Ecological Importance of Viral Lysis as a Loss Factor of Phytoplankton in the Amundsen Sea
Abstract
:1. Introduction
2. Materials and Methods
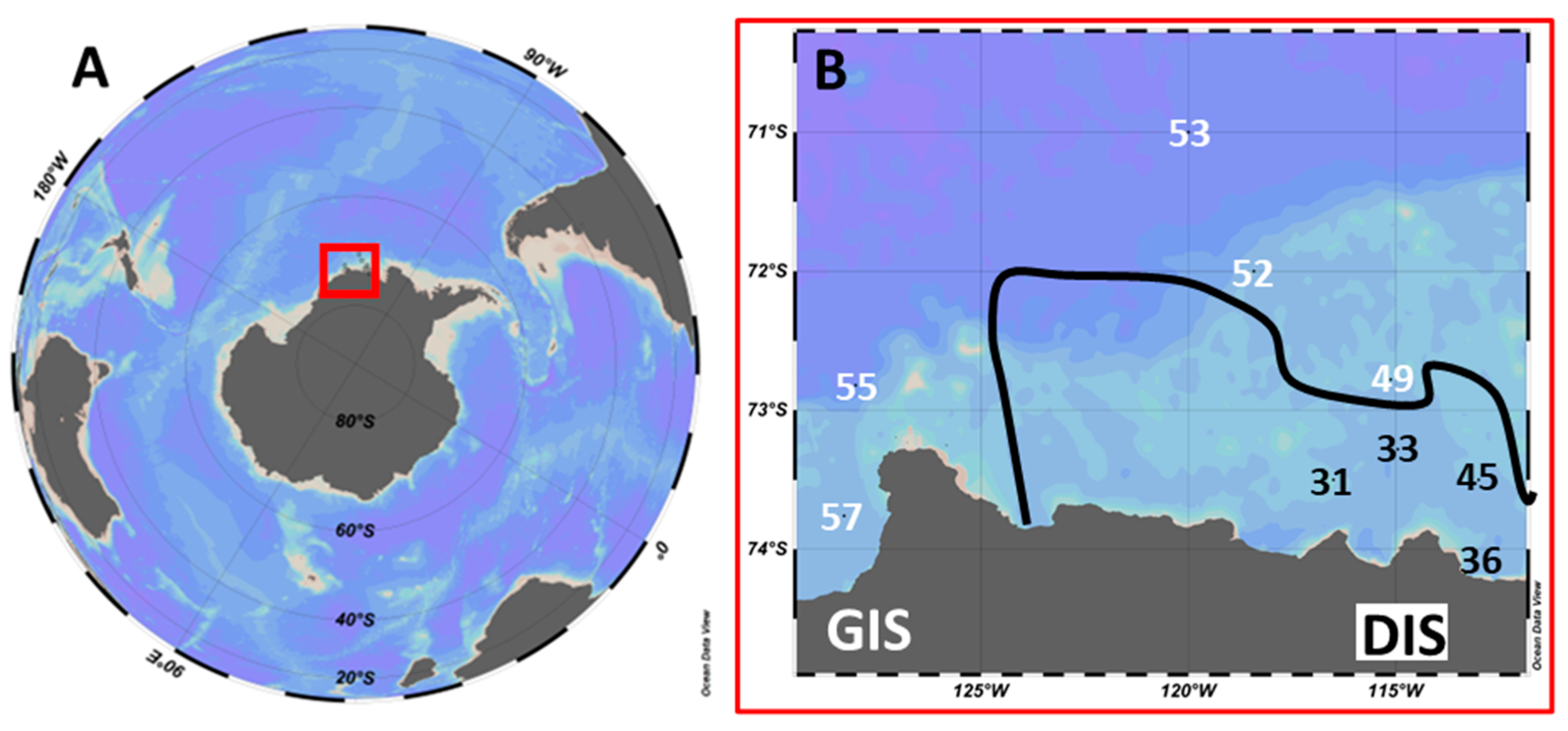
2.1. Study Area and Sampling
2.2. Nutrient Analysis
| Station | Lat. (°S) | Long. (°W) | Depth (m) | Temp. (°C) | Sal. (PSS) | PO4 (µM) | Si(OH4) (µM) | dFe (nM) |
|---|---|---|---|---|---|---|---|---|
| 31 | 73.5 | 116.5 | 15 | −0.56 | 33.99 | 1.78 | 84.7 | 0.36 |
| 33 | 73.3 | 115.0 | 18 | −0.32 | 33.95 | 1.57 | 78.1 | 0.09 |
| 36 | 74.2 | 113.3 | 28 | −1.51 | 33.89 | 2.20 | 98.0 | 0.23 |
| 45 | 73.5 | 113.0 | 15 | −0.69 | 33.85 | 1.51 | 77.7 | 0.19 |
| 49 | 72.8 | 115.1 | 21 | −0.83 | 33.68 | 1.46 | 75.5 | 0.29 |
| 52 | 72.0 | 118.4 | 36 | −1.58 | 33.89 | 2.07 | 78.5 | 0.11 |
| 53 | 71.0 | 120.0 | 30 | −1.31 | 33.83 | 1.93 | 77.2 | 0.16 |
| 55 | 72.8 | 128.0 | 24 | −1.54 | 33.50 | 1.29 | 70.3 | 0.08 |
| 57 | 73.8 | 128.3 | 11 | −0.96 | 33.64 | 1.88 | 78.4 | 0.22 |
2.3. Phytoplankton Community Taxonomy
2.4. Phytoplankton Abundances
2.5. Loss Rates
2.6. Statistical Analyses
3. Results and Discussion
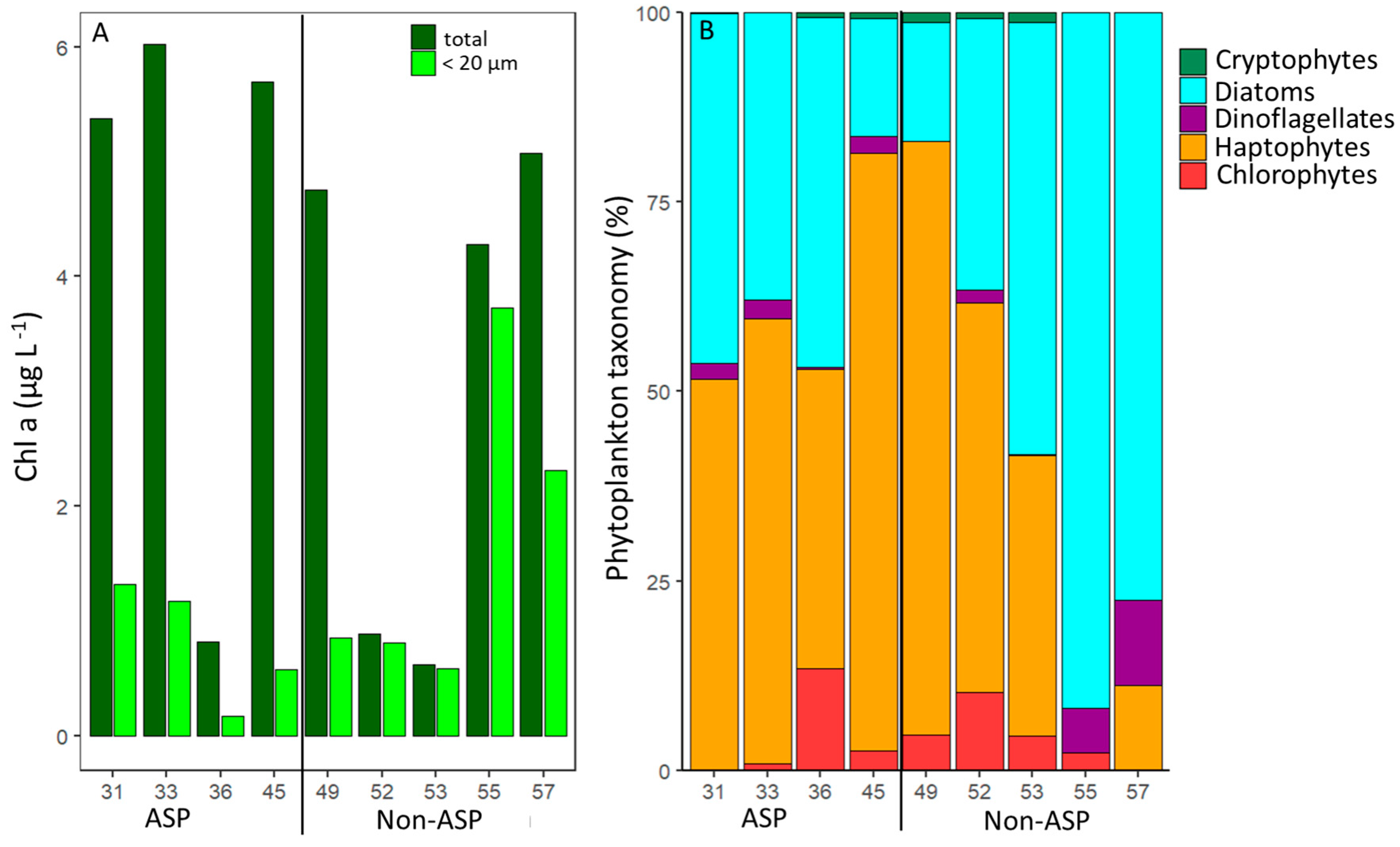
3.1. Phytoplankton Community Composition
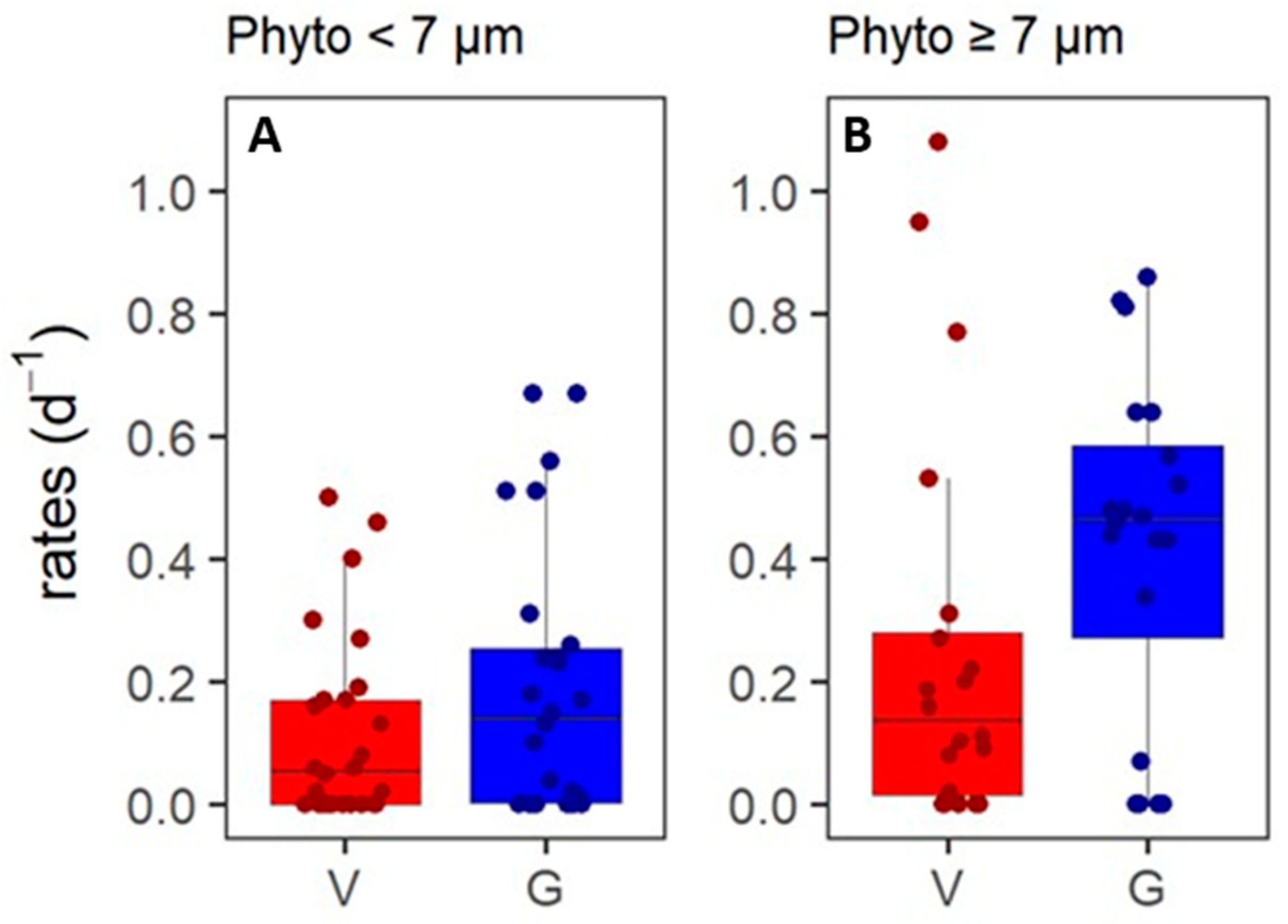
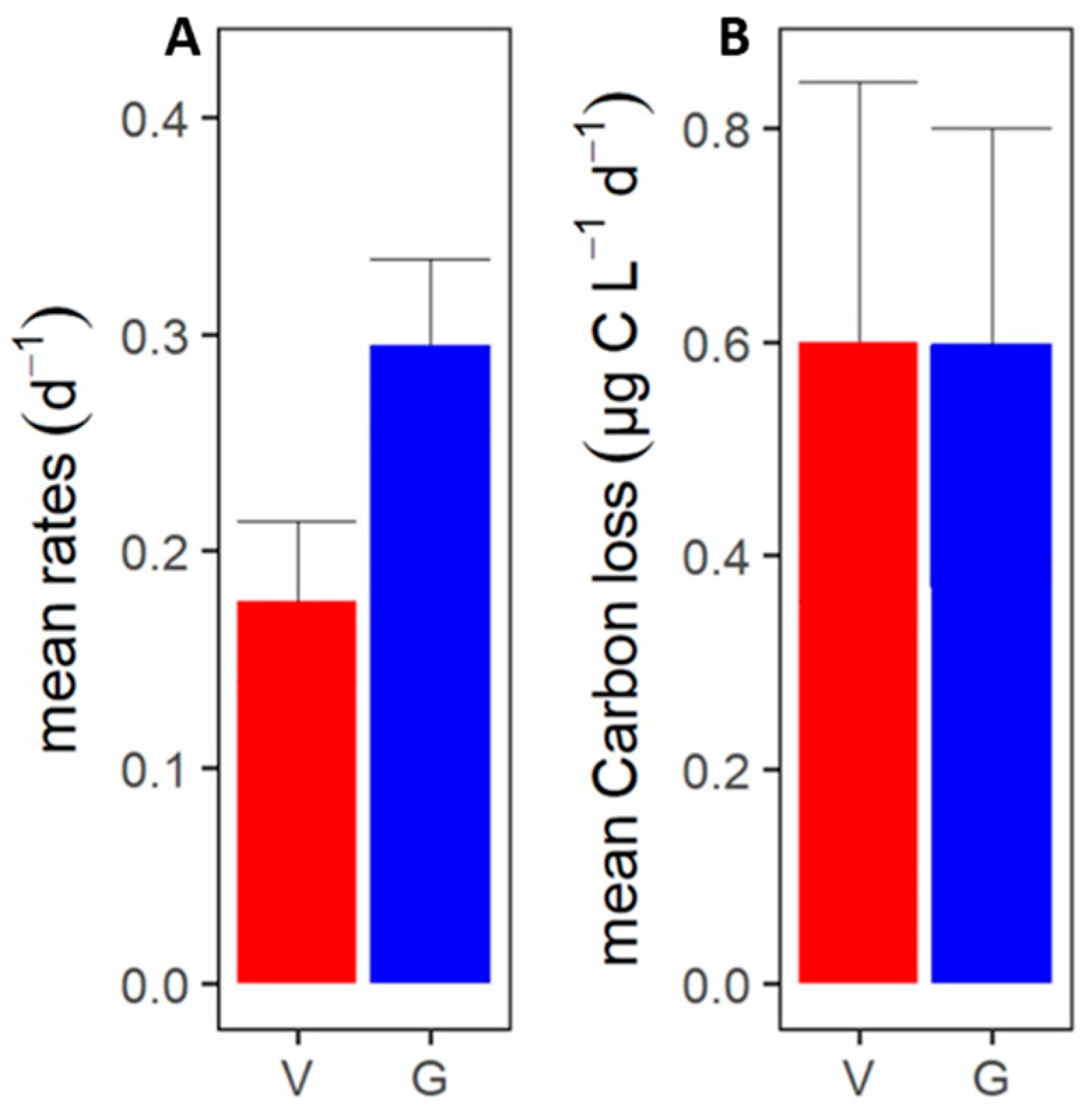
3.2. Phytoplankton Mortality Rates
3.3. Phytoplankton Carbon Loss
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frölicher, T.L.; Sarmiento, J.L.; Paynter, D.J.; Dunne, J.P.; Krasting, J.P.; Winton, M. Dominance of the Southern Ocean in Anthropogenic Carbon and Heat Uptake in CMIP5 Models. J. Clim. 2015, 28, 862–886. [Google Scholar] [CrossRef]
- Moreau, S.; Schloss, I.R.; Mostajir, B.; Demers, S.; Almandoz, G.O.; Ferrario, M.E.; Ferreyra, G.A. Influence of Microbial Community Composition and Metabolism on Air.Sea ΔpCO2 Variation off the Western Antarctic Peninsula. Mar. Ecol. Prog. Ser. 2012, 446, 45–59. [Google Scholar] [CrossRef] [Green Version]
- DiTullio, G.R.; Grebmeier, J.M.; Arrigo, K.R.; Lizotte, M.P.; Robinson, D.H.; Leventer, A.; Barry, J.P.; VanWoert, M.L.; Dunbar, R.B. Rapid and Early Export of Phaeocystis antarctica Blooms in the Ross Sea, Antarctica. Nature 2000, 404, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Weber, L.H.; El-Sayed, S.Z. Contributions of the Net, Nano- and Picoplankton to the Phytoplankton Standing Crop and Primary Productivity in the Southern Ocean. J. Plankton Res. 1987, 9, 973–994. [Google Scholar] [CrossRef]
- Irion, S.; Christaki, U.; Berthelot, H.; L’Helguen, S.; Jardillier, L. Small Phytoplankton Contribute Greatly to CO2-Fixation after the Diatom Bloom in the Southern Ocean. ISME J. 2021, 15, 2509–2522. [Google Scholar] [CrossRef]
- Lim, Y.J.; Kim, T.W.; Lee, S.H.; Lee, D.; Park, J.; Kim, B.K.; Kim, K.; Jang, H.K.; Bhavya, P.S.; Lee, S.H. Seasonal Variations in the Small Phytoplankton Contribution to the Total Primary Production in the Amundsen Sea, Antarctica. J. Geophys. Res. Ocean. 2019, 124, 8324–8341. [Google Scholar] [CrossRef]
- Arrigo, K.R.; van Dijken, G.L.; Bushinsky, S. Primary Production in the Southern Ocean, 1997–2006. J. Geophys. Res. Ocean. 2008, 113, 1997–2006. [Google Scholar] [CrossRef]
- Thomalla, S.J.; Fauchereau, N.; Swart, S.; Monteiro, P.M.S. Regional Scale Characteristics of the Seasonal Cycle of Chlorophyll in the Southern Ocean. Biogeosciences 2011, 8, 2849–2866. [Google Scholar] [CrossRef] [Green Version]
- Arrigo, K.R.; van Dijken, G.L. Phytoplankton Dynamics within 37 Antarctic Coastal Polynya Systems. J. Geophys. Res. Ocean. 2003, 108, 27-1–27-18. [Google Scholar] [CrossRef]
- Arrigo, K.R.; Lowry, K.E.; van Dijken, G.L. Annual Changes in Sea Ice and Phytoplankton in Polynyas of the Amundsen Sea, Antarctica. Deep Res. Part II Top. Stud. Oceanogr. 2012, 71–76, 5–15. [Google Scholar] [CrossRef]
- Nitsche, F.O.; Jacobs, S.S.; Larter, R.D.; Gohl, K. Bathymetry of the Amundsen Sea Continental Shelf: Implications for Geology, Oceanography, and Glaciology. Geochem. Geophys. Geosyst. 2007, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Yang, E.J.; Park, J.; Jung, J.; Kim, T.W.; Lee, S.H. Physical-Biological Coupling in the Amundsen Sea, Antarctica: Influence of Physical Factors on Phytoplankton Community Structure and Biomass. Deep Res. Part I Oceanogr. Res. Pap. 2016, 117, 51–60. [Google Scholar] [CrossRef]
