
- Wollanke, B. Die equine rezidivierende Uveitis (ERU) als intraokulare Leptospirose [Equine recurrent uveitis (ERU) as an intraocular leptospirosis]. Habilitation Thesis, Ludwig-Maximilians-University (LMU), Munich, Germany, July 2002. [Google Scholar] [CrossRef]
- Wollanke, B.; Gerhards, H.; Brem, S.; Meyer, P.; Kopp, H. Ätiologie der equinen rezidivierenden Uveitis (ERU): Autoimmunkrankheit oder intraokulare Leptospireninfektion [Etiology of equine recurrent uveitis (ERU): Autoimmune disease or intraocular leptospiral infection?]. Pferdeheilkunde 2004, 20, 327–340. [Google Scholar] [CrossRef]
- Wollanke, B.; Gerhards, H.; Brem, S.; Geiger, T.; Wiehen, L.E. Leptospira serovars in Germany and neighbouring countries in horses suffering from recurrent uveitis looking at intraocular and serum samples. In Proceedings of the 3rd ELS Scientific Meeting on leptospirosis and other rodent borne haemorrhagic fevers, Alghero, Italy, 24–26 May 2018; p. 51. [Google Scholar]
- Kenngott, R.; Ackermann, K.; Settles, M.; Maierl, J.; Schick, B.; Gerhards, H.; Wollanke, B. Is there an intraocular biofilm production in horses suffering from recurrent leptospiral uveitis? In Proceedings of the 11th International Leptospirosis Society (ILS) Meeting, Vancouver, BC, Canada, 8–12 July 2019; pp. 13–14. [Google Scholar]
- Tsirouki, T.; Dastiridou, A.; Symeonidis, C.; Tounakaki, O.; Brazitikou, I.; Kalogeropoulos, C.; Androudi, S. A Focus on the Epidemiology of Uveitis. Ocul. Immunol. Inflamm. 2018, 26, 2–16. [Google Scholar] [CrossRef]
- Hurtienne, H. Klinische Diagnostik bei Glaskörperveranderungen des Pferdes [Clinical diagnostics of vitreous changes in the horse]. DTW Dtsch. Tierarztl. Wochenschr. 1972, 79, 537–539. [Google Scholar]
- Dimock, W.W.; Bruner, D.W.; Edwards, P.R. Periodic ophthalmia of horses and mules. Kentucky Agric. Exper. Sta. Bull. 1948, 512, 3–35. [Google Scholar]
- Gelatt, K. Ophthalmoscopic studies in the normal and diseased ocular fundi of horses. J. Am. Anim. Hosp. Assoc. 1971, 7, 158–167. [Google Scholar]
- Gelatt, K.N. The eye. In Equine Medinice and Surgery, 2nd ed.; Catcott, E.J., Smithcors, J.F., Eds.; American Veterinary Publications: Wheaton, IL, USA, 1972; pp. 399–432. [Google Scholar]
- Spiess, B.M. Zur equinen rezidivierenden Uveitis (ERU) [Equine recurrent uveitis (ERU)]. Schweiz. Arch. Tierheilkd. 1997, 139, 126–133. [Google Scholar]
- Gerhards, H.; Wollanke, B. Uveitis bei Pferden—Diagnose und Therapie [Uveitis in horses—Diagnosis and therapy]. Pferdeheilkunde 2001, 17, 319–329. [Google Scholar] [CrossRef][Green Version]
- Angelos, J.; Oppenheim, Y.; Rebhun, W.; Mohammed, H.; Antczak, D. Evaluation of breed as a risk factor for sarcoid and uveitis in horses. Anim. Genet. 1988, 19, 417–425. [Google Scholar] [CrossRef]
- Baumgart, A. Cyclosporin A und dessen möglicher Einsatz bei der Tigerschecken-Uveitis [Cyclosporine A and its potential use in leopard coat pattern uveitis]. Ph.D. Thesis, Ludwig-Maximilians-University (LMU), Munich, Germany, 2014. [Google Scholar] [CrossRef]
- Bistner, S.; Wiebe, E. Traumatic panophthalmitis in a horse. Cornell Vet. 1971, 61, 415–422. [Google Scholar]
- Pinto, N.I.; McMullen Jr, R.J.; Linder, K.E.; Cullen, J.M.; Gilger, B.C. Clinical, histopathological and immunohistochemical characterization of a novel equine ocular disorder: Heterochromic iridocyclitis with secondary keratitis in adult horses. Vet. Ophthalmol. 2015, 18, 443–456. [Google Scholar] [CrossRef]
- Burgess, E.C.; Gillette, D.; Pickett, J.P. Arthritis and panuveitis as manifestations of Borrelia burgdorferi infection in a Wisconsin pony. J. Am. Vet. Med. Assoc. 1986, 189, 1340–1342. [Google Scholar]
- Priest, H.L.; Irby, N.L.; Schlafer, D.H.; Divers, T.J.; Wagner, B.; Glaser, A.L.; Chang, Y.F.; Smith, M.C. Diagnosis of Borrelia-associated uveitis in two horses. Vet. Ophthalmol. 2012, 15, 398–405. [Google Scholar] [CrossRef]
- Wollanke, B.; Gerhards, H.; Kaufmann, S. Untersuchungen zur Beteiligung von Borrelien an der Ätiologie der equinen rezidivierenden Uveitis (ERU) [Studies on the involvement of Borrelia in the etiology of equine recurrent uveitis (ERU)]. Pferdeheilkunde—Equine Med. 2017, 33, 447–451. [Google Scholar] [CrossRef]
- Tarancón, I.; Leiva, M.; Jose-Cunilleras, E.; Ríos, J.; Peña, T. Ophthalmologic findings associated with Rhodococcus equi bronchopneumonia in foals. Vet. Ophthalmol. 2019, 22, 660–665. [Google Scholar] [CrossRef]
- Leiva, M.; Peña, T.; Armengou, L.; Cesarini, C.; Monreal, L. Uveal inflammation in septic newborn foals. J. Vet. Intern. Med. 2010, 24, 391–397. [Google Scholar] [CrossRef][Green Version]
- Barr, B.S. Rhodococcus equi pneumonia in a foal. Vet. Clin. N. Am. Equine Pract. 2006, 22, 239–246. [Google Scholar] [CrossRef]
- Sanchez, L.C. Equine neonatal sepsis. Vet. Clin. N. Am. Equine Pract. 2005, 21, 273–293. [Google Scholar] [CrossRef] [PubMed]
- Delph, K.; Sharpe, E.; Beard, L.; Rankin, A. Haemolytic anaemia and bilateral uveitis associated with leptospirosis in a 6-year-old Quarter Horse gelding. Equine Vet. Educ. 2018, 30, 132–136. [Google Scholar] [CrossRef]
- Wollanke, B.; Gerhards, H.; Schäffer, E. Keratouveitis und Makrohaematurie bei einer Infektion mit Micronema deletrix bei einem Pferd [Keratouveitis and macrohaematuria associated with infection with Micronema deletrix in a horse]. Pferdeheilkunde 2000, 16, 23–29. [Google Scholar] [CrossRef][Green Version]
- Rames, D.S.; Miller, D.K.; Barthel, R.; Craig, T.M.; Dziezyc, J.; Helman, R.G.; Mealey, R. Ocular Halicephalobus (syn. Micronema) deletrix in a horse. Vet. Pathol. 1995, 32, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Wollanke, B. Untersuchungen zur Ätiologie der equinen rezidivierenden Uveitis (ERU) [Studies on the aetiology of equine recurrent uveitis (ERU)]. Ph.D. Thesis, Ludwig-Maximilians-University, Munich, Germany, 1995. [Google Scholar]
- Gerhards, H.; Wollanke, B. Antikörpertiter gegen Borrelien bei Pferden im Serum und im Auge und Vorkommen der equinen rezidivierenden Uveitis (ERU) [Antibody titers against Borrelia in horses in serum and in eyes and occurrence of equine recurrent uveitis]. Berl. Munch. Tierarztl. Wochenschr. 1996, 109, 273–278. [Google Scholar]
- Wollanke, B.; Gerhards, H.; Brem, S.; Wolf, E.; Kopp, H.; Meyer, P. Zur Leptospirenätiologie der equinen rezidivierenden Uveitis (ERU)—Ergebnisse der Untersuchungen von Serum- und Glaskörperproben [Leptospiral aetiology of equine recurrent uveitis (ERU)—Results of studies on vitreous and serum samples]. Tierarztl. Prax. Ausgabe G Grosstiere/Nutztiere 2000, 28, 153–158. [Google Scholar]
- Wilcock, B.P.; Brooks, D.E.; Latimer, C.A. Glaucoma in horses. Vet. Pathol. 1991, 28, 74–78. [Google Scholar] [CrossRef]
- Wilkie, D.A.; Gilger, B.C. Equine glaucoma. Vet. Clin. N. Am. Equine Pract. 2004, 20, 381–391, vii. [Google Scholar] [CrossRef]
- Wilkie, D.A. Equine glaucoma: State of the art. Equine Vet. J. Suppl. 2010, 37, 62–68. [Google Scholar] [CrossRef]
- Kellner, R. Ein Beitrag zu Erbfehlerstudien [A contribution to genetic defect studies]. Dtsch. Landwirtsch. Tierz. 1934, 38, 209–211. [Google Scholar]
- Cross, R.S. Equine periodic ophthalmia. Vet. Rec. 1966, 78, 8–13. [Google Scholar] [CrossRef][Green Version]
- Bayer, J. Mondblindheit oder periodische Augenentzündung [Moon blindness or periodic ophthalmia]. In Handbuch der Tierärztlichen Chirurgie und Geburtshilfe, 2nd ed.; Bayer, J., Ed.; Wilhelm Braumüller: Vienna, Austria; Leipzig, Germany, 1906; Volume V, pp. 427–484. [Google Scholar]
- Fritz, K.L.; Kaese, H.J.; Valberg, S.J.; Hendrickson, J.A.; Rendahl, A.K.; Bellone, R.R.; Dynes, K.M.; Wagner, M.L.; Lucio, M.A.; Cuomo, F.M.; et al. Genetic risk factors for insidious equine recurrent uveitis in Appaloosa horses. Anim. Genet. 2014, 45, 392–399. [Google Scholar] [CrossRef]
- Bellone, R.R. Genetics of Equine Ocular Disease. Vet. Clin. N. Am. Equine Pract. 2020, 36, 303–322. [Google Scholar] [CrossRef]
- Rockwell, H.; Mack, M.; Famula, T.; Sandmeyer, L.; Bauer, B.; Dwyer, A.; Lassaline, M.; Beeson, S.; Archer, S.; McCue, M.; et al. Genetic investigation of equine recurrent uveitis in Appaloosa horses. Anim. Genet. 2020, 51, 111–116. [Google Scholar] [CrossRef]
- Deeg, C.A.; Marti, E.; Gaillard, C.; Kaspers, B. Equine recurrent uveitis is strongly associated with the MHC class I haplotype ELA-A9. Equine Vet. J. 2004, 36, 73–75. [Google Scholar] [CrossRef]
- Kulbrock, M.; Lehner, S.; Metzger, J.; Ohnesorge, B.; Distl, O. A genome-wide association study identifies risk loci to equine recurrent uveitis in German warmblood horses. PLoS ONE 2013, 8, e71619. [Google Scholar] [CrossRef]
- Ackermann, K.; Kenngott, R.; Settles, M.; Gerhards, H.; Maierl, J.; Wollanke, B. In Vivo Biofilm Formation of Pathogenic Leptospira spp. in the Vitreous Humor of Horses with Recurrent Uveitis. Microorganisms 2021, 9, 1915. [Google Scholar] [CrossRef]
- Zwierzchowski, J. Klinik und Therapie der Leptospirosen der Haus- und Nutztiere [Clinic and therapy of leptospirosis of domestic and farm animals]. In Leptospiren und Leptospirosen; Kathe, J., Mochmann, H., Eds.; Gustav Fischer Verlag: Jena, Germany, 1967; Volume I, pp. 79–137. [Google Scholar]
- Ellis, W.A. Animal leptospirosis. Curr. Top. Microbiol. Immunol. 2015, 387, 99–137. [Google Scholar] [CrossRef]
- Cibulski, S.; Wollanke, B. Untersuchungen von wildlebenden Kleinsäugern und Wasserproben von Pferdebetrieben auf DNA pathogener Leptospiren mittels real-time PCR [Examination of wild small mammals and water samples from horse farms for DNA of pathogenic leptospires by real-time PCR]. Pferdeheilkunde 2016, 32, 635–641. [Google Scholar] [CrossRef]
- Kathe, J.; Popp, L.; Mochmann, H. 3. Deutschland (1886–1945, DBR, DDR) [3. Germany (1886–1945, West Germany and East Germany)]. In Leptospiren und Leptospirosen; Kathe, J., Mochmann, H., Eds.; Gustav Fischer Verlag: Jena, Germany, 1967; pp. 661–696. [Google Scholar]
- Obaidat, M.M.; Malania, L.; Bani Salman, A.E.; Dreyfus, A.; Arner, R.J.; Roess, A.A. Seroprevalence and risk factors of Leptospira sp. among different groups in the Jordanian population: First study. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 1260–1264. [Google Scholar] [CrossRef]
- Divers, T.J.; Chang, Y.F.; Irby, N.L.; Smith, J.L.; Carter, C.N. Leptospirosis: An important infectious disease in North American horses. Equine Vet. J. 2019, 51, 287–292. [Google Scholar] [CrossRef]
- Donahue, J.M.; Smith, B.J.; Donahoe, J.K.; Rigsby, C.L.; Tramontin, R.R.; Poonacha, K.B.; Wilson, M.A. Prevalence and serovars of Leptospira involved in equine abortions in central Kentucky during the 1990 foaling season. J. Vet. Diagn. Investig. 1992, 4, 279–284. [Google Scholar] [CrossRef]
- Donahue, J.M.; Smith, B.J.; Poonacha, K.B.; Donahoe, J.K.; Rigsby, C.L. Prevalence and serovars of Leptospira involved in equine abortions in central Kentucky during the 1991–1993 foaling seasons. J. Vet. Diagn. Investig. 1995, 7, 87–91. [Google Scholar] [CrossRef]
- Von Borstel, M.V.; Oey, L.; Strutzberg-Minder, K.; Boevé, M.H.; Ohnesorge, B. Direkter und indirekter Nachweis von Leptospiren aus Glaskörperproben von Pferden mit ERU [Direct and indirect detection of leptospires in vitreal samples of horses with ERU]. Pferdeheilkunde 2010, 26, 219–225. [Google Scholar] [CrossRef]
- Tömördy, E.; Hässig, M.; Spiess, B.M. The outcome of pars plana vitrectomy in horses with equine recurrent uveitis with regard to the presence or absence of intravitreal antibodies against various serovars of Leptospira interrogans. Pferdeheilkunde 2010, 26, 251–254. [Google Scholar] [CrossRef][Green Version]
- Voelter, K.; Vial, Z.; Pot, S.A.; Spiess, B.M. Leptospiral antibody prevalence and surgical treatment outcome in horses with Equine Recurrent Uveitis (ERU) in Switzerland. Vet. Ophthalmol. 2020, 23, 648–658. [Google Scholar] [CrossRef]
- Baake, E.I.; von Borstel, M.; Rohn, K.; Ohnesorge, B. Detection of intraocular leptospiral DNA, antibodies and Leptospira spp. in horses with equine recurrent uveitis in different laboratories. Pferdeheilkunde 2016, 32, 346–356. [Google Scholar] [CrossRef][Green Version]
- Roberts, S.J.; York, C.J.; Robinson, J.W. An outbreak of leptospirosis in horses on a small farm. J. Am. Vet. Med. Assoc. 1952, 121, 237–242. [Google Scholar] [PubMed]
- Brem, S.; Grabner, A.; Hänichen, T.; Kopp, H.; Meyer, P. Leptospireninfektion (Leptospira grippotyphosa) als Ursache einer hämolytischen Anämie bei einem Pferd [Leptospiral infection (Leptospira grippotyphosa) as a cause of hemolytic anemia in a horse]. Pferdeheilkunde 1992, 8, 297–301. [Google Scholar] [CrossRef][Green Version]
- Frellstedt, L.; Slovis, N. Acute renal disease from Leptospira interrogans in three yearlings from the same farm. Equine Vet. Educ. 2009, 21, 478–484. [Google Scholar] [CrossRef]
- Fouché, N.; Graubner, C.; Lanz, S.; Schweighauser, A.; Francey, T.; Gerber, V. Acute kidney injury due to Leptospira interrogans in 4 foals and use of renal replacement therapy with intermittent hemodiafiltration in 1 foal. J. Vet. Int. Med. 2020, 34, 1007–1012. [Google Scholar] [CrossRef]
- Ellis, W.A. Leptospirosis as a cause of reproductive failure. Vet. Clin. N. Am. Food Anim. Pract. 1994, 10, 463–478. [Google Scholar] [CrossRef]
- Shapiro, J.L.; Prescott, J.F.; Henry, G. Equine abortions in eastern Ontario due to leptospirosis. Can. Vet. J. 1999, 40, 350–351. [Google Scholar] [PubMed]
- Donahue, J.M.; Williams, N.M. Emergent causes of placentitis and abortion. Vet. Clin. N. Am. Equine Pract. 2000, 16, 443–456, viii. [Google Scholar] [CrossRef]
- Lu, K.G.; Morresey, P.R. Reproductive tract infections in horses. Vet. Clin. N. Am. Equine Pract. 2006, 22, 519–552. [Google Scholar] [CrossRef]
- Shanahan, L.M.; Slovis, N.M. Leptospira interrogans associated with hydrallantois in 2 pluriparous Thoroughbred mares. J. Vet. Intern. Med. 2011, 25, 158–161. [Google Scholar] [CrossRef]
- Bernard, W.V.; Bolin, C.; Riddle, T.; Durando, M.; Smith, B.J.; Tramontin, R.R. Leptospiral abortion and leptospiruria in horses from the same farm. J. Am. Vet. Med. Assoc. 1993, 202, 1285–1286. [Google Scholar]
- Hong, C.B.; Donahue, J.M.; Giles, R.C., Jr.; Petrites-Murphy, M.B.; Poonacha, K.B.; Roberts, A.W.; Smith, B.J.; Tramontin, R.R.; Tuttle, P.A.; Swerczek, T.W. Equine abortion and stillbirth in central Kentucky during 1988 and 1989 foaling seasons. J. Vet. Diagn. Investig. 1993, 5, 560–566. [Google Scholar] [CrossRef]
- Poonacha, K.B.; Donahue, J.M.; Giles, R.C.; Hong, C.B.; Petrites-Murphy, M.B.; Smith, B.J.; Swerczek, T.W.; Tramontin, R.R.; Tuttle, P.A. Leptospirosis in equine fetuses, stillborn foals, and placentas. Vet. Pathol. 1993, 30, 362–369. [Google Scholar] [CrossRef]
- Szeredi, L.; Haake, D.A. Immunohistochemical identification and pathologic findings in natural cases of equine abortion caused by leptospiral infection. Vet. Pathol. 2006, 43, 755–761. [Google Scholar] [CrossRef]
- Hamond, C.; Pestana, C.P.; Rocha-de-Souza, C.M.; Cunha, L.E.; Brandão, F.Z.; Medeiros, M.A.; Lilenbaum, W. Presence of leptospires on genital tract of mares with reproductive problems. Vet. Microbiol. 2015, 179, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Ellis, W.A.; O’Brien, J.J.; Cassells, J.A.; Montgomery, J. Leptospiral infection in horses in Northern Ireland: Serological and microbiological findings. Equine Vet. J. 1983, 15, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Myers, D.M. Serological studies and isolations of serotype hardjo and Leptospira biflexa strains from horses of Argentina. J. Clin. Microbiol. 1976, 3, 548–555. [Google Scholar] [CrossRef]
- Rocha, T.; Ellis, W.A.; Montgomery, J.; Gilmore, C.; Regalla, J.; Brem, S. Microbiological and serological study of leptospirosis in horses at slaughter: First isolations. Res. Vet. Sci. 2004, 76, 199–202. [Google Scholar] [CrossRef]
