Effect of Polydextrose on the Growth of Pediococcus pentosaceus as Well as Lactic Acid and Bacteriocin-like Inhibitory Substances (BLIS) Production
Abstract
1. Introduction
2. Material and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Experimental Design
2.3. Analytical Procedures
2.4. Determination of BLIS Antimicrobial Activity
2.5. Calculation of Fermentation Parameters
2.6. Statistical Study
3. Results and Discussion
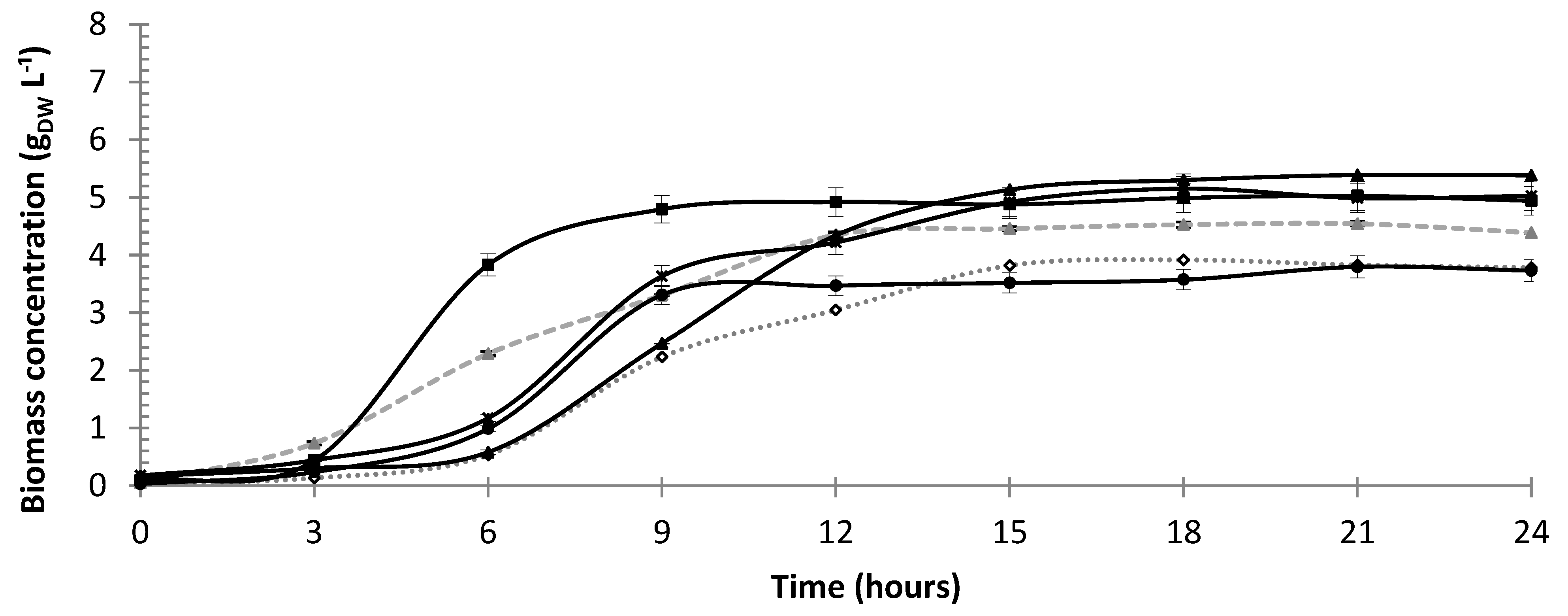
3.1. Cell Growth
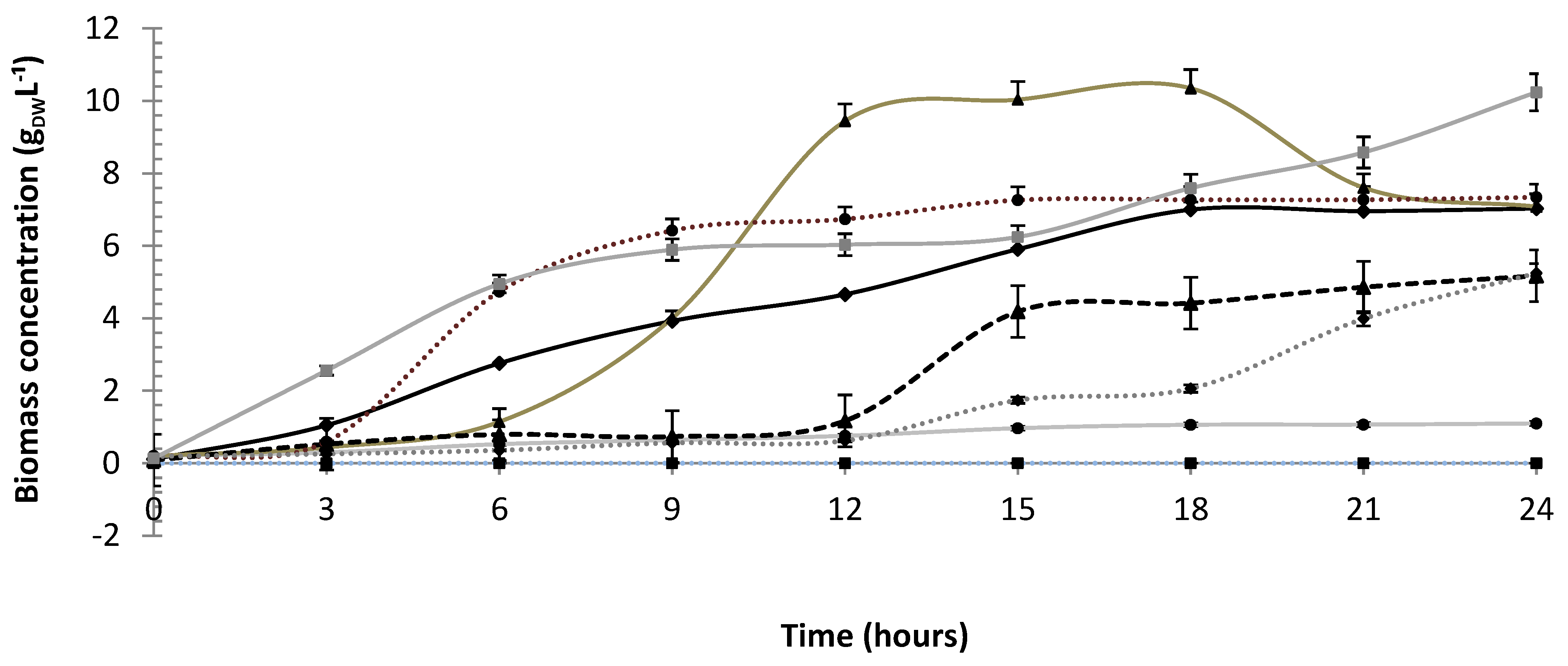
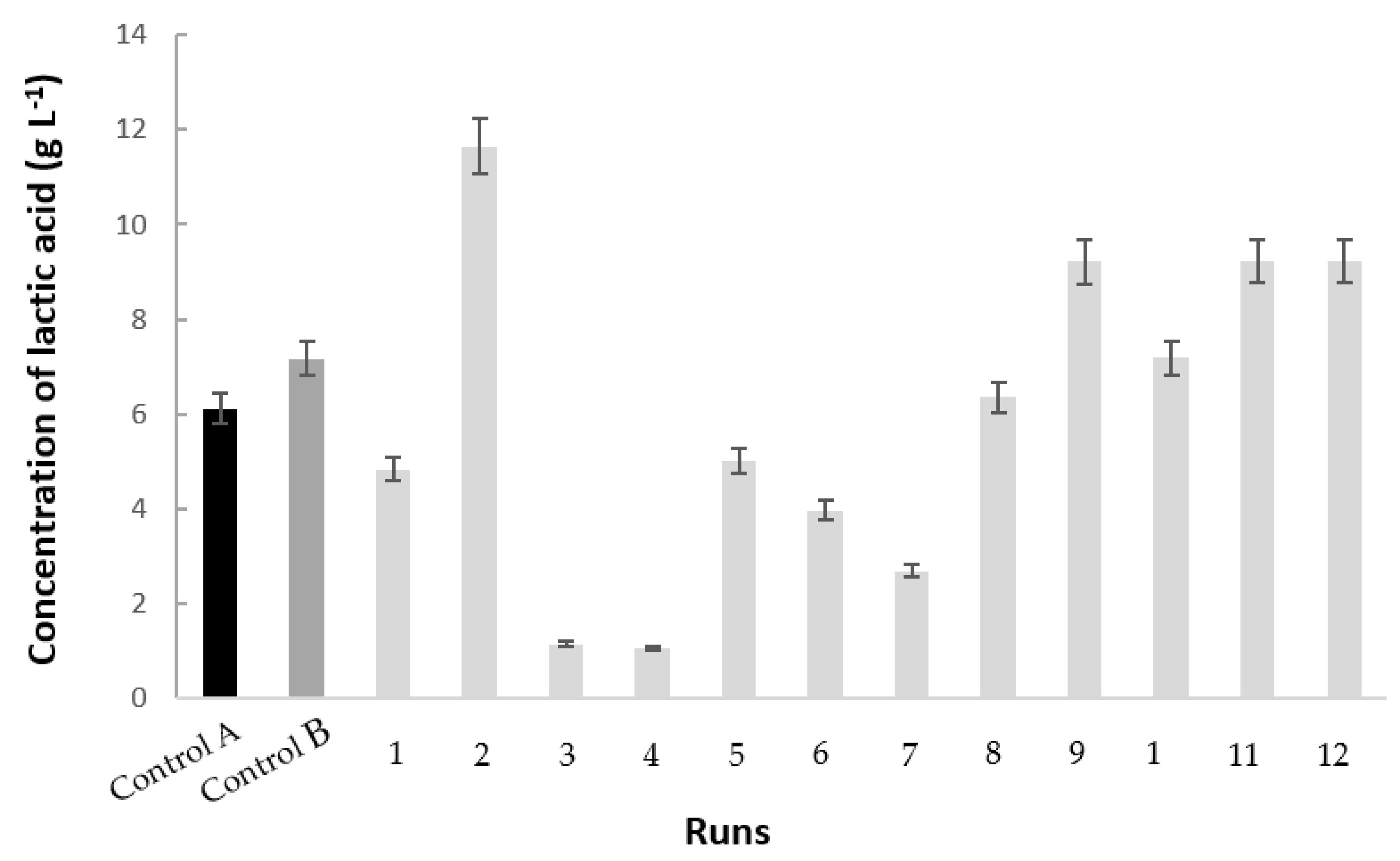
3.2. Polydextrose/Glucose Consumption and Lactic Acid Production
3.3. Antimicrobial Activity of BLIS
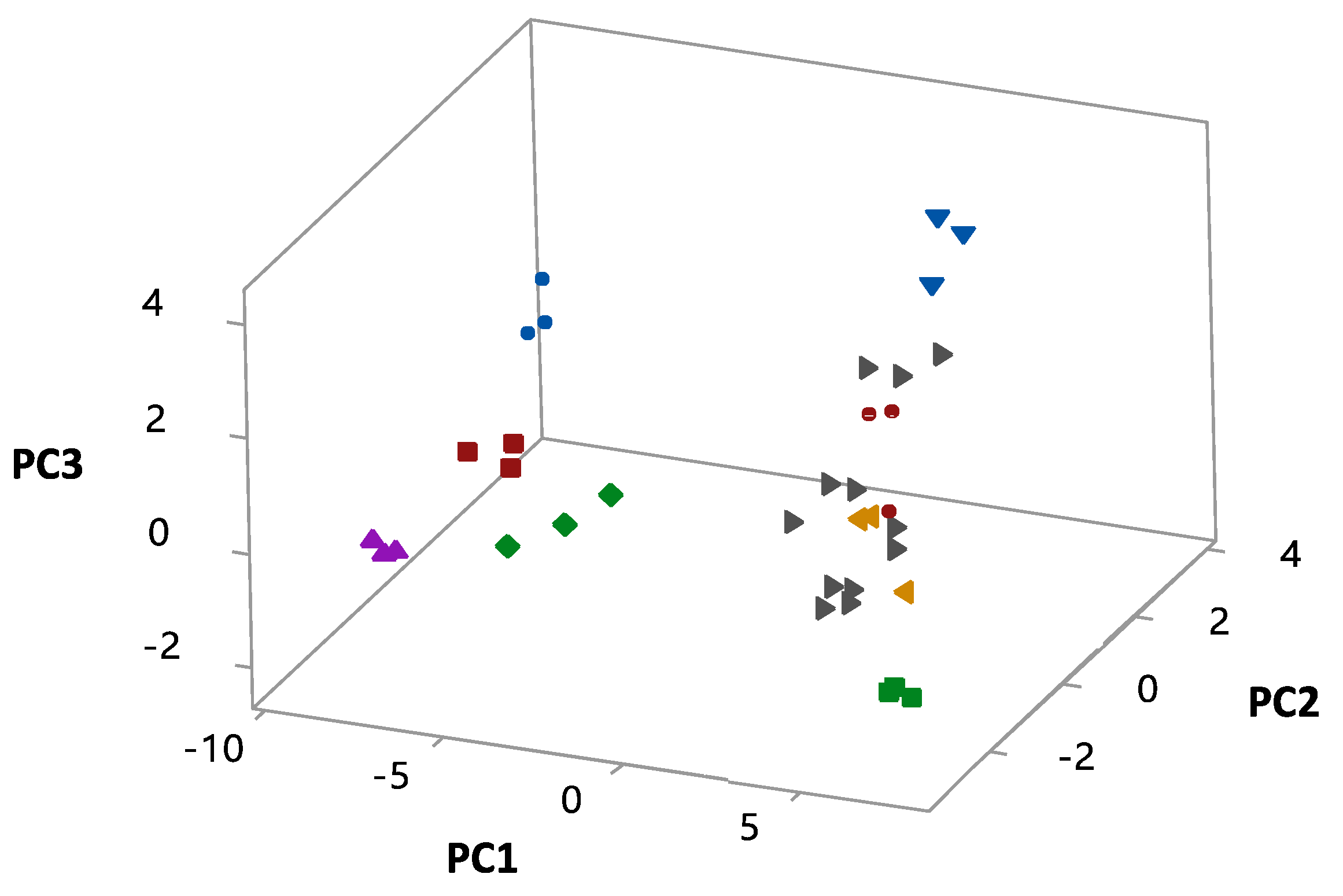
3.4. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raza, G.S.; Putaala, H.; Hibberd, A.; Alhoniemi, E.; Tiihonen, K.; Mäkelä, K.A.; Herzig, K.H. Polydextrose changes the gut microbiome and attenuates fasting triglyceride and cholesterol levels in Western diet fed mice. Sci. Rep. 2017, 7, 5294. [Google Scholar] [CrossRef] [PubMed]
- Aquilina, G.; Castle, L.; Engel, K.H.; Fowler, P.; Fernandez, M.J.F.; Fürst, P.; Gürtler, R.; Gundert-Remy, U.; Husøy, T.; Manco, M.; et al. Re-evaluation of polydextrose (E1200) as food additive. EFSA J. 2021, 19, e06363. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdanipour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasem, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Noonan, S.; Zaveri, M.; Macninch, E.; Martyn, K. Food & mood: A review of supplementary prebiotic and prebiotic interventions in the treatment of anxiety and depression in adults. BMJ Nutr. Prev. Health 2020, 3, e000053. [Google Scholar] [CrossRef]
- Hooda, S.; Boler, B.M.; Serao, M.C.; Brulc, J.M.; Staeger, M.A.; Boileau, T.W.; Dowd, S.E.; Fahey, G.C., Jr.; Swanson, K.S. 454-pyrosequencing reveals a shift in fecal microbiota of healthy adult men consuming polydextrose or soluble corn fiber. J. Nutr. 2012, 142, 1259–1265. [Google Scholar] [CrossRef]
- Hernot, D.C.; Boileau, T.W.; Bauer, L.L.; Middelbos, I.S.; Murphy, M.R.; Swanson, K.S.; Fahey, G.C., Jr. In vitro fermentation profiles, gas production rates, and microbiota modulation as affected by certain fructans, galactooligosaccharides, and polydextrose. J. Agric. Food Chem. 2009, 57, 1354–1361. [Google Scholar] [CrossRef]
- Röytio, H.; Owehand, A.C. The fermentation of polydextrose in the large intestine and its beneficial effects. Benef. Microbes 2014, 5, 305–314. [Google Scholar] [CrossRef]
- Mäkeläinen, H.; Ottman, N.; Forssten, S.; Saarinen, M.; Rautonen, N.; Ouwehand, A.C. Synbiotic effects of GOS, PDX and Bifidobacterium lactis Bi-07 in vitro. Int. J. Probiotics Prebiotics 2010, 5, 203–210. [Google Scholar]
- Michael, J.; Sajid, A.; Quentin, J.; Walton, S.; Webb, P. Enhancing the Nutrient Bioavailability of Food Aid Products; Report to USAID; Tufts University: Boston, MA, USA, 2019. [Google Scholar]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability of nutraceuticals: Role of the food matrix, processing conditions, the gastrointestinal tract, and nanodelivery systems. Compr. Rev. Food Sci. Food Saf. 2020, 19, 954–994. [Google Scholar] [CrossRef]