- Mills, M.M.; Alderkamp, A.C.; Thuróczy, C.E.; van Dijken, G.L.; Laan, P.; de Baar, H.J.W.; Arrigo, K.R. Phytoplankton Biomass and Pigment Responses to Fe Amendments in the Pine Island and Amundsen Polynyas. Deep Res. Part II Top. Stud. Oceanogr. 2012, 71–76, 61–76. [Google Scholar] [CrossRef]
- Schofield, O.; Miles, T.; Alderkamp, A.C.; Lee, S.H.; Haskins, C.; Rogalsky, E.; Sipler, R.; Sherrell, R.M.; Yager, P.L. In Situ Phytoplankton Distributions in the Amundsen Sea Polynya Measured by Autonomous Gliders. Elementa 2015, 3, 000073. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.C.; Park, M.O.; Jung, J.; Yang, E.J.; Lee, S.H. Taxonomic Variability of Phytoplankton and Relationship with Production of CDOM in the Polynya of the Amundsen Sea, Antarctica. Deep Res. Part II Top. Stud. Oceanogr. 2016, 123, 30–41. [Google Scholar] [CrossRef]
- Meredith, M.P.; Jullion, L.; Brown, P.J.; Garabato, A.C.N.; Couldrey, M.P. Dense Waters of the Weddell and Scotia Seas: Recent Changes in Properties and Circulation. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372, 20130041. [Google Scholar] [CrossRef]
- Montes-Hugo, M. Recent Changes in Phytoplankton. Science 2009, 1470, 1470–1473. [Google Scholar] [CrossRef]
- Saba, G.K.; Fraser, W.R.; Saba, V.S.; Iannuzzi, R.A.; Coleman, K.E.; Doney, S.C.; Ducklow, H.W.; Martinson, D.G.; Miles, T.N.; Patterson-Fraser, D.L.; et al. Winter and Spring Controls on the Summer Food Web of the Coastal West Antarctic Peninsula. Nat. Commun. 2014, 5, 4318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rignot, E. Evidence for Rapid Retreat and Mass Loss of Thwaites Glacier, West Antarctica. J. Glaciol. 2001, 47, 213–222. [Google Scholar] [CrossRef] [Green Version]
- Fragoso, G.M.; Smith, W.O. Influence of Hydrography on Phytoplankton Distribution in the Amundsen and Ross Seas, Antarctica. J. Mar. Syst. 2012, 89, 19–29. [Google Scholar] [CrossRef]
- Joughin, I.; Smith, B.E.; Medley, B. Marine Ice Sheet Collapse Potentially under Way for Thwaites Glacier Basin, West Antarctica. Science 2014, 344, 735–739. [Google Scholar] [CrossRef]
- Yang, E.J.; Jiang, Y.; Lee, S.H. Microzooplankton Herbivory and Community Structure in the Amundsen Sea, Antarctica. Deep Res. Part II Top. Stud. Oceanogr. 2015, 123, 58–68. [Google Scholar] [CrossRef]
- Petrou, K.; Kranz, S.A.; Trimborn, S.; Hassler, C.S.; Ameijeiras, S.B.; Sackett, O.; Ralph, P.J.; Davidson, A.T. Southern Ocean Phytoplankton Physiology in a Changing Climate. J. Plant Physiol. 2016, 203, 135–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thoma, M.; Jenkins, A.; Holland, D.; Jacobs, S. Modelling Circumpolar Deep Water Intrusions on the Amundsen Sea Continental Shelf, Antarctica. Geophys. Res. Lett. 2008, 35, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Holland, P.R.; Jenkins, A.; Holland, D.M. Ice and Ocean Processes in the Bellingshausen Sea, Antarctica. J. Geophys. Res. Ocean. 2010, 115, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, S.S.; Jenkins, A.; Giulivi, C.F.; Dutrieux, P. Stronger Ocean Circulation and Increased Melting under Pine Island Glacier Ice Shelf. Nat. Geosci. 2011, 4, 519–523. [Google Scholar] [CrossRef]
- Gille, S.T. Decadal-Scale Temperature Trends in the Southern Hemisphere Ocean. J. Clim. 2008, 21, 4749–4765. [Google Scholar] [CrossRef] [Green Version]
- Thompson, D.W.J.; Solomon, S. Interpretation of Recent Southern Hemisphere Climate Change. Science 2002, 296, 895–899. [Google Scholar] [CrossRef] [Green Version]
- Spence, P.; Griffes, S.M.; England, M.H.; Hogg, A.M.C.; Saenko, O.A.; Jourdain, N.C. Rapid Subsurface Warming and Circulation Changes of Antarctic Coastal Waters by Poleward Shifting Winds. Geophys. Res. Lett. 2014, 41, 4601–4610. [Google Scholar] [CrossRef] [Green Version]
- Raphael, M.N.; Marshall, G.J.; Turner, J.; Fogt, R.L.; Schneider, D.; Dixon, D.A.; Hosking, J.S.; Jones, J.M.; Hobbs, W.R. The Amundsen Sea Low: Variability, Change, and Impact on Antarctic Climate. Bull. Am. Meteorol. Soc. 2016, 97, 111–121. [Google Scholar] [CrossRef]