- Hamond, C.; Martins, G.; Lawson-Ferreira, R.; Medeiros, M.A.; Lilenbaum, W. The role of horses in the transmission of leptospirosis in an urban tropical area. Epidemiol. Infect. 2013, 141, 33–35. [Google Scholar] [CrossRef]
- Wollanke, B.; Gerhards, H. LipL32 real-time PCR using 50 urine samples from horses suffering from uveitis. In Proceedings of the 10th Conference of the International Leptospirosis Society (ILS), Palmerston North, New Zealand, 27 November–1 December 2017; p. 225. [Google Scholar]
- Gsell, O.; Rehsteiner, K.; Verrey, F. Iridocyclitis als Spätfolge von Leptospirosis Pomona (Schweinehüterkrankheit). Agglutinin- und Lymphozytose-Befund im Kammerwasser [Iridocyclitis as a late consequence of Leptospirosis Pomona (porter’s disease): Agglutinin and lymphocytosis in the aqueous humor]. Ophthalmologica 1946, 112, 320–334. [Google Scholar] [CrossRef]
- Moro, F. Les Uvéites leptospirosiques. Doc. Ophthalmol. 1960, 14, 383–398. [Google Scholar] [CrossRef]
- Rathinam, S.R. Ocular leptospirosis. Curr. Opin. Ophthalmol. 2002, 13, 381–386. [Google Scholar] [CrossRef]
- Rathinam, S.R.; Rathnam, S.; Selvaraj, S.; Dean, D.; Nozik, R.A.; Namperumalsamy, P. Uveitis associated with an epidemic outbreak of leptospirosis. Am. J. Ophthalmol. 1997, 124, 71–79. [Google Scholar] [CrossRef]
- Pappachan, J.M.; Mathew, S.; Thomas, B.; Renjini, K.; Scaria, C.K.; Shukla, J. The incidence and clinical characteristics of the immune phase eye disease in treated cases of human leptospirosis. Indian J. Med. Sci. 2007, 61, 441–447. [Google Scholar] [CrossRef][Green Version]
- Shukla, D.; Rathinam, S.R.; Cunningham, E.T., Jr. Leptospiral uveitis in the developing world. Int. Ophthalmol. Clin. 2010, 50, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Stevenson, B. Leptospiral uveitis—There is more to it than meets the eye! Zoonoses Public Health 2012, 59 (Suppl. 2), 132–141. [Google Scholar] [CrossRef] [PubMed]
- Bryans, J.T. Studies on equine leptospirosis. Cornell Vet. 1955, 45, 16–50. [Google Scholar]
- Roberts, S.J. Sequelae of leptospirosis in horses on a small farm. J. Am. Vet. Med. Assoc. 1958, 133, 189–194. [Google Scholar]
- Sova, Z. Prispevek k onemocneni koni Weilovou chorobou. Beitrag zur Forschung der Weilschen Krankheit bei Pferden. [Contribution to research of Weil’s disease in horses]. Vet. Med. 1962, 7, 859–866. [Google Scholar]
- Sova, Z. Febris grippotyphosa u koni [Grippotyphosa-fever in horses]. Cesk. Parazitol. 1963, 10, 147–161. [Google Scholar]
- Bolte, H.F. Uveitis, a sequela to experimentally induced Leptospira pomona infection in the Shetland pony. Master’s Thesis, Purdue University, West Lafayette, IN, USA, 1966. [Google Scholar]
- Williams, R.D.; Morter, R.L.; Freeman, M.J.; Lavignette, A.M. Experimental chronic uveitis. Ophthalmic signs following equine leptospirosis. Investig. Ophthalmol. 1971, 10, 948–954. [Google Scholar]
- Bernard, W.V. Leptospirosis. Vet. Clin. N. Am. Equine Pract. 1993, 9, 435–444. [Google Scholar] [CrossRef]
- Spiess, B.M. Equine recurrent uveitis: The European viewpoint. Equine Vet. J. Suppl. 2010, 37, 50–56. [Google Scholar] [CrossRef]
- Braun, D. Die Geschichte der Erforschung und Behandlung der “periodischen Augenentzündung“ des Pferdes im deutschsprachigen Raum von 1750–1950 [The history of research and treatment of equine “periodic ophthalmitis” in German-speaking countries from 1750–1950]. Ph.D. Thesis, Ludwig-Maximilians-University (LMU), Munich, Germany, 1994. [Google Scholar]
- Pettigrew, T.J. Biographical Memoirs of the Most Celebrated Physicians, Surgeons, etc. Who Have Contributed to the Advencement of Medical Science; Fisher, Son & Co: London, UK, 1838; Volume 2. [Google Scholar]
- Paglia, D.T.; Miller, P.E.; Dubielzig, R.R. James Wardrop and equine recurrent uveitis. Arch. Ophthalmol. 2004, 122, 1218–1223. [Google Scholar] [CrossRef][Green Version]
- Bayer, J. Die Mondblindheit oder periodische Augenentzündung [The moon blindness or periodic ophthalmia]. Monatshefte Tierheilkunde 1900, 10, 433–488. [Google Scholar]
- Fröhner, E. Die Mondblindheit oder periodische Augenentzündung [The moon blindness or periodic ophthalmia]. In Kompendium der Speziellen Chirurgie für Tierärzte, 4th ed.; Fröhner, E., Ed.; Ferdinand Enke Verlag: Stuttgart, Germany, 1910; pp. 31–35. [Google Scholar]
- Möller, H. Iridochorioiditis and Cyclitis [Iridochoroiditis and cyclitis]. In Lehrbuch der Augenheilkunde für Tierärzte, 4th ed.; Möller, H., Ed.; Ferdinand Enke Verlag: Stuttgart, Germany, 1910; pp. 149–203. [Google Scholar]
- Schleich, G. §73 Die periodische, intermittierende Augenentzündung, die Mondblindheit des Pferdes, Iridochorioiditis recidiva equi, Moonblindness. In Handbuch der gesamten Augenheilkunde, 2nd ed.; Graefe, A., Saemisch, T., Eds.; Tier-Augenheilkunde; Springer: Berlin, Germany, 1922; pp. 141–151. [Google Scholar]
- Zipperlen, W. Der illustrierte Hausthierarzt für Landwirthe und Hausthierbesitzer; Ebner’sche: Ulm, Germany, 1877. [Google Scholar]
- Zündel, A. Die Mondblindheit der Pferde. Der Gesundheitszustand der Hausthiere in Elsass-Lothringen vom 1. April 1880 bis 1. April 1881 [Moon blindness in horses. The state of health of domestic animals in Alsace-Lorraine from April 1, 1880 to April 1, 1881.]. Zeitschrift fuer vergleichende Augenheilkunde 1882, 1, 153. [Google Scholar]
- Schwarznecker, G. Die periodische Augenentzündung im Saargebiet [Periodc ophthalmia in the Saar region]. Z. Veterinaerk. 1892, 4, 1–10. [Google Scholar]
- Jones, T.C. Equine periodic ophthalmia. Am. J. Vet. Res. 1942, 3, 45–71. [Google Scholar]
- Heusser, H. Die Periodische Augenentzündung, Eine Leptospirose? Schweiz. Arch. Tierheilkd. 1948, 90, 287–312. [Google Scholar]
- Kathe, J.; Engelhardt, K.; Greve, O. Allergische Erkrankungen nach Leptospirose [Allergic diseases after leptospirosis]. Zbl. Bakteriol. Parasitenk. 1952, 158, 333–336. [Google Scholar]
- Komar, G.; Szutter, L. Die innere periodische Augenentzündung (“Mondblindheit“) der Pferde [The internal periodic eye inflammation (“moon blindness”) of horses]. In Tieraerztliche Augenheilkunde; Komar, G., Szutter, L., Eds.; Paul Parey: Berlin, Germany, 1968; pp. 231–237. [Google Scholar]
- Heusser, H. Zur Ätiologie der periodischen Augenentzündung [On the etiology of periodic ophthalmia]. Schweiz. Arch. Tierheilkd. 1952, 94, 296–306. [Google Scholar]
- Witmer, R.; Löhrer, J.; Wiesmann, E. Zur Ätiologie, Diagnose und Therapie der periodischen Augenentzündung (p. A.) des Pferdes [On the etiology, diagnosis, and therapy of equine periodic ocular inflammation (p. A.)]. Schweiz. Arch. Tierheilkd. 1953, 95, 419–439. [Google Scholar]
- Rossi, P.; Kolochine-Erber, B. Iridocyclites des équidés et leptospiroses [Iridocyclitis of horses and leptospirosis]. Rev. Pathol. Gen. Physiol. Clin. 1954, 54, 432–477. [Google Scholar]
- Witmer, R. Periodic ophthalmia in horses. Am. J. Ophthalmol. 1954, 37, 243–253. [Google Scholar] [CrossRef]
- Kemenes, F.; Surjan, J.; Vizy, L. Leptospira as the cause of periodic ophthalmia in horses. Vet. Bull. 1961, 31, 12. [Google Scholar]
- Bürki, F.; Egli, P.; Wiesmann, E. Experimentelle Infektion von Pferden mit Leptospira pomona [Experimental infection of horses with Leptospira pomona]. Berl. Munch. Tierarztl. Wschr. 1963, 76, 265–269. [Google Scholar]
- Hartwigk, H.; Stoebbe, E. Kultureller Nachweis von Leptospiren bei Hund und Pferd [Cultural detection of leptospires in dogs and horses]. Berl. Munch. Tierarztl. Wschr. 1952, 65, 188–190. [Google Scholar]
- Popovic, B.; Bordjoski, M. Les leptospiroses des chevaux dans la valle de la Morava [Leptospirosis of horses in the Morava Valley]. Bull. Off. Int. Epiz. 1957, 1–2, 95–105. [Google Scholar]
- Williams, R.D. The Presence and Duration of Persistence of Leptospira Pomona in Equine Ocular Tissues Following Experimentally Induced Systemic Infection. Master’s Thesis, Purdue University, West Lafayette, IN, USA, 1968. [Google Scholar]
- Bohl, E.; Ferguson, L. Leptospirosis in domestic animals. J. Am. Vet. Med. Assoc. 1952, 121, 421–428. [Google Scholar]
- Kemenes, F.; Tamas, L. Ist die fibrinöse Iridozyklitis der Pferde eine Leptospirose? [Is equine fibrinous iridocyclitis leptospirosis?]. Acta Veterinaria 1952, 2, 327–336. [Google Scholar]
- Tomasek, V. Etiology and treatment of equine periodic ophthalmia. Vet. Glasnik 1954, 8, 243. [Google Scholar]
- Morter, R.L.; Herschler, R.C.; Fessler, J.F.; Lavignette, A. Experimental equine leptospirosis (Leptospira pomona). Proc. Annu. Meet. U.S. Anim. Health Assoc. 1964, 68, 147–152. [Google Scholar]
- Gelatt, K.; Peiffer, R., Jr.; Gwin, R.; Williams, L. The status of equine ophthalmology. J. Equine Med. Surg. 1977, 1, 13–19. [Google Scholar]
- Halliwell, R.E.; Hines, M.T. Studies on equine recurrent uveitis. I: Levels of immunoglobulin and albumin in the aqueous humor of horses with and without intraocular disease. Curr. Eye Res. 1985, 4, 1023–1031. [Google Scholar] [CrossRef]
- Kalsow, C.M.; Hainworth, S.A.; Russell, N.S.; Dwyer, A.E. Retinal autoimmunity in leptospiral associated uveitis. Investig. Ophthalmol. Vis. Sci. 1998, 39, S782, B3619–B3445. [Google Scholar]
- Gilger, B.C.; Malok, E.; Cutter, K.V.; Stewart, T.; Horohov, D.W.; Allen, J.B. Characterization of T-lymphocytes in the anterior uvea of eyes with chronic equine recurrent uveitis. Vet. Immunol. Immunopathol. 1999, 71, 17–28. [Google Scholar] [CrossRef]
- Sillerud, C.L.; Bey, R.F.; Ball, M.; Bistner, S.I. Serologic correlation of suspected Leptospira interrogans serovar pomona-induced uveitis in a group of horses. J. Am. Vet. Med. Assoc. 1987, 191, 1576–1578. [Google Scholar]
- Malalana, F.; Stylianides, A.; McGowan, C. Equine recurrent uveitis: Human and equine perspectives. Vet. J. 2015, 206, 22–99. [Google Scholar] [CrossRef]
- Allbaugh, R. Equine recurrent uveitis: A review of clinical assessment and management. Equine Vet. Educ. 2017, 29, 279–288. [Google Scholar] [CrossRef]
- Severin, G.A. Equine recurrent uveitis: Etiology signs and management. In Proceedings of the Bain-Fallon Memorial Lectures, Artamon, NSW, Australia, 12–16 May 1986; pp. 58–61, ISBN 9780959397741. [Google Scholar]
- Mair, T.; Crispin, S. Immunological mechanisms in uveitis. Equine Vet. J. 1989, 21, 391–393. [Google Scholar] [CrossRef]
- Hines, M.T.; Halliwell, R. Autoimmunity to retinal S-antigen in horses with equine recurrent uveitis. Prog. Vet. Comp. Ophthalmol. 1991, 1, 283–290. [Google Scholar]
- Maxwell, S.; Hurt, D.; Brightman, A.; Takemoto, D. Humoral responses to retinal proteins in horses with recurrent uveitis. Prog. Vet. Comp. Ophthalmol. 1991, 1, 155–161. [Google Scholar]
- Kalsow, C.M.; Dwyer, A.E. Retinal immunopathology in horses with uveitis. Ocul. Immunol. Inflamm. 1998, 6, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Romeike, A.; Brügmann, M.; Drommer, W. Immunohistochemical studies in equine recurrent uveitis (ERU). Vet. Pathol. 1998, 35, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.E. Uvea. In Slatter’s Fundamentals of Veterinary Ophthamology, 4th ed.; Maggs, D.J., Miller, P.E., Ofri, R., Slatter, D.H., Eds.; WB Saunders: St. Louis, MO, USA, 2008; pp. 203–229. [Google Scholar]
- Deeg, C.A.; Ehrenhofer, M.; Thurau, S.R.; Reese, S.; Wildner, G.; Kaspers, B. Immunopathology of recurrent uveitis in spontaneously diseased horses. Exp. Eye Res. 2002, 75, 127–133. [Google Scholar] [CrossRef]
- Parma, A.E.; Santisteban, C.G.; Villalba, J.S.; Bowden, R.A. Experimental demonstration of an antigenic relationship between Leptospira and equine cornea. Vet. Immunol. Immunopathol. 1985, 10, 215–224. [Google Scholar] [CrossRef]
- Parma, A.E.; Fernández, A.S.; Santisteban, C.G.; Bowden, R.A.; Cerone, S.I. Tears and aqueous humor from horses inoculated with Leptospira contain antibodies which bind to cornea. Vet. Immunol. Immunopathol. 1987, 14, 181–185. [Google Scholar] [CrossRef]
- Parma, A.E.; Cerone, S.I.; Sansinanea, S.A. Biochemical analysis by SDS-PAGE and western blotting of the antigenic relationship between Leptospira and equine ocular tissues. Vet. Immunol. Immunopathol. 1992, 33, 179–185. [Google Scholar] [CrossRef]
- Parma, A.E.; Cerone, S.I.; Sansinanea, S.A.; Ghezzi, M. C3 fixed in vivo to cornea from horses inoculated with Leptospira interrogans. Vet. Immunol. Immunopathol. 1992, 34, 181–187. [Google Scholar] [CrossRef]
- Parma, A.E.; Sanz, M.E.; Lucchesi, P.M.; Mazzonelli, J.; Petruccelli, M.A. Detection of an antigenic protein of Leptospira interrogans which shares epitopes with the equine cornea and lens. Vet. J. 1997, 153, 75–79. [Google Scholar] [CrossRef]
- Lucchesi, P.M.; Parma, A.E. A DNA fragment of Leptospira interrogans encodes a protein which shares epitopes with equine cornea. Vet. Immunol. Immunopathol. 1999, 71, 173–179. [Google Scholar] [CrossRef]
- Williams, R.D. Equine Uveitis: A model system for study of immunologically-mediated tissue injury. Dis. Abst. 1973, 33B, 4578–4579. [Google Scholar]
- Verma, A.; Kumar, P.; Babb, K.; Timoney, J.F.; Stevenson, B. Cross-reactivity of antibodies against leptospiral recurrent uveitis-associated proteins A and B (LruA and LruB) with eye proteins. PLoS Negl. Trop. Dis. 2010, 4, e778. [Google Scholar] [CrossRef]
- Deeg, C.A.; Amann, B.; Raith, A.J.; Kaspers, B. Inter- and intramolecular epitope spreading in equine recurrent uveitis. Investig. Ophthalmol. Vis. Sci. 2006, 47, 652–656. [Google Scholar] [CrossRef]
- Pleyer, U.; Foster, C.S. Uveitis and Immunological Disorders; Springer: Berlin, Germany, 2007. [Google Scholar]
- Wang, L.; Wang, F.S.; Gershwin, M.E. Human autoimmune diseases: A comprehensive update. J. Intern. Med. 2015, 278, 369–395. [Google Scholar] [CrossRef]
- Deeg, C.A.; Gerhards, H.; Kaspers, B.; Thurau, S.R.; Wollanke, B.; Wildner, G. T cell mediated autoreactivity is a pathophysiological mechanism in equine recurrent uveitis. IOVS 1999, 40, S137. [Google Scholar]
- Deeg, C.A.; Kaspers, B.; Gerhards, H.; Thurau, S.R.; Wollanke, B.; Wildner, G. Immune responses to retinal autoantigens and peptides in equine recurrent uveitis. Investig. Ophthalmol. Vis. Sci. 2001, 42, 393–398. [Google Scholar]
- Deeg, C.A.; Pompetzki, D.; Raith, A.J.; Hauck, S.M.; Amann, B.; Suppmann, S.; Goebel, T.W.; Olazabal, U.; Gerhards, H.; Reese, S.; et al. Identification and functional validation of novel autoantigens in equine uveitis. Mol. Cell Proteomics 2006, 5, 1462–1470. [Google Scholar] [CrossRef]
- Deeg, C.A.; Hauck, S.M.; Amann, B.; Kremmer, E.; Stangassinger, M.; Ueffing, M. Major retinal autoantigens remain stably expressed during all stages of spontaneous uveitis. Mol. Immunol. 2007, 44, 3291–3296. [Google Scholar] [CrossRef]
- Deeg, C.A.; Raith, A.J.; Amann, B.; Crabb, J.W.; Thurau, S.R.; Hauck, S.M.; Ueffing, M.; Wildner, G.; Stangassinger, M. CRALBP is a highly prevalent autoantigen for human autoimmune uveitis. Clin. Dev. Immunol. 2007, 2007, 39245. [Google Scholar] [CrossRef]
- Deeg, C.A. Ocular immunology in equine recurrent uveitis. Vet. Ophthalmol. 2008, 11 (Suppl. 1), 61–65. [Google Scholar] [CrossRef]
- Deeg, C.A.; Hauck, S.M.; Amann, B.; Pompetzki, D.; Altmann, F.; Raith, A.; Schmalzl, T.; Stangassinger, M.; Ueffing, M. Equine recurrent uveitis—A spontaneous horse model of uveitis. Ophthalmic Res. 2008, 40, 151–153. [Google Scholar] [CrossRef]
- Zipplies, J.K.; Hauck, S.M.; Schoeffmann, S.; Amann, B.; Stangassinger, M.; Ueffing, M.; Deeg, C.A. Serum PEDF levels are decreased in a spontaneous animal model for human autoimmune uveitis. J. Proteome Res. 2009, 8, 992–998. [Google Scholar] [CrossRef]