- Hull, S.; Re, R.; Tijhonen, K.; Viscione, L.; Wickham, M. Consuming polydextrose in a mid-morning snack increases acute satiety measurements and reduces subsequent energy intake at lunch in healthy human subjects. Appetite 2012, 59, 706–712. [Google Scholar] [CrossRef]
- Covián-Rios, D.; Ruas-Madiedo, P.; Margalles, A.; Gueimonde, M.; Reyes-Gavilan, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, A.; Pelipyagina, T.; Rueffer, M.; Evans, M.; Ouwehand, A.C. Efficacy of polydextrose supplementation on colon transit time, bowel movements, and gastrointestinal symptoms in adults: A double-blind, randomized, placebo-controlled trial. Nutrients 2019, 11, 439. [Google Scholar] [CrossRef]
- Farinha, L.L.; Sabo, S.S.; Porto, M.C.; Souza, E.C.; Oliveira, M.N.; Oliveira, R.P.S. Influence of prebiotic ingredients on the growth kinetics and bacteriocin production of Lactococcus lactis. Chem. Eng. Trans. 2015, 43, 313–318. [Google Scholar] [CrossRef]
- Heba, H.; Abd-Rabou, H.S.; Saad, M.A. The Impact of Incorporating Lactobacillus acidophilus Bacteriocin with Inulin and FOS on Yogurt Quality. Sci. Rep. 2022, 12, 13401. [Google Scholar] [CrossRef]
- Reis, J.A.; Paula, A.T.; Casarotti, S.N.; Penna, A.L.B. Lactic acid bacteria antimicrobial compounds: Characteristics and applications. Food Eng. Rev. 2012, 4, 124–140. [Google Scholar] [CrossRef]
- Ünlü, G.; Nielsen, B.; Ionita, C. Production of antilisterial bacteriocins from lactic acid bacteria in dairy-based media: A comparative study. Probiotics Antimicrob. Proteins 2015, 7, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Sidek, N.L.; Halim, M.; Tan, J.S.; Abbasiliasi, S.; Mustafa, S.; Ariff, A.B. Stability of bacteriocin-like inhibitory substance (BLIS) produced by Pediococcus acidilactici kp10 at different extreme conditions. BioMed. Res. Int. 2018, 2018, 5973484. [Google Scholar] [CrossRef]
- Jawan, R.; Abbasiliasia, S.; Mustafa, M.R.; Kapri, M.; Halim, M.; Ariff, A.B. In vitro evaluation of potential probiotic strain Lactococcus lactis Gh1 and its bacteriocin-like inhibitory substance for potential use in the food industry. Probiotics Antimicrob. Proteins 2021, 13, 422–440. [Google Scholar] [CrossRef]
- Alvarez-Sieiro, P.; Montalbán-López, M.; Mu, D.; Kuipers, O.P. Bacteriocins of lactic acid bacteria: Extending the family. Appl. Microbiol. Biotechnol. 2016, 100, 2939–2951. [Google Scholar] [CrossRef]
- Piazentin, A.C.M.; Mendonça, C.M.N.; Vallejo, M.; Mussato, S.; Oliveira, R.P.S. Bacteriocin-like inhibitory substances production by Enterococcus faecium 135 in co-culture with Ligilactobacillus salivarius and Limosilactobacillus reuteri. Braz. J. Microbiol. 2022, 53, 131–141. [Google Scholar] [CrossRef]
- Melia, S.; Juliyarsi, I.; Kurnia, Y.F.; Pratama, Y.E.; Pratama, D.R. The quality of fermented goat milk produced by Pediococcus acidilactici BK01 on refrigerator temperature. Biodivers. J. Biol. Divers. 2020, 21, 4591–4596. [Google Scholar] [CrossRef]
