Pathogens and Passengers: Roles for Crustacean Zooplankton Viruses in the Global Ocean
Abstract
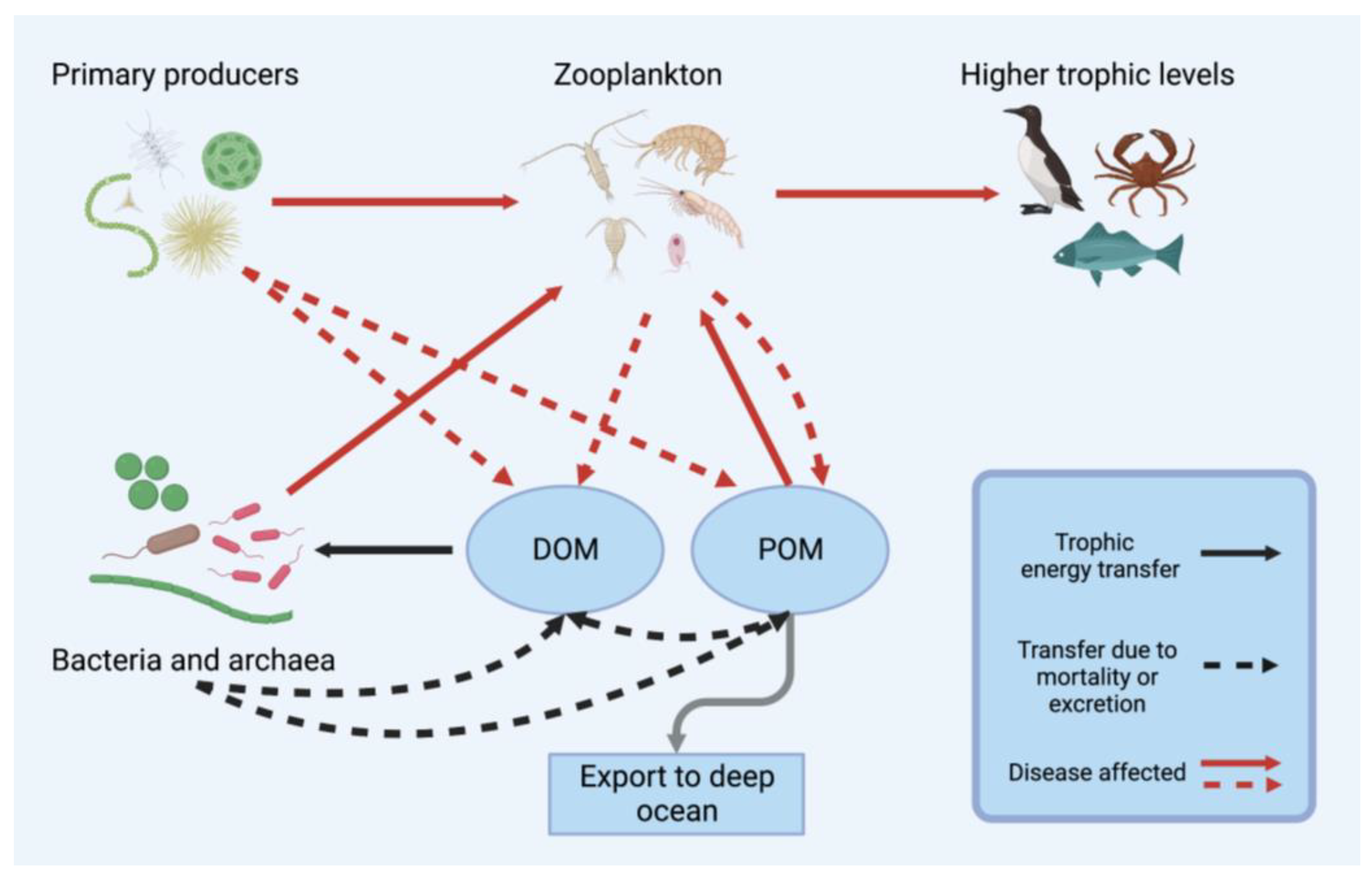
1. The Roles of Viruses and Planktonic Crustaceans in Marine Ecosystems
2. The Pathogens: Viral Disease in Crustacean Zooplankton and Its Impacts
2.1. The Viruses of Crustacean Zooplankton
| Virus Name | Virus Order, Family | Associated Organism Order—Family, Genus or Species | Evidence of Replication | Disease Caused | References |
|---|---|---|---|---|---|
| Daphnia iridescent virus 1 (DIV-1) | Pimascovirales, Iridoviridae | Branchiopoda—Daphnia sp. | Yes | White fat cell disease | [47] |
| Unnamed circo-like viruses | Cirlivirales, Circoviridae | Amphipoda—Diporeia sp. | No | ND | [48,58] |
| Labidocera aestiva copepod circovirus (LaCopCV) | Cirlivirales, Circoviridae | Copepoda—Labidocera aestiva | Yes | ND | [56] |
| Acartia tonsa copepod circovirus (AtCopCV) | Cirlivirales, Circoviridae | Copepoda—Acartia tonsa | Yes | ND | [56] |
| Zooplankton invertebrate iridescent virus (ZoopIIV(HR)) | Pimascovirales, Iridoviridae | Copepoda—Gladioferens pectinatus, Boeckella triarticulata, Gippslandia estuarina | Yes | Zooplankton iridoviral disease | [46] |
| White spot syndrome virus (WSSV) | Unclassified, Nimaviridae | Copepoda—Acartidae, Oithonidae, Tachididae, Centropagidae, Corycaeidae, Temoridae, Calanidae, Paracalanidae, Pontellidae, Peltididae, Metrididae, Miraciidae, Cyclopidae, Ameiridae, Pseudodiaptomidae, Ergasilus manicatus Amphipoda—Caprellidae, Gammaridea Cirripedia—undocumented family Branchiopoda—Artemiidae Mysida—Mysidae Euphausiacea—Euphausiidae | Variable | White spot disease | [33,35,38,39,59,60,61,62,63,64] |
| Viral haemorrhagic septicaemia virus (VHSV) | Mononegavirales, Rhabdoviridae | Amphipoda—Diporeia sp. | No | Viral haemorrhagic septicaemia | [32] |
| Unnamed mononega-like viruses | Mononegavirales, Artoviridae | Copepoda—Caligus spp., Lepeophtheiris spp., Tracheliastes sp., Pseudodiaptomus sp. Amphipoda—Eulimnogammarus sp., Ommatogammarus sp., Hyatellopsis sp. | No | ND | [49] |
| Unnamed mononega-like viruses | Mononegavirales, Rhabdoviridae | Copepoda—Caligus spp. Amphipoda—Carinurus bicarinatus, Gammarus spp. | No | ND | [49] |
| Unnamed chu-like viruses | Jingchuvirales, Chuviridae | Copepoda—Cosmocalanus darwinii, Pleuromamma sp., Platychelipus littoralis Amphipoda—Hyatellopsis sp., Gammarus sp. | No | ND | [49] |
| Unnamed ghabri-botybri-like viruses | Ghabrivirales, Totiviridae | Copepoda—C. darwinii, Lepeophtheiris spp., Caligus spp., Tigriopus californicus, Labidocera madurae, Tracheliastes polycolpus Amphipoda—Gammarus sp., Eogammarus sp., Echinogammarus sp. | No | ND | [49] |
| Unnamed tymo-like viruses | Tymovirales, Tymoviridae | Copepoda—C. darwinii | No | ND | [49] |
