Role of the Spore Coat Proteins CotA and CotB, and the Spore Surface Protein CDIF630_02480, on the Surface Distribution of Exosporium Proteins in Clostridioides difficile 630 Spores
Abstract
1. Introduction
2. Material and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Whole-Genome Sequencing and Mutant Confirmation
2.3. Plasmid Conjugation
2.4. Spore Purification
2.5. Spore Immunofluorescence
2.6. Transmission Electron Microscopy
2.7. Statistical Analysis
3. Results
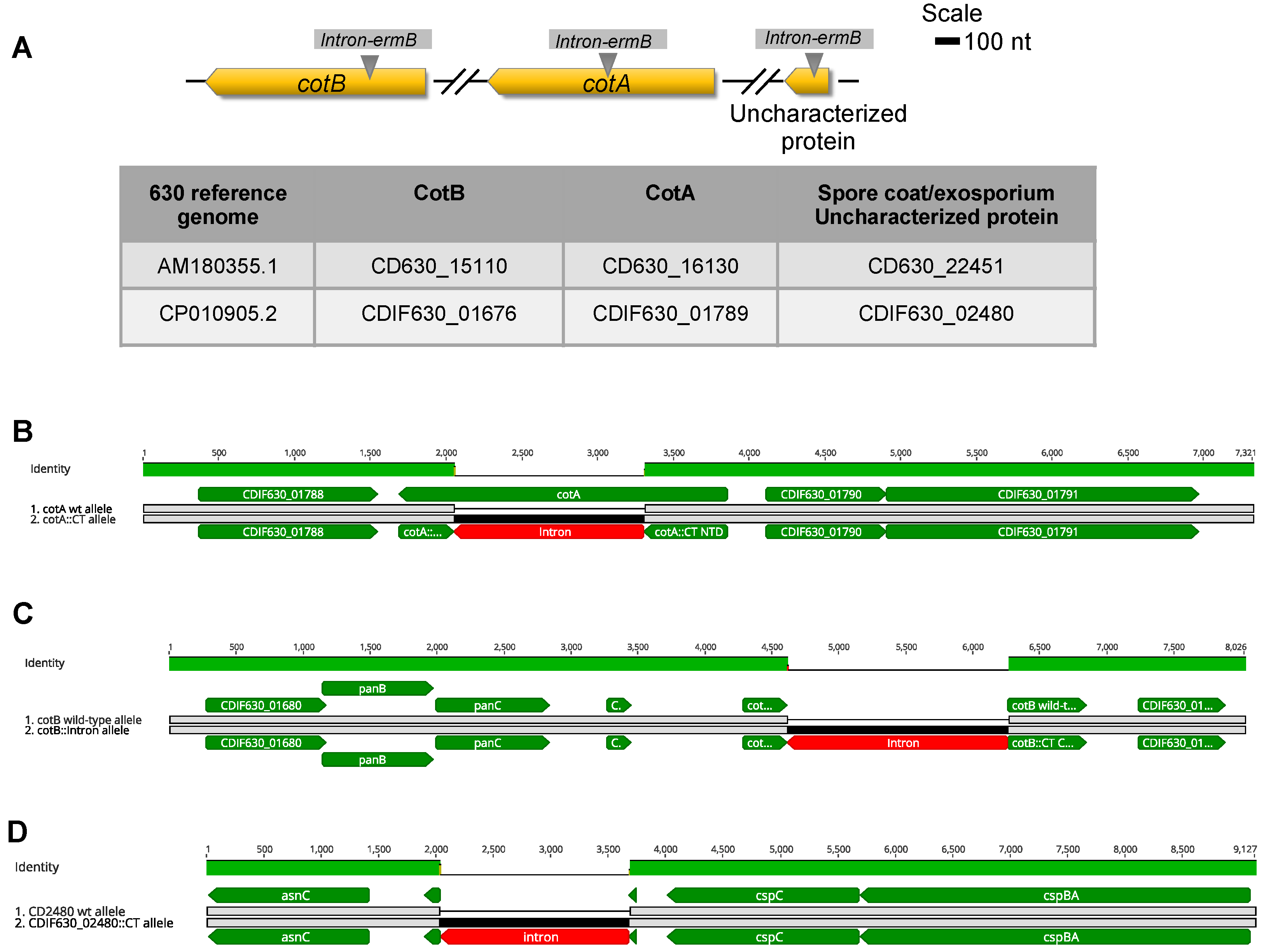
3.1. In Silico Analysis of CDIF630_02480 and Whole-Genome Analysis of Clotron-Insertional Mutants
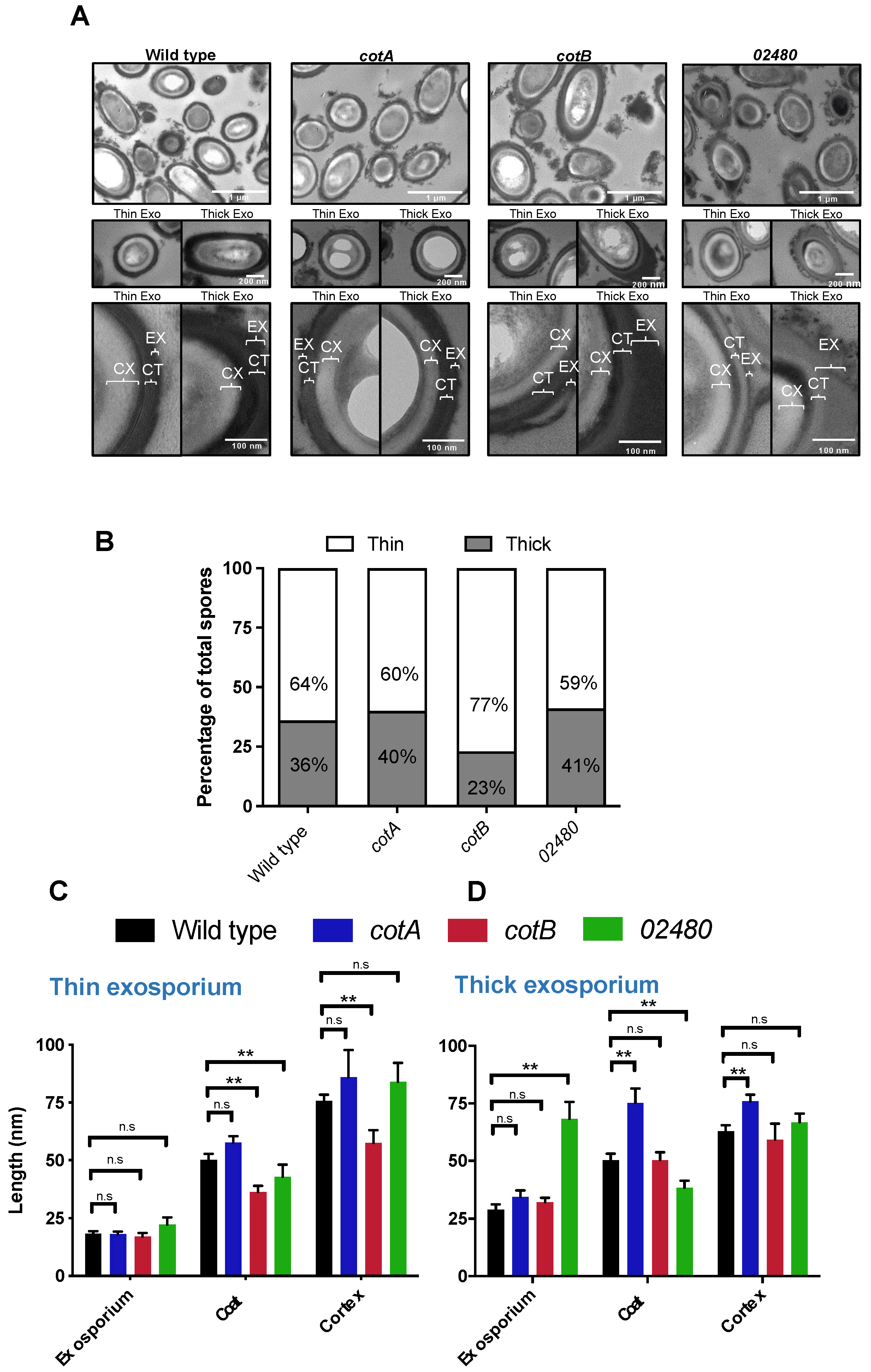
3.2. Inactivation of CotA, CotB and CDIF630_02480 Genes Produces Changes in the Ultrastructure of the Spore
3.3. Inactivation of CotA, CotB and CDIF630_02480 Genes Alters Spore Layers Thickness
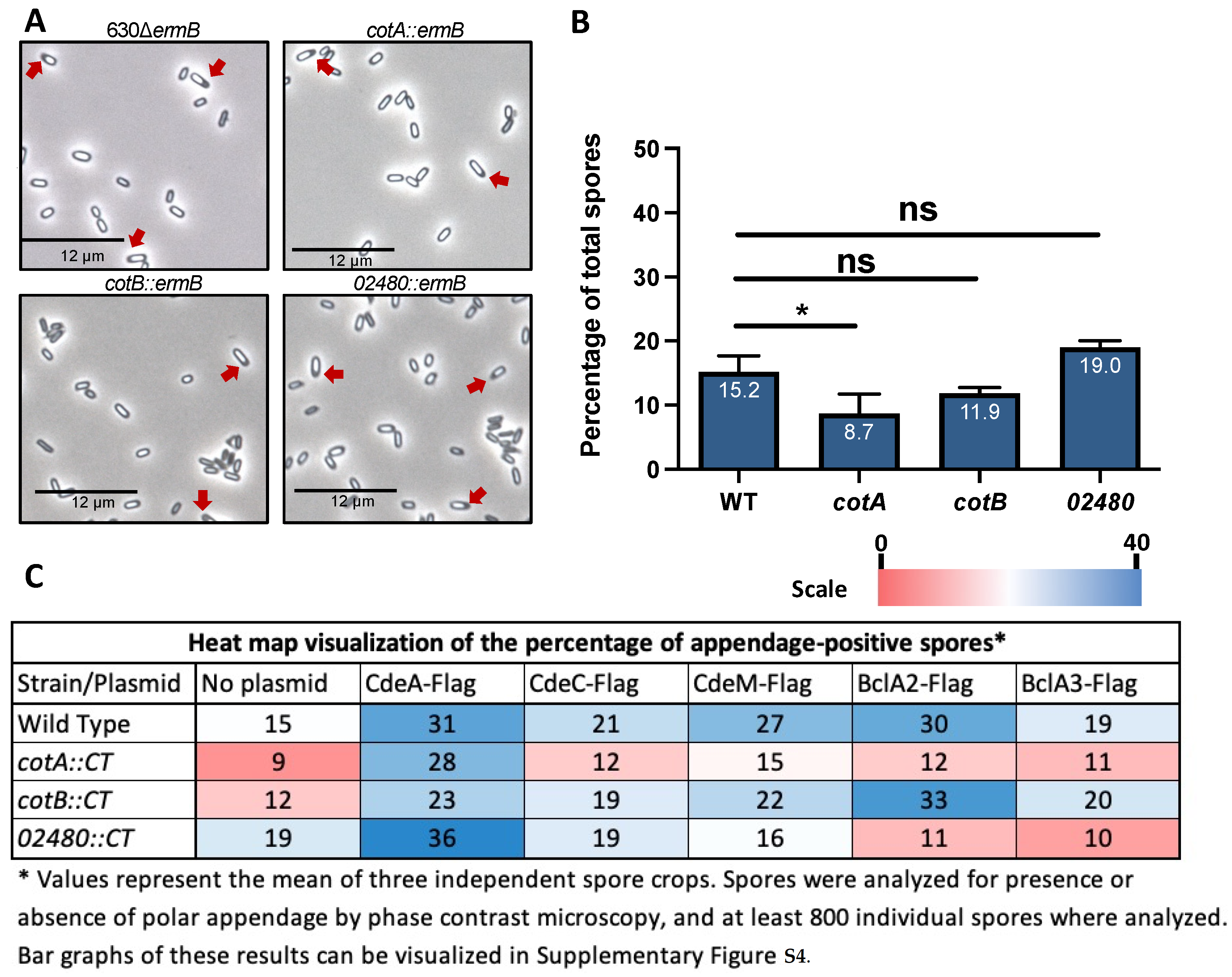
3.4. CotA, CotB and CDIF630_02480 Proteins in Spore Appendage
3.5. Absence of Coat Proteins Differentially Affects the Accessibility of Cysteine-Rich and Collagen-like Proteins to Antibodies
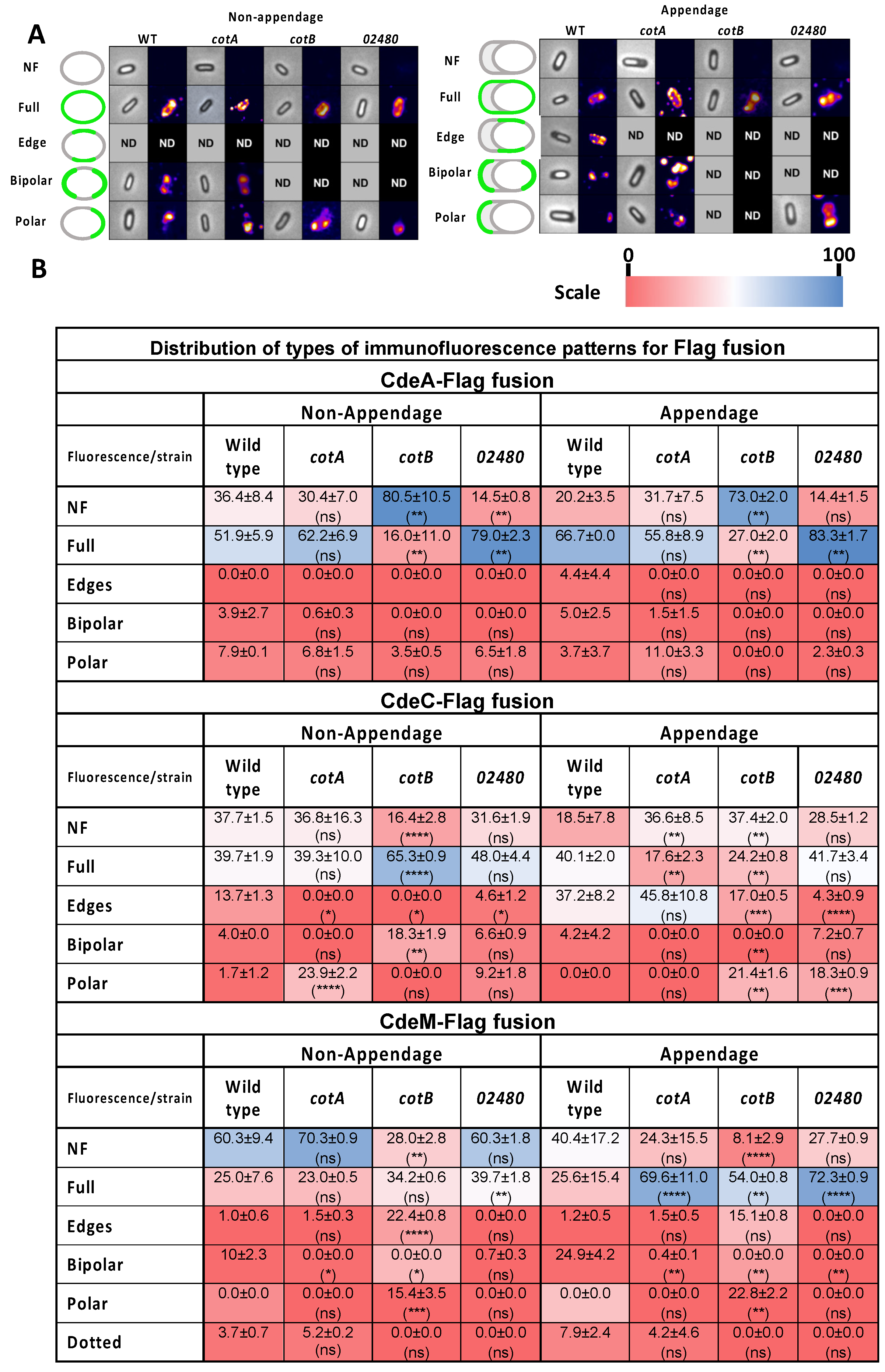
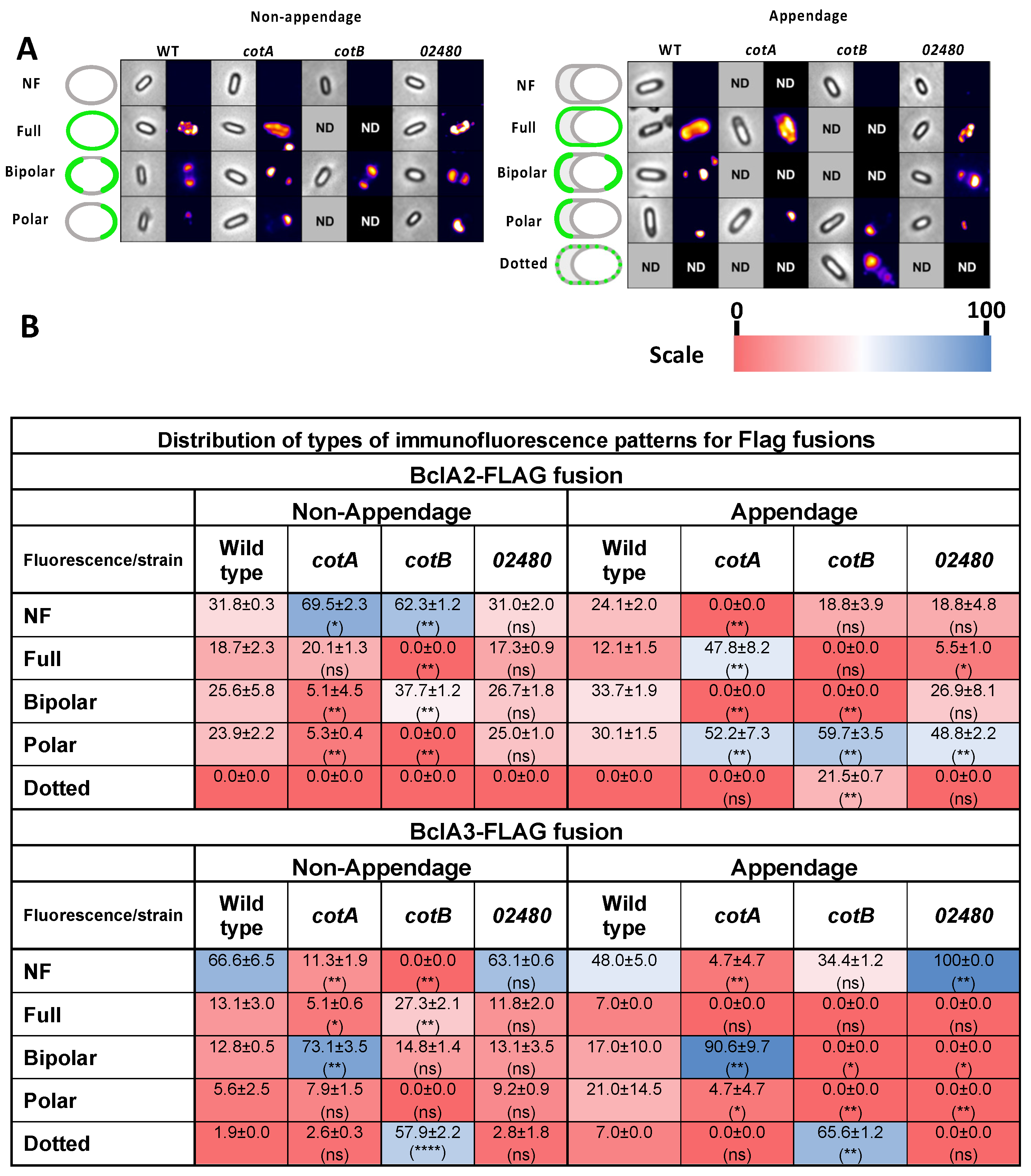
3.6. Effect of Insertional Inactivation of Spore Coat Proteins on the Immunofluorescence Distribution of Cystein-Rich Flag Fusion Proteins in the Presence/Absence of Appendage
3.7. Effect of Insertional Inactivation of Spore Coat on the Immunofluorescence Distribution of Collagen-like Exosporium Flag Fusion Proteins in the Presence/Absence of Appendage
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lessa, F.C.; Mu, Y.; Bamberg, W.M.; Beldavs, Z.G.; Dumyati, G.K.; Dunn, J.R.; Farley, M.M.; Holzbauer, S.M.; Meek, J.I.; Phipps, E.C.; et al. Burden of Clostridium difficile infection in the United States. N. Engl. J. Med. 2015, 372, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.E.; Simbartl, L.A.; Kralovic, S.M.; Jain, R.; Roselle, G.A. Clostridium difficile infections in Veterans Health Administration acute care facilities. Infect. Control Hosp. Epidemiol. 2014, 35, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Bouza, E. Consequences of Clostridium difficile infection: Understanding the healthcare burden. Clin. Microbiol. Infect. 2012, 18 (Suppl. 6), 5–12. [Google Scholar] [CrossRef] [PubMed]