- Khorshidian, N.; Khanniri, E.; Mohammadi, M.; Mortazavian, A.M.; Yousefi, M. Antibacterial activity of pediocin and pediocin-producing bacteria against Listeria monocytogenes in meat products. Front. Microbiol. 2021, 12, 709959. [Google Scholar] [CrossRef] [PubMed]
- Bédard, F.; Hammani, R.; Zirah, S.; Rebuffat, S.; Fliss, I.; Biron, E. Synthesis, antimicrobial activity, and conformational analysis of the class IIa bacteriocin pediocin PA-1 and analogs thereof. Sci. Rep. 2018, 8, 9029. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, Y.; Bai, D.; Wei, Y.; Sun, J.; Luo, Y.; Zhao, J.; Liu, Y.; Wang, Q. Synergistic inhibitors mechanism of pediocin PA-1 and L-lactic acid against Aeromonas hydrophila. BBA Biomembr. 2020, 1862, 183346. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadou, S.; Papagianni, M.; Filiousis, G.; Ambrosiadis, I.; Koidis, P. Growth and metabolism of a meat isolated strain of Pediococcus pentosaceus in submerged fermentation. Purification, characterization and properties of the produced pediocin SM-1. J. Elsevier Enz. Microb. Technol. 2008, 43, 448–454. [Google Scholar] [CrossRef]
- Osmanagaoglu, O.; Kiran, F.; Nes, I.F. A probiotic bacterium, Pediococcus pentosaceus OZF, isolated from human breast milk produces pediocin AcH-PA-1. Afr. J. Biotechnol. 2011, 10, 2070–2079. [Google Scholar]
- Devi, S.M.; Halami, P.M. Detection and Characterization of Pediocin PA-1/AcH like bacteriocin Producing Lactic Acid Bacteria. Curr. Microbiol. 2011, 63, 181–185. [Google Scholar] [CrossRef]
- Piva, A.; Headon, D.R. Pediocin A, a bacteriocin produced by Pediococcus pentosaceus FBB61. Microbiology 1994, 140, 697–702. [Google Scholar] [CrossRef][Green Version]
- Porto, M.C.W.; Kuniyoshi, T.M.; Azevedo, P.O.S.; Vitolo, M.; Oliveira, R.P.S. Pediococcus spp.: An important genus of lactic acid bacteria and pediocin producers. Biotechnol. Adv. 2017, 35, 361–374. [Google Scholar] [CrossRef]
- Azevedo, P.O.S.; Converti, A.; Gierus, M.; Oliveira, R.P.S. Antimicrobial activity of bacteriocin-like inhibitory substance produced by Pediococcus pentosaceus: From shake flasks to bioreactor. Mol. Biol. Rep. 2018, 45, 461–469. [Google Scholar] [CrossRef]
- Azevedo, P.O.S.; Mendonça, C.M.N.; Seibert, L.; Domínguez, J.M.; Converti, A.; Gierus, M.; Oliveira, R.P.S. Bacteriocin-like inhibitory substance of Pediococcus pentosaceus as a biopreservative for Listeria sp. control in ready-to-eat pork ham. Braz. J. Microbiol. 2020, 51, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Danilčenko, H. Changes in the Quality of Inulin-Based Products During Storage. In Jerusalem Artichoke Food Science and Technology; Sawicka, B., Krochmal-Marczak, B., Eds.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Bertuccioli, A.; Bressan, A.; Neri, M.; Vergoni, D.; Zonzini, G. Specific prebiotic composition for precision bacterial therapy in patients with irritable bowel syndrome. Nutrafoods 2022, 1, 331–336. [Google Scholar] [CrossRef]