| Unnamed bunya-like viruses | Bunyavirales, unnamed families | Copepoda—Lepeophtheirus sp., C. darwinii Amphipoda—Talitrus sp., Gammarus sp., Eogammarus sp., Hyatellopsis sp. | No | ND | [49] |
| Unnamed orthomyxo-like viruses | Orthomyxsovirales, unnamed families | Copepoda—T. californicus Amphipoda—Gammaroporeia sp., Platychelipus sp., Echinogammarus sp., Marinogammarus sp. | No | ND | [49] |
| Unnamed qin-like viruses | Muvirales, Qinviridae | Copepoda—Apocyclops royi Amphipoda—Gammarus sp. | No | ND | [49] |
| Unnamed partiti-like viruses | Durnavirales, Partitiviridae | Copepoda—C. darwinii, Caligus spp., T. californicus, Euchaeta spp., Eucalanus bungii Amphipoda—Eugammarus sp., Echinogammarus sp. | No | ND | [49] |
| Unammed picobirna-like viruses | Durnavirales, Partitiviridae | Copepoda—Eurytemora affinis Amphipoda—Talitrus saltator | No | ND | [49] |
| Unnamed durna-like viruses | Durnavirles, unnamed family | Amphipoda—Gammarus sp. | No | ND | [49] |
| Unnamed martelli-like viruses | Martellivirales, Endornaviridae | Copepoda—Caligus sp. | No | ND | [49] |
| Unnamed picorna-like viruses | Picornavirales, unnamed family | Copepoda—Caligus spp., Lepeophtheirus sp., E. bungii, Eucyclops serrulatus, Calanus finmarchicus Amphipoda—Gammarus sp., Echinogammarus sp., Ommatogammarus sp., Macropereiopus parvus | No | ND | [49] |
| Taura syndrome virus | Picornavirales, Dicistroviridae | Copepoda—E. manicatus | Yes | Taura syndrome | [35] |
| Unnamed flavi-like viruses | Amarillovirales, Flaviviridae | Amphipoda—Gammarus sp. | No | ND | [49] |
| Unnamed noda-barna-like viruses | Nodamuvirales, Nodaviridae | Copepoda—T. californicus, Pleuromamma abdominalis, E. affinis Amphipoda—Gammarus spp. | No | ND | [49] |
| Unnamed noda-barna-like viruses | Sobelivirales, Solemoviridae | Copepoda—Caligus spp. | No | ND | [49] |
| Unnamed nido-like viruses | Nidovirales, unnamed family | Copepoda—E. affinis | No | ND | [49] |
| Unnamed birna-permutotetra-like viruses | Durnavirales, Birnaviridae | Copepoda—E. affinis | No | ND | [49] |
2.2. Viral Disease Likely Accounts for a Portion of Non-Consumptive Mortality in Crustacean Zooplankton
2.3. Implications of Viral Disease for Food Webs and Biogeochemical Cycling
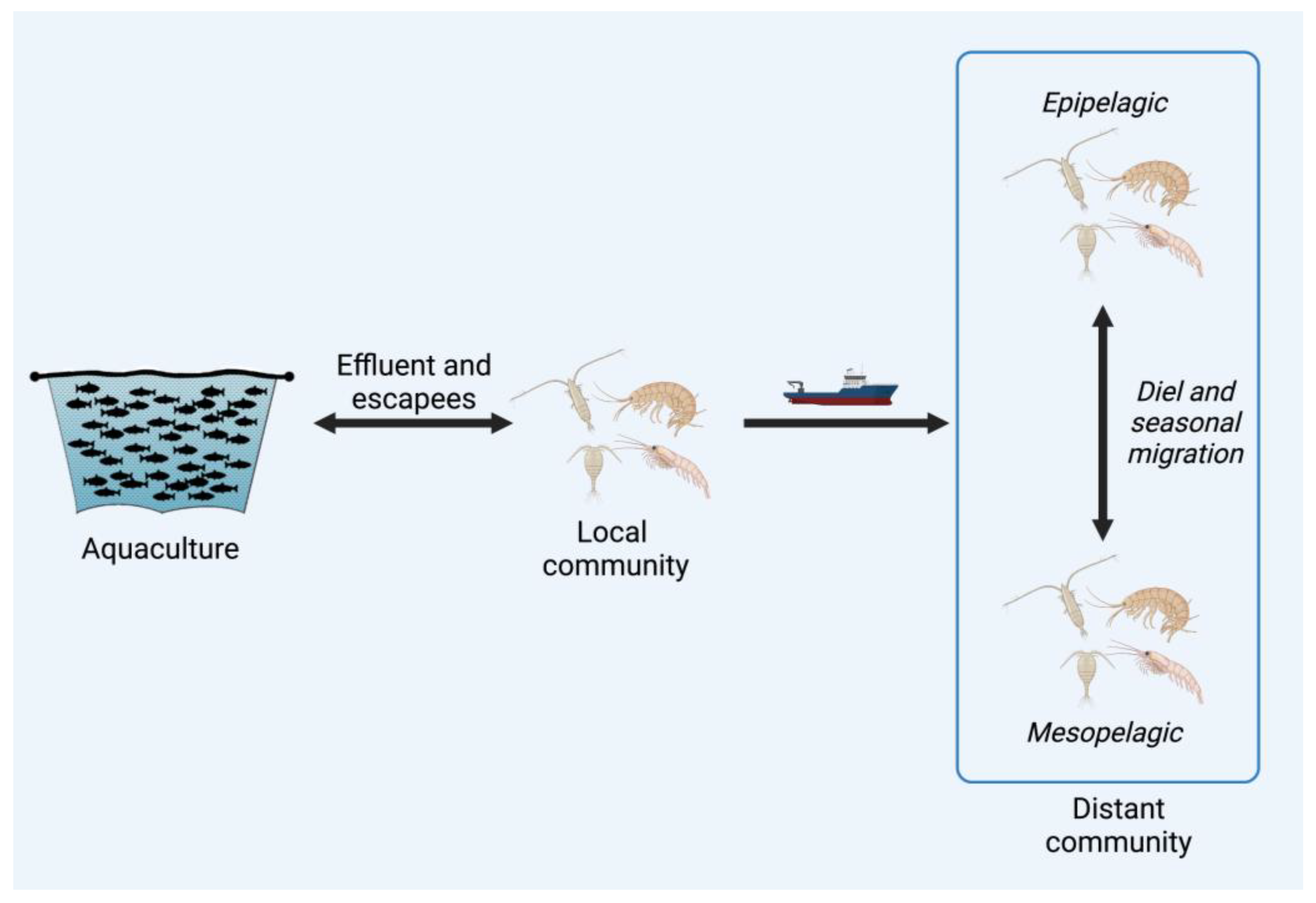
3. The Passengers: Viruses Spread by Crustacean Zooplankton
4. Lifting the Veil on the Identity and Impacts of Zooplankton Viruses
- I.
- Virus discovery in crustacean zooplankton with a focus on the establishment of host–virus relationships as well as a link between infection, disease and mortality.
- II.
- An assessment of viral prevalence, distribution and seasonal dynamics in crustacean host populations.
- III.