- Alderkamp, A.C.; Mills, M.M.; van Dijken, G.L.; Laan, P.; Thuróczy, C.E.; Gerringa, L.J.A.; de Baar, H.J.W.; Payne, C.D.; Visser, R.J.W.; Buma, A.G.J.; et al. Iron from Melting Glaciers Fuels Phytoplankton Blooms in the Amundsen Sea (Southern Ocean): Phytoplankton Characteristics and Productivity. Deep Res. Part II Top. Stud. Oceanogr. 2012, 71–76, 32–48. [Google Scholar] [CrossRef]
- Gerringa, L.J.A.; Alderkamp, A.C.; Laan, P.; Thuróczy, C.E.; De Baar, H.J.W.; Mills, M.M.; van Dijken, G.L.; van Haren, H.; Arrigo, K.R. Iron from Melting Glaciers Fuels the Phytoplankton Blooms in Amundsen Sea (Southern Ocean): Iron Biogeochemistry. Deep Res. Part II Top. Stud. Oceanogr. 2012, 71–76, 16–31. [Google Scholar] [CrossRef]
- Martin, J.H.; Gordon, R.M.; Fitzwater, S.E. Iron in Antarctic Waters. Nature 1990, 345, 156–158. [Google Scholar] [CrossRef]
- Park, J.; Kuzminov, F.I.; Bailleul, B.; Yang, E.J.; Lee, S.; Falkowski, P.G.; Gorbunov, M.Y. Light Availability Rather than Fe Controls the Magnitude of Massive Phytoplankton Bloom in the Amundsen Sea Polynyas, Antarctica. Limnol. Oceanogr. 2017, 62, 2260–2276. [Google Scholar] [CrossRef]
- Lee, Y.; Jung, J.; Kim, T.W.; Yang, E.J.; Park, J. Phytoplankton Growth Rates in the Amundsen Sea (Antarctica) during Summer: The Role of Light. Environ. Res. 2021, 207, 112165. [Google Scholar] [CrossRef] [PubMed]
- Henson, S.A.; Cael, B.B.; Allen, S.R.; Dutkiewicz, S. Future Phytoplankton Diversity in a Changing Climate. Nat. Commun. 2021, 12, 5372. [Google Scholar] [CrossRef]
- Deppeler, S.L.; Davidson, A.T. Southern Ocean Phytoplankton in a Changing Climate. Front. Mar. Sci. 2017, 4, 40. [Google Scholar] [CrossRef] [Green Version]
- Arteaga, L.A.; Boss, E.; Behrenfeld, M.J.; Westberry, T.K.; Sarmiento, J.L. Seasonal Modulation of Phytoplankton Biomass in the Southern Ocean. Nat. Commun. 2020, 11, 5364. [Google Scholar] [CrossRef] [PubMed]
- Smetacek, V.; Assmy, P.; Henjes, J. The Role of Grazing in Structuring Southern Ocean Pelagic Ecosystems and Biogeochemical Cycles. Antarct. Sci. 2004, 16, 541–558. [Google Scholar] [CrossRef]
- Garzio, L.M.; Steinberg, D.K.; Erickson, M.; Ducklow, H.W. Microzooplankton Grazing along the Western Antarctic Peninsula. Aquat. Microb. Ecol. 2013, 70, 215–232. [Google Scholar] [CrossRef]
- Brussaard, C.P.D.; Timmermans, K.R.; Uitz, J.; Veldhuis, M.J.W. Virioplankton Dynamics and Virally Induced Phytoplankton Lysis versus Microzooplankton Grazing Southeast of the Kerguelen (Southern Ocean). Deep Res. Part II Top. Stud. Oceanogr. 2008, 55, 752–765. [Google Scholar] [CrossRef]
- Evans, C.; Brussaard, C.P.D. Viral Lysis and Microzooplankton Grazing of Phytoplankton throughout the Southern Ocean. Limnol. Oceanogr. 2012, 57, 1826–1837. [Google Scholar] [CrossRef] [Green Version]
- Biggs, T.E.G.; Huisman, J.; Brussaard, C.P.D. Viral Lysis Modifies Seasonal Phytoplankton Dynamics and Carbon Flow in the Southern Ocean. ISME J. 2021, 15, 3615–3622. [Google Scholar] [CrossRef]
- Suttle, C.A. Viruses in the Sea. Nature 2005, 437, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.W.; Suttle, C.A. Viruses and Nutrient Cycles in the Sea. Bioscience 1999, 49, 781–788. [Google Scholar] [CrossRef] [Green Version]
- Suttle, C.A. Marine Viruses—Major Players in the Global Ecosystem. Nat. Rev. Microbiol. 2007, 5, 801–812. [Google Scholar] [CrossRef]
- Brussaard, C.P.D.; Wilhelm, S.W.; Thingstad, F.; Weinbauer, M.G.; Bratbak, G.; Heldal, M.; Kimmance, S.A.; Middelboe, M.; Nagasaki, K.; Paul, J.H.; et al. Global-Scale Processes with a Nanoscale Drive: The Role of Marine Viruses. ISME J. 2008, 2, 575–578. [Google Scholar] [CrossRef] [Green Version]
- Weinbauer, M.G. Ecology of Prokaryotic Viruses. FEMS Microbiol. Rev. 2004, 28, 127–181. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, H.; Blanc-Mathieu, R.; Endo, H.; Chaffron, S.; Delmont, T.O.; Gaia, M.; Henry, N.; Hernández-Velázquez, R.; Nguyen, C.H.; Mamitsuka, H.; et al. Eukaryotic Virus Composition Can Predict the Efficiency of Carbon Export in the Global Ocean. iScience 2021, 24, 102002. [Google Scholar] [CrossRef]
- Zimmerman, A.E.; Howard-Varona, C.; Needham, D.M.; John, S.G.; Worden, A.Z.; Sullivan, M.B.; Waldbauer, J.R.; Coleman, M.L. Metabolic and Biogeochemical Consequences of Viral Infection in Aquatic Ecosystems. Nat. Rev. Microbiol. 2020, 18, 21–34. [Google Scholar] [CrossRef]