- Sorg, J.A.; Sonenshein, A.L. Bile salts and glycine as cogerminants for Clostridium difficile spores. J. Bacteriol. 2008, 190, 2505–2512. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Sabja, D.; Shen, A.; Sorg, J.A. Clostridium difficile spore biology: Sporulation, germination, and spore structural proteins. Trends Microbiol. 2014, 22, 406–416. [Google Scholar] [CrossRef]
- Deakin, L.J.; Clare, S.; Fagan, R.P.; Dawson, L.F.; Pickard, D.J.; West, M.R.; Wren, B.W.; Fairweather, N.F.; Dougan, G.; Lawley, T.D. The Clostridium difficile spo0A gene is a persistence and transmission factor. Infect. Immun. 2012, 80, 2704–2711. [Google Scholar] [CrossRef]
- Mora-Uribe, P.; Miranda-Cardenas, C.; Castro-Cordova, P.; Gil, F.; Calderon, I.; Fuentes, J.A.; Rodas, P.I.; Banawas, S.; Sarker, M.R.; Paredes-Sabja, D. Characterization of the Adherence of Clostridium difficile Spores: The Integrity of the Outermost Layer Affects Adherence Properties of Spores of the Epidemic Strain R20291 to Components of the Intestinal Mucosa. Front. Cell Infect. Microbiol. 2016, 6, 99. [Google Scholar] [CrossRef]
- Castro-Córdova, P.; Mora-Uribe, P.; Reyes-Ramírez, R.; Cofré-Araneda, G.; Orozco-Aguilar, J.; Brito-Silva, C.; Mendoza-León, M.J.; Kuehne, S.A.; Minton, N.P.; Pizarro-Guajardo, M.; et al. Entry of spores into intestinal epithelial cells contributes to recurrence of Clostridioides difficile infection. Nat. Commun. 2021, 12, 1140. [Google Scholar] [CrossRef]
- Paredes-Sabja, D.; Cid-Rojas, F.; Pizarro-Guajardo, M. Assembly of the exosporium layer in Clostridioides difficile spores. Curr. Opin. Microbiol. 2022, 67, 102137. [Google Scholar] [CrossRef]
- Aguzzi, A.; Altmeyer, M. Phase Separation: Linking Cellular Compartmentalization to Disease. Trends. Cell Biol. 2016, 26, 547–558. [Google Scholar] [CrossRef]
- Mitrea, D.M.; Kriwacki, R.W. Phase separation in biology; functional organization of a higher order. Cell Commun. Signal. 2016, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. Intrinsically disordered proteins in overcrowded milieu: Membrane-less organelles, phase separation, and intrinsic disorder. Curr. Opin. Struct. Biol. 2017, 44, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.; Plannic, J.; Isticato, R.; Pelosi, A.; Zilhão, R.; Serrano, M.; Baccigalupi, L.; Ricca, E.; Elsholz, A.K.W.; Losick, R.; et al. A protein phosphorylation module patterns the Bacillus subtilis spore outer coat. Mol. Microbiol. 