- A comprehensive assessment of viral ‘passengers’, mechanisms of transmission and associated risks.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wilhelm, S.W.; Suttle, C.A. Viruses and Nutrient Cycles in the Sea. Bioscience 1999, 49, 781–788. [Google Scholar] [CrossRef]
- Suttle, C.A. Marine Viruses-Major Players in the Global Ecosystem. Nat. Rev. Microbiol. 2007, 5, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Gustavsen, J.A.; Winget, D.M.; Tian, X.; Suttle, C.A. High Temporal and Spatial Diversity in Marine RNA Viruses Implies That They Have an Important Role in Mortality and Structuring Plankton Communities. Front. Microbiol. 2014, 5, 703. [Google Scholar] [CrossRef]
- Weinbauer, M.G.; Rassoulzadegan, F. Are Viruses Driving Microbial Diversification and Diversity? Environ. Microbiol. 2004, 6, 1–11. [Google Scholar] [CrossRef]
- Thingstad, T.F. Elements of a Theory for the Mechanisms Controlling Abundance, Diversity, and Biogeochemical Role of Lytic Bacterial Viruses in Aquatic Systems. Limnol. Oceanogr. 2000, 45, 1320–1328. [Google Scholar] [CrossRef]
- Korytowski, D.A.; Smith, H. Permanence and Stability of a Kill the Winner Model in Marine Ecology. Bull. Math. Biol. 2017, 79, 995–1004. [Google Scholar] [CrossRef]
- Flynn, K.J.; Mitra, A.; Wilson, W.H.; Kimmance, S.A.; Clark, D.R.; Pelusi, A.; Polimene, L. ‘Boom-and-Busted’ Dynamics of Phytoplankton–Virus Interactions Explain the Paradox of the Plankton. New Phytol. 2022, 234, 990–1002. [Google Scholar] [CrossRef]
- Bratbak, G.; Egge, J.K.; Heldal, M. Viral Mortality of the Marine Alga Emiliania Huxleyi (Haptophyceae) and Termination of Algal Blooms. Mar. Ecol. Prog. Ser. 1993, 93, 39–48. [Google Scholar] [CrossRef]
- Rhodes, C.J.; Truscott, J.E.; Martin, A.P. Viral Infection as a Regulator of Oceanic Phytoplankton Populations. J. Mar. Syst. 2008, 74, 216–226. [Google Scholar] [CrossRef]
- Lønborg, C.; Middelboe, M.; Brussaard, C.P.D. Viral Lysis of Micromonas Pusilla: Impacts on Dissolved Organic Matter Production and Composition. Biogeochemistry 2013, 116, 231–240. [Google Scholar] [CrossRef]
- Kaneko, H.; Blanc-Mathieu, R.; Endo, H.; Chaffron, S.; Delmont, T.O.; Gaia, M.; Henry, N.; Hernández-Velázquez, R.; Nguyen, C.H.; Mamitsuka, H.; et al. Eukaryotic Virus Composition Can Predict the Efficiency of Carbon Export in the Global Ocean. iScience 2021, 24, 102002. [Google Scholar] [CrossRef]
- Weitz, J.S.; Stock, C.A.; Wilhelm, S.W.; Bourouiba, L.; Coleman, M.L.; Buchan, A.; Follows, M.J.; Fuhrman, J.A.; Jover, L.F.; Lennon, J.T.; et al. A Multitrophic Model to Quantify the Effects of Marine Viruses on Microbial Food Webs and Ecosystem Processes. ISME J. 2015, 9, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Shelford, E.J.; Middelboe, M.; Møller, E.F.; Suttle, C.A. Virus-Driven Nitrogen Cycling Enhances Phytoplankton Growth. Aquat. Microb. Ecol. 2012, 66, 41–46. [Google Scholar] [CrossRef]
- Roossinck, M.J. The Good Viruses: Viral Mutualistic Symbioses. Nat. Rev. Microbiol. 2011, 9, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Song, K.; Deng, C.; Ahlgren, N.A.; Fuhrman, J.A.; Li, Y.; Xie, X.; Poplin, R.; Sun, F. Identifying Viruses from Metagenomic Data Using Deep Learning. Quant. Biol. 2020, 8, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Lin, X.D.; Tian, J.H.; Chen, L.J.; Chen, X.; Li, C.X.; Qin, X.C.; Li, J.; Cao, J.P.; Eden, J.S.; et al. Redefining the Invertebrate RNA Virosphere. Nature 2016, 540, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Zayed, A.A.; Wainaina, J.M.; Dominguez-Huerta, G.; Pelletier, E.; Guo, J.; Mohssen, M.; Tian, F.; Pratama, A.A.; Bolduc, B.; Zablocki, O.; et al. Cryptic and Abundant Marine Viruses at the Evolutionary Origins of Earth’s RNA Virome. Science 2022, 376, 156–162. [Google Scholar] [CrossRef]
- Edwards, R.A.; McNair, K.; Faust, K.; Raes, J.; Dutilh, B.E. Computational Approaches to Predict Bacteriophage-Host Relationships. FEMS Microbiol. Rev. 2016, 40, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Mokili, J.L.; Rohwer, F.; Dutilh, B.E. Metagenomics and Future Perspectives in Virus Discovery. Curr. Opin. Virol. 2012, 2, 63–77. [Google Scholar] [CrossRef]
- Uritskiy, G.; Press, M.; Sun, C.; Huerta, G.D.; Zayed, A.A.; Wiser, A.; Grove, J.; Auch, B.; Eacker, S.M.; Sullivan, S.; et al. Accurate Viral Genome Reconstruction and Host Assignment with Proximity-Ligation Sequencing. BioRxiv 2021. [Google Scholar] [CrossRef]
- Bernot, J.P.; Owen, C.L.; Wolfe, J.M.; Meland, K.; Olesen, J.; Crandall, K.A. Major Revisions in Pancrustacean Phylogeny with Recommendations for Resolving Challenging Nodes. BioRxiv 2022. [Google Scholar] [CrossRef]
- Lomartire, S.; Marques, J.C.; Gonçalves, A.M.M. The Key Role of Zooplankton in Ecosystem Services: A Perspective of Interaction between Zooplankton and Fish Recruitment. Ecol. Indic. 2021, 129, 107867. [Google Scholar] [CrossRef]
- Steinberg, D.K.; Landry, M.R. Zooplankton and the Ocean Carbon Cycle. Ann. Rev. Mar. Sci. 2017, 9, 413–444. [Google Scholar] [CrossRef] [PubMed]
- Banse, K. Biomass and Production Measurements Zooplankton: Pivotal Role in the Control of Ocean Production. ICES J. Mar. Sci. 1995, 52, 265–277. [Google Scholar] [CrossRef]
- Tang, K.W.; Turk, V.; Grossart, H.P. Linkage between Crustacean Zooplankton and Aquatic Bacteria. Aquat. Microb. Ecol. 2010, 61, 261–277. [Google Scholar] [CrossRef]
- Nowicki, M.; DeVries, T.; Siegel, D.A. Quantifying the Carbon Export and Sequestration Pathways of the Ocean’s Biological Carbon Pump. Glob. Biogeochem. Cycles 2022, 36, e2021GB007083. [Google Scholar] [CrossRef]
- Pinti, J.; DeVries, T.; Norin, T.; Serra-Pompei, C.; Proud, R.; Siegel, D.A.; Kiørboe, T.; Petrik, C.M.; Andersen, K.H.; Brierley, A.S.; et al. Model Estimates of Metazoans’ Contributions to the Biological Carbon Pump. Biogeosciences 2023, 20, 997–1009. [Google Scholar] [CrossRef]
- Costalago, D.; Forster, I.; Nemcek, N.; Neville, C.; Perry, R.I.; Young, K.; Hunt, B.P.V. Seasonal and Spatial Dynamics of the Planktonic Trophic Biomarkers in the Strait of Georgia (Northeast Pacific) and Implications for Fish. Sci. Rep. 2020, 10, 8517. [Google Scholar] [CrossRef]
- Bateman, K.S.; Stentiford, G.D. A Taxonomic Review of Viruses Infecting Crustaceans with an Emphasis on Wild Hosts. J. Invertebr. Pathol. 2017, 147, 86–110. [Google Scholar] [CrossRef]