- Swadzba, M.E.; Hirmer, S.; Amann, B.; Hauck, S.M.; Deeg, C.A. Vitreal IgM autoantibodies target neurofilament medium in a spontaneous model of autoimmune uveitis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 294–300. [Google Scholar] [CrossRef]
- Zipplies, J.K.; Hauck, S.M.; Eberhardt, C.; Hirmer, S.; Amann, B.; Stangassinger, M.; Ueffing, M.; Deeg, C.A. Miscellaneous vitreous-derived IgM antibodies target numerous retinal proteins in equine recurrent uveitis. Vet. Ophthalmol. 2012, 15 (Suppl. 2), 57–64. [Google Scholar] [CrossRef] [PubMed]
- Merl, J.; Deeg, C.A.; Swadzba, M.E.; Ueffing, M.; Hauck, S.M. Identification of autoantigens in body fluids by combining pull-downs and organic precipitations of intact immune complexes with quantitative label-free mass spectrometry. J. Proteome Res. 2013, 12, 5656–5665. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, L.; Cywinska, A.; Paschalis-Trela, K.; Crisman, M.; Kita, J. Multiple etiologies of equine recurrent uveitis—A natural model for human autoimmune uveitis: A brief review. Comp. Immunol. Microbiol. Infect. Dis. 2016, 44, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Degroote, R.L.; Deeg, C.A. Immunological Insights in Equine Recurrent Uveitis. Front. Immunol. 2020, 11, 609855. [Google Scholar] [CrossRef] [PubMed]
- Degroote, R.L.; Uhl, P.B.; Amann, B.; Krackhardt, A.M.; Ueffing, M.; Hauck, S.M.; Deeg, C.A. Formin like 1 expression is increased on CD4+ T lymphocytes in spontaneous autoimmune uveitis. J. Proteomics 2017, 154, 102–108. [Google Scholar] [CrossRef]
- Lorenz, L.; Amann, B.; Hirmer, S.; Degroote, R.L.; Hauck, S.M.; Deeg, C.A. NEU1 is more abundant in uveitic retina with concomitant desialylation of retinal cells. Glycobiology 2021, 31, 873–883. [Google Scholar] [CrossRef]
- Barfüßer, C.; Wiedemann, C.; Hoffmann, A.L.C.; Hirmer, S.; Deeg, C.A. Altered Metabolic Phenotype of Immune Cells in a Spontaneous Autoimmune Uveitis Model. Front. Immunol. 2021, 12, 601619. [Google Scholar] [CrossRef]
- Kalsow, C.M.; Dubielzig, R.R.; Dwyer, A.E. Immunopathology of pineal glands from horses with uveitis. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1611–1615. [Google Scholar]
- Verma, A.; Matsunaga, J.; Artiushin, S.; Pinne, M.; Houwers, D.J.; Haake, D.A.; Stevenson, B.; Timoney, J.F. Antibodies to a novel leptospiral protein, LruC, in the eye fluids and sera of horses with Leptospira-associated uveitis. Clin. Vaccine Immunol. 2012, 19, 452–456. [Google Scholar] [CrossRef]
- Gilger, B.C.; Deeg, C.A. Equine recurrent uveitis. In Equine Ophthalmology, 2nd ed.; Gilger, B.C., Ed.; Elsevier Saunders: Maryland Heights, MO, USA, 2011; pp. 317–349. [Google Scholar]
- O’Connor, G.R. Endogenous uveitis. Bacterial infections. In Uveitis. Pathophysiology and Therapy., 2nd ed.; Kraus-Mackiw, E., O’Connor, G.R., Eds.; Thieme: Stuttgart, Germany, 1986; pp. 63–68. [Google Scholar]
- Kotb, M. Infection and autoimmunity: A story of the host, the pathogen, and the copathogen. Clin. Immunol. Immunopathol. 1995, 74, 10–22. [Google Scholar] [CrossRef]
- Girschick, H.J.; Guilherme, L.; Inman, R.D.; Latsch, K.; Rihl, M.; Sherer, Y.; Shoenfeld, Y.; Zeidler, H.; Arienti, S.; Doria, A. Bacterial triggers and autoimmune rheumatic diseases. Clin. Exp. Rheumatol. 2008, 26, S12–S17. [Google Scholar]
- Smyk, D.S.; Koutsoumpas, A.L.; Mytilinaiou, M.G.; Rigopoulou, E.I.; Sakkas, L.I.; Bogdanos, D.P. Helicobacter pylori and autoimmune disease: Cause or bystander. World J. Gastroenterol. 2014, 20, 613–629. [Google Scholar] [CrossRef]
- Christen, U. Pathogen infection and autoimmune disease. Clin. Exp. Immunol. 2019, 195, 10–14. [Google Scholar] [CrossRef]
- Liu, Y.; Sawalha, A.H.; Lu, Q. COVID-19 and autoimmune diseases. Curr. Opin. Rheumatol. 2021, 33, 155–162. [Google Scholar] [CrossRef]
- Voigt, V.; Wikstrom, M.E.; Kezic, J.M.; Schuster, I.S.; Fleming, P.; Makinen, K.; Daley, S.R.; Andoniou, C.E.; Degli-Esposti, M.A.; Forrester, J.V. Ocular antigen does not cause disease unless presented in the context of inflammation. Sci. Rep. 2017, 7, 14226. [Google Scholar] [CrossRef]
- Bovey, E.H.; Herbort, C.P. Vitrectomy in the management of uveitis. Ocul. Immunol. Inflamm. 2000, 8, 285–291. [Google Scholar] [CrossRef]
- Heimann, K.; Tavakolian, U.; Paulmann, H.; Morris, R. Pars-plana Vitrektomie zur Behandlung der chronischen Uveitis [Pars plana vitrectomy for the treatment of chronic uveitis]. Ber. Dtsch. Ophthalmol. Ges. 1981, 78, 249–251. [Google Scholar]
- Klöti, R. Vitrektomie bei chronischer Uveitis und anderen Eintrübungen des Glaskörpers [Vitrectomy for chronic uveitis and other inflammatory opacities of the vitreous body]. Ber. Dtsch. Ophthalmol. Ges. 1981, 78, 233–241. [Google Scholar]
- Werry, H.; Honegger, H. Pars-plana Vitrektomie bei chronischer Uveitis [Pars plana vitrectomy in chronic uveitis]. Klin. Monatsbl. Augenheilkd. 1987, 191, 9–12. [Google Scholar] [CrossRef]
- Klöti, R. Pars-plana Vitrektomie bei chronischer Uveitis [Pars-plana vitrectomy for chronic uveitis]. Klin. Monatsbl. Augenheilkd. 1988, 192, 425–429. [Google Scholar] [CrossRef]
- Berg, P.; Kroll, P.; Busse, H. Operative therapie bei Uveitis [Surgical therapy for uveitis]. Klin. Monatsbl. Augenheilkd. 1990, 197, 373–377. [Google Scholar] [CrossRef]
- Lightman, S.; Towler, H.M. Fundamentals of Clinical Ophthalmology: Uveitis; BMJ Books: London, UK, 1998. [Google Scholar]
- Dinning, W. Treatment of uveitis. Trans. Ophthalmol. Soc. UK 1981, 101, 391–393. [Google Scholar]
- Gerhards, H.; Wollanke, B. Surgical treatment of equine recurrent uveitis: Trans-pars-plana vitrectomy in horses. In Equine Ophthalmology, 1st ed.; Gilger, B.C., Ed.; Elsevier Saunders: St. Louis, MO, USA, 2005; pp. 314–319. [Google Scholar]
- Deeg, C.A.; Altmann, F.; Hauck, S.M.; Schoeffmann, S.; Amann, B.; Stangassinger, M.; Ueffing, M. Down-regulation of pigment epithelium-derived factor in uveitic lesion associates with focal vascular endothelial growth factor expression and breakdown of the blood-retinal barrier. Proteomics 2007, 7, 1540–1548. [Google Scholar] [CrossRef]
- Deeg, C.A.; Thurau, S.R.; Gerhards, H.; Ehrenhofer, M.; Wildner, G.; Kaspers, B. Uveitis in horses induced by interphotoreceptor retinoid-binding protein is similar to the spontaneous disease. Eur. J. Immunol. 2002, 32, 2598–2606. [Google Scholar] [CrossRef]
- Loibl, J.K. Immunologische und mikrobiologische Untersuchungen zur intraokular persistierenden Leptospireninfektion bei Pferden mit rezidivierender Uveitis [Immunologic and microbiologic studies of intraocular persistent leptospiral infection in horses with recurrent uveitis]. Ph.D. Thesis, Ludwig-Maximilians-University (LMU), Munich, Germany, 2009. [Google Scholar] [CrossRef]
- Loibl, J.K.; Gerhards, H.; Brem, S.; Wollanke, B. Improving the laboratory diagnosis of leptospiral uveitis in horses by using an indirect ELISA for the detection of antibodies against Leptospira spp. in intraocular samples. Pferdeheilkunde—Equine Med. 2018, 34, 267–277. [Google Scholar] [CrossRef]
- Popp, M.; Gerhards, H.; Wollanke, B. Enrofloxacinkonzentrationen im Glaskörper und im Serum an equiner rezidivierender Uveitis (ERU) erkrankter Pferde nach wiederholter intravenöser Verabreichung [Enrofloxacin concentrations in vitreous and serum of horses affected with equine recurrent uveitis (ERU) after repeated intravenous administration]. Pferdeheilkunde—Equine Med. 2013, 29, 574–580. [Google Scholar] [CrossRef][Green Version]
- Wiehen, L.E. Retrospektive Analyse zum Vorkommen der Equinen rezidivierenden Uveitis—Unter Beruecksichtigung der Leptospireninfektion—An der LMU München von 01/2005 bis 06/2010 [Retrospective analysis of the incidence of equine recurrent uveitis—Considering leptospiral infection—At the LMU Munich from 01/2005 to 06/2010]. Ph.D. Thesis, Ludwig-Maximilians-University (LMU), Munich, Germany, 2012. [Google Scholar] [CrossRef]
- Geiger, T. Evaluierung des SNAP-Lepto® (ELISA-Schnelltest) für den Nachweis von Antikörpern gegen LipL32 in Serum und intraokularem Probenmaterial von Pferden als Diagnostikum der Leptospiren-induzierten equinen rezidivierenden Uveitis (ERU) [Evaluation of a rapid ELISA test (SNAP-Lepto®) for the detection of antibodies against LipL32 in serum and intraocular specimen material from horses as a diagnostic tool for equine recurrent uveitis]. Ph.D. Thesis, Ludwig-Maximilians-University (LMU), Munich, Germany, 2019. [Google Scholar] [CrossRef]
- Wollanke, B.; Geiger, T.; Gerhards, H. Evaluation of “SNAP® Lepto”-ELISA and comparison with MAT and PCR results for diagnosis of leptospiral uveitis in horses using intraocular samples. Pferdeheilkunde—Equine Med. 2018, 34, 508–516. [Google Scholar] [CrossRef]
- Hines, M.T. Immunologically mediated ocular disease in the horse. Vet. Clin. N. Am. Large Anim. Pract. 1984, 6, 501–512. [Google Scholar] [CrossRef]
- Sanders, R.J.; Seery, C.M.; Weiter, J.J. Peripheral uveitis: An infectious etiology? Int. Ophthalmol. Clin. 1990, 30, 318–321. [Google Scholar] [CrossRef]
- Albert, D.; Jakobiec, F. Albert and Jakobiec’s Principles and Practice of Ophthalmology; Albert, D., Jakobiec, F., Eds.; Saunders: Philadelphia, PA, USA, 1994; Volume 5. [Google Scholar]
- Nussenblatt, R.; Whitcup, S.; Palestine, A. Uveitis, Fundamentals and Clinical Practice, 2nd ed.; Mosby: St. Louis, MO, USA, 1996; ISBN 9780815164463. [Google Scholar]
- Okada, A.A.; Forrester, J.V. Ocular inflammatory disease in the new millennium. Arch. Ophthalmol. 2000, 118, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Samson, C.M.; Foster, C.S. Masquerade Syndromes: Endophthalmitis. In Diagnosis and Treatment of Uveitis; Foster, C.S., Vitale, A.T., Eds.; W.B. Saunders Company: Philadelphia, PA, USA, 2002; pp. 528–536. [Google Scholar]
- Vitale, A.T.; Foster, C.S. Corticosteroids. In Diagnosis and Treatment of Uveitis; Foster, C.S., Vitale, A.T., Eds.; W.B. Saunders Company: Philadelphia, PA, USA, 2002; pp. 142–157. [Google Scholar]
- Rathinam, S.R. Ocular manifestations of leptospirosis. J. Postgrad. Med. 2005, 51, 189–194. [Google Scholar] [PubMed]
- Forrester, J.V.; Kuffova, L.; Dick, A.D. Autoimmunity, Autoinflammation, and Infection in Uveitis. Am. J. Ophthalmol. 2018, 189, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Odaka, M.; Yuki, N. [Bacterial infection-induced autoimmune disease]. Ryoikibetsu Shokogun Shirizu 1999, 407–411. [Google Scholar]
- Siffrin, V. Von der Infektion zur Autoimmunität [From infection to autoimmunity]. Ph.D. Thesis, Humboldt-University, Berlin, Germany, 2005. [Google Scholar] [CrossRef]
- Aujla, S.J.; Dubin, P.J.; Kolls, J.K. Th17 cells and mucosal host defense. Semin. Immunol. 2007, 19, 377–382. [Google Scholar] [CrossRef]
- Sugita, S.; Takase, H.; Kawaguchi, T.; Taguchi, C.; Mochizuki, M. Cross-reaction between tyrosinase peptides and cytomegalovirus antigen by T cells from patients with Vogt-Koyanagi-Harada disease. Int. Ophthalmol. 2007, 27, 87–95. [Google Scholar] [CrossRef]
- Berthelot, J.M.; Le Goff, B.; Neel, A.; Maugars, Y.; Hamidou, M. NETosis: At the crossroads of rheumatoid arthritis, lupus, and vasculitis. Joint Bone Spine 2017, 84, 255–262. [Google Scholar] [CrossRef]
- Nicastro, L.; Tükel, Ç. Bacterial Amyloids: The Link between Bacterial Infections and Autoimmunity. Trends Microbiol. 2019, 27, 954–963. [Google Scholar] [CrossRef]
- Qiu, C.C.; Caricchio, R.; Gallucci, S. Triggers of Autoimmunity: The Role of Bacterial Infections in the Extracellular Exposure of Lupus Nuclear Autoantigens. Front. Immunol. 2019, 10, 2608. [Google Scholar] [CrossRef]
- Tóth, J.; Hollerieder, J.; Sótoni, P. Augenheilkunde beim Pferd—Lehrbuch und Atlas [Equine Ophthalmology—Textbook and Atlas]; Schattauer GmbH: Stuttgart, Germany, 2010. [Google Scholar]
- Gerhards, H.; Wollanke, B. Equine rezidivierende Uveitis [Equine recurrent uveitis]. In [Handbook Equine Practice]; Dietz, O., Huskamp, B., Eds.; Enke Verlag: Stuttgart, Germany, 2006; pp. 775–786. [Google Scholar]
- Gilger, B.C.; Wilkie, D.A.; Clode, A.B.; McMullen, R.J., Jr.; Utter, M.E.; Komaromy, A.M.; Brooks, D.E.; Salmon, J.H. Long-term outcome after implantation of a suprachoroidal cyclosporine drug delivery device in horses with recurrent uveitis. Vet. Ophthalmol. 2010, 13, 294–300. [Google Scholar] [CrossRef]
- Barnett, K.C. Uveitis. In Equine Ophthalmology—An Atlas and Text, 2nd ed.; Barnett, K.C., Crispin, S.M., Lavach, J.D., Matthews, A.G., Eds.; Saunders: Edinburgh, UK, 2004; pp. 191–197. [Google Scholar]
- Sivakumar, R.; Balakrishnan, V.; Gowri, P.; Visalakshi, J. Leptospiral Uveitis: Usefulness of Clinical Signs as Diagnostic Predictors. Ocul. Immunol. Inflamm. 2018, 26, 569–576. [Google Scholar] [CrossRef]
- Wollanke, B.; Gerhards, H. Differential diagnosis of equine recurrent uveitis: The importance of a paracentesis of the anterior chamber and aqueous analysis. In Proceedings of the International Society of Veterinary Ophthalmology (IVO) Pre-Congress Programme of the 30th WSAVA Congress, Mexico City, Mexico, 9–10 May 2005. [Google Scholar]
- Gesell, S.; Wollanke, B.; Brem, S.; Gerhards, H. Vergleich der Antikörpertiter gegen Leptospiren in Kammerwasser- und Glaskörperproben bei Pferden mit rezidivierender Uveitis [Comparison of anti-Leptospira antibody titers in aqueous humor and vitreous samples in horses with recurrent uveitis]. In Proceedings of the 19th DVG-Fachtagung Pferdekrankheiten, Hanover, Germany, 10–11 February 2006; pp. 239–241. [Google Scholar]
- Schinagl, C. Pars-Plana-Vitrektomie bei Equiner Rezidivierender Uveitis: Langzeitergebnisse zu Rezidivfreiheit, Sehfähigkeit und Bulbuserhalt bei 654 Augen von 549 Pferden [Pars plana vitrectomy for equine recurrent uveitis: Long-term results on absence of recurrence, vision, and preservation of the globe in 654 eyes of 549 horses]. Ph.D. Thesis, Ludwig-Maximilians University (LMU), Munich, Germany, 2017. [Google Scholar]
- Cielewicz, M.-B. Histologische Untersuchungen von an Glaukom erkrankten Pferdeaugen [Histological examinations of equine eyes affected by glaucoma]. Ph.D. Thesis, Ludwig-Maximilians University (LMU), Munich, Germany, 2014. [Google Scholar] [CrossRef]
- Pickett, J.; Ryan, J. Equine glaucoma: A retrospective study of 11 cases from 1988 to 1993. Vet. Med. USA 1993, 88, 756–763. [Google Scholar]
- Grahn, B.H.; Cullen, C.L. Equine phacoclastic uveitis: The clinical manifestations, light microscopic findings, and therapy of 7 cases. Can. Vet. J. 2000, 41, 376–382. [Google Scholar]
- Drießen, F. Untersuchungen zum Glaukom beim Pferd [Investigations of glaucoma in horses]. Ph.D. Thesis, Ludwig-Maximilians-University (LMU), Munich, Germany, 2009. [Google Scholar] [CrossRef]
- Meehan, S.; Berry, Y.; Luisi, B.; Dobson, C.M.; Carver, J.A.; MacPhee, C.E. Amyloid fibril formation by lens crystallin proteins and its implications for cataract formation. J. Biol. Chem. 2004, 279, 3413–3419. [Google Scholar] [CrossRef]
- Habin, D.J. Equine traumatic uveitis. Equine Vet. Educ. 1994, 6, 122–127. [Google Scholar] [CrossRef]
- Nussenblatt, R.B.; Gery, I. Experimental autoimmune uveitis and its relationship to clinical ocular inflammatory disease. J. Autoimmun. 1996, 9, 575–585. [Google Scholar] [CrossRef]
- Zierhut, M.; Wild, U.; Roser, R.; Wiggert, B.; Thiel, H.J.; Stiemer, R.E. Experimentelle Autoimmun-Uveitis. Charakterisierung der die Retina infiltrierenden Zellen [Experimental autoimmune uveitis. Characterization of retina infiltrating cells]. Ophthalmologe 1999, 96, 252–256. [Google Scholar] [CrossRef]
- Schwink, K.L. Equine uveitis. Vet. Clin. N. Am. Equine Pract. 1992, 8, 557–574. [Google Scholar] [CrossRef]
- Pfleghaar, S.; Schäffer, E. Die linseninduzierte Uveitis (endophthalmitis phakoanaphylactica) beim Haustier [Lens-induced uveitis (endophthalmitis phacoanaphylactica) in the domestic animal]. Tierarztl. Prax. 1992, 20, 7–18. [Google Scholar]