2020, 114, 934–951. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Gonzalez, F.; Milano, M.; Olguin-Araneda, V.; Pizarro-Cerda, J.; Castro-Cordova, P.; Tzeng, S.C.; Maier, C.S.; Sarker, M.R.; Paredes-Sabja, D. Protein composition of the outermost exosporium-like layer of Clostridium difficile 630 spores. J. Proteomics 2015, 123, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Romero-Rodríguez, A.; Troncoso-Cotal, S.; Guerrero-Araya, E.; Paredes-Sabja, D. The cysteine-rich exosporium morphogenetic protein, CdeC, exhibits self-assembly properties that lead to organized inclusion bodies in Escherichia coli. mSphere 2020, 5, e01065-01020. [Google Scholar] [CrossRef]
- Pizarro-Guajardo, M.; Calderón, P.; Romero-Rodriguez, A.; Paredes-Sabja, D. Characterization of exosporium layer variability of Clostridioides difficile spores in the epidemically relevant strain R20291. Front. Microbiol. 2020, 11, 1345. [Google Scholar] [CrossRef]
- Pizarro-Guajardo, M.; Calderon-Romero, P.; Paredes-Sabja, D. Ultrastructure Variability of the Exosporium Layer of Clostridium difficile Spores from Sporulating Cultures and Biofilms. Appl. Environ. Microbiol. 2016, 82, 5892–5898. [Google Scholar] [CrossRef]
- Pizarro-Guajardo, M.; Calderon-Romero, P.; Castro-Cordova, P.; Mora-Uribe, P.; Paredes-Sabja, D. Ultrastructural Variability of the Exosporium Layer of Clostridium difficile Spores. Appl. Environ. Microbiol. 2016, 82, 2202–2209. [Google Scholar] [CrossRef]
- Barra-Carrasco, J.; Olguin-Araneda, V.; Plaza-Garrido, A.; Miranda-Cardenas, C.; Cofre-Araneda, G.; Pizarro-Guajardo, M.; Sarker, M.R.; Paredes-Sabja, D. The Clostridium difficile exosporium cysteine (CdeC)-rich protein is required for exosporium morphogenesis and coat assembly. J. Bacteriol. 2013, 195, 3863–3875. [Google Scholar] [CrossRef]
- Calderon-Romero, P.; Castro-Cordova, P.; Reyes-Ramirez, R.; Milano-Cespedes, M.; Guerrero-Araya, E.; Pizarro-Guajardo, M.; Olguin-Araneda, V.; Gil, F.; Paredes-Sabja, D. Clostridium difficile exosporium cysteine-rich proteins are essential for the morphogenesis of the exosporium layer, spore resistance, and affect C. difficile pathogenesis. PLoS Pathog. 2018, 14, e1007199. [Google Scholar] [CrossRef]