- Stentiford, G.D.; Neil, D.M.; Peeler, E.J.; Shields, J.D.; Small, H.J.; Flegel, T.W.; Vlak, J.M.; Jones, B.; Morado, F.; Moss, S.; et al. Disease Will Limit Future Food Supply from the Global Crustacean Fishery and Aquaculture Sectors. J. Invertebr. Pathol. 2012, 110, 141–157. [Google Scholar] [CrossRef]
- Small, H.J.; Pagenkopp, K.M. Reservoirs and Alternate Hosts for Pathogens of Commercially Important Crustaceans: A Review. J. Invertebr. Pathol. 2011, 106, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Winters, A.D. Detection of Viral Hemorrhagic Septicemia Virus (VHSV) from Diporeia spp. (Pontoporeiidae, Amphipoda) in the Laurentian Great Lakes, USA. Parasit Vectors 2011, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Diggles, B.K. Survey for WSSV Vectors in the Moreton Bay White Spot Biosecurity Area Survey for WSSV Vectors in the Moreton Bay White Spot Biosecurity Area; Deakin: Melbourne, Australia, 2020. [Google Scholar]
- Yanuhar, U.; Arfiati, D. Opportunity Plankton as Vector Transmission of Koi Herpes Virus Infection on Carp (Cyprinus Carpio). AACL Bioflux 2018, 11, 1869–1881. [Google Scholar]
- Overstreet, R.M.; Jovonovich, J.; Ma, H. Parasitic Crustaceans as Vectors of Viruses, with an Emphasis on Three Penaeid Viruses. Integr. Comp. Biol. 2009, 49, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Cano, F.; Sánchez-Paz, A.; Terán-Díaz, B.; Galván-Alvarez, D.; Encinas-García, T.; Enríquez-Espinoza, T.; Hernández-López, J. The Endemic Copepod Calanus Pacificus Californicus as a Potential Vector of White Spot Syndrome Virus. J. Aquat. Anim. Health 2014, 26, 113–117. [Google Scholar] [CrossRef]
- Jakob, E.; Barker, D.E.; Garver, K.A. Vector Potential of the Salmon Louse Lepeophtheirus Salmonis in the Transmission of Infectious Haematopoietic Necrosis Virus (IHNV). Dis. Aquat. Organ. 2011, 97, 155–165. [Google Scholar] [CrossRef]
- Zhang, J.S.; Dong, S.L.; Dong, Y.W.; Tian, X.L.; Hou, C.Q. Bioassay Evidence for the Transmission of WSSV by the Harpacticoid Copepod Nitocra sp. J. Invertebr. Pathol. 2008, 97, 33–39. [Google Scholar] [CrossRef]
- Porchas-Cornejo, M.A.; Álvarez-Ruiz, P.; Álvarez-Tello, F.J.; Martínez-Porchas, M.; Martínez-Córdova, L.R.; López-Martínez, J.; García-Morales, R. Detection of the White Spot Syndrome Virus in Zooplankton Samples Collected off the Coast of Sonora, Mexico. Aquac. Res. 2018, 49, 48–56. [Google Scholar] [CrossRef]
- Priyangha, S.J.; Gopalakrishnan, A.; Muhil Vannan, S.; Gunasekaran, T.; Somasundaram, S.T. First Report of Pedunculate Barnacle (Octolasmis neptuni), as Potential Asymptomatic Carrier of White Spot Syndrome Virus (WSSV). Comp. Clin. Path 2020, 29, 631–638. [Google Scholar] [CrossRef]
- Ng, T.H.; Chiang, Y.A.; Yeh, Y.C.; Wang, H.C. Review of Dscam-Mediated Immunity in Shrimp and Other Arthropods. Dev. Comp. Immunol. 2014, 46, 129–138. [Google Scholar] [CrossRef]
- Kulkarni, A.; Krishnan, S.; Anand, D.; Kokkattunivarthil Uthaman, S.; Otta, S.K.; Karunasagar, I.; Kooloth Valappil, R. Immune Responses and Immunoprotection in Crustaceans with Special Reference to Shrimp. Rev. Aquac. 2021, 13, 431–459. [Google Scholar] [CrossRef]
- Tetreau, G.; Dhinaut, J.; Gourbal, B.; Moret, Y. Trans-Generational Immune Priming in Invertebrates: Current Knowledge and Future Prospects. Front. Immunol. 2019, 10, 1938. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.F.J.; Redman, R.M.; Pantoja, C.R.; Le Groumellec, M.; Duraisamy, P.; Lightner, D.V. Identification of an Iridovirus in Acetes Erythraeus (Sergestidae) and the Development of in Situ Hybridization and PCR Method for Its Detection. J. Invertebr. Pathol. 2007, 96, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; He, J.; Li, C. Decapod Iridescent Virus 1: An Emerging Viral Pathogen in Aquaculture. Rev. Aquac. 2022, 14, 1779–1789. [Google Scholar] [CrossRef]
- Roennfeldt, R.-L. Iridoviruses of Copepods: Their Identification, Estuarine Ecology and Host Histopathology. Ph.D. Thesis, Deakin University, Warrnambool, Australia, 2013. [Google Scholar]
- Toenshoff, E.R.; Fields, P.D.; Bourgeois, Y.X.; Ebert, D. The End of a 60-Year Riddle: Identification and Genomic Characterization of an Iridovirus, the Causative Agent of White Fat Cell Disease in Zooplankton. G3 Genes Genomes Genet. 2018, 8, 1259–1272. [Google Scholar] [CrossRef]
- Bistolas, K.S.I.; Jackson, E.W.; Watkins, J.M.; Rudstam, L.G.; Hewson, I. Distribution of Circular Single-Stranded DNA Viruses Associated with Benthic Amphipods of Genus Diporeia in the Laurentian Great Lakes. Freshw. Biol. 2017, 62, 1220–1231. [Google Scholar] [CrossRef]
- Chang, T.; Hirai, J.; Hunt, B.P.v.; Suttle, C.A. Arthropods and the Evolution of RNA Viruses. BioRxiv 2021. [Google Scholar] [CrossRef]
- Bateman, K.S. Viruses Infecting Crustaceans. In Studies in Viral Ecology; Hurst, C.J., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 305–340. [Google Scholar]
- Kibenge, F.S. Emerging Viruses in Aquaculture. Curr. Opin. Virol. 2019, 34, 97–103. [Google Scholar] [CrossRef]
- Elliott, D.T.; Tang, K.W. Spatial and Temporal Distributions of Live and Dead Copepods in the Lower Chesapeake Bay (Virginia, USA). Estuaries Coasts 2011, 34, 1039–1048. [Google Scholar] [CrossRef]
- McConaugha, J.R. Decapod Larvae: Dispersal, Mortality and Ecology. A Working Hypothesis. Am. Zool. 1992, 32, 512–523. [Google Scholar] [CrossRef]
- Bojko, J.; Jennings, L.A.; Behringer, D.C. A Novel Positive Single-Stranded RNA Virus from the Crustacean Parasite, Probopyrinella Latreuticola (Peracarida: Isopoda: Bopyridae). J. Invertebr. Pathol. 2020, 177, 107494. [Google Scholar] [CrossRef] [PubMed]
- Kuris, A.M.; Poinar, G.O.; Hess, R.; Morris, T.J. Virus Particles in an Internal Parasite, Portunion Conformis (Crustacea: Isopoda: Entoniscidae), and Its Marine Crab Host, Hemigrapsus Oregonensis. J. Invertebr. Pathol. 1979, 34, 26–31. [Google Scholar] [CrossRef]
- Dunlap, D.S.; Ng, T.F.F.; Rosario, K.; Barbosa, J.G.; Greco, A.M.; Breitbart, M.; Hewson, I. Molecular and Microscopic Evidence of Viruses in Marine Copepods. Proc. Natl. Acad. Sci. USA 2013, 110, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Eaglesham, J.B.; Hewson, I. Widespread Detection of Circular Replication Initiator Protein (Rep)-Encoding SsDNA Viral Genomes in Estuarine, Coastal and Open Ocean Net Plankton. Mar. Ecol. Prog. Ser. 2013, 494, 65–72. [Google Scholar] [CrossRef]