- Quentin, C.D.; Reiber, H. Fuchs heterochromic cyclitis: Rubella virus antibodies and genome in aqueous humor. Am. J. Ophthalmol. 2004, 138, 46–54. [Google Scholar] [CrossRef]
- Becker, M.D.; Zierhut, M. Das Fuchs’sche Uveitis Syndrom—Die Heterochromie ist keine Conditio sine qua non [Fuchs uveitis syndrome—Heterochromia is no “conditio sine qua non”]. Ophthalmologe 2005, 102, 733–742. [Google Scholar] [CrossRef]
- De Groot-Mijnes, J.D.; De Visser, L.; Rothova, A.; Schuller, M.; Van Loon, A.M.; Weersink, A.J. Rubella virus is associated with fuchs heterochromic iridocyclitis. Am. J. Ophthalmol. 2006, 141, 212–214. [Google Scholar] [CrossRef]
- Barr, B.S. Pneumonia in weanlings. Vet. Clin. N. Am. Equine Pract. 2003, 19, 35–49. [Google Scholar] [CrossRef]
- Blogg, J.; Barton, M.; Graydon, R.; Cust, R. Blindness caused by Rhodococcus equi infection in a foal. Equine Vet. J. 1983, 15, 25–26. [Google Scholar] [CrossRef]
- Naylor, J.M. Severe metabolic acidemia, hypoglycemia, and sepsis in a 3-week-old quarter horse foal. Vet. Clin. N. Am. Equine Pract. 2006, 22, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.M.; Del Piero, F.; Habecker, P.L.; Langohr, I.M. A retrospective histologic study of 140 cases of clinically significant equine ocular disorders. J. Vet. Diagn. Investig. 2020, 32, 382–388. [Google Scholar] [CrossRef]
- Reuss, S.M.; Chaffin, M.K.; Cohen, N.D. Extrapulmonary disorders associated with Rhodococcus equi infection in foals: 150 cases (1987–2007). J. Am. Vet. Med. Assoc. 2009, 235, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.D. Rhodococcus equi foal pneumonia. Vet. Clin. N. Am. Equine Pract. 2014, 30, 609–622. [Google Scholar] [CrossRef]
- Müller-Hermelink, H.K.; Daus, W. Recent topics in the pathology of uveitis. In Uveitis. Pathophysiology and Therapy, 2nd ed.; Kraus-Mackiw, E., O’Conner, G.R., Eds.; Thieme: Stuttgart, Germany, 1986; pp. 155–203. [Google Scholar]
- Murphy, J.; Young, S. Intraocular melanoma in a horse. Vet. Pathol. 1979, 16, 539–542. [Google Scholar] [CrossRef]
- Matthews, A.; Barry, D. Bilateral melanoma of the iris in a horse. Equine Vet. J. 1987, 19, 358–360. [Google Scholar] [CrossRef] [PubMed]
- Barnett, K.C.; Platt, H. Intraocular melanomata in the horse. Equine Vet. J. Suppl. 1990, 22, 76–82. [Google Scholar] [CrossRef]
- Davidson, H.J.; Blanchard, G.L.; Wheeler, C.A.; Render, J.A. Anterior uveal melanoma, with secondary keratitis, cataract, and glaucoma, in a horse. J. Am. Vet. Med. Assoc. 1991, 199, 1049–1050. [Google Scholar] [PubMed]
- Riis, R.C.; Scherlie, P.H., Jr.; Rebhun, W.C. Intraocular medulloepithelioma in a horse. Equine Vet. J. Suppl. 1990, 22, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Bistner, S.I. Medullo-epithelioma of the iris and ciliary body in a horse. Cornell Vet. 1974, 64, 588–595. [Google Scholar] [PubMed]
- Eagle, R.C., Jr.; Font, R.L.; Swerczek, T.W. Malignant medulloepithelioma of the optic nerve in a horse. Vet. Pathol. 1978, 15, 488–494. [Google Scholar] [CrossRef]
- Szymanski, C.M. Malignant teratoid medulloepithelioma in a horse. J. Am. Vet. Med. Assoc. 1987, 190, 301–302. [Google Scholar]
- Leiva, M.; Felici, F.; Carvalho, A.; Ramis, A.; Peña, T. Benign intraocular teratoid medulloepithelioma causing glaucoma in an 11-year-old Arabian mare. Vet. Ophthalmol. 2013, 16, 297–302. [Google Scholar] [CrossRef]
- Monk, C.S.; Craft, W.F.; Abbott, J.R.; Farina, L.L.; Reuss, S.M.; Czerwinski, S.L.; Brooks, D.E.; Plummer, C.E. Clinical behavior of intraocular teratoid medulloepithelioma in two-related Quarter Horses. Vet. Ophthalmol. 2017, 20, 551–559. [Google Scholar] [CrossRef]
- Trope, G.D.; McCowan, C.I.; Tyrrell, D.; Lording, P.M.; Maggs, D.J. Solitary (primary) uveal T-cell lymphoma in a horse. Vet. Ophthalmol. 2014, 17, 139–145. [Google Scholar] [CrossRef]
- Rebhun, W.C.; Del Piero, F. Ocular lesions in horses with lymphosarcoma: 21 cases (1977–1997). J. Am. Vet. Med. Assoc. 1998, 212, 852–854. [Google Scholar]
- Germann, S.E.; Richter, M.; Schwarzwald, C.C.; Wimmershoff, J.; Spiess, B.M. Ocular and multicentric lymphoma in a young racehorse. Vet. Ophthalmol. 2008, 11 (Suppl. 1), 51–56. [Google Scholar] [CrossRef]
- Kinde, H.; Mathews, M.; Ash, L.; St Leger, J. Halicephalobus gingivalis (H. deletrix) infection in two horses in southern California. J. Vet. Diagn Investig. 2000, 12, 162–165. [Google Scholar] [CrossRef]
- Anderson, R.; Bemrick, W. Micronema deletrix n. sp., a saprophagous nematode inhabiting a nasal tumor of a horse. Proc. Helminthol. Soc. Wash. 1965, 32, 74–75. [Google Scholar]
- Johnson, K.H.; Johnson, D.W. Granulomas associated with Micronema deletrix in the maxillae of a horse. J. Am. Vet. Med. Assoc. 1966, 149, 155–159. [Google Scholar]
- Stone, W.M.; Stewart, T.B.; Peckham, J.C. Micronema deletrix Anderson and Bemrick, 1965 in the central nervous system of a pony. J. Parasitol. 1970, 56, 986–987. [Google Scholar] [CrossRef]
- Ferris, D.H.; Levine, N.D.; Beamer, P.D. Micronema deletrix in equine brain. Am. J. Vet. Res. 1972, 33, 33–38. [Google Scholar]
- Rubin, H.L.; Woodard, J.C. Equine infection with Micronema deletrix. J. Am. Vet. Med. Assoc. 1974, 165, 256–258. [Google Scholar]
- Jordan, W.H.; Gaafar, S.M.; Carlton, W.W. Micronema deletrix in the brain of a horse. Vet. Med. Small Anim. Clin. 1975, 70, 707–709. [Google Scholar]
- Powers, R.D.; Benz, G.W. Micronema deletrix in the central nervous system of a horse. J. Am. Vet. Med. Assoc. 1977, 170, 175–177. [Google Scholar]
- Alstad, A.D.; Berg, I.E.; Samuel, C. Disseminated Micronema deletrix infection in the horse. J. Am. Vet. Med. Assoc. 1979, 174, 264–266. [Google Scholar]
- Keg, P.R.; Mirck, M.H.; Dik, K.J.; Vos, J.H. Micronema deletrix infection in a Shetland pony stallion. Equine Vet. J. 1984, 16, 471–475. [Google Scholar] [CrossRef]
- Yoshihara, T.; Kanemaru, T.; Hasegawa, M.; Tomioka, Y.; Kaneko, M.; Kiryu, K.; Wada, R.; Watanebe, O. Micronema deletrix infection in the central nervous system of a horse. Bull. Equine Res. Inst. 1985, 1985, 30–37. [Google Scholar]
- Blunden, A.S.; Khalil, L.F.; Webbon, P.M. Halicephalobus deletrix infection in a horse. Equine Vet. J. 1987, 19, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Darien, B.J.; Belknap, J.; Nietfeld, J. Cerebrospinal fluid changes in two horses with central nervous system nematodiasis (Micronema deletrix). J. Vet. Intern. Med. 1988, 2, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.M.; Hodgin, E.C.; Cho, D.Y. Micronema deletrix-induced granulomatous osteoarthritis in a lame horse. J. Comp. Pathol. 1988, 99, 347–351. [Google Scholar] [CrossRef]
- Liebler, E.M.; Gerhards, H.; Denkhaus, M.; Pohlenz, J. Micronema deletrix als Ursache einer granulomatösen Nephritis bei einem Pferd [Micronema deletrix as the cause of a granulomatous nephritis in a horse]. DTW Dtsch. Tierarztl. Wochenschr. 1989, 96, 223–224. [Google Scholar]
- Spalding, M.G.; Greiner, E.C.; Green, S.L. Halicephalobus (Micronema) deletrix infection in two half-sibling foals. J. Am. Vet. Med. Assoc. 1990, 196, 1127–1129. [Google Scholar]
- Angus, K.W.; Roberts, L.; Archibald, D.R.; Fraser, D.G.; Jackson, F.; Gibbons, L.M. Halicephalobus deletrix infection in a horse in Scotland. Vet. Rec. 1992, 131, 495. [Google Scholar] [CrossRef]
- Reifinger, M. Severe encephalitis in a horse caused by free-living nematodes. Wien. Tieraerztl. Monatsschr. 1993, 80, 239–243. [Google Scholar]
- Ruggles, A.J.; Beech, J.; Gillette, D.M.; Midla, L.T.; Reef, V.B.; Freeman, D.E. Disseminated Halicephalobus deletrix infection in a horse. J. Am. Vet. Med. Assoc. 1993, 203, 550–552. [Google Scholar]
- Kreuder, C.; Kirker-Head, C.A.; Rose, P.; Gliatto, J. What is your diagnosis? Severe granulomatous osteomyelitis associated with Micronema deletrix infection in a horse. J. Am. Vet. Med. Assoc. 1996, 209, 1070–1071. [Google Scholar]
- Teifke, J.P.; Schmidt, E.; Traenckner, C.M.; Bauer, C. [Halicephalobus (Syn. Micronema) deletrix as a cause of granulomatous gingivitis and osteomyelitis in a horse]. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 1998, 26, 157–161. [Google Scholar]
- Onyiche, T.E.; Okute, T.O.; Oseni, O.S.; Okoro, D.O.; Biu, A.A.; Mbaya, A.W. Parasitic and zoonotic meningoencephalitis in humans and equids: Current knowledge and the role of Halicephalobus gingivalis. Parasite Epidemiol. Control 2018, 3, 36–42. [Google Scholar] [CrossRef]
- Pintore, M.D.; Cerutti, F.; D’Angelo, A.; Corona, C.; Gazzuola, P.; Masoero, L.; Colombo, C.; Bona, R.; Cantile, C.; Peletto, S.; et al. Isolation and molecular characterisation of Halicephalobus gingivalis in the brain of a horse in Piedmont, Italy. Parasit. Vectors 2017, 10, 135. [Google Scholar] [CrossRef]
- Brooks, D.; Matthews, A. Acquired disorders of the anterior chamber. In Equine Ophthalmology—An Atlas and Text, 2nd ed.; Barnett, K.C., Crispin, S.M., Lavach, J.D., Matthews, A.G., Eds.; Saunders: Edinburgh, UK, 2004; pp. 151–155. [Google Scholar]
- Hernandez-Da Mota, S.E.; Guerrero-Naranjo, J.L.; Dalma-Weiszhausz, J.; Velez-Montoya, R.; Gonzalez-Cortes, J.H. Acute Postoperative Infectious Endophthalmitis: Advances in Diagnosis and Treatment. In Infectious Eye Diseases. Recent Advances in Diagnosis and Treatment; Rodriguez-Garcia, A., Hernandez-Camarena, J.C., Eds.; IntechOpen: London, UK, 2021; pp. 94–145. [Google Scholar]
- Werry, H.; Gerhards, H. Möglichkeiten der und Indikationen zur chirurgischen Behandlung der equinen rezidivierenden Uveitis (ERU) [Technique and indications for surgical treatment of equine recurrent uveitis]. Pferdeheilkunde 1991, 7, 321–331. [Google Scholar] [CrossRef]
- Werry, H.; Gerhards, H. Zur operativen Therapie der equinen rezidivierenden Uveitis [The surgical therapy of equine recurrent uveitis]. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 1992, 20, 178–186. [Google Scholar]
- Frühauf, B.; Ohnesorge, B.; Deegen, E.; Boevé, M. Surgical management of equine recurrent uveitis with single port pars plana vitrectomy. Vet. Ophthalmol. 1998, 1, 137–151. [Google Scholar] [CrossRef]
- Gerhards, H.; Wollanke, B.; Winterberg, A.; Werry, H. Technique for and results with vitrectomy in horses with recurrent uveitis. In Proceedings of the 29th Annual ACVO-Meeting (American College of Veterinary Ophthalmologists), Seattle, WA, USA, 21–24 October 1998; p. 30. [Google Scholar]
- Winterberg, A. Langzeitergebnisse der Pars-plana-Vitrektomie bei equiner rezidivierender Uveitis [Long-term results of pars plana vitrectomy for equine recurrent uveitis]. Ph.D. Thesis, Ludwig-Maximilians-University (LMU), Munich, Germany, 1997. [Google Scholar]
- Winterberg, A.; Gerhards, H. Langzeitergebnisse der Pars-plana-Vitrektomie bei equiner rezidivierender Uveitis [Longterm-results of pars-plana-vitrectomy in equine recurrent uveitis]. Pferdeheilkunde—Equine Med. 1997, 13, 377–383. [Google Scholar] [CrossRef][Green Version]
- Baake, E.I.; Borstel, M.v.; Rohn, K.; Boeve, M.H.; Ohnesorge, B. Long-term ophthalmologic examinations of eyes with equine recurrent uveitis after pars plana vitrectomy. Pferdeheilkunde—Equine Med. 2019, 35, 220–233. [Google Scholar] [CrossRef]
- Von Borstel, M.; Von Oppen, T.; Glitz, F.; Fruhauf, B.; Deegen, E.; Boeve, M.; Ohnesorge, B. Long-term results of pars-plana (double-port) vitrectomy in equine recurrent uveitis. Pferdeheilkunde—Equine Med. 2005, 21, 13–18. [Google Scholar] [CrossRef][Green Version]
- Dorrego-Keiter, E.; Tóth, J.; Dikker, L.; Sielhorst, J.; Schusser, G. Langzeitergebnisse der Pars-Plana-Vitrektomie in Abhängigkeit vom Leptospiren-Antikörper-Nachweis im Glaskörper bei 118 Pferden mit Equiner Rezidivierender Uveitis (ERU) [Long-term results of pars plana vitrectomy in relation to leptospiral antibody detection in the vitreous in 118 horses with equine recurrent uveitis (ERU)]. Pferdeheilkunde—Equine Med. 2017, 33, 112–118. [Google Scholar] [CrossRef][Green Version]
- Waid, H.; Tóth, J.; Buijs, L.; Schusser, G.F. Clinical experiences after the insertion of a Cyclosporine-A drug delivery device in horses with Equine Recurrent Uveitis. Pferdeheilkunde—Equine Med. 2018, 34, 113–120. [Google Scholar] [CrossRef]
- Fischer, B.M.; McMullen, R.J., Jr.; Reese, S.; Brehm, W. Intravitreal injection of low-dose gentamicin for the treatment of recurrent or persistent uveitis in horses: Preliminary results. BMC Vet. Res. 2019, 15, 29. [Google Scholar] [CrossRef]
- Pinard, C.; Piètrement, E.; Macieira, S.; Tremblay, D. Intravitreal injections of gentamicin for the treatment of Leptospira-associated equine recurrent uveitis. In Proceedings of the 36th Annual Meeting of the ACVO, Nashville, TN, USA; 2005. [Google Scholar]
- Kleinpeter, A.; Göpfert, A.; Köhler, E.; Brehm, W. Intravitreale Low-Dose-Gentamicininjektion zur Behandlung ERU-erkrankter Pferde [Intravitreal injection of low-dose gentamicin for the treatment of ERU-affected horses]. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 2019, 47, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Launois, T.; González Hilarión, L.M.; Barbe, F.; Leurquin, C.; Bihin, B.; Hontoir, F.; Dugdale, A.; Vandeweerd, J.M. Use of Intravitreal Injection of Gentamicin in 71 Horses With Equine Recurrent Uveitis. J. Equine Vet. Sci. 2019, 77, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.; Ohnesorge, B. Die intravitreale Gentamicin-Injektion zur Behandlung der Equinen rezidivierenden Uveitis (ERU)—Status quo [The intravitreal gentamicin-injection for treatment of the equine recurrent uveitis (ERU)—Status quo]. Pferdeheilkunde—Equine Med. 2021, 37, 302–311. [Google Scholar] [CrossRef]
- Dixon, P.; Coppack, R. Equine recurrent uveitis. Vet. Rec. 2002, 150, 556. [Google Scholar]
- Divers, T.J.; Irby, N.L.; Mohammed, H.O.; Schwark, W.S. Ocular penetration of intravenously administered enrofloxacin in the horse. Equine Vet. J. 2008, 40, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Popp, M.K. Enrofloxacin im Glaskörper an equiner rezidivierender Uveitis erkrankter Pferde [Enrofloxacin in the vitreous of equine recurrent uveitis diseased horses]. Ph.D. Thesis, Ludwig-Maximilians-University (LMU), Munich, Germany, 2011. [Google Scholar] [CrossRef]
- Sallam, A.B.; Kirkland, K.A.; Barry, R.; Soliman, M.K.; Ali, T.K.; Lightman, S. A Review of Antimicrobial Therapy for Infectious Uveitis of the Posterior Segment. Med. Hypothesis Discov. Innov. Ophthalmol. 2018, 7, 140–155. [Google Scholar] [PubMed]
- Ng, H.R.; Cheong, M.Y.; Mustapha, M. Ocular leptospirosis in four patients: A diagnostic dilemma. Med. J. Malaysia 2021, 76, 569–572. [Google Scholar]
- Gilger, B.C.; Salmon, J.H.; Wilkie, D.A.; Cruysberg, L.P.; Kim, J.; Hayat, M.; Kim, H.; Kim, S.; Yuan, P.; Lee, S.S.; et al. A novel bioerodible deep scleral lamellar cyclosporine implant for uveitis. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2596–2605. [Google Scholar] [CrossRef]
- Wollanke, B.; Gerhards, H. Cyclosporine A (CsA) concentrations in aqueous and vitreous humour samples and clinical and ophthalmological findings in 16 equine eyes after implantation of a sustainedrelease CsA delivery device. Pferdeheilkunde—Equine Med. 2021, 37, 234–242. [Google Scholar] [CrossRef]
- Yi, N.Y.; Davis, J.L.; Salmon, J.H.; Gilger, B.C. Ocular distribution and toxicity of intravitreal injection of triamcinolone acetonide in normal equine eyes. Vet. Ophthalmol. 2008, 11 (Suppl. 1), 15–19. [Google Scholar] [CrossRef] [PubMed]
- Hathaway, S.C.; Little, T.W.; Finch, S.M.; Stevens, A.E. Leptospiral infection in horses in England: A serological study. Vet. Rec. 1981, 108, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, R.E.; Brim, T.A.; Hines, M.T.; Wolf, D.; White, F.H. Studies on equine recurrent uveitis. II: The role of infection with Leptospira interrogans serovar pomona. Curr. Eye Res. 1985, 4, 1033–1040. [Google Scholar] [CrossRef]