- Antunes, W.; Pereira, F.C.; Feliciano, C.; Saujet, L.; Vultos, T.d.; Couture-Tosi, E.; Pechine, S.; Bruxelle, J.-F.; Janoir, C.; Melo, L.; et al. Structure and assembly of a Clostridioides difficile spore polar appendage. bioRxiv 2018, 468637. [Google Scholar] [CrossRef]
- Escobar-Cortes, K.; Barra-Carrasco, J.; Paredes-Sabja, D. Proteases and sonication specifically remove the exosporium layer of spores of Clostridium difficile strain 630. J. Microbiol. Methods 2013, 93, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Driks, A.; Eichenberger, P. The Spore Coat. Microbiol. Spectr. 2016, 4, 179–200. [Google Scholar] [CrossRef] [PubMed]
- Henriques, A.O.; Moran, C.P., Jr. Structure, assembly, and function of the spore surface layers. Annu. Rev. Microbiol. 2007, 61, 555–588. [Google Scholar] [CrossRef] [PubMed]
- Permpoonpattana, P.; Phetcharaburanin, J.; Mikelsone, A.; Dembek, M.; Tan, S.; Brisson, M.C.; La Ragione, R.; Brisson, A.R.; Fairweather, N.; Hong, H.A.; et al. Functional characterization of Clostridium difficile spore coat proteins. J. Bacteriol. 2013, 195, 1492–1503. [Google Scholar] [CrossRef] [PubMed]
- Permpoonpattana, P.; Tolls, E.H.; Nadem, R.; Tan, S.; Brisson, A.; Cutting, S.M. Surface layers of Clostridium difficile endospores. J. Bacteriol. 2011, 193, 6461–6470. [Google Scholar] [CrossRef]
- Saggese, A.; Isticato, R.; Cangiano, G.; Ricca, E.; Baccigalupi, L. CotG-Like Modular Proteins Are Common among Spore-Forming Bacilli. J. Bacteriol. 2016, 198, 1513–1520. [Google Scholar] [CrossRef]
- Lawley, T.D.; Clare, S.; Walker, A.W.; Goulding, D.; Stabler, R.A.; Croucher, N.; Mastroeni, P.; Scott, P.; Raisen, C.; Mottram, L.; et al. Antibiotic treatment of Clostridium difficile carrier mice triggers a supershedder state, spore-mediated transmission, and severe disease in immunocompromised hosts. Infect. Immun. 2009, 77, 3661–3669. [Google Scholar] [CrossRef]
- Sebaihia, M.; Wren, B.W.; Mullany, P.; Fairweather, N.F.; Minton, N.; Stabler, R.; Thomson, N.R.; Roberts, A.P.; Cerdeño-Tárraga, A.M.; Wang, H.; et al. The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nat. Genet. 2006, 38, 779–786. [Google Scholar] [CrossRef]
- Riedel, T.; Bunk, B.; Thürmer, A.; Spröer, C.; Brzuszkiewicz, E.; Abt, B.; Gronow, S.; Liesegang, H.; Daniel, R.; Overmann, J. Genome Resequencing of the Virulent and Multidrug-Resistant Reference Strain Clostridium difficile 630. Genome Announc. 2015, 3, e00276-15. [Google Scholar] [CrossRef]