- Yamada, Y.; Tomaru, Y.; Fukuda, H.; Nagata, T. Aggregate Formation during the Viral Lysis of a Marine Diatom. Front. Mar. Sci. 2018, 5, 167. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, J.M.; Sherr, E.B.; Sherr, B.F. Size-Selective Grazing on Bacteria by Natural Assemblages of Estuarine Flagellates and Ciliates. Appl. Environ. Microbiol. 1990, 56, 583–589. [Google Scholar] [CrossRef] [Green Version]
- Thingstad, T.F. Elements of a Theory for the Mechanisms Controlling Abundance, Diversity, and Biogeochemical Role of Lytic Bacterial Viruses in Aquatic Systems. Limnol. Oceanogr. 2000, 45, 1320–1328. [Google Scholar] [CrossRef]
- Thingstad, T.F. Eutrophication in Planktonic Ecosystems: Food Web Dynamics and Elemental Cycling; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar] [CrossRef]
- De Baar, H.J.W.; Timmermans, K.R.; Laan, P.; De Porto, H.H.; Ober, S.; Blom, J.J.; Bakker, M.C.; Schilling, J.; Sarthou, G.; Smit, M.G.; et al. Titan: A New Facility for Ultraclean Sampling of Trace Elements and Isotopes in the Deep Oceans in the International Geotraces Program. Mar. Chem. 2008, 111, 4–21. [Google Scholar] [CrossRef]
- Rijkenberg, M.J.A.; Fischer, A.C.; Kroon, J.J.; Gerringa, L.J.A.; Timmermans, K.R.; Wolterbeek, H.T.; De Baar, H.J.W. The Influence of UV Irradiation on the Photoreduction of Iron in the Southern Ocean. Mar. Chem. 2005, 93, 119–129. [Google Scholar] [CrossRef]
- Rijkenberg, M.J.A.; de Baar, H.J.W.; Bakker, K.; Gerringa, L.J.A.; Keijzer, E.; Laan, M.; Laan, P.; Middag, R.; Ober, S.; van Ooijen, J.; et al. “PRISTINE”, a New High Volume Sampler for Ultraclean Sampling of Trace Metals and Isotopes. Mar. Chem. 2015, 177, 501–509. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.-A.; van Manen, M.; Wille, F.; Jung, J.; Lee, S.H.; Kim, T.-W.; Aoki, S.; Eich, C.; Brussaard, C.P.D.; Reichart, G.-J.; et al. The Biogeochemistry of Zinc and Cadmium in the Amundsen Sea, Coastal Antarctica. Mar. Chem. 2022; in revison. [Google Scholar]
- Jeon, M.H.; Jung, J.; Park, M.O.; Aoki, S.; Kim, T.W.; Kim, S.K. Tracing Circumpolar Deep Water and Glacial Meltwater Using Humic-like Fluorescent Dissolved Organic Matter in the Amundsen Sea, Antarctica. Mar. Chem. 2021, 235, 104008. [Google Scholar] [CrossRef]
- Spreen, G.; Kaleschke, L.; Heygster, G. Sea Ice Remote Sensing Using AMSR-E 89-GHz Channels. J. Geophys. Res. Ocean. 2008, 113, C02S03. [Google Scholar] [CrossRef] [Green Version]
- Gordon, L.I.; Jennings, J.C.; Ross Andrew, A.J.; Krest, J.M. A Suggested Protocol for Continuous Flow Automated Analysis of Seawater Nutrients (Phosphate, Nitrate, Nitrite and Silicic Acid) in the WOCE Hydrographic Program and the Joint Global Ocean Fluxes Study; WOCE Hydrographic Program Office: Southampton, UK, 1993; pp. 1–55. [Google Scholar]
- van Manen, M.; Aoki, S.; Brussaard, C.P.D.; Conway, T.M.; Eich, C.; Gerringa, L.; Jung, J.; Kim, T.-W.; Lee, S.H.; Lee, Y.; et al. The Role of the Dotson Ice Shelf and Circumpolar Deep Water as Driver and Source of Dissolved and Particulate Iron and Manganese in the Amundsen Sea Polynya, Southern Ocean. Mar. Chem. 2022; in revison. [Google Scholar]
- van Leeuwe, M.A.; Villerius, L.A.; Roggeveld, J.; Visser, R.J.W.; Stefels, J. An Optimized Method for Automated Analysis of Algal Pigments by HPLC. Mar. Chem. 2006, 102, 267–275. [Google Scholar] [CrossRef]
- Van Heukelem, L.; Thomas, C.S. Computer-Assisted High-Performance Liquid Chromatography Method Development with Applications to the Isolation and Analysis of Phytoplankton Pigments. J. Chromatogr. A 2001, 910, 31–49. [Google Scholar] [CrossRef]
- Mackey, M.D.; Mackey, D.J.; Higgins, H.W.; Wright, S.W. CHEMTAX—A Program for Estimating Class Abundances from Chemical Markers: Application to HPLC Measurements of Phytoplankton. Mar. Ecol. Prog. Ser. 1996, 144, 265–283. [Google Scholar] [CrossRef] [Green Version]
- Selz, V.; Lowry, K.E.; Lewis, K.M.; Joy-Warren, H.L.; Van De Poll, W.; Nirmel, S.; Tong, A.; Arrigo, K.R. Distribution of Phaeocystis Antarctica-Dominated Sea Ice Algal Communities and Their Potential to Seed Phytoplankton across the Western Antarctic Peninsula in Spring. Mar. Ecol. Prog. Ser. 2018, 586, 91–112. [Google Scholar] [CrossRef]