- Malalana, F.; Blundell, R.J.; Pinchbeck, G.L.; McGowan, C.M. The role of Leptospira spp. in horses affected with recurrent uveitis in the UK. Equine Vet. J. 2017, 49, 706–709. [Google Scholar] [CrossRef]
- Geiger, T.; Gerhards, H.; Wollanke, B. Detection of Anti-LipL32 Antibodies in Serum Samples from Horses with Chronic Intraocular Infection with Leptospira spp. Pathogens 2021, 10, 1325. [Google Scholar] [CrossRef]
- Khurana, S.; Malik, P.; Nandal, A.; Srivastava, S. Seroprevalence of Leptospirosis in Equines in India. Indian J. Comp. Microbiol. Immunol. Infect. Dis. 2003, 24, 93–95. [Google Scholar]
- Sohail, M.; Khan, M.; Ijaz, M.; Fatima, Z.; Naseer, O.; Ahamad, W.; Ahmad, A. Seroprevalence of Leptospira spp. in horses of distinct climatic regions of Punjab, Pakistan. In Proceedings of the 10th International Leptospirosis Society Conferecnce (ILS 2017), Palmerston North, New Zealand, 27 November–1 December 2017; p. 237. [Google Scholar]
- Schebitz, H.; Dedié, K. Zur Bewertung von Leptospirentitern im Pferdeserum [Evaluation of anti-leptospiral antibody titers in equine serum]. Zentralbl. Veterinaermed. 1955, 2, 522–543. [Google Scholar] [CrossRef]
- Zaharija, I.; Marlot, J.; Cermak, K.; Andrasic, N.; Sankovi, F. Leptospirose und periodische Augenentzündung beim Pferd [Leptospirosis and periodic ophthalmia in horses]. Arch. f. Tierheilkunde 1960, 7, 102. [Google Scholar]
- Davidson, M.G.; Nasisse, M.P.; Roberts, S.M. Immunodiagnosis of leptospiral uveitis in two horses. Equine Vet. J. 1987, 19, 155–157. [Google Scholar] [CrossRef]
- Wollanke, B.; Gerhards, H.; Brem, S.; Kopp, H.; Meyer, P. Intraokulare und Serumantikörpertiter gegen Leptospiren bei 150 wegen equiner rezidivierender Uveitis (ERU) vitrektomierten Pferden [Intraocular and serum antibody titers to Leptospira in 150 horses with equine recurrent uveitis (ERU) subjected to vitrectomy]. Berl. Munch. Tierarztl. Wochenschr. 1998, 111, 134–139. [Google Scholar]
- Dorrego-Keiter, E.; Tóth, J.; Dikker, L.; Sielhorst, J.; Schusser, G.F. Kultureller Nachweis von Leptospiren in Glaskörperflüssigkeit und Antikörpernachweis gegen Leptospiren in Glaskörperflüssigkeit und Serum von 225 Pferden mit equiner rezidivierender Uveitis (ERU) [Detection of Leptospira by culture of vitreous humor and detection of antibodies against Leptospira in vitreous humor and serum of 225 horses with equine recurrent uveitis]. Berl. Munch. Tierarztl. Wochenschr. 2016, 129, 209–215. [Google Scholar]
- Gilger, B.C.; Salmon, J.H.; Yi, N.Y.; Barden, C.A.; Chandler, H.L.; Wendt, J.A.; Colitz, C.M. Role of bacteria in the pathogenesis of recurrent uveitis in horses from the southeastern United States. Am. J. Vet. Res. 2008, 69, 1329–1335. [Google Scholar] [CrossRef]
- Finger, M.A.; de Barros Filho, I.R.; Leutenegger, C.; Estrada, M.; Ullmann, L.S.; Langoni, H.; Kikuti, M.; Dornbush, P.T.; Deconto, I.; Biondo, A.W. Serological and molecular survey of Leptospira spp. among cart horses from an endemic area of human leptospirosis in Curitiba, southern Brazil. Rev. Inst. Med. Trop. Sao Paulo 2014, 56, 473–476. [Google Scholar] [CrossRef]
- Jung, B.Y.; Lee, K.W.; Ha, T.Y. Seroprevalence of Leptospira spp. in clinically healthy racing horses in Korea. J. Vet. Med. Sci. 2010, 72, 197–201. [Google Scholar] [CrossRef][Green Version]
- Blatti, S.; Overesch, G.; Gerber, V.; Frey, J.; Hüssy, D. Seroprevalence of Leptospira spp. in clinically healthy horses in Switzerland. Schweiz. Arch. Tierheilkd. 2011, 153, 449–456. [Google Scholar] [CrossRef]
- Simbizi, V.; Saulez, M.N.; Potts, A.; Lötter, C.; Gummow, B. A study of leptospirosis in South African horses and associated risk factors. Prev. Vet. Med. 2016, 134, 6–15. [Google Scholar] [CrossRef]
- Wasiński, B.; Paschalis-Trela, K.; Trela, J.; Czopowicz, M.; Kita, J.; Żychska, M.; Cywińska, A.; Markowska-Daniel, I.; Carter, C.; Witkowski, L. Serological Survey of Leptospira Infection in Arabian Horses in Poland. Pathogens 2021, 10, 688. [Google Scholar] [CrossRef]
- Calderón, J.C.; Astudillo, M.; Romero, M.H. Epidemiological characterization of Leptospira spp. infection in working horses and in an occupationally exposed population in six Colombian police stations. Biomedica 2019, 39, 19–34. [Google Scholar] [CrossRef]
- Ismail, Z.B.; Abutarbush, S.M.; Al-Majali, A.M.; Gharaibeh, M.H.; Al-Khateeb, B. Seroprevalence and risk factors of Leptospira serovar Pomona and Leptospira serovar Hardjo infection in dairy cows in Jordan. J. Infect. Dev. Countries 2019, 13, 473–479. [Google Scholar] [CrossRef]
- Vera, E.; Taddei, S.; Cavirani, S.; Schiavi, J.; Angelone, M.; Cabassi, C.S.; Schiano, E.; Quintavalla, F. Leptospira Seroprevalence in Bardigiano Horses in Northern Italy. Animals 2019, 10, 23. [Google Scholar] [CrossRef]
- Da Silva, A.S.; Jaguezeski, A.M.; Laber, I.F.; von Laer, A.E.; Lovato, L.T.; da Silva, M.O.; de Moura, A.B. Leptospira spp. in horses in southern Brazil: Seroprevalence, infection risk factors, and influence on reproduction. Comp. Immunol. Microbiol. Infect. Dis. 2020, 73, 101552. [Google Scholar] [CrossRef]
- Fagre, A.C.; Mayo, C.E.; Pabilonia, K.L.; Landolt, G.A. Seroprevalence of Leptospira spp. in Colorado equids and association with clinical disease. J. Vet. Diagn. Investig. 2020, 32, 718–721. [Google Scholar] [CrossRef]
- Chu, K.M.; Rathinam, R.; Namperumalsamy, P.; Dean, D. Identification of Leptospira species in the pathogenesis of uveitis and determination of clinical ocular characteristics in south India. J. Infect. Dis. 1998, 177, 1314–1321. [Google Scholar] [CrossRef]
- Polle, F.; Storey, E.; Eades, S.; Alt, D.; Hornsby, R.; Zuerner, R.; Carter, R. Role of intraocular Leptospira infections in the pathogenesis of equine recurrent uveitis in the southern United States. J. Equine Vet. Sci. 2014, 34, 1300–1306. [Google Scholar] [CrossRef]
- Kitson-Piggot, A.W.; Prescott, J.F. Leptospirosis in horses in Ontario. Can. J. Vet. Res. 1987, 51, 448–451. [Google Scholar]
- Båverud, V.; Gunnarsson, A.; Engvall, E.O.; Franzén, P.; Egenvall, A. Leptospira seroprevalence and associations between seropositivity, clinical disease and host factors in horses. Acta Vet. Scand. 2009, 51, 15. [Google Scholar] [CrossRef]
- Tsegay, K.; Potts, A.; Aklilu, N.; Lötter, C.; Gummow, B. Circulating serovars of Leptospira in cart horses of central and southern Ethiopia and associated risk factors. Prev. Vet. Med. 2016, 125, 106–115. [Google Scholar] [CrossRef]
- Habus, J.; Persic, Z.; Spicic, S.; Vince, S.; Stritof, Z.; Milas, Z.; Cvetnic, Z.; Perharic, M.; Turk, N. New trends in human and animal leptospirosis in Croatia, 2009–2014. Acta Trop. 2017, 168, 1–8. [Google Scholar] [CrossRef]
- Peixoto Ribeiro, T.M.; Correia, L.; Hofstaetter Spohr, K.A.; Aguiar, D.M.; Martins, G.; de Sá Jayme, V. Risk Factors Associated With Seroreactivity Against Leptospira sp. in Horses From Brazilian Amazon. J. Equine Vet. Sci. 2018, 68, 59–62. [Google Scholar] [CrossRef]
- Siqueira, C.C.; Fraga, D.B.M.; Chagas-Junior, A.D.; Athanazio, D.A.; Silva, M.M.N.; Cerqueira, R.B.; da, C.M.F.W.; Pinna, M.H.; Ayres, M.C.C. Seroprevalence and risk factors associated with equine leptospirosis in the metropolitan region of Salvador and Recôncavo Baiano region, Bahia state (NE Brazil). Trop. Anim. Health Prod. 2020, 52, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Witmer, R. Clinical implications of aqueous humor studies in uveitis. Am. J. Ophthalmol. 1978, 86, 39–44. [Google Scholar] [CrossRef]
- Haut, J.; Roman, S.; Morin, Y.; Monin, C.; Morel, C. Recherche étiologique de cent dix uvéites. Intérêt des ponctions d’humeur aqueuse et du vitré [Search for etiology in 110 cases of uveitis. Value of punctures of the aqueous humor and vitreous body]. J. Fr. Ophtalmol. 1995, 18, 292–304. [Google Scholar] [PubMed]
- Rothova, A.; de Boer, J.H.; Ten Dam-van Loon, N.H.; Postma, G.; de Visser, L.; Zuurveen, S.J.; Schuller, M.; Weersink, A.J.; van Loon, A.M.; de Groot-Mijnes, J.D. Usefulness of aqueous humor analysis for the diagnosis of posterior uveitis. Ophthalmology 2008, 115, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Sivakolundu, S.; Sivakumar, R.R.; Chidambaranathan, G.P.; Sritharan, M. Serological diagnosis of leptospiral uveitis by HbpA IgG ELISA. J. Med. Microbiol. 2012, 61, 1681–1687. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Butler, N.J.; Furtado, J.M.; Winthrop, K.L.; Smith, J.R. Ocular toxoplasmosis II: Clinical features, pathology and management. Clin. Exp. Ophthalmol. 2013, 41, 95–108. [Google Scholar] [CrossRef]
- Brockie, R.E.; Till, D.G. Leptospira ballum isolated from hedgehogs. N. Z. Vet. J. 1977, 25, 28–30. [Google Scholar] [CrossRef]
- Hathaway, S.C.; Blackmore, D.K. Ecological aspects of the epidemiology of infection with leptospires of the Ballum serogroup in the black rat (Rattus rattus) and the brown rat (Rattus norvegicus) in New Zealand. J. Hyg. 1981, 87, 427–436. [Google Scholar] [CrossRef]
- Libonati, H.; Pinto, P.S.; Lilenbaum, W. Seronegativity of bovines face to their own recovered leptospiral isolates. Microb. Pathog. 2017, 108, 101–103. [Google Scholar] [CrossRef]
- Remky, H.; Kuchle, H.J.; Vollberchtshausen, R. Quantitative serologische Untersuchungen bei toxoplasmoseverdächtigen Augenerkrankungen [Quantitative serological studies in suspected toxoplasmosis of the eye]. Klin. Monatsbl. Augenheilkd. Augenarztl. Fortbild. 1957, 130, 794–800. [Google Scholar]
- Dussaix, E.; Cerqueti, P.M.; Pontet, F.; Bloch-Michel, E. New approaches to the detection of locally produced antiviral antibodies in the aqueous of patients with endogenous uveitis. Ophthalmologica 1987, 194, 145–149. [Google Scholar] [CrossRef]
- De Visser, L. Infectious uveitis—New developments in etiology and pathogenesis, Chapter 1: Etiology and diagnosis of infectious uveitis. In Intraocular Fluid Analysis; Proefschrift, Utrecht University: Utrecht, The Netherlands, 2009; Available online: https://dspace.library.uu.nl/handle/1874/36951 (accessed on 5 December 2021).
- Pleyer, U.; Ruokonen, P. Kammerwasseranalyse in der Diagnostik intraokularer Entzündungen [Aqueous humor analysis: A diagnostic tool in intraocular inflammation]. Klin. Monbl. Augenheilkd. 2010, 227, 953–960. [Google Scholar] [CrossRef]
- Chen, K.; Li, X.; Wang, D.; Ma, Y.; Chen, B.; Wang, Q.; Ma, J.; Guan, M. The diagnostic value of IL-10 and IL-6 level in vitreous fluid and aqueous humor for vitreoretinal lymphoma. Clin. Chim. Acta 2021, 515, 21–26. [Google Scholar] [CrossRef]
- Lappin, M.R.; Roberts, S.M.; Davidson, M.G.; Powell, C.C.; Reif, J.S. Enzyme-linked immunosorbent assays for the detection of Toxoplasma gondii-specific antibodies and antigens in the aqueous humor of cats. J. Am. Vet. Med. Assoc. 1992, 201, 1010–1016. [Google Scholar]
- Maggs, D.J.; Lappin, M.R.; Nasisse, M.P. Detection of feline herpesvirus-specific antibodies and DNA in aqueous humor from cats with or without uveitis. Am. J. Vet. Res. 1999, 60, 932–936. [Google Scholar]
- De Groot-Mijnes, J.D.; Rothova, A.; Van Loon, A.M.; Schuller, M.; Ten Dam-Van Loon, N.H.; De Boer, J.H.; Schuurman, R.; Weersink, A.J. Polymerase chain reaction and Goldmann-Witmer coefficient analysis are complimentary for the diagnosis of infectious uveitis. Am. J. Ophthalmol. 2006, 141, 313–318. [Google Scholar] [CrossRef]
- Santos, H.; Ferracioli-Oda, E.; Barbosa, T.S.; Otani, C.S.V.; Tanaka, T.; Silva, L.; Lopes, G.O.; Doi, A.; Hirata, C.E.; Yamamoto, J.H. Usefulness of aqueous and vitreous humor analysis in infectious uveitis. Clinics 2020, 75, e1498. [Google Scholar] [CrossRef]
- Van Gelder, R.N. Cme review: Polymerase chain reaction diagnostics for posterior segment disease. Retina 2003, 23, 445–452. [Google Scholar] [CrossRef]
- Oahalou, A.; Schellekens, P.A.; de Groot-Mijnes, J.D.; Rothova, A. Diagnostic pars plana vitrectomy and aqueous analyses in patients with uveitis of unknown cause. Retina 2014, 34, 108–114. [Google Scholar] [CrossRef]
- Matet, A.; Paris, L.; Fardeau, C.; Terrada, C.; Champion, E.; Fekkar, A.; Cassoux, N.; Touitou, V.; LeHoang, P.; Bodaghi, B. Clinical and Biological Factors Associated With Recurrences of Severe Toxoplasmic Retinochoroiditis Confirmed by Aqueous Humor Analysis. Am. J. Ophthalmol. 2019, 199, 82–93. [Google Scholar] [CrossRef]
- Greigert, V.; Di Foggia, E.; Filisetti, D.; Villard, O.; Pfaff, A.W.; Sauer, A.; Candolfi, E. When biology supports clinical diagnosis: Review of techniques to diagnose ocular toxoplasmosis. Br. J. Ophthalmol. 2019, 103, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Minkus, C.L.; Bispo, P.J.M.; Papaliodis, G.N.; Sobrin, L. Real-Time Multiplex PCR Analysis in Infectious Uveitis. Semin. Ophthalmol. 2019, 34, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Quentin, C.D.; Reiber, H. Kammerwasseranalytik bei intraokularer Toxoplasmose [Analysis of aqueous humor in intraocular toxoplasmosis]. Ophthalmologe 1997, 94, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Liekfeld, A.; Schweig, F.; Jaeckel, C.; Wernecke, K.D.; Hartmann, C.; Pleyer, U. Intraocular antibody production in intraocular inflammation. Graefes Arch. Clin. Exp. Ophthalmol. 2000, 238, 222–227. [Google Scholar] [CrossRef]
- Bertelmann, T.; Kičová, N.; Kohlberger, L.; Spychalska, M.; Strodthoff, S.; Irle, S.; Mennel, S. Sampling aqueous humor: Anterior segment anatomy, anesthetic and surgical technique, and rates of yield. Ophthalmic Res. 2012, 47, 214–219. [Google Scholar] [CrossRef]
- Harper, T.W.; Miller, D.; Schiffman, J.C.; Davis, J.L. Polymerase chain reaction analysis of aqueous and vitreous specimens in the diagnosis of posterior segment infectious uveitis. Am. J. Ophthalmol. 2009, 147, 140–147.e2. [Google Scholar] [CrossRef]
- Davis, J.L.; Miller, D.M.; Ruiz, P. Diagnostic testing of vitrectomy specimens. Am. J. Ophthalmol. 2005, 140, 822–829. [Google Scholar] [CrossRef]
- De Groot-Mijnes, J.D.; Rothova, A. Diagnostic testing of vitrectomy specimens. Am. J. Ophthalmol. 2006, 141, 982, author reply 982–983. [Google Scholar] [CrossRef]
- Margolis, R.; Brasil, O.F.; Lowder, C.Y.; Singh, R.P.; Kaiser, P.K.; Smith, S.D.; Perez, V.L.; Sonnie, C.; Sears, J.E. Vitrectomy for the diagnosis and management of uveitis of unknown cause. Ophthalmology 2007, 114, 1893–1897. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Xia, S.; Chen, Y.X. Role of diagnostic pars plana vitrectomy in determining the etiology of uveitis initially unknown. Retina 2020, 40, 359–369. [Google Scholar] [CrossRef]
- Bodaghi, B.; LeHoang, P. Testing ocular fluids in uveitis. Ophthalmol. Clin. N. Am. 2002, 15, 271–279. [Google Scholar] [CrossRef]
- Jeroudi, A.; Yeh, S. Diagnostic vitrectomy for infectious uveitis. Int. Ophthalmol. Clin. 2014, 54, 173–197. [Google Scholar] [CrossRef]
- Gelatt, K.N. Ophthalmic examination and diagnostic procedures. In Veterinary Ophthalmology; Gelatt, K.N., Ed.; Lea and Febiger: Philadelphia, PA, USA, 1981; pp. 219–221. [Google Scholar]
- Schwink, K.; Crisman, M.; Rigg, D. Chronic recurrent uveitis in a horse with an elevated aqueous humor antibody titer to Leptospira interrogans serovar autumnalis. Equine Pract. 1989, 11, 41–43. [Google Scholar]
- Sauvage, A.C.; Monclin, S.J.; Elansary, M.; Hansen, P.; Grauwels, M.F. Detection of intraocular Leptospira spp. by real-time polymerase chain reaction in horses with recurrent uveitis in Belgium. Equine Vet. J. 2019, 51, 299–303. [Google Scholar] [CrossRef]
- Pearce, J.W.; Galle, L.E.; Kleiboeker, S.B.; Turk, J.R.; Schommer, S.K.; Dubielizig, R.R.; Mitchell, W.J.; Moore, C.P.; Giuliano, E.A. Detection of Leptospira interrogans DNA and antigen in fixed equine eyes affected with end-stage equine recurrent uveitis. J. Vet. Diagn. Investig. 2007, 19, 686–690. [Google Scholar] [CrossRef]