- Davis, J.J.; Wattam, A.R.; Aziz, R.K.; Brettin, T.; Butler, R.; Butler, R.M.; Chlenski, P.; Conrad, N.; Dickerman, A.; Dietrich, E.M.; et al. The PATRIC Bioinformatics Resource Center: Expanding data and analysis capabilities. Nucleic Acids Res. 2020, 48, D606–D612. [Google Scholar] [CrossRef] [PubMed]
- Brettin, T.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Olsen, G.J.; Olson, R.; Overbeek, R.; Parrello, B.; Pusch, G.D.; et al. RASTtk: A modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci. Rep. 2015, 5, 8365. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Sebaihia, M.; Lawley, T.D.; Stabler, R.A.; Dawson, L.F.; Martin, M.J.; Holt, K.E.; Seth-Smith, H.M.; Quail, M.A.; Rance, R.; et al. Evolutionary dynamics of Clostridium difficile over short and long time scales. Proc. Natl. Acad. Sci. USA 2010, 107, 7527–7532. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- McWilliam, H.; Li, W.; Uludag, M.; Squizzato, S.; Park, Y.M.; Buso, N.; Cowley, A.P.; Lopez, R. Analysis Tool Web Services from the EMBL-EBI. Nucleic Acids Res. 2013, 41, W597–W600. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Pizarro-Guajardo, M.; Ortega-Lizárraga, C.; Cid-Rojas, F.; Inostroza, A.; Paredes-Sabja, D. Role of collagen-like protein BclA3 in the assembly of the exosporium layer of Clostridioides difficile spores. bioRxiv 2021. [Google Scholar] [CrossRef]
- Saujet, L.; Pereira, F.C.; Serrano, M.; Soutourina, O.; Monot, M.; Shelyakin, P.V.; Gelfand, M.S.; Dupuy, B.; Henriques, A.O.; Martin-Verstraete, I. Genome-wide analysis of cell type-specific gene transcription during spore formation in Clostridium difficile. PLoS Genet. 2013, 9, e1003756. [Google Scholar] [CrossRef]
- Fimlaid, K.A.; Bond, J.P.; Schutz, K.C.; Putnam, E.E.; Leung, J.M.; Lawley, T.D.; Shen, A. Global analysis of the sporulation pathway of Clostridium difficile. PLoS Genet. 2013, 9, e1003660. [Google Scholar] [CrossRef]
- Heap, J.T.; Pennington, O.J.; Cartman, S.T.; Minton, N.P. A modular system for Clostridium shuttle plasmids. J. Microbiol. Methods 2009, 78, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Pishdadian, K.; Fimlaid, K.A.; Shen, A. SpoIIID-mediated regulation of σK function during Clostridium difficile sporulation. Mol. Microbiol. 2015, 95, 189–208. [Google Scholar] [CrossRef] [PubMed]