- Martinez, F.A.; Dominguez, J.M.; Converti, A.; Oliveira, R.P. Production of bacteriocin-like inhibitory substance by Bifidobacterium lactis in skim milk supplemented with additives. J. Dairy Res. 2015, 82, 350–355. [Google Scholar] [CrossRef]
- Fukuda, I.M.; Francini, C.F.; Pinto, P.; Moreira, C.S.; Saviano, A.M.; Lourenço, F.R. Design of Experiments (DoE) applied to Pharmaceutical and Analytical Quality by Design (QbD). Braz. J. Pharm. Sci. 2018, 54, e01006. [Google Scholar] [CrossRef]
- Andersen, J.M.; Barrangou, R.; Hachem, M.A.; Lantinen, S.J.; Goh, Y.J.; Svensson, B.; Klaenhammer, T.R. Transcriptional Analysis of Prebiotic Uptake and Catabolism by Lactobacillus acidophilus NCFM. PLoS ONE 2012, 7, e44409. [Google Scholar] [CrossRef]
- Anastasiadou, S.; Papagianni, M.; Filiousis, G.; Ambrosiadis, I.; Koidis, P. Pediocin SA-1, an antimicrobial peptide from Pediococcus accidilactici NRRL B 5627: Production conditions, purification and characterization. J. Bioresour. Technol. 2008, 99, 5384–5390. [Google Scholar] [CrossRef]
- Papagianni, M.; Anastasiadou, S. Pediocins: The bacteriocins of Pediococci. Sources, production, properties and applications. Microb. Cell Fact. 2009, 8, 3. [Google Scholar] [CrossRef]
- Huys, G.; Leisner, J.; Bjorkroth, J. The lesser LAB gods: Pediococcus, Leuconostoc, Weissella, Carnobacterium and affiliated genera. In Lactic Acid Bacteria: Microbiological and Functional Aspects, 4th ed.; Lahtinen, S., Ouwehand, A.C., Salminen, S., Wright, A.V., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 94–98. [Google Scholar]
- Halami, P.M.; Badarinath, V.; Devi, S.M.; Vijayendra, S.V.N. Partial characterization of heat-stable, antilisterial and cell lytic bacteriocin of Pediococcus pentosaceus CFR SIII isolated from a vegetable source. Ann. Microbiol. 2010, 61, 323–330. [Google Scholar] [CrossRef][Green Version]
- Pinto, A.L.; Fernandes, M.; Pinto, C.; Albano, H.; Castilho, F.; Teixeira, P.; Gibbs, P.A. Characterization of anti-Listeria bacteriocins isolated from shellfish: Potential antimicrobials to control non-fermented seafood. Int. J. Food Microbiol. 2009, 129, 50–58. [Google Scholar] [CrossRef]
- Lehninger, A.L.; Nelson, D.L.; Cox, M.M. Principles of Biochemistry, 2nd ed.; Worth: New York, USA, 1993; pp. 415–418. [Google Scholar]
- Gao, C.; Ma, C.; Xu, P. Biotechnological routes based on lactic acid production from biomass. Biotechnol. Adv. 2011, 29, 930–939. [Google Scholar] [CrossRef]
- Lahtinen, S.J.; Knoblock, K.; Drakoularakou, A.; Jacob, M.; Stowell, J.; Gibson, G.R.; Ouwehand, A.C. Effect of molecule branching and glycosidic linkage on the degradation of polydextrose by gut microbiota. Biosci. Biotechnol. Biochem. 2010, 74, 2016–2021. [Google Scholar] [CrossRef] [PubMed]
- Fava, F.; Mäkivuokko, H.; Siljander-Rasi, H.; Putaala, H.; Tiihonen, K.; Stowell, J.; Tuohy, K.; Gibson, G.R.; Rautonen, N. Effect of polydextrose on intestinal microbes and immune functions in pigs. Br. J. Nutr. 2007, 98, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Costalibe, A.; Fava, F.; Röytiö, H.; Forssten, S.D.; Olli, K.; Klievink, J.; Rowland, I.R.; Ouwehand, A.C.; Rastall, R.A.; Gibson, G.R.; et al. Impact of polydextrose on the faecal microbiota: A double-blind, crossover, placebo-controlled feeding study in healthy human subjects. Br. J. Nutr. 2012, 108, 471–481. [Google Scholar] [CrossRef]