- Wollanke, B.; Gerhards, H.; Loibl, J.K.; Brem, S. Laboratory Diagnosis of Leptospiral Uveitis in Horses. In Proceedings of the 10th Conference of the International Leptospirosis Society (ILS), Palmerston North, New Zealand, 27 November–1 December 2017; p. 143. [Google Scholar]
- Brem, S. Unpublished data, Personal Communication. 1996. [Google Scholar]
- Brem, S.; Gerhards, H.; Wollanke, B.; Meyer, P.; Kopp, H. Intraokularer Leptospirennachweis bei 4 Pferden mit rezidivierender Uveitis (ERU) [Demonstration of intraocular Leptospira in 4 horses suffering from equine recurrent uveitis (ERU)]. Berl. Munch. Tierarztl. Wochenschr. 1998, 111, 415–417. [Google Scholar]
- Brem, S.; Gerhards, H.; Wollanke, B.; Meyer, P.; Kopp, H. 35 Leptospirenisolationen aus Glaskörpern von 32 Pferden mit rezidivierender Uveitis (ERU) [35 Leptospira isolated from the vitreous body of 32 horses with recurrent uveitis (ERU)]. Berl. Munch. Tierarztl. Wochenschr. 1999, 112, 390–393. [Google Scholar]
- Wessling, B.E. Klinischer Vergleich der Wirkung von Phenylbutazon, Flunixin und Vedaprofen bei equinen Vitrektomie-Patienten sowie Bestimmung der Wirkstoffspiegel in Serum- und Glaskörperproben [Clinical comparison of the effects of phenylbutazone, flunixin, and vedaprofen in equine vitrectomy patients and determination of drug levels in serum and vitreous samples]. Ph.D. Thesis, Ludwig-Maximilians-University (LMU), Munich, Germany, 2004. [Google Scholar]
- Hartskeerl, R.A.; Goris, M.G.; Brem, S.; Meyer, P.; Kopp, H.; Gerhards, H.; Wollanke, B. Classification of Leptospira from the eyes of horses suffering from recurrent uveitis. J. Vet. Med. B Infect. Dis. Vet. Public Health 2004, 51, 110–115. [Google Scholar] [CrossRef]
- Da Silva, M.V.; Nakamura, P.M.; Camargo, E.D.; Batista, L.; Vaz, A.J.; Romero, E.C.; Brandao, A.P. Immunodiagnosis of human leptospirosis by dot-ELISA for the detection of IgM, IgG, and IgA antibodies. Am. J. Trop. Med. Hyg. 1997, 56, 650–655. [Google Scholar] [CrossRef]
- Wagner, B.; Brandt, K.; Sheoran, A.; Holmes, M.A.; Deegen, E.; Leibold, W. Nachweis von lmmunglobulinisotypen im Glaskörper als Beitrag zur Ätiologie der equinen rezidivierenden Uveitis [Demonstration of immunoglobulin isotypes in the vitreous body as a contribution to the etiology of recurrent equine uveitis]. Dtsch Tierarztl Wochenschr 1997, 104, 467–470. [Google Scholar]
- Garweg, J.G.; Garweg, S.D.; Flueckiger, F.; Jacquier, P.; Boehnke, M. Aqueous humor and serum immunoblotting for immunoglobulin types G, A, M, and E in cases of human ocular toxoplasmosis. J. Clin. Microbiol. 2004, 42, 4593–4598. [Google Scholar] [CrossRef]
- Ronday, M.J.; Ongkosuwito, J.V.; Rothova, A.; Kijlstra, A. Intraocular anti-Toxoplasma gondii IgA antibody production in patients with ocular toxoplasmosis. Am. J. Ophthalmol. 1999, 127, 294–300. [Google Scholar] [CrossRef]
- Verma, A.; Artiushin, S.; Matsunaga, J.; Haake, D.A.; Timoney, J.F. LruA and LruB, novel lipoproteins of pathogenic Leptospira interrogans associated with equine recurrent uveitis. Infect. Immun. 2005, 73, 7259–7566. [Google Scholar] [CrossRef]
- Pedersen, S.S.; Shand, G.H.; Hansen, B.L.; Hansen, G.N. Induction of experimental chronic Pseudomonas aeruginosa lung infection with P. aeruginosa entrapped in alginate microspheres. APMIS 1990, 98, 203–211. [Google Scholar] [CrossRef]
- Høiby, N.; Krogh Johansen, H.; Moser, C.; Song, Z.; Ciofu, O.; Kharazmi, A. Pseudomonas aeruginosa and the in vitro and in vivo biofilm mode of growth. Microbes Infect. 2001, 3, 23–35. [Google Scholar] [CrossRef]
- Aanaes, K.; Johansen, H.K.; Poulsen, S.S.; Pressler, T.; Buchwald, C.; Høiby, N. Secretory IgA as a diagnostic tool for Pseudomonas aeruginosa respiratory colonization. J. Cyst. Fibros. 2013, 12, 81–87. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Moser, C.; Bassi, G.L.; Coenye, T.; Donelli, G.; Hall-Stoodley, L.; Holá, V.; Imbert, C.; Kirketerp-Møller, K.; et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin. Microbiol. Infect. 2015, 21 (Suppl. 1), 1–25. [Google Scholar] [CrossRef]
- Gesell-May, S.; Brem, S.; Wollanke, B.; Gerhards, H. Untersuchung gesunder Pferdeaugen auf eine intraokulare Leptospireninfektion [Examination of equine healthy eyes for intraocular leptospiral infection]. Pferdeheilkunde—Equine Med. 2021, 37, 215–224. [Google Scholar] [CrossRef]
- Gesell, S. Gibt es eine asymptomatische intraokulare Leptospireninfektion beim Pferd? [Is there an asymptomatic intraocular leptospiral infection in horses?]. Ph.D. Thesis, Ludwig-Maximilians-University (LMU), Munich, Germany, 2004. [Google Scholar] [CrossRef]
- Hartmann, C.; Severin, M.; Ritter, D.; Schädlich, H. Der diagnostische Wert von immunologischen Kammerwasser-und Glaskörperuntersuchungen bei entzündlichen Netzhaut-/Aderhauterkrankungen [The diagnostic value of immunological aqueous humor and vitreous examinations in inflammatory retinal/choroidal diseases]. Proc. 153. Versammlung des Vereins der Rheinisch-Westfälischen Augenärzte 1991, 153, 187–195. [Google Scholar]
- Novales, M.; Lopez, R.; Ginel, P.; Martin, E.; Moreno, P.; Molleda, J. Les effets de l’uveite sur la concentration des proetines totales dans l’humeur aqueuse de chiens atteints de leishmaniose [Effects of uveitis on the concentration of total proetins in the aqueous humor of dogs with leishmaniasis]. Rev. Med. Vet. 1994, 145, 257–260. [Google Scholar]
- Altmann, F. Differenzielle Expression von Proteinen im Glaskörper gesunder und an ERU erkrankter Pferde [Differential expression of proteins in the vitreous of healthy and ERU diseased horses]. Ph.D. Thesis, Ludwig-Maximilians-University (LMU), Munich, Germany, 2007. [Google Scholar] [CrossRef]
- Gesell-May, S. Die equine rezidivierende Uveitis (ERU): Diskussion von Untersuchungs-und Therapieergebnissen, insbesondere in Bezug auf die Vitrektomie und den Einsatz von Gentamicin und Cyclosporin [Equine recurrent uveitis (ERU): Discussion of investigational and therapeutic findings, particularly in relation to vitrectomy and the use of gentamicin and cyclosporine]. Der Praktische Tierarzt 2020, 101, 560–566. [Google Scholar] [CrossRef]
- Irvine, R.; Irvine, A.R.; Irvine, M.D. A study of aqueous humor as an aid to understanding uveitis and certain related conditions. Am. J. Ophthalmol. 1942, 25, 150–163. [Google Scholar] [CrossRef]
- Dernouchamps, J.P.; Michiels, J. Molecular sieve properties of the blood-aqueous barrier in uveitis. Exp. Eye Res. 1977, 25, 25–31. [Google Scholar] [CrossRef]
- Witmer, R. Antikörperbildung im Auge [Antibody formation in the eye]. Schweiz. Med. Wochenschr. 1955, 85, 332–333. [Google Scholar] [PubMed]
- Goldmann, H.; Witmer, R. Antikörper im Kammerwasser [Antibodies in the aqueous humor]. Ophthalmologica 1954, 127, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Witmer, R.H. Ätiologische Diagnostik der Uveitis. I. Klinische und experimentelle Ergebnisse an Mensch und Tier [Etiological diagnosis of uveitis. I. Clinical and experimental results in man and animal]. Albrecht Von Graefes Arch. Ophthalmol. 1955, 156, 235–260. [Google Scholar] [CrossRef]
- Kleinwort, K.J.; Amann, B.; Hauck, S.M.; Feederle, R.; Sekundo, W.; Deeg, C.A. Immunological Characterization of Intraocular Lymphoid Follicles in a Spontaneous Recurrent Uveitis Model. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4504–4511. [Google Scholar] [CrossRef]
- Witmer, R. Serologie der Uveitis [Serology of uveitis]. Ophthalmologica 1962, 143, 357–362. [Google Scholar] [CrossRef]
- O’Connor, G.R. Factors related to the initiation and recurrence of uveitis. XL Edward Jackson memorial lecture. Am. J. Ophthalmol. 1983, 96, 577–599. [Google Scholar] [CrossRef]
- Kijlstra, A.; Luyendijk, L.; Baarsma, G.S.; Rothova, A.; Schweitzer, C.M.; Timmerman, Z.; de Vries, J.; Breebaart, A.C. Aqueous humor analysis as a diagnostic tool in toxoplasma uveitis. Int. Ophthalmol. 1989, 13, 383–386. [Google Scholar] [CrossRef]
- Robert-Gangneux, F.; Binisti, P.; Antonetti, D.; Brezin, A.; Yera, H.; Dupouy-Camet, J. Usefulness of immunoblotting and Goldmann-Witmer coefficient for biological diagnosis of toxoplasmic retinochoroiditis. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 34–38. [Google Scholar] [CrossRef]
- Kalogeropoulos, D.; Sakkas, H.; Mohammed, B.; Vartholomatos, G.; Malamos, K.; Sreekantam, S.; Kanavaros, P.; Kalogeropoulos, C. Ocular toxoplasmosis: A review of the current diagnostic and therapeutic approaches. Int. Ophthalmol. 2022, 42, 295–321. [Google Scholar] [CrossRef]
- De Boer, J.H.; Luyendijk, L.; Rothova, A.; Baarsma, G.S.; de Jong, P.T.; Bollemeijer, J.G.; Rademakers, A.J.; Van der Lelij, A.; Zaal, M.J.; Kijlstra, A. Detection of intraocular antibody production to herpesviruses in acute retinal necrosis syndrome. Am. J. Ophthalmol. 1994, 117, 201–210. [Google Scholar] [CrossRef]
- Abe, T.; Tsuchida, K.; Tamai, M. A comparative study of the polymerase chain reaction and local antibody production in acute retinal necrosis syndrome and cytomegalovirus retinitis. Graefes Arch. Clin. Exp. Ophthalmol. 1996, 234, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Desmonts, G. Definitive serological diagnosis of ocular toxoplasmosis. Arch. Ophthalmol. 1966, 76, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Garweg, J.G.; Jacquier, P.; Boehnke, M. Early aqueous humor analysis in patients with human ocular toxoplasmosis. J. Clin. Microbiol. 2000, 38, 996–1001. [Google Scholar] [CrossRef]
- Hermans, L.E.; Oosterheert, J.J.; Kampschreur, L.M.; Ossewaarde-van Norel, J.; Dekkers, J.; Rothova, A.; de Groot-Mijnes, J.D. Molecular and Serological Intraocular Fluid Analysis of Coxiella burnetii-seropositive Patients with Concurrent Idiopathic Uveitis. Ocul. Immunol. Inflamm. 2016, 24, 77–80. [Google Scholar] [CrossRef]
- Baarsma, G.S.; Luyendijk, L.; Kijlstra, A.; de Vries, J.; Peperkamp, E.; Mertens, D.A.; van Meurs, J.C. Analysis of local antibody production in the vitreous humor of patients with severe uveitis. Am. J. Ophthalmol. 1991, 112, 147–150. [Google Scholar] [CrossRef]
- Kijlstra, A.; van den Horn, G.J.; Luyendijk, L.; Baarsma, G.S.; Schweitzer, C.M.; Zaal, M.J.; Timmerman, Z.; Beintema, M.; Rothova, A. Laboratory tests in uveitis. New developments in the analysis of local antibody production. Doc. Ophthalmol. 1990, 75, 225–231. [Google Scholar] [CrossRef]
- De Boer, J.H.; Verhagen, C.; Bruinenberg, M.; Rothova, A.; de Jong, P.T.; Baarsma, G.S.; Van der Lelij, A.; Ooyman, F.M.; Bollemeijer, J.G.; Derhaag, P.J.; et al. Serologic and polymerase chain reaction analysis of intraocular fluids in the diagnosis of infectious uveitis. Am. J. Ophthalmol. 1996, 121, 650–658. [Google Scholar] [CrossRef]
- Gilger, B.C. Association of acute leptospirosis with systemic disease and uveitis in horses. Equine Vet. Educ. 2018, 30, 137–138. [Google Scholar] [CrossRef]
- Faber, N.A.; Crawford, M.; LeFebvre, R.B.; Buyukmihci, N.C.; Madigan, J.E.; Willits, N.H. Detection of Leptospira spp. in the aqueous humor of horses with naturally acquired recurrent uveitis. J. Clin. Microbiol. 2000, 38, 2731–2733. [Google Scholar] [CrossRef]
- Brem, S. Unpublished data, Personal communication. 2021. [Google Scholar]
- Majumder, P.D.; Ghosh, A.; Biswas, J. Infectious uveitis: An enigma. Middle East Afr. J. Ophthalmol. 2017, 24, 2–10. [Google Scholar] [CrossRef]
- Roczek, A. Entwicklung einer quantitativen PCR zum Nachweis von DNA pathogener Leptospiren in Glaskörper- und Kammerwasserproben von Pferden [Development of a quantitative PCR for the detection of DNA from pathogenic leptospires in vitreous- and aqueous humor samples of horses]. Ph.D. Thesis, Ludwig-Maximilians-University (LMU), Munich, Germany, 2008. [Google Scholar] [CrossRef]
- Westeneng, A.C.; Rothova, A.; de Boer, J.H.; de Groot-Mijnes, J.D. Infectious uveitis in immunocompromised patients and the diagnostic value of polymerase chain reaction and Goldmann-Witmer coefficient in aqueous analysis. Am. J. Ophthalmol. 2007, 144, 781–785. [Google Scholar] [CrossRef]
- Errera, M.H.; Goldschmidt, P.; Batellier, L.; Degorge, S.; Héron, E.; Laroche, L.; Sahel, J.A.; Westcott, M.; Chaumeil, C. Real-time polymerase chain reaction and intraocular antibody production for the diagnosis of viral versus toxoplasmic infectious posterior uveitis. Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 1837–1846. [Google Scholar] [CrossRef]
- Merien, F.; Perolat, P.; Mancel, E.; Persan, D.; Baranton, G. Detection of Leptospira DNA by polymerase chain reaction in aqueous humor of a patient with unilateral uveitis. J. Infect. Dis. 1993, 168, 1335–1336. [Google Scholar] [CrossRef]
- Kalogeropoulos, D.; Asproudis, I.; Stefaniotou, M.; Moschos, M.; Gartzonika, C.; Bassukas, I.; Konitsiotis, S.; Milionis, H.; Gaitanis, G.; Malamos, K.; et al. Spirochetal uveitis: Spectrum of clinical manifestations, diagnostic and therapeutic approach, final outcome and epidemiological data. Int. Ophthalmol. 2021. [Google Scholar] [CrossRef]
- Eule, J.C.; Wagner, B.; Leibold, W.; Deegen, E. Vorkommen verschiedener lmmunglobulinisotypen bei Pferden mit equiner rezidivierender Uveitis (ERU) [Occurrence of various immunoglobulin isotopes in horses with equine recurrent uveitis (ERU)]. Berl. Munch. Tierarztl. Wochenschr. 2000, 113, 253–257. [Google Scholar]
- Kongyai, N.; Pathanapitoon, K.; Sirirungsi, W.; Kunavisarut, P.; de Groot-Mijnes, J.D.; Rothova, A. Infectious causes of posterior uveitis and panuveitis in Thailand. Jpn. J. Ophthalmol. 2012, 56, 390–395. [Google Scholar] [CrossRef]
- Niedermaier, G.; Wollanke, B.; Hoffmann, R.; Matiasek, K.; Gerhards, H. Darstellung der Glaskörperstruktur von augengesunden Pferden und von Pferden mit equiner rezidivierender Uveitis (ERU) mittels Transmissions-Elektronenmikroskopie [Depiction of the structure of the vitreous body in horses without ocular diseases and in horses with equine recurrent uveitis (ERU) using transmission electron microscopy]. DTW Dtsch. Tierarztl. Wochenschr. 2006, 113, 211–217. [Google Scholar]
- Niedermaier, G. Elektronenmikroskopische Untersuchungen des Glaskörpers des Pferdes mit equiner rezidivierender Uveitis [Electron microscopic studies of the vitreous body of the horse with equine recurrent uveitis]. Ph.D. Thesis, Ludwig-Maximilians-University (LMU), Munich, Germany, 2002. [Google Scholar]
- Niedermaier, G.; Wollanke, B.; Hoffmann, R.; Brem, S.; Gerhards, H. Darstellung von Leptospiren im Glaskörper augengesunder und an ERU erkrankter Pferde mittels Transmissions-Elektronenmikroskopie [Detection of Leptospira in the vitreous body of horses without ocular diseases and of horses with equine recurrent uveitis (ERU) using transmission-electron microscopy]. DTW Dtsch. Tierarztl. Wochenschr. 2006, 113, 418–422. [Google Scholar]
- Brandes, K.; Wollanke, B.; Niedermaier, G.; Brem, S.; Gerhards, H. Recurrent uveitis in horses: Vitreal examinations with ultrastructural detection of leptospires. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2007, 54, 270–275. [Google Scholar] [CrossRef]
- Geißler, P.; Wollanke, B. Biofilm formation in persistent infections and its role in the pathogenesis of equine recurrent uveitis (ERU)—A literature review. Pferdeheilkunde—Equine Med. 2021, 37, 225–233. [Google Scholar] [CrossRef]
- Dubielzig, R.; Render, J.; Morreale, R. Distinctive morphologic features of the ciliary body in equine recurrent uveitis. Vet. Comp. Ophthalmol. 1997, 7, 163–167. [Google Scholar]
- Linke, R.P.; Brandes, K.; Cielewicz, M.B.; Gerhards, H.; Wollanke, B. Ocular leptospiral infection leads to ciliary induction and local AA-amyloidosis in horses. Amyloid 2019, 26, 127–128. [Google Scholar] [CrossRef]