- Pizarro-Guajardo, M.; Olguin-Araneda, V.; Barra-Carrasco, J.; Brito-Silva, C.; Sarker, M.R.; Paredes-Sabja, D. Characterization of the collagen-like exosporium protein, BclA1, of Clostridium difficile spores. Anaerobe 2014, 25, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Phetcharaburanin, J.; Hong, H.A.; Colenutt, C.; Bianconi, I.; Sempere, L.; Permpoonpattana, P.; Smith, K.; Dembek, M.; Tan, S.; Brisson, M.C.; et al. The spore-associated protein BclA1 affects the susceptibility of animals to colonization and infection by Clostridium difficile. Mol. Microbiol. 2014, 92, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Barra-Carrasco, J.; Paredes-Sabja, D. Clostridium difficile spores: A major threat to the hospital environment. Future Microbiol. 2014, 9, 475–486. [Google Scholar] [CrossRef]
- Paredes-Sabja, D.; Sarker, M.R. Adherence of Clostridium difficile spores to Caco-2 cells in culture. J. Med. Microbiol. 2012, 61, 1208–1218. [Google Scholar] [CrossRef]
- Hong, H.A.; Ferreira, W.T.; Hosseini, S.; Anwar, S.; Hitri, K.; Wilkinson, A.J.; Vahjen, W.; Zentek, J.; Soloviev, M.; Cutting, S.M. The Spore Coat Protein CotE Facilitates Host Colonization by Clostridium difficile. J. Infect. Dis. 2017, 216, 1452–1459. [Google Scholar] [CrossRef]
- Abhyankar, W.; Pandey, R.; Ter Beek, A.; Brul, S.; de Koning, L.J.; de Koster, C.G. Reinforcement of Bacillus subtilis spores by cross-linking of outer coat proteins during maturation. Food Microbiol. 2015, 45, 54–62. [Google Scholar] [CrossRef]
- Alves Feliciano, C.; Douché, T.; Giai Gianetto, Q.; Matondo, M.; Martin-Verstraete, I.; Dupuy, B. CotL, a new morphogenetic spore coat protein of Clostridium difficile. Environ. Microbiol. 2019, 21, 984–1003. [Google Scholar] [CrossRef]
- Ng, Y.K.; Ehsaan, M.; Philip, S.; Collery, M.M.; Janoir, C.; Collignon, A.; Cartman, S.T.; Minton, N.P. Expanding the repertoire of gene tools for precise manipulation of the Clostridium difficile genome: Allelic exchange using pyrE alleles. PLoS ONE 2013, 8, e56051. [Google Scholar] [CrossRef]
- McAllister, K.N.; Bouillaut, L.; Kahn, J.N.; Self, W.T.; Sorg, J.A. Using CRISPR-Cas9-mediated genome editing to generate C. difficile mutants defective in selenoproteins synthesis. Sci. Rep. 2017, 7, 14672. [Google Scholar] [CrossRef] [PubMed]