- Hewson, I.; Eaglesham, J.B.; Höök, T.O.; LaBarre, B.A.; Sepúlveda, M.S.; Thompson, P.D.; Watkins, J.M.; Rudstam, L.G. Investigation of Viruses in Diporeia spp. from the Laurentian Great Lakes and Owasco Lake as Potential Stressors of Declining Populations. J. Great Lakes Res. 2013, 39, 499–506. [Google Scholar] [CrossRef]
- Desrina; Prayitno, S.B.; Verdegem, M.C.J.; Verreth, J.A.J.; Vlak, J.M. White Spot Syndrome Virus Host Range and Impact on Transmission. Rev. Aquac. 2022, 14, 1843–1860. [Google Scholar] [CrossRef]
- Zhang, J.S.; Dong, S.L.; Dong, Y.W.; Tian, X.L.; Cao, Y.C.; Li, Z.J.; Yan, D.C. Assessment of the Role of Brine Shrimp Artemia in White Spot Syndrome Virus (WSSV) Transmission. Vet. Res. Commun. 2010, 34, 25–32. [Google Scholar] [CrossRef]
- Yan, D.-C.; Dong, S.-L.; Huang, J.; Yu, X.-M.; Feng, M.-Y.; Liu, X.-Y. White Spot Syndrome Virus (WSSV) Detected by PCR in Rotifers and Rotifer Resting Eggs from Shrimp Pond Sediments. Dis. Aquat. Organ. 2004, 59, 69–73. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, S.; Tian, X.; Dong, Y.; Liu, X.; Yan, D. Virus-Phytoplankton Adhesion: A New WSSV Transmission Route to Zooplankton. Acta Oceanol. Sin. 2007, 26, 109–115. [Google Scholar]
- Lo, C.-F.; Ho, C.-H.; Peng, S.-E.; Chen, C.-H.; Hsu, H.-C.; Chiu, Y.-L.; Chang, C.-F.; Liu, K.-F.; Su, M.-S.; Wang, C.-H.; et al. White Spot Syndrome Baculovirus (WSBV) Detected in Cultured and Captured Shrimp, Crabs and Other Arthropods. Dis. Aquat. Organ. 1996, 27, 215–225. [Google Scholar] [CrossRef]
- Xu, T.; Shan, X.; Li, Y.; Yang, T.; Teng, G.; Wu, Q.; Wang, C.; Tang, K.F.J.; Zhang, Q.; Jin, X. White Spot Syndrome Virus (WSSV) Prevalence in Wild Crustaceans in the Bohai Sea. Aquaculture 2021, 542, 736810. [Google Scholar] [CrossRef]
- Johnson, P.T. Viral Diseases of Marine Invertebrates. Helgol. Meeresunters 1984, 37, 65–98. [Google Scholar] [CrossRef]
- Takahashi, H.; Fukuhara, T.; Kitazawa, H.; Kormelink, R. Virus Latency and the Impact on Plants. Front. Microbiol. 2019, 10, 2764. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.S.; Li, G.; Gulbudak, H.; Cortez, M.H.; Whitaker, R.J. Viral Invasion Fitness across a Continuum from Lysis to Latency. Virus Evol. 2019, 5, vez006. [Google Scholar] [CrossRef]
- Hick, P.; Becker, J.; Whittington, R. Iridoviruses of Fish. In Aquaculture Virology; Kibenge, F., Godoy, M., Eds.; Academic Press: London, UK, 2016; pp. 127–152. ISBN 9780128017548. [Google Scholar]
- Leibovitz, L.; Koulish, S. A Viral Disease of the Ivory Barnacle, Balanus Eburneus, Gould (Crustacea, Cirripedia). Biol. Bull. 1989, 176, 301–307. [Google Scholar] [CrossRef]
- Canuti, M.; Large, G.; Verhoeven, J.T.P.; Dufour, S.C. A Novel Iridovirus Discovered in Deep-Sea Carnivorous Sponges. Viruses 2022, 14, 1595. [Google Scholar] [CrossRef]
- Engelstädter, J.; Fortuna, N.Z. The Dynamics of Preferential Host Switching: Host Phylogeny as a Key Predictor of Parasite Distribution. Evolution 2019, 73, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, M.J.; Weiller, G.F. Evidence That a Plant Virus Switched Hosts to Infect a Vertebrate and Then Recombined with a Vertebrate-Infecting Virus. Proc. Natl. Acad. Sci. USA 1999, 96, 8022–8027. [Google Scholar] [CrossRef]
- Hirst, A.G.; Kiørbe, T. Mortality of Marine Planktonic Copepods: Global Rates and Patterns. Mar. Ecol. Prog. Ser. 2002, 230, 195–209. [Google Scholar] [CrossRef]
- Daase, M.; Søreide, J.E. Seasonal Variability in Non-Consumptive Mortality of Arctic Zooplankton. J. Plankton Res. 2021, 43, 565–585. [Google Scholar] [CrossRef]
- Maud, J.L.; Hirst, A.G.; Atkinson, A.; Lindeque, P.K.; McEvoy, A.J. Mortality of Calanus Helgolandicus: Sources, Differences between the Sexes and Consumptive and Nonconsumptive Processes. Limnol. Oceanogr. 2018, 63, 1741–1761. [Google Scholar] [CrossRef]
- Daase, M.; Varpe, O.; Falk-Petersen, S. Non-Consumptive Mortality in Copepods: Occurrence of Calanus spp. Carcasses in the Arctic Ocean during Winter. J. Plankton Res. 2014, 36, 129–144. [Google Scholar] [CrossRef]
- Elliott, D.T.; Tang, K.W. Influence of Carcass Abundance on Estimates of Mortality and Assessment of Population Dynamics in Acartia Tonsa. Mar. Ecol. Prog. Ser. 2011, 427, 1–12. [Google Scholar] [CrossRef]
- Giesecke, R.; Vallejos, T.; Sanchez, M.; Teiguiel, K. Plankton Dynamics and Zooplankton Carcasses in a Mid-Latitude Estuary and Their Contributions to the Local Particulate Organic Carbon Pool. Cont. Shelf Res. 2017, 132, 58–68. [Google Scholar] [CrossRef]
- Terazaki, M.; Wada, M. Occurrence of Large Numbers of Carcasses of the Large, Grazing Copepod Calanus Cristatus from the Japan Sea. Mar. Biol. 1988, 97, 177–183. [Google Scholar] [CrossRef]
- Tsuda, A. Starvation Tolerance of a Planktonic Marine Copepods. J. Exp. Mar. Biol. Ecol. 1994, 181, 81–89. [Google Scholar] [CrossRef]
- Roman, M.R.; Brandt, S.B.; Houde, E.D.; Pierson, J.J. Interactive Effects of Hypoxia and Temperature on Coastal Pelagic Zooplankton and Fish. Front. Mar. Sci. 2019, 6, 139. [Google Scholar] [CrossRef]
- Byers, J.E. Marine Parasites and Disease in the Era of Global Climate Change. Ann. Rev. Mar. Sci. 2020, 13, 397–420. [Google Scholar] [CrossRef]
- Harvell, C.D.; Mitchell, C.E.; Ward, J.R.; Altizer, S.; Dobson, A.P.; Ostfeld, R.S.; Samuel, M.D. Climate Warming and Disease Risks for Terrestrial and Marine Biota. Sci. Rev. Ecol. 2002, 296, 2158–2162. [Google Scholar] [CrossRef]
- Ratnarajah, L.; Abu-Alhaija, R.; Atkinson, A.; Batten, S.; Bax, N.J.; Bernard, K.S.; Canonico, G.; Cornils, A.; Everett, J.D.; Grigoratou, M.; et al. Monitoring and Modelling Marine Zooplankton in a Changing Climate. Nat. Commun. 2023, 14, 564. [Google Scholar] [CrossRef]
- Cripps, G.; Lindeque, P.; Flynn, K.J. Have We Been Underestimating the Effects of Ocean Acidification in Zooplankton? Glob. Chang. Biol. 2014, 20, 3377–3385. [Google Scholar] [CrossRef] [PubMed]
- McLaskey, A.K.; McElhany, P.; Busch, D.S.; Maher, M.; Winans, A.K.; Keister, J.E. Early Life Stages of Calanus Pacificus Are Neither Exposed nor Sensitive to Low PH Waters. J. Plankton Res. 2019, 41, 893–896. [Google Scholar] [CrossRef]
- Bednaršek, N.; Feely, R.A.; Beck, M.W.; Alin, S.R.; Siedlecki, S.A.; Calosi, P.; Norton, E.L.; Saenger, C.; Štrus, J.; Greeley, D.; et al. Exoskeleton Dissolution with Mechanoreceptor Damage in Larval Dungeness Crab Related to Severity of Present-Day Ocean Acidification Vertical Gradients. Sci. Total Environ. 2020, 716, 136610. [Google Scholar] [CrossRef] [PubMed]