- Roth, T.; Brandes, K.; Gerhards, H.; Giving, E.; Wollanke, B. Histologische Untersuchungen des Glaskörpers bei Pferden mit equiner rezidivierender Uveitis [Histologic studies of the vitreous in horses with equine recurrent uveitis]. Pferdeheilkunde 2014, 30, 512–520. [Google Scholar] [CrossRef][Green Version]
- Roth, T. Histologische Untersuchungen des Glaskörpers bei an equiner rezidivierender Uveitis erkrankten Pferden [Histological studies of the vitreous in horses suffering from equine recurrent uveitis]. Ph.D. Thesis, Ludwig-Maximilians-University (LMU), Munich, Germany, 2013. [Google Scholar] [CrossRef]
- Deeg, C.A. A proteomic approach for studying the pathogenesis of spontaneous equine recurrent uveitis (ERU). Vet. Immunol. Immunopathol. 2009, 128, 132–136. [Google Scholar] [CrossRef]
- Fingerhut, L.; Ohnesorge, B.; von Borstel, M.; Schumski, A.; Strutzberg-Minder, K.; Mörgelin, M.; Deeg, C.A.; Haagsman, H.P.; Beineke, A.; von Köckritz-Blickwede, M.; et al. Neutrophil Extracellular Traps in the Pathogenesis of Equine Recurrent Uveitis (ERU). Cells 2019, 8, 1528. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef]
- Mahajan, A.; Grüneboom, A.; Petru, L.; Podolska, M.J.; Kling, L.; Maueröder, C.; Dahms, F.; Christiansen, S.; Günter, L.; Krenn, V.; et al. Frontline Science: Aggregated neutrophil extracellular traps prevent inflammation on the neutrophil-rich ocular surface. J. Leukoc. Biol. 2019, 105, 1087–1098. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Branzk, N.; Lubojemska, A.; Hardison, S.E.; Wang, Q.; Gutierrez, M.G.; Brown, G.D.; Papayannopoulos, V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 2014, 15, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Fingerhut, L.; Dolz, G.; De Buhr, N. What Is the Evolutionary Fingerprint in Neutrophil Granulocytes? Int. J. Mol. Sci. 2020, 21, 4523. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Laube, B.; Abu Abed, U.; Goosmann, C.; Zychlinsky, A. Neutrophil extracellular traps: How to generate and visualize them. J. Vis. Exp. 2010, 36, 1724. [Google Scholar] [CrossRef] [PubMed]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 2014, 9, 181–218. [Google Scholar] [CrossRef]
- Yipp, B.G.; Kubes, P. NETosis: How vital is it? Blood 2013, 122, 2784–2794. [Google Scholar] [CrossRef]
- Tan, C.; Aziz, M.; Wang, P. The vitals of NETs. J. Leukoc. Biol. 2021, 110, 797–808. [Google Scholar] [CrossRef]
- Yousefi, S.; Mihalache, C.; Kozlowski, E.; Schmid, I.; Simon, H.U. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009, 16, 1438–1444. [Google Scholar] [CrossRef]
- Mutua, V.; Gershwin, L.J. A Review of Neutrophil Extracellular Traps (NETs) in Disease: Potential Anti-NETs Therapeutics. Clin. Rev. Allergy Immunol. 2021, 61, 194–211. [Google Scholar] [CrossRef]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Haensch, G.M. Host defence against bacterial biofilms: “Mission Impossible”? Int. Scholarly Res. Not. 2012, 2012. [Google Scholar] [CrossRef]
- Thanabalasuriar, A.; Kubes, P. Rise and shine: Open your eyes to produce anti-inflammatory NETs. J. Leukoc. Biol. 2019, 105, 1083–1084. [Google Scholar] [CrossRef]
- Thanabalasuriar, A.; Scott, B.N.V.; Peiseler, M.; Willson, M.E.; Zeng, Z.; Warrener, P.; Keller, A.E.; Surewaard, B.G.J.; Dozier, E.A.; Korhonen, J.T.; et al. Neutrophil Extracellular Traps Confine Pseudomonas aeruginosa Ocular Biofilms and Restrict Brain Invasion. Cell Host Microbe 2019, 25, 526–536.e4. [Google Scholar] [CrossRef]
- Papayannopoulos, V. Neutrophils Facing Biofilms: The Battle of the Barriers. Cell Host Microbe 2019, 25, 477–479. [Google Scholar] [CrossRef]
- Tsuprun, V.; Shibata, D.; Paparella, M.M.; Cureoglu, S. Formations of Host Fibers and Bacteria in Human Temporal Bones With Otitis Media. Otol. Neurotol. 2021, 42, e949–e957. [Google Scholar] [CrossRef]
- Jacobsen, S.; Andersen, P. The acute phase protein serum amyloid A (SAA) as a marker of inflammation in horses. Equine Vet. Educ. 2007, 19, 38–46. [Google Scholar] [CrossRef]
- Petersen, H.H.; Nielsen, J.P.; Heegaard, P.M. Application of acute phase protein measurements in veterinary clinical chemistry. Vet. Res. 2004, 35, 163–187. [Google Scholar] [CrossRef]
- Labelle, A.L.; Hamor, R.E.; Macneill, A.L.; Lascola, K.M.; Breaux, C.B.; Tolar, E.L. Effects of ophthalmic disease on concentrations of plasma fibrinogen and serum amyloid A in the horse. Equine Vet. J. 2011, 43, 460–465. [Google Scholar] [CrossRef]
- Waldner, J.; Gerhards, H.; Wollanke, B. Investigations into the occurrence of serum amyloid A in the equine eye. Pferdeheilkunde—Equine Med. 2018, 34, 461–467. [Google Scholar] [CrossRef]
- Waldner, J.S. Untersuchungen zum Vorkommen von Serum Amyloid A im Pferdeauge [Studies on the occurrence of serum amyloid A in the horse eye]. Ph.D. Thesis, Ludwig-Maximilians-University (LMU), Munich, Germany, 2017. [Google Scholar] [CrossRef]
- Müller, A.C.; Büttner, K.; Röcken, M. Systemic serum amyloid A in early (<24 h) diagnosis of acute synovial structure involvement in horses with penetrating limb injuries. Vet. J. 2021, 277, 105759. [Google Scholar] [CrossRef]
- Lachmann, H.J.; Goodman, H.J.; Gilbertson, J.A.; Gallimore, J.R.; Sabin, C.A.; Gillmore, J.D.; Hawkins, P.N. Natural history and outcome in systemic AA amyloidosis. N. Engl. J. Med. 2007, 356, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Ostevik, L.; de Souza, G.A.; Wien, T.N.; Gunnes, G.; Sørby, R. Characterization of amyloid in equine recurrent uveitis as AA amyloid. J. Comp. Pathol. 2014, 151, 228–233. [Google Scholar] [CrossRef]
- Huang, Y.; Nasir, S.; Challa, S.R.; Peng, C.C.; Stashek, K.; Fanaroff, R.; Hu, S. Gastric AA amyloidosis secondary to chronic infection presenting with hematemesis: A case report. Clin. J. Gastroenterol. 2020, 13, 1070–1073. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Matsumae, T.; Murakami, Y.; Abe, Y.; Sasatomi, Y.; Nagayoshi, I.; Ueda, K.; Nakashima, H. Chronic renal failure due to amyloid nephropathy caused by chronic infection after total hip replacement. CEN Case Rep. 2014, 3, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.O.; Hengge, R. Bacterial Multicellularity: The Biology of Escherichia coli Building Large-Scale Biofilm Communities. Annu. Rev. Microbiol. 2021, 75, 269–290. [Google Scholar] [CrossRef] [PubMed]
- Taglialegna, A.; Lasa, I.; Valle, J. Amyloid Structures as Biofilm Matrix Scaffolds. J. Bacteriol. 2016, 198, 2579–2588. [Google Scholar] [CrossRef] [PubMed]
- Hengge, R. Targeting Bacterial Biofilms by the Green Tea Polyphenol EGCG. Molecules 2019, 24, 2403. [Google Scholar] [CrossRef]
- Erskine, E.; MacPhee, C.E.; Stanley-Wall, N.R. Functional amyloid and other protein fibers in the biofilm matrix. J. Mol. Biol. 2018, 430, 3642–3656. [Google Scholar] [CrossRef]
- Blanco, L.P.; Evans, M.L.; Smith, D.R.; Badtke, M.P.; Chapman, M.R. Diversity, biogenesis and function of microbial amyloids. Trends Microbiol. 2012, 20, 66–73. [Google Scholar] [CrossRef]
- Vidakovic, L.; Singh, P.K.; Hartmann, R.; Nadell, C.D.; Drescher, K. Dynamic biofilm architecture confers individual and collective mechanisms of viral protection. Nat. Microbiol. 2018, 3, 26–31. [Google Scholar] [CrossRef]
- Thibeaux, R.; Soupé-Gilbert, M.E.; Kainiu, M.; Girault, D.; Bierque, E.; Fernandes, J.; Bähre, H.; Douyère, A.; Eskenazi, N.; Vinh, J.; et al. The zoonotic pathogen Leptospira interrogans mitigates environmental stress through cyclic-di-GMP-controlled biofilm production. NPJ Biofilms Microbiomes 2020, 6, 24. [Google Scholar] [CrossRef]
- O’Toole, G.; Kaplan, H.B.; Kolter, R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000, 54, 49–79. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, Q.; Zhang, D.; Yan, W. Mechanisms and Control Strategies of Antibiotic Resistance in Pathological Biofilms. J. Microbiol. Biotechnol. 2021, 31, 1–7. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Stoodley, P.; Kathju, S.; Høiby, N.; Moser, C.; Costerton, J.W.; Moter, A.; Bjarnsholt, T. Towards diagnostic guidelines for biofilm-associated infections. FEMS Immunol. Med. Microbiol. 2012, 65, 127–145. [Google Scholar] [CrossRef]
- Høiby, N. A short history of microbial biofilms and biofilm infections. APMIS 2017, 125, 272–275. [Google Scholar] [CrossRef]
- Stewart, P.S. Theoretical aspects of antibiotic diffusion into microbial biofilms. Antimicrob. Agents Chemother. 1996, 40, 2517–2522. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Nielsen, S.M.; Nørskov-Lauritsen, N.; Bjarnsholt, T.; Meyer, R.L. Achromobacter Species Isolated from Cystic Fibrosis Patients Reveal Distinctly Different Biofilm Morphotypes. Microorganisms 2016, 4, 33. [Google Scholar] [CrossRef]
- Høiby, N.; Henneberg, K.; Wang, H.; Stavnsbjerg, C.; Bjarnsholt, T.; Ciofu, O.; Johansen, U.R.; Sams, T. Formation of Pseudomonas aeruginosa inhibition zone during tobramycin disk diffusion is due to transition from planktonic to biofilm mode of growth. Int. J. Antimicrob. Agents 2019, 53, 564–573. [Google Scholar] [CrossRef]
- Bjarnsholt, T. Introduction to biofilms. In Biofilm Infections; Bjarnsholt, T., Moser, C., Ostrup Jensen, P., Hoiby, N., Eds.; Springer: New York, NY, USA, 2011; pp. 1–10. [Google Scholar]
- Høiby, N. A personal history of research on microbial biofilms and biofilm infections. Pathog. Dis. 2014, 70, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N. Pseudomonas aeruginosa infection in cystic fibrosis. Diagnostic and prognostic significance of Pseudomonas aeruginosa precipitins determined by means of crossed immunoelectrophoresis. A survey. Acta Pathol. Microbiol. Scand. Suppl. 1977, 1–96. [Google Scholar]
- Costerton, J.W.; Geesey, G.G.; Cheng, K.J. How bacteria stick. Sci. Am. 1978, 238, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Vinod Kumar, K.; Lall, C.; Vimal Raj, R.; Vedhagiri, K.; Vijayachari, P. Molecular detection of pathogenic leptospiral protein encoding gene (LipL32) in environmental aquatic biofilms. Lett. Appl. Microbiol. 2016, 62, 311–315. [Google Scholar] [CrossRef]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. APMIS Suppl. 2013, 1–51. [Google Scholar] [CrossRef]
- Jefferson, K.K. What drives bacteria to produce a biofilm? FEMS Microbiol. Lett. 2004, 236, 163–173. [Google Scholar] [CrossRef]
- Høiby, N.; Ciofu, O.; Johansen, H.K.; Song, Z.J.; Moser, C.; Jensen, P.; Molin, S.; Givskov, M.; Tolker-Nielsen, T.; Bjarnsholt, T. The clinical impact of bacterial biofilms. Int. J. Oral Sci. 2011, 3, 55–65. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Kaplan, J.B.; Izano, E.A.; Gopal, P.; Karwacki, M.T.; Kim, S.; Bose, J.L.; Bayles, K.W.; Horswill, A.R. Low levels of β-lactam antibiotics induce extracellular DNA release and biofilm formation in Staphylococcus aureus. mBio 2012, 3, e00198-12. [Google Scholar] [CrossRef]
- Johnson, L.; Mulcahy, H.; Kanevets, U.; Shi, Y.; Lewenza, S. Surface-localized spermidine protects the Pseudomonas aeruginosa outer membrane from antibiotic treatment and oxidative stress. J. Bacteriol. 2012, 194, 813–826. [Google Scholar] [CrossRef]
- Moser, C.; Pedersen, H.T.; Lerche, C.J.; Kolpen, M.; Line, L.; Thomsen, K.; Høiby, N.; Jensen, P. Biofilms and host response—Helpful or harmful. APMIS 2017, 125, 320–338. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Buhlin, K.; Dufrêne, Y.F.; Gomelsky, M.; Moroni, A.; Ramstedt, M.; Rumbaugh, K.P.; Schulte, T.; Sun, L.; Åkerlund, B.; et al. Biofilm formation—What we can learn from recent developments. J. Intern. Med. 2018, 284, 332–345. [Google Scholar] [CrossRef]
- Mehrotra, N.; Singh, S. Periodontitis. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Palmer, R.J.; Darveau, R.; Lamont, R.J.; Nyvad, B.; Teles, R.P. Human Oral Biofilms: Composition, Dynamics, and Pathogenesis. In Biofilm Infections; Bjarnsholt, T., Moser, C., Ostrup Jensen, P., Hoiby, N., Eds.; Springer: New York, NY, USA, 2011; pp. 35–68. [Google Scholar]
- Schaudinn, C.; Gorur, A.; Keller, D.; Sedghizadeh, P.P.; Costerton, J.W. Periodontitis: An archetypical biofilm disease. J. Am. Dent. Assoc. 2009, 140, 978–986. [Google Scholar] [CrossRef]
- Høiby, N.; Krogh Johansen, H.; Moser, C.; Ciofu, O.; Ostrup Jensen, P.; Kolpen, M.; Mandsberg, L.; Givskov, M.; Molin, S.; Bjarnsholt, T. Pseudomonas aeruginosa Biofilms in the Lungs of Cystic Fibrosis Patients. In Biofilm Infections; Bjarnsholt, T., Moser, C., Ostrup Jensen, P., Hoiby, N., Eds.; Springer: New York, NY, USA, 2011; pp. 167–184. [Google Scholar]
- Høiby, N.; Bjarnsholt, T.; Moser, C.; Jensen, P.; Kolpen, M.; Qvist, T.; Aanaes, K.; Pressler, T.; Skov, M.; Ciofu, O. Diagnosis of biofilm infections in cystic fibrosis patients. APMIS 2017, 125, 339–343. [Google Scholar] [CrossRef]
- Jørgensen, E.; Bjarnsholt, T.; Jacobsen, S. Biofilm and Equine Limb Wounds. Animals 2021, 11, 2825. [Google Scholar] [CrossRef]
- Kirketerp-Moller, K.; Zulkowski, K.; James, G. Chronic wound colonization, infection, and biofilms. In Biofilm Infections; Bjarnsholt, T., Moser, C., Ostrup Jensen, P., Hoiby, N., Eds.; Springer: New York, NY, USA, 2011; pp. 11–24. [Google Scholar]
- Zimmerli, W.; Sendi, P. Orthopaedic biofilm infections. APMIS 2017, 125, 353–364. [Google Scholar] [CrossRef]
- O’May, G.A.; Brady, R.A.; Prabhakara, R.; Leid, J.G.; Calhoun, J.H.; Shirtliff, M.E. Osteomyelitis. In Biofilm Infections; Bjarnsholt, T., Moser, C., Ostrup Jensen, P., Hoiby, N., Eds.; Springer: New York, NY, USA, 2011; pp. 111–138. [Google Scholar]
- Guo, P.; Xue, H.Y.; Buttaro, B.A.; Tran, N.T.; Wong, H.L. Enhanced eradication of intracellular and biofilm-residing methicillin-resistant Staphylococcus aureus (MRSA) reservoirs with hybrid nanoparticles delivering rifampicin. Int. J. Pharm. 2020, 589, 119784. [Google Scholar] [CrossRef]
- Singh, S.; Tan, C.L.; Ahmad, A.R. Explaining Osteomyelitis and Prosthetic Joint Infections (PJI) in terms of Biofilm—A Review. Malays. Orthop. J. 2021, 15, 1–8. [Google Scholar] [CrossRef]
- Homoe, P.; Krogh Johansen, H. The relation of biofilms to chronic otitis media and other ear-related chronic infections. In Biofilm Infections; Bjarnsholt, T., Moser, C., Ostrup Jensen, P., Hoiby, N., Eds.; Springer: New York, NY, USA, 2011; pp. 25–34. [Google Scholar]
- Lerche, C.J.; Schwartz, F.; Theut, M.; Fosbøl, E.L.; Iversen, K.; Bundgaard, H.; Høiby, N.; Moser, C. Anti-biofilm Approach in Infective Endocarditis Exposes New Treatment Strategies for Improved Outcome. Front. Cell Dev. Biol. 2021, 9, 643335. [Google Scholar] [CrossRef]
- Sapi, E.; Balasubramanian, K.; Poruri, A.; Maghsoudlou, J.S.; Socarras, K.M.; Timmaraju, A.V.; Filush, K.R.; Gupta, K.; Shaikh, S.; Theophilus, P.A.; et al. Evidence of In Vivo Existence of Borrelia Biofilm in Borrelial Lymphocytomas. Eur. J. Microbiol. Immunol. (Bp) 2016, 6, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Sapi, E.; Theophilus, P.A.; Pham, T.V.; Burugu, D.; Luecke, D.F. Effect of RpoN, RpoS and LuxS Pathways on the Biofilm Formation and Antibiotic Sensitivity of Borrelia Burgdorferi. Eur. J. Microbiol. Immunol. 2016, 6, 272–286. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, E.G.; Cavallo, I.; Bordignon, V.; D’Agosto, G.; Pontone, M.; Trento, E.; Gallo, M.T.; Prignano, G.; Pimpinelli, F.; Toma, L.; et al. The Emerging Role of Microbial Biofilm in Lyme Neuroborreliosis. Front. Neurol. 2018, 9, 1048. [Google Scholar] [CrossRef] [PubMed]
- Dowsett, C. Biofilms: A practice-based approach to identification and treatment. Wounds UK 2013, 9, 68–72. [Google Scholar]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Alhede, M.; Alhede, M.; Eickhardt-Sørensen, S.R.; Moser, C.; Kühl, M.; Jensen, P.; Høiby, N. The in vivo biofilm. Trends Microbiol. 2013, 21, 466–474. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Calum, H. APMIS pandemic editorial. APMIS 2021, 129, 319. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S. Battling biofilms. Sci. Am. 2001, 285, 74–81. [Google Scholar] [CrossRef]