- Marie, D.; Partensky, F.; Vaulot, D.; Brussaard, C.P.D. Enumeration of Phytoplankton, Bacteria, and Viruses in Marine Samples. Curr. Protoc. Cytom. 1999, 10, 11.11.1–11.11.15. [Google Scholar] [CrossRef] [PubMed]
- Veldhuis, M.J.W.; Kraay, G.W. Phytoplankton in the Subtropical Atlantic Ocean: Towards a Better Assessment of Biomass and Composition. Deep Res. Part I Oceanogr. Res. Pap. 2004, 51, 507–530. [Google Scholar] [CrossRef]
- Zingone, A.; Forlani, G.; Percopo, I.; Montresor, M. Morphological Characterization of Phaeocystis antarctica (Prymnesiophyceae). Phycologia 2011, 50, 650–660. [Google Scholar] [CrossRef]
- Schoemann, V.; Becquevort, S.; Stefels, J.; Rousseau, V.; Lancelot, C. Phaeocystis Blooms in the Global Ocean and Their Controlling Mechanisms: A Review. J. Sea Res. 2005, 53, 43–66. [Google Scholar] [CrossRef]
- Sow, S.L.S.; Trull, T.W.; Bodrossy, L. Oceanographic Fronts Shape Phaeocystis Assemblages: A High-Resolution 18S RRNA Gene Survey from the Ice-Edge to the Equator of the South Pacific. Front. Microbiol. 2020, 11, 1847. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.E.M.; Lancelot, C.; Brandini, F.P.; Sakshaug, E.; John, D.M. The Taxonomic Identity of the Cosmopolitan Prymnesiophyte Phaeocystis: A Morphological and Ecophysiological Approach. J. Mar. Syst. 1994, 5, 5–22. [Google Scholar] [CrossRef]
- Arrigo, K.R.; Robinson, D.H.; Worthen, D.L.; Dunbar, R.B.; DiTullio, G.R.; VanWoert, M.; Lizotte, M.P. Phytoplankton Community Structure and the Drawdown of Nutrients and CO2 in the Southern Ocean. Science 1999, 283, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Kimmance, S.A.; Brussaard, C.P.D. Estimation of Viral-Induced Phytoplankton Mortality Using the Modified Dilution Method. Limnol. Oceanogr. Methods 2010, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Mojica, K.D.A.; Huisman, J.; Wilhelm, S.W.; Brussaard, C.P.D. Latitudinal Variation in Virus-Induced Mortality of Phytoplankton across the North Atlantic Ocean. ISME J. 2016, 10, 500–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrison, D.L.; Gowing, M.M.; Hughes, M.P.; Campbell, L.; Caron, D.A.; Dennett, M.R.; Shalapyonok, A.; Olson, R.J.; Landry, M.R.; Brown, S.L.; et al. Microbial Food Web Structure in the Arabian Sea: A US JGOFS Study. Deep Res. Part II Top. Stud. Oceanogr. 2000, 47, 1387–1422. [Google Scholar] [CrossRef]
- Worden, A.Z.; Nolan, J.K.; Palenik, B. Assessing the Dynamics and Ecology of Marine Picophytoplankton: The Importance of the Eukaryotic Component. Limnol. Oceanogr. 2004, 49, 168–179. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Williams, C.M.; Dupont, A.M.; Loevenich, J.; Post, A.F.; Dinasquet, J.; Yager, P.L. Pelagic Microbial Heterotrophy in Response to a Highly Productive Bloom of Phaeocystis antarctica in the Amundsen Sea Polynya, Antarctica. Elem. Sci. Anthr. 2016, 4, 000102. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Kim, B.K.; Yun, M.S.; Joo, H.T.; Yang, E.J.; Kim, Y.N.; Shin, H.C.; Lee, S.H. Spatial Distribution of Phytoplankton Productivity in the Amundsen Sea, Antarctica. Polar Biol. 2012, 35, 1721–1733. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, B.K.; Lim, Y.J.; Joo, H.T.; Kang, J.J.; Lee, D.; Park, J.; Ha, S.Y.; Lee, S.H. Small Phytoplankton Contribution to the Standing Stocks and the Total Primary Production in the Amundsen Sea. Biogeosciences 2017, 14, 3705–3713. [Google Scholar] [CrossRef] [Green Version]
- Wolf, C.; Frickenhaus, S.; Kilias, E.S.; Peeken, I.; Metfies, K. Regional Variability in Eukaryotic Protist Communities in the Amundsen Sea. Antarct. Sci. 2013, 25, 741–751. [Google Scholar] [CrossRef]
- Coello-Camba, A.; Agustí, S. Thermal Thresholds of Phytoplankton Growth in Polar Waters and Their Consequences for a Warming Polar Ocean. Front. Mar. Sci. 2017, 4, 168. [Google Scholar] [CrossRef] [Green Version]
- Sherman, E.; Moore, J.K.; Primeau, F.; Tanouye, D. Global Biogeochemical Cycles Community Growth Rates. Glob. Biogeochem. Cycles 2016, 30, 550–559. [Google Scholar] [CrossRef]