- McLaskey, A.K.; Keister, J.E.; McElhany, P.; Olson, M.B.; Busch, D.S.; Maher, M.; Winans, A.K. Development of Euphausia pacifica (Krill) Larvae Is Impaired under pCO2 Levels Currently Observed in the Northeast Pacific. Mar. Ecol. Prog. Ser. 2016, 555, 65–78. [Google Scholar] [CrossRef]
- Wyeth, A.C.; Grünbaum, D.; Keister, J.E. Effects of Hypoxia and Acidification on Calanus Pacificus: Behavioral Changes in Response to Stressful Environments. Mar. Ecol. Prog. Ser. 2022, 697, 15–29. [Google Scholar] [CrossRef]
- Keil, K.E.; Klinger, T.; Keister, J.E.; McLaskey, A.K. Comparative Sensitivities of Zooplankton to Ocean Acidification Conditions in Experimental and Natural Settings. Front. Mar. Sci. 2021, 86, 613778. [Google Scholar] [CrossRef]
- Stalder, L.C.; Marcus, N.H. Zooplankton Responses to Hypoxia: Behavioral Patterns and Survival of Three Species of Calanoid Copepods. Mar. Biol. 1997, 127, 599–607. [Google Scholar] [CrossRef]
- Roman, M.R.; Gauzens, A.L.; Rhinehart, W.K.; White, J.R. Effects of Low Oxygen Waters on Chesapeake Bay Zooplankton. Limnol. Oceanogr. 1993, 38, 1603–1614. [Google Scholar] [CrossRef]
- Marcus, N.H. Zooplankton: Responses to and Consequences of Hypoxia. In Coastal Hypoxia: Consequences for Living Resources and Ecosystems; Rabalais, N.N., Turner, E.R., Eds.; American Geophysical Union: Washington, DC, USA, 2001; pp. 49–60. [Google Scholar]
- Richmond, C.; Marcus, N.H.; Sedlacek, C.; Miller, G.A.; Oppert, C. Hypoxia and Seasonal Temperature: Short-Term Effects and Long-Term Implications for Acartia Tonsa Dana. J. Exp. Mar. Biol. Ecol. 2006, 328, 177–196. [Google Scholar] [CrossRef]
- Zajączkowski, M.J.; Legeżyńska, J. Estimation of Zooplankton Mortality Caused by an Arctic Glacier Outflow Arctic Plankton Mortality Glaciers. Oceanologia 2001, 43, 341–351. [Google Scholar]
- Lance, J. The Salinity Tolerance Of Some Estuarine Planktonic Copepods. Limnol. Oceanogr. 1963, 8, 440–449. [Google Scholar] [CrossRef]
- Dutz, J.; Christensen, A.M. Broad Plasticity in the Salinity Tolerance of a Marine Copepod Species, Acartia Longiremis, in the Baltic Sea. J. Plankton Res. 2018, 40, 342–355. [Google Scholar] [CrossRef]
- Koski, M.; Klein Breteler, W. Influence of Diet on Copepod Survival in the Laboratory. Mar. Ecol. Prog. Ser. 2003, 264, 73–82. [Google Scholar] [CrossRef]
- Sampei, M.; Sasaki, H.; Forest, A.; Fortier, L. A Substantial Export Flux of Particulate Organic Carbon Linked to Sinking Dead Copepods during Winter 2007–2008 in the Amundsen Gulf (Southeastern Beaufort Sea, Arctic Ocean). Limnol. Oceanogr. 2012, 57, 90–96. [Google Scholar] [CrossRef]
- Ceballos, S.; Kiørboe, T. Senescence and Sexual Selection in a Pelagic Copepod. PLoS ONE 2011, 6, e18870. [Google Scholar] [CrossRef]
- Mckean, K.A.; Nunney, L. Increased Sexual Activity Reduces Male Immune Function in Drosophila Melanogaster. Proc. Natl. Acad. Sci. USA 2001, 98, 7904–7909. [Google Scholar] [CrossRef]
- Wedekind, C.; Jakobsen, P.J. Male-Biased Susceptibility to Helminth Infection: An Experimental Test with a Copepod. Oikos 1998, 81, 458–462. [Google Scholar] [CrossRef]
- Avery, D.E.; Altland, K.K.; Dam, H.G. Sex-Related Differential Mortality of a Marine Copepod Exposed to a Toxic Dinoflagellate. Limnol. Oceanogr. 2008, 53, 2627–2635. [Google Scholar] [CrossRef]
- Kiørboe, T.; Ceballos, S.; Thygesen, U.H. Interrelations between Senescence, Life-History Traits, and Behavior in Planktonic Copepods. Ecology 2015, 96, 2225–2235. [Google Scholar] [CrossRef]
- Ianora, A.; Miralto, A.; Poulet, S.; Carotenuto, Y.; Buttino, I.; Romano, G.; Casotti, R.; Pohnert, G.; Wichard, T.; Colucci-D’Amato, L.; et al. Aldehyde Suppression of Copepod Recruitment in Blooms of a Ubiquitous Planktonic Diatom. Nature 2004, 429, 403–407. [Google Scholar] [CrossRef]
- Chakraborty, S.; Moorthi, S.D.; Karnatak, R.; Feudel, U. Irregular Harmful Algal Blooms Triggered by Feedback between Toxin Production and Zooplankton Feeding. Ecol. Modell. 2022, 473, 110120. [Google Scholar] [CrossRef]
- Fulton, R.S.; Paerl, H.W. Toxic and Inhibitory Effects of the Blue-Green Alga Microcystis Aeruginosa on Herbivorous Zooplankton. J. Plankton Res. 1987, 9, 837–855. [Google Scholar] [CrossRef]
- Delgado, M.; Alcaraz, M. Interactions between Red Tide Microalgae and Herbivorous Zooplankton: The Noxious Effects of Gyrodinium corsicum (Dinophyceae) on Acartia grani (Copepoda: Calanoida). J. Plankton Res. 1999, 21, 2361–2371. [Google Scholar] [CrossRef]
- Savage, R.-L.; Maud, J.L.; Kellogg, C.T.E.; Hunt, B.P.V.; Tai, V. Symbiont Diversity in the Eukaryotic Microbiomes of Marine Crustacean Zooplankton. J. Plankton Res. 2023, fbad003. [Google Scholar] [CrossRef]
- Kimmerer, W.J.; Mckinnon, A.D. High Mortality in a Copepod Population Caused by a Parasitic Dinoflagellate. Mar. Biol. 1990, 107, 449–452. [Google Scholar] [CrossRef]
- Zamora-Terol, S.; Novotny, A.; Winder, M. Molecular Evidence of Host-Parasite Interactions between Zooplankton and Syndiniales. Aquat. Ecol. 2021, 55, 125–134. [Google Scholar] [CrossRef]
- Gómez-Gutiérrez, J.; Peterson, W.T.; de Robertis, A.; Brodeur, R.D. Mass Mortality of Krill Caused by Parasitoid Ciliates. Science 2003, 301, 339. [Google Scholar] [CrossRef]
- Ohtsuka, S.; Hora, M.; Suzaki, T.; Arikawa, M.; Omura, G.; Yamada, K. Morphology and Host-Specificity of the Apostomeciliate Vampyrophrya pelagica infecting Pelagiccopepods in the Seto Inland Sea, Japan. Mar. Ecol. Prog. Ser. 2004, 282, 129–142. [Google Scholar] [CrossRef]
- Burns, C.W. Fungal Parasitism in a Copepod Population: The Effects of Aphanomyces on the Population Dynamics of Boeckella Dilatata Sars. J. Plankton Res. 1985, 7, 201–205. [Google Scholar] [CrossRef]
- Bass, D.; Rueckert, S.; Stern, R.; Cleary, A.C.; Taylor, J.D.; Ward, G.M.; Huys, R. Parasites, Pathogens, and Other Symbionts of Copepods. Trends Parasitol. 2021, 37, 875–889. [Google Scholar] [CrossRef]
- Ohtsuka, S.; Hanamura, Y.; Harada, S.; Michitaka, S. Recent Advances in Studies of Parasites on Mysid Crustaceans. Bull. Plankton Soc. Jpn. 2006, 53, 37–44. [Google Scholar]
- Johnson, P.T.J.; Stanton, D.E.; Preu, E.R.; Forshay, K.J.; Carpenter, S.R. Dining on Disease: How Interactions between Infection and Environment Affect Predation Risk. Ecology 2006, 87, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.A.; Housley, J.M.; Penczykowski, R.M.; Cáceres, C.E.; Hall, S.R. Unhealthy Herds: Indirect Effects of Predators Enhance Two Drivers of Disease Spread. Funct. Ecol. 2011, 25, 945–953. [Google Scholar] [CrossRef]