- Høiby, N.; Ciofu, O.; Bjarnsholt, T. Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol. 2010, 5, 1663–1674. [Google Scholar] [CrossRef]
- Dieltjens, L.; Appermans, K.; Lissens, M.; Lories, B.; Kim, W.; Van der Eycken, E.V.; Foster, K.R.; Steenackers, H.P. Inhibiting bacterial cooperation is an evolutionarily robust anti-biofilm strategy. Nat. Commun. 2020, 11, 107. [Google Scholar] [CrossRef]
- Lee, J.; Zhang, L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 2015, 6, 26–41. [Google Scholar] [CrossRef]
- Paluch, E.; Rewak-Soroczyńska, J.; Jędrusik, I.; Mazurkiewicz, E.; Jermakow, K. Prevention of biofilm formation by quorum quenching. Appl. Microbiol. Biotechnol. 2020, 104, 1871–1881. [Google Scholar] [CrossRef]
- He, N.; Hu, J.; Liu, H.; Zhu, T.; Huang, B.; Wang, X.; Wu, Y.; Wang, W.; Qu, D. Enhancement of vancomycin activity against biofilms by using ultrasound-targeted microbubble destruction. Antimicrob. Agents Chemother. 2011, 55, 5331–5337. [Google Scholar] [CrossRef]
- Sauer, K.; Camper, A.K.; Ehrlich, G.D.; Costerton, J.W.; Davies, D.G. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 2002, 184, 1140–1154. [Google Scholar] [CrossRef]
- Chao, Y.; Marks, L.R.; Pettigrew, M.M.; Hakansson, A.P. Streptococcus pneumoniae biofilm formation and dispersion during colonization and disease. Front. Cell Infect. Microbiol. 2014, 4, 194. [Google Scholar] [CrossRef]
- Goarant, C.; Trueba, G.; Bierque, E.; Thibeaux, R.; Davis, B.; De la Pena-Moctezuma, A. Leptospira and Leptospirosis. In Water and Sanitation for the 21st Century: Health and Microbiological Aspects of Excreta and Wastewater Management (Global Water Pathogen Project); Pruden, A., Ashbolt, N., Miller, J., Eds.; Michigan State University: East Lansing, MI, USA; UNESCO: Paris, France, 2019; pp. 1–33. [Google Scholar] [CrossRef]
- Barragan, V.A.; Mejia, M.E.; Trávez, A.; Zapata, S.; Hartskeerl, R.A.; Haake, D.A.; Trueba, G.A. Interactions of Leptospira with environmental bacteria from surface water. Curr. Microbiol. 2011, 62, 1802–1806. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef]
- Parsek, M.R.; Singh, P.K. Bacterial biofilms: An emerging link to disease pathogenesis. Annu. Rev. Microbiol. 2003, 57, 677–701. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Stoodley, P. Evolving concepts in biofilm infections. Cell Microbiol. 2009, 11, 1034–1043. [Google Scholar] [CrossRef]
- Monahan, A.M.; Callanan, J.J.; Nally, J.E. Review paper: Host-pathogen interactions in the kidney during chronic leptospirosis. Vet. Pathol. 2009, 46, 792–799. [Google Scholar] [CrossRef]
- Brihuega, B.; Samartino, L.; Auteri, C.; Venzano, A.; Caimi, K. In vivo cell aggregations of a recent swine biofilm-forming isolate of Leptospira interrogans strain from Argentina. Rev. Argent. Microbiol. 2012, 44, 138–143. [Google Scholar]
- Yamaguchi, T.; Higa, N.; Okura, N.; Matsumoto, A.; Hermawan, I.; Yamashiro, T.; Suzuki, T.; Toma, C. Characterizing interactions of Leptospira interrogans with proximal renal tubule epithelial cells. BMC Microbiol. 2018, 18, 64. [Google Scholar] [CrossRef]
- Santos, A.A.N.; Ribeiro, P.D.S.; da França, G.V.; Souza, F.N.; Ramos, E.A.G.; Figueira, C.P.; Reis, M.G.; Costa, F.; Ristow, P. Leptospira interrogans biofilm formation in Rattus norvegicus (Norway rats) natural reservoirs. PLoS Negl. Trop. Dis. 2021, 15, e0009736. [Google Scholar] [CrossRef]
- Nally, J.E.; Hornsby, R.L.; Alt, D.P. Antigen-Specific Urinary Immunoglobulin in Reservoir Hosts of Leptospirosis. Vet. Sci. 2021, 8, 178. [Google Scholar] [CrossRef]
- Kobayashi, H. Airway biofilm disease. Int. J. Antimicrob. Agents 2001, 17, 351–356. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Jensen, P.; Fiandaca, M.J.; Pedersen, J.; Hansen, C.R.; Andersen, C.B.; Pressler, T.; Givskov, M.; Høiby, N. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr. Pulmonol. 2009, 44, 547–558. [Google Scholar] [CrossRef]
- Radic, M. Clearance of Apoptotic Bodies, NETs, and Biofilm DNA: Implications for Autoimmunity. Front. Immunol. 2014, 5, 365. [Google Scholar] [CrossRef]
- Lou, H.; Pickering, M.C. Extracellular DNA and autoimmune diseases. Cell Mol. Immunol. 2018, 15, 746–755. [Google Scholar] [CrossRef]
- Singh, R.; Stine, O.C.; Smith, D.L.; Spitznagel, J.K., Jr.; Labib, M.E.; Williams, H.N. Microbial diversity of biofilms in dental unit water systems. Appl. Environ. Microbiol. 2003, 69, 3412–3420. [Google Scholar] [CrossRef] [PubMed]
- Trueba, G.; Zapata, S.; Madrid, K.; Cullen, P.; Haake, D. Cell aggregation: A mechanism of pathogenic Leptospira to survive in fresh water. Int. Microbiol. 2004, 7, 35–40. [Google Scholar] [PubMed]
- Ristow, P.; Bourhy, P.; Kerneis, S.; Schmitt, C.; Prevost, M.C.; Lilenbaum, W.; Picardeau, M. Biofilm formation by saprophytic and pathogenic leptospires. Microbiology 2008, 154, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Vinod Kumar, K.; Lall, C.; Raj, R.V.; Vedhagiri, K.; Sunish, I.P.; Vijayachari, P. In Vitro Antimicrobial Susceptibility of Pathogenic Leptospira Biofilm. Microb. Drug Resist. 2016, 22, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Villanueva, S.Y.; Chakraborty, A.; Miyahara, S.; Segawa, T.; Asoh, T.; Ozuru, R.; Gloriani, N.G.; Yanagihara, Y.; Yoshida, S. Comparative analysis of Leptospira strains isolated from environmental soil and water in the Philippines and Japan. Appl. Environ. Microbiol. 2013, 79, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Thibeaux, R.; Geroult, S.; Benezech, C.; Chabaud, S.; Soupé-Gilbert, M.E.; Girault, D.; Bierque, E.; Goarant, C. Seeking the environmental source of Leptospirosis reveals durable bacterial viability in river soils. PLoS Negl. Trop. Dis. 2017, 11, e0005414. [Google Scholar] [CrossRef]
- Andre-Fontaine, G.; Aviat, F.; Thorin, C. Waterborne Leptospirosis: Survival and Preservation of the Virulence of Pathogenic Leptospira spp. in Fresh Water. Curr. Microbiol. 2015, 71, 136–142. [Google Scholar] [CrossRef]
- Bierque, E.; Thibeaux, R.; Girault, D.; Soupé-Gilbert, M.E.; Goarant, C. A systematic review of Leptospira in water and soil environments. PLoS ONE 2020, 15, e0227055. [Google Scholar] [CrossRef]
- Kumar, K.V.; Lall, C.; Raj, R.V.; Vedhagiri, K.; Vijayachari, P. Coexistence and survival of pathogenic leptospires by formation of biofilm with Azospirillum. FEMS Microbiol. Ecol. 2015, 91, fiv051. [Google Scholar] [CrossRef]
- Vinod Kumar, K.; Lall, C.; Raj, R.V.; Vijayachari, P. Coaggregation and biofilm formation of Leptospira with Staphylococcus aureus. Microbiol. Immunol. 2019, 63, 147–150. [Google Scholar] [CrossRef]
- Richer, L.; Potula, H.H.; Melo, R.; Vieira, A.; Gomes-Solecki, M. Mouse model for sublethal Leptospira interrogans infection. Infect. Immun. 2015, 83, 4693–4700. [Google Scholar] [CrossRef]
- Ratet, G.; Veyrier, F.J.; Fanton d’Andon, M.; Kammerscheit, X.; Nicola, M.A.; Picardeau, M.; Boneca, I.G.; Werts, C. Live imaging of bioluminescent leptospira interrogans in mice reveals renal colonization as a stealth escape from the blood defenses and antibiotics. PLoS Negl. Trop. Dis. 2014, 8, e3359. [Google Scholar] [CrossRef]
- Geißler, P. Biofilm-Bildung als Pathogenitätsmechanismus bei persistierenden Infektionen und ihre mögliche Rolle bei der Equinen Rezidivierenden Uveitis eine Literaturstudie [Biofilm formation as a pathogenicity mechanism in persistent infections and its possible role in Equine Recurrent Uveitis a literature review]. Ph.D. Thesis, Ludwig-Maximilians-University (LMU), Munich, Germany, 2021. [Google Scholar] [CrossRef]
- Ackermann, K. Untersuchungen von Glaskörperproben aus an equiner rezidivierender Uveitis (ERU) erkrankten Augen im Hinblick auf Leptospiren und deren Biofilmbildung [Investigation of vitreous samples from eyes affected with equine recurrent uveitis (ERU) with focus on leptospires and their biofilm formation]. Ph.D. Thesis, Ludwig-Maximilians-University (LMU), Munich, Germany, 2021. [Google Scholar] [CrossRef]
- Sebag, J.; Balazs, E.A. Morphology and ultrastructure of human vitreous fibers. Investig. Ophthalmol. Vis. Sci. 1989, 30, 1867–1871. [Google Scholar]
- Snowden, J.M.; Swann, D.A. Vitreous structure. V. The morphology and thermal stability of vitreous collagen fibers and comparison to articular cartilage (type II) collagen. Investig. Ophthalmol. Vis. Sci. 1980, 19, 610–618. [Google Scholar]
- Grisanti, S. Das Immunprivileg des Auges [Immune privilege of the eye]. Ophthalmologe 1998, 95, 124–135. [Google Scholar] [CrossRef]
- Zhou, R.; Caspi, R.R. Ocular immune privilege. F1000 Biol. Rep. 2010, 2. [Google Scholar] [CrossRef]
- Dietrich, M. Fibrin- versus Plasma-Gel Scaffolds—Und der Einfluss von TGF-ß und bFGF auf Myofibroblasten und die Gewebeneogenese [Fibrin versus plasma gel scaffolds—And the influence of TGF-ß and bFGF on myofibroblasts and tissue neogenesis]. Ph.D. Thesis, Rheinisch-Westfälische Technische Hochschule Aachen, Aachen, Germany, 2015. Available online: https://publications.rwth-aachen.de/record/466223/files/466223.pdf (accessed on 27 December 2021).
- Kollmer, M.; Meinhardt, K.; Haupt, C.; Liberta, F.; Wulff, M.; Linder, J.; Handl, L.; Heinrich, L.; Loos, C.; Schmidt, M.; et al. Electron tomography reveals the fibril structure and lipid interactions in amyloid deposits. Proc. Natl. Acad. Sci. USA 2016, 113, 5604–5609. [Google Scholar] [CrossRef]
- Techdico. Baudelocque’s Diameter. Available online: https://de.techdico.com/uebersetzung/englisch-deutsch/baudelocque%27s+diameter.html (accessed on 18 December 2021).
- Babudieri, B. Schutzimpfungen gegen Leptospirosen [Vaccinations against leptospirosis]. In Leptospiren und Leptospirosen; Kathe, J., Mochmann, H., Eds.; Gustac Fischer: Jena, Germany, 1967; Volume I, pp. 1090–1105. [Google Scholar]
- Rohrbach, B.W.; Ward, D.A.; Hendrix, D.V.; Cawrse-Foss, M.; Moyers, T.D. Effect of vaccination against leptospirosis on the frequency, days to recurrence and progression of disease in horses with equine recurrent uveitis. Vet. Ophthalmol. 2005, 8, 171–179. [Google Scholar] [CrossRef]
- Goldstein, S.F.; Charon, N.W. Motility of the spirochete Leptospira. Cell Motil. Cytoskeleton 1988, 9, 101–110. [Google Scholar] [CrossRef]
- Matthews, A. Ophthalmic therapeutics. In Equine Clinical Pharmacology; Bertone, J.J., Horspool, L.J.I., Eds.; Saunders: Edinburgh, UK, 2004; p. 240. ISBN 978-0-7020-2484-9. [Google Scholar]
- Phaneuf, L.P.; Ruckebusch, Y. Physiological, pharmacological and therapeutic aspects of some gastrointestinal disorders in the horse. In Veterinary Pharmacology and Toxicology; Ruckebusch, Y., Toutain, P.-L., Koritz, G.D., Eds.; MTP Press: Boston, MA, USA, 1983; pp. 371–380. [Google Scholar]
- Ström, L.; Dalin, F.; Domberg, M.; Stenlund, C.; Bondesson, U.; Hedeland, M.; Toutain, P.L.; Ekstrand, C. Topical ophthalmic atropine in horses, pharmacokinetics and effect on intestinal motility. BMC Vet. Res. 2021, 17, 149. [Google Scholar] [CrossRef]
- Kawasaki, K.; Mochizuki, K.; Torisaki, M.; Yamashita, Y.; Shirao, Y.; Wakabayashi, K.; Tanabe, J. Electroretinographical changes due to antimicrobials. Lens Eye Toxic Res. 1990, 7, 693–704. [Google Scholar]
- D’Amico, D.J.; Libert, J.; Kenyon, K.R.; Hanninen, L.A.; Caspers-Velu, L. Retinal toxicity of intravitreal gentamicin. An electron microscopic study. Investig. Ophthalmol. Vis. Sci. 1984, 25, 564–572. [Google Scholar]
- Zachary, I.G.; Forster, R.K. Experimental intravitreal gentamicin. Am. J. Ophthalmol. 1976, 82, 604–611. [Google Scholar] [CrossRef]
- D’Amico, D.J.; Caspers-Velu, L.; Libert, J.; Shanks, E.; Schrooyen, M.; Hanninen, L.A.; Kenyon, K.R. Comparative toxicity of intravitreal aminoglycoside antibiotics. Am. J. Ophthalmol. 1985, 100, 264–275. [Google Scholar] [CrossRef]
- Friedrich, S.; Cheng, Y.L.; Saville, B. Drug distribution in the vitreous humor of the human eye: The effects of intravitreal injection position and volume. Curr. Eye Res. 1997, 16, 663–669. [Google Scholar] [CrossRef]
- Shirodkar, A.R.; Pathengay, A.; Flynn, H.W., Jr. Intravitreal Gentamicin-Induced Macular Infarction: SD-OCT Features. Ophthalmic Surg. Lasers Imaging 2011, 42, e67–e70. [Google Scholar] [CrossRef] [PubMed]
- McDonald, H.R.; Schatz, H.; Allen, A.W.; Chenoweth, R.G.; Cohen, H.B.; Crawford, J.B.; Klein, R.; May, D.R.; Snider, J.D., 3rd. Retinal toxicity secondary to intraocular gentamicin injection. Ophthalmology 1986, 93, 871–877. [Google Scholar] [CrossRef]
- Conway, B.P.; Campochiaro, P.A. Macular infarction after endophthalmitis treated with vitrectomy and intravitreal gentamicin. Arch. Ophthalmol. 1986, 104, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Conway, B.P.; Tabatabay, C.A.; Campochiaro, P.A.; D’Amico, D.J.; Hanninen, L.A.; Kenyon, K.R. Gentamicin toxicity in the primate retina. Arch. Ophthalmol. 1989, 107, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Peyman, G.A.; Paque, J.T.; Meisels, H.I.; Bennett, T.O. Postoperative endophthalmitis: A comparison of methods for treatment and prophlaxis with gentamicin. Ophthalmic Surg. 1975, 6, 45–55. [Google Scholar]
- Kathe, J. Über die Gelbsucht und die Sterblichkeit bei Schlamm-Feldfieber [About jaundice and mortality in mud field fever]. Klin. Wochenschr. 1942, 21, 791–794. [Google Scholar] [CrossRef]
- Pagani, M. Pathogénie des symptomes oculaires dans la leptospirose [Pathogenesis of ocular manifestations in leptospirosis]. XVI. Concil. Ophthalm. Britannia Acta 1950, 1, 336. [Google Scholar]
- Alexander, A.; Baer, A.; Fair, J.R.; Gochenour, W.S., Jr.; King, J.H., Jr.; Yager, R.H. Leptospiral uveitis; report of a bacteriologically verified case. AMA Arch. Ophthalmol. 1952, 48, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Cimbal, O. Chronische Leptospiren-Uveitis [Chronic leptospiral uveitis]. Klin. Monatsbl. Augenheilkd. 1952, 119, 649. [Google Scholar]
- Okamune, S.; Yamamoto, T. Leptospiral Uveitis in Kochi Prefecture, Japan. Acta XVII Conc. Ophth. 1954, 3, 1695–1700. [Google Scholar]
- Fischer, A.; Freytag, B.; Remky, H. [Clinical, bacteriological and serological findings in leptospiral uveitis]. Albrecht Von Graefes Arch. Ophthalmol. 1955, 156, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Kemenes, F.; Surján, J.; Vizy, L. Leptospira as the cause of periodic ophthalmia in horses. Magyar Allatorvosok Lapja 1960, 15, 253–257. [Google Scholar]
- Merien, F.; Baranton, G.; Perolat, P. Comparison of polymerase chain reaction with microagglutination test and culture for diagnosis of leptospirosis. J. Infect. Dis. 1995, 172, 281–285. [Google Scholar] [CrossRef] [PubMed]
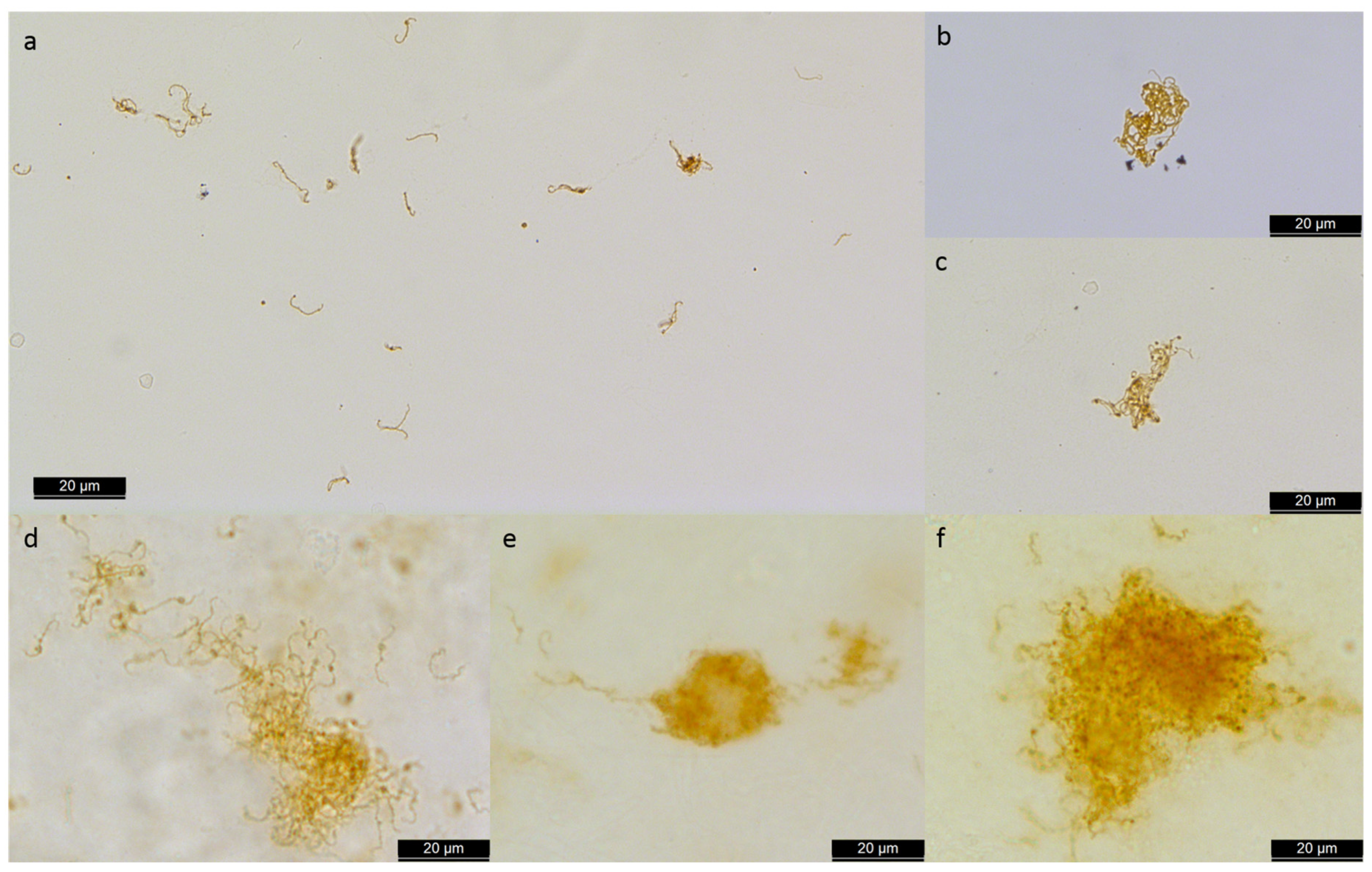

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wollanke, B.; Gerhards, H.; Ackermann, K. Infectious Uveitis in Horses and New Insights in Its Leptospiral Biofilm-Related Pathogenesis. Microorganisms 2022, 10, 387. https://doi.org/10.3390/microorganisms10020387
Wollanke B, Gerhards H, Ackermann K. Infectious Uveitis in Horses and New Insights in Its Leptospiral Biofilm-Related Pathogenesis. Microorganisms. 2022; 10(2):387. https://doi.org/10.3390/microorganisms10020387
Chicago/Turabian StyleWollanke, Bettina, Hartmut Gerhards, and Kerstin Ackermann. 2022. "Infectious Uveitis in Horses and New Insights in Its Leptospiral Biofilm-Related Pathogenesis" Microorganisms 10, no. 2: 387. https://doi.org/10.3390/microorganisms10020387
APA StyleWollanke, B., Gerhards, H., & Ackermann, K. (2022). Infectious Uveitis in Horses and New Insights in Its Leptospiral Biofilm-Related Pathogenesis. Microorganisms, 10(2), 387. https://doi.org/10.3390/microorganisms10020387