- Azevedo, P.O.S.; Converti, A.; Domínguez, J.M.; Oliveira, R.P.S. Stimulating effects of sucrose and inulin on growth, lactate and bacteriocin Productions by Pediococcus pentosaceus. Probiotics Antimicrob. Proteins 2017, 9, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Kingcha, Y.; Tosukhowong, A.; Zendo, T.; Roytrakuls, S.; Luxananil, P.; Chareonpornsook, K.; Valyasevi, R.; Jang, S.; Lee, J.; Jung, U.; et al. Anti-listeria activity of Pediococcus pentosaceus BCC 3772 and application as starter culture for Nham, a traditional fermented pork sausage. Food Control 2012, 25, 190–196. [Google Scholar] [CrossRef]
- Huang, Y.; Luo, Y.; Zhai, Z.; Zhang, H.; Yang, C.; Tian, H.; Li, Z.; Feng, J.; Liu, H.; Hao, Y. Characterization and application of an anti-Listeria bacteriocin produced by Pediococcus pentosaceus 05-10 isolated from Sichuan Pickle, a traditionally fermented vegetable product from China. Food Control 2009, 20, 1030–1035. [Google Scholar] [CrossRef]
- Abrams, D.; Barbosa, J.; Albano, H.; Silva, J.; Gibbs, A.P.; Teixeira, P. Characterization of bacPPK34 a bacteriocin produced by Pediococcus pentosaceus strain K34 isolated from “Alheira”. Food Control 2011, 22, 940–946. [Google Scholar] [CrossRef]
- Papagianni, M.; Sergelidis, D. Chemostat production of pediocin Sm-1 by Pediococcus Mees 1934. AIChE J. 2015, 31, 1481–1486. [Google Scholar] [CrossRef]
- Engelhardt, H.; Albano, G.; Kiskó, G.; Farkas, C.M.; Teixeira, P. Antilisterial activity of bacteriocinogenic Pediococcus acidilactici HA 6111-2 and Lactobacillus plantarum ESB 202 grown under pH and osmotic stress conditions. J. Food Microbiol. 2015, 48, 109–115. [Google Scholar] [CrossRef]
- Mointville, T.J.; Chen, Y. Mechanistic action of pediocin and nisin: Recent progress and unresolved questions. Appl. Microbiol. Biotechnol. 1998, 50, 511–519. [Google Scholar] [CrossRef]
- Moll, G.N.; Konings, W.N.; Driessen, A.J.M. Bacteriocins: Mechanism of membrane insertion and pore formation. Antonie Leeuwenhoek 1999, 76, 185–198. [Google Scholar] [CrossRef]
- Synder, A.B.; Worobo, R.W. Chemical and genetic characterization of bacteriocins: Antimicrobial peptides for food safety. J. Sci. Food Agric. 2014, 94, 28–44. [Google Scholar] [CrossRef]
- Kramer, N.F.; Smith, J.L.; Hillman, J.D.; Zachariah, C.; Kuipers, O.P.; Kruijff, B.; Breukink, E. An alternative bactericidal mechanism of action for lantibiotic peptides that target lipid II. Science 2006, 313, 1636–1637. [Google Scholar] [CrossRef]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef]
- Madigan, M.T.; Martinko, J.M.; Clark, D.P. Microbiologia de Brock, 12th ed.; Artmed: Porto Alegre, RS, Brazil, 2010. [Google Scholar]
- Tiwari, S.K.; Noll, K.S.; Cavera, V.L.; Chikindas, M.L. Improved antimicrobial activities of synthetic hybrid bacteriocins designed from enterocin E50-52 and pediocin PA-1. Appl. Environ. Microbiol. 2015, 81, 1661–1667. [Google Scholar] [CrossRef]