| Strain or Plasmid | Relevant Characteristic | Source/Reference |
|---|---|---|
| C. difficile | ||
| 630ΔermB | An erythromycin-sensitive derivative of C. difficile strain 630 | [14] |
| 630ΔermB(pDP345) | 630∆ermB carrying cdeC-FLAG fusion in pMTL | [14] |
| 630ΔermB(pDP360) | 630∆ermB carrying cdeM-FLAG fusion | [14] |
| 630ΔermB(pDP363) | 630∆ermB carrying bclA3-FLAG fusion | [14] |
| 630ΔermB(pDP365) | 630∆ermB carrying cdeA-FLAG fusion | [14] |
| 630ΔermB(pDP369) | 630∆ermB carrying bclA2-FLAG fusion | [14] |
| 630ΔermB cotA | 630 ΔermB cotA::intron ermB | [25] |
| 630ΔermB cotA(pDP345) | 630∆ermB cotA::intron carrying cdeC-FLAG fusion | This work |
| 630ΔermB cotA (pDP360) | 630∆ermB cotA::intron carrying cdeM-FLAG fusion | This work |
| 630ΔermB cotA (pDP363) | 630∆ermB cotA::intron carrying bclA3-FLAG fusion | This work |
| 630ΔermB cotA (pDP369) | 630∆ermB cotA::intron carrying bclA2-FLAG fusion | This work |
| 630ΔermB cotA (pDP365) | 630∆ermB cotA::intron carrying cdeA-FLAG fusion | This work |
| 630ΔermB cotB | 630 ΔermB cotB::intron ermB | [20,25] |
| 630ΔermB cotB (pDP345) | 630∆ermB cotB::intron carrying cdeC-FLAG fusion | This work |
| 630ΔermB cotB (pDP360) | 630∆ermB cotB::intron carrying cdeM-FLAG fusion | This work |
| 630ΔermB cotB (pDP361) | 630∆ermB cotB::intron carrying bclA1-FLAG fusion | This work |
| 630ΔermB cotB (pDP363) | 630∆ermB cotB::intron carrying bclA3-FLAG fusion | This work |
| 630ΔermB cotB (pDP369) | 630∆ermB cotB::intron carrying bclA2-FLAG fusion | This work |
| 630ΔermB cotB (pDP365) | 630∆ermB cotB::intron carrying cdeA-FLAG fusion | This work |
| 630ΔermB CDIF630_02480 | 630 ΔermB CDIF630_02480::intron ermB; 630 strain reference genome CP010905.2 (CD2245.1 of the strain 630 reference genome AM180355.1). | [25] and This work. |
| 630ΔermB CDIF630_02480 (pDP345) | 630∆ermB CDIF630_02480::intron carrying cdeC-FLAG fusion | This work |
| 630ΔermB CDIF630_02480 (pDP360) | 630∆ermB CDIF630_02480::intron carrying cdeM-FLAG fusion | This work |
| 630ΔermB CDIF630_02480 (pDP361) | 630∆ermB CDIF630_02480::intron carrying bclA1-FLAG fusion | This work |
| 630ΔermB CDIF630_02480 (pDP363) | 630∆ermB CDIF630_02480::intron carrying bclA3-FLAG fusion | This work |
| 630ΔermB CDIF630_02480 (pDP369) | 630∆ermB CDIF630_02480::intron carrying bclA2-FLAG fusion | This work |