- Delmont, T.O.; Hammar, K.M.; Ducklow, H.W.; Yager, P.L.; Post, A.F. Phaeocystis antarctica Blooms Strongly Influence Bacterial Community Structures in the Amundsen Sea Polynya. Front. Microbiol. 2014, 5, 646. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Lee, Y.; Lee, S.H. Trophic Interactions of Micro- and Mesozooplankton in the Amundsen Sea Polynya and Adjacent Sea Ice Zone during Austral Late Summer. Prog. Oceanogr. 2018, 174, 117–130. [Google Scholar] [CrossRef]
- Lannuzel, D.; Vancoppenolle, M.; Van Der Merwe, P.; De Jong, J.; Meiners, K.M.; Grotti, M.; Nishioka, J.; Schoemann, V. Iron in Sea Ice: Review & New Insights. Elementa 2016, 4, 000130. [Google Scholar] [CrossRef] [Green Version]
- Marañón, E.; Cermeño, P.; Rodríguez, J.; Zubkov, M.V.; Harris, R.P. Scaling of Phytoplankton Photosynthesis and Cell Size in the Ocean. Limnol. Oceanogr. 2007, 52, 2190–2198. [Google Scholar] [CrossRef]
- Yager, P.; Sherrell, R.; Stammerjohn, S.; Ducklow, H.; Schofield, O.; Ingall, E.; Wilson, S.; Lowry, K.; Williams, C.; Riemann, L.; et al. A Carbon Budget for the Amundsen Sea Polynya, Antarctica: Estimating Net Community Production and Export in a Highly Productive Polar Ecosystem. Elem. Sci. Anthr. 2016, 4, 000140. [Google Scholar] [CrossRef] [Green Version]
- Biggs, T.E.G.; Alvarez-Fernandez, S.; Evans, C.; Mojica, K.D.A.; Rozema, P.D.; Venables, H.J.; Pond, D.W.; Brussaard, C.P.D. Antarctic Phytoplankton Community Composition and Size Structure: Importance of Ice Type and Temperature as Regulatory Factors. Polar Biol. 2019, 42, 1997–2015. [Google Scholar] [CrossRef] [Green Version]
- Baudoux, A.C.; Noordeloos, A.A.M.; Veldhuis, M.J.W.; Brussaard, C.P.D. Virally Induced Mortality of Phaeocystis globosa during Two Spring Blooms in Temperate Coastal Waters. Aquat. Microb. Ecol. 2006, 44, 207–217. [Google Scholar] [CrossRef]
- Evans, C.; Archer, S.D.; Jacquet, S.; Wilson, W.H. Direct Estimates of the Contribution of Viral Lysis and Microzooplankton Grazing to the Decline of a Micromonas spp. Population. Aquat. Microb. Ecol. 2003, 30, 207–219. [Google Scholar] [CrossRef] [Green Version]
- Pasulka, A.L.; Samo, T.J.; Landry, M.R. Grazer and Viral Impacts on Microbial Growth and Mortality in the Southern California Current Ecosystem. J. Plankton Res. 2015, 37, 320–336. [Google Scholar] [CrossRef]
- Mojica, K.D.A.; Behrenfeld, M.J.; Clay, M.; Brussaard, C.P.D. Spring Accumulation Rates in North Atlantic Phytoplankton Communities Linked to Alterations in the Balance between Division and Loss. Front. Microbiol. 2021, 12, 706137. [Google Scholar] [CrossRef]
- Baudoux, A.C.; Veldhuis, M.J.W.; Noordeloos, A.A.M.; Van Noort, G.; Brussaard, C.P.D. Estimates of Virus- vs. Grazing Induced Mortality of Picophytoplankton in the North Sea during Summer. Aquat. Microb. Ecol. 2008, 52, 69–82. [Google Scholar] [CrossRef]
- Staniewski, M.A.; Short, S.M. Methodological Review and Meta-Analysis of Dilution Assays for Estimates of Virus- and Grazer-Mediated Phytoplankton Mortality. Limnol. Oceanogr. Methods 2018, 16, 649–668. [Google Scholar] [CrossRef]
- Brussaard, C.P.D. Viral Control of Phytoplankton Populations—A Review. J. Eucaryotic Microbiol. 2004, 51, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Nagasaki, K. Dinoflagellates, Diatoms, and Their Viruses. J. Microbiol. 2008, 46, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Arsenieff, L.; Simon, N.; Rigaut-Jalabert, F.; Le Gall, F.; Chaffron, S.; Corre, E.; Com, E.; Bigeard, E.; Baudoux, A.C. First Viruses Infecting the Marine Diatom Guinardia Delicatula. Front. Microbiol. 2019, 10, 3235. [Google Scholar] [CrossRef]
- Evans, C.; Wilson, W.H. Preferential Grazing of Oxyrrhis marina on Virus-Infected Emiliania huxleyi. Limnol. Oceanogr. 2008, 53, 2035–2040. [Google Scholar] [CrossRef]
- Vermont, A.I.; Martínez Martínez, J.; Waller, J.D.; Gilg, I.C.; Leavitt, A.H.; Floge, S.A.; Archer, S.D.; Wilson, W.H.; Fields, D.M. Virus Infection of Emiliania huxleyi Deters Grazing by the Copepod Acartia tonsa. J. Plankton Res. 2016, 38, 1194–1205. [Google Scholar] [CrossRef] [Green Version]
- Staniewski, M.A.; Short, S.M. Potential Viral Stimulation of Primary Production Observed during Experimental Determinations of Phytoplankton Mortality. Aquat. Microb. Ecol. 2014, 71, 239–256. [Google Scholar] [CrossRef] [Green Version]