- Cizeikiene, D.; Juodeikeine, G.; Paskevicius, A.; Bartkiene, E. Antimicrobial activity of lactic acid bacteria against pathogenic and spoilage microorganism isolated from food and their control in wheat bread. Food Control 2013, 31, 539–545. [Google Scholar] [CrossRef]
- Nahid, A.; Chapman, B.; Hunter, A.; Bellgard, M. 1.31-Metabolomics–The Combination of Analytical Biochemistry, Biology, and Informatics. In Comprehensive Biotechnology, 3rd ed.; Moo-Young, M., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 1, pp. 435–447. [Google Scholar] [CrossRef]

| Run | pH | Polydextrose Concentration (%) | Rotational Speed (rpm) | Temperature (°C) |
|---|---|---|---|---|
| Control A a | 5.0 | 0 | 100 | 30 |
| Control B b | 6.0 | 0 | 100 | 30 |
| 1 | 4.0 | 0.50 | 50 | 25 |
| 2 | 6.0 | 0.50 | 50 | 35 |
| 3 | 4.0 | 1.50 | 50 | 35 |
| 4 | 6.0 | 1.50 | 50 | 25 |
| 5 | 4.0 | 0.50 | 150 | 35 |
| 6 | 6.0 | 0.50 | 150 | 25 |
| 7 | 4.0 | 1.50 | 150 | 25 |
| 8 | 6.0 | 1.50 | 150 | 35 |
| 9 c | 5.0 | 1.00 | 100 | 30 |
| 10 c | 5.0 | 1.00 | 100 | 30 |
| 11 c | 5.0 | 1.00 | 100 | 30 |
| 12 c | 5.0 | 1.00 | 100 | 30 |
| Run | X (gDW L−1) a | µmax (h−1) b | tg (h) c | YX/S (gX gS−1) d | YP/X (gP gX−1) e | QX (gX L−1 h−1) f |
|---|---|---|---|---|---|---|
| Control A g | 3.78 ± 0.06 | 0.94 ± 0.01 | 0.75 ± 0.02 | 0.46 ± 0.00 | 1.63 ± 0.07 | 0.16 ± 0.00 |
| Control B h | 4.38 ± 0.01 | 0.89 ± 0.03 | 0.89 ± 0.03 | 0.38 ± 0.02 | 1.65 ± 0.13 | 0.18 ± 0.00 |
| 1 | 5.00 ± 0.23 | 0.93 ± 0.02 | 0.76 ± 0.02 | 0.61 ± 0.15 | 1.02 ± 0.05 | 0.20 ± 0.01 |
| 2 | 7.00 ± 1.11 | 0.87 ± 0.01 | 0.83 ± 0.01 | 0.46 ± 0.08 | 1.56 ± 0.20 | 0.29 ± 0.04 |
| 3 | 1.09 ± 0.34 | 0.84 ± 0.16 | 0.88 ± 0.21 | 0.52 ± 0.22 | 1.52 ± 0.10 | 0.37 ± 0.01 |
| 4 | 7.08 ± 0.41 | 0.99 ± 0.01 | 0.70 ± 0.00 | 2.96 ± 0.34 | 0.15 ± 0.12 | 0.29 ± 0.02 |
| 5 | 5.17 ± 0.27 | 0.84 ± 0.06 | 0.87 ± 0.07 | 0.75 ± 0.18 | 0.98 ± 0.08 | 0.21 ± 0.01 |
| 6 | 7.34 ± 0.70 | 0.71 ± 0.04 | 1.03 ± 0.06 | 2.40 ± 0.42 | 0.55 ± 0.02 | 0.21 ± 0.17 |
| 7 | 5.25 ± 0.03 | 0.95 ± 0.02 | 0.75 ± 0.03 | 1.10 ± 0.51 | 0.53 ± 0.35 | 0.21 ± 0.00 |
| 8 | 10.24 ± 0.16 | 0.77 ± 0.04 | 0.95 ± 0.06 | 1.14 ± 0.14 | 0.63 ± 0.05 | 0.42 ± 0.01 |
| 9 i | 4.94 ± 0.26 | 0.68 ± 0.01 | 1.07 ± 0.02 | 0.61 ± 0.02 | 1.90 ± 0.12 | 0.20 ± 0.01 |
| 10 i | 3.69 ± 0.08 | 0.90 ± 0.02 | 0.80 ± 0.02 | 0.61 ± 0.25 | 1.94 ± 0.37 | 0.15 ± 0.00 |
| 11 i | 5.38 ± 0.13 | 0.97 ± 0.00 | 0.72 ± 0.00 | 0.66 ± 0.05 | 1.76 ± 0.01 | 0.22 ± 0.00 |
| 12 i | 5.02 ± 0.21 | 0.92 ± 0.03 | 0.77 ± 0.03 | 0.61 ± 0.06 | 1.89 ± 0.07 | 0.20 ± 0.01 |
| Mean j | 4.75 ± 0.17 | 0.87 ± 0.01 | 0.84 ± 0.02 | 0.62 ± 0.09 | 1.87 ± 0.14 | 0.19 ± 0.01 |
| Run | pH Decrease | S (gS L−1) a | P (gP L−1) b | qS (gS gX−1 h−1) c | qP (gP gX−1 h−1) d | YP/S (gP gS−1) e | QP (gP L−1 h−1) f |
|---|---|---|---|---|---|---|---|
| Control A g | 1.07 ± 0.04 | 8.08 ± 0.08 | 6.12 ± 0.33 | 0.09 ± 0.00 | 0.07 ± 0.00 | 0.76 ± 0.05 | 0.26 ± 0.01 |
| Control B h | 1.89 ± 0.03 | 11.44 ± 0.58 | 7.16 ± 0.58 | 0.11 ± 0.00 | 0.07 ± 0.01 | 0.64 ± 0.07 | 0.30 ± 0.02 |