- Grey, J.; Jones, R. Seasonal Changes in the Importance of the Source of Organic Matter to the Diet of Zooplankton in Loch Ness, as Indicated by Stable Isotope Analysis. Limnol. Oceanogr. 2001, 46, 505–513. [Google Scholar] [CrossRef]
- Robinson, C.L.K. The Consumption of Euphausiids by the Pelagic Fish Community off Southwestern Vancouver Island, British Columbia. J. Plankton Res. 2000, 22, 1649–1662. [Google Scholar] [CrossRef]
- Duffy, J.; Stachowicz, J. Why Biodiversity Is Important to Oceanography: Potential Roles of Genetic, Species and Trophic Diversity in Pelagic Ecosystem Processes. Mar. Ecol. Prog. Ser. 2006, 311, 179–189. [Google Scholar] [CrossRef]
- Sampei, M.; Sasaki, H.; Hattori, H.; Forest, A.; Fortier, L. Significant Contribution of Passively Sinking Copepods to Downward Export Flux in Arctic Waters. Limnol. Oceanogr. 2009, 54, 1894–1900. [Google Scholar] [CrossRef]
- Frangoulis, C.; Skliris, N.; Lepoint, G.; Elkalay, K.; Goffart, A.; Pinnegar, J.K.; Hecq, J.H. Importance of Copepod Carcasses versus Faecal Pellets in the Upper Water Column of an Oligotrophic Area. Estuar. Coast Shelf Sci. 2011, 92, 456–463. [Google Scholar] [CrossRef]
- Halfter, S.; Cavan, E.L.; Butterworth, P.; Swadling, K.M.; Boyd, P.W. “Sinking Dead”—How Zooplankton Carcasses Contribute to Particulate Organic Carbon Flux in the Subantarctic Southern Ocean. Limnol. Oceanogr. 2022, 67, 13–25. [Google Scholar] [CrossRef]
- Wigington, C.H.; Sonderegger, D.; Brussaard, C.P.D.; Buchan, A.; Finke, J.F.; Fuhrman, J.A.; Lennon, J.T.; Middelboe, M.; Suttle, C.A.; Stock, C.; et al. Re-Examination of the Relationship between Marine Virus and Microbial Cell Abundances. Nat. Microbiol. 2016, 1, 15024. [Google Scholar] [CrossRef]
- Coutinho, F.H.; Silveira, C.B.; Gregoracci, G.B.; Thompson, C.C.; Edwards, R.A.; Brussaard, C.P.D.; Dutilh, B.E.; Thompson, F.L. Marine Viruses Discovered via Metagenomics Shed Light on Viral Strategies throughout the Oceans. Nat. Commun. 2017, 8, 15955. [Google Scholar] [CrossRef] [PubMed]
- Short, S.M. The Ecology of Viruses That Infect Eukaryotic Algae. Environ. Microbiol. 2012, 14, 2253–2271. [Google Scholar] [CrossRef] [PubMed]
- Stentiford, G.D. Diseases in Aquatic Crustaceans: Problems and Solutions for Global Food Security. J. Invertebr. Pathol. 2012, 110, 139. [Google Scholar] [CrossRef]
- Frada, M.J.; Vardi, A. Algal Viruses Hitchhiking on Zooplankton across Phytoplankton Blooms. Commun. Integr. Biol. 2015, 8, e1029210. [Google Scholar] [CrossRef] [PubMed]
- Frada, M.J.; Schatz, D.; Farstey, V.; Ossolinski, J.E.; Sabanay, H.; Ben-Dor, S.; Koren, I.; Vardi, A. Zooplankton May Serve as Transmission Vectors for Viruses Infecting Algal Blooms in the Ocean. Curr. Biol. 2014, 24, 2592–2597. [Google Scholar] [CrossRef]
- Kitamura, S.; Kamata, S.; Nakano, S.; Suzuki, S. Detection of Marine Birnavirus Genome in Zooplankton Collected from the Uwa Sea, Japan. Dis. Aquat. Organ. 2003, 54, 69–72. [Google Scholar] [CrossRef]
- Ismail, N.S.; Olive, M.; Fernandez-Cassi, X.; Bachmann, V.; Kohn, T. Viral Transfer and Inactivation through Zooplankton Trophic Interactions. Environ. Sci. Technol. 2020, 54, 9418–9426. [Google Scholar] [CrossRef]
- Joseph, T.C.; James, R.; Anbu Rajan, L.; Surendran, P.K.; Lalitha, K.V. Occurrence of Viral Pathogens in Penaeus Monodon Post-Larvae from Aquaculture Hatcheries. Data Brief 2015, 4, 170–176. [Google Scholar] [CrossRef]
- Mishra, S.; Das, R.; Swain, P. Status of Fish Diseases in Aquaculture and Assessment of Economic Loss Due to Disease; Nagaraja, P.R., Pandey, B., Pandey, P., Joshi, B., Eds.; Today and Tomorrow’s Printers and Publishers: New Delhi, India, 2018; Volume 1. [Google Scholar]
- Lafferty, K.D.; Harvell, C.D.; Conrad, J.M.; Friedman, C.S.; Kent, M.L.; Kuris, A.M.; Powell, E.N.; Rondeau, D.; Saksida, S.M. Infectious Diseases Affect Marine Fisheries and Aquaculture Economics. Ann. Rev. Mar. Sci. 2015, 7, 471–496. [Google Scholar] [CrossRef]
- Shea, D. The Influence of Aquaculture on Marine Microbiota and Pathogen Communities in a British Columbia Coastal Ecosystem. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 2022. [Google Scholar]
- Teffer, A.K.; Carr, J.; Tabata, A.; Schulze, A.; Bradbury, I.; Deschamps, D.; Gillis, C.-A.; Brunsdon, E.B.; Mordecai, G.; Miller, K.M. A Molecular Assessment of Infectious Agents Carried by Atlantic Salmon at Sea and in Three Eastern Canadian Rivers, Including Aquaculture Escapees and North American and European Origin Wild Stocks. FACETS 2020, 5, 234–263. [Google Scholar] [CrossRef]
- Bateman, A.W.; Teffer, A.K.; Bass, A.; Ming, T.; Kaukinen, K.; Hunt, B.P.V.; Krkošek, M.; Miller, K.M. Atlantic Salmon Farms Are a Likely Source of Tenacibaculum Maritimum Infection in Migratory Fraser River Sockeye Salmon. Can. J. Fish. Aquat. Sci. 2022, 79, 1225–1240. [Google Scholar] [CrossRef]
- Erazo, N.G.; Bowman, J.S. Sensitivity of the Mangrove-Estuarine Microbial Community to Aquaculture Effluent. iScience 2021, 24, 102204. [Google Scholar] [CrossRef] [PubMed]
- Primavera, J.H. Overcoming the Impacts of Aquaculture on the Coastal Zone. Ocean Coast Manag. 2006, 49, 531–545. [Google Scholar] [CrossRef]
- Mordecai, G.J.; Miller, K.M.; Bass, A.L.; Bateman, A.W.; Teffer, A.K.; Caleta, J.M.; DiCicco, E.; Schulze, A.D.; Kaukinen, K.H.; Li, S.; et al. Aquaculture Mediates Global Transmission of a Viral Pathogen to Wild Salmon. Sci. Adv. 2021, 7, eabe2592. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Martínez, J.G.; Aguirre-Guzmán, G.; Mejía-Ruíz, H. White Spot Syndrome Virus in Cultured Shrimp: A Review. Aquac. Res. 2007, 38, 1339–1354. [Google Scholar] [CrossRef]
- Bir, J.; Ray, S.; Howlader, P.; Sultana, S.; Ibrahim Khalil, S.M.; Reza Banu, G. A Critical Review on White Spot Syndrome Virus (WSSV): A Potential Threat to Shrimp Farming in Bangladesh. Int. J. Microbiol. 2017, 6, 39–48. [Google Scholar]
- Moser, J.R.; Álvarez, D.A.G.; Cano, F.M.; Garcia, T.E.; Molina, D.E.C.; Clark, G.P.; Marques, M.R.F.; Barajas, F.J.M.; López, J.H. Water Temperature Influences Viral Load and Detection of White Spot Syndrome Virus (WSSV) in Litopenaeus Vannamei and Wild Crustaceans. Aquaculture 2012, 326–329, 9–14. [Google Scholar] [CrossRef]
- Oidtmann, B.; Dixon, P.; Way, K.; Joiner, C.; Bayley, A.E. Risk of Waterborne Virus Spread–Review of Survival of Relevant Fish and Crustacean Viruses in the Aquatic Environment and Implications for Control Measures. Rev. Aquac. 2018, 10, 641–669. [Google Scholar] [CrossRef]