- Ruardij, P.; Veldhuis, M.J.W.; Brussaard, C.P.D. Modeling the Bloom Dynamics of the Polymorphic Phytoplankter Phaeocystis globosa: Impact of Grazers and Viruses. Harmful Algae 2005, 4, 941–963. [Google Scholar] [CrossRef]
- Brussaard, C.P.D.; Bratbak, G.; Baudoux, A.C.; Ruardij, P. Phaeocystis and Its Interaction with Viruses. Biogeochemistry 2007, 83, 201–215. [Google Scholar] [CrossRef]
- Lee, D.B.; Choi, K.H.; Ha, H.K.; Yang, E.J.; Lee, S.H.; Lee, S.H.; Shin, H.C. Mesozooplankton Distribution Patterns and Grazing Impacts of Copepods and Euphausia crystallorophias in the Amundsen Sea, West Antarctica, during Austral Summer. Polar Biol. 2013, 36, 1215–1230. [Google Scholar] [CrossRef]
- Short, S.M. The Ecology of Viruses That Infect Eukaryotic Algae. Environ. Microbiol. 2012, 14, 2253–2271. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Landry, M.R.; Huang, B.; Liu, H. Does Warming Enhance the Effect of Microzooplankton Grazing on Marine Phytoplankton in the Ocean? Limnol. Oceanogr. 2012, 57, 519–526. [Google Scholar] [CrossRef]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a Metabolic Theory of Ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Rose, J.M.; Caron, D.A. Does Low Temperature Constrain the Growth Rates of Heterotrophic Protists? Evidence and Implications for Algal Blooms in Cold Waters. Limnol. Oceanogr. 2007, 52, 886–895. [Google Scholar] [CrossRef]
- Mojica, K.D.A.; Brussaard, C.P.D. Factors Affecting Virus Dynamics and Microbial Host-Virus Interactions in Marine Environments. FEMS Microbiol. Ecol. 2014, 89, 495–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- St-Laurent, P.; Yager, P.L.; Sherrell, R.M.; Oliver, H.; Dinniman, M.S.; Stammerjohn, S.E. Modeling the Seasonal Cycle of Iron and Carbon Fluxes in the Amundsen Sea Polynya, Antarctica. J. Geophys. Res. Ocean. 2019, 124, 1544–1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Hwang, J.; Ducklow, H.W.; Hahm, D.; Lee, S.H.; Kim, D.; Hyun, J.H.; Park, J.; Ha, H.K.; Kim, T.W.; et al. Evidence of Minimal Carbon Sequestration in the Productive Amundsen Sea Polynya. Geophys. Res. Lett. 2017, 44, 7892–7899. [Google Scholar] [CrossRef]
- Venables, H.J.; Buma, A.G.J.; Rozema, P.D.; van de Poll, W.H.; Clarke, A.; Meredith, M.P. Interannual Variability in Phytoplankton Biomass and Species Composition in Northern Marguerite Bay (West Antarctic Peninsula) Is Governed by Both Winter Sea Ice Cover and Summer Stratification. Limnol. Oceanogr. 2016, 62, 235–252. [Google Scholar] [CrossRef] [Green Version]
- Vaqué, D.; Boras, J.A.; Torrent-Llagostera, F.; Agustí, S.; Arrieta, J.M.; Lara, E.; Castillo, Y.M.; Duarte, C.M.; Sala, M.M. Viruses and Protists Induced-Mortality of Prokaryotes around the Antarctic Peninsula during the Austral Summer. Front. Microbiol. 2017, 8, 241. [Google Scholar] [CrossRef]
- Sullivan, M.B.; Weitz, J.S.; Wilhelm, S. Viral Ecology Comes of Age. Environ. Microbiol. Rep. 2017, 9, 33–35. [Google Scholar] [CrossRef]
- Kranzler, C.F.; Brzezinski, M.A.; Cohen, N.R.; Lampe, R.H.; Maniscalco, M.; Till, C.P.; Mack, J.; Latham, J.R.; Bruland, K.W.; Twining, B.S.; et al. Impaired Viral Infection and Reduced Mortality of Diatoms in Iron-Limited Oceanic Regions. Nat. Geosci. 2021, 14, 231–237. [Google Scholar] [CrossRef]
- Poulton Alex, J. Shunt or Shuttle. Nat. Geosci. 2021, 14, 180–181. [Google Scholar] [CrossRef]
- La, H.S.; Lee, H.; Fielding, S.; Kang, D.; Ha, H.K.; Atkinson, A.; Park, J.; Siegel, V.; Lee, S.H.; Shin, H.C. High Density of Ice Krill (Euphausia crystallorophias) in the Amundsen Sea Coastal Polynya, Antarctica. Deep Res. Part I Oceanogr. Res. Pap. 2015, 95, 75–84. [Google Scholar] [CrossRef]
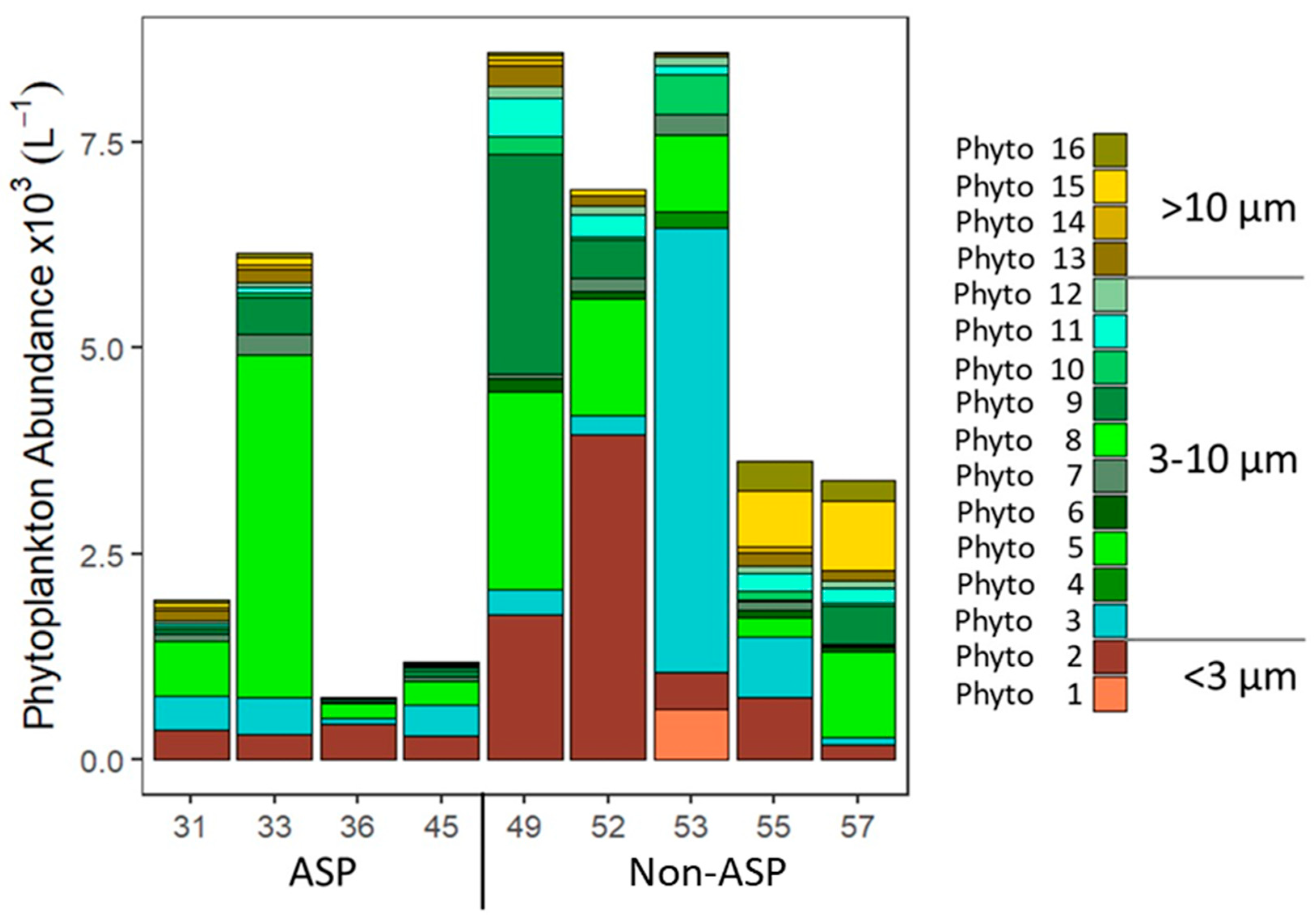

| Phyto | Average Diameter (µm) |
|---|---|
| 1 | 1.0 |
| 2 | 2.0 |
| 3 | 3.3 |
| 4 | 3.3 |
| 5 | 4.2 |
| 6 | 5.2 |
| 7 | 8.6 |
| 8 | 8.7 |
| 9 | 9.1 |
| 10 | 9.6 |
| 11 | 10.0 |
| 12 | 10.0 |
| 13 | 14.3 |
| 14 | 15.4 |
| 15 | 19.0 |
| 16 | 19.8 |
| T | Dep | Expl | H | df | p | Mean ± SD | Median | n | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| in | out | in | out | in | out | ||||||
| 1 | Chl a | ASP | 45.9 | 1 | 1.2 × 10−11 | 4.5 ± 2.1 | 3.1 ± 2.0 | 5.5 | 4.3 | 4 | 5 |