| 630ΔermB CDIF630_02480 (pDP365) | 630∆ermB CDIF630_02480::intron carrying cdeA-FLAG fusion | This work |
| Plasmids | ||
| pDP345 | pMTL82151 carrying cdeC630 labeled at the C-terminal with FLAG as a reporter tag in NdeI/HindIII sites. | [19] |
| pDP360 | pMTL82151 carrying cdeM630 labeled at the C-terminal with FLAG as a reporter tag in KpnI/SalI sites. | [14] |
| pDP363 | pMTL82151 carrying bclA3630 labeled at the C-terminal with FLAG as a reporter tag in KpnI/SalI sites. | [14] |
| pDP365 | pMTL82151 carrying cdeA630 labeled at the C-terminal with FLAG as a reporter tag in KpnI/SalI sites. | [14] |
| pDP369 | pMTL82151 carrying bclA2630 labeled at the C-terminal with FLAG as a reporter tag in KpnI/SalI sites. | [14] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montes-Bravo, N.; Romero-Rodríguez, A.; García-Yunge, J.; Medina, C.; Pizarro-Guajardo, M.; Paredes-Sabja, D. Role of the Spore Coat Proteins CotA and CotB, and the Spore Surface Protein CDIF630_02480, on the Surface Distribution of Exosporium Proteins in Clostridioides difficile 630 Spores. Microorganisms 2022, 10, 1918. https://doi.org/10.3390/microorganisms10101918
Montes-Bravo N, Romero-Rodríguez A, García-Yunge J, Medina C, Pizarro-Guajardo M, Paredes-Sabja D. Role of the Spore Coat Proteins CotA and CotB, and the Spore Surface Protein CDIF630_02480, on the Surface Distribution of Exosporium Proteins in Clostridioides difficile 630 Spores. Microorganisms. 2022; 10(10):1918. https://doi.org/10.3390/microorganisms10101918
Chicago/Turabian StyleMontes-Bravo, Nicolás, Alba Romero-Rodríguez, José García-Yunge, César Medina, Marjorie Pizarro-Guajardo, and Daniel Paredes-Sabja. 2022. "Role of the Spore Coat Proteins CotA and CotB, and the Spore Surface Protein CDIF630_02480, on the Surface Distribution of Exosporium Proteins in Clostridioides difficile 630 Spores" Microorganisms 10, no. 10: 1918. https://doi.org/10.3390/microorganisms10101918
APA StyleMontes-Bravo, N., Romero-Rodríguez, A., García-Yunge, J., Medina, C., Pizarro-Guajardo, M., & Paredes-Sabja, D. (2022). Role of the Spore Coat Proteins CotA and CotB, and the Spore Surface Protein CDIF630_02480, on the Surface Distribution of Exosporium Proteins in Clostridioides difficile 630 Spores. Microorganisms, 10(10), 1918. https://doi.org/10.3390/microorganisms10101918