- Kanchanaphum, P.; Wongteerasupaya, C.; Sitidilokratana, N.; Boonsaeng, V.; Panyim, S.; Tassanakajon, A.; Withyachumnarnkal, B.; Flege, T. Experimental Transmission of White Spot Syndrome Virus (WSSV) from Crabs to Shrimp Penaeus Monodon. Dis. Aquat. Organ. 1998, 34, 1–7. [Google Scholar] [CrossRef]
- Bain, M.B.; Cornwell, E.R.; Hope, K.M.; Eckerlin, G.E.; Casey, R.N.; Groocock, G.H.; Getchell, R.G.; Bowser, P.R.; Winton, J.R.; Batts, W.N.; et al. Distribution of an Invasive Aquatic Pathogen (Viral Hemorrhagic Septicemia Virus) in the Great Lakes and Its Relationship to Shipping. PLoS ONE 2010, 5, e10156. [Google Scholar] [CrossRef]
- Garver, K.A.; Traxler, G.S.; Hawley, L.M.; Richard, J.; Ross, J.P.; Lovy, J. Molecular Epidemiology of Viral Haemorrhagic Septicaemia Virus (VHSV) in British Columbia, Canada, Reveals Transmission from Wild to Farmed Fish. Dis. Aquat. Organ. 2013, 104, 93–104. [Google Scholar] [CrossRef]
- Oelckers, K.; Vike, S.; Duesund, H.; Gonzalez, J.; Wadsworth, S.; Nylund, A. Caligus Rogercresseyi as a Potential Vector for Transmission of Infectious Salmon Anaemia (ISA) Virus in Chile. Aquaculture 2014, 420–421, 126–132. [Google Scholar] [CrossRef]
- DiBacco, C.; Humphrey, D.B.; Nasmith, L.E.; Levings, C.D. Ballast Water Transport of Non-Indigenous Zooplankton to Canadian Ports. ICES J. Mar. Sci. 2012, 69, 483–491. [Google Scholar] [CrossRef]
- Briski, E.; Wiley, C.J.; Bailey, S.A. Role of Domestic Shipping in the Introduction or Secondary Spread of Nonindigenous Species: Biological Invasions within the Laurentian Great Lakes. J. Appl. Ecol. 2012, 49, 1124–1130. [Google Scholar] [CrossRef]
- Chan, F.T.; Briski, E.; Bailey, S.A.; MacIsaac, H.J. Richness-Abundance Relationships for Zooplankton in Ballast Water: Temperate versus Arctic Comparisons. ICES J. Mar. Sci. 2014, 71, 1876–1884. [Google Scholar] [CrossRef]
- Choi, K.H.; Kimmerer, W.; Smith, G.; Ruiz, G.M.; Lion, K. Post-Exchange Zooplankton in Ballast Water of Ships Entering the San Francisco Estuary. J. Plankton Res. 2005, 27, 707–714. [Google Scholar] [CrossRef]
- Drake, L.A.; Doblin, M.A.; Dobbs, F.C. Potential Microbial Bioinvasions via Ships’ Ballast Water, Sediment, and Biofilm. Mar. Pollut. Bull. 2007, 55, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, D.B. Characterising Ballast Water as a Vector for Nonindigenous Zooplankton Transport. Master’s Thesis, Unversity of British Columbia, Vancouver, BC, Canada, 2008. [Google Scholar]
- Gollasch, S.; Rosenthal, H.; Botnen, H.; Hamer, J.; Laing, I.; Leppäkoski, E.; Macdonald, E.; Minchin, D.; Nauke, M.; Olenin, S.; et al. Fluctuations of Zooplankton Taxa in Ballast Water during Short-Term and Long-Term Ocean-Going Voyages. Int. Rev. Hydrobiol. 2000, 85, 597–608. [Google Scholar] [CrossRef]
- Winder, M.; Jassby, A.D. Shifts in Zooplankton Community Structure: Implications for Food Web Processes in the Upper San Francisco Estuary. Estuaries Coasts 2011, 34, 675–690. [Google Scholar] [CrossRef]
- Carney, K.J.; Minton, M.S.; Holzer, K.K.; Miller, A.W.; McCann, L.D.; Ruiz, G.M. Evaluating the Combined Effects of Ballast Water Management and Trade Dynamics on Transfers of Marine Organisms by Ships. PLoS ONE 2017, 12, e0172468. [Google Scholar] [CrossRef]
- Bradie, J.N.; Drake, D.A.R.; Ogilvie, D.; Casas-Monroy, O.; Bailey, S.A. Ballast Water Exchange plus Treatment Lowers Species Invasion Rate in Freshwater Ecosystems. Environ. Sci. Technol. 2021, 55, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.A.; Brydges, T.; Casas-Monroy, O.; Kydd, J.; Linley, R.D.; Rozon, R.M.; Darling, J.A. First Evaluation of Ballast Water Management Systems on Operational Ships for Minimizing Introductions of Nonindigenous Zooplankton. Mar. Pollut. Bull. 2022, 182, 113947. [Google Scholar] [CrossRef] [PubMed]
- Longhurst, A.R.; Harrison, W.G. Vertical Nitrogen Flux from the Oceanic Photic Zone by Diel Migrant Zooplankton and Nekton. Deep Sea Res. 1988, 35, 881–889. [Google Scholar] [CrossRef]
- Steinberg, D.K.; Steinberg, D.K.; Carlson, C.A.; Bates, N.R.; Goldthwait, S.A.; Madin, L.P.; Michaels, A.F. Zooplankton Vertical Migration and the Active Transport of Dissolved Organic and Inorganic Carbon in the Sargasso Sea. Deep Sea Res. 2000, 47, 137–158. [Google Scholar] [CrossRef]
- Cavan, E.L.; le Moigne, F.A.C.; Poulton, A.J.; Tarling, G.A.; Ward, P.; Daniels, C.J.; Fragoso, G.M.; Sanders, R.J. Attenuation of Particulate Organic Carbon Flux in the Scotia Sea, Southern Ocean, Is Controlled by Zooplankton Fecal Pellets. Geophys. Res. Lett. 2015, 42, 821–830. [Google Scholar] [CrossRef]
- Pinti, J.; Jónasdóttir, S.H.; Record, N.R.; Visser, A.W. The Global Contribution of Seasonally Migrating Copepods to the Biological Carbon Pump. Limnol. Oceanogr. 2023. [Google Scholar] [CrossRef]
- Visser, A.W.; Jónasdóttir, S.H. Lipids, Buoyancy and the Seasonal Vertical Migration of Calanus Finmarchicus. Fish. Oceanogr. 1999, 8, 100–106. [Google Scholar] [CrossRef]
- Park, J.I.; Kang, C.K.; Suh, H.L. Ontogenetic Diet Shift in the Euphausiid Euphausia pacifica Quantified Using Stable Isotope Analysis. Mar. Ecol. Prog. Ser. 2011, 429, 103–109. [Google Scholar] [CrossRef]
- Koonin, E.V.; Dolja, V.V.; Krupovic, M.; Varsani, A.; Wolf, Y.I.; Yutin, N.; Zerbini, F.M.; Kuhn, J.H. Global Organization and Proposed Megataxonomy of TheVirus World. Microbiol. Mol. Biol. Rev. 2020, 84, e00061-19. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roberts, A.J.; Suttle, C.A. Pathogens and Passengers: Roles for Crustacean Zooplankton Viruses in the Global Ocean. Microorganisms 2023, 11, 1054. https://doi.org/10.3390/microorganisms11041054
Roberts AJ, Suttle CA. Pathogens and Passengers: Roles for Crustacean Zooplankton Viruses in the Global Ocean. Microorganisms. 2023; 11(4):1054. https://doi.org/10.3390/microorganisms11041054
Chicago/Turabian StyleRoberts, Alastair J., and Curtis A. Suttle. 2023. "Pathogens and Passengers: Roles for Crustacean Zooplankton Viruses in the Global Ocean" Microorganisms 11, no. 4: 1054. https://doi.org/10.3390/microorganisms11041054
APA StyleRoberts, A. J., & Suttle, C. A. (2023). Pathogens and Passengers: Roles for Crustacean Zooplankton Viruses in the Global Ocean. Microorganisms, 11(4), 1054. https://doi.org/10.3390/microorganisms11041054



_Brussaard.png)