| 2 | dFe | ASP | 23.0 | 1 | 1.6 × 10−6 | 0.22 ± 0.10 | 0.17 ± 0.08 | 0.21 | 0.16 | 4 | 5 |
| 3 | Chl a < 20 µm | ASP | 20.4 | 1 | 6.3 × 10−6 | 0.8 ± 0.5 | 1.7 ± 1.2 | 0.9 | 0.9 | 4 | 5 |
| 4 | G | ASP | 5.4 | 1 | 0.02 | 0.42 ± 0.26 | 0.23 ± 0.25 | 0.44 | 0.13 | 16 | 30 |
| 5 | Abun | ASP | 81.6 | 1 | 2.2 × 10−16 | 2.5 ± 2.2 | 6.2 ± 2.3 | 1.6 | 6.9 | 4 | 5 |
| 6 | Temp | ASP | 62.5 | 1 | 2.7 × 10−15 | −0.8 ± 0.5 | −1.2 ± 0.3 | −0.6 | −1.3 | 4 | 5 |
| 7 | TL | ASP | 13.0 | 1 | 0.0003 | 0.62 ± 0.27 | 0.39 ± 0.27 | 0.57 | 0.37 | 16 | 30 |
| T | Dep | Expl | H | df | p | G | V | G | V | G | V |
| 8 | TL P7 | G or V | 6.0 | 1 | 0.01 | 0.47 ± 0.25 | 0.09 ± 0.09 | 0.47 | 0.08 | 7 | 7 |
| 9 | TL P9 | G or V | 3.9 | 1 | 0.05 | 0.71 ± 0.22 | 0.16 ± 0.16 | 0.82 | 0.16 | 3 | 3 |
| 10 | TL P10 | G or V | 3.9 | 1 | 0.05 | 0.53 ± 0.10 | 0.13 ± 0.13 | 0.52 | 0.09 | 3 | 3 |
| T | Dep | Expl | H | df | p | <7 | ≥7 | <7 | ≥7 | <7 | ≥7 |
| 11 | G | size | 5.5 | 1 | 0.01 | 0.20 ± 0.21 | 0.42 ± 0.28 | 0.24 | 0.47 | 26 | 20 |
| 12 | G ASP | size | 0.8 | 1 | 0.01 | 0.26 ± 0.19 | 0.59 ± 0.21 | 0.23 | 0.51 | 8 | 8 |
| T | Dep | Expl | H | df | p | G | V | G | V | G | V |
| 13 | TL | G or V | 4.7 | 1 | 0.03 | 0.29 ± 0.27 | 0.18 ± 0.25 | 0.24 | 0.19 | 46 | 46 |
| 14 | TL C | G or V | 3.6 | 1 | 0.06 | 0.60 ± 1.36 | 0.61 ± 1.67 | 0.2 | 0.07 | 46 | 46 |
| T | Dep | Expl | slope | r2 | p | n |
|---|---|---|---|---|---|---|
| 1 | G | Size | 0.03 | 0.12 | 0.01 | 46 |
| 2 | G | Temp | 0.26 | 0.18 | 0.004 | 46 |
| 3 | TL | Gross growth | 0.41 | 0.21 | 0.001 | 46 |
| 4 | TL | Abun | −9.4 × 10−5 | 0.09 | 0.04 | 46 |
| Station | Gate | Lysis (d−1) | Grazing (d−1) | Station | Gate | Lysis (d−1) | Grazing (d−1) |
|---|---|---|---|---|---|---|---|
| 31 | Phyto 2 | 0.08 | 0.51 | 52 | Phyto 2 | 0 | 0.13 |
| Phyto 3 | 0.19 | 0.26 | Phyto 3 | 0 | 0 | ||
| Phyto 5 | 0 | 0.23 | Phyto 5 | 0 | 0 | ||
| Phyto 7 | 0 | 0.81 | Phyto 7 | 0 | 0.48 | ||
| Phyto 9 | 0.31 | 0.46 | Phyto 11 | 0 | 0 | ||
| 33 | Phyto 2 | 0.4 | 0.56 | Phyto 13 | 0.19 | 0 | |
| Phyto 3 | 0.17 | 0.15 | 53 | Phyto 1 | 0.27 | 0.02 | |
| Phyto 5 | 0.3 | 0 | Phyto 2 | 0.17 | 0 | ||
| Phyto 9 | 0 | 0.82 | Phyto 3 | 0 | 0.18 | ||
| 36 | Phyto 2 | 0.46 | 0.1 | Phyto 4 | 0.02 | 0.04 | |
| Phyto 9 | 0.16 | 0.86 | Phyto 5 | 0.02 | 0.01 | ||
| 45 | Phyto 6 | 0 | 0.24 | Phyto 7 | 0 | 0 | |
| Phyto 7 | 0.11 | 0.44 | 55 | Phyto 2 | 0 | 0.24 | |
| Phyto 10 | 0.09 | 0.43 | Phyto 3 | 0.06 | 0 | ||
| Phyto 11 | 0.77 | 0.34 | Phyto 5 | 0 | 0.51 | ||
| Phyto 14 | 0.1 | 0.57 | Phyto 6 | 0.05 | 0 | ||
| 49 | Phyto 2 | 0 | 0.17 | Phyto 7 | 0.08 | 0.43 | |
| Phyto 3 | 0.16 | 0.31 | Phyto 11 | 0.53 | 0 | ||
| Phyto 5 | 0.5 | 0 | 57 | Phyto 5 | 0 | 0.1 | |
| Phyto 6 | 0.13 | 0.67 | Phyto 6 | 0.06 | 0.67 | ||
| Phyto 7 | 0.22 | 0.64 | Phyto 7 | 0.2 | 0.47 | ||
| Phyto 10 | 0.02 | 0.64 | Phyto 10 | 0.27 | 0.52 | ||
| Phyto 11 | 1.08 | 0.48 | Phyto 12 | 0.95 | 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eich, C.; Biggs, T.E.G.; van de Poll, W.H.; van Manen, M.; Tian, H.-A.; Jung, J.; Lee, Y.; Middag, R.; Brussaard, C.P.D. Ecological Importance of Viral Lysis as a Loss Factor of Phytoplankton in the Amundsen Sea. Microorganisms 2022, 10, 1967. https://doi.org/10.3390/microorganisms10101967
Eich C, Biggs TEG, van de Poll WH, van Manen M, Tian H-A, Jung J, Lee Y, Middag R, Brussaard CPD. Ecological Importance of Viral Lysis as a Loss Factor of Phytoplankton in the Amundsen Sea. Microorganisms. 2022; 10(10):1967. https://doi.org/10.3390/microorganisms10101967
Chicago/Turabian StyleEich, Charlotte, Tristan E. G. Biggs, Willem H. van de Poll, Mathijs van Manen, Hung-An Tian, Jinyoung Jung, Youngju Lee, Rob Middag, and Corina P. D. Brussaard. 2022. "Ecological Importance of Viral Lysis as a Loss Factor of Phytoplankton in the Amundsen Sea" Microorganisms 10, no. 10: 1967. https://doi.org/10.3390/microorganisms10101967
APA StyleEich, C., Biggs, T. E. G., van de Poll, W. H., van Manen, M., Tian, H.-A., Jung, J., Lee, Y., Middag, R., & Brussaard, C. P. D. (2022). Ecological Importance of Viral Lysis as a Loss Factor of Phytoplankton in the Amundsen Sea. Microorganisms, 10(10), 1967. https://doi.org/10.3390/microorganisms10101967





_Brussaard.png)