| 1 | 0.39 ± 0.09 | 7.75 ± 2.18 | 4.84 ± 0.43 | 0.07 ± 0.02 | 0.04 ± 0.00 | 0.65 ± 0.15 | 0.20 ± 0.02 |
| 2 | 1.87 ± 0.02 | 15.00 ± 1.34 | 11.65 ± 0.47 | 0.09 ± 0.02 | 0.06 ± 0.01 | 0.71 ± 0.04 | 0.44 ± 0.02 |
| 3 | 0.21 ± 0.06 | 1.72 ± 0.20 | 1.14 ± 0.53 | 0.09 ± 0.03 | 0.06 ± 0.04 | 0.67 ± 0.35 | 0.048 ± 0.02 |
| 4 | 1.90 ± 0.06 | 2.32 ± 0.12 | 1.06 ± 0.84 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.46 ± 0.38 | 0.044 ± 0.04 |
| 5 | 0.64 ± 0.01 | 6.76 ± 1.67 | 5.01 ± 0.07 | 0.06 ± 0.06 | 0.04 ± 0.04 | 0.76 ± 0.13 | 0.21 ± 0.03 |
| 6 | 2.05 ± 0.03 | 2.98 ± 0.58 | 3.97 ± 0.45 | 0.01 ± 0.02 | 0.02 ± 0.02 | 1.35 ± 0.17 | 0.16 ± 0.02 |
| 7 | 0.30 ± 0.01 | 4.58 ± 1.77 | 2.69 ± 1.80 | 0.04 ± 0.01 | 0.02 ± 0.01 | 0.53 ± 0.03 | 0.11 ± 0.08 |
| 8 | 1.94 ± 0.01 | 8.82 ± 0.09 | 6.35 ± 0.50 | 0.04 ± 0.00 | 0.02 ± 0.00 | 0.73 ± 0.12 | 0.26 ± 0.02 |
| 9 i | 1.14 ± 0.03 | 7.90 ± 1.42 | 9.21 ± 0.28 | 0.01 ± 0.01 | 0.08 ± 0.00 | 0.59 ± 0.05 | 0.38 ± 0.01 |
| 10 i | 1.26 ± 0.02 | 6.06 ± 2.77 | 7.18 ± 1.33 | 0.07 ± 0.03 | 0.08 ± 0.01 | 1.27 ± 0.29 | 0.30 ± 0.06 |
| 11 i | 1.21 ± 0.11 | 7.90 ± 0.56 | 9.22 ± 0.21 | 0.06 ± 0.01 | 0.07 ± 0.00 | 1.17 ± 0.09 | 0.38 ± 0.01 |
| 12 i | 1.26 ± 0.04 | 7.93 ± 0.56 | 9.22 ± 0.21 | 0.07 ± 0.007 | 0.08 ± 0.003 | 1.15 ± 0.089 | 0.38 ± 0.009 |
| Mean j | 1.22 ± 0.05 | 7.45 ± 1.33 | 8.70 ± 0.50 | 0.05 ± 0.016 | 0.08 ± 0.006 | 1.04 ± 0.131 | 0.36 ± 0.02 |
| Time (h) | 0 | 3 | 4 | 6 | 9 | 12 | 15 | 18 | 21 | 24 |
|---|---|---|---|---|---|---|---|---|---|---|
| Biomass Concentration | ||||||||||
| PC1 | −0.020 | 0.088 | 0.122 | 0.170 | 0.198 | 0.171 | 0.172 | 0.166 | 0.140 | |
| PC2 | −0.388 | −0.061 | 0.060 | 0.077 | −0.063 | −0.055 | −0.062 | −0.033 | −0.044 | |
| PC3 | −0.073 | 0.302 | 0.230 | 0.054 | −0.044 | 0.022 | 0.099 | 0.192 | 0.224 | |
| Substrate concentration | ||||||||||
| PC1 | −0.103 | −0.188 | −0.199 | −0.199 | −0.199 | −0.183 | −0.181 | −0.132 | −0.177 | |
| PC2 | 0.198 | 0.022 | −0.055 | −0.071 | −0.089 | −0.122 | −0.177 | −0.122 | −0.202 | |
| PC3 | 0.297 | 0.166 | 0.123 | 0.101 | 0.073 | 0.073 | 0.022 | 0.111 | 0.098 | |
| Lactic acid concentration | ||||||||||
| PC1 | 0.144 | 0.177 | 0.191 | 0.202 | 0.199 | 0.177 | 0.163 | 0.161 | 0.166 | |
| PC2 | −0.270 | −0.211 | −0.033 | 0.024 | 0.095 | 0.200 | 0.223 | 0.233 | 0.254 | |
| PC3 | −0.155 | −0.077 | −0.044 | 0.022 | 0.093 | 0.140 | 0.073 | 0.054 | 0.099 | |
| pH | ||||||||||
| PC1 | 0.193 | 0.194 | 0.199 | 0.185 | 0.173 | 0.170 | 0.170 | 0.176 | 0.161 | |
| PC2 | −0.023 | −0.033 | −0.055 | −0.022 | −0.202 | −0.199 | −0.202 | −0.207 | −0.199 | |
| PC3 | 0.092 | −0.041 | −0.116 | −0.116 | −0.061 | −0.077 | −0.055 | 0.043 | 0.088 | |
| BLIS activity against E. faecium 101 | ||||||||||
| PC1 | −0.022 | −0.044 | ||||||||
| PC2 | 0.221 | −0.303 | ||||||||
| PC3 | −0.064 | 0.326 | ||||||||
| BLIS activity against E. coli | ||||||||||
| PC1 | 0.033 | 0.072 | ||||||||
| PC2 | −0.163 | 0.022 | ||||||||
| PC3 | 0.422 | 0.414 | ||||||||
| Response | Model | R2 | R2 Adj | R2 Pred |
|---|---|---|---|---|
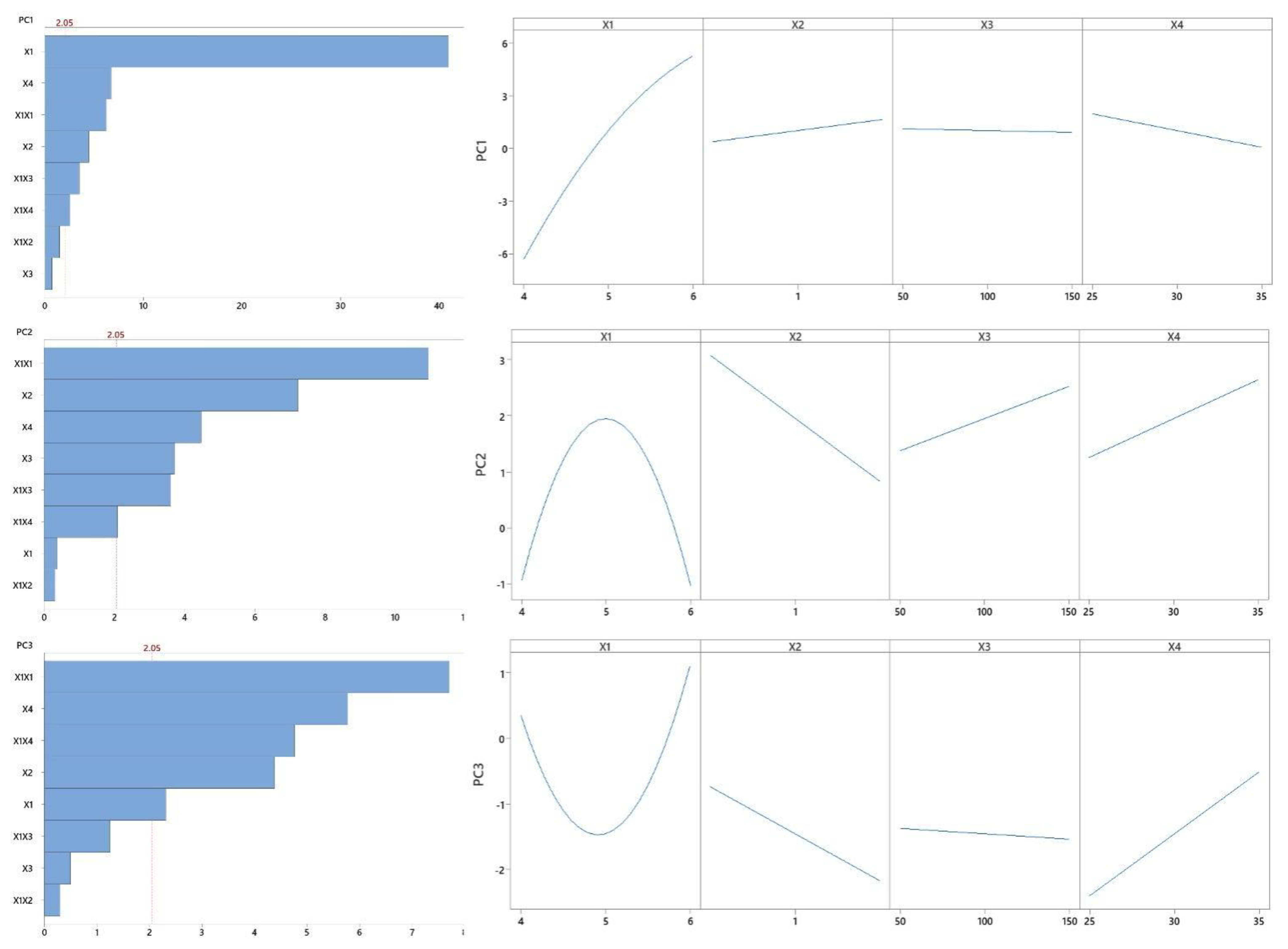
| PC1 | PC1 = −53.21 + 19.40 X1 − 0.87 X2 + 0.055 X3 − 0.554 X4 − 1.532 X12 + 0.433 X1X2 − 0.001 X1X3 + 0.073 X1X4 | 0.9852 | 0.9809 | 0.9794 |
| PC2 | PC2 = −70.32 + 28.46 X1 − 1.75 X2 + 0.077 X3 − 0.184 X4 − 2.932 X12 − 0.101 X1X2 − 0.011 X1X3 + 0.076 X1X4 | 0.8919 | 0.8599 | 0.8536 |
| PC3 | PC3 = 67.87 − 25.57 X1 − 0.94 X2 + 0.022 X3 − 0.606 X4 + 2.188 X12 − 0.101 X1X2 – 0.004 X1X3 + 0.166 X1X4 | 0.8402 | 0.7928 | 0.7582 |
| PC1 | PC2 | PC3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | df | SS | p | df | SS | p | df | SS | p |
| Model | 8 | 858.20 | 0.00 | 8 | 127.80 | 0.00 | 8 | 90.80 | 0.00 |
| Linear | 4 | 829.50 | 0.00 | 4 | 49.39 | 0.00 | 4 | 37.23 | 0.00 |
| pH | 1 | 797.90 | 0.00 | 1 | 0.08 | 0.72 | 1 | 3.43 | 0.03 |
| Polydextrose | 1 | 9.50 | 0.00 | 1 | 30.00 | 0.00 | 1 | 12.29 | 0.00 |
| Rotational speed | 1 | 0.61 | 0.47 | 1 | 7.87 | 0.00 | 1 | 0.16 | 0.62 |
| Temperature | 1 | 21.70 | 0.00 | 1 | 11.48 | 0.00 | 1 | 21.35 | 0.00 |
| Quadratic | 1 | 18.60 | 0.00 | 1 | 68.42 | 0.00 | 1 | 38.00 | 0.00 |
| pH × pH | 1 | 18.60 | 0.00 | 1 | 68.42 | 0.00 | 1 | 38.00 | 0.00 |
| Interactions | 3 | 10.20 | 0.00 | 3 | 10.01 | 0.00 | 3 | 15.57 | 0.00 |
| pH × Polydextrose | 1 | 1.09 | 0.14 | 1 | 0.06 | 0.76 | 1 | 0.06 | 0.77 |
| pH × R. speed | 1 | 5.89 | 0.00 | 1 | 7.45 | 0.00 | 1 | 1.00 | 0.22 |
| pH × Temperature | 1 | 3.17 | 0.02 | 1 | 2.50 | 0.05 | 1 | 14.51 | 0.00 |
| Error | 27 | 12.86 | 27 | 15.50 | 27 | 17.27 | |||
| Total | 35 | 871.1 | 35 | 143.3 | 35 | 108.1 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wanderley Porto, M.C.; de Souza de Azevedo, P.O.; Lourenço, F.R.; Converti, A.; Vitolo, M.; Oliveira, R.P.d.S. Effect of Polydextrose on the Growth of Pediococcus pentosaceus as Well as Lactic Acid and Bacteriocin-like Inhibitory Substances (BLIS) Production. Microorganisms 2022, 10, 1898. https://doi.org/10.3390/microorganisms10101898
Wanderley Porto MC, de Souza de Azevedo PO, Lourenço FR, Converti A, Vitolo M, Oliveira RPdS. Effect of Polydextrose on the Growth of Pediococcus pentosaceus as Well as Lactic Acid and Bacteriocin-like Inhibitory Substances (BLIS) Production. Microorganisms. 2022; 10(10):1898. https://doi.org/10.3390/microorganisms10101898
Chicago/Turabian StyleWanderley Porto, Maria Carolina, Pamela Oliveira de Souza de Azevedo, Felipe Rebello Lourenço, Attilio Converti, Michele Vitolo, and Ricardo Pinheiro de Souza Oliveira. 2022. "Effect of Polydextrose on the Growth of Pediococcus pentosaceus as Well as Lactic Acid and Bacteriocin-like Inhibitory Substances (BLIS) Production" Microorganisms 10, no. 10: 1898. https://doi.org/10.3390/microorganisms10101898
APA StyleWanderley Porto, M. C., de Souza de Azevedo, P. O., Lourenço, F. R., Converti, A., Vitolo, M., & Oliveira, R. P. d. S. (2022). Effect of Polydextrose on the Growth of Pediococcus pentosaceus as Well as Lactic Acid and Bacteriocin-like Inhibitory Substances (BLIS) Production. Microorganisms, 10(10), 1898. https://doi.org/10.3390/microorganisms10101898







